Abstract
Aim
The multicentre non-interventional AVANTI study assessed safety, effectiveness and patient-reported outcomes with approved first-line bevacizumab-containing regimens for HER2-negative locally recurrent/metastatic breast cancer (LR/MBC) in German routine oncology practice.
Methods
Eligible patients had HER2-negative LR/MBC, no bevacizumab contraindications and no prior chemotherapy for LR/MBC. Chemotherapy schedule, diagnostics and follow-up were at physicians’ discretion. Data were collected for 1 year after starting bevacizumab, then every 6 months for 1.5 years (maximum follow-up: 2.5 years). Patients and physicians rated treatment satisfaction. Subgroup analyses were prespecified in clinically relevant populations, including triple-negative breast cancer (TNBC).
Results
Between November 1, 2009 and April 30, 2016, 2065 eligible patients at 346 centres received bevacizumab with paclitaxel or capecitabine. Patients receiving bevacizumab–capecitabine were less likely to have de novo disease and more likely to have TNBC, age ≥60 years and prior anthracycline/taxane and/or endocrine therapy. Median PFS was 12.6 (95% CI 11.9–13.2) months (12.8 with bevacizumab–paclitaxel, 10.5 with bevacizumab–capecitabine); median OS was 23.9 (95% CI 22.2–25.1) months. Outcomes were worse in patients with TNBC, prior anthracycline/taxane or prior endocrine therapy. Grade ≥3 adverse events occurred in 27% of patients. Treatment was discontinued for adverse events in 15%. Treatment satisfaction was rated as good or better by 304/394 responding patients (77%) at week 54 and in 1393/2065 patients (67%) by physicians overall.
Conclusions
In routine clinical practice, effectiveness and safety of first-line bevacizumab-containing therapy for LR/MBC were consistent with experience from phase III trials. Patient and physician treatment satisfaction showed high concordance.
Keywords: Bevacizumab, Metastatic breast cancer, Non-interventional study
Highlights
-
•
AVANTI assessed 1st-line bevacizumab-based therapy for LR/MBC in routine practice.
-
•
Median progression-free and overall survival were 12.6 and 23.9 months respectively.
-
•
Treatment satisfaction was rated as good or better by 77% of patients at week 54.
-
•
Physician- and patient-rated treatment satisfaction showed high concordance.
-
•
Effectiveness and safety were consistent with experience from phase III trials.
1. Introduction
For many years, the standard first-line treatment for patients with HER2-negative metastatic breast cancer (MBC) has been chemotherapy, with or without the anti-angiogenic agent bevacizumab. In Europe, bevacizumab is approved in combination with either paclitaxel or capecitabine based on results from the randomised phase III E2100 [1] and RIBBON-1 [2] trials, respectively. Both demonstrated significantly improved progression-free survival (PFS) but not overall survival (OS) with the addition of bevacizumab to chemotherapy. The subsequent randomised phase III TURANDOT trial demonstrated non-inferior OS with bevacizumab plus capecitabine (BEV–CAP) versus bevacizumab plus paclitaxel (BEV–PAC) [3]. In clinical practice, the decision to use bevacizumab may depend on disease and patient characteristics, and many factors may influence the chemotherapy partner.
In Germany, bevacizumab is used routinely, based on European and German guidelines [[4], [5], [6], [7]]. We report final results from AVANTI, a non-interventional post-marketing surveillance study of bevacizumab combined with paclitaxel or capecitabine in patients with MBC.
2. Patients and methods
The single-arm multicentre non-interventional AVANTI study was designed to assess treatment decision making, selection criteria, safety, effectiveness, patient-reported outcomes (PROs) and treatment satisfaction in patients receiving first-line bevacizumab-containing therapy for HER2-negative MBC in routine oncology practice in Germany.
Eligible patients were female, aged ≥18 years, and eligible for first-line BEV–PAC or BEV–CAP for locally recurrent or metastatic breast cancer (LR/MBC). Patients with contraindications for bevacizumab according to the approved indication in Europe [8] were excluded. Patients were enrolled by oncologists and gynaecologists in clinics or outpatient clinics and by office-based physicians specialising in oncology. All patients provided written informed consent before study documentation began. The treatment regimen and schedule were chosen by treating physicians; patients were not randomised between BEV–PAC and BEV–CAP.
There was no specific primary objective; however, predefined questions of particular importance included: treatment selection criteria; safety (especially in elderly patients); response and time-related endpoints overall and in clinically relevant subgroups; treatment impact on PROs; and patient and physician treatment satisfaction. Patients were assessed at baseline and underwent regular detailed documentation for the first 12 months, followed by 6-monthly follow-up documentation for 1.5 years thereafter (according to German regulations). Data were captured in an electronic case report form. Adverse events (AEs) were coded using the latest version of the Medical Dictionary for Regulatory Activities (MedDRA). Investigators assessed response according to standard local practice. PFS was defined as the interval between first bevacizumab dose and disease progression or death from any cause, censoring patients who were alive without progression at the date of last follow-up or start of subsequent anti-neoplastic therapy, whichever was earlier. OS was defined as the interval between first bevacizumab dose and death from any cause; patients not known to have died by the data cut-off were censored at the date they were last known to be alive. PROs were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) at baseline and at weeks 9, 15, 33 and 54. Overall treatment satisfaction was evaluated by patients and physicians using a 5-point Likert-type questionnaire, completed at the same timepoints as PROs by patients and at the end of treatment by physicians. Patients also recorded their subjective experience of side effects at the end of the 12-month documentation period (or end of therapy if earlier) using a study-specific questionnaire.
When the study was initially designed, the target sample size was 3000 patients based on detection of rare serious AEs with BEV–PAC or bevacizumab plus docetaxel, which was an approved regimen when the study began. The analysis population for efficacy and safety included all patients who had received at least one bevacizumab dose, providing at least the initial dose was in accordance with the approved indication. Those who received ‘off-label’ bevacizumab-containing regimens from the outset were excluded. The BEV–PAC subpopulation was defined as all patients qualifying for analysis who received at least one dose of paclitaxel with bevacizumab. The BEV–CAP subpopulation was defined as all patients qualifying for analysis who received at least one dose of capecitabine with bevacizumab. Patients switching between these regimens were analysed in both subpopulations in subgroup analyses (but were not double counted in analyses of the overall population). Kaplan–Meier estimates and Cox regression were used to estimate PFS and OS. Change from baseline was calculated at each timepoint for PROs. Descriptive statistics were used for all baseline parameters and safety analyses. Predefined analyses included subgroups according to hypertension (hypertensive vs normotensive), triple-negative breast cancer (TNBC; yes vs no), age (<60 vs ≥ 60 years), number of metastatic sites (<3 vs ≥ 3), previous anthracycline/taxane (yes vs no), previous endocrine therapy (yes vs no) and ‘urgency to treat’ (defined as fulfilling at least three of the following: ≥3 metastatic sites, liver metastasis, prior [neo]adjuvant anthracycline/taxane, TNBC). There was no adjustment for multiple testing.
3. Results
3.1. Patient population and treatment decision making
Between November 1, 2009 and April 30, 2016, 2065 eligible patients enrolled at 346 centres in Germany received at least one dose of bevacizumab-containing therapy: BEV–PAC in 1821 patients (88%) and BEV–CAP in 295 patients (14%; recruitment starting July 1, 2011). Fifty-one patients (2%) switched between regimens and are thus analysed in both subpopulations. Appendix Fig. A1 shows reasons for exclusion from the analysis population. Treatment documentation was completed in 1996 (97%) out of 2065 patients. The most common reasons for ending documentation were tumour progression (766 patients; 37%), end of the documentation period (505; 24%), physician decision (290; 14%), patient's wish (200; 10%), death (183; 9%) and tumour remission (103; 5%).
Table 1 shows baseline characteristics overall and by selected chemotherapy. The BEV–CAP subpopulation included higher proportions of patients who were aged ≥60 years, had TNBC, and had received prior taxane/anthracycline and/or endocrine therapy, but a lower proportion with de novo MBC, emphasising the different patient populations enrolled in these two non-randomised groups. Treatment decisions were typically made by a tumour board (1294 patients; 63%) or an office-based oncologist (338 patients; 16%). The most commonly cited reasons for treatment choice were efficacy (65% overall, 67% for BEV–PAC vs 58% for BEV–CAP), guidelines (57% of patients overall; 58% vs 50% for BEV–PAC and BEV–CAP, respectively) and tolerability (41% overall and in both subpopulations). Previous therapy was more commonly cited as a reason for choosing BEV–CAP (40%) than BEV–PAC (30%).
Table 1.
Baseline characteristics overall and according to selected chemotherapy.
| Characteristic | All patients (n = 2065) | BEV–PAC (n = 1821) | BEV–CAP (n = 295) |
|---|---|---|---|
| Median age, years (range) | 60 (24–87) | 60 (24–86) | 61 (29–87) |
| Age ≥60 years, n (%) | 1019 (49) | 891 (49) | 158 (54) |
| TNBC, n (%) | 425 (21) | 363 (20) | 74 (25) |
| De novo metastatic breast cancer, n (%) | 609 (29) | 570 (31) | 48 (16) |
| ≥3 metastatic sites, n (%) | 414 (20) | 380 (21) | 47 (16) |
| Visceral metastases | 1548 (75) | 1384 (76) | 211 (72) |
| ECOG performance status, n (%) | |||
| 0 | 953 (46) | 840 (46) | 139 (47) |
| 1 | 817 (40) | 725 (40) | 108 (37) |
| 2 | 132 (6) | 124 (7) | 12 (4) |
| 3 | 10 (<1) | 9 (<1) | 1 (<1) |
| 4 | 4 (<1) | 2 (<1) | 2 (1) |
| Missing | 149 (7) | 121 (7) | 33 (11) |
| Prior (neo)adjuvant chemotherapy, n (%) | 1147 (56) | 976 (54) | 202 (68) |
| Taxane | 671 (32) | 536 (29) | 147 (50) |
| Anthracycline | 1008 (49) | 856 (47) | 179 (61) |
| Prior endocrine therapy | |||
| Adjuvant setting | 752 (36) | 649 (36) | 126 (43) |
| Metastatic setting | 364 (18) | 312 (17) | 61 (21) |
BEV–CAP, bevacizumab plus capecitabine; BEV–PAC, bevacizumab plus paclitaxel; ECOG, Eastern Cooperative Oncology Group; TNBC, triple-negative breast cancer. Patients who received at least one dose of BEV–CAP and at least one dose of BEV–PAC (switched between regimens) were analysed in both subpopulations; consequently, the sum of these two subgroups is larger than the total number of patients.
3.2. Treatment exposure
At the data cut-off (February 3, 2020), the median bevacizumab duration was 6.0 months (95% confidence interval [CI] 5.6–6.3 months) and the median chemotherapy duration was 4.2 months (95% CI 4.0–4.2 months). There was no difference in chemotherapy exposure according to chemotherapy partner; median bevacizumab duration was 6.2 months (95% CI 5.8–6.7 months) in the BEV–PAC subpopulation and 5.6 months (95% CI 5.1–6.6 months) in the BEV–CAP subpopulation. Some patients received maintenance therapy with bevacizumab (25%), capecitabine (10% of the BEV–CAP subpopulation, 2% of the BEV–PAC subpopulation) or endocrine therapy.
The most commonly administered second-line therapies following disease progression were eribulin (10%; 9% after BEV–PAC vs 14% after BEV–CAP), capecitabine (9%; 10% vs 4%, respectively), doxorubicin (9%) and vinorelbine (9%) (Appendix Fig. A2).
3.3. Effectiveness
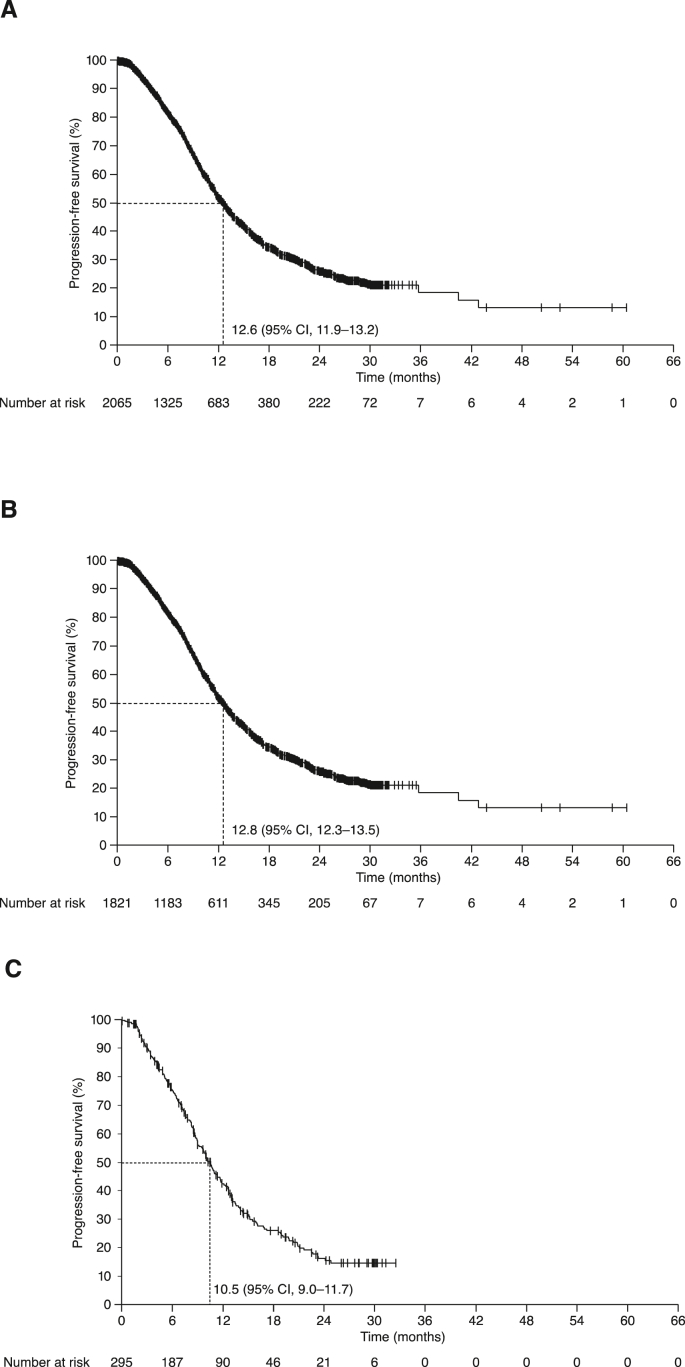
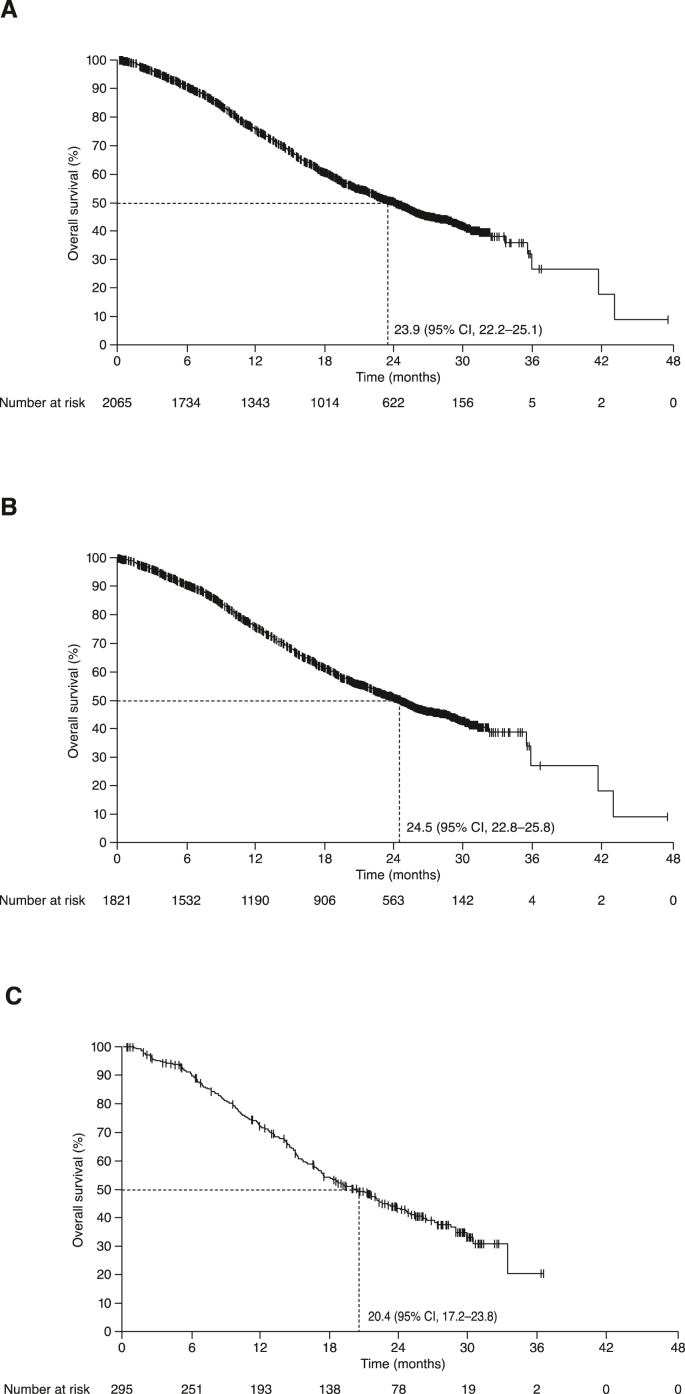
The overall response rate (ORR) was 49% (95% CI 47–51%) overall, including complete responses in 6% of patients. ORRs were 51% (95% CI 48–53%) with BEV–PAC and 39% (95% CI 34–45%) with BEV–CAP. At the data cut-off, PFS events had been recorded in 53% of patients (51% vs 65% in the BEV–PAC and BEV–CAP subpopulations, respectively). Median PFS was 12.6 (95% CI 11.9–13.2) months in the overall population, 12.8 months with BEV–PAC and 10.5 months with BEV–CAP (Fig. 1). Median OS after events in 48% of patients was 23.9 (95% CI 22.2–25.1) months overall, 24.5 months with BEV–PAC and 20.4 months with BEV–CAP (Fig. 2).
Fig. 1.
Progression-free survival in: (A) All patients; (B) BEV–PAC subpopulation; and (C) BEV–CAP subpopulation. BEV–CAP, bevacizumab plus capecitabine; BEV–PAC, bevacizumab plus paclitaxel; CI, confidence interval.
Fig. 2.
Overall survival in: (A) All patients; (B) BEV–PAC subpopulation; and (C) BEV–CAP subpopulation. BEV–CAP, bevacizumab plus capecitabine; BEV–PAC, bevacizumab plus paclitaxel; CI, confidence interval.
Table 2 summarises PFS and OS in clinically relevant subgroups. Generally, prognostic effects observed in the overall population were replicated in the BEV–PAC and BEV–CAP subpopulations. However, subgroup analyses according to age showed marked differences, with apparently more favourable outcomes in patients aged ≥60 than <60 years receiving BEV–CAP (Appendix Fig. A3). Conversely, the apparently worse prognosis among patients with versus without prior endocrine therapy was driven almost entirely by the BEV–PAC subpopulation (Appendix Fig. A3).
Table 2.
Overview of effectiveness by subgroup.
| Subgroup | PFS |
OS |
|||
|---|---|---|---|---|---|
| Median, months | Hazard ratio (95% CI) | Median, months | Hazard ratio (95% CI) | ||
| Baseline hypertension | Hypertensivea vs normotensive | 13.6 vs 11.9 | 0.88 (0.77–1.00) | 25.1 vs 23.2 | 0.88 (0.76–1.01) |
| TNBCb | Yes vs no | 10.3 vs 12.9 | 1.44 (1.24–1.67) | 16.8 vs 25.2 | 1.53 (1.30–1.80) |
| Age, years | ≥60 vs < 60 | 12.8 vs 12.3 | 1.09 (0.96–1.23) | 21.9 vs 25.4 | 1.26 (1.11–1.44) |
| Metastatic sites | ≥3 vs < 3 | 11.6 vs 12.8 | 1.06 (0.88–1.28) | 19.3 vs 24.9 | 1.15 (0.96–1.39) |
| Prior anthracycline/taxane | Yes vs no | 11.5 vs 14.3 | 1.32 (1.16–1.50) | 20.8 vs 27.4 | 1.25 (1.09–1.43) |
| Prior ET | Yes vs no | 10.7 vs 13.2 | 1.56 (1.33–1.82) | 17.6 vs 25.1 | 1.56 (1.33–1.82) |
| Urgency to treatc | Yes vs no | 9.9 vs 12.9 | 1.38 (1.09–1.74) | 14.8 vs 25.0 | 1.38 (1.09–1.75) |
CI, confidence interval; ET, endocrine therapy; OS, overall survival; PFS, progression-free survival; TNBC, triple-negative breast cancer.
Defined as: documented pre-existing arterial hypertension; documented blood pressure >150/100 mmHg at baseline; at least one anti-hypertensive drug with indication hypertension and documented start date before the first dose of bevacizumab; or documented ongoing hypertension in medical history screening with start date before the first date of bevacizumab.
Unknown in 127 patients.
Fulfilling at least three of the following: ≥3 metastatic sites, liver metastasis, prior [neo]adjuvant anthracycline/taxane therapy, TNBC.
3.4. PROs and treatment satisfaction
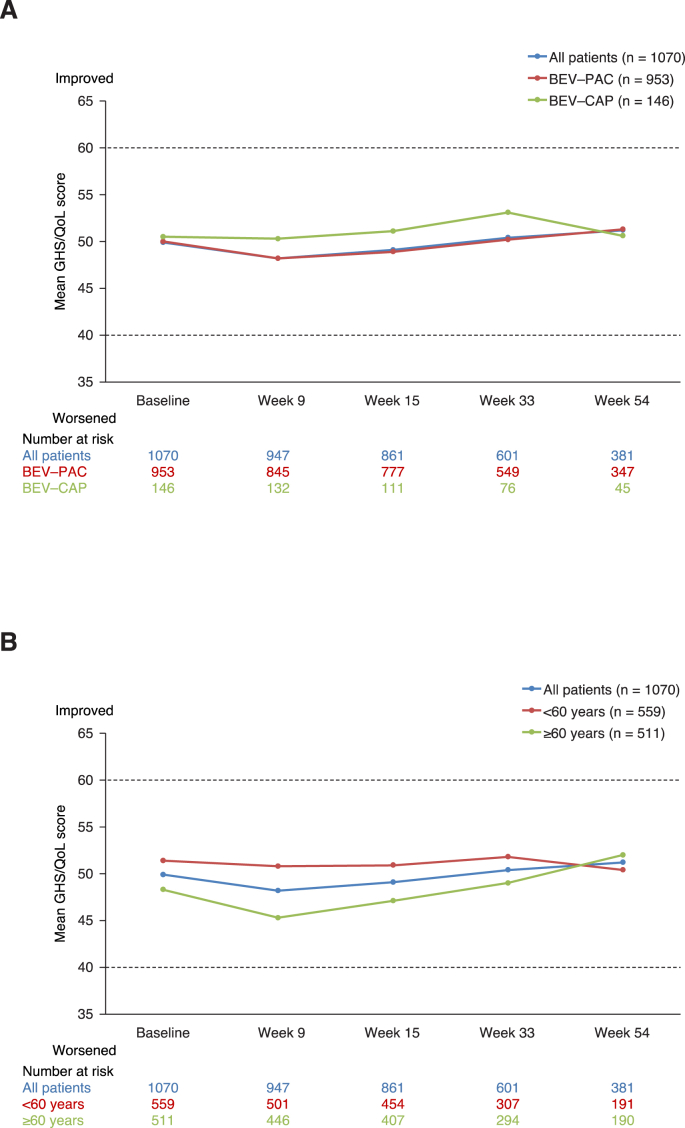
Approximately 90% of patients completed at least one question of QLQ-C30 at baseline. Mean global health status/quality of life (GHS/QoL) scores were higher (representing better GHS/QoL) at baseline in patients aged <60 than ≥60 years, irrespective of chemotherapy partner. Compliance with questionnaire completion diminished only slightly over time: among those who received a questionnaire at week 54, 74% completed at least one question. Mean change from baseline GHS/QoL showed no relevant change in the overall population, nor in subgroups according to chemotherapy or age (Fig. 3). Similar patterns were seen for functioning and symptom subscales, except for fatigue, which increased substantially from baseline to week 9, but decreased thereafter (data not shown).
Fig. 3.
Patient-reported global health status/quality of life (GHS/QoL) over time: (A) By chemotherapy partner and (B) By age. BEV–CAP, bevacizumab plus capecitabine; BEV–PAC, bevacizumab plus paclitaxel.
Patient-reported experience of side effects and interference with daily activities showed little difference according to chemotherapy partner or age. At week 54, most patients (78%) completing therapy reported “some impairment” to daily life overall but very few (4%) reported strong impairment (Appendix Fig. A4). A similar pattern was seen among those discontinuing treatment prematurely.
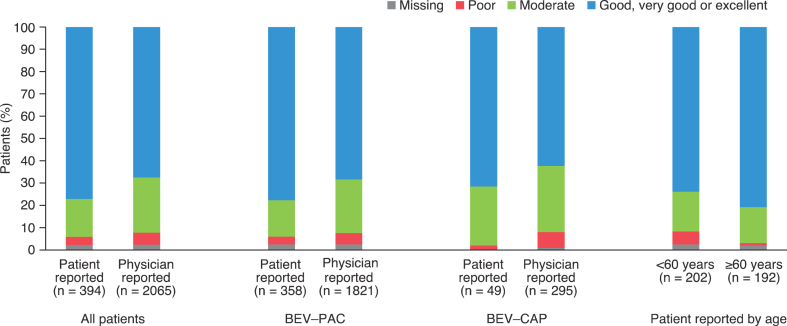
Most patients reported treatment satisfaction as good or better. Physician-reported treatment satisfaction was similar or slightly lower than patient-reported treatment satisfaction (Fig. 4).
Fig. 4.
Treatment satisfaction reported by patients at week 54 (n = 394) and (for all 2065 patients) reported by physicians at end of treatment. BEV–CAP, bevacizumab plus capecitabine; BEV–PAC, bevacizumab plus paclitaxel.
3.5. Safety
AEs of any grade were reported in 59% of patients (grade ≥3 in 27%, including grade 5 in 5%). Generally, incidences were slightly higher in older than younger patients, and with BEV–CAP (which included a higher proportion of older patients) versus BEV–PAC (Table 3). AEs recorded as fatal included a substantial proportion related to disease progression, and the 16 fatal AEs described by investigators as bevacizumab related included eight described as disease progression or comorbidities (Appendix Table A1).
Table 3.
Overview of safety overall and in subgroups according to selected chemotherapy and age.
| AE, n (%) | All patients (n = 2065) | BEV–PAC (n = 1821) | BEV–CAP (n = 295) | <60 years (n = 1046) | ≥60 years (n = 1019) |
|---|---|---|---|---|---|
| Any grade AE | 1214 (59) | 1055 (58) | 195 (66) | 585 (56) | 629 (62) |
| Bevacizumab related | 625 (30) | 558 (31) | 92 (31) | 304 (29) | 321 (32) |
| Grade ≥3 AE | 549 (27) | 480 (26) | 94 (32) | 247 (24) | 302 (30) |
| Fatal AEa | 111 (5) | 101 (6) | 12 (4) | 40 (4) | 71 (7) |
| Bevacizumab relatedb | 16 (1) | 14 (1) | 2 (1) | 5 (<1) | 11 (1) |
| AE leading to treatment discontinuation | 310 (15) | 272 (15) | 44 (15) | 132 (13) | 178 (17) |
AE, adverse event; BEV–CAP, bevacizumab plus capecitabine; BEV–PAC, bevacizumab plus paclitaxel. Patients who received at least one dose of BEV–CAP and at least one dose of BEV–PAC (switched between regimens) were analysed in both subpopulations; consequently, the sum of these two subgroups is larger than the total number of patients.
Documented cause of death: disease progression (n = 62), unknown (n = 24), comorbidity (n = 5), treatment associated (n = 2), other (n = 11), missing (n = 7). Fatal AEs: including unexplained death (n = 27), general physical health deterioration (n = 23), disease progression/metastases (n = 22).
Documented cause of death: disease progression (n = 6), comorbidity (n = 2), treatment associated (n = 1), unknown (n = 2), other (n = 4), missing (n = 1). Further details provided in Appendix Table A1.
Consistent with the known safety profile of bevacizumab, the most common all-grade AEs were hypertension, fatigue and polyneuropathy (Table 4). Proteinuria was reported in 2% of patients (grade 3 in eight patients [0.4%]; no grade 4). Palmar-plantar erythrodysaesthesia, diarrhoea and mucosal inflammation were more common with BEV–CAP, whereas fatigue, leucopenia and alopecia were less common. The only grade ≥3 AEs in ≥2% of patients were leucopenia, general physical health deterioration and hypertension (each in 2%). Prespecified subgroup analyses comparing hypertension in patients with versus without pre-existing hypertension at baseline showed marginally lower incidences in patients without pre-existing hypertension (132/1358 patients [10%] vs 109/707 patients [15%] with pre-existing hypertension). Within these subgroups, there were no differences in the incidence of hypertension according to age <60 versus ≥60 years or bevacizumab dose <2.5 versus 2.5–<5 versus ≥5 mg/kg/week. Similarly, there was no difference in the incidence of proteinuria according to age or bevacizumab dose.
Table 4.
Most common adverse events (any grade in ≥5% of any population; grade ≥3 in ≥1% of any population).
| Adverse event, n (%) | All patients (n = 2065) |
BEV–PAC (n = 1821) |
BEV–CAP (n = 295) |
|||
|---|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Hypertension | 241 (12) | 43 (2) | 214 (12) | 39 (2) | 36 (12) | 9 (3) |
| Fatigue | 210 (10) | 13 (0.6) | 195 (11) | 13 (0.7) | 22 (7) | 1 (0.3) |
| Polyneuropathy | 177 (9) | 15 (0.7) | 169 (9) | 15 (0.8) | 20 (7) | 2 (0.7) |
| Nausea | 145 (7) | 7 (0.3) | 120 (7) | 6 (0.3) | 29 (10) | 1 (0.3) |
| Leucopenia | 138 (7) | 51 (2) | 129 (7) | 48 (3) | 10 (3) | 4 (1) |
| Diarrhoea | 129 (6) | 14 (0.7) | 101 (6) | 11 (0.6) | 33 (11) | 3 (1) |
| Epistaxis | 109 (5) | 3 (0.1) | 103 (6) | 3 (0.2) | 10 (3) | 0 |
| Alopecia | 95 (5) | – | 94 (5) | – | 5 (2) | – |
| Palmar-plantar erythrodysaesthesia | 91 (4) | 14 (0.7) | 30 (2) | 3 (0.2) | 68 (23) | 13 (4) |
| Mucosal inflammation | 83 (4) | 8 (0.4) | 63 (3) | 3 (0.2) | 25 (8) | 5 (2) |
| Anaemia | 81 (4) | 19 (0.9) | 75 (4) | 16 (0.9) | 6 (2) | 3 (1) |
| General physical health deterioration | 67 (3) | 44 (2) | 60 (3) | 39 (2) | 8 (3) | 6 (2) |
| Neutropenia | 38 (2) | 24 (1) | 36 (2) | 23 (1) | 2 (1) | 1 (0.3) |
| Urinary tract infection | 36 (2) | 14 (0.7) | 30 (2) | 11 (0.6) | 8 (3) | 4 (1) |
| Pulmonary embolism | 34 (2) | 28 (1) | 31 (2) | 25 (1) | 5 (2) | 5 (2) |
| Back pain | 32 (2) | 5 (0.2) | 28 (2) | 3 (0.2) | 5 (2) | 3 (1) |
| Malignant neoplasm progression | 29 (1) | 29 (1) | 27 (1) | 27 (1) | 3 (1) | 3 (1) |
| Unexplained deatha | 28 (1) | 24 (1) | 24 (1) | 21 (1) | 4 (1) | 3 (1) |
| Aspartate aminotransferase increased | 25 (1) | 9 (0.4) | 20 (1) | 6 (0.3) | 5 (2) | 3 (1) |
| Thrombocytopenia | 24 (1) | 8 (0.4) | 18 (1) | 6 (0.3) | 7 (2) | 3 (1) |
| Pleural effusion | 23 (1) | 10 (0.5) | 17 (0.9) | 8 (0.4) | 7 (2) | 3 (1) |
BEV–CAP, bevacizumab plus capecitabine; BEV–PAC, bevacizumab plus paclitaxel. Patients who received at least one dose of BEV–CAP and at least one dose of BEV–PAC (switched between regimens) were analysed in both subpopulations; consequently, the sum of these two subgroups is larger than the total number of patients.
Grade missing in 3 patients in the BEV–PAC subpopulation and recorded as grade 2 in error in 1 patient in the BEV–CAP subpopulation; these patients are not counted as grade ≥3.
4. Discussion
In this non-interventional study in routine oncology practice in Germany, PFS and OS are consistent with results from numerous phase III trials (E2100, MERiDiAN, TURANDOT, CALGB 40502/NCCTG N063H, RIBBON-1, CARIN), which consistently reported median PFS of 11.0–11.4 months with BEV–PAC [[8], [9], [10], [11]] and 8.1–8.8 months with BEV–CAP [2,10,12] and median OS of 26.5–29.5 months [3,8,11,13] and 25.1–29.0 months, respectively [3,12,14] (Appendix Table A2). OS in AVANTI is more difficult to interpret given the relatively short follow-up (maximum 2.5 years), which biases towards early deaths in higher-risk patients, and the varied subsequent therapy, with predictable imbalances in capecitabine and taxane use. However, the feasibility of a broad range of available treatment options following progression on first-line bevacizumab-containing therapy is noteworthy.
AEs with both regimens were generally consistent with previous clinical trial experience, the well-established safety profile of bevacizumab-containing therapy for LR/MBC, and known paclitaxel and capecitabine side effects [[1], [2], [3],[8], [9], [10]]. Interestingly, patient-reported treatment satisfaction was at least as positive as physician-reported treatment satisfaction, although only a fraction of patients completed treatment satisfaction questionnaires, potentially leading to some bias.
A limitation of the trial is its single-arm design and non-standardised response assessment (according to local practice rather than Response Evaluation Criteria in Solid Tumours), which could affect both PFS and ORR evaluation. Additionally, there was extensive censoring for PFS in the first 6 months. Comparing effectiveness of BEV–PAC and BEV–CAP is challenging because of the different characteristics of these two non-randomised subpopulations. Chemotherapy choice was at the treating physician's discretion, and prior therapy was a clear contributor to treatment selection. BEV–CAP was selected more often in patients previously treated with anthracycline/taxane or endocrine therapy, and less often in those with de novo MBC. Imbalances in the patient population may also contribute to somewhat counterintuitive findings with regard to prognostic factors. For example, among patients receiving BEV–PAC, those previously treated with endocrine therapy appeared to have worse PFS and OS than those without prior endocrine therapy. However, as PFS and OS are calculated from the first dose of bevacizumab, those in the prior endocrine therapy subgroup could have received multiple lines of prior endocrine therapy between diagnosis of LR/MBC and entry into the AVANTI study at the time of chemotherapy eligibility, whereas in endocrine therapy-naïve patients, PFS is essentially calculated from their first diagnosis of LR/MBC. Similarly, patient selection and physician bias may have contributed to the apparently more favourable outcomes in patients aged ≥60 versus <60 years in the BEV–CAP subpopulation. It is plausible that in the BEV–CAP subpopulation, only relatively fit older patients with more indolent disease or perhaps a preference for oral chemotherapy were enrolled, whereas younger patients treated with BEV–CAP were perhaps frailer and more heavily pretreated.
AE reporting may represent another potential limitation. Non-interventional studies may be more susceptible to under-reporting of AEs, and reports that may be queried in more rigorously monitored prospective studies may not be queried in a non-interventional study. For example, a number of cases described as bevacizumab-related fatal AEs were also attributable to disease progression or comorbidities (Appendix Table A1), thus the true incidence of treatment-related fatal AEs may be <0.5%. As with the effectiveness comparisons, imbalances between the BEV–PAC and BEV–CAP populations could lead to misperceptions of safety. For example, superficially AEs appear to be more common with BEV–CAP, but this subpopulation is over-represented by older patients, who were also at increased risk of AEs.
Our data provide only limited information on subsequent therapy, partly because of the constraints of a non-interventional study. Detailed documentation is permitted for a maximum of 15 months, making it difficult to capture patterns of subsequent therapy, especially with median PFS approaching the maximum follow-up allowed. In addition, switch maintenance therapy is not approved in Germany (or indeed anywhere in Europe), so while the strategy of switching from bevacizumab plus a taxane to BEV–CAP after an induction period demonstrated a statistically significant OS benefit in the IMELDA trial [15], such an approach is used less often than may be expected in clinical practice because of regulatory and funding challenges.
A strength of the study is its real-world patient population, providing insight into everyday oncology practice. In addition, inclusion of PROs allowed assessment of quality of life over time, which did not deteriorate meaningfully either overall or in the subgroups defined by age or chemotherapy partner. These data complement real-world data from the ESME project, which used data recorded from patients receiving first-line therapy between 2008 and 2013 in French routine practice and elegant statistical methods to compare BEV–PAC with paclitaxel alone. In the ESME database, median PFS in 2127 patients treated with BEV–PAC for HER2-negative MBC was 8.1 months and median OS was 27.7 months [16].
In conclusion, in routine practice, BEV–PAC and BEV–CAP remain valid first-line treatment options for HER2-negative LR/MBC. Treatment options continue to evolve, particularly in the settings of hormone receptor-positive disease before initiation of chemotherapy and in PD-L1-positive or BRCA-mutated LR/MBC, but for the many patients not eligible for biomarker-selected therapies, bevacizumab-containing regimens remain an effective and tolerable therapy, irrespective of age.
Role of the funding source
The sponsor was involved in the design and conduct of the study; data collection, management, analysis, and interpretation; and preparation, review, and approval of the manuscript.
Author contributions
Volkmar Müller: Conceptualisation, investigation, resources, visualisation, writing – original draft, writing – review & editing. Markus Ruhnke: Investigation, resources, visualisation, writing – review & editing. Oliver Hoffmann: Investigation, resources, writing – review & editing. Andrea Grafe: Investigation, resources, writing – review & editing. Oliver Tomé: Investigation, resources, writing – review & editing. Werner Fett: Investigation, resources, writing – review & editing. Harald-Robert Bruch: Investigation, resources, writing – review & editing. Ann-Katrin Sommer: Writing – review & editing. Andreas Schneeweiss: Conceptualisation, investigation, resources, visualisation, writing – original draft, writing – review & editing.
Declaration of interest statement
V.M. reports honoraria from Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva and Seattle Genetics (for advisory boards) and from Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Tesaro, Seattle Genetics and Nektar (for consultancy); research funding to his institution from Novartis, Roche, Seattle Genetics and Genentech; and travel grants from Roche, Pfizer and Daiichi-Sankyo. O.H. reports honoraria from Roche, Novartis, Pfizer, MSD, Daiichi-Sankyo, AstraZeneca, Eisai, Hexal, Amgen and Riemser Pharma; advisory/consultancy roles for Roche, Novartis, MSD, Daiichi-Sankyo and Hexal; and travel/accommodation/expenses from Novartis, Pfizer, MSD, Daiichi-Sankyo, Eisai, Hexal and Amgen. A.-K.S.-J. is an employee of Roche Pharma AG and holds shares in Roche. A.S. reports honoraria from Roche, AstraZeneca, Celgene, Pfizer, Novartis, MSD, Tesaro and Lilly; expert testimony for Roche and AstraZeneca; research funding to his institution from Roche, Celgene and AbbVie; and travel/accommodation/expenses from Roche, Celgene and Pfizer. The remaining authors declare no conflicts of interest.
Acknowledgements
We are grateful to the patients participating in the study and their families, the investigators and staff at participating centres and the study team at Roche Pharma AG. This study was sponsored and funded by Roche Pharma AG, Grenzach-Wyhlen, Germany. Medical writing support was provided by Jennifer Kelly, MA (Medi-Kelsey Ltd, Ashbourne, UK), funded by Roche Pharma AG.
Footnotes
Sources of support: The AVANTI study and medical writing for this publication were funded by Roche Pharma AG, Grenzach-Wyhlen, Germany.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.08.014.
Contributor Information
Volkmar Müller, Email: v.mueller@uke.de.
Markus Ruhnke, Email: markus.ruhnke@helios-gesundheit.de.
Oliver Hoffmann, Email: oliver.hoffmann@uk-essen.de.
Andrea Grafe, Email: andrea.grafe@shk-ndh.de.
Oliver Tomé, Email: oliver.tome@vincentius-ka.de.
Werner Fett, Email: fett-onkologe@t-online.de.
Harald-Robert Bruch, Email: DRHRBRUCH@t-online.de.
Ann-Katrin Sommer-Joos, Email: ann-katrin.sommer@roche.com.
Andreas Schneeweiss, Email: Andreas.Schneeweiss@med.uni-heidelberg.de.
Data statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Miller K., Wang M., Gralow J., Dickler M., Cobleigh M., Perez E.A. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 2.Robert N.J., Diéras V., Glaspy J., Brufsky A.M., Bondarenko I., Lipatov O.N. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 3.Zielinski C., Láng I., Inbar M., Kahán Z., Greil R., Beslija S. TURANDOT investigators. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): primary endpoint results of a randomised, open-label, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:1230–1239. doi: 10.1016/S1470-2045(16)30154-1. [DOI] [PubMed] [Google Scholar]
- 4.Wöckel A., Festl J., Stüber T., Brust K., Krockenberger M., Heuschmann P.U. Interdisciplinary screening, diagnosis, therapy and follow-up of breast cancer. Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017) - Part 2 with recommendations for the therapy of primary, recurrent and advanced breast cancer. Geburtshilfe Frauenheilkd. 2018;78:1056–1088. doi: 10.1055/a-0646-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomssen C., Lüftner D., Untch M., Haidinger R., Würstlein R., Harbeck N. International consensus conference for advanced breast cancer, Lisbon 2019: ABC5 consensus - assessment by a German group of experts. Breast Care. 2020;15:82–95. doi: 10.1159/000505957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso F., Senkus E., Costa A., Papadopoulos E., Aapro M., André F. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onkopedia guidelines: breast cancer in women. https://www.onkopedia.com/de/onkopedia/guidelines/mammakarzinom-der-frau/@@guideline/html/index.html
- 8.Roche Pharma A.G. 2015. Avastin summary of product characteristics.https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf accessed. [Google Scholar]
- 9.Miles D., Cameron D., Bondarenko I., Manzyuk L., Alcedo J.C., Lopez R.I. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146–155. doi: 10.1016/j.ejca.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Lang I., Brodowicz T., Ryvo L., Kahan Z., Greil R., Beslija S. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer: interim efficacy results of the randomised, open-label, non-inferiority, phase 3 TURANDOT trial. Lancet Oncol. 2013;14:125–133. doi: 10.1016/S1470-2045(12)70566-1. [DOI] [PubMed] [Google Scholar]
- 11.Rugo H.S., Barry W.T., Moreno-Aspitia A., Lyss A.P., Cirrincione C., Leung E. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance) J Clin Oncol. 2015;33:2361–2369. doi: 10.1200/JCO.2014.59.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welt A., Marschner N., Lerchenmueller C., Decker T., Steffens C.C., Koehler A. Capecitabine and bevacizumab with or without vinorelbine in first-line treatment of HER2/neu-negative metastatic or locally advanced breast cancer: final efficacy and safety data of the randomised, open-label superiority phase 3 CARIN trial. Breast Cancer Res Treat. 2016;156:97–107. doi: 10.1007/s10549-016-3727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miles D., Cameron D., Hilton M., Garcia J., O’Shaughnessy J. Overall survival in MERiDiAN, a double-blind placebo-controlled randomised phase III trial evaluating first-line bevacizumab plus paclitaxel for HER2-negative metastatic breast cancer. Eur J Cancer. 2018;90:153–155. doi: 10.1016/j.ejca.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Robert N.J., Dieras V., Glaspy J., Brufsky A., Bondarenko I., Lipatov O. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) J Clin Oncol. 2009;27(suppl 15) doi: 10.1200/JCO.2010.28.0982. Abstract 1005. [DOI] [PubMed] [Google Scholar]
- 15.Gligorov J., Doval D., Bines J., Alba E., Cortes P., Pierga J.Y. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1351–1360. doi: 10.1016/S1470-2045(14)70444-9. [DOI] [PubMed] [Google Scholar]
- 16.Delaloge S., Pérol D., Courtinard C., Brain E., Asselain B., Bachelot T. Paclitaxel plus bevacizumab or paclitaxel as first-line treatment for HER2-negative metastatic breast cancer in a multicenter national observational study. Ann Oncol. 2016;27:1725–1732. doi: 10.1093/annonc/mdw260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.