Abstract
Thrombospondin-1 (TSP1) is the prototypical member of a family of secreted proteins that modulate cell behavior by engaging with molecules in the extracellular matrix and with receptors on the cell surface. CD47 is widely displayed on many, if not all, cell types and is a high-affinity TSP1 receptor. CD47 is a marker of self that limits innate immune cell activities, a feature recently exploited to enhance cancer immunotherapy. Another major role for CD47 in health and disease is to mediate TSP1 signaling. TSP1 acting through CD47 contributes to mitochondrial, metabolic, and endocrine dysfunction. Studies in animal models found that elevated TSP1 expression, acting in part through CD47, causes mitochondrial and metabolic dysfunction. Clinical studies established that abnormal TSP1 expression positively correlates with obesity, fatty liver disease, and diabetes. The unabated increase in these conditions worldwide and the availability of CD47 targeting drugs justify a closer look into how TSP1 and CD47 disrupt metabolic balance and the potential for therapeutic intervention.
Keywords: diabetes, fatty liver disease, metabolism, mitochondria, obesity
INTRODUCTION
Metabolic dysregulation characterizes numerous acquired diseases including metabolic syndrome, nonalcoholic fatty liver disease (NAFLD), obesity, and diabetes and contributes to vascular disease, hypertension, myocardial infarction, and stroke. The incidence of these diseases is associated with increase in weight coincident with minimal activity. As of 2016, the World Health Organization reported that close to 2 billion adults were overweight, and 34% of these were obese (1). That an estimated 340 million children were likewise obese indicates that this process begins early. More topically, obesity is one of the leading risk factors for COVID-19-related mortality (2).
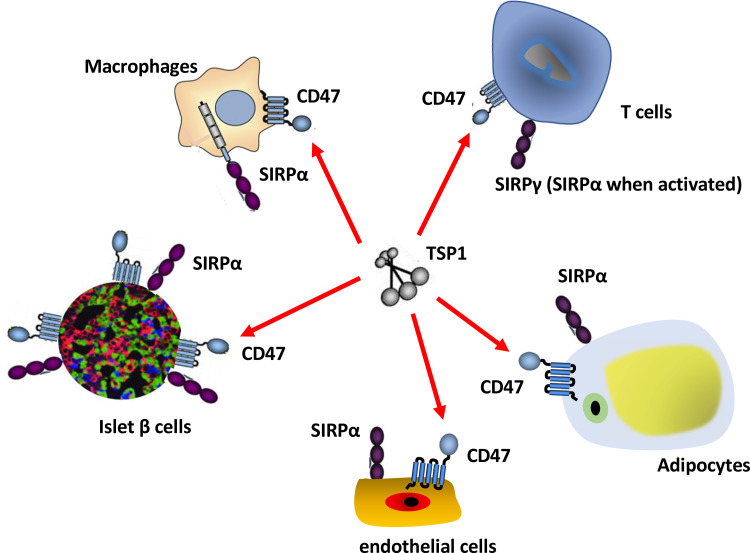
At the cellular level, metabolic health depends upon modulated delivery of oxygen and nutrients concurrent with removal of waste products such as excess nitrogen and carbon dioxide. For multicellular organisms, this requires complex layers of organization and systems with feed forward and backward regulation. Some relevant cues derive from the need of cells in multicellular organisms to adhere and respond to mechanical and chemical signals in their extracellular matrix environment. Cells build and constantly modify this extracellular matrix. Cells also secrete bioactive proteins that bind to the matrix and cell surface to provide yet another layer of regulation. Secreted proteins that serve as modulatory rather than structural links between the extracellular matrix and the cell surface are termed matricellular proteins (3). Thrombospondin-1 (TSP1) is a classic example of a function-modifying matricellular protein. The known regulatory functions of TSP1 have expanded from disrupting cell adhesion to modulating inflammation, responses to hypoxic and genotoxic stress, redox signaling, stem cell self-renewal, and autophagy. These functions of TSP1 are mediated by its interactions with several cell surface receptors, structural components of the extracellular matrix, and secreted factors such as transforming growth factor beta (TGF-β) (4). Among its cell surface receptors, a growing body of data indicate that TSP1 interactions with the cell surface receptor CD47 play a key role in regulating cell stress and fundamental metabolic processes (5) (Fig. 1).
Figure 1.
Direct and indirect effects of thrombospondin-1 (TSP1) on CD47 signaling. TSP1 is secreted under stress conditions and binds with high affinity to CD47, which is expressed on all cells. TSP1 binding induces CD47 signaling that alters cell metabolism, gene expression, and responses to stress. CD47 also interacts with signal regulatory protein-α (SIRPα) on different cells (trans interaction) and induces SIRPα signaling by altering the activity of phosphatases SHP1 and SHP2. Functional cis interactions between CD47 and SIRPα on the surface of a single cell have been reported but require further verification. TSP1 can also prevent cis and trans SIRPα signaling by inhibiting its binding to CD47. Conversely, therapeutic agents that target CD47 or SIRPα may also alter TSP1 interactions with CD47 to alter metabolism of glucose, fatty acids, and reactive oxygen species (ROS).
THROMBOSPONDIN-1 AND CD47 REGULATION OF MITOCHONDRIA
TSP1 is known to drive cell death through CD47, and this is associated with mitochondrial dysfunction. Treatment of human T cells with TSP1, a CD47 antibody (clone 1F7) or a TSP1-derived peptide that bound specifically to CD47, and a CD3 antibody led to cell death (6). Of note, T cells treated with the CD47 antibody showed a decrease in cyclic adenosine monophosphate (cAMP) levels, although it is not clear if the antibody employed in these studies blocked TSP1 binding to CD47. Cells lacking CD47 were resistant to cell death, whereas re-expression of CD47 in null cells promoted mitochondrial dysfunction and Fas antibody killing (7). TSP1 negatively regulates cAMP signaling in several cell types (8–11), and pretreatment of T cells with cell-permeant cyclic adenosine monophosphate (cAMP) provided protection from TSP1-CD47-mediated cell death (6). In smooth muscle cells, cAMP regulation is independent of phosphodiesterases (10). Treatment of leukemic cells with TSP1, or the C-terminus domain of TSP1 that was shown to bind CD47 (12), resulted in loss of mitochondrial membrane potential, increased mitochondrial reactive oxygen species (ROS) formation, and promoted cell death (13). This is likely of import, as mitochondria are a major source of intracellular ROS. In addition, the TSP1-mediated degradation of the mitochondrial membrane potential would be expected to disrupt normal mitochondrial oxidative phosphorylation and thus broadly impact cell metabolism. TSP1 and a CD47 antibody stimulated B cell death. The antibody treatment rapidly damaged mitochondria, in part, via translocation of the dynamin-related protein 1 (Drp1) from the cytoplasmic domain of CD47 to mitochondria (14). Here too, a human CD47 antibody (clone B6H12) induced loss of mitochondrial membrane potential and ROS production. B6H12 is known to block TSP1 binding to CD47 (12), but its ability to induce B cell death may be independent of that activity. Contrasting this, we found that B6H12 provides protection to cells and blood vessels to a range of stress (15–17), consistent with the agonist and antagonist activities in this and other CD47 antibodies (18). Another TSP1 receptor, CD36, likely mediated mitochondrial dysfunction through altering free fatty acid (FFA) uptake (19) and, as we found, TSP1, under some conditions, interfered with FFA uptake (20).
Mitochondrial biogenesis is stimulated via peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1α), which is increased by low concentrations of nitric oxide (NO) (21) and cAMP (22). We reported that TSP1, via CD47, potently inhibited NO production and signaling (23) and cAMP signaling (10). Consistent with this, skeletal muscle from young Cd47-null mice, which have increased basal NO/cyclic guanosine monophosphate (cGMP) signaling (24), showed increased mRNA and protein levels of mitochondrial-associated genes such as PGC-1α, cytochrome b and c, and nuclear respiratory factor 1 (NRF1) compared with muscle from wild-type controls (25). These differences were age-sensitive and tissue-specific and not found in the brain, liver, kidneys, heart, aorta, or brown fat. On electron microscopy, fast-twitch and slow-twitch skeletal muscle from young Thbs1- and Cd47-null mice showed more and larger mitochondria. Indeed, null skeletal muscle cells contained approximately two times more mitochondria. This is far reaching, as skeletal muscle harbors the bulk of all mitochondria in individuals, and metabolic dysfunction in skeletal muscle is a fixture of metabolic syndrome, obesity, and diabetes (26). Despite the differences in mitochondrial mass between control and Cd47-null skeletal muscles, the function of electron transport chain complexes and the ATP/O2 ratios were comparable between control and Cd47-null skeletal muscle mitochondria. Young Cd47-null mice had increased exercise capacity but consumed less oxygen compared with age-matched wild-type animals (25). Of relevance to age-related obesity, Cd47-null mice were leaner throughout the life cycle compared with controls. Volumetric analysis of fat from the null and control mice was not performed. This is relevant as visceral and subcutaneous fat expand and contract asynchronously. Similarly, white fat adipocytes from young Cd47-null mice showed increased levels of the downstream NO second messenger cGMP (27). However, baseline mitochondrial oxygen utilization was similar in null and wild-type white fat adipocytes (27). In contrast, brown fat adipocyte mitochondria from Cd47-null mice were larger and longer and, during fatty acid oxidation, consumed more oxygen compared with brown fat mitochondria from wild types. The latter enhancement is expected to increase the thermogenic activity of brown fat Cd47-null mitochondria in keeping with the role of brown fat to serve as a buffer against lowering of environmental temperature (28).
TSP1 AND CD47 IN METABOLISM
Mitochondria are essential for oxidative metabolism. In human T cells, loss of CD47 increased mitochondrial mass, oxygen consumption, and spare respiratory capacity (29). The implications of this on several metabolic pathways were demonstrated when a somatic T-cell mutant lacking CD47 (JinB8 cells) were subjected to metabolome analysis. More than 25% of assessed metabolite levels differed between CD47-deficient and control Jurkat T cells under standard normoxic growth conditions, and the CD47-deficient cells had a higher extracellular acidification rate (29). In addition, loss of Thbs1 altered basal metabolites in mouse livers and the response of null mice to dietary fat (30). In human colorectal cancer cell lines, CD47 overexpression was associated with increased mRNA levels of genes involved in glycolysis and gluconeogenesis (31). The mechanisms by which CD47 regulates basal metabolism remain to be defined. However, several findings suggest a role for the central transcription factor avian myelocytomatosis viral oncogene homolog (cMyc). TSP1-CD47 signaling, through regulation of phosphorylation, destabilized cMyc (16). As well, Thbs1- and Cd47-null cells had higher basal levels of cMyc mRNA and protein along with increased proliferation with asymmetric cell division that preserves stem cells (32). Furthermore, enhanced cMyc activity was maintained in Cd47-null cells and animals under stress conditions (16) and with normal aging (33). cMyc plays critical roles in regulating glycolysis, fatty acid homeostasis, and metabolism (34), suggesting that TSP1-CD47 widely and negatively controls cellular metabolism by decreasing the expression of cMyc.
In contrast to nonmalignant cells, Myc is not inhibited by TSP1-CD47 signaling in cancer cells where the MYC promoter is disrupted (32). In triple-negative breast cancer cells, a CD47 antibody that blocks TSP1 binding decreased epidermal growth factor (EGF) receptor signaling (35), and EGF itself regulates glycolysis (36). Mitochondria require oxygen for aerobic glucose metabolism, whereas oxygen, through effects upon the master transcription factor hypoxia inducible factor (HIF), controls TSP1 (37) and CD47 expression (38). The ability of TSP1-CD47 to control metabolism under hypoxic conditions requires verification. However, some effects of hypoxia-mediated induction of TSP1-CD47 signaling on gene expression in primary human cells persisted even after reoxygenation (39).
TSP1 AND CD47 IN METABOLIC SYNDROME, NONALCOHOLIC FATTY LIVER DISEASE, AND OBESITY
Metabolic Syndrome and Obesity
Excess calorie intake and inactivity are causative of several metabolic abnormalities that left unchecked, result in obesity, nonalcoholic fatty liver disease (NAFLD), and diabetes. These conditions rarely exist as isolated processes, and NAFLD is found in a majority of obese and diabetic individuals (40). As well, individuals with obesity have a markedly increased risk for developing diabetes (41). Metabolic syndrome encompasses earlier manifestations of all of these diseases and includes insulin resistance, along with elevated blood lipids and blood pressure and chronic inflammation (42). Roles of TSP1 and CD47 in each of these conditions have lately emerged (Fig. 2).
Figure 2.
Thrombospondin-1 (TSP1) impinges adversely on metabolic pathways and endocrine systems. TSP1 is upregulated by hypoxia, inflammation, hyperglycemia, high-fat intake, and age. Through activation of CD47 signaling, TSP1 adversely potentiates metabolic disease and diabetes via effects on mitochondrial homeostasis, metabolism, and survival in adipocytes and pancreatic islets. TSP1 signaling mediated by CD47 on vascular cells modulates angiogenesis and blood flow, and on macrophages and T cells modulates innate and adaptive immunity. CD47 interacting with signal regulatory protein-α (SIRPα) or SIRPγ further regulates innate and T cell immunity to impact metabolic disease.
Adipose tissue TSP1 mRNA levels were increased in high-fat diet (HFD)-fed mice with obesity (43). Interestingly, mice on a normal diet showed increased TSP1 transcript levels in adipose tissues simply with aging. TSP1 protein was increased largely in the perivascular areas of adipose tissues, and this was not associated with decreased vascularity (43). This latter observation is notable, given the primary role of TSP1, via CD47, to limit proangiogenic signals such as those induced by vascular endothelial growth factor (VEGF) (44) and NO (45–48). Conversely, lack of TSP1 protected from diet-induced weight gain and adiposity, especially in male mice, and was associated with fewer inflammatory cells in adipose tissue. The benefits noted in HFD-fed Thbs1-null mice were posited to be independent of activity and appetite since control and null animals on a standard chow diet showed similar energy expenditure and food intake. Interestingly, FFA and cholesterol levels were higher in HFD-fed Thbs1-null mice. Similarly, Thbs1-null mice had less HFD-mediated skeletal muscle fibrosis and more fat browning than controls (49). Noteworthy, muscle inflammation and fibrosis occur in individuals with obesity (50) which may adversely alter metabolism through decreased glucose uptake. Increased TSP1 expression was also noted in the visceral abdominal fat of genetically obese rats (51) and in the serum and adipose tissues of HFD-fed rats with obesity (52). Interestingly, isolated adipocytes from these animals showed high glucose-mediated suppression of TSP1. Although blood glucose levels were not characterized in the HFD-fed rats with obesity, the data in cultured adipocytes stand in contrast to results in multiple primary cell types that showed increased TSP1 expression under high-glucose culture conditions (53–55). Furthermore, differentiated murine adipocyte-like 3T3-L1 cells cultured under hypoxia showed decreased TSP1 mRNA and protein expression (56), again in contrast to the known effects of hypoxia to increase TSP1 in other cells types, animals (37, 57–60) and human arteries (15, 61). Together, these data suggest atypical control of TSP1 expression in rodent adipocytes. Localization of TSP1 to the perivascular area of murine subcutaneous adipose tissue was confirmed (62). Interestingly, HFD-fed mice lacking endothelial argonaute-1 (AGO1) had less TSP1 expression in adipose vascular endothelial cells along with less weight gain and more brown fat (62). The role adipocyte AGO1 plays in metabolic diseases such as fatty liver, and potential regulation of CD47 expression remains to be determined.
Nonobese individuals without overt chronic disease had comparable TSP1 mRNA expression in visceral and subcutaneous fat (63). Conversely, in individuals with severe obesity, especially women, TSP1 expression was higher in visceral compared with subcutaneous fat (64, 65). In Asian women, increased circulating TSP1 positively correlated with abdominal obesity, hyperglycemia, and hypertension (65). Adherence to a weight reduction diet for 6 mo tended to lower circulating TSP1. Consistent with this, extracellular vesicles (EVs) derived from the visceral fat of individuals with obesity expressed increased TSP1 protein compared with vesicles from subcutaneous fat from the same individuals (66). Further studies are needed to determine whether TSP1 on the surface of EVs engages receptors to actively signal. Separately, TSP1 mRNA expression in subcutaneous abdominal adipose tissue correlated positively with obesity, as defined by body mass index (BMI) (63). In these individuals, circulating levels of plasminogen activator inhibitor-1, a biomarker of metabolic syndrome (67), positively correlated with adipose TSP1 expression. Although the primary source(s) of circulating TSP1 under basal and disease conditions have not been confirmed, it should be pointed out that platelet alpha granules contain large amounts of preformed TSP1 (68) that is released with platelet activation, a common event during blood sampling. Thus, accurate assessment of soluble blood-borne TSP1 requires efforts to limit platelet activation before, and during, separation of the plasma from other blood components (69). Peripheral blood mononuclear cells (PBMCs) and abdominal wall subcutaneous adipose tissue from obese men showed increased TSP1 expression compared with cells and adnominal wall fat from lean individuals (70). Here too, TSP1 levels positively correlated with BMI. Exercise (40 min at 65%–80% of maximum heart rate daily for 3 mo) lowered TSP1 expression in PBMCs and fat from obese subjects while not altering BMI. Although the cohort of Nordic men in this study was small (38 obese and 11 lean subjects) and homogenous, the finding that moderate exercise, and possibly weight reduction (65), decreased circulating and tissue TSP1 justifies confirmation of these findings in a large clinical trial.
TSP1 and thrombospondin-2 (TSP2), which shares homology in its C-terminus to TSP1 and which binds CD47, albeit with less affinity (12), were increased in visceral and gonadal fat of diet- and genetic-driven mice with obesity (71). The implications of this remain unclear, as HFD-fed Thbs2-null mice, while weighing less than controls, showed no differences in adiposity, adipose-associated inflammatory cell numbers, and vascularization (72). We noted that Thbs2-null mice mimicked wild-type mice under ischemic stress (12). It is possible that TSP1 expression in Thbs2-null mice accounted for the lack of phenotypic differences in adipose tissue from HFD-fed control and Thbs2-null animals.
Young Cd47-null mice on a HFD showed less weight gain, less inflammation, and lighter livers compared with controls (73). Decreased adipose inflammatory cell numbers in the absence of CD47 is consistent with other reports that showed, under certain stress conditions, less inflammatory cell tissue invasion in Cd47-null mice (74). Circulating levels of cholesterol and FFAs were lower in HFD-fed Cd47-null mice. Lean muscle volume was comparable between groups, but controls showed more fat mass (73). We found that 18-mo-old Cd47-null mice resisted weight gain on a HFD compared with controls (33). It was not known if intake and activity varied between these groups. However, the latter is unlikely since we reported no variation in daily activity between young Cd47-null and control mice (75). This is germane as metabolic syndrome and nonimmune diabetes are diseases of aging, decreased activity, and excess caloric intake whereas TSP1 is maladaptively increased with aging (76). Both HFD-fed young (73) and aged (33) Cd47-null mice were more insulin sensitive compared with controls and showed less blood glucose excursion after glucose bolus. Thus, CD47 promotes metabolic imbalance throughout the life cycle.
Liver Disease
NAFLD is progressive and, through dysregulated lipid metabolism and chronic inflammation, results in hepatic sclerosis, organ failure, and death. To promote fatty liver disease, control and Thbs1-null mice were fed a choline deficient l-amino acid-defined high-fat diet (77). Inconsistent with other reports, body and liver weights of mice after 12-wk on the special liver fat-promoting diet were the same among groups. Unfortunately, animal age and sex were not reported. Still, Thbs1-null mice were protected from liver inflammation. Specifically, collagen protein and transforming growth factor-β (TGF-β) mRNA levels were less in null mice fed a fatty liver-promoting diet. The latter finding is consistent with the ability of TSP1 to activate latent TGF-β (78). Also, genes involved in metabolism, mitochondrial function, and cell proliferation varied between control and Thbs1-null mice. Expanding on this, mice with macrophage-specific loss of Thbs1 had less fatty liver disease on a HFD (79). Curiously, lack of adipocyte TSP1 did not alter the development of diet-driven NAFLD, or nonalcoholic steatosis (NASH) (79). Wild-type mice fed a HFD had increased TSP1 in macrophages and bile duct cholangiocytes. This latter finding warrants further inquiry given that cholangiocytes regulate bile cholesterol composition, likely, in part, through CD36 (80), another TSP1 receptor (81). Interestingly, in individuals with obesity, weight reduction after bariatric surgery is, in part, due to modifications of bile acid homeostasis (82) and the gut microbiome (83). However, contrasting these data, HFD-fed Thbs1-null/adenomatous polyposis coli-Min mutant (ApcMin/+) mice showed abnormal liver lipid accumulation (30). In keeping with data that found a link between activity and TSP1 expression, wild-type mice with diet-mediated NAFLD showed reversion of liver TSP1 expression following daily exercise (1 h of swimming for 10 wk) (84). Exercise-mediated suppression of TSP1 was linked to increased Ak strain transforming (AKT) signaling. Of note, AKT-mediated activation of endothelial nitric oxide synthase increases NO production, which suppresses TSP1 expression (85).
Individuals with NASH-associated cirrhosis showed markedly increased TSP1 expression in the liver (86). This finding was not specific to NASH, as individuals with alcohol- and hepatitis-associated cirrhosis also showed increased liver TSP1. Human hepatocyte cells (HuH7 cells), cultured with FFAs showed increased lipid accumulation, ROS, and TSP1 (87). Adipocyte-secreted adiponectin limits NAFLD (88) and increases insulin sensitivity. Parenthetically, TSP1 bound to adiponectin in blood samples from healthy individuals and diabetics (89), although the physiologic implications of this are not known. The role of CD47 in clinical NASH and NAFLD remains to be assessed.
TSP1 AND CD47 IN DIABETES
In the United States, 1 in 10 people have overt diabetes, whereas one in three have incipient disease (90). Worldwide, diabetes is the seventh leading cause of death, a primary driver of cardiovascular, ocular, and renal disease, and is predicted to increase worldwide by 48% (629 million) over the next 25 years (91). In 2021, the National Institute of Diabetes and Digestive and Kidney Diseases requested some $2 billion in support (Congressional Budget Justifications, NIDDK). Although implicated in metabolic diseases, little is known about the role of TSP1 and CD47 in diabetes, particularly at the level of human islets. This latter deficit in knowledge, to some degree, likely arises from difficulties in obtaining fresh human islets.
Among individuals with type 1 diabetes (T1D, n = 30, mean age 31 ± 3 yr, duration of diabetes 8 ± 4 yr) plasma TSP1 was significantly elevated (136.6 ± 14.2 ng/mL) compared with controls (91.2 ± 14.3 ng/mL) (n = 15; mean age 29 ± 2 yr) (92). Plasma TSP1 levels were even higher in the subset of individuals with T1D-associated complications (150.4 ± 23.7 ng/mL). The ∼59% elevation in plasma TSP1 levels above healthy subjects was striking. Importantly, subjects with T1D with tighter control of blood glucose, as defined by lower glycated hemoglobin A1c (HbA1c), had lower plasma TSP1 than those with poor blood glucose control (i.e., higher HbA1c). As well, individuals with type 2 diabetes (T2D, n = 149) had elevated circulating TSP1 compared with those without diabetes (n = 225) (93). In type 2 diabetics, elevated plasma TSP1 independently predicted coronary artery disease (CAD). The study cohort, while relatively large, was ethnically uniform. Still, the clinical finding that TSP1 predicted CAD in individuals with T2D is in line with preclinical data implicating TSP1, via CD47, as a driver of systemic (33, 94) and pulmonary (15, 57, 69) vasculopathy through limiting endothelial VEGF and NO while stimulating vascular remodeling and atherosclerosis. In contrast, women with polycystic ovary disease, elevated blood glucose, and insulin resistance had lower circulating TSP1 compared with controls (95). However, those individuals treated with metformin displayed increased circulating TSP1 levels (95). This later observation is interesting since metformin is administered for several diseases including diabetes.
Although these results show an association in people between increased TSP1 and diabetes and diabetes-related complications, they do not indicate what role TSP1 plays in the disease process. β-klotho-mediated suppression of TSP1 in adipose-derived stem cells from individuals with T2D resulted in increased cell proliferation and matrix production (96), suggesting improved wound healing capacity. It would be interesting to see if changes in klotho signaling associate with age-related increases in TSP1. Fifteen-week-old HFD-fed male Thbs1-null mice were more glucose tolerant and sensitive to insulin compared with wild-type HFD-fed mice (97). Null mice had fewer inflammatory cells in adipose tissue and lower blood triglyceride levels than wild types, but both groups gained weight equally on the HFD. Male Thbs1-null mice were more resistant to HFD weight gain, but these differences only appeared after longer exposure to the special diet (5 mo) (43). Bone marrow-derived macrophages from the HFD-fed Thbs1-null mice were less sensitive to lipopolysaccharide and migrated less (97). It was suggested that CD36 signaling did not account for the migratory deficit in Thbs1-null macrophages. However, this finding was based on macrophages from Cd36-null mice on a regular diet. In contrast, CD36 was noted to retard macrophage migration to oxidized low-density lipoprotein (LDL) (98), although what role TSP1 had in this was not tested. CD36 also promotes foam cell formation (99). Furthermore, we found that TSP1-CD47 signaling stimulated nicotinamide adenine dinucleotide phosphate oxidase-1 (Nox1) to increase macrophage uptake of native LDL (100). This TSP1-CD47 effect was associated with changes in the macrophage actin cytoskeleton. Related to this, increased cell stiffness, which is influenced by the rigidity of the extracellular matrix, overcame CD47 inhibition of phagocytosis (101). Noteworthy, macrophage phagocytosis may contribute to T1D (102). CD47 negatively regulated dermal TGF-β1 mRNA expression and TGF-β1 activation, and fibrosis (103), whereas pancreata from individuals with T1D showed increased fibrosis (104, 105). It would be useful to apply biomechanical methodologies to determine the stiffness of T1D and T2D pancreata and, if possible, islets. Although data under the metabolic stress of a HDF suggests a deleterious role for TSP1, information under basal conditions indicates differently. Pancreas weight was similar in young 12-wk-old wild-type and Thbs1-null mice. Furthermore, islet and β cell mass were greater in organs from Thbs1-null mice (106). Despite this, Thbs1-null mice had less basal insulin and more glucose intolerance compared with wild type, findings that did not change in 52-wk-old animals. It is unknown if TSP1 stimulates β cell insulin release.
Streptozotocin (STZ) is an alkylator that is toxic to several cell types, but especially to insulin-producing islet β cells, and animals treated with STZ develop diabetes (107). Young wild-type mice treated with STZ (40 mg/kg daily for 5 days) had elevated plasma glucose and lower insulin levels with slower increases in body weight compared with untreated mice (108). In pancreata from treated mice, islet CD47 expression was decreased along with increased numbers of pancreatic macrophages. As cell-specific CD47 expression or cell death was not determined, the meaning of lower CD47 expression is unclear. In vitro, STZ treatment also decreased islet CD47 expression and was judged to increase phagocytosis of endocrine-like Min6 cells. Although preliminary, these data suggest that rapid alteration in CD47 expression may prime islet cells for macrophage recognition. However, it is not clear if this is associated with altered interactions with TSP1, which can enhance phagocytosis (109). Interestingly, PBMCs from individuals with T2D displayed decreased levels of CD47 compared with cells from nondiabetics (110). Diabetic nephropathy is a primary source of renal failure. In endothelial nitric oxide synthase (Nos3)-null mice, STZ-induced diabetes was associated with worse nephropathy and increased expression of renal TSP1 compared with controls. Conversely, mice treated with cinaciguat, to activate the NO cytoplasmic receptor soluble guanylate cyclase and increase cGMP levels, showed less diabetic nephropathy and less renal TSP1 (111). The latter is consistent with our prior finding that human cells treated with low amounts of NO showed decreased TSP1 expression (85). Diabetes is also characterized by a general decrease in tissue vascularity. STZ-treated diabetic mice showed increased tissue TSP1 and decreased capillaries, findings which were partially reverted by hyperbaric oxygen (112). Related to this, hyperbaric oxygen increased endothelial Nos3 expression and putatively NO production (113).
In contrast to the finding that loss of CD47 was associated with diabetes, enhanced CD47 signaling through altered binding affinity with the cell membrane signal regulatory protein-α (SIRPα) was linked to development of autoimmune T1D in nonobese diabetic (NOD) mice (114). NOD SIRPα bound CD47 with higher affinity compared with SIRPα from nonobese diabetes resistant mice, which were protected from T1D. Stronger binding resulted in T cell activation and increased diabetes when cells were transplanted. Interestingly, single-polymorphism nucleotides in the SIRPγ locus were linked to increased human T cell activation and killing (115). This has importance, as CD47 binds both SIRPγ and SIRPα and builds on our finding that variation in CD47-SIRPα affinity dictated dendritic cell immune response to allo-antigen (116). These findings predict possible untoward effects from agents that target CD47 binding with interacting partners such as SIRPα (18). Antibodies that engage other immune cell inhibitory receptors such as CTLA-4 and PD-1 were associated with rapid onset type 1-like diabetes (117, 118). It remains to be seen if cell-to-cell trans CD47-SIRPα/SIRPγ interaction or CD47 acting in a cis manner with SIRPα on the same cell membrane also regulates such events (119). What role TSP1 plays in this should be queried given that TSP1 inhibits SIRPα binding with CD47 (12, 120).
In BioBreeding rats, which develop autoimmune T1D, skeletal muscle TSP1 protein levels were increased and muscle capillary density decreased (121). Little is known about the skeletal muscle microcirculation of individuals with T1D. As insulin and glucose delivery is predicated upon a robust functional capillary network, it would be useful to characterize skeletal muscle and islet capillarization along with TSP1 and CD47 in individuals with T1- and T2-diabetes.
Inflammation resulted in enzymatic shedding of the extracellular domain of SIRPα in monocytes (122), which suggests an alternative mechanism for immune cell activation that could be important in the inflammatory microenvironment of T1D and T2D. Incidentally, these findings cast doubt upon CD47-SIRPα signaling as a dominant inhibitor of phagocytosis. Expression of a mutant nonfunctional SIRPα that lacked recruitment capacity in the cytoplasmic domain led to lower plasma insulin levels in standard diet-fed mutant mice and this was exacerbated in HFD-fed animals (123). Furthermore, HFD-fed mutant mice had less glucose sensitivity. Treatment with an α2-adrenergic antagonist partially improved glucose sensitivity. Insulin levels in pancreata from mutant and control mice were comparable as was insulin release, suggesting a possible role for SIRPα in regulating peripheral glucose homeostasis. Interestingly, in skeletal muscle, SIRPα interacted with the insulin receptor to decrease insulin signaling (124). It is not known if this extends to β cells.
The function of TSP1 and CD47 in mammalian and human islets and β cells remains to be fully explored. Isolated human islets from donors without overt diseases and normal BMI (23.6 ± 1.8 kg/m2) showed increased TSP1 mRNA after culture in high-glucose medium (125). As well, relatively high amounts of TSP1 protein were noted in the endocrine and exocrine tissues of human pancreata (126, 127). In contrast, CD47 protein was less abundant in endocrine tissues of human pancreata (128). However, cell-specific characterization of TSP1 and CD47 in human pancreata and islets remains undone. To this end, single-cell mRNA sequencing and FACS analysis will be helpful.
Expression levels in tissue samples indicate the presence of TSP1 and CD47 in human pancreata and islets (Fig. 3), but what this means under basal function or in stress is not clear. Treatment with the free fatty acid palmitate stimulated β-like INS-1 cell death, which was less after TSP1 knockdown (129). These results are provocative, as we showed TSP1 limited free fatty acid uptake via CD36 (20). The effect of TSP1 in INS-1 cells and human islets was postulated to be secondary to limiting hydrogen peroxide-mediated injury. This is contrary to data that TSP1-CD47 promoted cell injury by stimulating increased Nox1-mediated superoxide production (48, 57). Pertinent to this, β cells express Nox1 (130). In rodent and human islets, TSP1 was proposed to limit cytokine- and thapsigargin-mediated injury (131). Islets isolated from pancreata from young Thbs1-null mice secreted more insulin under low, but not high, glucose conditions (106). Islets from wild-type mice, on transplantation into Thbs1-null mice, showed lower glucose-mediated insulin secretion (132). A deficit in islet mitochondrial function was postulated to explain the altered response of Thbs1-null islets. Given these findings, it would be enlightening to assess Thbs1-null islet oxygen consumption. Interestingly, islet endothelial cells from wild-type mice and rats showed markedly greater TSP1 mRNA levels compared with other islet cells, including β cells. Increased islet EC TSP1 may explain the decreased vascularity of islets from diabetic Zucker rats (133), the increased vascularity in Thbs1-null islets compared with wild types (134), the better post-transplantation engraftment of Thbs1-null islets (135), and the improved islet angiogenesis following TSP1 blockade (136). Separate from this, islets expressing a nonfunctional extracellular domain of CD47 showed improved engraftment (137), probably through suppression of post-transplantation innate immunity, a process that negatively impacts islet transplantation (138). Modifying CD47 expression as a means of decreasing immune activation in transplantation was reported in other organs (139). Lastly, in a number of studies using isolated islets, a signaling role for TSP1 was inferred through knockdown strategies rather than employing exogenous TSP1 to interrogate ligand-receptor interactions. This may explain the discordant results in relation to the large body of data showing an injurious role for TSP1-CD47 signaling (140–142).
Figure 3.
Thrombospondin-1 (TSP1) and CD47 in the pancreas. In isolated human islets, TSP1 is increased by elevated glucose, and CD47 is expressed in human pancreata. However, cell-specific expression analysis of both genes and their protein products is wanting. This is important, since islets are organoids with a unique vasculature and neural input, multiple endocrine cell types, and resident inflammatory cells. The signaling effects of altered TSP1 expression will depend on the source (endogenous vs. blood stream) and cell type. Given its immune modulatory actions, manipulation of TSP1 signaling with clinical antibodies targeting CD47 may unbalance innate immunity with possible adverse effects in pancreatic islets.
Loss of β cell function and mass is seen in T1D and T2D (143). Approaches to restore functional β cell mass include stimulation of proliferation and transformation of precursor cells into β-like insulin producing cells. Interestingly, the former approach was successful in isolated human islets and was associated with increased cMyc levels (144). How realistic this goal may be is uncertain, since, along with increased cMyc expression, proliferating β cells lose the ability to secrete insulin (145), which presumably would be restored with maturation. Nonetheless, TSP1-CD47 signaling potently suppresses cMyc and other self-renewal factors (Oxt3/4, Sox 2, Klf4) and could represent an inherent barrier to precursor cell transformation.
FUTURE DIRECTIONS
Preclinical data indicate a role for TSP1 and CD47 in metabolic homeostasis and disease (Table 1). Clinical data finds increased TSP1 expression associated with metabolic dysfunction and disease. However, some findings require confirmation or resolution of discrepancies, which may identify new questions to address. It is not clear if TSP1-CD47 signaling impedes mitochondrial function in all cells or just in those with increased metabolic function. Furthermore, do mechanisms in addition to DRP1 transduce the cell surface TSP1-CD47 signal to mitochondria? Bcl-2 homology 3 (BH3)-only protein 19 kDa interacting protein-3 (BNIP3) regulation of calcium is another possible mediator of CD47 signaling (146). In endothelial cells and cardiac myocytes, TSP1 altered calcium-mediated signaling (147, 148). Some CD47-dependent changes in metabolites under stress are independent of changes in mitochondrial function. It would be useful to test if therapeutic disruption of TSP1-CD47 signaling improves mitochondrial function. In relation to diet-mediated diseases such as obesity and FLD, the role of TSP1-CD47 may be dependent upon cross talk with CD36, as was noted in relationship to amyloid effects on cGMP signaling (149).
Table 1.
Cell and animal responses to TSP1, CD47, SIRPα, and/or metabolic stress
| Cell/Animal Type | Treatment/Stress | Responses |
|---|---|---|
| Human cells | ||
| T cells | TSP1, CD47 ab, TSP1 peptide | ↓ cAMP, proliferation, activation↑ Cell death |
| Leukemic B cells | TSP1, CD47 ab, C-terminal domain | ↓ Miitochondrial membrane potential↑ Cell death, reactive oxygen species |
| Adipocyte EVs | Obesity | ↑ TSP1 |
| Endothelial, vascular smooth muscle, retinal pigment epithelial, & renal tubular epithelial cells | High glucose | ↑ TSP1 |
| CD47-deficient T cells | ↑ Mitochondria mass↑ Respiratory capacity | |
| Rat cells | ||
| Adipocytes, mesangial cells | High glucose | ↑ TSP1 |
| INS-1 β-like cells | TSP1 KOpalmitate | ↓ Cell death |
| Murine cells/tissues | ||
| Islets | TSP1 KO | ↑ Insulin release to low glucose↑ Islet vascularity |
| Brown adipocytes | CD47 KO | ↑ Mitochondrial oxygen consumption |
| 3T3-L1 adipocytes | Hypoxia | ↓ TSP1 |
| Skeletal myocytes | TSP1 and CD47 KO | ↑ Mitochondria number and size↓ Reactive oxygen species (CD47 KO) |
| Endothelial cells | AGO1 KO | ↓ TSP1 |
| Animals | ||
| Mice | AGO1 endothelial KO | ↓ Weight gain |
| Mice | TSP1 KOHigh-fat dietLiver-injury diet | ↓ Weight gain, muscle fibrosis, liver inflammation, blood triglycerides↑ Glucose tolerance |
| Mice | CD47 KOHigh-fat diet | ↓ Weight gain, inflammation, cholesterol, free fatty acids↑ Insulin sensitivity |
| Mice | SIRPα nonfunctional mutationHigh-fat diet | ↓ Plasma insulin worsened by high-fat diet |
KO, knockout; SIRPα, signal regulatory protein-α; TSP1, thrombospondin-1.
Questions also remain in the realm of glucose balance and islet function. The cell-specific expression of TSP1 and CD47 should be defined in islets and in the exocrine pancreas. Especially relevant would be to determine islet and non-islet cell expression patterns in relation to the presence of disease and glucose control. Loss of pancreata neural innervation was noted in individuals with T1D (150). This suggests an area for further inquiry since astrocytes cultured in high glucose showed decreased TSP1 secretion despite elevated intracellular TSP1, which impaired neural synapse proteins in cocultures (151). However, the positive effect of TSP1 on synapse formation is mediated by α2δ1 rather than CD47 (152). Effects of TSP1 on glucose uptake by skeletal muscle cells may be through promoting muscle inflammation and fibrosis (49) or through effects on insulin-like growth factor (153), muscle growth, and metabolic demand. The effects of CD47 on islets and islet β cells require additional study. Changes in CD47 expression led to increased neural syntaxin-1 (154). Syntaxin-2 and syntaxin-4 are involved in β cell insulin secretion (155, 156), although they have yet to be related to CD47. Given the variations between human and rodent islets, future studies are best done in freshly isolated human islets. As well, immune cell contributions to T1D suggest that TSP1 and CD47, through suppressing immune cell functions in some situations, could participate in limiting cell-specific inflammation. Going forward, the implications of this may be revealed in individuals with cancer treated using CD47 targeting agents (18). These agents are intended to block CD47-SIRPα signaling, but effects upon TSP1-CD47 signaling may inadvertently alter metabolism (Fig. 3). Conversely, TSP1 and CD47 likely promote T2D via several mechanisms and almost certainly hasten disease complications if simply through deleterious effects on blood flow and vascularity (76, 157).
GRANTS
This work was supported by the Intramural Research Program of the NIH/National Cancer Institute (Project ZIA SC009172, D.D.R.).
DISCLOSURES
J. S. Isenberg serves as Chief Science Officer of Radiation Control Technologies, Inc. J. S. Isenberg and D. D. Roberts are co-inventors of CD47 technology patents assigned to the United States of America.
AUTHOR CONTRIBUTIONS
D.D.R. and J.S.I. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.World Health Organization. Obesity. Health Topics 2021. https://www.who.int/health-topics/obesity [2021 May 4]
- 2.Peters SAE, MacMahon S, Woodward M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK biobank: comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes Metab 23: 258–262, 2021. doi: 10.1111/dom.14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol 37: 1–14, 2014. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol 37: 83–91, 2014. doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Soto-Pantoja DR, Kaur S, Roberts DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol 50: 212–230, 2015. doi: 10.3109/10409238.2015.1014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manna PP, Frazier WA. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol 170: 3544–3553, 2003. doi: 10.4049/jimmunol.170.7.3544. [DOI] [PubMed] [Google Scholar]
- 7.Manna PP, Dimitry J, Oldenborg PA, Frazier WA. CD47 augments Fas/CD95-mediated apoptosis. J Biol Chem 280: 29637–29644, 2005. doi: 10.1074/jbc.M500922200. [DOI] [PubMed] [Google Scholar]
- 8.Guo N, Zabrenetzky VS, Chandrasekaran L, Sipes JM, Lawler J, Krutzsch HC, Roberts DD. Differential roles of protein kinase C and pertussis toxin-sensitive G-binding proteins in modulation of melanoma cell proliferation and motility by thrombospondin-1. Cancer Res 58: 3154–3162, 1998. [PubMed] [Google Scholar]
- 9.Wang XQ, Lindberg FP, Frazier WA. Integrin-associated protein stimulates α2β1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. J Cell Biol 147: 389–400, 1999. doi: 10.1083/jcb.147.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao M, Roberts DD, Isenberg JS. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res 63: 13–22, 2011. doi: 10.1016/j.phrs.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aburima A, Berger M, Spurgeon BEJ, Webb BA, Wraith KS, Febbraio M, Poole AW, Naseem KM. Thrombospondin-1 promotes hemostasis through modulation of cAMP signaling in blood platelets. Blood 137: 678–689, 2021. doi: 10.1182/blood.2020005382. [DOI] [PubMed] [Google Scholar]
- 12.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 284: 1116–1125, 2009. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/αvβ3 in promyelocytic leukemia NB4 cells. Blood 106: 658–667, 2005. doi: 10.1182/blood-2004-09-3585. [DOI] [PubMed] [Google Scholar]
- 14.Bras M, Yuste VJ, Roue G, Barbier S, Sancho P, Virely C, Rubio M, Baudet S, Esquerda JE, Merle-Beral H, Sarfati M, Susin SA. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol 27: 7073–7088, 2007. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers NM, Sharifi-Sanjani M, Yao M, Ghimire K, Bienes-Martinez R, Mutchler SM, Knupp HE, Baust J, Novelli EM, Ross M, St Croix C, Kutten JC, Czajka CA, Sembrat JC, Rojas M, Labrousse-Arias D, Bachman TN, Vanderpool RR, Zuckerbraun BS, Champion HC, Mora AL, Straub AC, Bilonick RA, Calzada MJ, Isenberg JS. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc Res 113: 15–29, 2017. doi: 10.1093/cvr/cvw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers NM, Zhang ZJ, Wang JJ, Thomson AW, Isenberg JS. CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Int 90: 334–347, 2016. doi: 10.1016/j.kint.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, DeGraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 1: 3ra7, 2009. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur S, Cicalese KV, Bannerjee R, Roberts DD. Preclinical and clinical development of therapeutic antibodies targeting functions of CD47 in the tumor microenvironment. Antib Ther 3: 179–192, 2020. doi: 10.1093/abt/tbaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med 49: e291, 2017. doi: 10.1038/emm.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem 282: 15404–15415, 2007. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci 119: 2855–2862, 2006. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA 103: 14379–14384, 2006. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol 31: 162–169, 2012. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 281: 26069–26080, 2006. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 25.Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol 30: 154–161, 2011. doi: 10.1016/j.matbio.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos PA, Lytle KA, Delivanis D, Nielsen S, LeBrasseur NK, Jensen MD. Insulin-stimulated muscle glucose uptake and insulin signaling in lean and obese humans. J Clin Endocrinol Metab 106: e1631–e1646, 2020. doi: 10.1210/clinem/dgaa919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norman-Burgdolf H, Li D, Sullivan P, Wang S. CD47 differentially regulates white and brown fat function. Biol Open 9: bio056747, 2020. doi: 10.1242/bio.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 29.Miller TW, Soto-Pantoja DR, Schwartz AL, Sipes JM, DeGraff WG, Ridnour LA, Wink DA, Roberts DD. CD47 globally regulates metabolic pathways that control resistance to ionizing radiation. J Biol Chem 290: 24858–24874, 2015. doi: 10.1074/jbc.M115.665752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soto-Pantoja DR, Sipes JM, Martin-Manso G, Westwood B, Morris NL, Ghosh A, Emenaker NJ, Roberts DD. Dietary fat overcomes the protective activity of thrombospondin-1 signaling in the Apc(Min/+) model of colon cancer. Oncogenesis 5: e230, 2016. doi: 10.1038/oncsis.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu T, Liu H, Liang Z, Wang F, Zhou C, Zheng X, Zhang Y, Song Y, Hu J, He X, Xiao J, King RJ, Wu X, Lan P. Tumor-intrinsic CD47 signal regulates glycolysis and promotes colorectal cancer cell growth and metastasis. Theranostics 10: 4056–4072, 2020. doi: 10.7150/thno.40860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Isenberg JS, Roberts DD. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 3: 1673, 2013. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghimire K, Li Y, Chiba T, Julovi SM, Li J, Ross MA, Straub AC, O'Connell PJ, Ruegg C, Pagano PJ, Isenberg JS, Rogers NM. CD47 promotes age-associated deterioration in angiogenesis, blood flow and glucose homeostasis. Cells 9: 1695, 2020. doi: 10.3390/cells9071695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goetzman ES, Prochownik EV. The role for Myc in coordinating glycolysis, oxidative phosphorylation, glutaminolysis, and fatty acid metabolism in normal and neoplastic tissues. Front Endocrinol (Lausanne) 9: 129, 2018. doi: 10.3389/fendo.2018.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaur S, Elkahloun AG, Singh SP, Chen QR, Meerzaman DM, Song T, Manu N, Wu W, Mannan P, Garfield SH, Roberts DD. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget 7: 10133–10152, 2016. doi: 10.18632/oncotarget.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung KH, Lee EJ, Park JW, Lee JH, Moon SH, Cho YS, Lee KH. EGF receptor stimulation shifts breast cancer cell glucose metabolism toward glycolytic flux through PI3 kinase signaling. PLoS One 14: e0221294, 2019. doi: 10.1371/journal.pone.0221294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrousse-Arias D, Castillo-Gonzalez R, Rogers NM, Torres-Capelli M, Barreira B, Aragones J, Cogolludo A, Isenberg JS, Calzada MJ. HIF-2α-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction. Cardiovasc Res 109: 115–130, 2016. doi: 10.1093/cvr/cvv243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA 112: E6215–E6223, 2015. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers NM, Thomson AW, Isenberg JS. Activation of parenchymal CD47 promotes renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 1538–1550, 2012. doi: 10.1681/ASN.2012020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 313: 2263–2273, 2015. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 41.Lenz M, Richter T, Muhlhauser I. The morbidity and mortality associated with overweight and obesity in adulthood: a systematic review. Dtsch Arztebl Int 106: 641–648, 2009. doi: 10.3238/arztebl.2009.0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C; National Heart, Lung, Blood Institute; American Heart Association. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109: 433–438, 2004. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 43.Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab 305: E439–E450, 2013. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits vascular endothelial growth factor receptor-2 signaling by disrupting its association with CD47. J Biol Chem 285: 38923–38932, 2010. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress. Matrix Biol 31: 162–169, 2012. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, Frazier WA, Roberts DD. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol 27: 2582–2588, 2007. doi: 10.1161/ATVBAHA.107.155390. [DOI] [PubMed] [Google Scholar]
- 47.Rogers NM, Roberts DD, Isenberg JS. Age-associated induction of cell membrane CD47 limits basal and temperature-induced changes in cutaneous blood flow. Ann Surg 258: 184–191, 2013. doi: 10.1097/SLA.0b013e31827e52e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol 32: 2966–2973, 2012. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue M, Jiang Y, Barnes RH 2nd, Tokunaga M, Martinez-Santibanez G, Geletka L, Lumeng CN, Buchner DA, Chun TH. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology 154: 4548–4559, 2013. doi: 10.1210/en.2013-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camastra S, Vitali A, Anselmino M, Gastaldelli A, Bellini R, Berta R, Severi I, Baldi S, Astiarraga B, Barbatelli G, Cinti S, Ferrannini E. Muscle and adipose tissue morphology, insulin sensitivity and β-cell function in diabetic and nondiabetic obese patients: effects of bariatric surgery. Sci Rep 7: 9007, 2017. [Erratum in Sci Rep 8: 8177, 2018]. doi: 10.1038/s41598-017-08444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hida K, Wada J, Zhang H, Hiragushi K, Tsuchiyama Y, Shikata K, Makino H. Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J Lipid Res 41: 1615–1622, 2000. [PubMed] [Google Scholar]
- 52.Garcia-Diaz DF, Arellano AV, Milagro FI, Moreno-Aliaga MJ, Portillo MP, Martinez JA, Campion J. Glucose and insulin modify thrombospondin 1 expression and secretion in primary adipocytes from diet-induced obese rats. J Physiol Biochem 67: 453–461, 2011. doi: 10.1007/s13105-011-0081-7. [DOI] [PubMed] [Google Scholar]
- 53.Lan CC, Huang SM, Wu CS, Wu CH, Chen GS. High-glucose environment increased thrombospondin-1 expression in keratinocytes via DNA hypomethylation. Transl Res 169: 91–101.e1-3, 2016. doi: 10.1016/j.trsl.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res 102: 1558–1565, 2008. doi: 10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem 282: 5704–5714, 2007. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 56.Laria AE, Messineo S, Arcidiacono B, Varano M, Chiefari E, Semple RK, Rocha N, Russo D, Cuda G, Gaspari M, Brunetti A, Foti DP. Secretome analysis of hypoxia-induced 3T3-L1 adipocytes uncovers novel proteins potentially involved in obesity. Proteomics 18: e1700260, 2018. doi: 10.1002/pmic.201700260. [DOI] [PubMed] [Google Scholar]
- 57.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res 93: 682–693, 2012. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green DE, Kang BY, Murphy TC, Hart CM. Peroxisome proliferator-activated receptor gamma (PPARgamma) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm Circ 2: 483–491, 2012. doi: 10.4103/2045-8932.105037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortiz-Masia D, Diez I, Calatayud S, Hernandez C, Cosin-Roger J, Hinojosa J, Esplugues JV, Barrachina MD. Induction of CD36 and thrombospondin-1 in macrophages by hypoxia-inducible factor 1 and its relevance in the inflammatory process. PLoS One 7: e48535, 2012. doi: 10.1371/journal.pone.0048535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phelan MW, Forman LW, Perrine SP, Faller DV. Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. J Lab Clin Med 132: 519–529, 1998. doi: 10.1016/S0022-2143(98)90131-7. [DOI] [PubMed] [Google Scholar]
- 61.Rogers NM, Yao M, Sembrat J, George MP, Knupp H, Ross M, Sharifi-Sanjani M, Milosevic J, St Croix C, Rajkumar R, Frid MG, Hunter KS, Mazzaro L, Novelli EM, Stenmark KR, Gladwin MT, Ahmad F, Champion HC, Isenberg JS. Cellular, pharmacological, and biophysical evaluation of explanted lungs from a patient with sickle cell disease and severe pulmonary arterial hypertension. Pulm Circ 3: 936–951, 2013. doi: 10.1086/674754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang X, Miao Y, Luo Y, Sriram K, Qi Z, Lin FM, Gu Y, Lai CH, Hsu CY, Peterson KL, Van Keuren-Jensen K, Fueger PT, Yeo GW, Natarajan R, Zhong S, Chen ZB. Suppression of endothelial AGO1 promotes adipose tissue browning and improves metabolic dysfunction. Circulation 142: 365–379, 2020. [Erratum in Circulation 143: e871, 2021]. doi: 10.1161/CIRCULATIONAHA.119.041231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ 3rd, Lee MJ, Fried SK, McGehee RE Jr, Peterson CA, Kern PA. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes 57: 432–439, 2008. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramis JM, Franssen-van Hal NL, Kramer E, Llado I, Bouillaud F, Palou A, Keijer J. Carboxypeptidase E and thrombospondin-1 are differently expressed in subcutaneous and visceral fat of obese subjects. Cell Mol Life Sci 59: 1960–1971, 2002. doi: 10.1007/pl00012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuo Y, Tanaka M, Yamakage H, Sasaki Y, Muranaka K, Hata H, Ikai I, Shimatsu A, Inoue M, Chun TH, Satoh-Asahara N. Thrombospondin 1 as a novel biological marker of obesity and metabolic syndrome. Metabolism 64: 1490–1499, 2015. doi: 10.1016/j.metabol.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Camino T, Lago-Baameiro N, Bravo SB, Molares-Vila A, Sueiro A, Couto I, Baltar J, Casanueva EF, Pardo M. Human obese white adipose tissue sheds depot-specific extracellular vesicles and reveals candidate biomarkers for monitoring obesity and its comorbidities. Transl Res In press. doi: 10.1016/j.trsl.2021.01.006.[33465489] [DOI] [PubMed] [Google Scholar]
- 67.Kanaya AM, Wassel Fyr C, Vittinghoff E, Harris TB, Park SW, Goodpaster BH, Tylavsky F, Cummings SR. Adipocytokines and incident diabetes mellitus in older adults: the independent effect of plasminogen activator inhibitor 1. Arch Intern Med 166: 350–356, 2006. doi: 10.1001/archinte.166.3.350. [DOI] [PubMed] [Google Scholar]
- 68.McLaren KM. Immunohistochemical localisation of thrombospondin in human megakaryocytes and platelets. J Clin Pathol 36: 197–199, 1983. doi: 10.1136/jcp.36.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Novelli EM, Little-Ihrig L, Knupp HE, Rogers NM, Yao M, Baust JJ, Meijles D, St Croix CM, Ross MA, Pagano PJ, DeVallance ER, Miles G, Potoka KP, Isenberg JS, Gladwin MT. Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 316: L1150–L1164, 2019. doi: 10.1152/ajplung.00302.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abu-Farha M, Tiss A, Abubaker J, Khadir A, Al-Ghimlas F, Al-Khairi I, Baturcam E, Cherian P, Elkum N, Hammad M, John J, Kavalakatt S, Warsame S, Behbehani K, Dermime S, Dehbi M. Proteomics analysis of human obesity reveals the epigenetic factor HDAC4 as a potential target for obesity. PLoS One 8: e75342, 2013. doi: 10.1371/journal.pone.0075342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146: 4545–4554, 2005. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 72.Van Hul M, Frederix L, Lijnen HR. Role of thrombospondin-2 in murine adipose tissue angiogenesis and development. Obesity (Silver Spring) 20: 1757–1762, 2012. doi: 10.1038/oby.2011.260. [DOI] [PubMed] [Google Scholar]
- 73.Maimaitiyiming H, Norman H, Zhou Q, Wang S. CD47 deficiency protects mice from diet-induced obesity and improves whole body glucose tolerance and insulin sensitivity. Sci Rep 5: 8846, 2015. doi: 10.1038/srep08846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azcutia V, Routledge M, Williams MR, Newton G, Frazier WA, Manica A, Croce KJ, Parkos CA, Schmider AB, Turman MV, Soberman RJ, Luscinskas FW. CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions. Mol Biol Cell 24: 3358–3368, 2013. doi: 10.1091/mbc.E13-01-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol 28: 110–119, 2009. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isenberg JS, Roberts DD. Thrombospondin-1 in maladaptive aging responses: a concept whose time has come. Am J Physiol Cell Physiol 319: C45–C63, 2020. doi: 10.1152/ajpcell.00089.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Min-DeBartolo J, Schlerman F, Akare S, Wang J, McMahon J, Zhan Y, Syed J, He W, Zhang B, Martinez RV. Thrombospondin-I is a critical modulator in non-alcoholic steatohepatitis (NASH). PLoS One 14: e0226854, 2019. doi: 10.1371/journal.pone.0226854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-β activation: a therapeutic target for fibrotic disease. Matrix Biol 68–69: 28–43, 2018. doi: 10.1016/j.matbio.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gwag T, Reddy Mooli RG, Li D, Lee S, Lee EY, Wang S. Macrophage-derived thrombospondin 1 promotes obesity-associated non-alcoholic fatty liver disease. JHEP Rep 3: 100193, 2021. doi: 10.1016/j.jhepr.2020.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary β-carotene and cholesterol. Biochemistry 44: 4517–4525, 2005. doi: 10.1021/bi0484320. [DOI] [PubMed] [Google Scholar]
- 81.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2: re3, 2009. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang W, Cheng Z, Wang Y, Dai Y, Zhang X, Hu S. Role of bile acids in bariatric surgery. Front Physiol 10: 374, 2019. doi: 10.3389/fphys.2019.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koulas SG, Stefanou CK, Stefanou SK, Tepelenis K, Zikos N, Tepetes K, Kapsoritakis A. Gut microbiota in patients with morbid obesity before and after bariatric surgery: a ten-year review study (2009–2019). Obes Surg 31: 317–326, 2021. doi: 10.1007/s11695-020-05074-2. [DOI] [PubMed] [Google Scholar]
- 84.Wu H, Jin M, Han D, Zhou M, Mei X, Guan Y, Liu C. Protective effects of aerobic swimming training on high-fat diet induced nonalcoholic fatty liver disease: regulation of lipid metabolism via PANDER-AKT pathway. Biochem Biophys Res Commun 458: 862–868, 2015. doi: 10.1016/j.bbrc.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 85.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA 102: 13147–13152, 2005. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smalling RL, Delker DA, Zhang Y, Nieto N, McGuiness MS, Liu S, Friedman SL, Hagedorn CH, Wang L. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am J Physiol Gastrointest Liver Physiol 305: G364–G374, 2013. doi: 10.1152/ajpgi.00077.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chavez-Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol 12: 20, 2012. doi: 10.1186/1471-230X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci 18: 1649, 2017. doi: 10.3390/ijms18081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Xu LY, Lam KS, Lu G, Cooper GJ, Xu A. Proteomic characterization of human serum proteins associated with the fat-derived hormone adiponectin. Proteomics 6: 3862–3870, 2006. doi: 10.1002/pmic.200500840. [DOI] [PubMed] [Google Scholar]
- 90.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html.
- 91.International Diabetes Federation. IDF Diabetes Atlas. Brussels, Belgium: International Diabetes Federation, 2019. [Google Scholar]
- 92.Bayraktar M, Dundar S, Kirazli S, Teletar F. Platelet factor 4, β-thromboglobulin and thrombospondin levels in type I diabetes mellitus patients. J Int Med Res 22: 90–94, 1994. doi: 10.1177/030006059402200204. [DOI] [PubMed] [Google Scholar]
- 93.Choi KY, Kim DB, Kim MJ, Kwon BJ, Chang SY, Jang SW, Cho EJ, Rho TH, Kim JH. Higher plasma thrombospondin-1 levels in patients with coronary artery disease and diabetes mellitus. Korean Circ J 42: 100–106, 2012. doi: 10.4070/kcj.2012.42.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Nanda V, Direnzo D, Ye J, Xiao S, Kojima Y, Howe KL, Jarr KU, Flores AM, Tsantilas P, Tsao N, Rao A, Newman AAC, Eberhard AV, Priest JR, Ruusalepp A, Pasterkamp G, Maegdefessel L, Miller CL, Lind L, Koplev S, Bjorkegren JLM, Owens GK, Ingelsson E, Weissman IL, Leeper NJ. Clonally expanding smooth muscle cells promote atherosclerosis by escaping efferocytosis and activating the complement cascade. Proc Natl Acad Sci USA 117: 15818–15826, 2020. doi: 10.1073/pnas.2006348117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan BK, Adya R, Chen J, Farhatullah S, Heutling D, Mitchell D, Lehnert H, Randeva HS. Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res 83: 566–574, 2009. doi: 10.1093/cvr/cvp131. [DOI] [PubMed] [Google Scholar]
- 96.Suku M, Laiva AL, O’Brien FJ, Keogh MB. Anti-ageing protein β-klotho rejuvenates diabetic stem cells for improved gene-activated scaffold based wound healing. J Pers Med 11: 4, 2020. doi: 10.3390/jpm11010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One 6: e26656, 2011. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park YM, Drazba JA, Vasanji A, Egelhoff T, Febbraio M, Silverstein RL. Oxidized LDL/CD36 interaction induces loss of cell polarity and inhibits macrophage locomotion. Mol Biol Cell 23: 3057–3068, 2012. doi: 10.1091/mbc.E11-12-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Y, Kennedy DJ, Ramakrishnan DP, Yang M, Huang W, Li Z, Xie Z, Chadwick AC, Sahoo D, Silverstein RL. Oxidized LDL-bound CD36 recruits an Na(+)/K(+)-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci Signal 8: ra91, 2015. [Erratum in Sci Signal 8: er7, 2015]. doi: 10.1126/scisignal.aaa9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Csanyi G, Feck DM, Ghoshal P, Singla B, Lin H, Nagarajan S, Meijles DN, Al Ghouleh I, Cantu-Medellin N, Kelley EE, Mateuszuk L, Isenberg JS, Watkins S, Pagano PJ. CD47 and Nox1 mediate dynamic fluid-phase macropinocytosis of native LDL. Antioxid Redox Signal 26: 886–901, 2017. doi: 10.1089/ars.2016.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sosale NG, Rouhiparkouhi T, Bradshaw AM, Dimova R, Lipowsky R, Discher DE. Cell rigidity and shape override CD47’s “self”-signaling in phagocytosis by hyperactivating myosin-II. Blood 125: 542–552, 2015. doi: 10.1182/blood-2014-06-585299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maree AF, Kublik R, Finegood DT, Edelstein-Keshet L. Modelling the onset of Type 1 diabetes: can impaired macrophage phagocytosis make the difference between health and disease? Philos Trans A Math Phys Eng Sci 364: 1267–1282, 2006. doi: 10.1098/rsta.2006.1769. [DOI] [PubMed] [Google Scholar]
- 103.Soto-Pantoja DR, Shih HB, Maxhimer JB, Cook KL, Ghosh A, Isenberg JS, Roberts DD. Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice. Matrix Biol 37: 25–34, 2014. doi: 10.1016/j.matbio.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wiberg A, Granstam A, Ingvast S, Harkonen T, Knip M, Korsgren O, Skog O. Characterization of human organ donors testing positive for type 1 diabetes-associated autoantibodies. Clin Exp Immunol 182: 278–288, 2015. doi: 10.1111/cei.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skog O, Korsgren O. On the dynamics of the human endocrine pancreas and potential consequences for the development of type 1 diabetes. Acta Diabetol 57: 503–511, 2020. doi: 10.1007/s00592-019-01420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Olerud J, Mokhtari D, Johansson M, Christoffersson G, Lawler J, Welsh N, Carlsson PO. Thrombospondin-1: an islet endothelial cell signal of importance for β-cell function. Diabetes 60: 1946–1954, 2011. doi: 10.2337/db10-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 70: 5.47.1–5.47.20, 2015. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Tan SB, Guo ZG. CD47 decline in pancreatic islet cells promotes macrophage-mediated phagocytosis in type I diabetes. World J Diabetes 11: 239–251, 2020. doi: 10.4239/wjd.v11.i6.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang F, Liu YH, Zhang T, Gao J, Xu Y, Xie GY, Zhao WJ, Wang H, Yang YG. Aging-associated changes in CD47 arrangement and interaction with thrombospondin-1 on red blood cells visualized by super-resolution imaging. Aging Cell 19: e13224, 2020. doi: 10.1111/acel.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Corbi SCT, de Vasconcellos JF, Bastos AS, Bussaneli DG, da Silva BR, Santos RA, Takahashi CS, de S Rocha C, Carvalho B. D S, Maurer-Morelli CV, Orrico SRP, Barros SP, Scarel-Caminaga RM. Circulating lymphocytes and monocytes transcriptomic analysis of patients with type 2 diabetes mellitus, dyslipidemia and periodontitis. Sci Rep 10: 8145, 2020. doi: 10.1038/s41598-020-65042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harloff M, Pruschenk S, Seifert R, Schlossmann J. Activation of sGC signalling with Cinaciguat improves impaired kidney function in diabetic mice. Br J Pharmacol In press. doi: 10.1111/bph.15425. [DOI] [PubMed] [Google Scholar]
- 112.Tanaka M, Kanazashi M, Matsumoto T, Kondo H, Ishihara A, Fujino H. Mild hyperbaric oxygen exposure attenuates rarefaction of capillary vessels in streptozotocin-induced diabetic soleus muscle in rats. Biomed Res 42: 1–11, 2021. doi: 10.2220/biomedres.42.1. [DOI] [PubMed] [Google Scholar]
- 113.Xu X, Wang Z, Li Q, Xiao X, Lian Q, Xu W, Sun X, Tao H, Li R. Endothelial nitric oxide synthase expression is progressively increased in primary cerebral microvascular endothelial cells during hyperbaric oxygen exposure. Oxid Med Cell Longev 2: 7–13, 2009. doi: 10.4161/oxim.2.1.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wong AS, Mortin-Toth S, Sung M, Canty AJ, Gulban O, Greaves DR, Danska JS. Polymorphism in the innate immune receptor SIRPα controls CD47 binding and autoimmunity in the nonobese diabetic mouse. J Immunol 193: 4833–4844, 2014. doi: 10.4049/jimmunol.1401984. [DOI] [PubMed] [Google Scholar]
- 115.Sinha S, Borcherding N, Renavikar PS, Crawford MP, Tsalikian E, Tansey M, Shivapour ET, Bittner F, Kamholz J, Olalde H, Gibson E, Karandikar NJ. An autoimmune disease risk SNP, rs2281808, in SIRPG is associated with reduced expression of SIRPγ and heightened effector state in human CD8 T-cells. Sci Rep 8: 15440, 2018. doi: 10.1038/s41598-018-33901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dai H, Friday AJ, Abou-Daya KI, Williams AL, Mortin-Toth S, Nicotra ML, Rothstein DM, Shlomchik WD, Matozaki T, Isenberg JS, Oberbarnscheidt MH, Danska JS, Lakkis FG. Donor SIRPα polymorphism modulates the innate immune response to allogeneic grafts. Sci Immunol 2: eaam6202, 2017. doi: 10.1126/sciimmunol.aam6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hassel JC, Heinzerling L, Aberle J, Bahr O, Eigentler TK, Grimm MO, Grunwald V, Leipe J, Reinmuth N, Tietze JK, Trojan J, Zimmer L, Gutzmer R. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat Rev 57: 36–49, 2017. doi: 10.1016/j.ctrv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 118.de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, Van der Auwera BJ, Gorus FK, Roep BO, Aspeslagh S, Neyns B, Velkeniers B, Kharagjitsingh AV. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol 181: 363–374, 2019. doi: 10.1530/EJE-19-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hayes BH, Tsai RK, Dooling LJ, Kadu S, Lee JY, Pantano D, Rodriguez PL, Subramanian S, Shin JW, Discher DE. Macrophages show higher levels of engulfment after disruption of cis interactions between CD47 and the checkpoint receptor SIRPα. J Cell Sci 133: jcs237800, 2020. doi: 10.1242/jcs.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yao M, Rogers NM, Csanyi G, Rodriguez AI, Ross MA, St Croix C, Knupp H, Novelli EM, Thomson AW, Pagano PJ, Isenberg JS. Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol 25: 1171–1186, 2014. [Erratum in J Am Soc Nephrol 25: 1884, 2014]. doi: 10.1681/ASN.2013040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aiken J, Mandel ER, Riddell MC, Birot O. Hyperglycaemia correlates with skeletal muscle capillary regression and is associated with alterations in the murine double minute-2/forkhead box O1/thrombospondin-1 pathway in type 1 diabetic BioBreeding rats. Diab Vasc Dis Res 16: 28–37, 2019. doi: 10.1177/1479164118805928. [DOI] [PubMed] [Google Scholar]
- 122.Londino JD, Gulick D, Isenberg JS, Mallampalli RK. Cleavage of signal regulatory protein α (SIRPα) enhances inflammatory signaling. J Biol Chem 290: 31113–31125, 2015. doi: 10.1074/jbc.M115.682914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobayashi M, Ohnishi H, Okazawa H, Murata Y, Hayashi Y, Kobayashi H, Kitamura T, Matozaki T. Expression of Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 in pancreatic β-cells and its role in promotion of insulin secretion and protection against diabetes. Endocrinology 149: 5662–5669, 2008. doi: 10.1210/en.2008-0236. [DOI] [PubMed] [Google Scholar]
- 124.Thomas SS, Dong Y, Zhang L, Mitch WE. Signal regulatory protein-α interacts with the insulin receptor contributing to muscle wasting in chronic kidney disease. Kidney Int 84: 308–316, 2013. doi: 10.1038/ki.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dubois S, Madec AM, Mesnier A, Armanet M, Chikh K, Berney T, Thivolet C. Glucose inhibits angiogenesis of isolated human pancreatic islets. J Mol Endocrinol 45: 99–105, 2010. doi: 10.1677/JME-10-0020. [DOI] [PubMed] [Google Scholar]
- 126.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 127.The Human Protein Atlas. THBS1. https://www.proteinatlas.org/ENSG00000137801-THBS1/tissue [2021 Mar 24].
- 128.The Human Protein Atlas. CD47. https://www.proteinatlas.org/ENSG00000196776-CD47/tissue.
- 129.Cunha DA, Cito M, Carlsson PO, Vanderwinden JM, Molkentin JD, Bugliani M, Marchetti P, Eizirik DL, Cnop M. Thrombospondin 1 protects pancreatic β-cells from lipotoxicity via the PERK-NRF2 pathway. Cell Death Differ 23: 1995–2006, 2016. doi: 10.1038/cdd.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weaver JR, Grzesik W, Taylor-Fishwick DA. Inhibition of NADPH oxidase-1 preserves β cell function. Diabetologia 58: 113–121, 2015. doi: 10.1007/s00125-014-3398-2. [DOI] [PubMed] [Google Scholar]
- 131.Cunha DA, Cito M, Grieco FA, Cosentino C, Danilova T, Ladriere L, Lindahl M, Domanskyi A, Bugliani M, Marchetti P, Eizirik DL, Cnop M. Pancreatic β-cell protection from inflammatory stress by the endoplasmic reticulum proteins thrombospondin 1 and mesencephalic astrocyte-derived neutrotrophic factor (MANF). J Biol Chem 292: 14977–14988, 2017. doi: 10.1074/jbc.M116.769877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cao Z, Wang X. The endocrine role between β cells and intra-islet endothelial cells. Endocr J 61: 647–654, 2014. doi: 10.1507/endocrj.ej14-0045. [DOI] [PubMed] [Google Scholar]
- 133.Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes 55: 2965–2973, 2006. doi: 10.2337/db06-0733. [DOI] [PubMed] [Google Scholar]
- 134.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SMF, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-b1 in vivo. Cell 93: 1159–1170, 1998. doi: 10.1016/S0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 135.Olerud J, Johansson M, Lawler J, Welsh N, Carlsson PO. Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes 57: 1870–1877, 2008. doi: 10.2337/db07-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johansson A, Olerud J, Johansson M, Carlsson PO. Angiostatic factors normally restrict islet endothelial cell proliferation and migration: implications for islet transplantation. Transpl Int 22: 1182–1188, 2009. doi: 10.1111/j.1432-2277.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 137.Shrestha P, Batra L, Tariq Malik M, Tan M, Yolcu ES, Shirwan H. Immune checkpoint CD47 molecule engineered islets mitigate instant blood-mediated inflammatory reaction and show improved engraftment following intraportal transplantation. Am J Transplant 20: 2703–2714, 2020. doi: 10.1111/ajt.15958. [DOI] [PubMed] [Google Scholar]
- 138.Reffet S, Thivolet C. Immunology of pancreatic islet transplantation. Diabetes Metab 32: 523–526, 2006. doi: 10.1016/s1262-3636(06)72805-1. [DOI] [PubMed] [Google Scholar]
- 139.Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation 19: 23–30, 2012. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu L, Zhang Y, Chen J, Xu Y. Thrombospondin-1: a key protein that induces fibrosis in diabetic complications. J Diabetes Res 2020: 8043135, 2020. doi: 10.1155/2020/8043135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.LeBlanc AJ, Kelm NQ. Thrombospondin-1, free radicals, and the coronary microcirculation: the aging conundrum. Antioxid Redox Signal 27: 785–801, 2017. doi: 10.1089/ars.2017.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rogers NM, Ghimire K, Calzada MJ, Isenberg JS. Matricellular protein thrombospondin-1 in pulmonary hypertension: multiple pathways to disease. Cardiovasc Res 113: 858–868, 2017. doi: 10.1093/cvr/cvx094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol 16: 349–362, 2020. doi: 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- 144.Aly H, Rohatgi N, Marshall CA, Grossenheider TC, Miyoshi H, Stappenbeck TS, Matkovich SJ, McDaniel ML. A novel strategy to increase the proliferative potential of adult human β-cells while maintaining their differentiated phenotype. PLoS One 8: e66131, 2013. doi: 10.1371/journal.pone.0066131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Puri S, Roy N, Russ HA, Leonhardt L, French EK, Roy R, Bengtsson H, Scott DK, Stewart AF, Hebrok M. Replication confers β cell immaturity. Nat Commun 9: 485, 2018. doi: 10.1038/s41467-018-02939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lamy L, Ticchioni M, Rouquette-Jazdanian AK, Samson M, Deckert M, Greenberg AH, Bernard A. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J Biol Chem 278: 23915–23921, 2003. doi: 10.1074/jbc.M301869200. [DOI] [PubMed] [Google Scholar]
- 147.Sharifi-Sanjani M, Shoushtari AH, Quiroz M, Baust J, Sestito SF, Mosher M, Ross M, McTiernan CF, St Croix CM, Bilonick RA, Champion HC, Isenberg JS. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc 3: e000670, 2014. doi: 10.1161/JAHA.113.000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 88: 471–481, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller T, Isenberg J, Shih H, Wang Y, Roberts D. Amyloid-β inhibits no-cGMP signaling in a CD36-and CD47-dependent manner. Plos One 5: e15686, 2010. doi: 10.1371/journal.pone.0015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mundinger TO, Mei Q, Foulis AK, Fligner CL, Hull RL, Taborsky GJ Jr.. Human type 1 diabetes is characterized by an early, marked, sustained, and islet-selective loss of sympathetic nerves. Diabetes 65: 2322–2330, 2016. doi: 10.2337/db16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao Y, Pu D, Sun Y, Chen J, Luo C, Wang M, Zhou J, Lv A, Zhu S, Liao Z, Zhao K, Xiao Q. High glucose-induced defective thrombospondin-1 release from astrocytes via TLR9 activation contributes to the synaptic protein loss. Exp Cell Res 363: 171–178, 2018. doi: 10.1016/j.yexcr.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 152.Risher WC, Kim N, Koh S, Choi JE, Mitev P, Spence EF, Pilaz LJ, Wang D, Feng G, Silver DL, Soderling SH, Yin HH, Eroglu C. Thrombospondin receptor α2δ-1 promotes synaptogenesis and spinogenesis via postsynaptic Rac1. J Cell Biol 217: 3747–3765, 2018. doi: 10.1083/jcb.201802057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moralez AM, Maile LA, Clarke J, Busby WH Jr, Clemmons DR. Insulin-like growth factor binding protein-5 (IGFBP-5) interacts with thrombospondin-1 to induce negative regulatory effects on IGF-I actions. J Cell Physiol 203: 328–334, 2005. doi: 10.1002/jcp.20343. [DOI] [PubMed] [Google Scholar]
- 154.Numakawa T, Ishimoto T, Suzuki S, Numakawa Y, Adachi N, Matsumoto T, Yokomaku D, Koshimizu H, Fujimori KE, Hashimoto R, Taguchi T, Kunugi H. Neuronal roles of the integrin-associated protein (IAP/CD47) in developing cortical neurons. J Biol Chem 279: 43245–43253, 2004. doi: 10.1074/jbc.M406733200. [DOI] [PubMed] [Google Scholar]
- 155.Zhu D, Xie L, Kang Y, Dolai S, Bondo Hansen J, Qin T, Xie H, Liang T, Rubin DC, Osborne L, Gaisano HY. Syntaxin 2 acts as inhibitory SNARE for insulin granule exocytosis. Diabetes 66: 948–959, 2017. doi: 10.2337/db16-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xie L, Zhu D, Dolai S, Liang T, Qin T, Kang Y, Xie H, Huang YC, Gaisano HY. Syntaxin-4 mediates exocytosis of pre-docked and newcomer insulin granules underlying biphasic glucose-stimulated insulin secretion in human pancreatic β cells. Diabetologia 58: 1250–1259, 2015. doi: 10.1007/s00125-015-3545-4. [DOI] [PubMed] [Google Scholar]
- 157.Rogers NM, Sharifi-Sanjani M, Csanyi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol 37: 92–101, 2014. doi: 10.1016/j.matbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]