Keywords: epithelium, immunolocalization, kidney, thick ascending limb, tight junction
Abstract
Functional properties of the paracellular pathway depend critically on the set of claudins (CLDN) expressed at the tight junction. Two syndromes are causally linked to loss-of-function mutations of claudins: hypohidrosis, electrolyte imbalance, lacrimal gland dysfunction, ichthyosis, and xerostomia (HELIX) syndrome caused by genetic variations in the CLDN10 gene and familial hypomagnesemia with hypercalciuria and nephrocalcinosis caused by genetic variations in the CLDN16 or CLDN19 genes. All three genes are expressed in the kidney, particularly in the thick ascending limb (TAL). However, localization of these claudins in humans and rodents remains to be delineated in detail. We studied the segmental and subcellular expression of CLDN10, CLDN16, and CLDN19 in both paraffin-embedded and frozen kidney sections from the adult human, mouse, and rat using immunohistochemistry and immunofluorescence, respectively. Here, CLDN10 was present in a subset of medullary and cortical TAL cells, localizing to basolateral domains and tight junctions in human and rodent kidneys. Weak expression was detected at the tight junction of proximal tubular cells. CLDN16 was primarily expressed in a subset of TAL cells in the cortex and outer stripe of outer medulla, restricted to basolateral domains and tight junctional structures in both human and rodent kidneys. CLDN19 predominantly colocalized with CLDN16 in tight junctions and basolateral domains of the TAL but was also found in basolateral and junctional domains in more distal sites. CLDN10 expression at tight junctions almost never overlapped with that of CLND16 and CLDN19, consistent with distinct junctional pathways with different permeation profiles in both human and rodent kidneys.
NEW & NOTEWORTHY This study used immunohistochemistry and immunofluorescence to investigate the distribution of claudin 10, 16, and 19 in the human, mouse, and rat kidney. The findings showed distinct junctional pathways in both human and rodent kidneys, supporting the existence of different permeation profiles in all species investigated.
INTRODUCTION
The kidneys are critical for maintaining water and electrolyte balance. Downstream of every glomerulus, the renal tubule, and collecting duct reabsorb the bulk of filtered ions and water. The mammalian nephron and collecting duct are composed of different segments, each one having specific transport properties (1, 2). Renal electrolyte transport studies have focused predominantly on transcellular transport pathways in the kidney, with the paracellular pathway being less intensely studied. However, studies have demonstrated that paracellular permeability is highly specific to each nephron segment, and recent studies have shown that junctional permeability can be tightly regulated (3–5). The permeability characteristics of the paracellular junction to various solutes are determined by claudins (CLDN1), which are integral transmembrane proteins expressed at the tight junction (TJ). The mammalian claudin gene family comprises at least 27 members (6). Every tubular segment and collecting duct expresses a select set of claudins, contributing to the permeation profile of the paracellular pathway (7, 8).
Genetic variants of three claudins have been linked to rare human genetic syndromes. Genetic variants of CLDN10b cause Hypohidrosis, Electrolyte disturbances, hypoLacrimia, Ichtyosis, and Xerostomia (HELIX) syndrome (9), characterized by renal NaCl wasting, hypermagnesemia, and hypokalemia (9–12). In contrast, variants of CLDN16 or CLDN19 genes cause Familial Hypomagnesemia with Hypercalciuria and NephroCalcinosis (FHHNC) (13), characterized by excessive urinary loss of calcium (Ca2+) and magnesium (Mg2+), resulting in hypomagnesemia and hypercalciuria. Patients with FHHNC also develop nephrocalcinosis and renal failure (for a review, see Ref. 16).
Functional studies have revealed that renal NaCl wasting in patients with HELIX results from decreased NaCl reabsorption in the thick ascending limb (TAL) of Henle’s loop (9). In contrast, patients with FHHNC with variants of the CLDN16 gene have a selective defect in paracellular Mg2+ and Ca2+ reabsorption in the TAL, with intact NaCl reabsorption (15). Patients bearing CLDN19 variants likely have a similar defect in TAL reabsorption of Mg2+ and Ca2+ as patients bearing CLDN16 variants, since their renal phenotype is very similar (16). Functional studies in humans cannot determine whether the medullary TAL (MTAL) and/or cortical TAL (CTAL) are the primary drivers of electrolyte disturbances in these diseases. The TAL is axially heterogeneous with many structural and functional differences between medullary and cortical parts (17, 18). At least in rodents, Mg2+ and Ca2+ are mostly reabsorbed in the CTAL (17) through the paracellular pathway (17, 18). The paracellular permeability ratio of Na+ over Cl− (PNa/PCl) and PNa may be higher in inner stripe of outer medulla (ISOM) TALs (MTALISOM) compared with outer stripe of outer medulla (OSOM) TALs (MTALOSOM) and the CTAL (19).
Recent studies have added to this complexity, showing two types of TJs with a mosaic pattern in the mouse TAL. One type of junction was found to be expressing CLDN16 and CLDN19 and plays a major role in the paracellular transport of divalent cations, and a second type expressing CLDN10 would determine the paracellular permeability ratio PNa/PCl (18–22). Whether the same mosaic pattern of expression exists in humans remains to be determined. The pattern of expression of CLDN10, CLDN16, and CLDN19 along the remainder of the mammalian renal tubule and collecting duct remains controversial. A list of discrepancies is provided in Supplemental Tables S1−S3 (all Supplemental Material is available athttps://doi.org/10.6084/m9.figshare.14501982.v1). No comprehensive study comparing expression of CLDN10, CLDN16, and CLDN19 proteins has been performed in the human kidney (9, 23–25). Most studies have been made in rodents (Supplemental Tables S1−S3). Some studies have analyzed gene but not protein expression (26–32). Few studies have determined protein expression by immunoblot or mass spectrometry experiments on isolated tubular segments (33, 34). The latter experiments have their own merit but do not allow determination of the subcellular location of proteins. As expression of claudins at TJs or in intracellular/basolateral compartments certainly do not provide the same function, the advantage of immunostaining that allows subcellular location must be considered.
We aimed to determine and compare the expression patterns of CLDN10, CLDN16, and CLDN19 along the renal tubule and collecting duct using costaining for specific tubular segment markers and two different preparations (paraffin-embedded and frozen tissue), as epitope retrieval can be differently affected by distinct tissue processing techniques (35). We investigated the subcellular expression and colocalization of these claudins and determined whether a mosaic pattern of expression is also found in the human TAL.
MATERIALS AND METHODS
Antibodies
Mouse monoclonal antibodies directed against the COOH-terminal epitope of human CLDN16 GVSMAKSYSAPRTETAKMYAVDTRV were generated, as previously described in detail (36). NMRI mice were immunized using synthetic peptide coupled onto inactivated diphtheria toxoid and mixed with GERBU adjuvant. Mice received two injections of the immunization mixture over a period of 14 days. An intravenous booster injection of the conjugated CLDN16 peptide and epinephrine was given to the mice 3 days before the fusion of spleen cells. The SP2 myeloma cell line was used as the fusion partner, as previously described in detail (37). Clones were screened against the immunized peptide by ELISA and subsequently cloned out by limiting dilution. Positive clones producing antibodies against CLDN16 were screened on formalin-fixed paraffin-embedded rat and mice kidney sections, and clone 18 was selected for further characterization and used throughout the study. The production of mouse monoclonal antibodies was performed in accordance with Danish Law (animal experimental permit no. 2014-15-0201-00043). The antibodies used for immunofluorescence of frozen sections and for immunohistochemistry in paraffin-embedded tissues are shown in Table 1. We used costaining for specific tubular segment markers (Supplemental Fig. S1). For select colocalization experiments with Na+-K+-2Cl− cotransporter (NKCC2), an antibody directed against the epitope DDNTDPPHYEETSFGDEAQKRL in human NKCC2 was produced, as described above. The antibody reacted against the immunized peptide on ELISA and showed positive staining exclusively in the TAL of mouse, rat, and human kidney cross sections.
Table 1.
Antibodies used for immunofluorescence on cryosections and for immunohistochemistry on paraffin-embedded tissues
| Antigen | Host | Epitope | Condition | Dilution |
Reference | ||
|---|---|---|---|---|---|---|---|
| Human | Mouse | Rat | |||||
| Claudin 16 | Mouse | Human | IF | 1/100 | 1/25 | 1/100 | Characterized in this study |
| IHC | 1/1–1/100 | 1/1–1/100 | 1/1–1/100 | ||||
| Claudin 19 | Rabbit | Mouse | IF | 1/1,000 | 1/750 | 1/500 | Provided by J. Hou (Washington University, St. Louis, MO) (30) |
| IHC | 1/100 | 1/100 | 1/100 | ||||
| Claudin 10 | Rabbit | Human/Mouse | IF | 1/400 | 1/250 | 1/400 | No. 38-8400, Invitrogen, Life Technologies (Villebon-Sur Yvette, France) |
| IHC | 1/2,000–1/200 | 1/2,000–1/500 | 1/2,000–1/200 | ||||
| Mouse | Human | IF | 1/200 | 1/200 | 1/200 | No. 41-5100 Invitrogen, Life Technologies | |
| Megalin/LDL receptor-related protein 2 | Sheep | Rat | IF | 1/10,000 | 1/10,000 | 1/10,000 | Provided by R. Kozyraki (Institut National de la Santé et de la Recherche Médicale, Paris, France) (38) |
| Uromodulin | Mouse | Human | IF | 1/2,500 | 1/1,500 | 1/1,500 | THP (B-2), sc-271022, Santa Cruz Biotechnology (Dallas, TX) |
| Na+-K+-2Cl− cotransporter isoform 2 | Mouse | Human | IF | 1/50 | Generated by H. Dimke* | ||
| Rabbit | Rat | IF | 1/2,000 | 1/7,500 | 1/6,000 | Provided by J. Loffing (University of Zurich, Zurich, Switzerland) (39) | |
| Na+-Cl− cotransporter | Mouse | Human | IF | 1/50 | 1/50 | 1/50 | Generated by H. Dimke (40) |
| Rabbit | Mouse | IF | 1/1,000 | Provided by D. H. Ellison (Oregon Health & Science University, Portland, OR) (41) | |||
| Rabbit | Rat | IF | 1/2,500 | 1/2,500 | Provided by J. Loffing (University of Zurich, Zurich, Switzerland) (42) | ||
| Na+/Ca2+ exchanger isoform 1 | Mouse | Dog | IF | 1/1,000 | 1/1,000 | 1/1,000 | Provided by O. Bonny (University of Lausanne, Lausanne, Switzerland) (43) |
| Aquaporin-2 | Goat | Human | IF | 1/1,500 | 1/1,000 | 1/1,800 | C-17, sc-9882, Santa Cruz Biotechnology |
| Mouse | Human | IF | 1/400 | E-2, sc-515770, Santa Cruz Biotechnology | |||
| Cl−/anion exchanger isoform 1 | Guinea pig | Mouse | IF | 1/5,000 | 1/5,000 | 1/5,000 | Provided by C. Wagner (University of Zurich, Zurich, Switzerland) (44) |
| Pendrin | Guinea pig | Mouse | IF | 1/2,500 | 1/2,500 | 1/2,500 | Provided by C. Wagner (University of Zurich, Zurich, Switzerland) (45) |
| Zonula occludens-1 | Rabbit | Human | IF | 1/100 | No. 61-7300, Invitrogen, Life Technologies | ||
| Rat | Rat | IF | 1/50 | 1/50 | 1/10 | ZO-1 (R40.76), sc-33725, Santa Cruz Biotechnology | |
IF, immunofluorescence on cryosections; IHC, immunohistochemical staining on paraffin-embedded tissues. *This antibody only stains the thick ascending limb of Henle’s loop in the mouse, rat, and human kidney and is positive to the immunizing peptide on ELISA.
Tissue Acquisition
All experiments were conducted in accordance with institutional guidelines and recommendations for the care and use of laboratory animals (animal experimental permit no. 2019-15-0201–01629, French project authorization nos. CEEA5-2012-084 and CEEA5-2017-3877). The following animal models were used: Sprague–Dawley rats (Charles River, France), homozygous Cldn16 knockout (Cldn16−/−) (46) and their wild-type littermates (Cldn16+/+), and conditional Cldn10 deficient due to Ksp-cadherin-driven Cre-recombinase expression in the distal nephron (Cldn10fl/fl;Ksp-Cre) and their control littermates (Cldn10fl/fl) (22). Human tissue was removed from kidneys due to renal carcinoma (license no. S-20140159 from the Biomedical Research Ethics Committee of Southern Denmark and CPP2012-08-09 Projet10BVE-OncoHEGP from Ethics Committee “Ile de France II”). The kidney tissue used was excised from the healthy part of the nephrectomized kidney not affected by tumor growth.
Immunofluorescence on Cryosections
Immunofluorescence staining was done on 7-µm cryosections of 4% paraformaldehyde-perfused mouse and rat kidneys and 4% paraformaldehyde-fixed human kidneys. Sections were fixed and permeabilized in cold methanol for 20 min at −20°C, heated in citrate buffer (pH 6, Dako) or Tris-EDTA (pH 9, Dako) [for Cldn19 and for zonula occludens (ZO)-1 staining] in a water bath at 94°C for 10 min for antigen retrieval, and then cooled for 20 min at room temperature. Nonspecific binding sites were blocked in 10% serum-1% BSA, and sections were incubated overnight at 4°C with primary antibody. Sections were then washed and incubated at room temperature with Cy5 or Alexa Fluor 488-, 555-, or 647-labeled secondary antibodies (Jackson ImmunoResearch or Invitrogen, Thermo Fisher Scientific). DAPI was used for staining nuclei. Images were recorded using a Zeiss LSM710 confocal microscope or a Zeiss Axio Scan Z1 scanning microscope (Carl Zeiss Jena).
Immunohistochemical Staining on Paraffin-Embedded Tissues
Tissue was fixed in 10% formalin or 4% paraformaldehyde and stored in PBS until being embedded with paraffin. Tissue-Tek Vacuum Infiltration Processor 6 (Sakura Finetek, Torrance, CA) was used to embed the tissue, and blocks were subsequently sectioned on a HM 355S Automatic Microtome (Thermo Fisher Scientific, Waltham, MA) at 2 µm. Paraffin-embedded kidney cross sections were stained, as previously described in detail with minor modifications (47). Sections were rehydrated using Tissue-Clear (Tissue-Tek, Sakura Sections) before being placed in a series of graded ethanols. Following rehydration, heat-induced antigen retrieval was performed using Tris-EGTA buffer (10 mM Tris and 0.5 mM EGTA, pH 9.0). Free aldehyde groups were blocked using 50 mM NH4Cl in PBS, and endogenous peroxidase enzymes were blocked with 0.6% H2O2. Sections were then incubated at 4°C with primary antibody overnight dissolved in 0.1% Triton X-100 in PBS. Following a wash, sections with CLDN10 and CLDN19 were incubated with goat antirabbit secondary antibodies conjugated to horseradish peroxidase (HRP; DakoCytomation, Glostrup, Denmark) and sections with CLDN16 with HRP-conjugated goat antimouse IgG1 γ1 heavy chain antibodies (No. 610-103-040, Rockland). The DAB+ Substrate Chromogen System (K3467, DakoCytomation) was used to visualize HRP activity. Sections were counterstained with hematoxylin before being mounted with Aquamount. Light microscopy was carried out using an Olympus BX51 microscope.
RESULTS
Characterization of Anti-CLDN10 and Anti-CLDN16 Antibodies
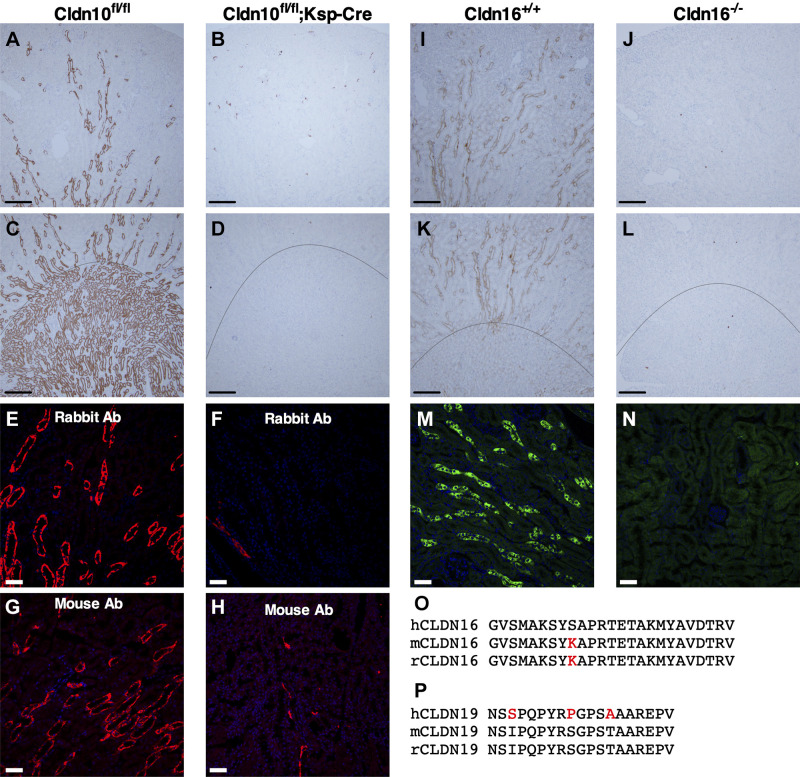
We confirmed the specificity of rabbit and mouse anti-CLDN10 antibodies (Fig. 1, A–H). Using rabbit anti-CLDN10 antibody, immunoreactivity was present in high abundance in the MTAL and CTAL of Cldn10fl/fl control mice (Fig. 1, A and C) and only in a few TALs in Cldn10fl/fl;Ksp-Cre mice due to mosaic excision of Ksp-Cre (Fig. 1, B and D). Very similar results were obtained in cryosections: intense immunoreactivity was present in the TAL of Cldn10fl/fl control mice with rabbit and mouse anti-CLDN10 antibodies (Fig. 1, E and G), whereas very few TALs showed immunoreactivity in Cldn10fl/fl;Ksp-Cre mice (Fig. 1, F and H). The sequences of the immunizing peptides for these commercial anti-CLDN10 antibodies are not available, but the antibody raised in the rabbit is directed toward the COOH-terminus of human/mouse claudin-10 protein and the mouse monoclonal antibody is generated by immunization with a peptide mixture from the middle and COOH-terminal regions of human CLDN10. Consequently, no antibody is available to distinguish between CLDN10a and CLDN10b proteins.
Figure 1.
Validation of anticlaudin (CLDN)16 and anti-CLDN10 antibodies (Ab) in wild-type (WT) mice and Cldn16- or Cldn10-specific knockout (KO) mice. A–D: immunohistochemistry for CLDN10 in paraffin-embedded cortical and medullary kidney tissue from WT (Cldn10fl/fl; A and C) and Cldn10 KO mice with kidney-specific deletion (Cldn10fl/fl;Ksp-Cre; B and D). A and B: cortex/outer stripe of the outer medulla (OSOM), C and D: outer medulla. Scale bars = 200 µm. E−H: immunofluorescence for CLDN10 in the outer medulla from WT (E: rabbit anti-CLDN10 antibody and G: mouse anti-CLDN10 antibody) and Cldn10 knockout mice with kidney-specific deletion (F: rabbit anti-CLDN10 antibody and H: mouse anti-CLDN10 antibody). Scale bars = 50 µm. I−L: immunohistochemistry for CLDN16 in paraffin-embedded cortical and medullary kidney tissue from WT (Cldn16+/+) mice [I: cortex/OSOM and K: OSOM/inner stripe of the outer medulla (ISOM)] and Cldn16 KO (Cldn16−/−) mice (J: cortex/OSOM and L: OSOM/ISOM). Scale bars = 200 µm. M and N: immunofluorescence for CLDN16 in frozen cortical kidney tissue from WT (M) and Cldn16 KO mice (N). Scale bars = 50 µm. The line indicates the border between the ISOM and OSOM. O and P: alignments of the parts of the amino acid sequence of CLDN16 (O) and CLDN19 (P) from the human, mouse, and rat used to generate antibodies: anti-CLDN16 antibody was generated against the human epitope and anti-CLDN19 antibody was generated against the mouse epitope. The differences between human and rodent sequences are shown in red.
The mouse monoclonal anti-CLDN16 antibody is directed against the COOH-terminal end of human CLDN16. The mouse and rat CLDN16 epitope differ only in one amino acid from the human (Fig. 1O). In the Cldn16+/+ mouse kidney, CLDN16 was found in the TAL (Fig. 1, I and K). Immunohistochemical staining on paraffin-embedded sections from the Cldn16−/− mouse kidney with monoclonal mouse anti-CLDN16 antibody and HRP-conjugated secondary IgG1 γ1 heavy chain-specific antibodies showed absence of staining (Fig. 1, J and L). Subsequent immunohistochemical stainings were done along with a no-primary/secondary-only control. For immunofluorescent staining on frozen sections, staining was observed in TALs of Cldn16+/+ mouse kidneys (Fig. 1M), which was absent in Cldn16−/− mice, and no obvious background could be detected in kidneys from Cldn16−/− mice (Fig. 1N).
No Cldn19-deficient mouse was available for validation of anti-CLDN19 antibody in our applications. Furthermore, no validation has been previously done using this antibody. Anti-CLDN19 antibody was raised against the mouse epitope, which does not differ between the rat and mouse but has a three-amino acid differences with the human epitope (Fig. 1P).
Expression of CLDN10 Along the Human, Mouse, and Rat Renal Tubule and Collecting Duct System
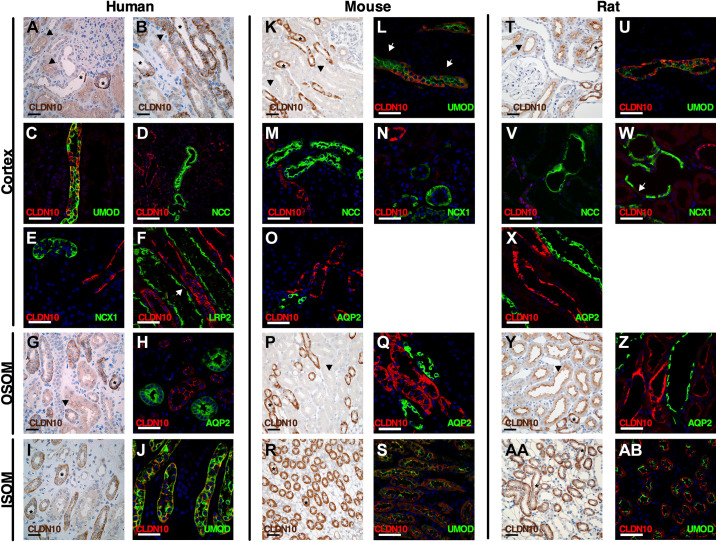
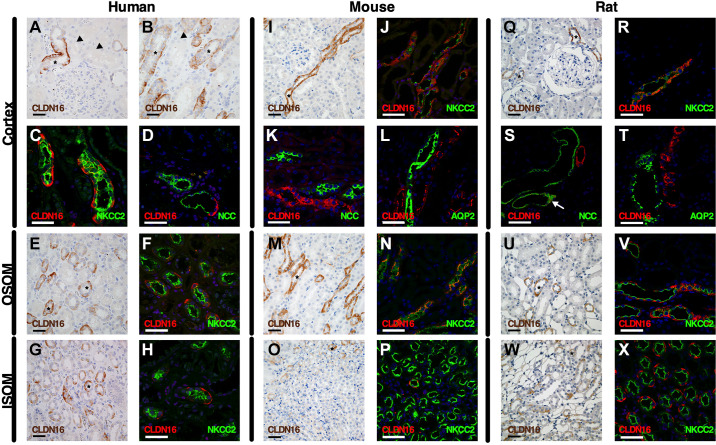
Figure 2 shows the findings on expression of CLDN10, CLDN16, and CLDN19 at the TJ in the three species studied and shown in the subsequent figures. CLDN10 was mostly expressed at the TJ and in basolateral membrane domains across the species tested. Immunohistochemical staining on 2-µm thin sections from paraffin-embedded sections from the human kidney revealed abundant CLDN10 immunoreactivity in TAL tubules in the cortex (Fig. 3, A and B). Lower intensity staining appeared to be present in the macula densa (Fig. 3A). Weak CLDN10 signal was present in apical domains of proximal convoluted tubules and straight tubules in medullary rays in the cortex (Fig. 3, A and B). Not all TAL cells appeared to express CLDN10 in paraffin-embedded human kidneys, and this was confirmed using colocalization with the TAL marker uromodulin (UMOD) in 7-µm frozen sections (Fig. 3C, Supplemental Fig. S2, C and D, Supplemental Fig. S3A, and Supplemental Fig. S4A). Macula densa cells showed basolateral and rare junctional staining (Supplemental Fig. S2, A and B, and Supplemental Fig. S3A). Junctional staining with CLDN10 was also only present between select CTAL cells (Fig. 4A). Colocalization between CLDN10 and a distal convoluted tubule (DCT) marker, the thiazide-sensitive NaCl cotransporter (NCC), showed that CLDN10 staining was absent from the DCT (Fig. 3D). Furthermore, CLDN10 was also absent from the remainder of the distal convolution [comprising the DCT, connecting tubule (CNT), and initial portion of the cortical collecting duct (CCD)], as determined by colocalization with the Na+/Ca2+ exchanger (NCX1; Fig. 3E). Furthermore, no CLDN10 staining was visible in the CCD, as determined using aquaporin (AQP)2 costaining (data not shown). In frozen sections, colocalization with megalin/LDL receptor-related protein 2 (LRP2) showed junctional expression of CLDN10 in the proximal tubule (Fig. 3F and Fig. 4B). Here, the staining intensity was much weaker than that observed for TAL segments, and proximal tubular staining was not observed in all frozen sections.
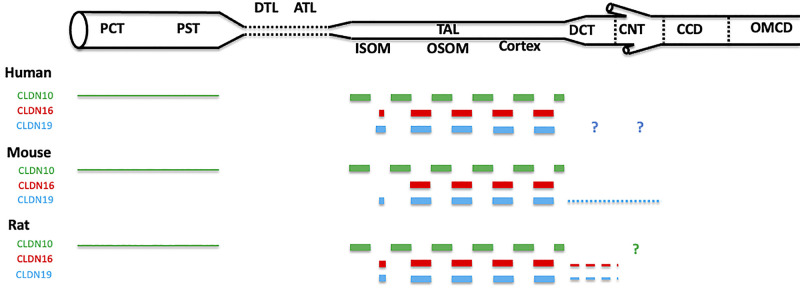
Figure 2.
Summary of expression at the tight junction of claudin (CLDN)10, CLDN16, and CLDN19 in human, mouse, and rat renal tubules and collecting ducts. Shown is the relative expression of a given claudin between different segments of the nephron in the same species. The dashed line indicates that some cells do not express the corresponding protein at the tight junction. The dotted line indicates that a minority of cells expresses the corresponding protein at the tight junction. The thickness of the line indicates the relative intensity of expression of the corresponding protein at the tight junction. Relative intensities of expression should not be compared between different claudins expressed by the same species nor between the same claudin expressed by different species. ATL, ascending thin limb; CCD, cortical collecting duct; CNT, connecting tubule; DCT, distal convoluted tubule; DTL, descending thin limb of Henle’s loop; ISOM, inner stripe of the outer medulla; OMCD, outer medullary collecting duct; OSOM, outer stripe of the outer medulla; PCT, proximal convoluted tubule; PST, proximal straight tubule; TAL, thick ascending limb of Henle’s loop. The expression of claudins in the inner medulla was not studied. ?, Expression at the tight junction could not be confirmed nor excluded.
Figure 3.
Expression of claudin (CLDN)10 in the human, mouse, and rat renal tubule and collecting duct. Immunostaining for CLDN10 in kidney tissue from humans (left), mice (middle), and rats (right) in the cortex, outer stripe of the outer medulla (OSOM), and inner stripe of the outer medulla (ISOM). Paraffin-embedded sections were stained from human (A, B, G, and I), mouse (K, P, and R), and rat (T, Y, and AA) kidneys using anti-CLDN10 antibody. Scale bars = 50 µm. Arrowheads indicate examples of proximal tubular staining. *Thick ascending limb (TAL) of Henle’s loop. Frozen sections were evaluated by colabeling human (C–F, H, and J), mouse (L–O, Q, and S), and rat (U–X, Z, and AB) kidneys using anti-CLDN10 antibody (red) with the following specific segment markers (green) along the renal tubule: megalin/LDL receptor-related protein 2 (LRP2), uromodulin (UMOD), Na+-Cl− cotransporter (NCC), Na+-Ca2+ exchanger (NCX1), and aquaporin-2 (AQP2). In F and L, the arrows show weak apical staining in the proximal tubule. This apical proximal tubular staining of CLDN10 is better visualized in Fig. 4, B, D, and E). In W, the arrow indicates weak apical staining in the connecting tubule. Scale bars = 50 µm.
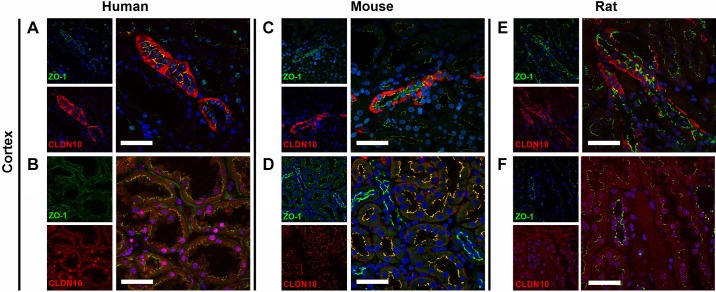
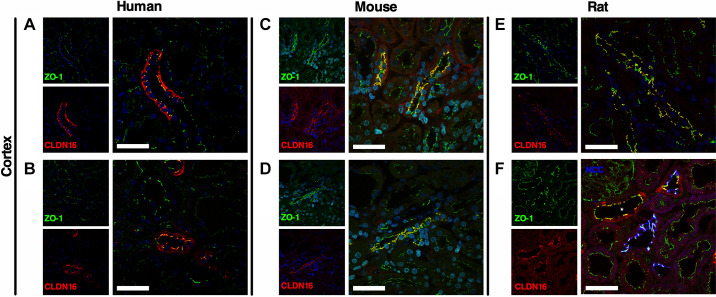
Figure 4.
Colocalization of claudin (CLDN)10 and zonula occludens-1 (ZO-1) in human, mouse, and rat kidneys. CLDN10 was colocalized with ZO-1 in the human (A and B), mouse (C and D), and rat (E and F) kidney cortex. CLDN10 was expressed at the tight junction in the thick ascending limb of Henle’s loop (A, C, and E) and in proximal tubules (B, D, and F). Scale bars = 50 µm.
In the OSOM of paraffin-embedded sections from the human kidney, strong CLDN10 staining intensity was again found in TAL tubules (MTALOSOM), in basolateral membrane domains, and at the TJ, whereas proximal tubular expression was lower and restricted to the TJ (Fig. 3G). On frozen sections, TJ strands were seen forming between some TAL cells, and double-labeling experiments with aquaporin-2 (AQP2) revealed that CLDN10 was absent from the collecting duct (Fig. 3H). Of note, in the human kidney, a clear boundary between the OSOM and ISOM within the outer medulla was difficult to identify (17). Similar findings were seen in the ISOM, where paraffin-embedded sections from the human kidney showed strong CLDN10 staining in MTALISOM (Fig. 3I). Staining appeared absent from the medullary collecting ducts. On frozen sections, CLDN10 coexpressed with the TAL marker UMOD (Fig. 3J, Supplemental Fig. S2, E and F, and Supplemental Fig. S4B). Here, it was clear that, as in the other TAL segments, CLDN10 expression was restricted to a subset of TAL cells and that junctions were formed between only some of these.
In paraffin-embedded sections from the mouse kidney, CLDN10 showed a similar expression pattern as that found in the human kidney with intense basolateral staining in some, but not all, cells of the CTAL and staining in the apical membrane domains of proximal tubules (Fig. 3K). Some punctuate background staining was observed in some tubules of the distal nephron (Fig. 3K); however, this staining was also present in Cldn10fl/fl;Ksp-Cre mice. Colocalization with UMOD and ZO-1 on frozen sections confirmed this and showed the formation of TJ strands between select CTAL cells (Fig. 3L, Fig. 4C, Supplemental Fig. S2, I and J, and Supplemental Fig. S5A). CLDN10 was expressed in some basolateral domains of mouse macula densa cells (Supplemental Fig. S2, G and H, and Supplemental Fig. S3I). CLDN10 was expressed at TJs in the proximal tubule (Fig. 4D). In most cases, no CLDN10 staining was observed on frozen sections in the mouse DCT when costained with NCC (Fig. 3M), in the CNT costained with NCX1 (Fig. 3N), and in the CCD costained with AQP2 (Fig. 3O). In the OSOM, intense staining was observed in the MTALOSOM on paraffin-embedded sections, with weaker staining in the apical membrane domains in the proximal tubules (Fig. 3P). Costaining with AQP2 showed no colocalization of expression on frozen sections. Again, not all MTALOSOM expressed CLDN10 and not all formed CLDN10-positive staining at the TJ (Fig. 3Q). In the ISOM, CLDN10 expression in paraffin-embedded sections showed most cells of the MTALISOM to express CLDN10 and no staining in collecting ducts (Fig. 3R). This was confirmed in frozen sections with colocalization of CLDN10 and UMOD and TJ staining in numerous MTALISOM cells (Fig. 3S, Supplemental Fig. S2, K and L, and Supplemental Fig. S5B).
CLDN10 distribution in the rat was similar to that in the mouse and human. In paraffin-embedded sections from the rat kidney, CLDN10 had intense expression in basolateral domains in the CTAL and staining in apical domains of the proximal tubules (Fig. 3T). On frozen sections, CLDN10 and UMOD were coexpressed in CTAL cells, where CLDN10 was expressed in basolateral domains in some, but not all, CTAL cells, and CLDN10 expression in TJs between some of these cells was also visible (Fig. 3U, Fig. 4E, Supplemental Fig. S2, O and P, and Supplemental Fig. S6A). CLDN10 was expressed in basolateral domains of some rat macula densa cells (Supplemental Fig. 2, M and N, and Supplemental Fig. S3N). No staining was observed with CLDN10 and NCC in the DCT (Fig. 3V). Occasionally, weak CLDN10 apical staining was seen in rat tubules expressing NCX1 on frozen sections (Fig. 3W). No staining was observed in the CCD when colocalized with AQP2 (Fig. 3X). In frozen sections in the rat kidney, CLDN10 was also seen at TJs in proximal tubules (Fig. 4F), but staining was less intense than in the TAL and variable between experiments. On paraffin-embedded sections, CLDN10 was found in a subset of MTALOSOM cells and in apical membrane domain of proximal tubular cells, as observed for mice and humans (Fig. 3Y). On frozen sections, CLDN10 was expressed at TJs and in basolateral domains of MTALOSOM cells; no staining was observed in AQP2-expressing cells (Fig. 3Z). In the ISOM, staining was similar to the mouse, with many MTALISOM cells expressing CLDN10 (Fig. 3AA). This was also observed when colocalized with UMOD (Fig. 3AB, Supplemental Fig. S2, Q and R, and Supplemental Fig. S6B). Data on expression and localization of CLDN10 are shown in Tables 2, 3 and 4 and Fig. 2.
Table 2.
Summary of immunostaining of CLDN10, CLDN16, and CLDN19 in the human renal tubules and collecting system
| Megalin/LRP2 |
UMOD |
NKCC2 |
NCC |
NCX1 |
AQP2 |
AE1 |
Pendrin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortex | OSOM | Cortex | OSOM | ISOM | Cortex | OSOM | ISOM | Cortex | Cortex | Cortex, OSOM, ISOM | Cortex, OSOM, ISOM | Cortex | |
| CLDN10 | * | * | *** | *** | *** | 0 | 0 | 0 | $ | $ | |||
| CLDN16 | 0 | 0 | *** | *** | * | 0 | 0 | $ | $ | ||||
| CLDN19 | 0/very rare | * | *** | *** | *** | *,£ | **,£ | *** | *** | *** | |||
Localization was determined by costaining for the following specific markers in immunofluorescence: megalin/LDL receptor-related protein 2 (LRP2), uromodulin (UMOD), Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), Na+/Ca2+ exchanger isoform 1 (NCX1), aquaporin-2 (AQP2), Cl−/
anion exchanger isoform 1 (AE1), and pendrin. ISOM, inner stripe of the outer medulla; OSOM, outer stripe of the outer medulla. *, **, and ***, Expression with increasing intensity. 0, Expression absent or below the limit of detection. $, Costainings for AE1-CLDN10, pendrin-CLDN10, AE1-CLDN16, and pendrin-CLDN16 were not performed in human kidney sections because we did not find any CLDN10 and CLDN16 staining in tubules expressing AQP2. £, We could not determine whether CLDN19 is expressed at tight junctions in the human distal convoluted tubule and connecting tubule for technical reasons and despite multiples tries.
Table 3.
Summary of immunostaining of CLDN10, CLDN16, and CLDN19 in the mouse renal tubule and collecting system
| Megalin/LRP2 |
UMOD |
NKCC2 |
NCC |
NCX1 |
AQP2 |
AE1 |
Pendrin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortex | OSOM | Cortex | OSOM | ISOM | Cortex | OSOM | ISOM | Cortex | Cortex | Cortex, OSOM, ISOM | Cortex, OSOM, ISOM | Cortex | |
| CLDN10 | £ | £ | ***, TJ: ** | ***, TJ: ** | ***, TJ: ** | 0 | 0 | 0 | $ | $ | |||
| CLDN16 | # | # | ***, TJ: *** | ***, TJ: *** | Very rare, TJ:? | 0 or unusual | 0 | $ | $ | ||||
| CLDN19 | 0 | 0 | ***, TJ: ** | ***, TJ: ** | ***TJ: very rare | **TJ: *, ∼50% of DCTs | **TJ: *, rare | ***no TJ staining | ***no TJ staining | ***no TJ staining | |||
Localization was determined by costaining for the following specific markers in immunofluorescence: megalin/LDL receptor-related protein 2 (LRP2), uromodulin (UMOD), Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), Na+/Ca2+ exchanger isoform 1 (NCX1), aquaporin-2 (AQP2), Cl−/
anion exchanger isoform 1 (AE1), and pendrin. DCT, distal convoluted tubule; ISOM, inner stripe of the outer medulla; OSOM, outer stripe of the outer medulla. *, **, and ***, Expression with increasing intensity. 0, Expression absent or below the limit of detection. TJ, Expression at the tight junction. £, Costaining of megalin-claudin (CLDN)10 was not performed in mouse kidney sections because the proximal tubule could be identified according its morphology. CLDN10 was expressed at tight junctions in the proximal tubule. The staining in the proximal tubule was weaker than that in the thick ascending limb of Henle’s loop. #, Costaining of megalin-CLDN16 was not performed in mouse kidney sections. However, no CLDN16 staining could be found in the proximal tubule that was identified according its morphology. $, Costainings of AE1-CLDN10, pendrin-CLDN10, AE1-CLDN16, and pendrin-CLDN16 were not performed in mouse kidney sections because we did not find any CLDN10 and CLDN16 staining in tubules expressing AQP2. ?, Expression at the tight junction could not be confirmed nor excluded.
Table 4.
Summary of immunostaining of CLDN10, CLDN16, and CLDN19 in rat renal tubules and collecting system
| Megalin/LRP2 |
UMOD |
NKCC2 |
NCC |
NCX1 |
AQP2 |
AE1 |
Pendrin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortex | OSOM | Cortex | OSOM | ISOM | Cortex | OSOM | ISOM | Cortex | Cortex | Cortex, OSOM, ISOM | Cortex, OSOM, ISOM | Cortex | |
| CLDN10 | £ | £ | ***,TJ: ** | ***, TJ: ** | ***, TJ: ** | 0 | *, rare, apical | 0 | $ | $ | |||
| CLDN16 | # | # | ***, TJ: ** | ***, TJ: ** | ***TJ: rare | *, ∼50% of DCTs, TJ: ∼50% of DCTs | 0 | $ | $ | ||||
| CLDN19 | 0 | 0 | ***,TJ: ** | ***, TJ: ** | ***TJ: rare | **, ∼50% of DCTs, TJ: ∼50% of DCTs | **no TJ staining | ***no TJ staining | ***no TJ staining | ***no TJ staining | |||
Localization was determined by costaining for the following specific markers in immunofluorescence: megalin/LDL receptor-related protein 2 (LRP2), uromodulin (UMOD), Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), Na+/Ca2+ exchanger isoform 1 (NCX1), aquaporin-2 (AQP2), Cl−/
anion exchanger isoform 1 (AE1), and pendrin. DCT, distal convoluted tubule; ISOM, inner stripe of the outer medulla; OSOM, outer stripe of the outer medulla. *, **, and ***, Expression with increasing intensity. 0, Expression absent or below the limit of detection. TJ, Expression at the tight junction. £, Costaining of megalin-claudin (CLDN)10 was not performed in rat kidney sections because the proximal tubule could be identified according its morphology. CLDN10 was expressed at tight junctions in the proximal tubule. CLDN10 staining in the proximal tubule was weaker than that in the thick ascending limb of Henle’s loop. #, Costaining of megalin-CLDN16 was not performed in rat kidney sections. However, no CLDN16 staining could be found in the proximal tubule that was identified according its morphology. $, Costainings of AE1-CLDN10, pendrin-CLDN10, AE1-CLDN16, and pendrin-CLDN16 were not performed in rat kidney sections because we did not find any CLDN10 and CLDN16 staining in tubules expressing AQP2.
Expression of CLDN16 Along the Human, Mouse, and Rat Renal Tubule and Collecting Duct System
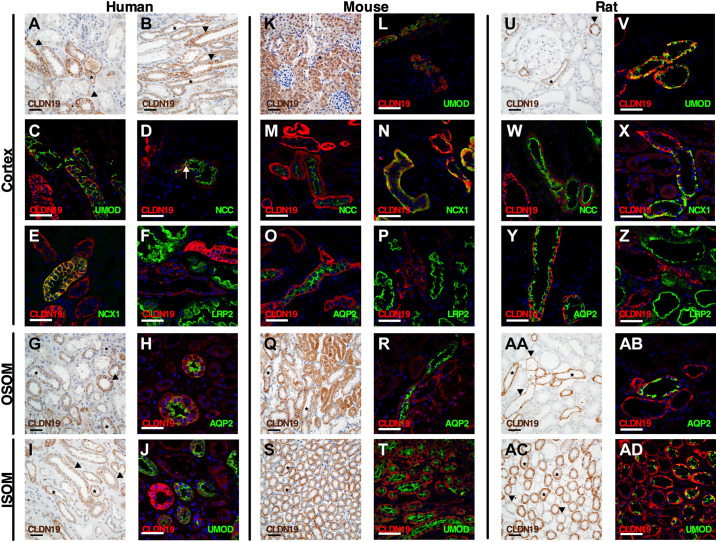
In the human, mouse, and rat cortex, CLDN16 was expressed in basolateral membrane domains and at TAL TJs. In the human kidney cortex, abundant expression of CLDN16 was observed in the TAL but only in a subset of CTAL cells (Fig. 5, A and B). No CLDN16 staining was apparent in the macula densa (Fig. 5A). Proximal tubular immunoreactivity was not seen using CLDN16 antibody in either proximal convoluted or straight tubules in the cortex (Fig. 5, A and B). CLDN16 expression in basolateral membrane domains was found in a subset of CTAL cells (Fig. 5C and Supplemental Fig. S4C). Similarly, immunoreactivity at TJs was found with CLDN16 only between a subset of CTAL cells in the tubule (Fig. 6, A and B). Colocalization with NCC and AQP2 revealed that CLDN16 was absent from the human DCT and CCD (Fig. 5D and Supplemental Fig. S3B).
Figure 5.
Expression of claudin (CLDN)16 in the human, mouse, and rat renal tubule and collecting duct. Shown is immunostaining for CLDN16 in kidney tissue from humans (left), mice (middle), and rats (right) in the cortex, outer stripe of the outer medulla (OSOM), and inner stripe of the outer medulla (ISOM). Paraffin-embedded sections were stained in human (A, B, E, and G), mouse (I, M, and O), and rat (Q, U, and W) kidneys using anti-CLDN16 antibody. Scale bars = 50 µm. *Examples of thick ascending limb (TAL) of Henle’s loop. Frozen sections were evaluated by colabeling human (C, D, F, and H), mouse (J –L, N, and P), and rat (R–T, V, and X) kidneys using anti-CLDN16 antibody (red) with the following specific segment markers (green) along the renal tubule: Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), and aquaporin-2 (AQP2). Since the CLDN16 antibody was raised in mice, we used a TAL marker, the furosemide-sensitive cotransporter (NKCC2), to perform colocalization experiments on frozen sections. In A and B, the arrowheads indicate proximal tubules. In S, the arrow shows CLDN16 expression at the tight junction of the distal convoluted tubule. Scale bars = 50 µm.
Figure 6.
Colocalization of claudin (CLDN)16 and zonula occludens-1 (ZO-1) in human, mouse, and rat kidneys. CLDN16 and ZO-1 were colocalized in humans (A and B), mice (C and D), and rats (E and F) in the kidney cortex. CLDN16 was expressed at tight junctions in the human, mouse, and rat thick ascending limb of Henle’s loop (A–F) and in the rat distal convoluted tubule (F). Colabeling on kidney sections with the Na+-Cl− cotransporter (NCC) was performed. *Thick ascending limb of Henle’s loop. Scale bars = 50 µm.
In paraffin-embedded sections from the human kidney, strong CLDN16 staining intensity was observed in a subset of MTALOSOM cells in basolateral membrane domains and TJs, as found in the cortex (Fig. 5E). On frozen sections, this was verified by costaining with NKCC2 (Fig. 5F); however, in the human outer medulla, CLDN16 was mostly expressed in human TALs that were close to the cortex. This was verified on frozen sections costaining with NKCC2, where TJ immunostaining could be readily seen between a subset of cells. No staining seemed apparent in proximal tubules and collecting ducts (data not shown). In the ISOM, staining was still visible in some cells of the MTALISOM on paraffin-embedded sections from the human kidney, albeit it appeared less frequent than that observed in the more cortical portions (Fig. 5G). On frozen sections, CLDN16 was restricted to very few MTALISOM cells and TJs in double-labeling experiments with NKCC2 (Fig. 5H and Supplemental Fig. S4D).
A similar expression pattern was observed in the mouse kidney. CLDN16 immunostaining on paraffin-embedded sections showed intense basolateral staining in a subset of CTAL cells in the basolateral membrane domain and near TJs (Fig. 5I). No apparent staining was visible at TJs in the macula densa segment. Double labeling with NKCC2 and CLDN16 confirmed this pattern of expression and showed TJ immunoreactivity between a subset of CTAL cells (Fig. 5J, Fig. 6, C and D, and Supplemental Fig. S5C). No staining was visible in the proximal tubules. CLDN16 staining could not be seen in the mouse DCT and CCD (Fig. 5, K and L, and Supplemental Fig. S3J). MTALOSOM staining was intense in paraffin-embedded mouse kidney sections, as before only in a subset of cells (Fig. 5M). Not all NKCC2-positive MTALOSOM cells expressed CLDN16 or showed immunoreactivity in TJs between cells (Fig. 5N and Supplemental Fig. S5D). Mouse MTALISOM cells showed immunoreactivity restricted to cells localized mainly in close proximity to the OSOM border, as evaluated on paraffin-embedded cross sections (Fig. 5O). Furthermore, in frozen sections, basolateral CLDN16 staining was observed in a very few MTALISOM cells and almost never in TJ strands (Fig. 5P). No expression was observed in the medullary collecting ducts.
Overall expression of CLDN16 in the rat resembled that in the human and mouse. In basolateral membrane domains of the CTAL, CLDN16 was abundantly expressed in a subset of cells. No expression was apparent in the macula densa or appeared visible in other tubular segments (Fig. 5Q). CLDN16 and NKCC2 were found to be coexpressed in a subset of CTAL cells on frozen sections, where TJ immunoreactivity was readily observed between numerous cells (Fig. 5R, Fig. 6E, and Supplemental Fig. S6C). Colocalization to some DCT cells was observed between CLDN16 and NCC, contrary to human and mouse DCT. CLDN16 immunoreactivity was seen in TJs but was absent from basolateral membrane domains (Fig. 5S and Fig. 6F). The morphology of the TJ in the DCT was different, straighter, than in the TAL. No colocalization was seen in CCD cells expressing AQP2 in frozen rat kidney sections (Fig. 5T). In paraffin-embedded sections, CLDN16 staining was seen in a subset of MTALOSOM cells of the rat kidney (Fig. 5U). This was confirmed on frozen sections, where a subset of NKCC2-positive cells was found to express CLDN16 and staining was visible in TJ domains as well between some, but not all, cells (Fig. 5V). CLDN16 staining in the ISOM of paraffin-embedded sections was found in multiple MTALISOM cells compared with the mouse (Fig. 5W). This was confirmed in frozen rat sections; however, CLDN16 was most often expressed in intracellular compartments rather than at the TJ (Fig. 5X and Supplemental Fig. S6D).
Data on the expression and localization of CLDN16 are shown in Tables 2, 3 and 4 and Fig. 2.
Expression of CLDN19 Along the Human, Mouse, and Rat Renal Tubule and Collecting Duct System
Immunohistochemical staining with anti-CLDN19 antibody on paraffin-embedded sections on human kidney sections revealed immunoreactivity in basolateral membrane domains of numerous cortical tubules (Fig. 7, A and B). CLDN19 immunoreactivity was absent from proximal tubules in the cortex but present in the distal convolution and CCD tubules (Fig. 7, A and B). Again, CLDN19 expression was observed in basolateral membrane domains in a subset of UMOD-positive CTAL cells (Fig. 7C and Supplemental Fig. S4E). Furthermore, CLDN19 staining was seen in TJs formed between a subset of CTAL cells (Fig. 8A). Weak CLDN19 immunoreactivity was detected in apical and basolateral membrane domains of some human DCT, as evidenced by colocalization with NCC (Fig. 7D). CLDN19 staining was observed in basolateral and lateral domains of the CNT, as observed when colocalized with NCX1 (Fig. 7E). Furthermore, additional CLDN19 staining was observed in the same pattern in the remainder of the human CCD, as evaluated by colocalization with AQP2, anion exchanger 1 (AE1), and pendrin (Supplemental Fig. S3, C−E). Likely, the lateral domains did not represent TJs of the collecting duct system (Fig. 8B). As with paraffin-embedded sections, frozen section from the human cortex showed almost no CLDN19 staining in proximal tubules, as evidenced when colabeled with the proximal tubular marker megalin/LRP2 (Fig. 7F).
Figure 7.
Expression of claudin (CLDN)19 in the human, mouse, and rat renal tubule and collecting duct. Immunostaining for CLDN19 in kidney tissue from humans (left), mice (middle), and rats (right) in the cortex, outer stripe of the outer medulla (OSOM), and inner stripe of the outer medulla (ISOM). Paraffin-embedded sections were stained in human (A, B, G, and I), mouse (K, Q, and S), and rat (U, AA, and AC) kidneys using anti-CLDN19 antibody. Scale bars = 50 µm. *Examples of thick ascending limb of Henle’s loop. Arrowheads show the collecting system. Colabeling on kidney sections from humans (C–F, H, and J), mouse (L–P, R, and T), and rat (V–Z, AB, and AD) kidneys using anti-CLDN19 antibody (red) with the following specific segment markers (green) along the renal tubule was performed: megalin/LDL receptor-related protein 2 (LRP2), uromodulin (UMOD), Na+-Cl− cotransporter (NCC), Na+/Ca2+ exchanger (NCX1), and aquaporin-2 (AQP2). In D, the arrow shows the expression of CLDN19 in the distal convoluted tubule. Scale bars = 50 µm.
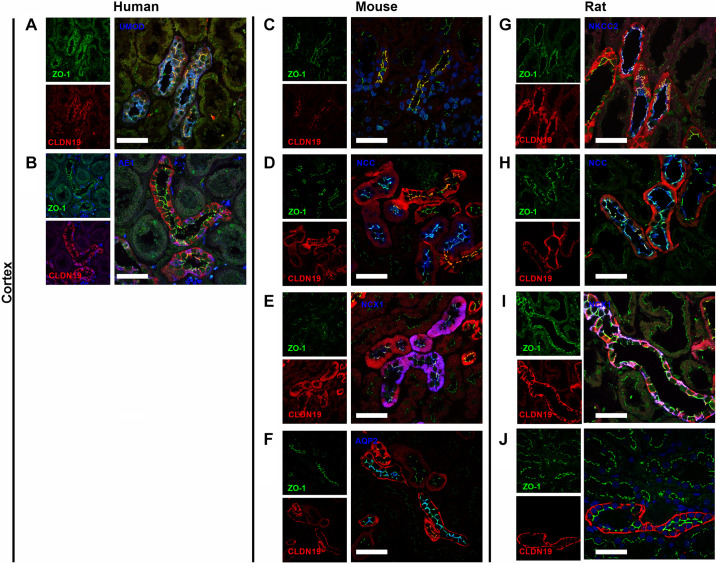
Figure 8.
Colocalization of claudin (CLDN)19 and zonula occludens-1 (ZO-1) in human, mouse, and rat kidneys. CLDN19 was colocalized with ZO-1 in human (A and B), mouse (C–F), and rat (G–J) kidneys. Colabeling on kidney sections with uromodulin (UMOD), Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), Na+/Ca2+ exchanger (NCX1), aquaporin-2 (AQP2), or Cl−/ anion exchanger isoform 1 (AE1) was performed. CLDN19 was expressed at the tight junction (TJ) in the thick ascending limb of Henle’s loop in humans (A), mice (C), and rats (G). CLDN19 was expressed at TJs in the distal convoluted tubule in mice (D) and rats (H). CLDN19 was expressed at a few TJs in the mouse connecting tubule (E) not in the rat connecting tubule (I). CLDN19 was not expressed at TJs in human (B), mouse (F), and rat cortical collecting ducts (J). Scale bars = 50 µm.
Strong CLDN19 immunoreactivity was also observed in a subset of MTALOSOM cells and collecting duct cells on paraffin-embedded sections from the human kidney. Staining was restricted primarily to basolateral membrane domains in the MTALOSOM and basolateral and lateral domains of the collecting duct (Fig. 7G). Costaining with UMOD verified this on frozen sections and showed CLDN19-positive staining in TJs between some MTALOSOM cells (data not shown). Unlike CLDN16, CLDN19 was expressed throughout the entire MTAL. Furthermore, CLDN19 expression was observed in AQP2-expressing OSOM collecting ducts (Fig. 7H). Occasionally, cells of the MTALISOM showed CLDN19 immunoreactivity on paraffin-embedded sections from the human kidney (Fig. 7I). On frozen sections, CLDN19 staining was observed in several MTALISOM cells and TJs (Fig. 7J and Supplemental Fig. S4F). In the outer medulla, most tubules expressing megalin/LRP2 showed CLDN19 staining in basolateral domains (Supplemental Fig. S3H).
Using anti-CLDN19 antibody on paraffin-embedded sections in the mouse kidney revealed that most tubules showed positive staining, which for some may appear to be background, but we were not able to evaluate this on Cldn19 knockout mice. CLDN19 immunostaining was found in proximal tubules and other tubules in the cortex (Fig. 7K). Colabeling CLDN19 with UMOD or ZO-1 on frozen mouse kidney sections revealed CLDN19 expression in most CTAL cells, both at the TJ and in basolateral membrane domains (Fig. 7L, Fig. 8C, and Supplemental Fig. S5E). Weak basolateral and TJ staining was seen in mouse DCTs when colabeling was performed with CLDN19 and NCC (Fig. 7M and Fig. 8D). Basolateral and TJ staining was seen in DCT2/CNT cells expressing NCX1 (Figs. 7N, Fig. 8E, and Supplemental Fig. S3K). Furthermore, CLDN19 staining in basal and lateral domains was seen in AQP2-, AE1-, and pendrin-expressing cells in the CCD (Fig. 7O, Fig. 8F, and Supplemental Fig. S3, L and M). In contrast to paraffin-embedded sections from mice, CLDN19 staining on frozen sections in mouse proximal tubules was not detected as validated by costaining with megalin/LRP2 (Fig. 7P). In paraffin-embedded mouse kidney sections, MTALOSOM staining was seen in the mouse kidney but there was also marked staining in the proximal tubules (Fig. 7Q). In frozen sections, some, but not all, UMOD-positive MTALOSOM cells expressed CLDN19 in the basolateral domains and TJs of the mouse kidney (data not shown). As observed with CLDN16, CLDN19 was expressed in basolateral compartments and TJs of MTALs along the entire OSOM. Furthermore, CLDN19 expression was observed in the medullary collecting duct, as evidenced by AQP2 costaining (Fig. 7R). In paraffin-embedded mouse kidney sections, staining was observed in basolateral membrane domains in MTALISOM cells and collecting duct cells in the ISOM (Fig. 7S). On frozen sections, mouse MTALISOM cells showed staining in basolateral membrane domains; however, very few showed CLDN19 immunoreactivity of the TJ strands. Small tubules, which could be thin limbs in the mouse, showed basolateral CLDN19 staining, and collecting ducts appeared to stain as well (Fig. 7T and Supplemental Fig. S5F).
In rat kidney sections, the staining was much cleaner and more in agreement between paraffin-embedded and frozen sections with respect to staining. In paraffin-embedded sections, CLDN19 was found in basolateral membrane domains of the majority of CTAL cells. Furthermore, expression was apparent in both the macula densa and cells of the collecting duct system (Fig. 7U). On frozen sections, CLDN19 were expressed in most CTAL cells, with TJ immunoreactivity readily apparent between numerous cells (Fig. 7V, Fig. 8G, and Supplemental Fig. S6E). CLDN19 colocalized with NCC to the DCT, where it stained basolateral domains and TJs in some DCT cells (Fig. 7W and Fig. 8H). Basolateral CLDN19 immunoreactivity was seen in the CNT, where it colocalized with NCX1 (Fig. 7X and Fig. 8I). This pattern of CLDN19 expression extended into AQP2-, AE1-, and pendrin-expressing cells of the CCD in frozen rat kidney sections (Fig. 7Y, Fig. 8J, and Supplemental Fig. S3, O and P). CLDN19 was not detectable in megalin/LRP2-expressing tubules in the rat cortex (Fig. 7Z). In MTALOSOM cells of the rat kidney in paraffin-embedded sections, CLDN19 staining was predominantly seen in basolateral membrane domains of the majority of cells (Fig. 7AA). CLDN19 expression in basolateral and TJ domains was confirmed in MTALOSOM cells in frozen sections and in basolateral domain of AQP2-expressing collecting duct cells (Fig. 7AB). In paraffin-embedded sections from the rat kidney, MTALISOM and collecting duct cells were found to abundantly express CLDN19 in basolateral membrane domains (Fig. 7AC). In rat kidney frozen sections, this was confirmed with colabeling with UMOD as a TAL marker (Fig. 7AD and Supplemental Fig. S6F); a few TJs expressed CLDN19 in the MTALISOM. As in the mouse ISOM, the small tubules expressing CLDN19 were likely thin limbs (Fig. 7AD and Supplemental Fig. S6F). Data on the expression and localization of CLDN19 are shown in Tables 2, 3 and 4 and Fig. 2.
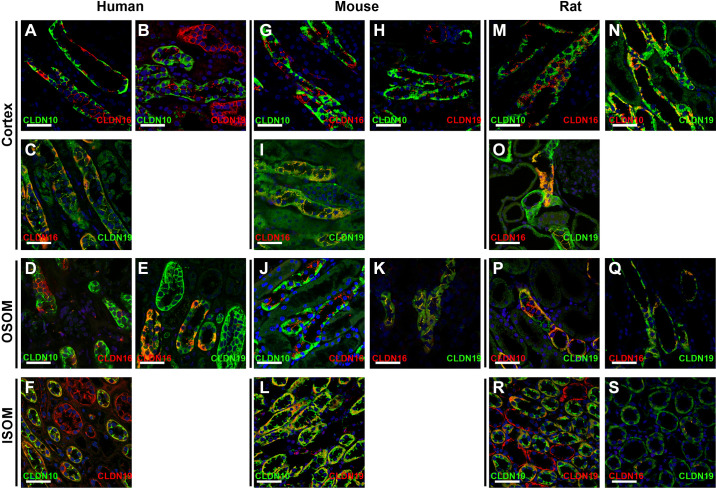
Colocalization of CLDN10, CLDN16, and CLDN19 in the TAL
In human kidney frozen sections, in the majority of CTAL cells, CLDN10 expression in the basolateral domains did not colocalize with CLDN16 (Fig. 9A). However, in a subset of CTAL cells, colocalization was observed in the basolateral domains (Supplemental Fig. S3F); importantly, CLDN10 and CLDN16 were not colocalized at TJs in the human CTAL. A similar coexpression pattern to basolateral membrane domains and TJs was observed in human frozen sections where CLDN10 was costained with CLDN19 (Fig. 9B). CLDN16 and CLDN19 were colocalized in TJs in the CTAL in frozen sections (Fig. 9C). In some cases, unusual TJ immunoreactivity was observed in cells expressing CLDN19 without CLDN16 (Supplemental Fig. S3G). In the MTALOSOM of tubules located near the corticomedullary boundary of the human kidney, CLDN10 and CLDN16 immunoreactivity (Fig. 9D) and CLDN16 and CLDN19 immunoreactivity (Fig. 9E) in frozen sections revealed a similar expression pattern as that observed in the CTAL. CLDN10 and CLDN19 were colocalized in few TJs in the human medulla (data not shown). In the ISOM, CLDN19 was expressed at TJs with or without CLDN16 (not shown). CLDN10 and CLDN19 were both found to be expressed in MTAL tubules, mostly in basolateral membrane domains, and also at TJs, where they colocalized sometimes (Fig. 9F).
Figure 9.
Colocalization of claudin (CLDN)10, CLDN16, and CLDN19 in the human, mouse, and rat renal tubule and collecting duct. Coimmunostaining for CLDN10 and CLDN16, CLDN10 and CLDN19, and CLDN16 and CLDN19 was performed in kidney tissue from humans (A–F, left), mice (G–L, middle), and rats (M–S, right) in the cortex, outer stripe of the outer medulla (OSOM), and inner stripe of the outer medulla (ISOM). Scale bars = 50 µm.
In the mouse kidney, colocalization of CLDN10 and CLDN16 on frozen sections revealed, as in humans, very rare colocalization in basolateral membrane domains of CTAL cells but not in TJs (Fig. 9G). A similar observation was made when CLDN10 and CLDN19 were stained (Fig. 9H). CLDN16 and CLDN19 showed almost complete colocalization at the TJ in mouse CTAL cells (Fig. 9I). In the MTALOSOM, CLDN10 and CLDN16 only colocalized in the basolateral domains of some cells, but not in TJs (Fig. 9J), whereas CLDN16 and CLDN19 showed mostly complete colocalization, with very few TJs expressing CLDN19 alone (Fig. 9K). Both CLDN10 and CLDN19 showed expression to the MTALISOM, but mostly not colocalized at TJs (Fig. 9L).
CLDN10 and CLDN16 were not colocalized in rat TJs in the CTAL (Fig. 9M) and the same was observed for CLDN10 and CLDN19 (Fig. 9N), whereas some cells expressed both in basolateral domains. In the rat, CLDN16 and CLDN19 were colocalized in TJs of the CTAL and DCT (Fig. 9O). In the MTALOSOM, the pattern was the same as that described in the mouse for colocalization between CLDN10 and CLDN19 (Fig. 9P) and CLDN16 and CLDN19 (Fig. 9Q). Both CLDN10 and CLDN19 were expressed in basolateral domains of the MTALISOM (Fig. 9R). CLDN16 and CLDN19 colocalized at TJs of a few cells in the rat MTALISOM (Fig. 9S). CLDN16 and CLDN19 were mostly not colocalized with CLDN10 at TJs.
DISCUSSION
The main purpose of our study was to determine the renal expression pattern of three claudins, CLDN10, CLDN16, and CLDN19, involved in HELIX and FHHNC genetic syndromes with a primary TAL phenotype and compare their expression in human, mouse, and rat kidneys. We used two different techniques for immunostaining allowing subcellular location: 2-µm thin sections from paraffin-embedded kidney tissue and 7-µm sections from frozen kidney tissue. Both allow determination of the cellular localization of claudins; however, 2-µm sections may allow better evaluation of the individual cells, whereas 7-µm frozen sections allow better visualization of TJs.
Here, we describe, for the first time, CLDN10 expression in both the human CTAL and MTAL, as only its expression in the CTAL was previously reported (9, 23), and confirm CLDN10 expression along the length of the mouse and rat MTAL and CTAL (14, 19, 21, 22, 48, 49). CLDN10 is expressed in both TJs and basolateral membrane domains in the human and murine MTAL and CTAL. CLDN10 expressed in basolateral domains may have its own function in the basolateral compartment or may be able to shuttle to the TJ to modify its permeability properties. CLDN10 expressed in the TJ likely plays a crucial role in paracellular electrolyte flux. Based on our observations from humans and others in mice (21), loss-of-function mutations of CLDN10 in HELIX syndrome likely affect paracellular NaCl transport in both the MTAL and CTAL; however, it is uncertain whether the defect in one segment predominates over the defect in the other segment. Noteworthy, we can see no evidence supporting a difference in cellular CLDN10 expression between the MTALISOM and MTALOSOM, or between the MTAL and CTAL, in any species. Consistently, ex vivo studies on the isolated perfused CTAL and MTAL from Cldn10fl/fl;Ksp-Cre mice showed a lower paracellular permeability ratio PNa/PCl than TALs from Cldn10fl/fl littermates (21, 22).
CLDN10 expression is found in human, mouse, and rat proximal tubules, similar to that already described in other studies (9, 22, 23, 48–50). Surprisingly, the staining is not found in all proximal tubules and in all experiments in frozen sections, whereas its expression is commonly seen in paraffin-embedded tissue. This highlights the importance of using the two techniques and the sensitivity of HRP-based systems. Unlike the findings reported by Van Itallie et al. (49), we did not observe any specific CLDN10 staining in the mouse DCT, CNT, CCD, outer medullary collecting duct, or inner medullary collecting duct, whatever the technique. In contrast to the present study, the specificity of the anti-CLDN10 antibody was not validated on kidneys from Cldn10-deficient animals.
The CLDN16 location has previously been studied in human cortex, where expression was noted in the CTAL; however, the authors also reported weak proximal tubular staining with their antibody (23). Here, we describe CLDN16 staining restricted largely to the human CTAL and MTAL. Importantly, we assessed the specificity of the antibody on CLDN16-deficient mice. These findings are in line with the previously reported expression of CLDN16 in the mouse and rat MTAL and CTAL (19, 48, 51, 52). CLDN16 is found to be expressed in basolateral membrane domains and in the TJ. As with CLDN10, the function of CLDN16 in the basolateral membrane domains remains to be determined, but a subset of these could provide a reservoir for trafficking to the TJ. In fact, in vitro experiments suggested that phosphorylation of CLDN16 can affect its trafficking to the TJ (53). It was recently published that CLDN16 was almost only expressed in the mouse MTALOSOM and CTAL (14, 19, 21) and that CLDN16 was only expressed in a maximum 1% of TJs in the mouse MTALISOM. The tubules with many CLDN16-positive TJs were found to be more permeable to Mg2+ than to Na+ (19). On paraffin-embedded human and rat sections, we did see expression of CLDN16 in basolateral membrane domains of the MTALISOM, whereas the mouse MTALISOM seemed to be more devoid of CLDN16 staining. When evaluated on frozen sections, CLDN16 expression in the MTALISOM rarely appeared in the TJ in either species. We could see no clear difference in CLDN16 expression either in basolateral domains or in TJs between the MTALOSOM and CTAL, whatever the species.
In a recent study, CLDN16 was localized by immunofluorescnce to the TAL in mouse and rat kidney sections (54). Furthermore, CLDN19 was colocalized with UMOD in the rat. Unlike the current study, the authors found that both CLDN16 and CLDN19 were evenly expressed over the length of the MTAL, with a localization pattern similar to that of UMOD. In their study, rabbit anti-CLDN primary antibodies were colocalized with goat anti-UMOD antibodies. However, the combination of secondary antibodies used in the study (donkey anti-goat and goat anti-rabbit) can lead to nonspecific cross-reactivity, which makes it difficult to interpret these data.
We did not confirm CLDN16 expression in the mouse DCT, previously reported in only one study so far (52) (Supplemental Table S2). A more recent study has suggested that CLDN16 protein phosphorylated at residue T303 can be expressed in the mouse DCT. This expression has been documented using a phospho-specific antibody and localized to the apical membrane, more than at the TJ (55). In contrast, we did not find substantial CLDN16 expression in the DCT of mouse and human kidneys. In fact, we consider the observed staining to mark the transition from the TAL to the DCT, rather than specific DCT staining. In the rat, the TJ staining stretched further into the DCT. The staining observed was restricted to the TJ, looked different from that in the TAL and involved neither apical nor basolateral surfaces of NCC-positive DCT cells. The specific location of the staining in the rat DCT needs to be better settled.
In the human, we found that CLDN19 was located at the TJ but also in basolateral/intracellular domains in the TAL. However, we must acknowledge that we were not able to confirm the specificity of the immunostaining with anti-CLDN19 antibody. No study has been made regarding CLDN19 expression in the human TAL. Lee et al. (24) reported CLDN19 staining in cortical distal tubules not expressing AQP1 or AQP2, which could be either the DCT or TAL and in medullary Henle’s loops, yet also found marked expression in proximal tubules. The staining found in the murine TAL with the antibody used in this study confirmed previous studies regarding CLDN19 expression in TJs and intracellular compartments in the mouse and rat MTALOSOM and CTAL (14, 19, 51, 52). The role of CLDN19 expression at the TJ has to be assessed by functional electrolyte transport studies in Cldn19-deficient mice, when they become available, to fully understand the respective contribution of CLDN16 and CLDN19 to paracellular Ca2+ and Mg2+ permeation. Several studies have reported that the mouse and rat MTALISOM do not reabsorb Ca2+ and Mg2+, whereas the mouse and rat CTAL reabsorb Ca2+ and Mg2+ along the paracellular pathway (17, 56, 57). We found expression of CLDN19 to be largely restricted to the intracellular compartment of the MTALISOM in mouse and rat kidneys; however, CLDN19 was also expressed at the TJ in the three species studied herein, but its specific function remains unsettled.
CLDN19 expression is found in the basolateral domains and at TJs in some mouse and rat DCT cells. It is expressed in a few cells and at a few TJs in the mouse CNT but not at TJs in the rat CNT. We could not determine whether CLDN19 can be expressed at some TJs in the human DCT and CNT. Expression of CLDN19 has been reported in other tubular segments by some, but not all, studies (Supplemental Table S3). We found mainly marked basolateral staining in the collecting duct, restricted to basolateral domains. This expression is not consistently observed between studies (Supplemental Table S3). CLDN19 protein expression has been reported in the rat CCD by immunoblot and mass spectrometry (33, 34) and in murine inner medullary collecting duct by immunoblot and immunofluorescence (34). Using another antibody, Angelow et al. (51) found CLDN19 expression in the mouse TAL and in ascending thin limbs in the inner medulla but not in the collecting duct. Expression of Cldn19 RNA has been found to be variable between different experiments and in gene expression databases using single-cell or whole nephron RNA sequencing. Such expression was absent from the collecting duct system as evaluated by RT-PCR in dissected nephron segments from the mouse (52) and in a single-cell transcriptomic study performed on microdissected CCDs (58). However, other RNA-sequencing databases have described Cldn19 expression in the rat and mouse CCD and in the dissected mouse CCD by RT-PCR (27–29, 32, 59). Very low levels of expression have been detected in the human CNT in some RNA-sequencing databases (60, 61). These findings suggest that Cldn19 may be expressed in the collecting system; however, its role remains unclear as it does not appear to be junctional. Largely, all databases show Cldn10 expressed in the proximal tubule and TAL and Cldn16 expression in the TAL. Patients with FHHNC bearing CLDN19 variants have a renal phenotype similar to that observed in patients with CLDN16 variants, which is not expressed in the collecting duct (13), as documented by our study and previous investigations (19). Therefore, we will not discuss the hypothetical role of CLDN19 in the human collecting duct before CLDN19 expression with this antibody is confirmed in Cldn19 knockout mice.
Colocalization of either CLDN10 with CLDN16 or CLDN10 with CLDN19 is rarely observed (19); a subset of TAL cells expresses both CLDN10 and CLDN19 proteins: in that case, coexpression is restricted to basolateral domains and does not extend to TJs in the cortex and OSOM, whereas CLDN16 and CLDN19 are colocalized at TJs of the human or rodent TAL. We conclude that the same mosaic pattern of either CLDN10 or CLDN16/CLDN19 in TJs exists in the human, mouse, and rat CTAL and OSOM TAL. We can assume that this mosaic can allow independent determination of paracellular permeability properties and allow us a better understanding of how mutations of different claudins give rise to syndromes with either divalent cations wasting as observed in FHHNC or NaCl wasting as observed in HELIX syndrome.
In summary, we documented the expression of CLDN10, CLDN16, and CLDN19 in human, mouse, and rat kidneys by immunostaining using two different techniques. The mosaic pattern of either CLDN10 or CLDN16 with CLDN19 is found in the TAL in all three species and helps to explain the molecular basis for understanding HELIX and FHHNC syndromes.
SUPPLEMENTAL DATA
Supplemental Tables S1–S3 and Supplemental Figs. S1–S6: https://doi.org/10.6084/m9.figshare.14501982.v1.
GRANTS
C.P.-B. was supported by the Fondation pour la Recherche Médicale (FRM FDT201904007918). This work was supported by grants from Agence Nationale de la Recherche (ANR-12-BSV1-0031-01 and ANR-17-CE14-0032 to P.H.), the Novo Nordisk Foundation, the Beckett Foundation, and Independent Research Fund Denmark (to H.D.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.P.-B., P.H., and H.D. conceived and designed research; C.P.-B., C.G., K.S., L.C., G.B., L.L., E.F., F.A., K.G., R.Z., N.M., L.F., T.B., D.M., P.B., and H.D. performed experiments; C.P.-B., C.G., K.S., R.Z., P.H., and H.D. analyzed data; C.P.-B., C.G., K.S., P.H., and H.D. interpreted results of experiments; C.P.-B., C.G., P.H., and H.D. prepared figures; C.P.-B., P.H., and H.D. drafted manuscript; C.P.-B., C.G., K.S., L.C., G.B., L.L., R.Z., T.B., P.H., and H.D. edited and revised manuscript; C.P.-B., C.G., K.S., L.C., G.B., L.L., E.F., F.A., K.G., R.Z., N.M., L.F., T.B., D.M., P.B., P.H., and H.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully thank many colleagues for providing antibodies and for fruitful discussion: C. Klein [Institut National de la Santé et de la Recherche Médicale (INSERM), Paris, France], J. Hou (Washington University, St. Louis, MO), R. Kozyraki (INSERM, Paris, France), J. Loffing (University of Zurich, Zurich, Switzerland), D. H. Ellison (Oregon Health & Science University, Portland, OR), O. Bonny (University of Lausanne, Lausanne, Switzerland), C. Wagner (University of Zurich, Zurich, Switzerland), S. Bourgeois (University of Zurich, Zurich, Switzerland), and N. Picard (LBTI UMR 5305 CNRS/UCBL, Lyon, France). The authors also thank Inger Nissen and Lars Vitved at the University of Southern Denmark for expert technical assistance and the Centre d’Histologie, d’Imagerie et de Cytométrie team (a core facility of Centre de Recherche des Cordeliers) for technical assistance.
Footnotes
Human and rodent protein symbols are capitalized. Human and rodent gene symbols are italicized; the initial letters of rodent gene symbols are uppercased.
REFERENCES
- 1.Chen L, Clark JZ, Nelson JW, Kaissling B, Ellison DH, Knepper MA. Renal-tubule epithelial cell nomenclature for single-cell RNA-sequencing studies. J Am Soc Nephrol 30: 1358–1364, 2019. [Erratum in J Am Soc Nephrol 30: 2475, 2019] doi: 10.1681/ASN.2019040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriz W, Bankir L. A standard nomenclature for structures of the kidney. The Renal Commission of the International Union of Physiological Sciences (IUPS). Kidney Int 33: 1–7, 1988. doi: 10.1038/ki.1988.1. [DOI] [PubMed] [Google Scholar]
- 3.Himmerkus N, Plain A, Marques RD, Sonntag SR, Paliege A, Leipziger J, Bleich M. AVP dynamically increases paracellular Na+ permeability and transcellular NaCl transport in the medullary thick ascending limb of Henle’s loop. Pflugers Arch 469: 149–158, 2017. doi: 10.1007/s00424-016-1915-5. [DOI] [PubMed] [Google Scholar]
- 4.Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest 122: 3355–3367, 2012. doi: 10.1172/JCI57407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morla L, Crambert G, Mordasini D, Favre G, Doucet A, Imbert-Teboul M. Proteinase-activated receptor 2 stimulates Na, K-ATPase and sodium reabsorption in native kidney epithelium. J Biol Chem 283: 28020–28028, 2008. doi: 10.1074/jbc.M804399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukita S, Tanaka H, Tamura A. The claudins: from tight junctions to biological systems. Trends Biochem Sci 44: 141–152, 2019. doi: 10.1016/j.tibs.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Muto S. Physiological roles of claudins in kidney tubule paracellular transport. Am J Physiol Renal Physiol 312: F9–F24, 2017. doi: 10.1152/ajprenal.00204.2016. [DOI] [PubMed] [Google Scholar]
- 8.Yu AS. Claudins and the kidney. J Am Soc Nephrol 26: 11–19, 2015. doi: 10.1681/ASN.2014030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadj-Rabia S, Brideau G, Al-Sarraj Y, Maroun RC, Figueres ML, Leclerc-Mercier S, Olinger E, Baron S, Chaussain C, Nochy D, Taha RZ, Knebelmann B, Joshi V, Curmi PA, Kambouris M, Vargas-Poussou R, Bodemer C, Devuyst O, Houillier P, El-Shanti H. Multiplex epithelium dysfunction due to CLDN10 mutation: the HELIX syndrome. Genet Med 20: 190–201, 2018. doi: 10.1038/gim.2017.71. [DOI] [PubMed] [Google Scholar]
- 10.Bongers E, Shelton LM, Milatz S, Verkaart S, Bech AP, Schoots J, Cornelissen EAM, Bleich M, Hoenderop JGJ, Wetzels JFM, Lugtenberg D, Nijenhuis T. A novel hypokalemic-alkalotic salt-losing tubulopathy in patients with CLDN10 mutations. J Am Soc Nephrol 28: 3118–3128, 2017. doi: 10.1681/ASN.2016080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klar J, Piontek J, Milatz S, Tariq M, Jameel M, Breiderhoff T, Schuster J, Fatima A, Asif M, Sher M, Mabert K, Fromm A, Baig SM, Gunzel D, Dahl N. Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet 13: e1006897, 2017. doi: 10.1371/journal.pgen.1006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyers N, Nelson-Williams C, Malaga-Dieguez L, Kaufmann H, Loring E, Knight J, Lifton RP, Trachtman H. Hypokalemia associated with a claudin 10 mutation: a case report. Am J Kidney Dis 73: 425–428, 2019. doi: 10.1053/j.ajkd.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godron A, Harambat J, Boccio V, Mensire A, May A, Rigothier C, Couzi L, Barrou B, Godin M, Chauveau D, Faguer S, Vallet M, Cochat P, Eckart P, Guest G, Guigonis V, Houillier P, Blanchard A, Jeunemaitre X, Vargas-Poussou R. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: phenotype-genotype correlation and outcome in 32 patients with CLDN16 or CLDN19 mutations. Clin J Am Soc Nephrol 7: 801–809, 2012. doi: 10.2215/CJN.12841211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plain A, Wulfmeyer VC, Milatz S, Klietz A, Hou J, Bleich M, Himmerkus N. Corticomedullary difference in the effects of dietary Ca2+ on tight junction properties in thick ascending limbs of Henle’s loop. Pflugers Arch 468: 293–303, 2016. doi: 10.1007/s00424-015-1748-7. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int 59: 2206–2215, 2001. doi: 10.1046/j.1523-1755.2001.00736.x. [DOI] [PubMed] [Google Scholar]
- 16.Prot-Bertoye C, Houillier P. Claudins in renal physiology and pathology. Genes (Basel) 11: 290, 2020. doi: 10.3390/genes11030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bankir L, Figueres L, Prot-Bertoye C, Bouby N, Crambert G, Pratt JH, Houillier P. Medullary and cortical thick ascending limb: similarities and differences. Am J Physiol Renal Physiol 318: F422–F442, 2019. doi: 10.1152/ajprenal.00261.2019. [DOI] [PubMed] [Google Scholar]
- 18.Dimke H, Schnermann J. Axial and cellular heterogeneity in electrolyte transport pathways along the thick ascending limb. Acta Physiol (Oxf) 223: e13057, 2018. doi: 10.1111/apha.13057. [DOI] [PubMed] [Google Scholar]
- 19.Milatz S, Himmerkus N, Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Muller D, Fromm M, Bleich M, Gunzel D. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc Natl Acad Sci USA 114: E219–E227, 2017. doi: 10.1073/pnas.1611684114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleich M, Wulfmeyer VC, Himmerkus N, Milatz S. Heterogeneity of tight junctions in the thick ascending limb. Ann N Y Acad Sci 1405: 5–15, 2017. doi: 10.1111/nyas.13400. [DOI] [PubMed] [Google Scholar]
- 21.Breiderhoff T, Himmerkus N, Drewell H, Plain A, Gunzel D, Mutig K, Willnow TE, Muller D, Bleich M. Deletion of claudin-10 rescues claudin-16-deficient mice from hypomagnesemia and hypercalciuria. Kidney Int 93: 580–588, 2018. doi: 10.1016/j.kint.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Muller D. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci USA 109: 14241–14246, 2012. [Erratum in Proc Natl Acad Sci USA 109: 15072, 2012]. doi: 10.1073/pnas.1203834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirk A, Campbell S, Bass P, Mason J, Collins J. Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol Dial Transplant 25: 2107–2119, 2010. doi: 10.1093/ndt/gfq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee NP, Tong MK, Leung PP, Chan VW, Leung S, Tam PC, Chan KW, Lee KF, Yeung WS, Luk JM. Kidney claudin-19: localization in distal tubules and collecting ducts and dysregulation in polycystic renal disease. FEBS Lett 580: 923–931, 2006. doi: 10.1016/j.febslet.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 26.Chabardes-Garonne D, Mejean A, Aude JC, Cheval L, Di Stefano A, Gaillard MC, Imbert-Teboul M, Wittner M, Balian C, Anthouard V, Robert C, Segurens B, Wincker P, Weissenbach J, Doucet A, Elalouf JM. A panoramic view of gene expression in the human kidney. Proc Natl Acad Sci USA 100: 13710–13715, 2003. doi: 10.1073/pnas.2234604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Lee JW, Chou C-L, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci USA 114: E9989–E9998, 2017. doi: 10.1073/pnas.1710964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheval L, Pierrat F, Dossat C, Genete M, Imbert-Teboul M, Duong Van Huyen JP, Poulain J, Wincker P, Weissenbach J, Piquemal D, Doucet A. Atlas of gene expression in the mouse kidney: new features of glomerular parietal cells. Physiol Genomics 43: 161–173, 2011. doi: 10.1152/physiolgenomics.00093.2010. [DOI] [PubMed] [Google Scholar]
- 29.Clark JZ, Chen L, Chou CL, Jung HJ, Lee JW, Knepper MA. Representation and relative abundance of cell-type selective markers in whole-kidney RNA-Seq data. Kidney Int 95: 787–796, 2019. doi: 10.1016/j.kint.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012. doi: 10.1038/emboj.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Muller D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci 122: 1507–1517, 2009. doi: 10.1242/jcs.040113. [DOI] [PubMed] [Google Scholar]
- 32.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limbutara K, Chou CL, Knepper MA. Quantitative proteomics of all 14 renal tubule segments in rat. J Am Soc Nephrol 31: 1255–1266, 2020. doi: 10.1681/ASN.2020010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziemens A, Sonntag SR, Wulfmeyer VC, Edemir B, Bleich M, Himmerkus N. Claudin 19 is regulated by extracellular osmolality in rat kidney inner medullary collecting duct cells. Int J Mol Sci 20: 4401, 2019. doi: 10.3390/ijms20184401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi SR, Liu C, Pootrakul L, Tang L, Young A, Chen R, Cote RJ, Taylor CR. Evaluation of the value of frozen tissue section used as “gold standard” for immunohistochemistry. Am J Clin Pathol 129: 358–366, 2008. doi: 10.1309/7CXUYXT23E5AL8KQ. [DOI] [PubMed] [Google Scholar]
- 36.Beggs MR, Appel I, Svenningsen P, Skjodt K, Alexander RT, Dimke H. Expression of transcellular and paracellular calcium and magnesium transport proteins in renal and intestinal epithelia during lactation. Am J Physiol Renal Physiol 313: F629–F640, 2017. doi: 10.1152/ajprenal.00680.2016. [DOI] [PubMed] [Google Scholar]
- 37.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol 174: 2453–2455, 2005. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 38.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner CA, Loffing-Cueni D, Yan Q, Schulz N, Fakitsas P, Carrel M, Wang T, Verrey F, Geibel JP, Giebisch G, Hebert SC, Loffing J. Mouse model of type II Bartter’s syndrome. II. Altered expression of renal sodium- and water-transporting proteins. Am J Physiol Renal Physiol 294: F1373–F1380, 2008. doi: 10.1152/ajprenal.00613.2007. [DOI] [PubMed] [Google Scholar]
- 40.Frische S, Chambrey R, Trepiccione F, Zamani R, Marcussen N, Alexander RT, Skjodt K, Svenningsen P, Dimke HH. H+-ATPase subunit localizes to thick ascending limb and distal convoluted tubule of rodent and human kidney. Am J Physiol Renal Physiol 315: F429–F444, 2018. doi: 10.1152/ajprenal.00539.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bostanjoglo M, Reeves WB, Reilly RF, Velázquez H, Robertson N, Litwack G, Morsing P, Dørup J, Bachmann S, Ellison DH, Bostonjoglo M. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol 9: 1347–1358, 1998. [Erratum in J Am Soc Nephrol 9: 2179, 1998]. doi: 10.1681/ASN.V981347. [DOI] [PubMed] [Google Scholar]
- 42.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B. Altered renal distal tubule structure and renal Na+ and Ca2+ handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15: 2276–2288, 2004. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 43.Moor MB, Haenzi B, Legrand F, Koesters R, Hynes NE, Bonny O. Renal memo1 differentially regulates the expression of vitamin D-dependent distal renal tubular calcium transporters. Front Physiol 9: 874, 2018. doi: 10.3389/fphys.2018.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl−/HCO3− exchanger (slc4a1). J Am Soc Nephrol 18: 1408–1418, 2007. doi: 10.1681/ASN.2006101072. [DOI] [PubMed] [Google Scholar]
- 45.Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA. Pendrin in the mouse kidney is primarily regulated by Cl− excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol 295: C1658–C1667, 2008. doi: 10.1152/ajpcell.00419.2008. [DOI] [PubMed] [Google Scholar]
- 46.Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Gunzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Muller D. Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am J Physiol Renal Physiol 298: F1152–F1161, 2010. doi: 10.1152/ajprenal.00499.2009. [DOI] [PubMed] [Google Scholar]
- 47.Alexander RT, Beggs MR, Zamani R, Marcussen N, Frische S, Dimke H. Ultrastructural and immunohistochemical localization of plasma membrane Ca2+-ATPase 4 in Ca2+-transporting epithelia. Am J Physiol Renal Physiol 309: F604–F616, 2015. [Erratum in Am J Physiol Renal Physiol 309: F991–F992]doi: 10.1152/ajprenal.00651.2014. [DOI] [PubMed] [Google Scholar]
- 48.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 49.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 291: F1288–F1299, 2006. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 50.Inai T, Sengoku A, Guan X, Hirose E, Iida H, Shibata Y. Heterogeneity in expression and subcellular localization of tight junction proteins, claudin-10 and -15, examined by RT-PCR and immunofluorescence microscopy. Arch Histol Cytol 68: 349–360, 2005. doi: 10.1679/aohc.68.349. [DOI] [PubMed] [Google Scholar]
- 51.Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol 293: F166–F177, 2007. doi: 10.1152/ajprenal.00087.2007. [DOI] [PubMed] [Google Scholar]
- 52.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79: 949–957, 2006. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikari A, Matsumoto S, Harada H, Takagi K, Hayashi H, Suzuki Y, Degawa M, Miwa M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J Cell Sci 119: 1781–1789, 2006. doi: 10.1242/jcs.02901. [DOI] [PubMed] [Google Scholar]
- 54.Oh IH, Jo CH, Kim S, Jo S, Chung S, Kim GH. Thick ascending limb claudins are altered to increase calciuria and magnesiuria in metabolic acidosis. Am J Physiol Renal Physiol 320: F418–F428, 2021. doi: 10.1152/ajprenal.00282.2020. [DOI] [PubMed] [Google Scholar]
- 55.Hou J, Renigunta V, Nie M, Sunq A, Himmerkus N, Quintanova C, Bleich M, Renigunta A, Wolf MTF. Phosphorylated claudin-16 interacts with Trpv5 and regulates transcellular calcium transport in the kidney. Proc Natl Acad Sci USA 116: 19176–19186, 2019. doi: 10.1073/pnas.1902042116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandon B, Siga E, Roinel N, de Rouffignac C. Ca2+, Mg2+ and K+ transport in the cortical and medullary thick ascending limb of the rat nephron: influence of transepithelial voltage. Pflügers Arch 424: 558–560, 1993. doi: 10.1007/BF00374924. [DOI] [PubMed] [Google Scholar]
- 57.Quamme GA, de Rouffignac C. Epithelial magnesium transport and regulation by the kidney. Front Biosci 5: D694–D711, 2000. doi: 10.2741/quamme. [DOI] [PubMed] [Google Scholar]
- 58.Ransick A, Lindstrom NO, Liu J, Zhu Q, Guo JJ, Alvarado GF, Kim AD, Black HG, Kim J, McMahon AP. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell 51: 399–413.e7, 2019. doi: 10.1016/j.devcel.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Susztak K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu H, Malone AF, Donnelly EL, Kirita Y, Uchimura K, Ramakrishnan SM, Gaut JP, Humphreys BD. Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol 29: 2069–2080, 2018. doi: 10.1681/ASN.2018020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23: 869–881.e8, 2018. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]