Abstract
Leukocyte recruitment is a critical step in the pathogenesis of inflammatory and immunological responses. Cell adhesion molecules (CAMs) are involved in controlling cell movements and the recruitment process, and the integrin family of CAMs plays a key role. During cell movement, integrin function is dynamically and precisely regulated. However, this balance might be broken under pathological conditions. Thus, the functional regulation and molecular mechanisms of integrins related to diseases are often a focus of research. Integrin β2 is one of the most commonly expressed integrins in leukocytes that mediate leukocyte adhesion and migration, and it plays an important role in immune responses and inflammation. In this review, we focus on specific functions of integrin β2 in leukocyte recruitment, the conformational changes and signal transduction of integrin β2 activation, the similarities between murine and human factors, and how new insights into these processes can inform future therapies for inflammation and immune diseases.
Keywords: β2 integrins, integrin activation, integrin adaptors, leukocyte recruitment
INTRODUCTION
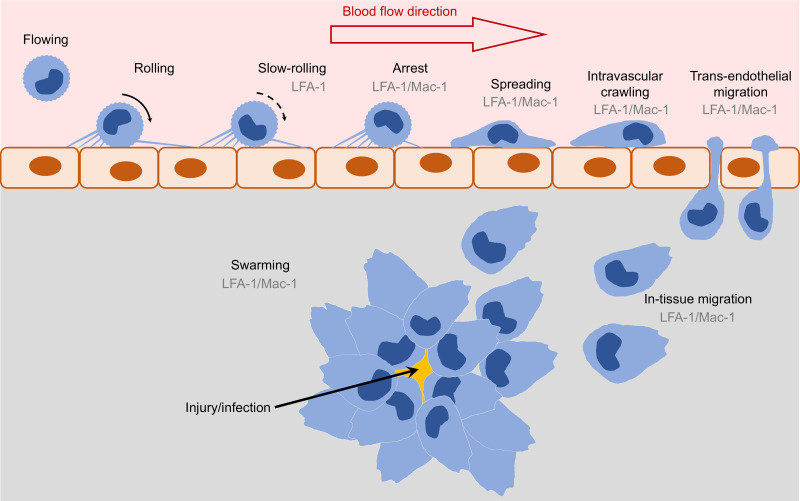
Blood leukocytes, which include neutrophils, monocytes, lymphocytes, and others, play critical roles in immune defense to pathogens and inflammatory responses. Blood leukocytes are recruited to tissues during inflammation and infections in a step-by-step process called the leukocyte recruitment cascade (1, 2) (Fig. 1). This includes rolling, slow-rolling, arrest, spreading, intravascular crawling, trans-endothelial migration, and migration to the site of inflammation. Integrins are αβ-heterodimer adhesion molecules expressed on leukocyte surfaces (Fig. 2A). β2 integrins, including lymphocyte function-associated antigen 1 (LFA-1, also known as αLβ2 and CD11a/CD18), macrophage-1 antigen (Mac-1, also known as αMβ2, CD11b/CD18, and complement receptor 3), αXβ2 (also known as CD11c/CD18 and complement receptor 4), and αDβ2 (also known as CD11d/CD18), are leukocyte-specific integrins (3, 4) and are involved in most steps of the leukocyte recruitment cascade, which will be introduced in detail in ROLE OF β2 INTEGRIN IN LEUKOCYTE RECRUITMENT. β2 integrin deficiencies in humans lead to leukocyte adhesion deficiency (LAD) syndromes (5). Mutations in β2 integrins cause LAD-I, which presents recurrent, life-threatening bacterial infections. Mutations in a critical integrin activation adaptor protein kindlin-3 result in LAD-III, characterized by severe bacterial infections and a severe bleeding disorder.
Figure 1.
Schematic showing the involvement of β2 integrins in the leukocyte recruitment cascade. Flowing leukocytes roll on the vascular endothelial surface. β2 integrins contribute to slow-rolling and firm adhesion (arrest). Leukocytes then spread on endothelial cells, perform intravascular crawling, and transmigrate through endothelial cells (paracellular or transcellular). Leukocytes may swarm during in-tissue migration to the site of inflammation or infection. LFA-1, lymphocyte function-associated antigen 1; Mac-1, macrophage-1 antigen.
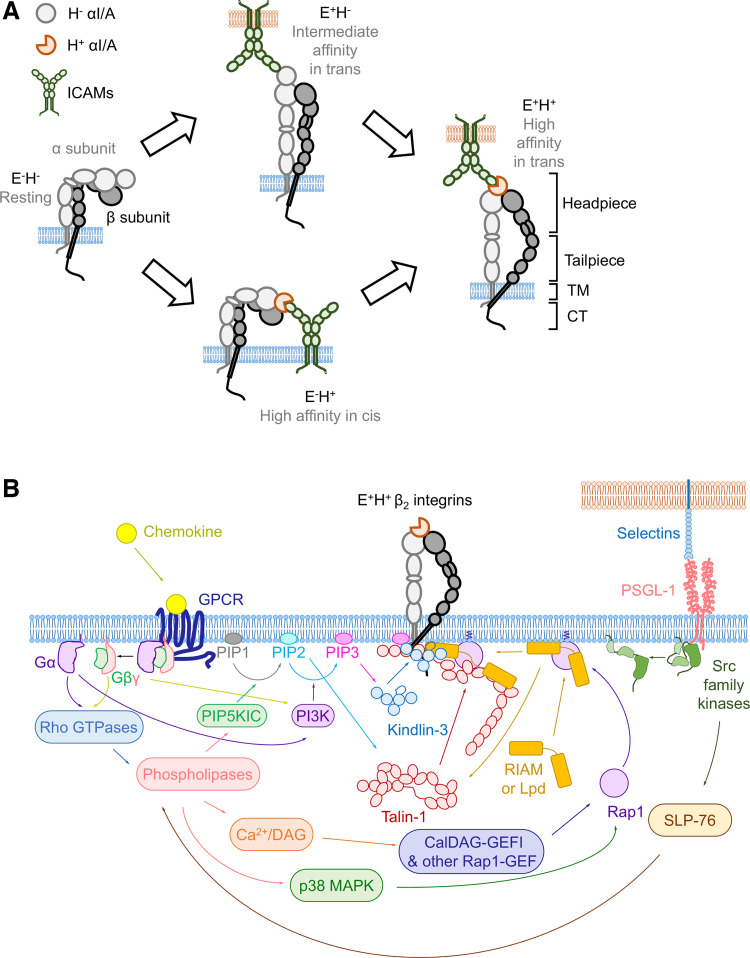
Figure 2.
Integrin conformational changes and signaling pathways during activation. A: schematic showing allosteric pathways of β2 integrin activation. B: schematic showing G protein-coupled receptor (GPCR) and P-selectin glycoprotein ligand-1 (PSGL-1)-triggered β2 integrin activation signaling pathways. CalDAG-GEFI, calcium and DAG-regulated guanine nucleotide exchange factor I; CT, cytoplasmic tail; DAG, diacylglycerol; E−H−, bent low-affinity; E+H−, extended low-affinity; E−H+, bent high-affinity; E+H+, extended high-affinity; Gα, G protein α subunit; Gβγ, G protein βγ complex; ICAMs, intercellular adhesion molecules; MAPK, mitogen-activated protein kinase; PIP1, phosphatidylinositol 4-phosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3–5)-trisphosphate; PIP5K1C, phosphatidylinositol-4-phosphate 5-kinase type-1-γ; PI3K, phosphoinositide 3-kinase; Rap1, Ras-proximate-1 or Ras-related protein 1; Rap1-GEF, Rap1 guanine nucleotide exchange factor; RIAM, Rap1-GTP-interacting adapter molecule; SLP-76, SH2 domain-containing leukocyte protein of 76 kDa; TM, transmembrane.
β2 INTEGRIN ACTIVATION AND CONFORMATIONAL CHANGES
Both integrin α and β subunits include a large ectodomain consisting of a headpiece and a tailpiece, a transmembrane domain, and a short flexible cytoplasmic tail (6) (Fig. 2A). The ligand-binding site of β2 integrins is on the αA/I domain. The conformational changes of β2 integrins are strictly regulated. When cells are not stimulated, β2 integrins are in a bent low-affinity conformation (E−H−), and the binding of E−H− β2 integrins and ligands is very weak. The “switchblade” model is a widely accepted model for integrin activation (6), and the activation process includes two steps: 1) the extension of integrin ectodomain (E+) and 2) the rearrangement of ligand-binding sites that acquire high affinity to ligands (H+). After activation, the β2 integrins present an extended high-affinity conformation (E+H+) (6). The conformation between the above two conformations is intermediate affinity, which has an extended low-affinity headpiece conformation (E+H−) (6, 7). The switchblade model suggests a sequence of conformational changes from E−H− to E+H− to E+H+ (6). During leukocyte migration, the transition from E+H− to E+H+ might involve intracellular forces applied on the integrin β-subunits through bond cytoskeletons (8).
Another integrin activation model is the “deadbolt” model (9), which involves a bent high-affinity conformation (E−H+) of integrin αVβ3 in a binding complex with fibronectin (10). In this model, a hairpin loop in the β tail domain acts as a deadbolt to restrain the displacement of the β-A/I domain β6-α7 loop and maintain integrin in the H− state. The movement of this loop results in E−H+ conformation and allows ligand binding. Then, the binding of ligands provides a pulling force that extends the ectodomain. This model is controversial in that the “deadbolt” works on Mac-1 (11) but not β3 integrins (12). However, E−H+ αXβ2 has been reported (13). Recent studies using quantitative dynamic foot printing (qDF) microscopy and stochastic optical reconstruction microscopy showed that primary human neutrophils express E−H+ β2 integrins that bind intercellular adhesion molecules (ICAMs) in cis and auto-inhibit integrin activation and neutrophil adhesion (7, 14). With qDF imaging, the conformational changes from E−H− to E+H− and then to E+H+ that favor the switchblade model and the conformational changes from E−H− to E−H+ and then to E+H+ that favor the deadbolt model were both observed. Another study showed that Mac-1 could bind Fc-γ receptor IIA (FcγRIIA, CD32) in cis and limit antibody-mediated neutrophil recruitment (15), further demonstrating that E−H+ Mac-1 exists. The mechanism of how this E−H+ activation occurs requires further investigation.
ROLE OF β2 INTEGRIN IN LEUKOCYTE RECRUITMENT
Leukocyte recruitment from blood (Fig. 1 and Table 1) is initiated by rolling on the vascular endothelium mediated by the interactions of leukocyte P-selectin glycoprotein ligand-1 (PSGL-1) and endothelial selectins (1, 2). β2 integrins are required to slow the rolling of cells, a process called “slow-rolling.” Slow-rolling is mediated by E+ integrins triggered by the interaction of PSGL-1 and selectins, a signaling pathway that will be introduced in a later section. Slow-rolling depends on LFA-1 but not Mac-1 interacting with intercellular adhesion molecule 1 (ICAM-1) in mouse and human neutrophils (Table 1).
Table 1.
Molecules involved in neutrophil recruitment cascades in mice and humans
| Recruitment Cascades | Human or Mouse | Involved β2 Integrins | Involved Ligands | Involved Integrin Adaptor Proteins | Integrin Activation State Required | References |
|---|---|---|---|---|---|---|
| Slow-rolling | Mouse | LFA-1 | ICAM-1 | Talin-1 | ND | (16–19) |
| Human | LFA-1 | ICAM-1 | Talin-1 | E+ | (20) | |
| Arrest | Mouse | LFA-1 | ICAM-1 | Talin-1, kindlin-3 | ND | (16, 17, 21–23) |
| Human | LFA-1, Mac-1 | ICAM-1 | Talin-1, kindlin-3 | E+H+ | (7, 24) | |
| Spreading | Mouse | Mac-1 | ICAM-1, immune complex | Kindlin-3 | ND | (22, 25, 26) |
| Human | ND | ICAM-1 | Talin-1, kindlin-3 | E+H+ | (27, 28) | |
| Intravascular crawling | Mouse | Mac-1 | ICAM-1 | NA | ND | (29) |
| Trans-endothelial migration | Mouse | LFA-1, Mac-1 | JAM-A, JAM-C, ICAM-1 | NA | ND | (30–33) |
| Human | LFA-1 | JAM-A | NA | ND | (34) | |
| Migration /chemotaxis | Human | LFA-1, Mac-1 | ICAM-1, collagen, fibronectin, β-glucan | Talin-1, kindlin-3 | ND | (35–38) |
| Swarming | Mouse | LFA-1, Mac-1 | ND | Talin-1 | ND | (39) |
ICAM-1, intercellular adhesion molecule 1; JAM-A, junctional adhesion molecule-A; JAM-C, junctional adhesion molecule-C; LFA-1, lymphocyte function-associated antigen 1; Mac-1, macrophage-1 antigen; NA, not applicable; ND, not determined.
When rolling leukocytes encounter chemokines expressed on endothelial surfaces or in blood, the G protein-coupled receptor (GPCR)-mediated signaling pathway (which will be discussed more in CHEMOKINE-GPCR-INDUCED SIGNALING PATHWAYS IN β2 INTEGRIN ACTIVATION) induces fully E+H+ β2 integrins that bind ligand in trans and stops rolling cells, a process called “arrest” (Fig. 1). LFA-1 but not Mac-1 interacting with ICAM-1 is important for mouse neutrophil arrest, and both LFA-1 and Mac-1 interacting with ICAM-1 are important for human neutrophil arrest (Table 1).
After arrest, round leukocytes undergo large morphological changes and spread on the endothelium surface, which requires β2 integrins as well (27). Then leukocytes crawl in the vascular lumen. Neutrophils use Mac-1 to perform intravascular crawling (Table 1). Both LFA-1 and Mac-1 are important for monocyte intravascular crawling (40).
Leukocyte trans-endothelial migration is the next step of recruitment. Trans-endothelial migration may happen at the junctions between endothelial cells (paracellular) or in the middle of an endothelial cell (transcellular) (30). A study using LFA-1-deficient mice and blocking antibodies in an in vitro system (cultured endothelial cell monolayers) showed that the trans-endothelial migration of neutrophils and T lymphocytes are LFA-1-dependent, whereas monocytes use both LFA-1 and Mac-1 for trans-endothelial migration (Table 1). However, later studies showed that Mac-1 is also important for neutrophil trans-endothelial migration by interacting with endothelial junctional adhesion molecule C (JAM-C or JAM-3, Table 1). Endothelial JAM-A (or JAM-1) is the ligand of LFA-1 involved in trans-endothelial migration of neutrophils and T lymphocytes (Table 1).
Leukocyte migration within tissues is a mechanistically different process than in the bloodstream that can occur without β2 integrins (41, 42). However, β2 integrins also play critical roles during in-tissue migration of leukocytes in some cases. Antibody blockade of Mac-1 can decrease neutrophil migration through low-concentration (0.4 mg/mL) but not high-concentration (>0.6 mg/mL) collagen gels (Table 1). Leukocytes migrate to the site of infection or sterile injury and form swarms, a process called “swarming.” Swarming is visualized by intravital microscopy of the dermis (Table 1). Using integrin β2, αL, αM, and talin-1 (a key adaptor of integrin activation; details will be discussed in INTEGRIN BINDING ADAPTOR PROTEINS) knockout mice, it was shown that both LFA-1 and Mac-1 are involved, and integrin activation is critical for neutrophil swarming (Table 1). It has also been shown that inhibition of LFA-1/ICAM-1 reduces the speed of interstitial T cell migration within ex vivo lymph nodes (43). Intravital imaging of lymph nodes showed that LFA-1 contributes to subtle cell elongation during native T cell migration (44). A T cell migration study using emission anisotropy total internal reflection fluorescence microscopy showed that cytoskeleton and ligand-bound integrins orient in the same direction as retrograde actin flow with their cytoskeleton-binding β-subunits tilted by the applied force, suggesting a molecular model of integrin activation (E+H− to E+H+) by cytoskeletal force (8).
CHEMOKINE-GPCR-INDUCED SIGNALING PATHWAYS IN β2 INTEGRIN ACTIVATION
Chemokines can regulate integrin-mediated cell adhesion and migration through the inside-out signaling pathway (Fig. 2B). This signaling is generally induced by chemokine stimulation through corresponding GPCRs, such as chemokine C-X-C motif ligand 8 [CXCL8, also known as interleukin-8 (IL-8)] and neutrophil C-X-C motif chemokine receptor 2 (CXCR2) (45), chemokine C-C motif chemokine ligand 2 [CCL2, also known as monocyte chemoattractant protein-1 (MCP-1)] and monocyte C-C motif chemokine receptor 2 (CCR2) (46), and CXCL12 [also known as stromal cell-derived factor 1 (SDF-1)] and lymphocyte CXCR4 (47). The activation of GPCRs dissociates G protein to Gα and Gβγ subunits (48). Gα activates downstream Ras homolog gene family (Rho) GTPases, such as Ras homolog gene family, member A (RhoA) and cell division control protein 42 (CDC42), and leads to β2 integrin activation (49). Blockade of the 23–40 RhoA effector region inhibits chemokine stimulation-induced high-affinity integrin αLβ2, reduces the adhesion of αLβ2 to high concentrations of ICAM-1, and blocks leukocyte homing to Peyer’s patches in vivo (50). Gβγ activates another Rho GTPase downstream, Ras-related C3 botulinum toxin substrate 1 (Rac1), then activates phospholipase C β2 (PLCβ2) and PLCβ3, induces intracellular Ca2+ flux, and activates β2 integrin (51). Other Rho GTPases, such as Rac2 (52), and phospholipases, such as PLCγ (53) and phospholipase D1 (PLD1) (4), may be involved in the integrin activation signaling pathway. Downstream of Rho GTPases, phospholipases, and intracellular Ca2+ flux in the integrin activation signaling pathway are Ras-related protein 1 (Rap1) GTPases (53–55), which are required for leukocyte adhesion (55). The activation of Rap1 by PLC through Ca2+ and diacylglycerol (DAG) depends on Rap1 guanine nucleotide exchange factors (Rap1-GEFs) (55) and calcium and DAG-regulated guanine nucleotide exchange factors (CalDAG-GEFs) (54). Another mediator between PLC and Rap1 may be p38 mitogen-activated protein kinase (MAPK) (56). N-formyl-Met-Leu-Phe stimulation activates neutrophil p38 MAPK and depends on PLC (57). Phosphatidylinositol-4-phosphate 5-kinase type I-γ (PIP5K1C) is downstream of PLD1 (21).
Rap1-GTP-interacting adaptor molecule (RIAM) is a Rap1 binding protein important for leukocyte β2 integrin activation. Loss of RIAM in mice results in impaired β2 integrin activation and leukocyte adhesion (58). RIAM binds the Src kinase-associated phosphoprotein 2 (Skap2), which is activated by phosphatidylinositol (3–5)-trisphosphate (PIP3) (59), and the adhesion and degranulation-promoting adapter protein (ADAP) module to promote membrane targeting of the RIAM-Rap1 complex, which is required for β2 integrin activation of T cells (60). RIAM binds talin-1, which is an integrin-binding activation adaptor (discussed in detail below) and recruits talin-1 to the membrane to bind and activate integrins (61). Lamellipodin (Lpd) is another bridge between Rap1 and talin-1 (61). RIAM and Lpd distinctly regulate integrin activation on effector and regulatory T cells, respectively (62).
Skap2, which is a RIAM-binding protein mentioned in the above paragraph, is important for regulating β2 integrin activation in neutrophils (63). Besides RIAM and ADAP, Skap2 was also found to bind to Wiskott–Aldrich syndrome protein (WASp), mediate actin polymerization, and be required for the recruitment of talin-1 and kindlin-3 to the β2 integrin cytoplasmic domain (63).
INTEGRIN BINDING ADAPTOR PROTEINS
Talin-1 is a key adaptor protein for integrin activation. It consists of a 50 kDa N-terminal head domain (THD) and a 220 kDa rod domain (64). The THD of talin-1 is a FERM domain (F for 4.1 protein, E for ezrin, R for radixin, and M for moesin) consisting of four subdomains, F0–F3. The F3 subdomain contains two binding sites (identified by three mutations, L325R, R358A, and W359A): one that binds to the membrane-proximal α-helix and one that binds to the membrane-proximal NPxY motif of the integrin β cytoplasmic tail (64). The W359A mutation eliminates the binding of talin-1 to β2 integrin and shows complete deficiencies of leukocyte slow-rolling and arrest (17), which is similarly observed in talin-1 knockout leukocytes (16). The talin-1 mutant L325R still binds to β2 integrin but shows a deficiency in leukocyte arrest but not slow-rolling (17), suggesting that talin-1 binding to the membrane-proximal α-helix of integrin β2 is required for H+ but not E+. The binding sites on the THD are hidden by the interaction with the rod domain of inactive talin-1 (65). Several molecules have been reported to disrupt the interactions between the THD and the rod domain, such as PIP5K1C-generated phosphatidylinositol 4,5-bisphosphate (PIP2, which is a cell membrane component) (66), calpain (67), and RIAM (68). Interaction of talin with the cell membrane is also essential for integrin activation. Mutation of K322 (K322D), which is predicted to be directed toward the membrane, but not K320 (K320D), which is predicted to point away from the membrane, on the talin-1 F3 domain abolished integrin activation (65). Mutations of the positively charged patch in the F2 domain of talin-1 (K256E/K272E/K274E/R277E) abolished the binding to phosphatidylcholine in vitro and αIIbβ3 integrin activation in Chinese hamster ovary (CHO) cells (69), suggesting the recruitment of talin-1 to cell membrane phosphatidylcholine is important for integrin activation. Another study showed that point mutations of the basic patch in the talin-1 F2 domain (K272A/K274Q/R277E) or pair-wise mutations of the lysine residues of the basic finger in the talin-1 F3 domain (K320A/K322A; K322A/K324A) reduced the association of talin-1 head domain with the PIP2-containing lipid surface up to sixfold, as measured by the response units at equilibrium (70). This study also showed that the talin-1 F2 domain K272A/K274Q/R277E mutation and F3 domain K322A/K324A mutation but not the F3 domain K320A/K322A mutation blocked integrin clustering (70), suggesting the role of PIP2 and talin-1 in integrin clustering. The lipid-binding-deficient talin-2 mutation (K272Q/K316Q/K324Q/E342Q/K343Q) failed to bind PIP2 in vitro (71). Experiments in CHO cells demonstrated that this mutant failed to be recruited to the cell membrane and activate αIIbβ3 integrins (71). However, all these studies were done on integrin αIIbβ3. The role of talin-PIP2 interaction needs to be further explored on leukocyte β2 integrins. Talin-1 is required for both β2 integrin E+ and H+, which are critical for leukocyte slow-rolling and arrest, respectively (16).
Kindlin-3 is another adaptor protein that binds to the NPxY motif of the β2 integrin cytoplasmic tail (72) and regulates β2 integrin activation in leukocytes (22). Similar to talin-1, kindlin-3 also contains a FERM domain that binds to the distal NPxY motif, the β cytoplasmic tail, and can be further divided into four subdomains, F0–F3. Kindlin-3-deficient mouse neutrophils showed less recruitment in a phorbol ester-treated ear inflammation model, similar rolling velocity but less adhesion on postcapillary venules of cremaster muscles, and less adhesion and spreading on an ICAM-1-coated surface in vitro (22). In another study (16), kindlin-3-deficient mouse neutrophils showed less binding of soluble ICAM-1 after CXCL1 stimulation. Consistent with the previous study, kindlin-3-deficient mouse neutrophils showed similar rolling velocity on the substrate of both E-selectin and E-selectin plus ICAM-1 compared with wild-type controls. Similar to wild-type neutrophils, kindlin-3-deficient neutrophils showed slow-rolling by rolling slower on the substrate of P-selectin plus ICAM-1 compared with that of P-selectin only, indicating kindlin-3 is dispensable for β2 integrin E+. This was also confirmed by intravital cremaster muscle imaging showing that LFA-1 blockade increased the rolling velocity of both wild-type and kindlin-3 knockout neutrophils. Kindlin-3 is required for neutrophil arrest in vitro and in vivo, indicating that kindlin-3 is necessary for β2 integrin H+. Kindlin-3 knockdown in HL60 cells inhibited expression of H+ but not E+ β2 integrins after stimulation with constitutively active Rap1a peptide. A Q597A/W598A mutation in the F3 domain of mouse kindlin-3 (equivalent to human kindlin-3 Q599A/W600A) disables the interaction of kindlin-3 and β cytoplasmic tail and suppresses neutrophil adhesion (73). The pleckstrin homology (PH) domain is required for the recruitment of kindlin-3 to the cell membrane, which is essential for β2 integrin activation and adhesion of B cells (74) and neutrophils (24). The PH domain itself is sufficient to recruit kindlin-3 to the cell membrane in response to chemokine stimulation (24). Dynamic live-cell imaging showed that kindlin-3 is recruited to the cell membrane before H+ of β2 integrins (24). Transfecting kindlin-3 back into kindlin-3 knockout cells restores neutrophil adhesion in vivo (24). Kindlin-3 PH domain shows a high affinity to PIP3 but not PIP2 (75). PIP2 is phosphorylated at the 3' site by phosphoinositide 3-kinases (PI3Ks) to produce PIP3 (76). Chemokine binding to GPCRs activates PI3K through the release of Gβγ (76, 77). PI3K has been linked to neutrophil migration (77), and PI3Kγ knockout neutrophils showed a significant decrease of adhesion in vivo (78). Thus, PI3Kγ might be the upstream factor that regulates kindlin-3 membrane recruitment. It has been shown that pretreating cells with PI3K inhibitors wortmannin and LY294002 significantly inhibits chemokine-induced leukocyte adhesion on ICAM-1. Further tests showed that PI3K inhibition results in less integrin clustering but similar soluble ICAM-1 binding in response to chemokine stimulation, suggesting that PI3K is involved in regulating β2 integrin mobility/clustering/avidity but not affinity changes (79).
PSGL-1-SELECTINS INDUCE SIGNALING PATHWAYS IN β2 INTEGRIN ACTIVATION
PSGL-1, also known as CD162, is an adhesion molecule expressed on leukocytes (1, 2). PSGL-1 molecules interact with selectins, including P-selectin (CD62P), E-selectin (CD62E), and L-selectin (CD62L), and mediate leukocyte rolling on endothelial cells. It has been found that PSGL-1-selectin interaction can induce E+ of β2 integrins (Fig. 2B) (7, 20, 80). These intermediate-affinity E+H− β2 integrins interact with ICAM-1 and slow down leukocyte rolling (7, 20). This activation pathway involves activation of Src family kinases (SFKs), DNAX-activating protein of 12 kDa (DAP12), FcγR, spleen tyrosine kinase (Syk), and p38 MAPK (80). Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) and adhesion and degranulation promoting adaptor protein (ADAP) are involved in PSGL-1-E-selectin-mediated integrin activation (81). SLP-76 is required for the activation of Bruton’s tyrosine kinase (Btk), PLCγ2, PI3Kγ, and p38 MAPK (81), and is downstream of SFKs (82). Tyrosine 145, rather than tyrosines 112 and 128, on SLP-76 is phosphorylated in the PSGL-1-selectin-induced signaling pathway (82). Rap1a activation through CalDAG-EGFI and p38 MAPK has been shown in E-selectin-dependent integrin activation (56). L-selectin expressed on leukocytes can engage with PSGL-1 and favor PSGL-1-induced integrin activation (83). Similar to PSGL-1, L-selectins that directly bind to E-selectins during leukocyte rolling can trigger E+ of β2 integrins (84). Clustered L-selectins that bind with E-selectins can induce H+ of β2 integrins (84). As talin-1 is involved in PSGL-1/selectin-mediated slow-rolling (16), talin-1 recruitment to the β2 integrin cytoplasmic tail may happen in PSGL-1-selectin-induced signaling pathways.
CONCLUDING REMARKS
As β2 integrins are critical for leukocyte recruitment, drugs targeting β2 integrins have been developed to treat inflammatory diseases (85). However, pan-β2 integrin targeting results in severe side effects. Efalizumab, an LFA-1 monoclonal antibody to treat autoimmune diseases, has side effects including bacterial sepsis, viral meningitis, invasive fungal disease, and progressive multifocal leukoencephalopathy, and was withdrawn from the market in 2009 (85). Thus, drugs targeting specific integrin conformations or signaling regulators may be safer and effective.
Although many signaling regulators have been discovered, as we discussed above, identifying new signaling regulators as potential drug targets remains important. For example, E−H+ β2 integrins show an auto-inhibitory function in integrin activation and leukocyte recruitment (7, 15), but the signaling regulators for E–H+ β2 integrins are not known. Omics studies of β2 integrin activation loss-of-function and gain-of-function systems may be helpful. Monocytes from patients with cystic fibrosis showed a deficiency in β2 integrin activation (86). Thus, comparing the transcriptomes (87, 88) or proteomes (89) of cystic fibrosis and healthy monocytes might be a way to identify new signaling regulators of integrin activation. As a model of neutrophils, HL60 cells need to be differentiated to activate their β2 integrins. Therefore, transcriptomic (90, 91) or proteomic studies of HL60 may also provide further insight.
GRANTS
This research was supported by funding from the National Institutes of Health (NIH, R01HL145454) and a startup fund from UConn Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.F. prepared figures; H.S. drafted manuscript; H.S., L.H., and Z.F. edited and revised manuscript; Z.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Christopher “Kit” Bonin and Dr. Geneva Hargis from UConn School of Medicine for help with the scientific writing and editing of this manuscript.
REFERENCES
- 1.Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, Catz SD. Neutrophils: new insights and open questions. Sci Immunol 3: eaat4579, 2018. doi: 10.1126/sciimmunol.aat4579. [DOI] [PubMed] [Google Scholar]
- 2.Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev 99: 1223–1248, 2019. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 3.Fagerholm SC, Guenther C, Asens ML, Savinko T, Uotila LM. Beta2-integrins and interacting proteins in leukocyte trafficking, immune suppression, and immunodeficiency disease. Front Immunol 10: 254, 2019. doi: 10.3389/fimmu.2019.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun H, Zhi K, Hu L, Fan Z. The activation and regulation of beta2 integrins in phagocytes and phagocytosis. Front Immunol 12: 633639, 2021. doi: 10.3389/fimmu.2021.633639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol 55: 49–58, 2013. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol 25: 619–647, 2007. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Z, McArdle S, Marki A, Mikulski Z, Gutierrez E, Engelhardt B, Deutsch U, Ginsberg M, Groisman A, Ley K. Neutrophil recruitment limited by high-affinity bent beta2 integrin binding ligand in cis. Nat Commun 7: 12658, 2016. doi: 10.1038/ncomms12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordenfelt P, Moore TI, Mehta SB, Kalappurakkal JM, Swaminathan V, Koga N, Lambert TJ, Baker D, Waters JC, Oldenbourg R, Tani T, Mayor S, Waterman CM, Springer TA. Direction of actin flow dictates integrin LFA-1 orientation during leukocyte migration. Nat Commun 8: 2047, 2017. doi: 10.1038/s41467-017-01848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol 21: 381–410, 2005. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 10.Adair BD, Xiong JP, Maddock C, Goodman SL, Arnaout MA, Yeager M. Three-dimensional EM structure of the ectodomain of integrin αVβ3 in a complex with fibronectin. J Cell Biol 168: 1109–1118, 2005. doi: 10.1083/jcb.200410068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta V, Gylling A, Alonso JL, Sugimori T, Ianakiev P, Xiong JP, Arnaout MA. The beta-tail domain (betaTD) regulates physiologic ligand binding to integrin CD11b/CD18. Blood 109: 3513–3520, 2007. doi: 10.1182/blood-2005-11-056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Boylan B, Luo BH, Newman PJ, Springer TA. Tests of the extension and deadbolt models of integrin activation. J Biol Chem 282: 11914–11920, 2007. doi: 10.1074/jbc.M700249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen M, Yuki K, Springer TA. An internal ligand-bound, metastable state of a leukocyte integrin, alphaXbeta2. J Cell Biol 203: 629–642, 2013. doi: 10.1083/jcb.201308083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Z, Kiosses WB, Sun H, Orecchioni M, Ghosheh Y, Zajonc DM, Arnaout MA, Gutierrez E, Groisman A, Ginsberg MH, Ley K. High-affinity bent beta2-integrin molecules in arresting neutrophils face each other through binding to ICAMs in cis. Cell Rep 26: 119–130.e5, 2019. doi: 10.1016/j.celrep.2018.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saggu G, Okubo K, Chen Y, Vattepu R, Tsuboi N, Rosetti F, Cullere X, Washburn N, Tahir S, Rosado AM, Holland SM, Anthony RM, Sen M, Zhu C, Mayadas TN. Cis interaction between sialylated FcgammaRIIA and the alphaI-domain of Mac-1 limits antibody-mediated neutrophil recruitment. Nat Commun 9: 5058, 2018. doi: 10.1038/s41467-018-07506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefort CT, Rossaint J, Moser M, Petrich BG, Zarbock A, Monkley SJ, Critchley DR, Ginsberg MH, Fassler R, Ley K. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood 119: 4275–4282, 2012. doi: 10.1182/blood-2011-08-373118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yago T, Petrich BG, Zhang N, Liu Z, Shao B, Ginsberg MH, McEver RP. Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. J Exp Med 212: 1267–1281, 2015. doi: 10.1084/jem.20142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med 205: 2339–2347, 2008. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity 26: 773–783, 2007. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood 116: 617–624, 2010. doi: 10.1182/blood-2010-01-266122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolomini-Vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, Martinello M, Constantin G, Laudanna C. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol 10: 185–194, 2009. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- 22.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, Wang HV, Sperandio M, Fassler R. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med 15: 300–305, 2009. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 23.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol 163: 5029–5038, 1999. [PubMed] [Google Scholar]
- 24.Wen L, Marki A, Roy P, McArdle S, Sun H, Fan Z, Gingras AR, Ginsberg MH, Ley K. Kindlin-3 recruitment to the plasma membrane precedes high affinity beta2 integrin and neutrophil arrest from rolling. Blood 137: 29–38, 2021. doi: 10.1182/blood.2019003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang T, Rosenkranz A, Assmann KJ, Goodman MJ, Gutierrez-Ramos JC, Carroll MC, Cotran RS, Mayadas TN. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med 186: 1853–1863, 1997. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Spriel AB, Leusen JH, van Egmond M, Dijkman HB, Assmann KJ, Mayadas TN, van de Winkel JG. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 97: 2478–2486, 2001. doi: 10.1182/blood.v97.8.2478. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Fan Z, Gingras AR, Lopez-Ramirez MA, Ginsberg MH, Ley K. Frontline science: a flexible kink in the transmembrane domain impairs beta2 integrin extension and cell arrest from rolling. J Leukoc Biol 107: 175–183, 2020. doi: 10.1002/JLB.1HI0219-073RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue ZH, Feng C, Liu WL, Tan SM. A role of kindlin-3 in integrin alphaMbeta2 outside-in signaling and the Syk-Vav1-Rac1/Cdc42 signaling axis. PLoS One 8: e56911, 2013. doi: 10.1371/journal.pone.0056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med 203: 2569–2575, 2006. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 12: 761–769, 2011. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrew DP, Spellberg JP, Takimoto H, Schmits R, Mak TW, Zukowski MM. Transendothelial migration and trafficking of leukocytes in LFA-1-deficient mice. Eur J Immunol 28: 1959–1969, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 32.Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs UJ, Nawroth PP, Preissner KT, Santoso S. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem 279: 55602–55608, 2004. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- 33.Henderson RB, Lim LH, Tessier PA, Gavins FN, Mathies M, Perretti M, Hogg N. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J Exp Med 194: 219–226, 2001. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol 3: 151–158, 2002. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 35.Harler MB, Wakshull E, Filardo EJ, Albina JE, Reichner JS. Promotion of neutrophil chemotaxis through differential regulation of beta 1 and beta 2 integrins. J Immunol 162: 6792–6799, 1999. [PubMed] [Google Scholar]
- 36.Heit B, Colarusso P, Kubes P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J Cell Sci 118: 5205–5220, 2005. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 37.Saltzman WM, Livingston TL, Parkhurst MR. Antibodies to CD18 influence neutrophil migration through extracellular matrix. J Leukoc Biol 65: 356–363, 1999. doi: 10.1002/jlb.65.3.356. [DOI] [PubMed] [Google Scholar]
- 38.Yamahashi Y, Cavnar PJ, Hind LE, Berthier E, Bennin DA, Beebe D, Huttenlocher A. Integrin associated proteins differentially regulate neutrophil polarity and directed migration in 2D and 3D. Biomed Microdevices 17: 100, 2015. doi: 10.1007/s10544-015-9998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498: 371–375, 2013. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol 185: 7057–7066, 2010. doi: 10.4049/jimmunol.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453: 51–55, 2008. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 42.Robker RL, Collins RG, Beaudet AL, Mersmann HJ, Smith CW. Leukocyte migration in adipose tissue of mice null for ICAM-1 and Mac-1 adhesion receptors. Obes Res 12: 936–940, 2004. doi: 10.1038/oby.2004.114. [DOI] [PubMed] [Google Scholar]
- 43.Katakai T, Habiro K, Kinashi T. Dendritic cells regulate high-speed interstitial T cell migration in the lymph node via LFA-1/ICAM-1. J Immunol 191: 1188–1199, 2013. doi: 10.4049/jimmunol.1300739. [DOI] [PubMed] [Google Scholar]
- 44.Hons M, Kopf A, Hauschild R, Leithner A, Gaertner F, Abe J, Renkawitz J, Stein JV, Sixt M. Chemokines and integrins independently tune actin flow and substrate friction during intranodal migration of T cells. Nat Immunol 19: 606–616, 2018. doi: 10.1038/s41590-018-0109-z. [DOI] [PubMed] [Google Scholar]
- 45.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem 273: 10095–10098, 1998. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 46.Wenstedt EF, Verberk SG, Kroon J, Neele AE, Baardman J, Claessen N, Pasaoglu OT, Rademaker E, Schrooten EM, Wouda RD, de Winther MP, Aten J, Vogt L, Van den Bossche J. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight 4: e130508, 2019. doi: 10.1172/jci.insight.130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laparidou M, Schlickenrieder A, Thoma T, Lengyel K, Schusser B. Blocking of the CXCR4-CXCL12 interaction inhibits the migration of chicken B cells into the bursa of Fabricius. Front Immunol 10: 3057, 2019. doi: 10.3389/fimmu.2019.03057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neves SR, Ram PT, Iyengar R. G protein pathways. Science 296: 1636–1639, 2002. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 49.Soede RD, Zeelenberg IS, Wijnands YM, Kamp M, Roos E. Stromal cell-derived factor-1-induced LFA-1 activation during in vivo migration of T cell hybridoma cells requires Gq/11, RhoA, and myosin, as well as Gi and Cdc42. J Immunol 166: 4293–4301, 2001. doi: 10.4049/jimmunol.166.7.4293. [DOI] [PubMed] [Google Scholar]
- 50.Giagulli C, Scarpini E, Ottoboni L, Narumiya S, Butcher EC, Constantin G, Laudanna C. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity 20: 25–35, 2004. doi: 10.1016/s1074-7613(03)00350-9. [DOI] [PubMed] [Google Scholar]
- 51.Block H, Stadtmann A, Riad D, Rossaint J, Sohlbach C, Germena G, Wu D, Simon SI, Ley K, Zarbock A. Gnb isoforms control a signaling pathway comprising Rac1, Plcbeta2, and Plcbeta3 leading to LFA-1 activation and neutrophil arrest in vivo. Blood 127: 314–324, 2016. doi: 10.1182/blood-2015-06-651034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arana E, Vehlow A, Harwood NE, Vigorito E, Henderson R, Turner M, Tybulewicz VL, Batista FD. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity 28: 88–99, 2008. doi: 10.1016/j.immuni.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Katagiri K, Shimonaka M, Kinashi T. Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-gamma1. J Biol Chem 279: 11875–11881, 2004. doi: 10.1074/jbc.M310717200. [DOI] [PubMed] [Google Scholar]
- 54.Ghandour H, Cullere X, Alvarez A, Luscinskas FW, Mayadas TN. Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood 110: 3682–3690, 2007. doi: 10.1182/blood-2007-03-077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, Fernandez-Borja M. Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol 80: 1542–1552, 2006. doi: 10.1189/jlb.0506357. [DOI] [PubMed] [Google Scholar]
- 56.Stadtmann A, Brinkhaus L, Mueller H, Rossaint J, Bolomini-Vittori M, Bergmeier W, Van Aken H, Wagner DD, Laudanna C, Ley K, Zarbock A. Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling. Eur J Immunol 41: 2074–2085, 2011. doi: 10.1002/eji.201041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen LW, Lin MW, Hsu CM. Different pathways leading to activation of extracellular signal-regulated kinase and p38 MAP kinase by formyl-methionyl-leucyl-phenylalanine or platelet activating factor in human neutrophils. J Biomed Sci 12: 311–319, 2005. doi: 10.1007/s11373-005-1704-1. [DOI] [PubMed] [Google Scholar]
- 58.Klapproth S, Sperandio M, Pinheiro EM, Prunster M, Soehnlein O, Gertler FB, Fassler R, Moser M. Loss of the Rap1 effector RIAM results in leukocyte adhesion deficiency due to impaired beta2 integrin function in mice. Blood 126: 2704–2712, 2015. doi: 10.1182/blood-2015-05-647453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson KD, Tang Y, Ceccarelli DF, Poy F, Sliwa JP, Neel BG, Eck MJ. The Skap-hom dimerization and PH domains comprise a 3'-phosphoinositide-gated molecular switch. Mol Cell 32: 564–575, 2008. doi: 10.1016/j.molcel.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menasche G, Kliche S, Chen EJ, Stradal TE, Schraven B, Koretzky G. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol Cell Biol 27: 4070–4081, 2007. doi: 10.1128/MCB.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH. RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J Biol Chem 284: 5119–5127, 2009. doi: 10.1074/jbc.M807117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun H, Lagarrigue F, Wang H, Fan Z, Lopez-Ramirez MA, Chang JT, Ginsberg MH. Distinct integrin activation pathways for effector and regulatory T cell trafficking and function. J Exp Med 218: e20201524, 2021. doi: 10.1084/jem.20201524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boras M, Volmering S, Bokemeyer A, Rossaint J, Block H, Bardel B, Van Marck V, Heitplatz B, Kliche S, Reinhold A, Lowell C, Zarbock A. Skap2 is required for beta2 integrin-mediated neutrophil recruitment and functions. J Exp Med 214: 851–874, 2017. doi: 10.1084/jem.20160647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klapholz B, Brown NH. Talin—the master of integrin adhesions. J Cell Sci 130: 2435–2446, 2017. doi: 10.1242/jcs.190991. [DOI] [PubMed] [Google Scholar]
- 65.Goksoy E, Ma YQ, Wang X, Kong X, Perera D, Plow EF, Qin J. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol Cell 31: 124–133, 2008. doi: 10.1016/j.molcel.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye X, McLean MA, Sligar SG. Phosphatidylinositol 4,5-bisphosphate modulates the affinity of talin-1 for phospholipid bilayers and activates its autoinhibited form. Biochemistry 55: 5038–5048, 2016. doi: 10.1021/acs.biochem.6b00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan B, Calderwood DA, Yaspan B, Ginsberg MH. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J Biol Chem 276: 28164–28170, 2001. doi: 10.1074/jbc.M104161200. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Zhu L, Zhang H, Hirbawi J, Fukuda K, Dwivedi P, Liu J, Byzova T, Plow EF, Wu J, Qin J. Conformational activation of talin by RIAM triggers integrin-mediated cell adhesion. Nat Commun 5: 5880, 2014. doi: 10.1038/ncomms6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anthis NJ, Wegener KL, Ye F, Kim C, Goult BT, Lowe ED, Vakonakis I, Bate N, Critchley DR, Ginsberg MH, Campbell ID. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J 28: 3623–3632, 2009. doi: 10.1038/emboj.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saltel F, Mortier E, Hytonen VP, Jacquier MC, Zimmermann P, Vogel V, Liu W, Wehrle-Haller B. New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control beta3-integrin clustering. J Cell Biol 187: 715–731, 2009. doi: 10.1083/jcb.200908134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chinthalapudi K, Rangarajan ES, Izard T. The interaction of talin with the cell membrane is essential for integrin activation and focal adhesion formation. Proc Natl Acad Sci USA 115: 10339–10344, 2018. doi: 10.1073/pnas.1806275115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morrison VL, MacPherson M, Savinko T, Lek HS, Prescott A, Fagerholm SC. The beta2 integrin-kindlin-3 interaction is essential for T-cell homing but dispensable for T-cell activation in vivo. Blood 122: 1428–1436, 2013. doi: 10.1182/blood-2013-02-484998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Z, Cai J, Gao J, White GC, Chen F, Ma Y-Q. Interaction of kindlin-3 and beta2-integrins differentially regulates neutrophil recruitment and NET release in mice. Blood 126: 373–377, 2015. doi: 10.1182/blood-2015-03-636720. [DOI] [PubMed] [Google Scholar]
- 74.Hart R, Stanley P, Chakravarty P, Hogg N. The kindlin 3 pleckstrin homology domain has an essential role in lymphocyte function-associated antigen 1 (LFA-1) integrin-mediated B cell adhesion and migration. J Biol Chem 288: 14852–14862, 2013. doi: 10.1074/jbc.M112.434621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ni T, Kalli AC, Naughton FB, Yates LA, Naneh O, Kozorog M, Anderluh G, Sansom MS, Gilbert RJ. Structure and lipid-binding properties of the kindlin-3 pleckstrin homology domain. Biochem J 474: 539–556, 2017. doi: 10.1042/BCJ20160791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol 10: 466–473, 2000. doi: 10.1016/s0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287: 1049–1053, 2000. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 78.Liu L, Puri KD, Penninger JM, Kubes P. Leukocyte PI3Kgamma and PI3Kdelta have temporally distinct roles for leukocyte recruitment in vivo. Blood 110: 1191–1198, 2007. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- 79.Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity 13: 759–769, 2000. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 80.Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, Coggeshall KM, McEver RP. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood 116: 485–494, 2010. doi: 10.1182/blood-2009-12-259556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Block H, Herter JM, Rossaint J, Stadtmann A, Kliche S, Lowell CA, Zarbock A. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J Exp Med 209: 407–421, 2012. doi: 10.1084/jem.20111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yago T, Liu Z, Ahamed J, McEver RP. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood 132: 1426–1437, 2018. doi: 10.1182/blood-2018-05-850859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stadtmann A, Germena G, Block H, Boras M, Rossaint J, Sundd P, Lefort C, Fisher CI, Buscher K, Gelschefarth B, Urzainqui A, Gerke V, Ley K, Zarbock A. The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J Exp Med 210: 2171–2180, 2013. doi: 10.1084/jem.20130664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morikis VA, Chase S, Wun T, Chaikof EL, Magnani JL, Simon SI. Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow. Blood 130: 2101–2110, 2017. doi: 10.1182/blood-2017-05-783027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov 15: 173–183, 2016. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sorio C, Montresor A, Bolomini-Vittori M, Caldrer S, Rossi B, Dusi S, Angiari S, Johansson JE, Vezzalini M, Leal T, Calcaterra E, Assael BM, Melotti P, Laudanna C. Mutations of cystic fibrosis transmembrane conductance regulator gene cause a monocyte-selective adhesion deficiency. Am J Respir Crit Care Med 193: 1123–1133, 2016. doi: 10.1164/rccm.201510-1922OC. [DOI] [PubMed] [Google Scholar]
- 87.Hisert KB, Birkland TP, Schoenfelt KQ, Long ME, Grogan B, Carter S, Liles WC, McKone EF, Becker L, Manicone AM, Gharib SA. CFTR modulator therapy enhances peripheral blood monocyte contributions to immune responses in people with cystic fibrosis. Front Pharmacol 11: 1219, 2020. doi: 10.3389/fphar.2020.01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schupp JC, Khanal S, Gomez JL, Sauler M, Adams TS, Chupp GL, Yan X, Poli S, Zhao Y, Montgomery RR, Rosas IO, Dela Cruz CS, Bruscia EM, Egan ME, Kaminski N, Britto CJ. Single-cell transcriptional archetypes of airway inflammation in cystic fibrosis. Am J Respir Crit Care Med 202: 1419–1429, 2020. doi: 10.1164/rccm.202004-0991OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hisert KB, Schoenfelt KQ, Cooke G, Grogan B, Launspach JL, Gallagher CG, Donnelly SC, Welsh MJ, Singh PK, McKone EF, Becker L. Ivacaftor-induced proteomic changes suggest monocyte defects may contribute to the pathogenesis of cystic fibrosis. Am J Respir Cell Mol Biol 54: 594–597, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramirez RN, El-Ali NC, Mager MA, Wyman D, Conesa A, Mortazavi A. Dynamic gene regulatory networks of human myeloid fifferentiation. Cell Syst 2017 Apr 26;4(4): 416–429.e3, 2017. doi: 10.1016/j.cels.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rincón E, Rocha-Gregg BL, Collins SR. A map of gene expression in neutrophil-like cell lines. BMC Genomics 19: 573, 2018. doi: 10.1186/s12864-018-4957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]