Abstract
PURPOSE:
Several professional organizations recommend universal genetic assessment for people with ovarian cancer as identifying pathogenic variants can affect treatment, prognosis and all-cause mortality for patients and relatives. We sought to evaluate the literature on genetic assessment for women with ovarian cancer and determine if any interventions or patient characteristics drive utilization of services.
METHODS:
We searched key electronic databases to identify trials that evaluated genetic assessment for people with ovarian cancer. Trials with the primary aim to evaluate utilization of genetic assessment with or without interventions were included. Eligible trials were subjected to meta-analysis and the moderating influence of health interventions on rates of genetic assessment were examined.
RESULTS:
A total of 35 studies were included (19 report on utilization of genetic services without an intervention, 7 with an intervention and 9 with both scenarios). Without an intervention, pooled estimates for referral to genetic counseling and completion of genetic testing were 39% [CI 27–53%] and 30% [CI 19–44%]. Clinician-facilitated interventions included: mainstreaming of genetic services (99% [CI 86–100%]), telemedicine (75% [CI 43–93%]), clinic-embedded genetic counselor (76% [CI 32–95%]), reflex tumor somatic genetic assessment (64% [CI 17–94%]), universal testing (57% [28–82%]) and referral forms (26% [CI 10–53%]). Random-effects pooled proportions demonstrated that Black vs. White race was associated with a lower rate of genetic testing (26%[CI 17–38%] vs. 40% [CI 25–57%]) as was being un-insured vs. insured (23% [CI 18–28%] vs. 38% [CI 26–53%]).
CONCLUSIONS:
Reported rates of genetic testing for people with ovarian cancer remain well below the goal of universal testing. Interventions such as mainstreaming can improve testing uptake. Strategies aimed at improving utilization of genetic services should consider existing disparities in race and insurance status.
Keywords: ovarian cancer, hereditary cancer syndromes, genetic testing, genetic counseling, cascade testing
Introduction
Approximately 25% of ovarian cancers are hereditary, with BRCA1/2 accounting for 15–18% of cases.1 Identifying a pathogenic variant has important health implications, as patients with BRCA1/2 variants have the option for targeted therapies and improved prognosis.2–6 Knowledge of the variant also allows for cascade testing of at-risk relatives, which can result in cancer screening and preventative strategies that decrease cancer incidence and mortality.7–10 The American Society of Clinical Oncology (ASCO), US Preventive Services Task Force (USPSTF), National Comprehensive Cancer Network (NCCN), Society of Gynecologic Oncology (SGO) and American College of Obstetricians and Gynecologists (ACOG) all recommend universal genetic counseling and testing for women diagnosed with epithelial ovarian cancer.11–16
Despite this recommendation, patients are not receiving genetic services consistently or equitably.17 The rates of reported guideline-compliant referral to genetics services in this population range from as low as 7% to as high as 90%, while rates of completion of genetic testing range from 3% to 85%.18–20 There are several potential factors contributing to disparate rates including limited provider identification of patients at hereditary cancer risk and referral for genetic counseling and testing and patient level factors such as mistrust, cost, competing health issues and other social determinants of health. Underutilization of genetic services can have perilous health implications. Attempts to synthesize the literature on rates of genetic testing among ovarian cancer patients, evidence-based interventions aimed at improving utilization and associations with specific patient demographics are lacking. The primary objective of the current study was to conduct a systematic review and meta-analysis of the literature on completion of genetic testing by women with ovarian cancer. Secondary aims were to explore referral to and completion of genetic counseling, the influence of interventions aimed at improving utilization and the influence of race, ethnicity and insurance status on referral patterns and successful completion of genetic testing.
Materials and Methods
Overview
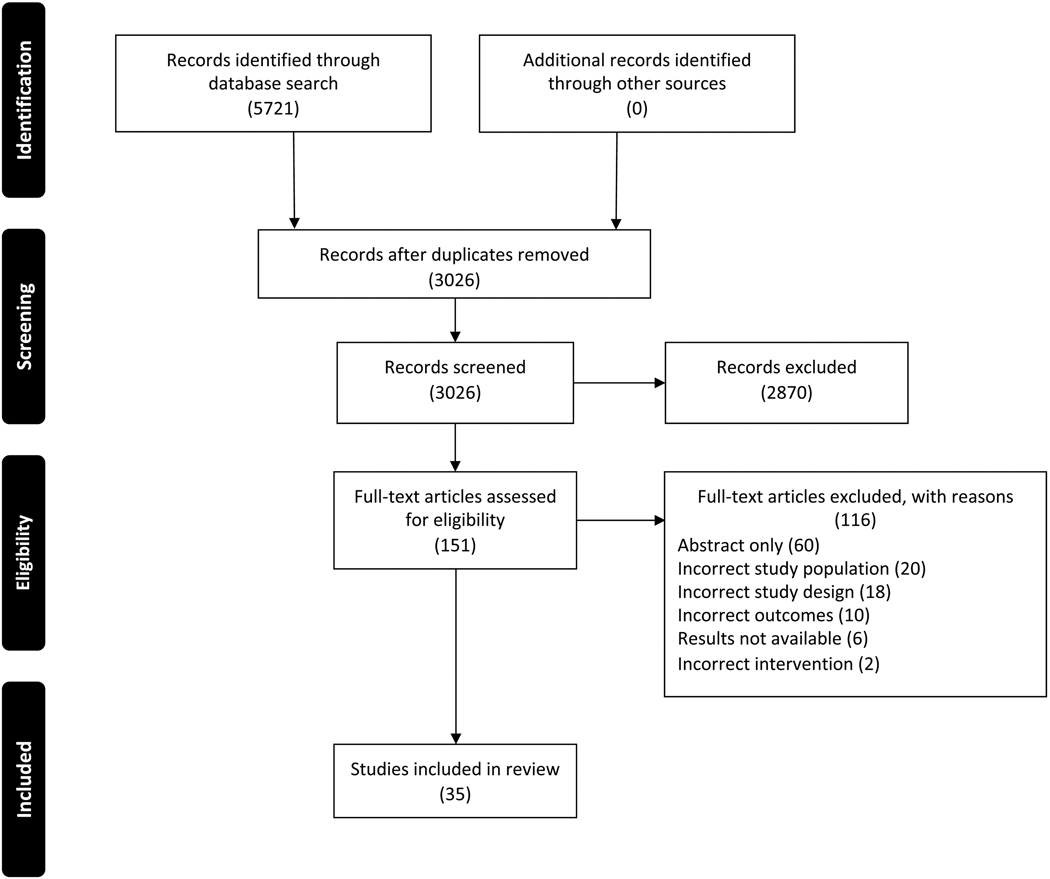
The current study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was preregistered with PROSPERO (registration no.: CRD42020192766).21 Search concepts included ovarian cancer, genetic testing or genetic counseling and comprehensive search strategies including backward searches (snowballing) of reference lists of identified articles and earlier systematic reviews and forward searches (citation tracking) were developed with the assistance of a medical librarian. Ovid MEDLINE (ALL: 1946 to present), Ovid EMBASE (1974 to present), and the Cochrane Library (Wiley) were searched from their inception through July 2020. There were no language, publication date or article type restrictions on the search strategy. The literature search yielded 3,026 studies after removal of duplicates and studies were screened by title and abstract against predetermined inclusion and exclusion criteria using Covidence. One hundred and fifty-one articles were selected for full-text review. Both reference and relevant article lists for these studies were gathered and deduplicated, producing 448 additional citations for review. From the full-text review, 35 articles met the inclusion criteria for this study (Supplementary Table 1 and Figure 1).
Figure 1. PRISMA flow diagram.
Inclusion Criteria
Eligible manuscripts included all primary research studies with the primary objective focusing on referral and/or completion of genetic counseling and/or genetic testing among women with ovarian cancer. Studies were evaluated to determine if a clinical intervention was employed that aimed at improving referral to and/or utilization of genetic services. Studies with and without interventions were included.
Exclusion Criteria
Excluded studies included published abstracts without published manuscripts (abstract only), studies evaluating genetic testing in at-risk patients without a diagnosis of ovarian cancer (incorrect study population), studies evaluating genetic testing for patients with multiple cancer types when data for the ovarian cancer population alone was not available (incorrect study population), meta-analyses or reviews (incorrect study design), studies evaluating variant percentages in a cohort where all subjects received genetic testing (incorrect outcomes), studies evaluating psychological factors associated with genetic testing (incorrect outcomes), clinical trials without results (results not available) and studies with interventions not aimed at increasing referral or testing rates (incorrect intervention) (Figure 1).
Data Extraction
Manuscripts were independently evaluated by two authors and disagreements were discussed with a third author. Data were extracted by one author and checked by a second author. Studies were coded according to a priori-specified characteristics, including study type, intervention, participant characteristics and risk of bias.
Risk of Bias and Analytic Strategy
The Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) was applied to intervention trials to assess the risk of bias.22 The Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data was applied to retrospective studies without interventions to determine the extent to which studies addressed risk of bias in their design, conduct and analysis.23 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system was used to rate the quality of evidence for each intervention included in the meta-analysis.24, 25 All risk of bias and ratings assessments were independently assessed by two reviewers and disagreements were discussed with a third author.
Statistical Analysis
Meta-analyses for the proportion of individuals referred to counseling and who completed genetic testing were conducted with the use of R software (Version 3.6.1[07/05/19], R Foundation for Statistical Computing, Vienna, Austria). Meta-analyses were conducted for all patients and by race and insurance type. Statistical heterogeneity was tested through the chi-square test (i.e., Cochrane Q test) and a P value ≤ 0.20 was used to indicate the presence of heterogeneity. Statistical heterogeneity was also assessed by the inconsistency statistic (I2). A random effects analysis was used to calculate the pooled proportions and means. The random effects analysis is more conservative and allows for more variability in the individual study proportion estimates when generating the pooled proportion. The pooled proportion was calculated using the Freeman-Tukey Double arcsine transformation and the 95% confidence interval was calculated using the Clopper-Pearson interval. To estimate the between-study variance, the DerSimonian-Laird estimator was used. For the outcome proportions of interest, the results of each study were expressed as binary proportions with exact 95% confidence intervals. For each meta-analysis, a funnel plot was constructed, displaying study proportion against study precision, estimated by the standard error, to assess for publication bias.
Results
Study characteristics
Thirty-five studies of original research were included. The studies included 15 retrospective studies without an intervention, 8 retrospective studies implementing an intervention, 8 prospective studies implementing an intervention, and 4 prospective studies without an intervention.
Patient characteristics
A total of 16,891 patients with ovarian cancer were evaluated. Study publication dates spanned 1999–2019 and included 9 countries: United States (15), Canada (6), Australia (3), United Kingdom, (3), South Korea (2), the Netherlands (2), New Zealand (2), Italy and Thailand (1 each). Across studies, the median reported age at cancer diagnosis was 60.5 years. Reported stage distribution was as follows: early stage (stages I/II), 24.0%, late stage (stages III/IV), 69.6%, and stage unknown, 6.4%. Reported histologies included serous, 57.9%, adenocarcinoma not otherwise specified, 14.9%, other/unknown histology, 11.3%, mixed, 7.4%, endometrioid, 3.7%, clear cell, 3.2%, mucinous, 1.1%, and carcinosarcoma, 0.4%. Further study characteristics are reported in Supplementary Table 1.
Utilization of genetics services without an intervention
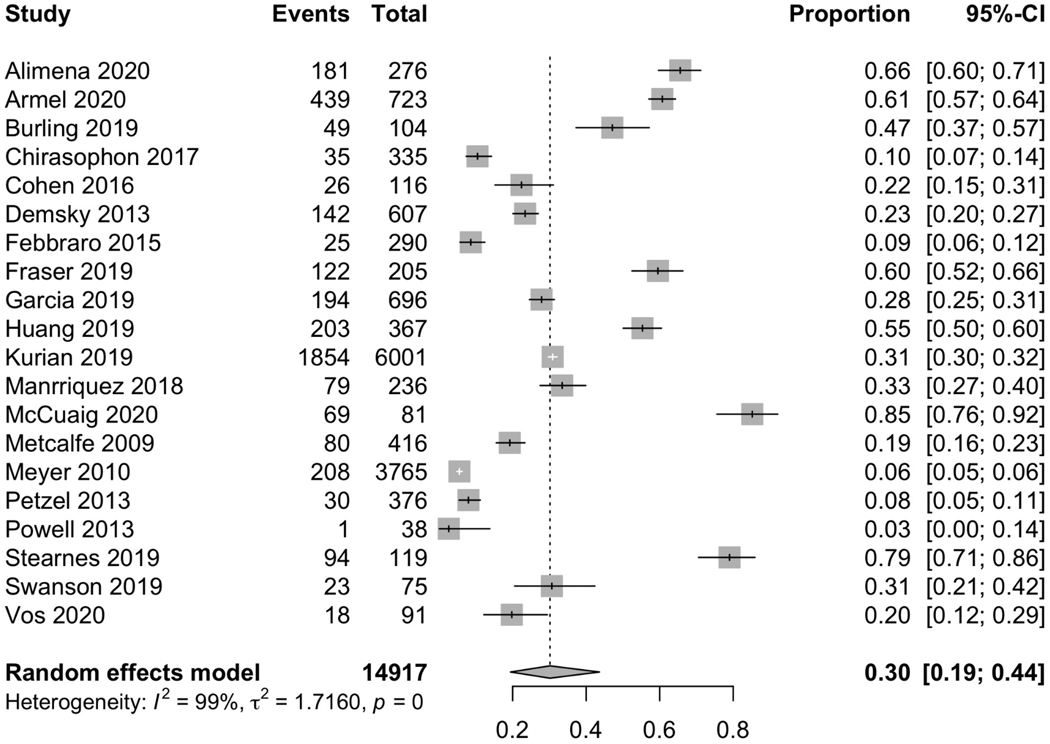
Nineteen papers (including the historic or non-intervention cohorts of 6 studies implementing an intervention) addressed referral to genetic counseling without a specified intervention and 20 (including the historic or non-intervention cohorts of 5 studies implementing an intervention) addressed completion of genetic testing without a specified intervention. Among these 20 studies, there were 14,917 women with ovarian cancer and 30% [CI 19–44%] underwent genetic testing (Figure 2). Among 8,403 women with referral data available, 39% [CI 27–53%] received a referral to genetic counseling. Among the 20 papers, 14 included information on the rates of completion of genetic counseling following a referral and found that, among 1,686 women referred for counseling, 81% [CI 69–90%] completed counseling. Fifteen papers reported on rates of completion of genetic testing following referral and found that, among 1,810 women referred to counseling, 78% [CI 68–86%] completed genetic testing.
Figure 2. Rates of completion of genetic testing for studies without an intervention.
A separate analysis was performed excluding studies with data collected exclusively in 2014 or earlier to reflect potential practice changes after the 2014 SGO clinical practice statement recommending that women diagnosed with epithelial ovarian, tubal and peritoneal cancers receive genetic counseling and be offered genetic testing, even in the absence of a family history.16 A cut-off of 2015 was utilized to reflect the time to adjust to the guideline change as adaptation to guidelines was unlikely to occur directly after publication of the statement. This cut-off also represents the approval of pharmacologic inhibitors of the enzyme poly-ADP ribose polymerase (PARP) in December 2014. For studies with data collected exclusively in 2014 or earlier, the pooled proportion of referral to genetic counseling was found to be 28% [CI 17–43%] and the pooled proportion of completion of genetic testing was found to be 16% [CI 10–24%]. For ten studies with any data collected in 2015 or later, the pooled proportion of referral to genetic counseling was found to be 56% [CI 38–73%] and the pooled proportion of completion of genetic testing was found to be 55% [CI 39–69%]. However, six of these studies also included data collected prior to and including 2015. An additional analysis of the four studies that included data exclusively collected during and following 2015 was performed. Among the three studies with data on referral to genetic counseling, the pooled proportion of patients referred was 64% [CI 26–90%]. Among the four studies reporting on the uptake of genetic testing, the pooled proportion of completion of genetic testing was 63% [CI 34–85%].
Interventions
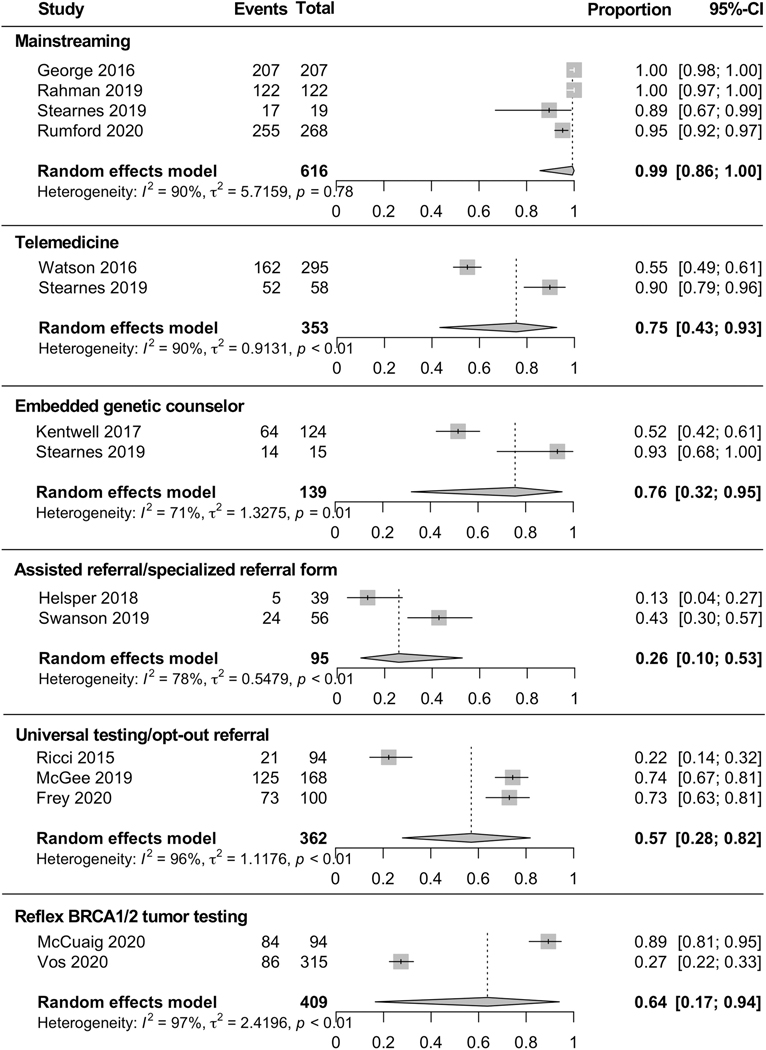
Among the 35 included studies, 16 reported on rates of referral to genetic counseling and/or completion of genetic testing after the implementation of one or more clinical interventions designed to improve utilization of genetic services (Figure 3).
Figure 3. Rates of completion of genetic testing after implementation of clinical intervention.
Mainstreaming
Mainstreaming is the process of providing testing/counseling in an oncology clinic by non-genetics specialists following upskilling to consent, order, interpret and deliver results.26 Nurses and physicians are trained to provide genetics services to patients, identify at-risk individuals, initiate genetics discussions and order genetic testing, thus bypassing referral to a separate genetics specialist.27 Four papers described mainstreaming and found, among 616 patients, 99% [CI 86–100%] completed genetic testing.26, 28–30 None of the studies reported rates of referral or genetic testing uptake prior to the intervention.
Telemedicine
Telemedicine is the delivery of healthcare services via electronic communication such as telecounseling or video counseling.31 Two studies reported genetic testing uptake with telemedicine and found that, among 353 patients, 75% [CI 43–93%] completed genetic testing.30, 32 Stearnes et al.30 utilized telephone counseling by genetic counselors and Watson et al.32 utilized a standardized 7-minute video created by a genetic counselor in place of an in-person counseling appointment. While Stearnes et al. did not evaluate pre-intervention testing uptake, Watson et al. reported uptake of 28.8% pre-intervention vs. 54.9% post-intervention.
Embedded genetic counselor
Embedded genetic counselors are counselors that offer genetic counseling and testing within the oncology clinic.33 Two studies described referral to genetic counseling with an embedded genetic counselor in a gynecologic oncology clinic and found, among 526 patients, 56% [CI 39–72%] were referred to counseling.33, 34 Both studies included data on referral to genetic testing prior to and following the intervention (34.4% pre-intervention vs. 67.9% post-intervention33 and 20.9% pre-intervention vs. 43.8%. post-intervention34). Two studies described completion of genetic testing after embedding a genetic counselor in the gynecologic oncology clinic and found that, among 139 patients, 76% [CI 32–95%] completed testing.30, 33 Testing uptake data prior to the interventions were not available.
Assisted referral/specialized referral form
Assisted referral/specialized referral forms are strategies implemented with the goal to remind physicians to refer their patients to genetic counseling. Four studies reported on referral to genetic counseling after implementing an assisted referral or specialized referral form and found, among 188 patients, 61% [CI 36–81%] were referred to counseling.18, 35–37 Three of these studies included data on referral to genetic counseling prior to and following the intervention (17.4% pre-intervention vs. 30.1% post-intervention36; 18.4% pre-intervention vs. 50.0% post-intervention18; 44.0% pre-intervention vs. 71.4% post-intervention37). Two studies reported on the completion of genetic testing following this intervention and found, among 95 patients, 26% [CI 10–53%] completed genetic testing.35, 37 Swanson et al.37 included data on genetic testing uptake prior to and following the intervention (30.7%, pre-intervention vs. 42.9% post-intervention). No other studies reported pre-intervention testing uptake.
Universal testing/opt-out referral
Universal testing is the clinical strategy whereby all ovarian cancer patients undergo genetic counseling as part of usual care unless the patient declines genetic cancer risk assessment.38 Three studies reported on this intervention and found that, among 362 patients, 96% [42–100%] were referred to genetic counseling.38–40 Among 362 patients included in the studies evaluating universal testing, 57% [CI 28–82%] completed genetic testing. None of the studies reported rates of pre-intervention referral or uptake.
Reflex BRCA1/2 tumor testing
Reflex BRCA1/2 tumor testing is a strategy whereby patients undergo somatic genomic profiling of their ovarian tumor and those found to harbor tumor BRCA1/2 pathogenic variants are then triaged to germline genetic testing.41 Two studies reported genetic testing completion rates and among 409 patients, 64% [CI 17–94%] completed genetic testing.19, 42 Both studies included data on testing uptake prior to and following the intervention (85.2% pre-intervention vs. 89.4% post-intervention19 and 19.8%, pre-intervention vs. 27.3% post-intervention42).
The quality of evidence was evaluated for each intervention using the GRADE system. There was high quality of evidence to suggest that mainstreaming may improve rates of genetic testing when compared to no intervention. For the remainder of the interventions, high study heterogeneity resulted in large confidence intervals in the pooled proportions of interest and the quality of evidence was low to very low.
Genetic testing results
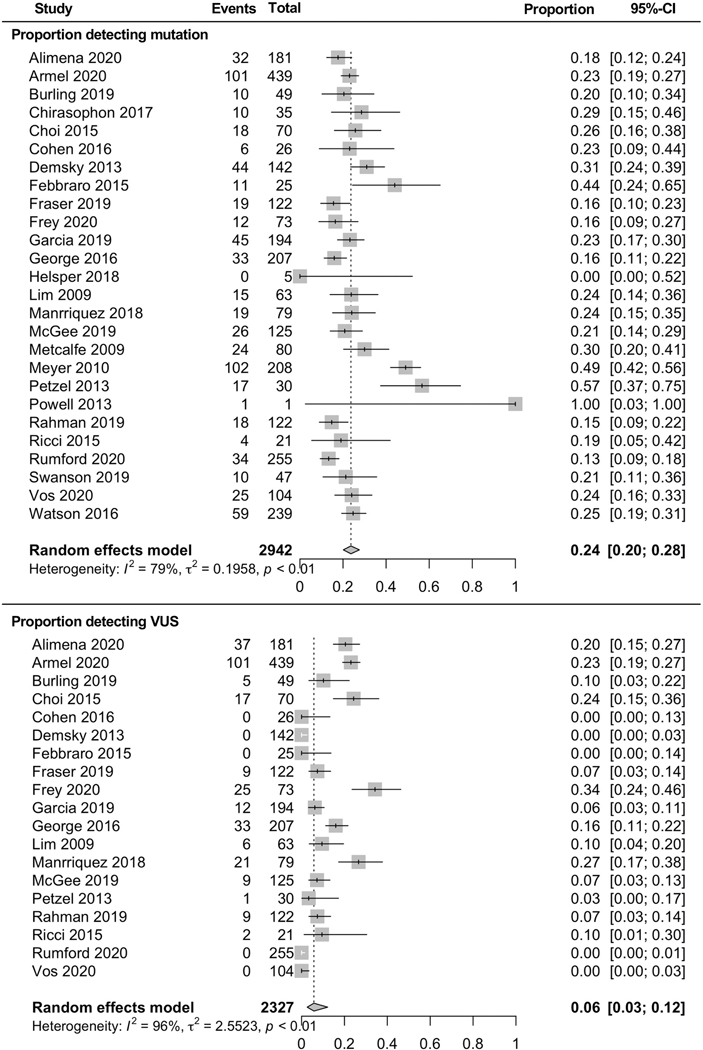
Twenty-six studies reported genetic testing results. Among 2,942 patients completing genetic testing, 24% [CI 20–28%] were found to have a pathogenic variant (Figure 4). Nineteen studies reported identification of variants of unknown significance (VUS), finding that among 2,327 patients, 6% [CI 3–12%] had a VUS (Figure 4).
Figure 4. Results of genetic testing.
Race and ethnicity
Eight studies included analysis by self-reported race.43–50 Among 7,862 patients, 6,469 (82%) reported being White, 599 (8%) Black and 794 (10%) Asian. Random-effects pooled proportions by race demonstrated that, among 2,310 total patients with data available on referral to genetic counseling, 43% [CI 26–62%] of White patients, 24% [CI 13–42%] of Black patients and 23% [CI 2–83%] of Asian patients were referred to genetic counseling. Among 6,428 total patients included in studies with available data on race and completion of genetic testing, 40% [CI 25–57%] of White patients, 26% [CI 17–38%] of Black patients and 14% [CI 2–51%] of Asian patients completed genetic testing (Table 1). Of note, for Asian patients, only two studies included data on referral to genetic counseling44, 49 and completion of genetic testing,47, 50 with the majority of patient data coming from a single study. None of the studies included analysis exclusively by ethnicity separate from race. Small sample size and high study heterogeneity observed in studies evaluating race and genetic testing resulted in large confidence intervals in the pooled proportions of interest.
Table 1.
Pooled proportions of referral to genetic counseling and completion of genetic testing by race and insurance status.
| % Referred to counseling (95% CI) | % Completed genetic testing (95% CI) | |
|---|---|---|
| Race | ||
| White | 43% (26–62%) | 40% (25–57%) |
| Black | 24% (13–42%) | 26% (17–38% |
| Asian | 23% (2–83%) | 14% (2–51%) |
| Insurance status | ||
| Private insurance | 39% (26–54%) | 47% (30–64%) |
| Medicare/Medicaid | 27% (18–38%) | 26% (16–40%) |
| Uninsured | 24% (13–51%) | 23% (18–28%) |
Insurance status
Six studies included analysis by insurance status.44–49 Among 7,681 patients, 5,320 (69%) had private insurance, 2,078 (27%) Medicare/Medicaid, and 283 (4%) were uninsured. Random-effects pooled proportions by insurance status demonstrated that, among 2,241 total patients, 39% [CI 26–54%] of private insurance patients, 27% [CI 18–38%] of Medicare/Medicaid patients and 24% [CI 13–41%] of uninsured patients were referred to genetic counseling. Among 6,127 total patients in studies with available data on insurance data and completion of genetic testing, 47% [CI 30–64%] of private insurance patients, 26% [CI 16–40%] of Medicare/Medicaid patients and 23% [CI 18–28%] of uninsured patients completed testing (Table 1). Small sample size and high study heterogeneity observed in studies evaluating insurance status and genetic testing resulted in large confidence intervals in the pooled proportions of interest.
U.S. based studies vs. non-U.S. based studies
Among ten U.S based studies with data on referral to genetic counseling, 25% [CI 16–38%] of 6,560 patients were referred to genetic counseling while, among nine non-U.S. based studies with data on referral to genetic counseling, 58% [CI 40–74%] of 1,843 patients were referred to genetic counseling. Among nine U.S. based studies with data on completion of genetic testing, 20% [CI 10–37%] of 11,884 patients completed genetic testing while, among eleven non-U.S based studies with data on completion of genetic testing, 40% [25–57%] of 3,033 patients completed genetic testing.
Discussion
Our systematic review and meta-analysis confirms that utilization of genetic services among people with ovarian cancer is well below the recommendation for universal genetic testing.11–14 Among studies that reported completion of genetic testing without an improvement-aimed intervention, only 39% of patients were referred to genetic counseling and 30% completed genetic testing. A separate analysis revealed that among the eighteen studies performed prior to the 2014 SGO clinical practice statement, only 29% of patients were referred to genetic counseling and 16% completed genetic testing. For studies including data collected during and beyond 2015, following the SGO guidelines and approval of PARP inhibitors for patients with BRCA1/2 pathogenic variants, 56% were referred to genetic counseling and 55% completed genetic testing. Although all studies in this separate analysis included data from 2015, some included data prior to 2015. A further analysis including only the four studies with data collected following 2014 found that 64% of patients were referred to genetic counseling and 63% completed genetic testing. These separate sub-analyses suggest that referral to genetic counseling and completion of genetic testing have increased significantly following the SGO guidelines for universal testing but still remain well below 100%. It is evident that successful expansion of germline testing among patients with ovarian cancer will require a scalable and integrated multidisciplinary approach.51 Due to a limited number of cancer-specific genetic counselors, the current system of genetic counseling visits for all germline testing is not sustainable.52
We identified multiple studies that engaged streamlined clinical pathways aimed to improve access and uptake of genetic assessment for women with ovarian cancer.51, 53 The most effective was mainstreaming, resulting in genetic testing for 99% of patients, followed by embedding a genetic counselor in the clinic (76%), telemedicine genetics appointments (75%) and reflex tumor somatic testing for triage to germline testing (64%). The quality of evidence to suggest that mainstreaming may improve rates of genetic testing when compared to no intervention was graded as high; however, the quality of evidence for all other interventions was graded as low or very low due to varying concerns about risk of bias, inconsistency, indirectness and imprecision.
Our results suggest that mainstreaming is likely to improve genetic testing uptake if implemented successfully. However, mainstreaming requires significant engagement and commitment of physicians and nurses that may not be feasible on a larger scale. Providers must undergo specific training in order to consent patients and interpret and share results in addition to their other responsibilities. Implementing such interventions would require significant resources and commitment not only of providers, but also of genetics specialists who must subsequently train non-genetics specialists. In addition to this, White et al.27 suggests that physicians and nurses are underprepared to integrate genetics services into routine clinical care due to certain barriers: limited genetic knowledge and skill, low confidence initiating genetic discussions, lack of resources and guidelines, and concerns about patient discrimination and psychological harm.
Two interventions resulted in genetic testing uptake for less than 60% of the studied population: assisted referral (26%) and universal testing / opt-out (57%). Low uptake for assisted referral is likely due to study heterogeneity as only two studies reported completion of genetic testing (Swanson et al., 43%, Helsper et al., 13%).35, 37 Helsper et al. reported that despite the described intervention of an assisted referral strategy, many patients did not follow-up for the genetics appointment or declined the initial recommendation for genetic assessment. Among the three studies utilizing the universal testing / opt-out strategy, both McGee et al.38 and Frey et al.54 reported similar uptake of 74% and 73% respectively, while Ricci et al.40 reported uptake of only 22%. Ricci et al. suggested that despite the intended universal intervention, physicians did not actually refer all patients, potentially due to limited knowledge on hereditary cancer risk and lack of specific training. The low uptake rate reported by Ricci et al. decreased the overall pooled proportion of completion of genetic testing to 57%, emphasizing a limitation of pooled proportions. The pooled proportion of referral to genetic counseling for universal testing / opt-out strategy was 96%. Although the pooled referral and testing uptake proportions were lower for these interventions, the individual studies still reported success in increasing referral and uptake rates when comparing pre- and post-intervention rates at their own institutions.
With germline and somatic BRCA1/2 pathogenic variants incorporated in the approval for PARP inhibitor maintenance therapy in ovarian cancer, many clinicians are now incorporating somatic tumor genetic testing into routine clinical care. Universal tumor genetic testing has also been proposed as a strategy for genetic prescreening, tailoring genetic counseling to those with a high a priori risk of a hereditary pathogenic variant. McCuaig et al.19 retrospectively evaluated patient uptake of germline genetic services before and after implementation of reflex BRCA1/2 tumor testing and found a significantly elevated rate of genetics referral following the intervention. However, in the post-intervention group, 82% of patients referred to genetics were referred prior to the availability of their somatic tumor testing results, leading the authors to conclude that improved rates of genetics referral were likely a reflection of patient and provider preferences, rather than a result of tumor testing itself. Vos et al.42 evaluated a similar algorithm and found that some patients with negative tumor testing results or those who did not have successful tumor testing were still referred to germline genetic testing. The strategy of germline genetic testing as a reflex to identification of somatic mutations may become more popular with the growing availability of time-efficient and affordable tumor genetic testing.
Research completed over the past two decades suggests that many factors influence a patient’s decision about genetic testing including race, ethnicity, gender, education level, affordability, insurance and concerns about discrimination.55–62 Our study found that Black race, compared to White race, was associated with decreased rates of referral to genetic counseling and completion of genetic testing among women with ovarian cancer. Unfortunately, only eight studies included data on race and ethnicity. Armstrong et al.63 showed that Black women with a family history of breast or ovarian cancer were significantly less likely to undergo BRCA1/2 counseling, a disparity not explained by differences in probability of carrying a pathogenic variant, socioeconomic status, cancer risk perception and worry, attitudes about BRCA1/2 testing or primary care physician discussions about genetic assessment.64, 65 More recently, Chapman-Davis et al.66 reported that minority patients are more likely to utilize genetics services only after a cancer diagnosis and not based on family history.66 Our results, along with the limited prior literature, highlight significant limitations in the existing data on potential racial and ethnic disparities and should implore researchers to include such variables in future study design.
Analysis of genetic counseling and testing by geographic location suggests higher rates of referral to genetic counseling and completion of genetic testing outside of the U.S. (58% vs. 25% and 40% vs. 20%, respectively). It is unclear what factors contributed to this inconsistency; however, national healthcare plans, physician training and cultural differences may play a role. Studies suggest that genetic counselors in the United Kingdom, Australia and South Africa practice a more patient-centered psychotherapeutic process compared to counselors in the U.S. and Canada, who tend to practice in a more didactic, teaching model style. Interestingly, counseling-based models focusing on the psychotherapeutic aspects of the work may result in improved patient utilization of genetic services.67
Likewise, insurance and affordability continue to be barriers to genetic assessment. Patients without insurance and those with either Medicare or Medicaid were less likely to use genetics services. Though the cost to patients for genetic testing has decreased dramatically over the past twenty years (previously exceeding $3,000 and currently approximately $250 for most self-pay options when not covered by insurance),58 many studies continue to cite cost and insurance status as a barrier.55, 56 In response, the American College of Medical Genetics and Genomics has published a policy statement challenging payors and health care providers to increase their coverage of genetic and genomic testing.68
Our results should be viewed in light of several limitations. We included studies with heterogeneity in study design. It is not possible to discern how rates of reported genetic assessment compare to utilization of genetic services in general practice. Additionally, the funnel plots suggest that the meta-analyses included did not have proper representation of smaller studies. It is possible that smaller studies are not reporting their findings, contributing to bias in the results. The funnel plots also confirm high heterogeneity among the studies included in the review. Finally, it should be noted that authors on this publication contributed to studies included in the review, which might raise concern for bias. However, all authors agreed to the final protocol and manuscript, and screening and data extraction were performed by authors who had not been principal investigators for any of the reviewed studies.
In conclusion, this systematic review and meta-analysis demonstrates that the current rates of genetic testing for women with ovarian cancer remain well below the accepted goal of universal testing. Importantly, race and insurance status influence utilization of genetic services and strategies aimed at improving access must take these disparities into consideration. There are promising interventions, such as mainstreaming genetic care, telemedicine and embedding genetic counselors in the clinic, that might improve genetic testing uptake. While mainstreaming did demonstrate a 99% rate of genetic testing and was supported by a high level of evidence, this strategy requires significant engagement and commitment of non-genetics specialists who must undergo specific training and take on the responsibilities of consenting patients and interpreting and sharing results, and therefore may not be successful on a large, more universal scale. Among the other strategies described, a clearly superior approach was not demonstrated by our study, as outcomes were sub-par and heterogeneous results diminished the quality of evidence. Additional well thought out interventions that can demonstrate a cost-effective, sustainable and scalable improvement in genetic testing rates are still needed to meet the increased demand for testing.
Supplementary Material
Highlights.
Reported rates of genetic testing for people with ovarian cancer remain well below the goal of universal testing
Race and insurance status influence utilization of genetic services by women with ovarian cancer
Promising interventions include mainstreaming genetic care, telemedicine and embedding genetic counselors in the clinic
The increased demand for testing coupled with the decreased supply of genetic counselors calls for novel approaches
Acknowledgments
Funding Support
Melissa K. Frey is supported by the following grant: NIH/NCATS Grant # KL2-TR-002385. Ravi N. Sharaf was supported by the following grants: National Cancer Institute Grant # K07CA216326 and R01CA211723 and Patient Centered Outcomes Research Institute Grant # IHS-2017C3-9211.
Ying Liu was supported by the NIH/National Cancer Institute award P30 CA008748.
Paul J. Christos and Charlene Thomas were supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
Steven Lipkin was supported by the following grant: National Cancer Institute Grant #U01CA233056.
Kevin Holcomb serves as a consultant for Johnson and Johnson and receives research support from Fujirebio Diagnostics, outside the submitted work.
Footnotes
Conflict of Interest Disclosures
The other authors made no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. November2011;108(44):18032–7. doi: 10.1073/pnas.1115052108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 122018;379(26):2495–2505. doi: 10.1056/NEJMoa1810858 [DOI] [PubMed] [Google Scholar]
- 3.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. Jul 2012;30(21):2654–63. doi: 10.1200/JCO.2011.39.8545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9(5):e95285. doi: 10.1371/journal.pone.0095285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 062017;317(23):2402–2416. doi: 10.1001/jama.2017.7112 [DOI] [PubMed] [Google Scholar]
- 6.Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. Jan 2015;121(2):269–75. doi: 10.1002/cncr.29041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey MK, Kahn RM, Chapman-Davis E, et al. Prospective Feasibility Trial of a Novel Strategy of Facilitated Cascade Genetic Testing Using Telephone Counseling. J Clin Oncol. January2020:JCO1902005. doi: 10.1200/JCO.19.02005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ring KL, Garcia C, Thomas MH, Modesitt SC. Current and future role of genetic screening in gynecologic malignancies. Am J Obstet Gynecol. 112017;217(5):512–521. doi: 10.1016/j.ajog.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 9.Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. Mar 2006;7(3):223–9. doi: 10.1016/S1470-2045(06)70585-X [DOI] [PubMed] [Google Scholar]
- 10.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. September2010;304(9):967–75. doi: 10.1001/jama.2010.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Committee on Practice Bulletins–Gynecology CoG, S.ciety of Gynecologic Oncology. Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 092017;130(3):e110–e126. doi: 10.1097/AOG.0000000000002296 [DOI] [PubMed] [Google Scholar]
- 12.Daly MB, Pilarski R, Yurgelun MB, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw. 042020;18(4):380–391. doi: 10.6004/jnccn.2020.0017 [DOI] [PubMed] [Google Scholar]
- 13.Randall LM, Pothuri B, Swisher EM, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol. 082017;146(2):217–224. doi: 10.1016/j.ygyno.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 14.Konstantinopoulos PA, Lacchetti C, Annunziata CM. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline Summary. JCO Oncol Pract. Aug 2020;16(8):e835–e838. doi: 10.1200/JOP.19.00773 [DOI] [PubMed] [Google Scholar]
- 15.Owens DK, Davidson KW, Krist AH, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 082019;322(7):652–665. doi: 10.1001/jama.2019.10987 [DOI] [PubMed] [Google Scholar]
- 16.SGO Clinical Practice Statement: Genetic Testing For Ovarian Cancer (SGO, October 14). 2014. https://www.sgo.org/resources/genetic-testing-for-ovarian-cancer/
- 17.Hinchcliff EM, Bednar EM, Lu KH, Rauh-Hain JA. Disparities in gynecologic cancer genetics evaluation. Gynecol Oncol. 042019;153(1):184–191. doi: 10.1016/j.ygyno.2019.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell CB, Littell R, Hoodfar E, Sinclair F, Pressman A. Does the diagnosis of breast or ovarian cancer trigger referral to genetic counseling? Int J Gynecol Cancer. March2013;23(3):431–6. doi: 10.1097/IGC.0b013e318280f2b4 [DOI] [PubMed] [Google Scholar]
- 19.McCuaig JM, Care M, Ferguson SE, Kim RH, Stockley TL, Metcalfe KA. Year 1: Experiences of a tertiary cancer centre following implementation of reflex BRCA1 and BRCA2 tumor testing for all high-grade serous ovarian cancers in a universal healthcare system. Gynecol Oncol. September2020;158(3):747–753. doi: 10.1016/j.ygyno.2020.06.507 [DOI] [PubMed] [Google Scholar]
- 20.Meyer LA, Anderson ME, Lacour RA, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: missed opportunities. Obstet Gynecol. May2010;115(5):945–52. doi: 10.1097/AOG.0b013e3181da08d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. Jul 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. October2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. September2015;13(3):147–53. doi: 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 24.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020. (developed by Evidence Prime, Inc.). gradepro.org. [Google Scholar]
- 25.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. April2011;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 26.George A, Riddell D, Seal S, et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep. 2016;6:29506. doi: 10.1038/srep29506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White S, Jacobs C, Phillips J. Mainstreaming genetics and genomics: a systematic review of the barriers and facilitators for nurses and physicians in secondary and tertiary care. Genet Med. 072020;22(7):1149–1155. doi: 10.1038/s41436-020-0785-6 [DOI] [PubMed] [Google Scholar]
- 28.Rahman B, Lanceley A, Kristeleit RS, et al. Mainstreamed genetic testing for women with ovarian cancer: first-year experience. J Med Genet. 032019;56(3):195–198. doi: 10.1136/jmedgenet-2017-105140 [DOI] [PubMed] [Google Scholar]
- 29.Rumford M, Lythgoe M, McNeish I, et al. Oncologist-led BRCA ‘mainstreaming’ in the ovarian cancer clinic: A study of 255 patients and its impact on their management. Sci Rep. 022020;10(1):3390. doi: 10.1038/s41598-020-60149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stearnes G, Nichols CB, Schofield L, O’Sullivan S, Pachter N, Cohen PA. Uptake of testing for germline. Int J Gynecol Cancer. 072019;29(6):1038–1042. doi: 10.1136/ijgc-2019-000389 [DOI] [PubMed] [Google Scholar]
- 31.Serper M, Volk ML. Current and Future Applications of Telemedicine to Optimize the Delivery of Care in Chronic Liver Disease. Clin Gastroenterol Hepatol. 022018;16(2):157–161.e8. doi: 10.1016/j.cgh.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson CH, Ulm M, Blackburn P, et al. Video-assisted genetic counseling in patients with ovarian, fallopian and peritoneal carcinoma. Gynecol Oncol. 102016;143(1):109–112. doi: 10.1016/j.ygyno.2016.07.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kentwell M, Dow E, Antill Y, et al. Mainstreaming cancer genetics: A model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol. 042017;145(1):130–136. doi: 10.1016/j.ygyno.2017.01.030 [DOI] [PubMed] [Google Scholar]
- 34.Senter L, O’Malley DM, Backes FJ, et al. Genetic consultation embedded in a gynecologic oncology clinic improves compliance with guideline-based care. Gynecol Oncol. 102017;147(1):110–114. doi: 10.1016/j.ygyno.2017.07.141 [DOI] [PubMed] [Google Scholar]
- 35.Helsper CW, Van Vliet LM, Velthuizen ME, et al. Identifying patients with a history of ovarian cancer for referral for genetic counselling: non-randomised comparison of two case-finding strategies in primary care. Br J Gen Pract. Nov 2018;68(676):e750–e756. doi: 10.3399/bjgp18X699533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petzel SV, Vogel RI, Bensend T, Leininger A, Argenta PA, Geller MA. Genetic risk assessment for women with epithelial ovarian cancer: referral patterns and outcomes in a university gynecologic oncology clinic. J Genet Couns. October2013;22(5):662–73. doi: 10.1007/s10897-013-9598-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson CL, Kumar A, Maharaj JM, et al. Increasing genetic counseling referral rates through bundled interventions after ovarian cancer diagnosis. Gynecol Oncol. 042018;149(1):121–126. doi: 10.1016/j.ygyno.2018.01.033 [DOI] [PubMed] [Google Scholar]
- 38.McGee J, Peart TM, Foley N, et al. Direct Genetics Referral Pathway for High-Grade Serous Ovarian Cancer Patients: The “Opt-Out” Process. J Oncol. 2019;2019:6029097. doi: 10.1155/2019/6029097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frey MK, Lee S, Gerber D, Schwartz Z, Levine D, Blank SV. Facilitated referral pathway for genetic testing at the time of ovarian cancer diagnosis: uptake of genetic counseling and testing and impact on patient-reported stress, anxiety and depression. Gynecologic Oncology. 2020;In press. [DOI] [PubMed] [Google Scholar]
- 40.Ricci MT, Sciallero S, Mammoliti S, et al. Referral of Ovarian Cancer Patients for Genetic Counselling by Oncologists: Need for Improvement. Public Health Genomics. 2015;18(4):225–32. doi: 10.1159/000431352 [DOI] [PubMed] [Google Scholar]
- 41.Frey MK, Pothuri B. Homologous recombination deficiency (HRD) testing in ovarian cancer clinical practice: a review of the literature. Gynecol Oncol Res Pract. 2017;4:4. doi: 10.1186/s40661-017-0039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vos JR, Fakkert IE, de Hullu JA, et al. Universal Tumor DNA BRCA1/2 Testing of Ovarian Cancer: Prescreening PARPi Treatment and Genetic Predisposition. J Natl Cancer Inst. 022020;112(2):161–169. doi: 10.1093/jnci/djz080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alimena S, Scarpetti L, Blouch EL, et al. Factors associated with referral and completion of genetic counseling in women with epithelial ovarian cancer. Int J Gynecol Cancer. September2020;30(9):1397–1403. doi: 10.1136/ijgc-2019-001168 [DOI] [PubMed] [Google Scholar]
- 44.Febbraro T, Robison K, Wilbur JS, et al. Adherence patterns to National Comprehensive Cancer Network (NCCN) guidelines for referral to cancer genetic professionals. Gynecol Oncol. July2015;138(1):109–14. doi: 10.1016/j.ygyno.2015.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia C, Harrison K, Ring KL, Sullivan MW, Rauh LA, Modesitt SC. Genetic counseling referral for ovarian cancer patients: a call to action. Fam Cancer. April2019;doi: 10.1007/s10689-019-00129-5 [DOI] [PubMed] [Google Scholar]
- 46.Huang M, Kamath P, Schlumbrecht M, et al. Identifying disparities in germline and somatic testing for ovarian cancer. Gynecol Oncol. 052019;153(2):297–303. doi: 10.1016/j.ygyno.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 47.Kurian AW, Ward KC, Howlader N, et al. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J Clin Oncol. 052019;37(15):1305–1315. doi: 10.1200/JCO.18.01854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallen AR, Conley CC, Townsend MK, et al. Patterns and predictors of genetic referral among ovarian cancer patients at a National Cancer Institute-Comprehensive Cancer Center. Clin Genet. 022020;97(2):370–375. doi: 10.1111/cge.13654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manrriquez E, Chapman JS, Mak J, Blanco AM, Chen LM. Disparities in genetics assessment for women with ovarian cancer: Can we do better? Gynecol Oncol. 042018;149(1):84–88. doi: 10.1016/j.ygyno.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 50.Metcalfe KA, Fan I, McLaughlin J, et al. Uptake of clinical genetic testing for ovarian cancer in Ontario: a population-based study. Gynecol Oncol. January2009;112(1):68–72. doi: 10.1016/j.ygyno.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajagopal PS, Catenacci DVT, Olopade OI. The Time for Mainstreaming Germline Testing for Patients With Breast Cancer Is Now. J Clin Oncol. 082019;37(24):2177–2178. doi: 10.1200/JCO.19.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milliron KJ, Griggs JJ. Advances in Genetic Testing in Patients With Breast Cancer, High-Quality Decision Making, and Responsible Resource Allocation. J Clin Oncol. 022019;37(6):445–447. doi: 10.1200/JCO.18.01952 [DOI] [PubMed] [Google Scholar]
- 53.Gleeson M, Kentwell M, Meiser B, et al. The development and evaluation of a nationwide training program for oncology health professionals in the provision of genetic testing for ovarian cancer patients. Gynecol Oncol. Aug 2020;158(2):431–439. doi: 10.1016/j.ygyno.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 54.Frey MK, Lee SS, Gerber D, et al. Facilitated referral pathway for genetic testing at the time of ovarian cancer diagnosis: uptake of genetic counseling and testing and impact on patient-reported stress, anxiety and depression. Gynecol Oncol. 042020;157(1):280–286. doi: 10.1016/j.ygyno.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 55.Hayden S, Mange S, Duquette D, Petrucelli N, Raymond VM, Partners BCN. Large, Prospective Analysis of the Reasons Patients Do Not Pursue BRCA Genetic Testing Following Genetic Counseling. J Genet Couns. August2017;26(4):859–865. doi: 10.1007/s10897-016-0064-5 [DOI] [PubMed] [Google Scholar]
- 56.Armstrong K, Calzone K, Stopfer J, Fitzgerald G, Coyne J, Weber B. Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev. November2000;9(11):1251–4. [PubMed] [Google Scholar]
- 57.Ropka ME, Wenzel J, Phillips EK, Siadaty M, Philbrick JT. Uptake rates for breast cancer genetic testing: a systematic review. Cancer Epidemiol Biomarkers Prev. May2006;15(5):840–55. doi: 10.1158/1055-9965.EPI-05-0002 [DOI] [PubMed] [Google Scholar]
- 58.Kieran S, Loescher LJ, Lim KH. The role of financial factors in acceptance of clinical BRCA genetic testing. Genet Test. 2007;11(1):101–10. doi: 10.1089/gte.2006.9999 [DOI] [PubMed] [Google Scholar]
- 59.Godard B, Pratte A, Dumont M, Simard-Lebrun A, Simard J. Factors associated with an individual’s decision to withdraw from genetic testing for breast and ovarian cancer susceptibility: implications for counseling. Genet Test. 2007;11(1):45–54. doi: 10.1089/gte.2006.9998 [DOI] [PubMed] [Google Scholar]
- 60.Thompson HS, Valdimarsdottir HB, Duteau-Buck C, et al. Psychosocial predictors of BRCA counseling and testing decisions among urban African-American women. Cancer Epidemiol Biomarkers Prev. December2002;11(12):1579–85. [PubMed] [Google Scholar]
- 61.Olaya W, Esquivel P, Wong JH, et al. Disparities in BRCA testing: when insurance coverage is not a barrier. Am J Surg. October2009;198(4):562–5. doi: 10.1016/j.amjsurg.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 62.Hallowell N, Ardern-Jones A, Eeles R, et al. Men’s decision-making about predictive BRCA1/2 testing: the role of family. J Genet Couns. June2005;14(3):207–17. doi: 10.1007/s10897-005-0384-3 [DOI] [PubMed] [Google Scholar]
- 63.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. April2005;293(14):1729–36. doi: 10.1001/jama.293.14.1729 [DOI] [PubMed] [Google Scholar]
- 64.Peters N, Rose A, Armstrong K. The association between race and attitudes about predictive genetic testing. Cancer Epidemiol Biomarkers Prev. March2004;13(3):361–5. [PubMed] [Google Scholar]
- 65.Rose A, Peters N, Shea JA, Armstrong K. Development and testing of the health care system distrust scale. J Gen Intern Med. January2004;19(1):57–63. doi: 10.1111/j.1525-1497.2004.21146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman-Davis E, Zhou ZN, Fields JC, et al. Racial and Ethnic Disparities in Genetic Testing at a Hereditary Breast and Ovarian Cancer Center. J Gen Intern Med. July2020;doi: 10.1007/s11606-020-06064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ormond KE, Laurino MY, Barlow-Stewart K, et al. Genetic counseling globally: Where are we now? Am J Med Genet C Semin Med Genet. 032018;178(1):98–107. doi: 10.1002/ajmg.c.31607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Directors ABo. Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet Med. June2015;17(6):505–7. doi: 10.1038/gim.2015.41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.