Abstract
A polycondensation reaction of the orthotungstate anion WO42–, buffered at pH 7.5 in a TRIS-HCl (0.15 M) solution, results in the first example of a discrete polyoxotungstate anion, with just two W ions stabilized with TRIS ligands. It was isolated and characterized as Na2[WVI2O6(C4O3NH10)2]·6H2O by single-crystal and powder X-ray diffraction, FT-IR spectroscopy, thermogravimetrical analysis (TGA), and elemental analysis in solid state and by electro-spray ionization mass spectrometry (ESI-MS), 13C, and 183W NMR, as well as Raman spectroscopy in solution. This synthesis demonstrates the crucial and new role of the added tris-alkoxy ligand in the development of a new hybrid TRIS-isopolytungstate with the lowest known nuclearity (so far) and the terminal oxygens substituted with two nitrogen atoms arising from amines of the TRIS ligands.
Short abstract
We report on the synthesis and characterization of a new hybrid isopolytungstate Na2[WVI2O6(C4O3NH10)2]·6H2O with the, so far, lowest known nuclearity and the terminal oxygen atoms substituted with nitrogen arising from amine.
Polyoxometalates (POMs) are discrete anionic molecular metal-oxide clusters that are usually composed of group V and VI transition metals in their highest oxidation states and exist at the unique interface between monomeric oxometalates and polymeric metal oxides1 exhibiting a wide range of applications.2 There are three main criteria by which a metal oxide can be called a POM: (1) addenda ions have quasi-octahedral-coordination and form dπ-pπ bonds with oxygen atoms,1 (2) two octahedra are connected via sharing the edge, and (3) each octahedral unit has just two terminal O atoms.3 Oxygen atoms are the primary ligands for the addenda metals in POMs; however, replacing them with other elements while maintaining the structure is possible. So far, many synthesis procedures of oxo-replaced POM structures require water-free organic solvents and the overwhelming majority of POMs with nitrogen atoms bound to the addenda ion are polyoxomolybdates (POMos)4 or polyoxovanadates (POVs).5 Attempts to directly functionalize [W6O19]2– polyoxotungstate (POT) have failed when applying imido approaches suitable for POMos and POVs, since [W6O19]2– does not react with phosphinimines, isocyanates, or primary amines.4
In solution, small and stable POMs are interesting as useful building blocks for constructing huge metal–oxo clusters.6 In many cases, polycondensation reactions of [MOx]n- (M = addenda ion) immediately lead to larger structures, and POMs with low nuclearity are quite elusive.1 In acidified solutions of WO42–, both in the presence or absence of heteroions, anions with less than six W are underrepresented.7−10 The smallest discrete isopolytungstates (IPOT) verified to exist both in the solid state and in solutions are the Lindqvist hexatungstate [W6O19]2– in nonaqueous media and heptatungstate [W7O24]6– in water.1,7 Discrete binuclear complexes of pentavalent tungsten [WV2O4(Y)]2− (Y4− = hexadentate ligand, e.g., ethylenediaminetetraacetate) in octahedral coordination are known;11 however, they have not been obtained by acidification of orthotungstate. The strategy of using organic ligands to inhibit the formation of large clusters has previously been applied to POMos12 and POVs13 while it has not been reported for POTs. Tris(hydroxymethyl)aminomethane ((HOCH2)3CNH2, TRIS) has been recently utilized to stabilize and isolate elusive heptavanadate.13 While in biochemistry, TRIS (pKa 8.06) is used as pH buffer between 7.0 and 9.2,14 in POM chemistry, TRIS is usually used for covalent organic functionalization via alkoxo-groups −CH2OH attachment to the POM,15−17 and −NH2 plays the key role for postfunctionalization through amide bond formation.18−20 Recently, we expanded the role of TRIS by showing that TRIS, as part of a buffer solution that is normally considered unimportant, plays a defining role in the formation of a new member of the Keggin family.21 In POM chemistry, TRIS has never acted as a primary amine by replacing oxo-ligands and thus as a protective ligand to prevent the formation of POTs with a higher nuclearity.
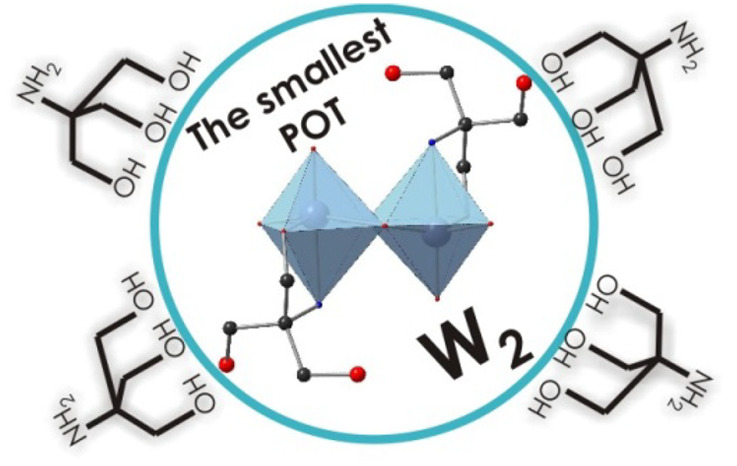
A discrete small anion, which is additionally organically functionalized for better stability, could be an ideal candidate for its use as a building block. To synthesize such an anion, we investigated an IPOT formation in a TRIS buffered solution (pH 7.5, 0.15 M) of WO42–. Herein, we report for the first time the successful synthesis of the discrete [WVI2O6(C4O3NH10)2]2– (W2), which meets all the requirements to be called a POM1 (WVI has quasi-octahedral-coordination; two octahedra are connected via sharing the edge; WVI has just two terminal O atoms) and is the smallest POT hybridized with TRIS known so far. W2 is a representative of a POM family with replaced oxygen ions by other nonmetals and the first POT functionalized directly by a primary amine in aqueous solution.
Initially, 12 mL of an aqueous solution of WO42– (0.188 M) was acidified with HCl (1 M) to pH 4.4 followed by the addition of TRIS (0.3 g, 2.5 mmol, 0.15 M) that led to an increase in pH to 7.5 (Figure 1A). After the final mixture was heated for 1 h at 90 °C and kept at room temperature, colorless crystals of Na2[WVI2O6(C4O3NH10)2]·6H2O (Na2W2) were formed. Single crystal structure analysis of W2 revealed that TRIS acts not only as a buffer component but also as a shielding ligand, preventing formation of IPOTs with a higher nuclearity in unbuffered WO42– solution, namely, Hx[WVI12O40(OH)2](10-x)– (x = 0–3) and [H2WVI12O40]6– between pH 2 and 5 as well as [WVI7O24]6– and [WVI12O40(OH)2]10– at pH 7.5 (Figure 1B).7−9
Figure 1.
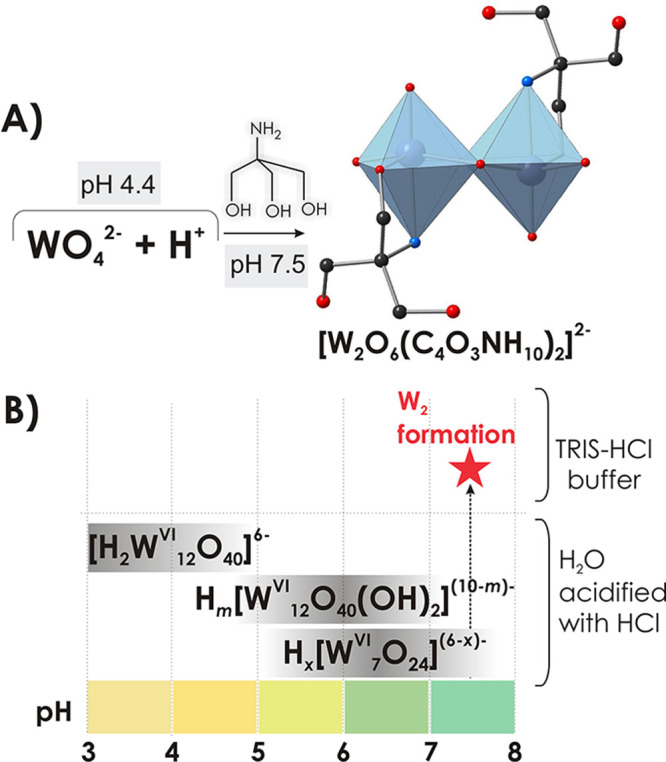
Synthetic route to prepare W2 (A) and the speciation diagram in buffered and unbuffered7 aqueous solution of WO42− in the pH range from 3 to 8 (B). Color code: {WO6}, light blue; O, red; N, blue; C, black.
Na2W2 crystallizes in the triclinic space group P1̅ (CCDC 2078090). The structure is composed of a [WVI2O6(C4O3NH10)2]2– and two Na+, connected to the tungsten cluster via O–CH2 groups of TRIS and forming a {NaO5} polyhedron (Figure 2). The coordination environment of each WVI consists of two terminal Ot (d(W1–O4) = 1.766 Å, d(W1–O5) = 1.767 Å), two bridging μ2-O (d(W1–O6) = 1.858 and 2.167 Å), one oxygen atom, and one nitrogen atom (d(W1–N1) = 2.344 Å) from one TRIS molecule (Figure 2). The W–N bond lengths in Na2W2 are slightly longer than those previously reported in POTs (2.13 to 2.17 Å),22,23 indicating the weaker π contribution of the bonds.24 The W–W bond length is 3.194 Å, which is shorter than that in classical IPOTs.9,21
Figure 2.
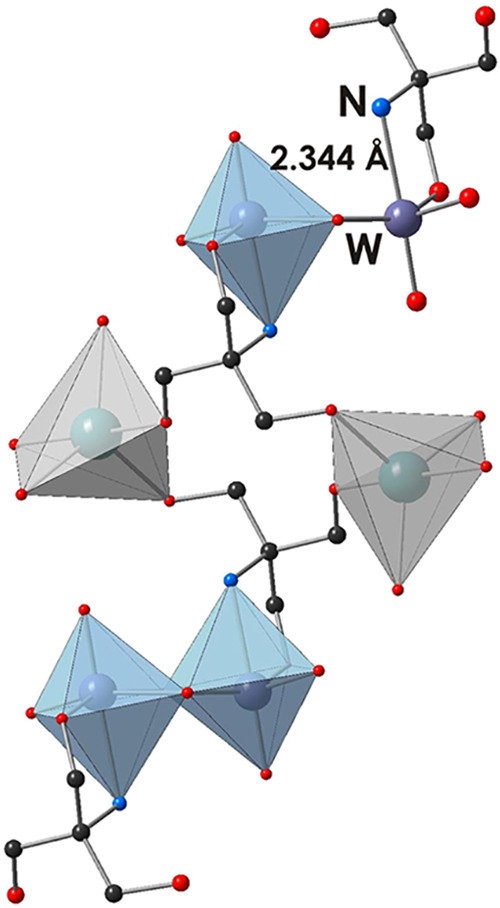
Binding of W2 to Na+ through the −CH2OH groups of TRIS ligands. Color code: W, purple; {WO6}, blue; {NaO5}, gray; O, red; N, blue; C, black.
The stretching vibrations of the W=O units are present at 919 cm–1 in the IR spectrum of Na2W2 (Figure S1). The bands at 894 cm–1 and in the region from 470 to 750 cm–1 correspond to the antisymmetric and symmetric deformation vibrations of W–O–W. The three bands at 1084, 1059, and 1040 cm–1 are assigned to C–O stretching vibrations, indicating the successful grafting of TRIS. TGA was used to examine the weight loss and thermal stability of the synthesized hybrid POT. The TG curve shows four weight-loss regions up to 700 °C due to dehydration followed by disintegration of TRIS (Figure S2). The experimental and simulated X-ray diffraction patterns of Na2W2 (Figure S3) fit perfectly in the range from 10 to 50° 2θ confirming its homogeneity.
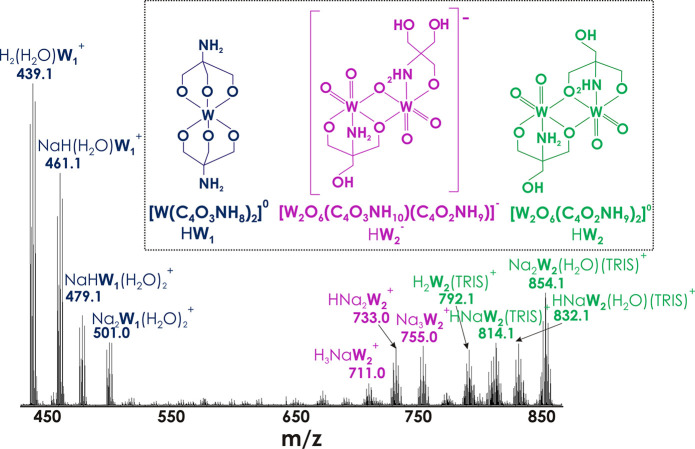
The stability of W2 in H2O at pH 6.8 was investigated by ESI-MS (Figures 3, S4, S5, and Table S2). The ESI-MS spectrum recorded in positive mode exhibits three series of peaks’ envelopes at m/z between 400 and 850, which can be unambiguously assigned to the singly charged cations (Figure 3, Table S2). The first group of signals (m/z = 439.1, 461.1, 479.1, 501.0) corresponds to the monomeric complex [WVI(C4O3NH8)2]0 in which two TRIS are coordinated to WVI via −CH2O- groups (blue in Figure 3). The signals at 711.0, 733.0, and 755.0 m/z correspond to the W2 (purple in Figure 3) in which one more –CH2OH fragment is attached to one of the equivalent WVI. The W2 with the symmetrical attachment of two additional –CH2OH (green in Figure 3) gives signals at 792.1, 814.1, 832.1, and 854.1 m/z. The attachment of TRIS ligands through three functional groups to the trimeric face of WVI ions is a known strategy in POM chemistry for elusive anion stabilization13 and may be the reason that these compounds are detected in solution. The ESI-MS spectrum of Na2W2 recorded in the negative mode (Figure S4) demonstrates the absence of signals from any IPOTs.
Figure 3.
ESI mass spectrum of Na2W2 in H2O (pH 6.8) recorded in the positive mode in the range from 430 to 860 m/z. Three types of species are shown in blue, purple, and green. Figure S5 shows the spectrum in the range from 100 to 1000 m/z, and Table S2 provides all species with experimental and theoretical m/z values.
To further examine the solution behavior of W2, 13C and 183W NMR spectroscopic studies were performed in D2O at pH 7.5 (Figures S6 and S7). The 183W NMR spectrum of Na2W2 (0.058 M) in D2O shows one intense signal with a chemical shift at −3.4 ppm and one minor signal at −91.9 ppm (Figure S6A). Since there exist no reference spectra for POTs with two equivalent W atoms and the chemical shift in Na2W2 spectrum is very close to 0 ppm (reference Na2WO4), a 183W NMR spectrum for the equimolar mixture of W2 and WO42– was acquired. The spectrum of the mixture demonstrates the same two signals at −2.8 and −91.6 ppm and one additional signal at −53.4 ppm (Figure S6B). The signal at −91.6 ppm can be attributed to [WVI7O24]6–, which gives three signals at 268.8, −90.9, and −180.2 (Figure S6D). The spectrum of W2 recorded in NaOAc/50% D2O at pH 6 (Figure S6C), where no signal for WO42– should be observed (Figure 1B), shows a very intense signal at −2.1 ppm. DFT calculations have been performed to assign the peaks to tungsten species (Table S3). A comparison of calculated and experimental values for [WVI6O19]2–, WX6 (X = F–, Cl–, CO) and for W2 shows that the calculated shift of −17 ppm can be attributed to the signal around 0 ppm if assigned to W2. The 13C NMR spectrum of Na2W2 in D2O (pH 7.5) shows two signals at 59.8 and 60.8 ppm (Figure S7B), which do not correspond to the W2 structure observed in the solid state, where three types of carbons are present (Figure 2). However, the signals at 59.8 and 60.8 ppm also cannot be attributed to the free TRIS with signals at 56.4 and 63.0 ppm (Figure S7A). In the Na2W2 solution at pH 6, the 13C NMR spectrum shows three signals at 59.3, 61.4, and 63.4 ppm, which indicate potential ligand binding according to the W2 structure (Figure 2). Raman spectroscopy was performed in the solid state and in solution to support ESI-MS and NMR spectroscopic data. The Raman spectrum of Na2W2 in H2O (pH 7.5) points to its dissociation to orthotungstate WO42– (Figure 4). On the basis of DFT calculations, ESI-MS, and NMR and Raman spectroscopy, we argue that W2 is unstable in an aqueous solution at pH 7.5; however, some intermediates detected by ESI-MS (Figure 3) with another type of TRIS attachment are at least partially present in slightly acidic solutions.
Figure 4.
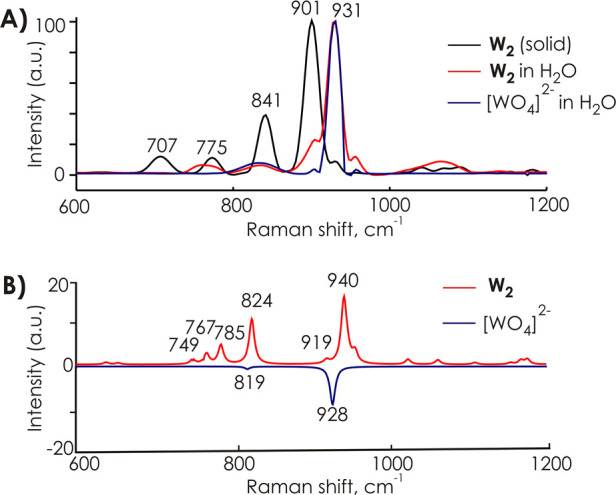
Experimental (A) and computed (B) Raman spectra of Na2[WVI2O6(C4O3NH10)2]·6H2O (Na2W2) and Na2WO4 in the range from 600 to 1200 cm–1. Solution spectra were recorded in H2O with pH 7.5 in Na2W2.
Considering the facile and reproducible synthesis of W2, as well as its small size, there are two scenarios for the use of W2 in the future for the formation of novel metal-oxide-based materials. One possible route is based on the rigid W2 nature, which is similar to that of the dinuclear [MV2S2O2(H2O)6]2+ (M = Mo or W),25,26 rendering W2 a potential connecting building block. The second scenario aims to expand the existing oxo-replaced POM chemistry. The strategy using the reaction of lacunary Keggin anion [PW11O39]7– with the mononuclear imido-tungsten precursor [WVI(NC6H5)Cl4] was successfully applied27 and can be taken as a model for W2.
In conclusion, the existence of the anion just with two tungsten ions, which fulfills all criteria to be called POM, has been demonstrated for the first time, where W(VI) ion is coordinated to two TRIS molecules through W–N and W–O chemical bonds. Full characterizations in the solid state and in solution elucidate the composition and solution behavior of the W2 anion.
Acknowledgments
We thank Ao.Univ.-Prof. Dr. Markus Galanski for great support with 183W NMR spectroscopic data collection at the NMR Core Facility, Elias Tanuhadi, MSc for PXRD measurements, Ass.-Prof. Dr. Peter Unfried for TGA and Mag. A. Fabisikova for ESI-MS measurements at the MS Center, Faculty of Chemistry, University of University of Vienna, and Florian Gregor Covi, BSc for speciation studies during his practical course.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.1c01188.
Synthetic details and spectroscopic data along with structural characterizations of the new compound (PDF)
Accession Codes
CCDC 2078090 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This research was funded by the Austrian Science Fund (FWF): P33927 (N.G.) and P33089 (A.R.) and the University of Vienna.
The authors declare no competing financial interest.
Dedication
Dedicated to Dr. sc. nat. Hans-Joachim Lunk on the occasion of his 80th birthday.
Supplementary Material
References
- Pope M. T.Inorganic Chemistry Concepts. Heteropoly and Isopoly Oxometalates; Springer: Berlin, 1983; Vol. 8. [Google Scholar]
- a Bijelic A.; Aureliano M.; Rompel A. Polyoxometalates as potential next-generation metallodrugs in the combat against cancer. Angew. Chem., Int. Ed. 2019, 58, 2980–2999. 10.1002/anie.201803868. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 3008 - 3029.10.1002/ange.201803868; b Bijelic A.; Aureliano M.; Rompel A. The antibacterial activity of polyoxometalates: structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. 10.1039/C7CC07549A. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang S. S.; Yang G. Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. 10.1021/cr500390v. [DOI] [PubMed] [Google Scholar]; d Horn M. R.; Singh A.; Alomari S.; Goberna-Ferrón S.; Benages-Vilau R.; Chodankar N.; Motta N.; Ostrikov K.; MacLeod J.; Sonar P.; Gomez-Romero P.; Dubal D. Polyoxometalates (POMs): from electroactive clusters to energy materials. Energy Environ. Sci. 2021, 14, 1652–1700. 10.1039/D0EE03407J. [DOI] [Google Scholar]
- Lipscomb W. N. Paratungstate ion. Inorg. Chem. 1965, 4, 132–134. 10.1021/ic50023a039. [DOI] [Google Scholar]
- Zhang J.; Xiao F.; Hao J.; Wei Y. The chemistry of organoimido derivatives of polyoxometalates. Dalton Trans. 2012, 41, 3599–3615. 10.1039/c2dt11948j. [DOI] [PubMed] [Google Scholar]
- Khan M. I.; Tabussum S.; Doedens R. J.; Golub V. O.; O’Connor C. J. Functionalized metal oxide clusters: Synthesis, characterization, crystal structures, and magnetic properties of a novel series of fully reduced heteropolyoxovanadium cationic clusters decorated with organic ligands- [MVIV6O6{(OCH2CH2)2N(CH2CH2OH)}6]X (M = Li, X = Cl·LiCl; M = Na, X = Cl·H2O; M = Mg, X = 2Br·H2O; M = Mn, Fe, X = 2Cl; M = Co, Ni, X = 2Cl·H2O). Inorg. Chem. 2004, 4, 5850–5859. 10.1021/ic049417m. [DOI] [PubMed] [Google Scholar]
- Miras H. N.; Yan J.; Long D.-L.; Cronin L. Structural evolution of “S”-shaped [H4W22O74]12– and “§”-Shaped [H10W34O116]18– isopolyoxotungstate clusters. Angew. Chem., Int. Ed. 2008, 47, 8420–8423. 10.1002/anie.200802109. [DOI] [PubMed] [Google Scholar]
- Gumerova N. I.; Rompel A. Polyoxometalates in solution: speciation under spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. 10.1039/D0CS00392A. [DOI] [PubMed] [Google Scholar]
- Hastings J. J.; Howarth O. W. A 183W, 1H and 17O nuclear magnetic resonance study of aqueous isopolytungstates. J. Chem. Soc., Dalton Trans. 1992, 209–215. 10.1039/dt9920000209. [DOI] [Google Scholar]
- Rozantsev G. M.; Radio S. V.; Gumerova N. I.. Strontium isopoly tungstates: Synthesis and properties. Pol. J. Chem. 2008, 82, 2067–2080 [Google Scholar]
- Liu Y.-J.; Jin M.-T.; Chen L.-J.; Zhao J.-W. Recent advances in isopolyoxotungstates and their derivatives. Acta Crystallogr., Sect. C: Struct. Chem. 2018, C74, 1202–1221. 10.1107/S2053229618012524. [DOI] [PubMed] [Google Scholar]
- a Novák J.; Podlaha J. Tungsten(V) complexes of ethylenediaminetetraacetic acid. J. Inorg. Nucl. Chem. 1974, 36, 1061–1065. 10.1016/0022-1902(74)80213-7. [DOI] [Google Scholar]; b Saito K.; Sasaki Y.; Hazama R. Doubly-bridged binuclear complexes of oxomolybdenum(V) and oxotungsten(V) containing sexadentate ligands. J. Cluster Sci. 1995, 6, 549–566. 10.1007/BF01165773. [DOI] [Google Scholar]
- Long D.-L.; Kögerler P.; Farrugia L. J.; Cronin L. Restraining symmetry in the formation of small polyoxomolybdates: New building blocks of unprecedented topology resulting from shrink-wrapping [H2Mo16O52]10– type clusters. Angew. Chem., Int. Ed. 2003, 42, 4180–4183. 10.1002/anie.200351615. [DOI] [PubMed] [Google Scholar]
- Fernández-Navarro L.; Nunes-Collado A.; Artetxe B.; Ruiz-Bilbao E.; San Felices L.; Reinoso S.; San José Wéry A.; Gutiérrez-Zorrilla J. M. Isolation of the Elusive Heptavanadate Anion with Trisalkoxide Ligands. Inorg. Chem. 2021, 60, 5442–5445. 10.1021/acs.inorgchem.1c00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb W. A.; Berthon H. A.; Kuchel P. W. Tris Buffer Reactivity with Low-Molecular-Weight Aldehydes: NMR Characterization of the Reactions of Glyceraldehyde-3-Phosphate. Bioorg. Chem. 1995, 23, 119–130. 10.1006/bioo.1995.1010. [DOI] [Google Scholar]
- Zhang J.; Huang Y.; Li G.; Wei Y. Recent advances in alkoxylation chemistry of polyoxometalates: From synthetic strategies, structural overviews to functional applications. Coord. Chem. Rev. 2019, 378, 395–414. 10.1016/j.ccr.2017.10.025. [DOI] [Google Scholar]
- a Blazevic A.; Al-Sayed E.; Roller A.; Giester G.; Rompel A. Tris–functionalized hybrid Anderson polyoxometalates: synthesis, characterization, hydrolytic stability and inversion of protein surface charge. Chem. - Eur. J. 2015, 21, 4762–4771. 10.1002/chem.201405644. [DOI] [PubMed] [Google Scholar]; b Gumerova N. I.; Roller A.; Rompel A. Synthesis and characterization of the first Ni(II)-centered single-side tris-functionalized Anderson-type polyoxomolybdate. Eur. J. Inorg. Chem. 2016, 36, 5507–5511. 10.1002/ejic.201601198. [DOI] [Google Scholar]
- a Gumerova N. I.; Roller A.; Rompel A. [Ni(OH)3W6O18(OCH2)3CCH2OH]4–: the first tris-functionalized Anderson-type heteropolytungstate. Chem. Commun. 2016, 52, 9263–9266. 10.1039/C6CC04326G. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gumerova N. I.; Caldera Fraile T.; Roller A.; Giester G.; Pascual-Borràs M.; Ohlin C. A.; Rompel A. The direct single- and double-side triol-functionalization of the mixed type Anderson polyoxotungstate [Cr(OH)3W6O21]6–. Inorg. Chem. 2019, 58, 106–113. 10.1021/acs.inorgchem.8b01740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyushin A. V.; Kondinski A.; Parac-Vogt T. N. Hybrid polyoxometalates as post-functionalization platforms: from fundamentals to emerging applications. Chem. Soc. Rev. 2020, 49, 382–432. 10.1039/C8CS00854J. [DOI] [PubMed] [Google Scholar]
- a Al-Sayed E.; Blazevic A.; Roller A.; Rompel A. The synthesis and characterization of aromatic hybrid Anderson-Evans POMs and their serum albumin interaction - The shift from polar to hydrophobic interactions. Chem. - Eur. J. 2015, 21, 17800–17807. 10.1002/chem.201502458. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gumerova N. I.; Blazevic A.; Caldera Fraile T.; Roller A.; Giester G.; Rompel A. Synthesis and Characterization of Hybrid Anderson Hexamolybdoaluminates (III) Functionalized with Indometacin or Cinnamic Acid. Acta Crystallogr., Sect. C: Struct. Chem. 2018, 74, 1378–1383. 10.1107/S2053229618012536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijelic A.; Dobrov A.; Roller A.; Rompel A. Binding of a fatty acid functionalized Anderson-type polyoxometalate to human serum albumin. Inorg. Chem. 2020, 58, 5243–5246. 10.1021/acs.inorgchem.9b03407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumerova N. I.; Roller A.; Giester G.; Krzystek J.; Cano J.; Rompel A. Incorporation of CrIII into a Keggin polyoxometalate as a chemical strategy to stabilize a labile {CrIIIO4} tetrahedral conformation and promote unattended single-ion magnet properties. J. Am. Chem. Soc. 2020, 142, 3336–3339. 10.1021/jacs.9b12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K.; Shinoe M.; Mizuno N. Synthesis and Reversible Transformation of Cun-Bridged (n = 1, 2, or 4) Silicodecatungstate Dimers. Inorg. Chem. 2012, 51, 11574–11581. 10.1021/ic301488a. [DOI] [PubMed] [Google Scholar]
- Suzuki K.; Minato T.; Tominaga N.; Okumo I.; Yonesato K.; Mizuno N.; Yamaguchi K. Hexavacant γ-Dawson-type phosphotungstates supporting an edge-sharing bis(square-pyramidal) {O2M(μ3-O)2(μ-OAc)MO2} core (M = Mn2+, Co2+, Ni2+, Cu2+, or Zn2+). Dalton Trans. 2019, 48, 7281–7289. 10.1039/C8DT04850A. [DOI] [PubMed] [Google Scholar]
- Li C.; Mizuno N.; Yamaguchi K.; Suzuki K. Self-Assembly of Anionic Polyoxometalate–Organic Architectures Based on Lacunary Phosphomolybdates and Pyridyl Ligands. J. Am. Chem. Soc. 2019, 141, 7687–7692. 10.1021/jacs.9b02541. [DOI] [PubMed] [Google Scholar]
- Cadot E.; Sokolov M. N.; Fedin V. P.; Simonnet-Jégat C.; Floquet S.; Sécheresse F. A building block strategy to access sulfur-functionalized polyoxometalate based systems using {Mo2S2O2} and {Mo3S4} as constitutional units, linkers or templates. Chem. Soc. Rev. 2012, 41, 7335–7353. 10.1039/c2cs35145e. [DOI] [PubMed] [Google Scholar]
- Béreau V.; Cadot E.; Bögge H.; Müller A.; Sécheresse F. Addition of {M2S2O2}2+, M = Mo, W, to A-α-[PW9O34]9-. Synthesis and structural characterizations in the solid state and in solution. Inorg. Chem. 1999, 38, 5803–5808. 10.1021/ic990666y. [DOI] [Google Scholar]
- Duhacek J. C.; Duncan D. C. Phenylimido Functionalization of α-[PW12O40]3-. Inorg. Chem. 2007, 46, 7253–7255. 10.1021/ic701024c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.