Abstract
Objective
To investigate the changes in serum growth hormone (GH), testosterone, and insulin-like growth factor 1 (IGF-1) during low-intensity resistance exercise under different cuff pressures.
Methods
We performed a single-blind, cross-over design study. Twenty-five healthy young men performed three exercise protocols as follows: 1) no blood flow restriction exercise (control group), 2) resistance exercise at 40% of arterial occlusion pressure (AOP) (low group), and 3) resistance exercise at 70% of AOP (high group). Blood lactate, GH, testosterone, and IGF-1 levels were measured at four time points.
Results
There were no differences in the indices before exercise. The blood flow restriction exercise under different pressures had different effects on each index and there was an interactive effect. GH levels were significantly higher in the high group than in the other groups after exercise. Immediately after exercise, IGF-1 and testosterone levels were significantly higher in the high group than in the other groups. At 15 minutes after exercise, testosterone levels were significantly higher in the high group than in the other groups.
Conclusions
Low-intensity resistance exercise combined with blood flow restriction effectively increases GH, IGF-1, and testosterone levels in young men. Increasing the cuff pressure results in greater levels of hormone secretion.
Keywords: Blood flow restriction, resistance exercise, cuff pressure, myogenic hormone, testosterone, insulin-like growth factor 1
Introduction
Blood flow restriction exercise is also known as compression exercise or “KAATSU”. This exercise is a training method that blocks part of the arterial blood flow for a certain period and allows exercise at a lower intensity, leading to stimulation of muscle growth and improved exercise function.1 The obstruction of venous blood flow during exercise causes a large number of metabolites to accumulate in the body.2 This accumulation results in severely stimulated receptors of the exercise system and the body, which leads to metabolic stress and an increase in accumulation of lactic acid.3 An increase in blood lactic acid concentrations may cause promotion of anabolic hormone secretion, such as growth hormone (GH), testosterone, and insulin-like growth factor 1 (IGF-1).4,5
Previous studies have shown that a reduction in blood flow volume by 40% to 70% using cuff pressure results in a similar blood flow restriction.6 Whether a higher cuff pressure can increase anabolic hormone levels by increasing lactic acid accumulation is unclear. The secretion of anabolic hormones is closely related to muscle function and muscle adaptation.6,7 Therefore, how the application of different cuff pressures in low-load resistance exercises affects GH and other hormones that are closely related to muscle synthesis is an important issue.
This study aimed to investigate the changes in serum GH, testosterone, and other synthetic hormone indicators that are closely related to muscle adaptation during low-intensity resistance exercises with different cuff pressures in young men. Our findings may provide coaches and researchers with a more scientific exercise plan and a theoretical basis for determining what degree of blood flow restriction to use.
Methods
Participants
Twenty-five healthy male college students, with no systematic training experience, except for a daily physical education class, participated in this study. Individuals with cardiopulmonary diseases and severe chronic diseases were excluded. Each participant was required to fill out the Physical Activity Readiness Questionnaire, and if the answer to all questions was “no”, they were allowed to participate in the research. The experiments were undertaken with the understanding and written consent of each participant. The study conformed with the Code of Ethics of the World Medical Association (Declaration of Helsinki), printed in the British Medical Journal (18 July 1964). This study was approved by the Ethics Committee of Zhengzhou Shengda University (approval number: SEC202034).
Study design
We performed a single-blind, cross-over design study. To improve the accuracy of the test, the participants performed a pre-test before the formal test, and practiced each movement five times. Before formal exercise, each participant was required to perform one repetition maximum (1-RM) test, then three protocols were randomly conducted by drawing lots. The three protocols were as follows: 1) resistance exercise without blood flow restriction (control group); (2) resistance exercise at 40% of arterial occlusion pressure (AOP) (low group); and (3) resistance exercise at 70% of AOP (high group). Each test was separated by 72 hours. To avoid the effect of the biological rhythm on hormones, a rhythmic test was carried out from 2:00 pm to 4:30 pm. Elbow vein blood was collected before exercise, immediately after exercise, 15 minutes after exercise, and 30 minutes after exercise, to test for blood biochemical indicators.
Maximum strength test
The participants’ 1-RM assessments were carried out in accordance with the American College of Sports Medicine exercise prescription guidelines.8 First, the participants were required to familiarize with the test procedure, and then perform several knee resistance exercises as a warm-up exercise. During the test, the participants selected the initial weight (50%–70% of 1-RM) according to the range of self-predictive ability. After each test was completed, the load was increased by 10% to 20% until the participant was unable to complete the predetermined number of repetitions. The same speed and joint range of motion were maintained, and 1-RM was obtained within four tests, with a rest period of 3 minutes between tests. The weight of the last completed test was recorded as the value of 1-RM.
Exercise plan
The control group used a ground exercise intensity of 30% of 1-RM, and each test required six sets of knee flexion and extension exercises using a sitting leg extension exerciser (HS-LE; Life Fitness, Schiller Park, IL, USA) and a sitting posture bending exerciser (HS-SLC; Life Fitness). The movement modes of the two devices are similar. For using these devices, the back of the body should be close to the seat, with both hands holding the handles, and the force point of the calf to the ankle joint should be adjusted. The concentric contraction and the eccentric contraction were 3 s each, with each set repeated 15 times, and the interval between sets was 1 minute. On this basis, participants in the low and high groups were required to wear a pneumatic cuff with 40% and 70% of AOP (SC12L; Hokanson, Bellevue, WA, USA). The cuff was straight with a nylon sleeve on the outside and was 12 × 124 cm in size, and was placed on the proximal end of the legs (approximately the upper third of the thigh) for exercise. The cuff was perpendicular to the limbs and close to the lower limbs when tied and was removed after completion of the exercise.9
Blood flow pressure setting
The blood flow occlusion pressures of the participants were tested with a blood flow restriction cuff and a Doppler probe (DV-600; Marted, Ribeirao Preto, Sao Paulo, Brazil) using a test method from a previous study.10 The participants remained in the supine position, and then the cuff was placed on the proximal end of their thighs and inflated in increments of 1 mmHg to a pressure where the arterial pulse could no longer be detected. The lowest pressure when the pulse disappeared was recorded as the AOP. The left and right legs were tested three times and the average value was calculated.
Blood samples
Elbow venous blood was collected before exercise, immediately after exercise, 15 minutes after exercise, and 30 minutes after exercise. Thirty minutes after the serum samples were collected, they were separated from blood cells by centrifugation (3000 rpm for 15 minutes) and stored at −80°C until further analysis. A double-antibody sandwich enzyme-linked immunosorbent assay was used to measure serum blood lactate (BLA), IGF-1, GH, and testosterone levels.
Statistical methods
All data are expressed as mean ± standard deviation. The Shapiro–Wilk normality test was used to determine the normal distribution of various index data. Two-way repeated measures analysis of variance was used to test the changes in GH, IGF-1, BLA, and testosterone levels at the four time points (i.e., pre-exercise, post-exercise, 15 minutes, and 30 minutes) in the main effects and interaction effects in each group. If the main effect or the interaction effect was significant, the Newman–Keuls method was used for multiple comparisons. Statistical analysis was performed using IBM SPSS version 24.0 software (IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant. G*power Version 3.1.9.2 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) was used for the sample size power test, with a post-hoc power (probability of error 1−β) of 0.99. We calculated the effect size of each group before and after exercise as described in a previous study: effect size = (post-mean − pre-mean)/pre standard deviation.11
Results
Characteristics of the participants
The male participants had a mean age of 19.8 ± 2.2 years, mean height of 176.7 ± 5.2 cm, mean weight of 67.5 ± 2.6 kg, and mean body mass index of 21.8 ± 0.8 kg/m2.
Effects of blood flow restriction exercise on BLA levels under different pressures
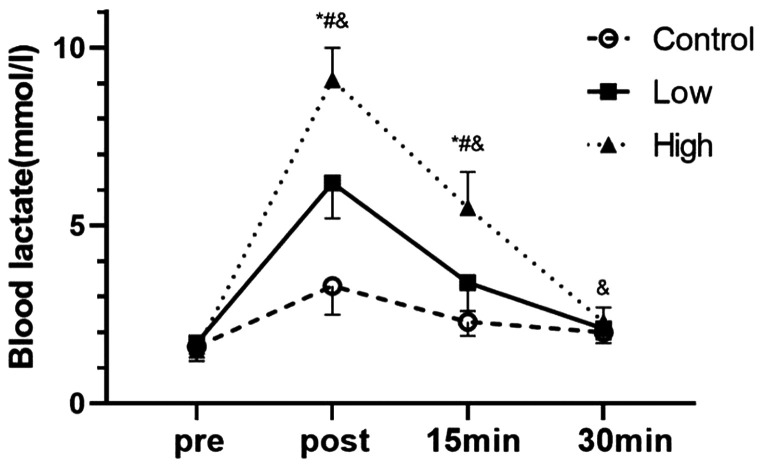
There was no significant difference in BLA levels between the groups before exercise (Figure 1). However, there was a difference in the effect of blood flow restriction at different pressures and the interaction effect was significant (P < 0.01). Furthermore, the effect of time on BLA levels was significant (P < 0.01). Immediately and 15 minutes after exercise, the mean BLA level in the high group was significantly higher than that in the low and control groups (all P < 0.01). Additionally, at these time points, the mean BLA level in the low group was significantly higher than that in the control group (both P < 0.01). At 30 minutes after exercise, the mean BLA level in the high group was significantly higher than that in the control group (P < 0.05). The effect size (%) increase in the control, low, and high groups after exercise compared with before exercise was 4.3 (106%), 15 (265%), and 25.3 (507%), respectively.
Figure 1.
Effects of blood flow restriction exercise on blood lactate levels under different pressures (n = 25).
*P < 0.05 between the control group and the low group; #P < 0.05 between the low group and the high group; &P < 0.05 between the high group and the control group.
Effects of blood flow restriction exercise on GH levels under different pressures
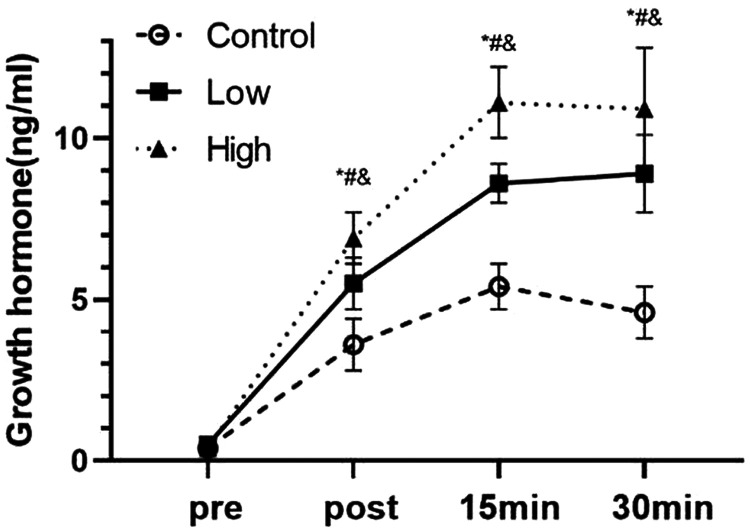
There was no significant difference in GH levels between the groups before exercise (Figure 2). However, there was a difference on the effect of blood flow restriction at different pressures and the interaction effect was significant (P < 0.01). Furthermore, the effect of time on GH levels was significant (P < 0.01). Immediately, 15 minutes, and 30 minutes after exercise, the mean GH level in the high group was significantly higher than that in the low group (all P < 0.01) and that in the low group was significantly higher than that in the control group (all P < 0.01). Additionally, the mean GH level in the high group was significantly higher than that in the control group after exercise at all time points (all P < 0.01). The effect size (%) increase in the control, low, and high groups after exercise compared with before exercise was 31 (775%), 49 (980%), and 65 (1625%), respectively.
Figure 2.
Effects of blood flow restriction exercise on growth hormone levels under different pressures (n = 25).
*P < 0.05 between the control group and the low group; #P < 0.05 between the low group and the high group; &P < 0.05 between the high group and the control group.
Effects of blood flow restriction exercise on IGF-1 levels under different pressures
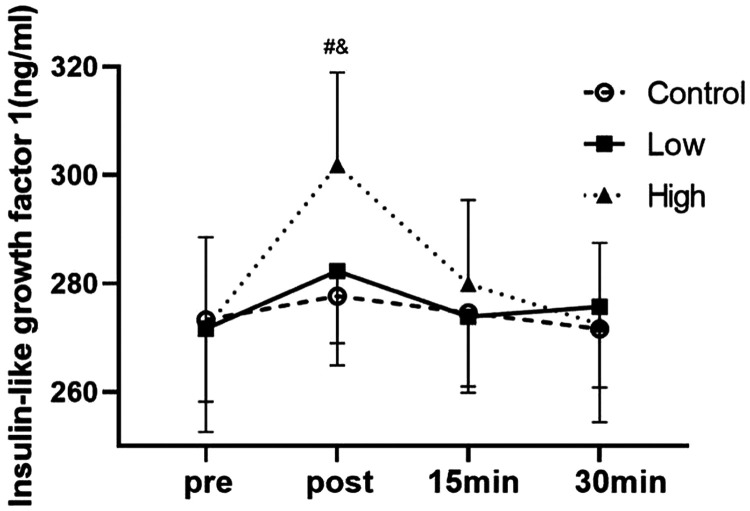
There was no significant difference in IGF-1 levels between the groups before exercise (Figure 3). However, there was a difference in the effect of blood flow restriction at different pressures and the interaction effect was significant (P < 0.05). Furthermore, the effect of time on IGF-1 levels was significant (P < 0.01). Immediately after exercise, the mean IGF-1 level in the high group was significantly higher than that in the low and control groups (both P < 0.01). There was no significant difference in the mean IGF-1 level between the groups at the other time points. The effect size (%) increase in the control, low, and high groups after exercise compared with before exercise was 0.3 (2%), 0.6 (4%), and 1.8 (11%), respectively.
Figure 3.
Effects of blood flow restriction exercise on insulin-like growth factor 1 under different pressures (n = 25).
#P < 0.05 between the low group and the high group; &P < 0.05 between the high group and the control group.
Effects of blood flow restriction exercise on testosterone levels under different pressures
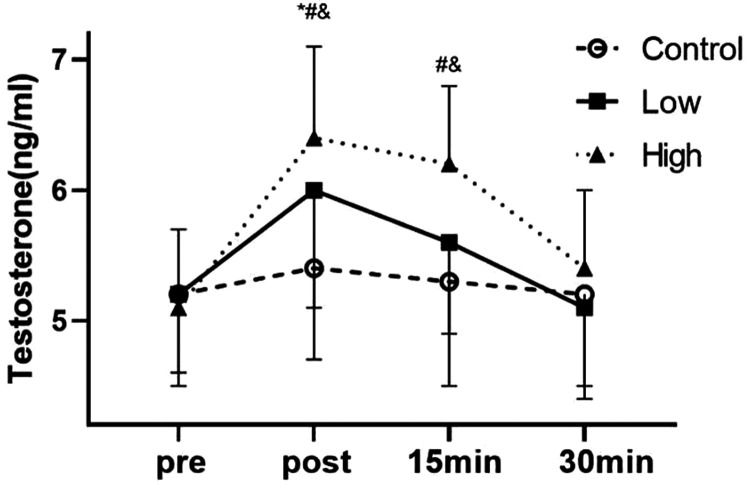
There was no significant difference in testosterone levels between the groups before exercise (Figure 4). However, there was a difference in the effect of blood flow restriction at different pressures and the interaction effect was significant (P < 0.05). Furthermore, the effect of time on testosterone levels was significant (P = 0.018). Immediately after exercise, the mean testosterone level in the high group was significantly higher than that in the low group (P < 0.05), and that in the low group was significantly higher than that in the control group (P < 0.01). At 15 minutes after exercise, the mean testosterone level in the high group was significantly higher than that in the low and control groups (both P < 0.01). There was no significant difference in the mean testosterone level between each group at 30 minutes after exercise. The effect size (%) increase in the control, low, and high groups after exercise compared with before exercise was 0.3 (4%), 1.1 (15%), and 2.2 (25%), respectively.
Figure 4.
Effects of blood flow restriction exercise on testosterone levels under different pressures (n = 25).
*P < 0.05 between the control group and the low group; #P < 0.05 between the low group and the high group; &P < 0.05 between the high group and the control group.
Discussion
This study investigated the effects of low-intensity resistance exercise combined with blood flow restriction under different pressures on the muscle growth-promoting hormones in healthy men. In previous studies on blood flow restriction training, a fixed value of cuff pressure was often used for training, but this resulted in a lack of individualization and appropriate cuff pressure.6 Blood pressure and blood flow in each person are heterogeneous. Some studies have proposed that the relative cuff pressure can be used to standardize the pressure of blood flow restriction.10 This standardization enables the degree of blood vessel obstruction to be relatively consistent in each participant, and this allows easier performance of quantitative training to evaluate the training effects. Participants in our study completed all tests and no adverse events occurred. This study showed that higher pressure blood flow restriction effectively stimulated the secretion of muscle-increasing synthetic hormones. Comparison of the three groups suggested that the amount of hormone secretion was associated with the pressure of blood flow restriction. To the best of our knowledge, no studies have compared the acute effects of different cuff pressures on muscle gain-related hormones. This study showed that under pressure, GH, IGF-1, and testosterone levels were significantly higher than those in a non-pressurized condition, which is consistent with a previous study.12 When we compared the three different pressurization protocols, we found that all of the measured hormones increased with increasing pressure.
GH and BLA
GH plays a synthetic role by promoting muscle and bone growth and development. Studies have shown that low-intensity resistance exercise combined with blood flow restriction effectively increases circulating GH levels in young people.13 Takarada et al. 14 reported that GH levels were increased by 290 times after blood flow restriction resistance exercise of knee joint extension in five groups. This increase is approximately 28 times higher than that in our study (the high group was approximately 28 times higher, the low group was approximately 18 times higher, and the control group was approximately 14 times higher) and that reported by Pierce et al.15 is even higher. We also found that GH levels may peak at 15 to 30 minutes, which is consistent with previous studies.17 Additionally, GH levels in the high group were higher than those in the low and control groups, which may have been due to increased metabolic stress. Resistance exercise itself can result in a certain amount of metabolic stress, and blood flow restriction can further increase this metabolic stress to stimulate the accumulation of metabolites and increase the secretion of GH.4 GH can also promote muscle growth and increase muscular adaptation with mediation by IGF-1.12 Therefore, an increase in GH levels during exercise may also affect the body’s IGF-1 levels to a certain extent.
In this study, BLA levels showed a similar trend as GH levels (high group > low group > control group). Blood flow restriction significantly increased BLA levels (the high group was approximately six times higher, the low group was approximately four times higher, and the control group was approximately times higher).
IGF-1
IGF-1, similar to GH, plays an important role in cell growth, but the acute response of resistance exercise to IGF-1 is still controversial. Some studies have shown that after low-intensity blood flow restriction exercise, serum IGF-1 levels are significantly increased,16 but this finding is in contrast to that in a study by Fujita et al. 17. In our study, the mean IGF-1 level in the high group was significantly higher than that in the other groups immediately after exercise. However, there was no significant difference in the mean IGF-1 level between the low and control groups, which suggested that 40% of AOP may not effectively affect the body’s IGF-1 levels. Although circulating GH levels in the body can stimulate the liver to secrete IGF-1,18 a sharp increase in IGF-1 levels is unlikely to be due to secretion of GH after exercise. This study showed that low-pressure blood flow restriction caused a significant increase in GH levels, but did not affect IGF-1 levels. Therefore, the reason for the increase in IGF-1 levels in the high group in this study is currently unclear. We speculate that IGF-1 secretion requires a pressure threshold and can be activated when that threshold is reached. The effect of blood flow restriction on IGF-1 levels still requires further studies.
Testosterone
Testosterone is a type of steroid that is derived from cholesterol and plays an important role in promoting muscle growth and synthesis. Testosterone also reduces muscle protein breakdown by inhibiting ubiquitin secretion and it has an anti-cortisol response.19,20 In this study, the level of testosterone secreted by the body gradually increased as the cuff pressure increased, which may have been due to the excessive pressure in the high group causing vascular occlusion of the moving limbs. Increased testosterone levels could have been caused by increased BLA levels. Lactic acid increases the production of cAMP in rat interstitial cells and stimulates the secretion of testosterone,21,22 while blood flow restriction effectively promotes the secretion of catecholamines.4
Limitations and outlook
There are many limitations to this study. First, the participating population was relatively single and only included young men. Therefore, whether our conclusions can be transferred to other populations is unknown. Additionally, this study failed to fully examine catecholamines and other stress hormones, and the results of this study can only be explained in a deductive form. Other studies have shown that exercise increases AOP and AOP at rest is positively correlated with AOP after exercise.23 Therefore, actual AOP during exercise may not be equal to the value measured before exercise, but due to current technical limitations, such as a failure to maintain the dynamic constant of the relative pressure of the cuff during exercise. Additionally, because AOP is tested in the supine position, it is different from the sports posture, which may cause errors in the actual AOP. Furthermore, whether an increase in synthetic hormones after exercise directly causes muscle hypertrophy is unclear. Some studies have shown that an increase in synthetic hormones cannot cause muscle hypertrophy.24,25 Other studies have shown that an increase in synthetic hormones improves muscle fitness.6,7 This inconsistency among studies hinders further interpretation of the results. In future studies, possible differences in the application of blood flow restriction in different populations should be further explored. The long-term effects of different types of blood flow restriction training should also be investigated. This information could provide coaches with more diverse exercise prescription options.
Conclusion
Low-intensity resistance exercise combined with blood flow restriction effectively increases GH, IGF-1, and testosterone levels in young men, and increasing the cuff pressure results in greater levels of hormone secretion. Low-intensity resistance exercise with blood flow restriction increases the anabolic potential of young men following exercise owing to an elevation in GH, IGF-1, and testosterone levels. Whether there is a direct association between changes in anabolic hormones and muscle hypertrophy in long-term training still needs to be investigated.
Table 1.
Stress and load during exercise (n = 25).
| Item | Value |
|---|---|
| 1-RM (lb) | 191.4 ± 19.4 |
| 30% 1-RM (lb) | 57.4 ± 5.8 |
| AOP (mmHg) | 196.9 ± 13.2 |
| 40% AOP (mmHg) | 78.8 ± 5.3 |
| 70% AOP (mmHg) | 137.8 ± 9.2 |
lb, pounds; 1-RM, one repetition maximum; AOP, arterial occlusion pressure.
Acknowledgment
The authors thank Ma Jiayuan for her suggestions regarding the research.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Li Shuoqi https://orcid.org/0000-0002-3678-9643
Author contributions: Conception: Li Shuoqi
Performance of work: Li Yinghao and Yang Jing
Interpretation or analysis of data: Wang Yongqi
Preparation of the manuscript: Li Yinghao and Zhou Jianming
Revision for important intellectual content: Li Yinghao, Li Shuoqi, and Tang Yiting
Supervision: Gao Zeng
References
- 1.Shen L, Li J, Chen Y, et al. L-carnitine's role in KAATSU training-induced neuromuscular fatigue. Biomed Pharmacother 2020; 125: 109899. [DOI] [PubMed] [Google Scholar]
- 2.Conceicao MS, Gaspari AF, Ramkrapes APB, et al. Anaerobic metabolism induces greater total energy expenditure during exercise with blood flow restriction. PloS One 2018; 13: e0194776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi S, Monazzami A, Nikousefat Z, et al. The acute and chronic effects of resistance training with blood flow restriction on hormonal responses in untrained young men: A comparison of frequency. Cell Mol Bio (Noisy-le-grand) 2020; 66: 1–8. [PubMed] [Google Scholar]
- 4.Madarame H, Sasaki K, Ishii N.Endocrine responses to upper- and lower-limb resistance exercises with blood flow restriction. Acta Physiol Hung 2010; 97: 192–200. [DOI] [PubMed] [Google Scholar]
- 5.Lixandrão ME, Ugrinowitsch C, Laurentino G, et al. Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur J Appl Physiol 2015; 115: 2471–2480. [DOI] [PubMed] [Google Scholar]
- 6.Falqueto H, Júnior JLR, Silvério MNO, et al. Can conditions of skeletal muscle loss be improved by combining exercise with anabolic–androgenic steroids? A systematic review and meta-analysis of testosterone-based interventions. Rev Endocr Metab Disord 2021; 22: 161–178. [DOI] [PubMed] [Google Scholar]
- 7.Gharahdaghi N, Phillips BE, Szewczyk NJ, et al. Links Between Testosterone, Oestrogen, and the Growth Hormone/Insulin-Like Growth Factor Axis and Resistance Exercise Muscle Adaptations. Front Physiol 2021; 11: 621226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D Riebe JKE, G, Liguori MM.ACSM's guidelines for exercise testing and prescription. 10th ed.Philadelphia: Wolters Kluwer, 2018, p.167–175. [Google Scholar]
- 9.Kjeldsen SS, Nss-Schmidt ET, Hansen GM, et al. Neuromuscular effects of dorsiflexor training with and without blood flow restriction. Heliyon 2019; 5: e02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues R, Ferraz RB, Kurimori CO, et al. Low-load resistance training with blood flow restriction increases muscle function, mass and functionality in women with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2020; 72: 787–797. [DOI] [PubMed] [Google Scholar]
- 11.Rhea MR.Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Cond Res 2004; 18: 918–920. [DOI] [PubMed] [Google Scholar]
- 12.Patterson SD, Leggate M, Nimmo MA, et al. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol 2013; 113: 713–719. [DOI] [PubMed] [Google Scholar]
- 13.Brumitt J, Hutchison MK, Kang D, et al. Blood Flow Restriction Training for the Rotator Cuff: A Randomized Controlled Trial. Int J Sports Physiol Perform 2020; 15: 1–6. [DOI] [PubMed] [Google Scholar]
- 14.Takarada Y, Nakamura Y, Aruga S, et al. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol 2000; 88: 61–65. [DOI] [PubMed] [Google Scholar]
- 15.Pierce JR, Clark BC, Ploutz-Snyder LL, et al. Growth hormone and muscle function responses to skeletal muscle ischemia. J Appl Physiol 2006, 101: 1588–1595. [DOI] [PubMed] [Google Scholar]
- 16.Takano H, Morita T, Iida H, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 2005; 95: 65–73. [DOI] [PubMed] [Google Scholar]
- 17.Fujita S, Abe T, Drummond M J, et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 2007; 103: 903–910. [DOI] [PubMed] [Google Scholar]
- 18.Scott CD, Martin JL, Baxter RC.Rat hepatocyte insulin-like growth factor I and binding protein: effect of growth hormone in vitro and in vivo. Endocrinol 1985; 116: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 19.Pires-Oliveira M, Maragno ALGC, Parreiras-e-Silva LT, et al. Testosterone represses ubiquitin ligases atrogin-1 and Murf-1 expression in an androgen-sensitive rat skeletal muscle in vivo. Eur J Appl Physiol 2010; 108: 266–273. [DOI] [PubMed] [Google Scholar]
- 20.Amani-Shalamzari S, Sarikhani A, Paton CD, et al. Occlusion Training During Specific Futsal Training Improves Aspects of Physiological and Physical Performance. J Sports Sci Med 2020; 19: 374–382. [PMC free article] [PubMed] [Google Scholar]
- 21.Lu SS, Lau CP, Tung YF, et al. Lactate and the effects of exercise on testosterone secretion: evidence for the involvement of a cAMP-mediated mechanism. Med Sci Sport Exer 1997; 29: 1048–1054. [DOI] [PubMed] [Google Scholar]
- 22.Lin H, Wang SW, Wang RY, et al. Stimulatory effect of lactate on testosterone production by rat Leydig cells. J Cell Biochem 2001; 83: 147–154. [DOI] [PubMed] [Google Scholar]
- 23.Jessee MB, Dankel SJ, Buckner SL, et al. The cardiovascular and perceptual response to very low load blood flow restricted exercise. Int J Sports Med 2017; 38: 597–603. [DOI] [PubMed] [Google Scholar]
- 24.West D, Phillips SM. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol 2012; 112: 2693–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morton RW, Oikawa SY, Wavell CG, et al. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J Appl Physiol (1985) 2016; 121: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]