Summary
In Alzheimer’s disease, soluble oligomers of the amyloid-β peptide (Aβo) trigger a cascade of events that includes abnormal hyperphosphorylation of the protein tau, which is essential for pathogenesis. However, the mechanistic link between these two key pathological proteins remains unclear. Using hippocampal slices, we show here that an Aβo-mediated increase in glutamate release probability causes enhancement of synaptically evoked N-methyl-d-aspartate subtype glutamate receptor (NMDAR)-dependent long-term depression (LTD). We also find that elevated glutamate release probability is required for Aβo-induced pathological hyperphosphorylation of tau, which is likewise NMDAR dependent. Finally, we show that chronic, repeated chemical or optogenetic induction of NMDAR-dependent LTD alone is sufficient to cause tau hyperphosphorylation without Aβo. Together, these results support a possible causal chain in which Aβo increases glutamate release probability, thus leading to enhanced LTD induction, which in turn drives hyperphosphorylation of tau. Our data identify a mechanistic pathway linking the two critical pathogenic proteins of AD.
Keywords: Alzheimer’s disease, amyloid-β, neurotransmitter release, tau, phosphorylation, long-term depression
Graphical abstract
Highlights
-
•
Amyloid-β oligomers mediate increased neuronal glutamate release probability (Pr)
-
•
Increased Pr enhances the magnitude of hippocampal long-term depression (LTD)
-
•
Enhanced LTD drives the accumulation of pathologically hyperphosphorylated tau
-
•
This mechanism links the two key pathogenic proteins of Alzheimer’s disease
How amyloid-β triggers the recruitment and hyperphosphorylation of tau in Alzheimer’s disease remains unclear. Taylor et al. show that an oligomeric amyloid-β-mediated increase in glutamate release probability enhances the magnitude of Hebbian long-term depression (LTD) in the hippocampus, which in turn drives the accumulation of pathologically hyperphosphorylated tau.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, with approximately 5.8 million people currently living with the disease in the United States alone. Consequently, total payments for AD health and social care in 2020 are estimated at $305 billion (Alzheimer’s Association, 2020), yet, despite the enormity of the burden, therapeutic options for AD remain extremely limited. AD is characterized pathologically by amyloid plaques, composed of aggregated amyloid β peptide (Aβ), and neurofibrillary tangles that represent intraneuronal deposits of insoluble tau (Hardy and Selkoe, 2002; Small and Duff, 2008). The dominant hypothesis explaining AD pathogenesis, the amyloid cascade hypothesis, proposes that changes in the level and/or activity of Aβ, in particular the accumulation of toxic small, soluble oligomers of Aβ (Aβo), trigger a cascade of events, including pathological changes in tau, that drive disease development (Hardy and Higgins, 1992). Changes in tau include hyperphosphorylation, necessary for the toxicity of tau in AD (Kopeikina et al., 2012), and eventual aggregation (Mandelkow and Mandelkow, 2012). The amyloid hypothesis is supported by many lines of genetic and experimental evidence (Mucke and Selkoe, 2012) and is compatible with the more recent demonstration that the presence of tau is necessary for full expression of the disease phenotype (Rapoport et al., 2002; Roberson et al., 2007). However, despite nearly 30 years having passed since the amyloid hypothesis was published, the mechanism by which the two signature proteins of AD co-operate to bring about pathogenesis remains a critical knowledge gap in the field (Pickett et al., 2019). It is still not known how Aβ recruits tau or how tau recruitment might lead to its hyperphosphorylation.
Rising levels of Aβo are associated with derangements in both neural circuit function and synaptic plasticity. Because these are thought to play an important role in pathogenesis (Busche and Konnerth, 2016; Mucke and Selkoe, 2012), it is important to understand both the underlying mechanisms and the role such disturbances might play in recruiting processes downstream in the pathogenic cascade. Aberrant enhancement of excitatory activity is one of the earliest changes observed in cortical and hippocampal circuits in patients with AD and model mice (Busche and Konnerth, 2016). Plasticity changes are another early signature of AD (Styr and Slutsky, 2018), and they include impairment of hippocampal long-term potentiation (LTP) and facilitation of long-term depression (LTD) (Hsieh et al., 2006; Li et al., 2009, 2011; Shankar et al., 2008; Walsh et al., 2002). It has been suggested that the plasticity changes contribute to synaptic weakening and loss in AD, although the evidence for this remains indirect (Spires-Jones and Hyman, 2014).
Both LTP impairment and facilitation of LTD appear to require the activation of extrasynaptic N-methyl-d-aspartate subtype glutamate receptors (NMDARs), which can result from Aβo-mediated impairments in the uptake and clearance of synaptically released glutamate (Li et al., 2009, 2011). However, several studies have indicated that Aβo can also enhance action potential-evoked synaptic glutamate release (Brito-Moreira et al., 2011; Dolev et al., 2013; Jeans et al., 2020; Kabogo et al., 2010; Russell et al., 2012), although this remains controversial (He et al., 2019; Nimmrich et al., 2008), and we hypothesized that such enhanced release could represent another source of excess glutamate that might potentially contribute to extrasynaptic NMDAR activation and plasticity changes. Under physiological conditions, certain forms of plasticity are associated with the phosphorylation of tau; specifically, glycogen synthase kinase 3β (GSK-3β)-mediated phosphorylation of tau at serine 396 is critical for both induction of hippocampal LTD and the reversal of learning in vivo (Kimura et al., 2013; Regan et al., 2015). Therefore, we further hypothesized that Aβo-associated changes in glutamate release and, consequently, plasticity might be able to recruit and phosphorylate tau via a similar mechanism.
We set out to explore these hypotheses and uncovered evidence for a functional pathway that links Aβo to hyperphosphorylation of tau via a well-defined mechanism, namely, enhanced release probability driving inappropriate or excessive induction of synaptic LTD, in turn driving hyperphosphorylation of tau. This study identifies a central axis of pathogenesis in AD and takes a major step toward filling a critical knowledge gap.
Results
Aβo enhance the probability of neurotransmitter release at CA3-CA1 synapses
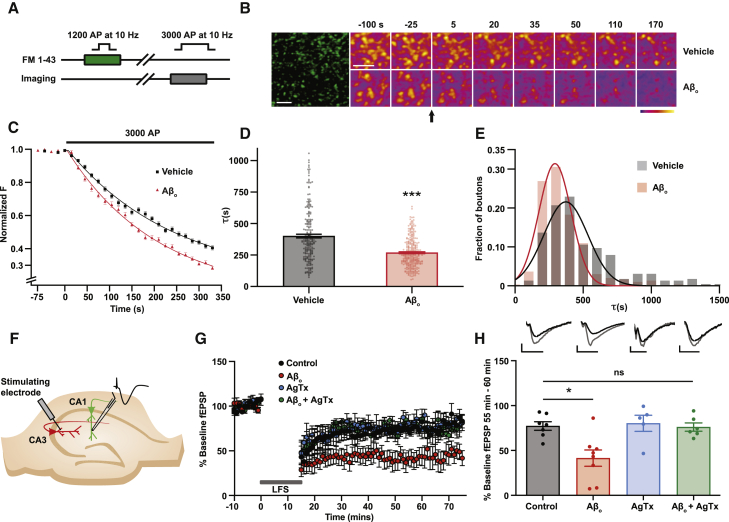
We carried out most of our experiments in organotypic hippocampal slices because they are particularly amenable to the chronic manipulations used in this study (Gogolla et al., 2006). We used a well-characterized Aβo preparation comprising mainly low-n oligomers such as trimers and tetramers (Chromy et al., 2003; Velasco et al., 2012) because these are known to be the most pathologically active species (Ferreira et al., 2015). Initially, we sought to confirm that glutamate release probability is elevated at CA3-CA1 (Schaffer collateral) synapses in this experimental model. Because α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor desensitization, which rapidly follows exposure to Aβo (Li et al., 2009), limits the ability of electrophysiological measurements to detect changes in release probability (Jeans et al., 2020), we used an optical method to measure release from the presynaptic bouton directly. FM1-43 is an activity-dependent dye label that allows for direct imaging of synaptic vesicle fusion (Kavalali and Jorgensen, 2014). Electrical stimulation of CA3 axons (10 Hz/120 s) turns over the total recycling pool of synaptic vesicles while dye is applied to CA1, where it is taken up by the vesicles as they are endocytosed. After washing to remove bound extracellular FM1-43, the same axons are stimulated at 10 Hz to unload the dye (Figure 1A). We monitored the rate of stimulus-evoked dye unloading, which is a reliable indicator of probability of evoked release (Zakharenko et al., 2001), at individual CA3-CA1 synapses, finding that it was increased following Aβo exposure (average time constant of fluorescence decay (τ) per punctum: control, 400.5 ± 14.6 s, n = 217 puncta from 6 slices; Aβo, 268.3 ± 7.0 s, n = 283 puncta from 6 slices) (Figures 1B–1E).
Figure 1.
Exposure to Aβo causes an increase in glutamate release probability that is required for the Aβo-dependent augmentation of hippocampal LTD
(A) Schematic showing protocol for determining kinetics of FM1-43 release from presynaptic terminals.
(B) Left panel: sample image of FM dye-labeled presynaptic terminals in CA1 of an organotypic hippocampal slice. Right panels: sample time-lapse images demonstrating stimulus-driven dye loss at synaptic puncta under conditions indicated. Arrow indicates onset of stimulation. Scale bars = 5 μm.
(C) Average FM1-43 destaining plots fitted with first-order exponential decay curves.
(D) Average time constants of destaining (control: n = 217 puncta from 6 slices, τ = 400.5 ± 14.6 s; Aβo: n = 283 puncta from 6 slices, τ = 268.3 ± 7.0 s).
(E) Frequency distribution of destaining time constants from individual puncta showing clear right shift following Aβo treatment.
(F) Experimental setup for plasticity experiments with acute hippocampal slices incubated in the indicated conditions for at least 2 h before recording field EPSPs from CA3-CA1 synapses.
(G) Summary traces showing LTD following a low-frequency stimulation protocol (900 × 1 Hz). Traces normalized to pre-induction baseline.
(H) Mean average fEPSP slopes calculated within a 55- to 60-min time window after LTD induction. Inset traces represent fEPSPs before (gray) and after (black) LTD induction; scale bars: 0.5 mV, 5 ms (control: n = 7, 77.26% ± 4.79% baseline fEPSP; Aβo: n = 8, 41.46% ± 9.08% baseline fEPSP; AgTx: n = 5, 80.26% ± 8.99% baseline fEPSP; AgTx + Aβo: n = 6, 76.08% ± 4.64% baseline fEPSP).
Kruskal-Wallis test followed by Dunn’s multiple comparison test versus control. Error bars represent ± SEM. ∗p < 0.05, ∗∗∗p < 0.001.
Aβo cause augmentation of LTD that can be rescued by partial suppression of presynaptic function
Increases in glutamate release probability have been associated with enhanced activation of extrasynaptic NMDAR (Arnth-Jensen et al., 2002; Lozovaya et al., 1999), which are critical for LTD induction in the hippocampus (Papouin et al., 2012), as well as for the effects of Aβo on both LTP and LTD (Li et al., 2009; Li et al., 2011). In addition, increasing release probability converts synapses into low-pass filters that preferentially release in response to single spikes or low-frequency trains but tend to depress release at higher frequencies of stimulation (Tong et al., 2020), strongly favoring the chronic, low-frequency synaptic activity that is associated with the induction of LTD (Lüscher and Malenka, 2012). Therefore, we hypothesized that Aβo-mediated enhancement of release probability might affect LTD induction. To test this, we first assessed the effects of Aβo on LTD in acute hippocampal slices. We induced LTD at CA3-CA1 synapses (Figure 1F) with a low-frequency stimulation (LFS) protocol of 900 stimuli at 1 Hz that is known to be NMDAR dependent (Lüscher and Malenka, 2012) and found that, under control conditions, LFS depressed field excitatory postsynaptic potentials (fEPSPs) to 77.26% ± 4.79% of their baseline value (n = 7). Incubation with Aβo (50 nM) robustly increased the magnitude of LTD (p = 0.033), depressing the fEPSP to 41.46% ± 9.08% of baseline (n = 8) (Figures 1G and 1H). If this enhanced LTD is indeed due to enhanced release probability, then normalizing presynaptic function should rescue the effect. We have previously shown that a low (50 nM) dose of the presynaptic CaV2.1 voltage-gated Ca2+ channel blocker ω-agatoxin IVA (AgTx) restores normal release probability in the presence of Aβo under these same experimental conditions (Jeans et al., 2020), and we confirmed that this treatment alone had no effect on LTD compared with control (80.26% ± 8.99% of baseline; n = 5). However, low-dose AgTx fully rescued the effect of Aβo on LTD (76.08% ± 4.64% of baseline; n = 6) (Figures 1G and 1H), suggesting that enhanced presynaptic function is required for the Aβo-mediated enhancement of LTD. To strengthen this conclusion, we repeated the experiment using a different manipulation, treatment with 20 μM adenosine, to normalize release probability (Figures S1A and S1B). This also restored LTD to control levels (70.08 ± 10.80% of baseline; n = 5) (Figures S1C and S1D).
Although we have so far focused on the role of NMDAR in Aβo-mediated alterations in LTD, metabotropic glutamate receptors (mGluRs) have also been implicated (Li et al., 2009; Shankar et al., 2008). Furthermore, the LFS induction protocol we used has been shown to induce a different form of LTD partly dependent on mGluRs if stimuli are given as paired pulses with a 50-ms interpulse interval (Kemp and Bashir, 2001). Because both paired-pulse stimulation and Aβo increase glutamate release, we asked whether some of the enhancement of LTD we saw following Aβo exposure might arise from the recruitment of an mGluR-dependent component. Although LTD induced in the presence of both Aβo and a pan mGluR blocker (100 μM LY341495) was slightly higher than with Aβo alone (40.38% ± 10.43%, n = 5 versus 37.08% ± 9.14%, n = 8), the difference was not significant (Figures S1E and S1F), indicating that the enhanced LTD was not substantially mGluR dependent.
Aβo-induced hyperphosphorylation of tau is NMDAR dependent and can be rescued by partial suppression of presynaptic function
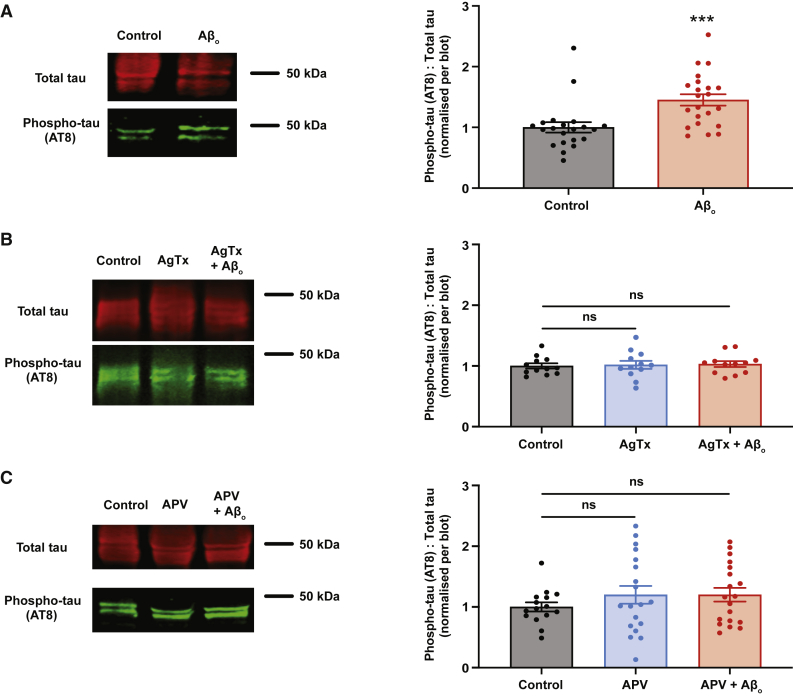
Given that Aβo enhance LTD and that LTD requires tau phosphorylation (Regan et al., 2015), we hypothesized that there may be a connection between synaptic changes induced by Aβo and tau hyperphosphorylation in AD, with LTD, or a pathological LTD-like process, serving as a potential mechanistic link between the two. This would also be consistent with studies that demonstrate mechanistic overlap between LTD and Aβo-induced synaptic decline in AD (Hsieh et al., 2006), and with the dependence of both processes on extrasynaptic NMDARs (Li et al., 2009; Papouin et al., 2012; Talantova et al., 2013). As a first step toward testing this hypothesis, we sought to confirm that we were able to detect pathological hyperphosphorylation of tau following chronic (7-day) Aβo incubation. For this, we used organotypic hippocampal slices that, after treatment, were lysed and subjected to electrophoretic separation and quantitative western blotting. We used an antibody to detect total tau alongside AT8, which specifically recognizes pathologically phosphorylated tau, binding to phosphorylated serine 202 and phosphorylated threonine 205 residues (Goedert et al., 1995). Phosphorylation of these residues is associated with tau misfolding into a pathological conformation (Bibow et al., 2011; Jeganathan et al., 2008) and is an early event in the formation of tau inclusions (Braak et al., 1994; Mondragón-Rodríguez et al., 2014). In addition, both of these residues can be phosphorylated by GSK-3β (Liu et al., 2002; Wang et al., 1998), which has been implicated in Aβo-associated tau phosphorylation in AD (Shipton et al., 2011; Takashima, 2006). Importantly, the residues probed by the AT8 antibody were explicitly shown not to be altered during normal LTD (Regan et al., 2015). Phosphorylation was quantified as a ratio of pathologically phosphorylated tau to total tau, with signals from all labeled antibodies normalized to control lanes within each blot to account for inter-experimental variation. Immunostaining for β-actin confirmed that none of the manipulations used in this study led to a change in total tau expression level (Figure S4F).
As expected, chronic Aβo incubation led to an increase in the levels of pathologically phosphorylated tau, as detected by AT8, compared with control (p = 0.001) (Figure 2A). To further validate this result, as well as to confirm the ability of AT8 to detect pathologically phosphorylated tau, we also performed the experiment with the antibody AT180, which detects tau phosphorylation at threonine 231. This is again an early event in AD, and levels of threonine 231-phosphorylated tau show a particularly strong association with pathological progression throughout the course of the disease (Neddens et al., 2018). As with the residues recognized by AT8, threonine 231 is also a substrate for GSK-3β (Billingsley and Kincaid, 1997). This corroborated results with AT8, showing an increase in tau phosphorylated at this residue following Aβo incubation (p = 0.034) (Figure S2).
Figure 2.
Aβo-mediated hyperphosphorylation of tau requires enhancement of glutamate release probability
(A) Western blot analysis of hippocampal slices treated for 7 days as indicated. Left panels show representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 21, 1.00 ± 0.09; Aβo: n = 22, 1.45 ± 0.09).
(B) Western blot analysis of hippocampal slices treated for 7 days as indicated. Low-dose (50 nM) ω-agatoxin IVA (AgTx) treatment restores elevated release probability to normal levels (Jeans et al., 2020). Left panels show representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 12, 1.00 ± 0.04; AgTx: n = 12, 1.02 ± 0.07; AgTx + Aβo: n = 12, 1.03 ± 0.05). One-way ANOVA with Dunnett’s multiple comparison test versus control.
(C) Western blot analysis of hippocampal slices treated for 7 days as indicated. Left panels show representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 15, 1.00 ± 0.08; APV: n = 19, 1.20 ± 0.15; APV + Aβo: n = 19, 1.20 ± 0.11).
One-way ANOVA with Dunnett’s multiple comparison test versus control. Error bars represent ± SEM. ∗∗∗p < 0.001. ns, not significant.
We then asked whether normalization of glutamate release probability with low-dose AgTx (Jeans et al., 2020), which rescues the enhancement of LTD by Aβo (Figures 1G and 1H), was also able to prevent the hyperphosphorylation of tau. Slices were incubated in AgTx alone, or AgTx with Aβo, and tau phosphorylation was examined as before. We found that incubation in AgTx alone had no effect on tau phosphorylation with respect to control, but AgTx was able to prevent Aβo-induced hyperphosphorylation of tau (Figure 2B). These results indicate that enhanced synaptic activity is necessary for the effects of Aβo on tau phosphorylation. Finally, we asked whether these effects of enhanced synaptic activity require NMDARs, which mediate the enhancement of LTD by Aβo and have been implicated in Aβo-associated synaptotoxicity (Hsieh et al., 2006). Chronic incubation in the NMDAR blocker 2-amino-5-phosphonovaleric acid (APV) (50 μM) alone had no effect on tau phosphorylation, but addition of APV was sufficient to restore tau phosphorylation in the presence of Aβo to control levels (Figure 2C), indicating the NMDAR dependence of this process.
Chronic induction of chemical LTD drives hyperphosphorylation of tau
So far, we have shown that Aβo-mediated increases in glutamate release probability are required for both the enhancement of LTD and the hyperphosphorylation of tau. Together, these findings suggest that Aβo-mediated increases in release probability tend synapses toward either inappropriate or excessive induction of LTD, which might in turn promote excessive tau phosphorylation. However, we have not yet explored whether there is a causal relationship between enhanced LTD and hyperphosphorylation of tau. In order to gain experimental support for such a relationship, we asked whether extreme LTD-inducing conditions are alone sufficient for hyperphosphorylation of tau. To accomplish this, we used conventional stimulation protocols for in vitro induction of LTD, but chronically applied over repeated cycles, reasoning that this would most closely approximate the effects of a sustained increase in release probability in vivo.
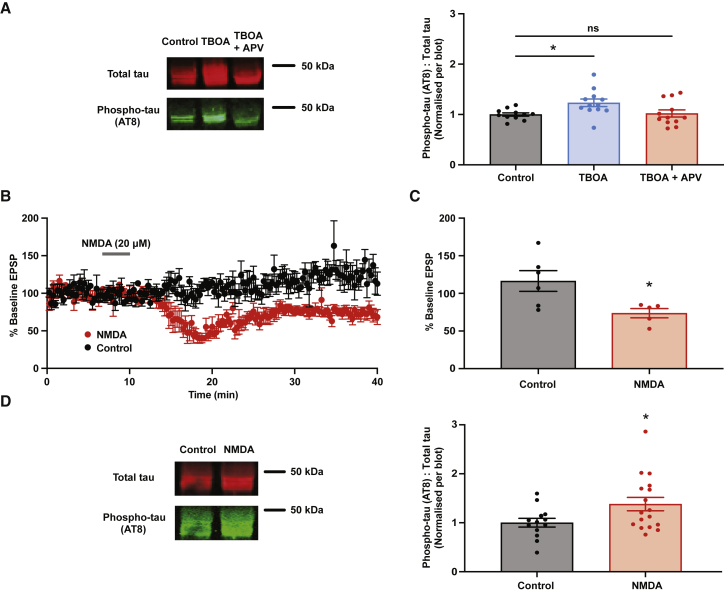
We first tested two chemical LTD protocols. The activation of extrasynaptic NMDAR in particular is necessary for LTD induction (Papouin et al., 2012), as well as for Aβo-associated synaptic depression (Li et al., 2009; Talantova et al., 2013). Activity of these receptors is partly controlled by reuptake of extracellular glutamate via astrocytic transporters (Papouin and Oliet, 2014), and application of the inhibitor of glial excitatory amino acid transporters dl-threo-β-benzyloxyaspartic acid (TBOA) (20 μM) alone induces a chemical LTD, likely via extrasynaptic NMDAR activation (Dutar and Potier, 2019). We therefore asked whether chronic incubation of slices with the same LTD-inducing concentration of TBOA could phenocopy the effects of Aβo on tau phosphorylation. Indeed, we found that chronic TBOA incubation led to enhanced tau phosphorylation (p = 0.032), and that this could be rescued by the inclusion of APV to inhibit NMDAR (Figure 3A). We then tested another chemical LTD induction protocol (20 μM NMDA for 3 min) (Lee et al., 1998) that is also NMDAR dependent and in addition is known to require tau (Kimura et al., 2013). We verified in patch-clamp recordings that it induced LTD in organotypic hippocampal slices, confirming a robust depression of the excitatory postsynaptic current (EPSP) following acute NMDA application (p = 0.027) (Figures 3B and 3C). To test the effect of chronic NMDA-LTD induction, 20 μM NMDA was presented to organotypic slices once per day for 3 min, repeated each day for 7 days, after which tau phosphorylation was examined as before. We found that this protocol indeed produced a significant (p = 0.037) increase in tau phosphorylation (Figure 3D). This indicates that chronic, repeated induction of NMDAR-dependent chemical LTD induces a pathological tau phosphorylation signature, as measured by increased AT8 immunoreactivity, which is distinct from that observed following typical induction protocols (Regan et al., 2015).
Figure 3.
Chronic induction of chemical LTD drives pathological hyperphosphorylation of tau
(A) Incubation with the inhibitor of glutamate uptake TBOA increases tau phosphorylation in an NMDAR-dependent manner. Western blot analysis of hippocampal slices treated for 7 days as indicated. Left panel shows representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 11, 1.00 ± 0.03; TBOA: n = 12, 1.23 ± 0.08; TBOA + APV: n = 12, 1.02 ± 0.07). One-way ANOVA with Bonferroni’s multiple comparisons test versus control.
(B) Summary traces of patch-clamp recordings showing slope of EPSP at CA3-CA1 synapses following addition of NMDA compared with control. Traces normalized to pre-addition baseline.
(C) Mean average EPSP slopes calculated within a 25- to 30-min time window after NMDA application (control: n = 6, 116.4% ± 13.75%; NMDA: n = 5, 73.63% ± 6.12%).
(D) Western blot analysis of hippocampal slices treated for 7 days as indicated. Left panel shows representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 13, 1.00 ± 0.09; NMDA: n = 17, 1.38 ± 0.14).
Error bars represent ± SEM. ∗p < 0.05.
Chronic optogenetic induction of LTD drives hyperphosphorylation of tau
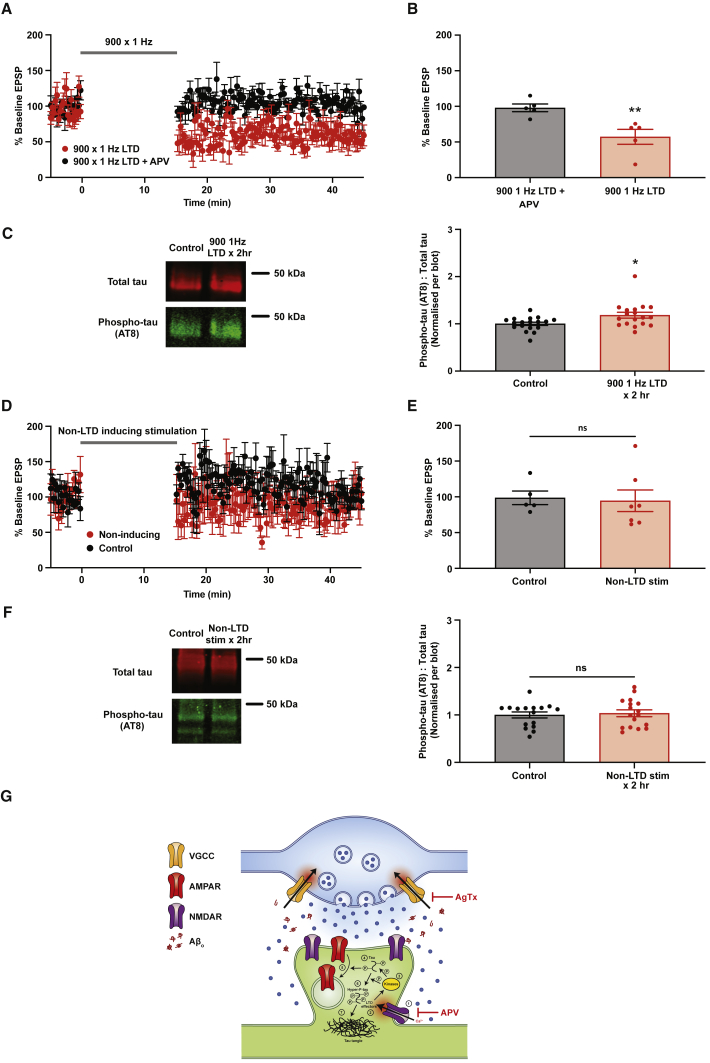
We then asked whether chronic LTD induced by synaptic activity would have similar effects. CA3 hippocampal neurons in organotypic slices were transfected with Channelrhodopsin 2 (ChR2) via adeno-associated virus to allow direct optical activation. As expected, we found robust expression of ChR2 at a high level, with transfected axons projecting to CA1 (Figure S3A). Control experiments established a minimum light intensity and pulse width that could reliably cause firing of a single action potential in CA3 neurons (Figures S3B and S3C). To confirm our ability to induce LTD via optical stimulation, slices were stimulated with blue light at either 900 × 1 Hz to induce NMDAR-dependent LTD or 500 × 1 Hz to induce a form of LTD dependent on mGluR activation. Both stimuli induced LTD (900 × 1 Hz: 51.49% ± 4.91% baseline EPSP, p = 0.008 (Figures 4A and 4B); 500 × 1 Hz: 57.24% ± 10.46% baseline EPSP, p = 0.008 (Figures S4A and S4B)). As expected, 500 × 1 Hz LTD was blocked by incubation with the pan mGluR blocker LY341495 (Figures S4A and S4B), while the 900 × 1 Hz LTD was blocked by APV, confirming its NMDAR dependence (Figures 4A and 4B). To study the effects of chronic electrophysiological LTD induction protocols on tau phosphorylation, we delivered optical stimulation to ChR2-expressing slices via a light-emitting diode (LED) array placed within the incubator. We stimulated slices once a day over 7 days, either with the mGluR-dependent protocol or with the NMDAR-dependent protocol. Neither condition significantly altered tau phosphorylation (Figures S4C and S4D), although the NMDAR-dependent protocol did show a small, but non-significant, increase (Figure S4D). We then asked whether increasing the number of induction cycles over the 7-day period might increase tau phosphorylation; this might also represent a better model of the pathological state, in which the enhancement of synaptic activity by Aβo would be constant. Accordingly, we delivered either the NMDAR-dependent or the mGluR-dependent protocol every 2 h for 7 days to see whether this had any further effect, finding that more frequent induction with the NMDAR-dependent protocol did indeed produce significant hyperphosphorylation of tau (p = 0.014) (Figure 4C), although with the mGluR-dependent protocol it did not (Figure S4E). In order to confirm that the increase in tau phosphorylation was dependent on repeated LTD-inducing stimuli, rather than simply elevated activity, we subjected slices to a stimulation protocol (three stimuli at 3 Hz, repeated every 3 s for 15 min) that included the same number of stimuli within the same time period as the NMDAR-dependent protocol but did not induce LTD (Figures 4D and 4E). We found that this protocol applied every 2 h for 7 days had no effect on tau phosphorylation (Figure 4F), confirming that it is induction of LTD that is important for the pathological tau phosphorylation we observe. Finally, we used propidium iodide staining to verify that chronic optogenetic stimulation (500 × 1 Hz every 2 h for 7 days) of hippocampal neurons did not impact the health of the slices (Figures S3D and S3E). Together, these results demonstrate that chronic induction of NMDAR-dependent LTD either chemically or electrophysiologically enhances the pathological phosphorylation of tau at residues distinct from serine 396 and 404, which are phosphorylated during physiological LTD.
Figure 4.
Chronic optogenetic induction of LTD drives pathological hyperphosphorylation of tau
(A) Summary traces of patch-clamp recordings showing slope of EPSP at CA3-CA1 synapses in ChR2-expressing slices following 900 × 1 Hz optical stimulation, with or without the NMDAR antagonist APV as indicated. Traces normalized to pre-stimulation baseline.
(B) Mean average EPSP slopes calculated within a 25- to 30-min time window after LTD induction (900 × 1 Hz: n = 5, 57.24% ± 10.46%; 900 × 1 Hz + APV: n = 5, 97.91% ± 5.36%). Mann-Whitney test.
(C) Western blot analysis of hippocampal slices treated for 7 days as indicated. Left panels show representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 18, 1.00 ± 0.03; 900 × 1 Hz LTD every 2 h: n = 18, 1.18 ± 0.06).
(D) Summary traces showing slope of EPSP at CA3-CA1 synapses in ChR2-expressing slices following optical stimulation with a non-LTD-inducing stimulus (three stimuli at 3 Hz, repeated every 3 s for 15 min). Traces normalized to pre-stimulation baseline.
(E) Mean average EPSP slopes calculated within a 25- to 30-min time window after delivery of non-inducing stimulus (control: n = 5, 98.61% ± 9.55%; non-LTD-inducing stimulus: n = 7, 94.52% ± 15.10%). Mann-Whitney test.
(F) Western blot analysis of hippocampal slices treated for 7 days as indicated. Non-LTD-inducing stimulation is three stimuli at 3 Hz, repeated every 3 s for 15 min, repeated every 2 h. Left panels show representative bands. The ratio of pathologically phosphorylated tau (AT8 antibody) to total tau was quantified and normalized to control within each blot (control: n = 16, 1.00 ± 0.06; non-LTD-inducing stimulus every 2 h: n = 17, 1.04 ± 0.07).
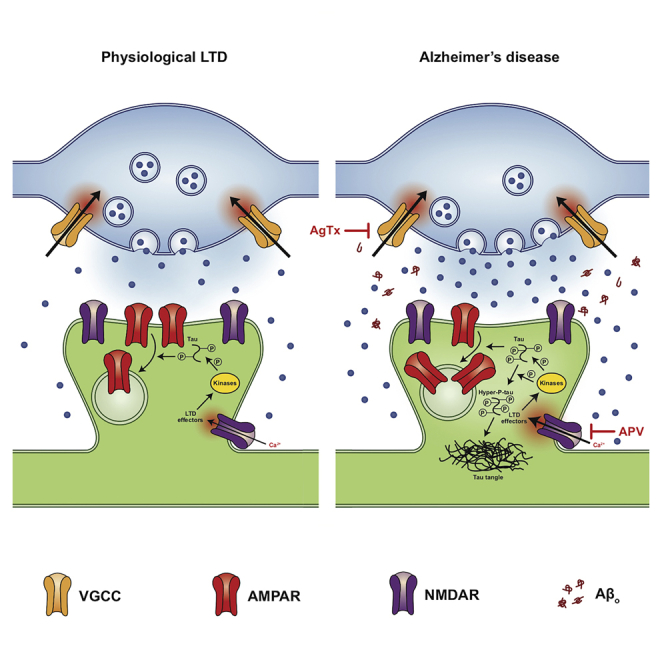
(G) Schematic diagram showing proposed mechanism of Aβo-induced tau hyperphosphorylation. Aβo enhance the probability of neurotransmitter release from the presynaptic terminal, resulting in increased low-frequency synaptic activity and/or activation of extrasynaptic NMDAR (1), thus promoting the induction of NMDAR-dependent LTD. LTD is initiated by an NMDAR-dependent Ca2+ influx that activates a variety of LTD effector proteins (2) that in turn activate LTD-associated kinases (3). During the physiological induction of LTD, these kinases phosphorylate tau (4), which alters its affinity for microtubules and helps to promote endocytosis and internalization of synaptic AMPAR (5). If the LTD induction stimulus is excessive or unusually prolonged, as a result of pathologically increased synaptic activity, kinase activation may be inappropriately sustained, potentially leading to hyperphosphorylation of tau at non-physiological residues (6). Hyperphosphorylated tau is in itself toxic and will eventually form stable aggregates that give rise to the histopathological inclusions (dystrophic neurites and neurofibrillary tangles) that are diagnostic of AD (7). Note that normalization of neurotransmitter release properties with a low dose of AgTx prevents the excessive induction of LTD and hyperphosphorylation of tau. Error bars represent ± SEM. ∗p < 0.05, ∗∗p < 0.01.
Discussion
Abnormal phosphorylation of tau is a critical event in AD pathophysiology, but how this is triggered by the oligomeric assemblies of Aβ that initiate the disease process is unknown. Here, we present evidence that Aβo-mediated augmentation of glutamate release probability is associated with both an increase in the magnitude of LTD induced by low-frequency synaptic stimulation and the pathological hyperphosphorylation of tau. Although physiological LTD induction recruits and phosphorylates tau at residues 396 and 404 only (Regan et al., 2015), we show that the chronic, sustained induction of LTD is sufficient to cause abnormal tau hyperphosphorylation at additional residues. Based on these findings, we propose a model in which pathogenic Aβo drive the recruitment and hyperphosphorylation of tau via enhanced neurotransmitter release probability and consequent inappropriate and/or sustained induction of LTD (Figure 4G). Hyperphosphorylated tau is neurotoxic (Kopeikina et al., 2012), and understanding the processes underlying its generation is of great importance, not only for the AD field, but also for other neurodegenerative diseases (tauopathies), in which aberrant tau phosphorylation plays a key role (Orr et al., 2017).
It is thought that the physiological role of tau is microtubule stabilization (Drubin and Kirschner, 1986; Lee et al., 1989), where the phosphorylation of tau tunes its ability to bind microtubules, thereby regulating axonal transport (Bramblett et al., 1993; Butner and Kirschner, 1991). Recent evidence that tau is also present in the dendrites of healthy neurons (Ittner et al., 2010; Kimura et al., 2013; Mondragón-Rodríguez et al., 2012; Swanson et al., 2017; Zempel et al., 2013) and, furthermore, that its phosphorylation is required for LTD (Kimura et al., 2013; Regan et al., 2015) raises the possibility that tau is additionally involved in trafficking of glutamate receptors at dendritic spines, a process critical for plasticity (Penn et al., 2017; Roth et al., 2020). Our data suggest that this process may be recruited by chronically elevated low-frequency synaptic activity during AD pathogenesis to drive tau phosphorylation. However, there is an important distinction between physiological phosphorylation of tau in LTD and pathological tau hyperphosphorylation as seen in AD, specifically that tau is phosphorylated at many more residues in AD than the two that are phosphorylated during induction of typical LTD (Regan et al., 2015). An explanation for this could be that excessive or prolonged activation of LTD-associated kinases arising from pathological, chronically enhanced synaptic activity might promote non-physiological phosphorylation of tau at additional residues. Indeed, one such kinase, GSK-3β, has already been implicated in both tau phosphorylation during LTD (Bradley et al., 2012; Kimura et al., 2013) and in the pathological phosphorylation events additionally required for the development of AD phenotypes (Deng et al., 2014; Jo et al., 2011; Shipton et al., 2011; Yi et al., 2018), including phosphorylation of tau residues recognized by the phosphorylation state-specific antibody AT8 (Liu et al., 2002; Wang et al., 1998). Several authors have proposed that GSK-3β plays a key role in AD pathogenesis in part because of this tau kinase activity (Hooper et al., 2008; Lauretti et al., 2020; Takashima, 2006), and GSK-3 inhibitors have been shown to have beneficial effects in AD models (Griebel et al., 2019; Hu et al., 2009; Morales-Garcia et al., 2012; Serenó et al., 2009). The current study adds mechanistic insight, further validating this therapeutic approach and suggesting that future work aimed at identifying tau kinases recruited by pathologically elevated synaptic activity might yield further potential targets for therapeutic intervention.
Overall, our work identifies a mechanistic link between the upstream Aβo-induced alterations in synaptic transmission and pathological tau phosphorylation. Many studies have indicated that Aβo accumulation triggers the downstream pathology of AD, including hyperphosphorylation of tau (Mucke and Selkoe, 2012), but no mechanism has previously been identified that clearly links these two critical players in AD pathology. This study, therefore, helps to fill a critical gap in our understanding of AD pathogenesis and in doing so implicates a plasticity process that offers a number of tractable targets for therapeutic intervention.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-phosphorylated tau (AT8) | Thermo Fisher Scientific | Cat# MN1020; RRID: AB_223647 |
| Rabbit anti-tau | Abcam | Cat# ab32057; RRID: AB_778254 |
| Mouse anti-phosphorylated tau (AT180) | Thermo Fisher Scientific | Cat# MN1040; RRID: AB_223649 |
| Mouse anti-β-actin | Insight Biotechnology | Cat# GTX629630; RRID: AB_2728646 |
| IRDye 800CW goat anti-mouse IgG | Li-Cor Biosciences | P/N 926-32210; RRID: AB_621842 |
| IRDye 680LT donkey anti-rabbit IgG | Li-Cor Biosciences | P/N 926-68023; RRID: AB_10706167 |
| Bacterial and virus strains | ||
| Adeno-associated virus for channelrhodopsin-2 expression - pAAV-EF1a-double floxed-hChR2(H134R)-EYFP-WPRE-HGHpA (AAV1) | Addgene; gift from Karl Deisseroth | 20298-AAV1; RRID: Addgene_20298 |
| Adeno-associated virus for Cre expression - pENN.AAV.hSyn.Cre.WPRE.hGH (AAV1) | Addgene; gift from James M. Wilson | 105553-AAV1; RRID: Addgene_105553 |
| Chemicals, peptides, and recombinant proteins | ||
| Human Aβ1-42 | Abcam | Cat# ab82795 |
| FM1-43 | Thermo Fisher Scientific | Cat# T3163 |
| ω-Agatoxin IVA | Alomone Labs | Cat# STA-500 |
| Adenosine | Santa Cruz Biotechnology | Cat# sc-291838 |
| LY341495 | Tocris Bioscience | Cat# 1209 |
| D-AP5 (APV) | Abcam | Cat# ab120003 |
| TBOA | Tocris Bioscience | Cat# 1223 |
| NMDA | Tocris Bioscience | Cat# 0114 |
| Propidium iodide | Sigma-Aldrich | Cat# P4170 |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice | Oxford University Biomedical Services | RRID:IMSR_JAX:000664 |
| Wistar rats | Charles River Laboratories UK | Strain code 003; RRID:RGD_737929 |
| Software and algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/; RRID: SCR_003070 |
| WinWCP | Strathclyde University | https://spider.science.strath.ac.uk/sipbs/software_ses.htm; RRID: SCR_014713 |
| Li-Cor Image Studio Lite | Li-Cor Biosciences | https://www.licor.com/bio/image-studio-lite/; RRID: SCR_013715 |
| Prism | GraphPad | https://www.graphpad.com/scientific-software/prism/; RRID: SCR_002798 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Alexander Jeans (alexander.jeans@pharm.ox.ac.uk).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Mice
Acute hippocampal slices were prepared from 7/8 week old C57BL/6 mice of either sex. Mice were housed in same-sex groups in individually ventilated cages (3-5 mice per cage) and maintained under standard, pathogen-free conditions on a 12 h light/dark cycle. Maintenance and procedures were fully compliant with both UK Home Office regulations and local institutional regulations.
Rats
Organotypic hippocampal slices were prepared from male P6 Wistar rat pups obtained directly from Charles River (UK). All procedures were were fully compliant with both UK Home Office regulations and local institutional regulations.
Method details
Preparation of acute hippocampal slices
7-8 week old C57BL/6 mice of either sex were sacrificed by dislocation of the cervix followed by decapitation, their brains extracted and placed in cold dissection media (65 mM sucrose, 85 mM NaCl, 2.5 mM KCl, 25 mM NaHCO3, 1.25 mM NaH2PO4, 10 mM d-glucose, 7 mM MgCl2⋅6H2O, and 0.5 mM CaCl2⋅6H2O). Coronal brain slices of 350 μm thickness were obtained using a Leica VT1000 S vibratome. Slices were allowed to recover for 1 h before use in a chamber containing oxygenated artificial cerebral spinal fluid (ACSF) (120 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 1.2 mM NaH2PO4, 11 mM d-glucose, 1 mM MgCl2⋅6H2O, and 2 mM CaCl2⋅6H2O).
Organotypic hippocampal slice preparation
Hippocampi from male Wistar rats (P6) were isolated in ice-cold Earle’s balanced salt solution with added 21 mM HEPES and 27.8 mM d-glucose (pH adjusted to 7.2-7.4 with NaOH) and then cut into coronal slices of 350 μm thickness using a McIlwain tissue chopper. Slices were placed onto Millicell CM culture plate inserts (polytetrafluoroethylene filter, pore size 0.4 μm, diameter 12 mm) in a six-well Millicell culture plate (both supplied by Merck Millipore) with 1 mL culture medium (78.8% minimum essential medium with GlutaMAX, 20% heat-inactivated horse serum, 1% B27 plus, with added 1 mM CaCl2, 30 mM HEPES, 26 mM d-glucose, 5.8 mM NaHCO3, and 2 mM MgSO4) and maintained at 34.5°C. Culture media were renewed every 3−4 days (Foster et al., 2018).
Synthesis and use of Aβ oligomers
Experiments were conducted using a single batch of Aβ1-42 peptide and oligomers were synthesized according to a validated protocol (Klein, 2002). Briefly, solid Aβ1-42 was dissolved in cold hexafluoro-2-propanol (HFIP). The peptide was incubated at room temperature for at least 1 h to establish monomerization and randomization of the structure. The HFIP was aliquoted and allowed to evaporate overnight, followed by 10 min in a Savant Speed Vac. The resulting peptide was stored as a film at −80°C. The film was dissolved in anhydrous dimethylsulfoxide (DMSO) (Sigma Aldrich) to 5 mM, diluted to approximately 100 μM with Ham’s F12 (without phenol red, with glutamine) (Caisson Laboratories, Logan, UT) and briefly vortexed. The solution was incubated at 4°C for 22−24 h, and soluble oligomers obtained by centrifugation at 14000 g for 10 min at 4°C. This preparation is well-characterized and highly reproducible, comprising mostly low-n oligomers such as tetramers and trimers (Chromy et al., 2003; Velasco et al., 2012).
Treatment and use of organotypic hippocampal slices
For drug incubation experiments, whether acute or chronic exposure, or applied alone or in combination, concentrations used were: Aβ oligomers 200 nM; ω-agatoxin IVA 50 nM; adenosine 20 μM; LY341495 100 μM; D-APV 50 μM; TBOA 20 μM; NMDA 20 μM. For chronic incubation experiments without electrophysiological recording, slices were lysed in RIPA buffer on DIV 14.
FM dye loading and unloading
Slices were transferred to a custom-made recording chamber mounted on an Olympus BX50WI microscope fitted with a BioRad Radiance 2000 confocal scanhead (BioRad/Zeiss) and were superfused at 35°C with oxygenated ACSF supplemented with 10 μM NBQX and 50 μM APV (both Tocris) to block recurrent activity. A patch pipette was filled with 20 μM of the styryl dye FM1-43 (Molecular Probes) in ACSF and placed in stratum radiatum of CA1 at a depth of approximately 100 μm. The dye was pressure applied for 3 min using a Picospritzer III (Intracel) before a 10 Hz train of 1200 stimuli (200 μA) was delivered to Schaffer collaterals using a glass stimulating electrode (4−8 MΩ) filled with 150 mM NaCl placed within 70 μm of the dye-filled pipette; the stimulating electrode was under the control of WIN WCP software (Strathclyde Electrophysiology Software) and a DS3 stimulation box (Digitimer). Pressure application of dye was maintained throughout the loading stimulus and for 2 min afterward to ensure completion of endocytosis. Slices were then perfused continuously in fresh ACSF for 20–25 min to wash residual FM dye from extracellular membranes. Imaging of labeled terminals was performed using a 63×, NA 0.9 LUMPlanFI objective (Olympus), a 488-nm Argon laser for excitation and a 500-nm long pass emission filter. Image stacks were acquired every 15 s throughout the unloading stimulus (3000 stimuli at 10 Hz). Each image stack comprised 4 images of 512 × 512 pixels acquired at 1 μm intervals in the z-axis, and a digital zoom of 3 x and 2 x Kalman averaging were applied. Images were acquired using Zeiss LaserSharp software and analyzed using ImageJ together with custom-written macros in Excel.
Electrophysiological field recordings in acute slices
Field excitatory postsynaptic potentials (fEPSPs) in the CA1 of acute hippocampal slices were recorded. Slices were placed in an interfaced recording chamber with oxygenated ACSF, and a bipolar stimulating electrode was placed in the Schaffer collaterals of CA3 neurons to deliver stimuli. A borosilicate glass recording electrode filled with ACSF was placed in the stratum radiatum of CA1 neurons. Neurons were stimulated every 25 s, with a paired pulse stimulation (50 ms after the first stimulus) delivered every four stimuli. Once a stable response was observed, a baseline recording was made for 10−15 min. Once a baseline had been recorded, LTD was induced using a 900 × 1 Hz LFS. For drug treatments, slices were incubated for >2 h in ω-agatoxin IVA (50 nM), adenosine (20 μM), Aβ oligomers (50 nM), or a combination as indicated, and recordings were performed in ACSF containing the same drugs.
Western blotting
Lysed samples were dissolved in 2X Laemmli sample buffer with 5% β–mercaptoethanol, heated to 60°C for 3 min and run on a precast 4%–20% gradient SDS-PAGE gel (Thermo Scientific). The separated samples were transferred to a nitrocellulose membrane (Bio-Rad) before blocking with 5% milk and 1% horse serum in TBS with 0.05% Tween-20 (TBST) and subsequent probing with the relevant combination of the following primary antibodies used at the following dilutions: anti-phosphorylated tau (AT8, 1:400); anti-tau phosphorylated at threonine 231 (AT180, 1:500); anti-total tau (1:1000); anti-β-actin (1:1000). All primary antibody incubations were carried out for 12 h (overnight) at 5°C. After three TBST washes, bound antibodies were incubated with the fluorescent-labeled secondaries IRDye 680LT donkey anti-rabbit IgG (1:20,000) and IRDye 800CW goat anti-mouse IgG (1:15,000) for 1 h at room temperature, washed three times in TBST and imaged on a Li-Cor Odyssey system. Images were analyzed quantitatively using Image Studio Lite software. The ratio of AT8 or AT180 to total tau, or tau to β-actin signals in each lane was measured. For phosphorylated tau blots, values were then normalized to the average of the corresponding control group to allow for discrepancies between runs.
Channelrhodopsin-2 expression
Organotypic slices were infected at 7 DIV using a patch pipette to deliver ∼1 μL aliquots directly into CA3, and used for experiments at DIV 12-19. CA3 was infected with recombinant AAV carrying double floxed and fluorescently tagged ChR2 (ChR2-eYFP), titer 3 × 1012 GC/mL, together with an AAV for Cre expression, titer 1 × 1013 GC/mL, after 7 days in vitro (DIV).
Electrophysiological patch-clamp experiments
Organotypic slices (10−14 DIV) were perfused (1 to 2 mL/min) with heated (32-34°C) artificial cerebral spinal fluid (ACSF) (145 mM NaCl, 2.5 mM KCl, 1.2 mM KH2PO4, 16.0 mM NaHCO3, 11.0 mM d-glucose, 3.0 mM CaCl2, and 2.0 mM MgCl2) aerated with 95% O2 and 5% CO2 (Foster et al., 2018). Whole-cell patch clamp recordings were performed on CA1 pyramidal neurons using low (4−8 MΩ) resistance patch electrodes filled with standard internal (135 mM KGluconate, 10 mM HEPES, 2 mM MgCl2, 2 mM Na2ATP, and 0.4 mM Na3GTP, pH 7.2−7.4). For chronic experiments, drugs were added to the culture medium of slices from 7 DIV for 7 days, and all drugs were used at the concentrations given above. Drugs were replaced each time slices were fed.
NMDA LTD
For NMDA LTD experiments, a glass stimulating electrode filled with ACSF was placed in stratum radiatum, nearer to CA3 than the recording electrode. Stimulation intensity was adjusted to evoke a 5-10 mV excitatory postsynaptic potential (EPSP). A 5 min baseline was recorded before perfusion of ACSF containing NMDA (20 μM) for 3 min and EPSPs were then recorded for another 32 min. EPSP slope was then measured, comparing slope post-NMDA addition with that of control (without any NMDA addition) at the same time point. For chronic chemical LTD experiments, from DIV 7 onward, NMDA (20 μM) was added for 3 min to the top of the slice in culture medium before being removed once a day for 7 days. Slices were then lysed in RIPA buffer on DIV 14.
Optogenetic LTD
For 500/900 × 1 Hz optogenetic LFS LTD, slices transfected in CA3 with ChR2 were used from DIV 12. To determine optimal stimulation intensity, a custom-built LED array was placed by the experimental bath and recordings were made from ChR2-expressing neurons. A stimulus intensity and duration of 0.3 mA, 20 ms was chosen because of its ability to reliably elicit one action potential (see Figures S3A–S3C). For LTD experiments, recordings were obtained from CA1 cells and EPSPs were elicited via optogenetic stimulation of CA3. After a 5 min baseline, LTD was induced, either with 500 × 1 Hz pulses or 900 × 1 Hz pulses, or a non-LTD-inducing stimulus (3 stimuli at 3 Hz, repeated every 3 s for 15 min) was given. EPSPs were then recorded for 30 mins post induction. This was repeated with LY351495 (100 μM) or APV (50 μM) for the 500 × 1 Hz protocol or 900 × 1 Hz protocol respectively. EPSP slopes were normalized to baseline, and average EPSP slope 25-30 min post induction was compared to the same time points for respective drug treatments. For chronic optogenetic LTD experiments, transfected slices were subjected to stimulation protocols as above every 2 h for 7 days from DIV 12, with optogenetic stimulation delivered by a custom-built array of blue LEDs in the incubator placed at the same distance from the slices as in the electrophysiological recording experiments. Slices were then lysed in RIPA buffer on DIV 19.
Cell viability assessment with propidium iodide
Following chronic optogenetic stimulation (500 × 1 Hz every 2 h for 7 days), organotypic slices were incubated in their culture wells with 5 μg/ml propidium iodide in culture medium for 30 min before imaging on a FLoid Cell Imaging Station (Life Technologies). Images of area CA1 were acquired and 3 circular regions of interest (ROI) were placed at evenly spaced intervals over the length of stratum pyramidale. For each slice, mean background-subtracted propidium iodide fluorescence in the three ROI was normalized to the control group mean.
Quantification and statistical analysis
All statistical tests were performed using Graphpad Prism software. Unless otherwise stated in the relevant figure legend, the two-tailed unpaired Student’s t test was used to determine the statistical significance of observed differences between conditions. Sample sizes (n) are reported in the relevant figure legends and p values are indicated in the figures themselves. Values of p < 0.05 were considered significant. Error bars in figures represent ± standard error of the mean.
Acknowledgments
A.F.J. was supported by an MRC Clinician Scientist Fellowship (G0802812). This work was also funded by research grants awarded to N.J.E. by the MRC (UK) (G0501572) and BBSRC (UK) (BB/J018724/1).
Author contributions
H.B.C.T. and A.F.J. planned and designed the study. H.B.C.T. and A.F.J. performed the experiments. N.J.E. provided oversight for the work. All authors contributed to the writing and reviewing of the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: August 31, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.109638.
Contributor Information
Nigel J. Emptage, Email: nigel.emptage@pharm.ox.ac.uk.
Alexander F. Jeans, Email: alexander.jeans@pharm.ox.ac.uk.
Supplemental information
Data and code availability
-
•
All data supporting the findings of this study are available either within the paper or from the Lead Contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.
References
- Alzheimer’s Association 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16:391–460. [Google Scholar]
- Arnth-Jensen N., Jabaudon D., Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat. Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- Bibow S., Ozenne V., Biernat J., Blackledge M., Mandelkow E., Zweckstetter M. Structural impact of proline-directed pseudophosphorylation at AT8, AT100, and PHF1 epitopes on 441-residue tau. J. Am. Chem. Soc. 2011;133:15842–15845. doi: 10.1021/ja205836j. [DOI] [PubMed] [Google Scholar]
- Billingsley M.L., Kincaid R.L. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem. J. 1997;323:577–591. doi: 10.1042/bj3230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak E., Braak H., Mandelkow E.M. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87:554–567. doi: 10.1007/BF00293315. [DOI] [PubMed] [Google Scholar]
- Bradley C.A., Peineau S., Taghibiglou C., Nicolas C.S., Whitcomb D.J., Bortolotto Z.A., Kaang B.K., Cho K., Wang Y.T., Collingridge G.L. A pivotal role of GSK-3 in synaptic plasticity. Front. Mol. Neurosci. 2012;5:13. doi: 10.3389/fnmol.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramblett G.T., Goedert M., Jakes R., Merrick S.E., Trojanowski J.Q., Lee V.M. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Brito-Moreira J., Paula-Lima A.C., Bomfim T.R., Oliveira F.B., Sepúlveda F.J., De Mello F.G., Aguayo L.G., Panizzutti R., Ferreira S.T. Aβ oligomers induce glutamate release from hippocampal neurons. Curr. Alzheimer Res. 2011;8:552–562. doi: 10.2174/156720511796391917. [DOI] [PubMed] [Google Scholar]
- Busche M.A., Konnerth A. Impairments of neural circuit function in Alzheimer’s disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016;371:20150429. doi: 10.1098/rstb.2015.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butner K.A., Kirschner M.W. Tau protein binds to microtubules through a flexible array of distributed weak sites. J. Cell Biol. 1991;115:717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromy B.A., Nowak R.J., Lambert M.P., Viola K.L., Chang L., Velasco P.T., Jones B.W., Fernandez S.J., Lacor P.N., Horowitz P. Self-assembly of Abeta(1-42) into globular neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- Deng Y., Xiong Z., Chen P., Wei J., Chen S., Yan Z. β-amyloid impairs the regulation of N-methyl-D-aspartate receptors by glycogen synthase kinase 3. Neurobiol. Aging. 2014;35:449–459. doi: 10.1016/j.neurobiolaging.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolev I., Fogel H., Milshtein H., Berdichevsky Y., Lipstein N., Brose N., Gazit N., Slutsky I. Spike bursts increase amyloid-β 40/42 ratio by inducing a presenilin-1 conformational change. Nat. Neurosci. 2013;16:587–595. doi: 10.1038/nn.3376. [DOI] [PubMed] [Google Scholar]
- Drubin D.G., Kirschner M.W. Tau protein function in living cells. J. Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Potier B. Susceptibility to Aβo and TBOA of LTD and Extrasynaptic NMDAR-Dependent Tonic Current in the Aged Rat Hippocampus. Neurochem. Res. 2019;44:692–702. doi: 10.1007/s11064-018-2677-6. [DOI] [PubMed] [Google Scholar]
- Ferreira S.T., Lourenco M.V., Oliveira M.M., De Felice F.G. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell. Neurosci. 2015;9:191. doi: 10.3389/fncel.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster W.J., Taylor H.B.C., Padamsey Z., Jeans A.F., Galione A., Emptage N.J. Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca2+ signaling. Sci. Signal. 2018;11:eaat9093. doi: 10.1126/scisignal.aat9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Gogolla N., Galimberti I., DePaola V., Caroni P. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat. Protoc. 2006;1:1165–1171. doi: 10.1038/nprot.2006.168. [DOI] [PubMed] [Google Scholar]
- Griebel G., Stemmelin J., Lopez-Grancha M., Boulay D., Boquet G., Slowinski F., Pichat P., Beeské S., Tanaka S., Mori A. The selective GSK3 inhibitor, SAR502250, displays neuroprotective activity and attenuates behavioral impairments in models of neuropsychiatric symptoms of Alzheimer’s disease in rodents. Sci. Rep. 2019;9:18045. doi: 10.1038/s41598-019-54557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- He Y., Wei M., Wu Y., Qin H., Li W., Ma X., Cheng J., Ren J., Shen Y., Chen Z. Amyloid β oligomers suppress excitatory transmitter release via presynaptic depletion of phosphatidylinositol-4,5-bisphosphate. Nat. Commun. 2019;10:1193. doi: 10.1038/s41467-019-09114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C., Killick R., Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Begum A.N., Jones M.R., Oh M.S., Beech W.K., Beech B.H., Yang F., Chen P., Ubeda O.J., Kim P.C. GSK3 inhibitors show benefits in an Alzheimer’s disease (AD) model of neurodegeneration but adverse effects in control animals. Neurobiol. Dis. 2009;33:193–206. doi: 10.1016/j.nbd.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B.C., Christie M.J., Napier I.A. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Jeans A., Padamsey Z., Taylor H., Weglinski C., Collins H., Bannerman D., Foster W.J., Klein W.L., van den Maagdenberg A., Emptage N. Partial Suppression of Cav2.1 Function Prevents Synaptic and Behavioral Impairments in Alzheimer’s Disease Models. SSRN. 2020 doi: 10.2139/ssrn.3701260. [DOI] [Google Scholar]
- Jeganathan S., Hascher A., Chinnathambi S., Biernat J., Mandelkow E.M., Mandelkow E. Proline-directed pseudo-phosphorylation at AT8 and PHF1 epitopes induces a compaction of the paperclip folding of Tau and generates a pathological (MC-1) conformation. J. Biol. Chem. 2008;283:32066–32076. doi: 10.1074/jbc.M805300200. [DOI] [PubMed] [Google Scholar]
- Jo J., Whitcomb D.J., Olsen K.M., Kerrigan T.L., Lo S.C., Bru-Mercier G., Dickinson B., Scullion S., Sheng M., Collingridge G., Cho K. Aβ(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat. Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Kabogo D., Rauw G., Amritraj A., Baker G., Kar S. ß-amyloid-related peptides potentiate K+-evoked glutamate release from adult rat hippocampal slices. Neurobiol. Aging. 2010;31:1164–1172. doi: 10.1016/j.neurobiolaging.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Kavalali E.T., Jorgensen E.M. Visualizing presynaptic function. Nat. Neurosci. 2014;17:10–16. doi: 10.1038/nn.3578. [DOI] [PubMed] [Google Scholar]
- Kemp N., Bashir Z.I. Long-term depression: a cascade of induction and expression mechanisms. Prog. Neurobiol. 2001;65:339–365. doi: 10.1016/s0301-0082(01)00013-2. [DOI] [PubMed] [Google Scholar]
- Kimura T., Whitcomb D.J., Jo J., Regan P., Piers T., Heo S., Brown C., Hashikawa T., Murayama M., Seok H. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;369:20130144. doi: 10.1098/rstb.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W.L. Abeta toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Kopeikina K.J., Hyman B.T., Spires-Jones T.L. Soluble forms of tau are toxic in Alzheimer’s disease. Transl. Neurosci. 2012;3:223–233. doi: 10.2478/s13380-012-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretti E., Dincer O., Praticò D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118664. doi: 10.1016/j.bbamcr.2020.118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Neve R.L., Kosik K.S. The microtubule binding domain of tau protein. Neuron. 1989;2:1615–1624. doi: 10.1016/0896-6273(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Kameyama K., Huganir R.L., Bear M.F. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Li S., Hong S., Shepardson N.E., Walsh D.M., Shankar G.M., Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Jin M., Koeglsperger T., Shepardson N.E., Shankar G.M., Selkoe D.J. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Iqbal K., Grundke-Iqbal I., Gong C.X. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3beta. FEBS Lett. 2002;530:209–214. doi: 10.1016/s0014-5793(02)03487-7. [DOI] [PubMed] [Google Scholar]
- Lozovaya N.A., Kopanitsa M.V., Boychuk Y.A., Krishtal O.A. Enhancement of glutamate release uncovers spillover-mediated transmission by N-methyl-D-aspartate receptors in the rat hippocampus. Neuroscience. 1999;91:1321–1330. doi: 10.1016/s0306-4522(98)00638-1. [DOI] [PubMed] [Google Scholar]
- Lüscher C., Malenka R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb. Perspect. Biol. 2012;4:a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E.M., Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragón-Rodríguez S., Trillaud-Doppia E., Dudilot A., Bourgeois C., Lauzon M., Leclerc N., Boehm J. Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. J. Biol. Chem. 2012;287:32040–32053. doi: 10.1074/jbc.M112.401240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragón-Rodríguez S., Perry G., Luna-Muñoz J., Acevedo-Aquino M.C., Williams S. Phosphorylation of tau protein at sites Ser(396-404) is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol. Appl. Neurobiol. 2014;40:121–135. doi: 10.1111/nan.12084. [DOI] [PubMed] [Google Scholar]
- Morales-Garcia J.A., Luna-Medina R., Alonso-Gil S., Sanz-Sancristobal M., Palomo V., Gil C., Santos A., Martinez A., Perez-Castillo A. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem. Neurosci. 2012;3:963–971. doi: 10.1021/cn300110c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L., Selkoe D.J. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J., Temmel M., Flunkert S., Kerschbaumer B., Hoeller C., Loeffler T., Niederkofler V., Daum G., Attems J., Hutter-Paier B. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2018;6:52. doi: 10.1186/s40478-018-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V., Grimm C., Draguhn A., Barghorn S., Lehmann A., Schoemaker H., Hillen H., Gross G., Ebert U., Bruehl C. Amyloid β oligomers (A β(1-42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J. Neurosci. 2008;28:788–797. doi: 10.1523/JNEUROSCI.4771-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M.E., Sullivan A.C., Frost B. A Brief Overview of Tauopathy: Causes, Consequences, and Therapeutic Strategies. Trends Pharmacol. Sci. 2017;38:637–648. doi: 10.1016/j.tips.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T., Oliet S.H. Organization, control and function of extrasynaptic NMDA receptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130601. doi: 10.1098/rstb.2013.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T., Ladépêche L., Ruel J., Sacchi S., Labasque M., Hanini M., Groc L., Pollegioni L., Mothet J.-P., Oliet S.H. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Penn A.C., Zhang C.L., Georges F., Royer L., Breillat C., Hosy E., Petersen J.D., Humeau Y., Choquet D. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature. 2017;549:384–388. doi: 10.1038/nature23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett E.K., Herrmann A.G., McQueen J., Abt K., Dando O., Tulloch J., Jain P., Dunnett S., Sohrabi S., Fjeldstad M.P. Amyloid Beta and Tau Cooperate to Cause Reversible Behavioral and Transcriptional Deficits in a Model of Alzheimer’s Disease. Cell Rep. 2019;29:3592–3604.e5. doi: 10.1016/j.celrep.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M., Dawson H.N., Binder L.I., Vitek M.P., Ferreira A. Tau is essential to beta-amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan P., Piers T., Yi J.-H., Kim D.-H., Huh S., Park S.J., Ryu J.H., Whitcomb D.J., Cho K. Tau phosphorylation at serine 396 residue is required for hippocampal LTD. J. Neurosci. 2015;35:4804–4812. doi: 10.1523/JNEUROSCI.2842-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Roth R.H., Cudmore R.H., Tan H.L., Hong I., Zhang Y., Huganir R.L. Cortical Synaptic AMPA Receptor Plasticity during Motor Learning. Neuron. 2020;105:895–908.e5. doi: 10.1016/j.neuron.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.L., Semerdjieva S., Empson R.M., Austen B.M., Beesley P.W., Alifragis P. Amyloid-β acts as a regulator of neurotransmitter release disrupting the interaction between synaptophysin and VAMP2. PLoS ONE. 2012;7:e43201. doi: 10.1371/journal.pone.0043201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serenó L., Coma M., Rodríguez M., Sánchez-Ferrer P., Sánchez M.B., Gich I., Agulló J.M., Pérez M., Avila J., Guardia-Laguarta C. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis. 2009;35:359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I., Brett F.M., Farrell M.A., Rowan M.J., Lemere C.A. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton O.A., Leitz J.R., Dworzak J., Acton C.E., Tunbridge E.M., Denk F., Dawson H.N., Vitek M.P., Wade-Martins R., Paulsen O., Vargas-Caballero M. Tau protein is required for amyloid beta-induced impairment of hippocampal long-term potentiation. J. Neurosci. 2011;31:1688–1692. doi: 10.1523/JNEUROSCI.2610-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S.A., Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones T.L., Hyman B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82:756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styr B., Slutsky I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat. Neurosci. 2018;21:463–473. doi: 10.1038/s41593-018-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson E., Breckenridge L., McMahon L., Som S., McConnell I., Bloom G.S. Extracellular Tau Oligomers Induce Invasion of Endogenous Tau into the Somatodendritic Compartment and Axonal Transport Dysfunction. J. Alzheimers Dis. 2017;58:803–820. doi: 10.3233/JAD-170168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 2006;9(Suppl 3):309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- Talantova M., Sanz-Blasco S., Zhang X., Xia P., Akhtar M.W., Okamoto S., Dziewczapolski G., Nakamura T., Cao G., Pratt A.E. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA. 2013;110:E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong R., Emptage N.J., Padamsey Z. A two-compartment model of synaptic computation and plasticity. Mol. Brain. 2020;13:79. doi: 10.1186/s13041-020-00617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco P.T., Heffern M.C., Sebollela A., Popova I.A., Lacor P.N., Lee K.B., Sun X., Tiano B.N., Viola K.L., Eckermann A.L. Synapse-binding subpopulations of Aβ oligomers sensitive to peptide assembly blockers and scFv antibodies. ACS Chem. Neurosci. 2012;3:972–981. doi: 10.1021/cn300122k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D.M., Klyubin I., Fadeeva J.V., Cullen W.K., Anwyl R., Wolfe M.S., Rowan M.J., Selkoe D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang J.Z., Wu Q., Smith A., Grundke-Iqbal I., Iqbal K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett. 1998;436:28–34. doi: 10.1016/s0014-5793(98)01090-4. [DOI] [PubMed] [Google Scholar]
- Yi J.H., Baek S.J., Heo S., Park H.J., Kwon H., Lee S., Jung J., Park S.J., Kim B.C., Lee Y.C. Direct pharmacological Akt activation rescues Alzheimer’s disease like memory impairments and aberrant synaptic plasticity. Neuropharmacology. 2018;128:282–292. doi: 10.1016/j.neuropharm.2017.10.028. [DOI] [PubMed] [Google Scholar]
- Zakharenko S.S., Zablow L., Siegelbaum S.A. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat. Neurosci. 2001;4:711–717. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]
- Zempel H., Luedtke J., Kumar Y., Biernat J., Dawson H., Mandelkow E., Mandelkow E.M. Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013;32:2920–2937. doi: 10.1038/emboj.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data supporting the findings of this study are available either within the paper or from the Lead Contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.