Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses a spike protein (S-protein) to recognize the receptor protein ACE2 of human cells and initiate infection, during which the conformational transition of the S-protein from inactive (down) state to active (up) state is one of the key molecular events determining the infectivity but the underlying mechanism remains poorly understood. In this work, we investigated the activation pathways and free energy landscape of the S-protein of SARS-CoV-2 and compared with those of the closely related counterpart SARS-CoV using molecular dynamics simulations. Our results revealed a large difference between the activation pathways of the two S-proteins. The transition from inactive to an active state for the S-protein of SARS-CoV-2 is more cooperative, involving simultaneous disruptions of several key interfacial hydrogen bonds, and the transition encounters a much higher free energy barrier. In addition, the conformational equilibrium of the SARS-CoV-2 S-protein is more biased to the inactive state compared to that of the SARS-CoV S-protein, suggesting the transient feature of the active state before binding to the receptor protein of the host cell. The key interactions contributing to the difference of the activation pathways and free energy landscapes were discussed. The results provide insights into the molecular mechanism involved in the initial stage of the SARS-CoV-2 infection.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a worldwide outbreak of the coronavirus disease 2019 (Covid-19).1−3 Similar to its closely related coronavirus SARS-CoV, which is the pathogen of another epidemic in 2002, the SARS-CoV-2 uses the spike protein (S-protein) to recognize the human receptor protein ACE2 and initiate the infection by mediating the membrane fusion.4,5 Therefore, the S-protein, which is exposed on the viral surface, has been the major target for the development of effective vaccines and therapeutics.6−9
The ectodomain of S-protein consists of two distinct functional subunits, one for recognizing and binding to the entry receptor (S1 subunit) and another for mediating membrane fusion (S2 subunit; Figure 1A).10,11 The S1 subunit is formed by the N-terminal domain (NTD), two subdomains (SD1 and SD2), and the receptor-binding domain (RBD). The RBD contains the ACE2-recognition motif and engages with ACE2 directly (Figure 1).12,13 Two different conformational states of the RBD exist in the prefusion stage, including the down-state and up-state.14,15 In the up-state, the ACE2-recognition motif is fully exposed and accessible to ACE binding, which therefore corresponds to the activated conformational state. Differently, the ACE2-recognition motif is partially buried in the (inactive) down-state and the ACE2 is not readily accessible. Therefore, the conformational transition of the S1 subunit from the inactive down-state to the active up-state through RBD opening is the prerequisite for membrane fusion and virus entry into the host cell (Figure 1B). The final infectivity of the virus not only depends on the RBD–ACE2 interactions but also on the conformational dynamics of the S-protein.16 As the SARS-CoV-2 and its counterpart SARS-CoV show different infectivities,17 it is important to investigate the differences between the conformational dynamics involved in the activation of the S-proteins.
Figure 1.
(A) Cartoon diagram illustrating the recognition of the ACE2 receptor of human cells by S-protein of the SARS-CoV-2. The locations of the NTD, S2, and RBD are labeled. (B) Structural illustration showing the down-to-up transition through RBD opening for the S-protein of the SARS-CoV-2. The RBD and ACE2 are shown in red and purple, respectively. The coordinates of the down and up conformations were taken from the protein data bank with the entries 6vxx and 6vyb.14 The location of the ACE2 was modeled based on the ACE2-bound structure (PDB ID: 7KNE).29
Recent experimental and molecular simulation studies have shown that the RBD domain of the SARS-CoV-2 S-protein (hereafter referred to as CoV2-S) binds to the receptor ACE2 much stronger compared to that of the SARS-CoV S-protein (CoV-S).15,18−23 However, a similar or even weaker affinity was observed for the binding between the ACE2 and the entire CoV2-S compared to that between the ACE2 and the entire CoV-S.14,24 More recently, Peng and co-workers showed that the RBD of the CoV-S can sample conformations with higher accessibility to ACE2 than that of the CoV2-S, which is helpful for the binding between the ACE2 and the entire S-protein.25 In addition, the effects of glycosylation of the CoV2-S have been extensively investigated based on computational and experimental studies.26,27 However, the conformational transition pathways and the key interactions contributing to the difference in the conformational dynamics for CoV2-S and CoV-S are not very clear, and directly simulating the whole activation pathways by conventional molecular dynamics is extremely challenging.
In this work, we investigated the conformational transition pathways of the two S-proteins using the parallel cascade selection molecular dynamics (PaCS-MD), which is a molecular simulation method based on the genetic-type algorithm and can sample rare events without introducing biasing potential.28 The PaCS-MD simulations revealed that the two S-proteins follow distinctive activation pathways. The transition from inactive to an active state for the CoV2-S is more cooperative, involving simultaneous disruptions of several key hydrogen bonds between the S-protein chains, and the conformational activation encounters a higher free energy barrier. In addition, we generated the reverse transition pathways from the up-state to the down-state, which are comparable for the two S-proteins. As a result, the conformational equilibrium of the CoV2-S is much more biased to the down-state compared with the CoV-S. Free energy landscapes constructed based on umbrella sampling simulations also demonstrated the narrower accessible conformational space in the activated state and higher activation free energy barrier for the CoV2-S. The key interactions contributing to the differences in the activation pathways and free energy landscapes between the two S-proteins were discussed. The results of this work provide insights into the molecular mechanism involved in the initial stage of the SARS-CoV-2 infection, which can be useful for drug design and vaccine development.
Results and Discussion
Activation Pathways of S-proteins in the SARS-CoV-2 and SARS-CoV
To investigate the activation process of the S-proteins, we generated conformational transition pathways from down-state (inactive) to up-state (active) via PaCS-MD simulations28 (see the Materials and Methods section). As shown in Figure 2A, the down-to-up conformational transition of the CoV2-S is much more difficult than that of the CoV-S. In four of the five independent PaCS-MD simulations starting from the down-state, the CoV-S arrives at the up-state (i.e., with the root mean square deviation (RMSD) from the up-state less than 0.5 nm) within 100 PaCS-MD cycles (red lines). In comparison, for the CoV2-S, even after 350 PaCS-MD cycles, only two of the five PaCS-MD simulations successfully sampled the full down-to-up transitions (blue lines). Similar results can be observed when the RMSD from the down-state was used as the reaction coordinate to characterize the transitions (Figure S1). Because the domain-wise fluctuation of the RBD can be relatively large, we used an RMSD cutoff value of 0.5 nm to assign the successful events of conformational transition. However, the significant differences in the conformational transitions between the CoV2-S and CoV-S can be observed from the features of the whole trajectories, which are independent of the RMSD cutoff value.
Figure 2.
Pathways of conformational transitions. (A) PaCS-MD trajectories for the S-proteins of the SARS-CoV-2 (blue) and SARS-CoV (red) characterized by the RMSD as a function of the PaCS-MD cycle for the down-to-up transitions. The RMSD is calculated with the structure of the up-state being used as a reference. The dashed line represents the threshold for accessing the up-state. (B) Same as (A) but for the up-to-down transitions. (C, D) Aligned conformations extracted from one representative PaCS-MD trajectory of the down-to-up transition for the S-proteins of SARS-CoV-2 (C) and SARS-CoV (D). The RBD is colored from red (down-state) to blue (up-state) along the trajectory.
The three-dimensional structures along the down-to-up transition extracted from PaCS-MD simulations are shown in Figure 2C,D. One can see that the RBD of the CoV-S can sample much more extended conformations in the up-state than that of the CoV2-S, which suggests that the RBD of the CoV-S can open its conformation to a larger extent. Such a result is relevant to the previous observation that tighter binding between the S-protein and the ACE2 requires more extended conformations of the RBD domain.25 In addition, the wider conformational space sampled in the open state may contribute to the lower free energy due to increased conformational entropy, as will be discussed in the subsequent subsection.
Using a similar strategy, we also generated the conformational transition pathways from the up-state to the down-state, and the results are shown in Figure 2B. One can see that the difference in the overall transition features for the two S-proteins is minor. Such results suggested that the differences in the overall conformational dynamics of the two S-proteins mainly arise from the down-to-up transition, and the conformational equilibrium is more biased to the down-state in the CoV2-S than that in the CoV-S. Such biased conformational equilibrium tends to destabilize the binding between the ACE2 and the CoV2-S.25 Consequently, although the ACE2 binds with the separated RBD of the CoV2-S much stronger, the overall affinity between the ACE2 and the entire CoV2-S can be similar or even weaker compared to that of the CoV-S, as demonstrated in the previous experimental work.14
As shown in the three-dimensional structures of the S-proteins (Figure 1), the RBD of chain A (which involves down-to-up transition) makes close contacts with the NTD domain and the S2 subunit of the neighboring chain (chain C), which need to be broken during the down-to-up transition. Therefore, to characterize the conformational transition pathways, we defined two reaction coordinates, including (1) the distance between the RBD of chain A and the S2 subunit of chain C (RRBD-S2) and (2) the distance between the RBD of chain A and the NTD of chain C (RRBD-NTD). In calculating the RRBD-S2 for the CoV2-S (CoV-S), the Cα atom of the S383 (S370) in chain A was used to represent the position of the RBD, and the centroid of the Cα atoms of the L981-L984 (L963-L966) segment in chain C were used to represent the position of the S2 subunit. Similarly, in calculating the RRBD-NTD for the CoV2-S (CoV-S), the Cα atom of the E465 (E452) in chain A was used to represent the position of the RBD, and the centroid of the Cα atoms of the G232-N234 (G225-N227) segment in chain C was used to represent the position of the NTD. The distances defined above are shown schematically in the three-dimensional structures of the two proteins in Figure S2.
In Figure 3, we projected the down-to-up transition pathways for the PaCS-MD trajectories with successful transitions onto the conformational space defined by the above two reaction coordinates. One can see that the activation pathways of the two S-proteins show obvious differences. For the CoV2-S, the breaking up of the interactions between two interfaces are relatively more cooperative with the simultaneous separation of the two interfaces. In comparison, for the CoV-S, the activation tends to occur sequentially with the interaction between the RBD and S2 subunit being broken up first, which is followed by the separation of the RBD–NTD interface. Apparently, the simultaneous breaking up of the two interfaces in the down-to-up transition of the CoV2-S tends to make the activation more difficult, which may lead to the above observation that the successful down-to-up transition events in the PaCS-MD simulations of the CoV2-S are rare.
Figure 3.
Down-to-up transition pathways for the PaCS-MD trajectories of the CoV2-S (A) and CoV-S (B). The trajectories were projected onto the conformational space formed by the reaction coordinates RRBD-NTD and RRBD-S2. The lines with different colors represent different trajectories for the successful down-to-up transition events.
Free Energy Landscapes of S-proteins
To more quantitatively characterize the activation dynamics of the two S-proteins, we conducted umbrella sampling simulations starting from the structures along the transition pathways by the above PaCS-MD simulations. As demonstrated in previous works,30−34 the PaCS-MD simulations can sample more realistic transition pathways compared to other methods with biasing potential and the umbrella sampling simulations initiating from the structures sampled by PaCS-MD simulations can cover the conformational space relevant to the conformational transition, which is essential for accurate estimation of the free energy landscapes. More details of the umbrella simulations are given in the Materials and Methods section.
As shown in Figure 4A, the transition from down-state to up-state involves a higher free energy barrier for the CoV2-S, and the up-state of the CoV2-S is less stable compared to that of the CoV-S, which are consistent with the above discussions based on direct simulations of the conformational transitions. Interestingly, the CoV-S shows much wider basins around the up-state (RMSD > 1.5 nm), demonstrating again that the up-state of the CoV-S is more extended and dynamic. Previous computational work showed that the bending angle of the RBD is a key parameter characterizing the accessibility of the ACE2.25 Therefore, we also constructed the two-dimensional free energy landscapes for the two S-proteins along the RMSD and the RBD bending angle. The RBD bending angle of the CoV2-S (CoV-S) is defined by the angle formed by the Cα atoms of three consecutive residues D405-V622-V991 (D392-T608-V973). According to the results of Peng et al., when the RBD bending angle is larger than 52.2°,25 the ACE2 is accessible. As shown in Figure 4B,C, both S-proteins can sample the ACE2-accessible conformations in the up-state by the PaCS-MD and umbrella simulations. Notably, the ACE2-accessible region in the conformational space at the up-state for the CoV2-S is much narrower than that for the CoV-S, which may lead to the decreased binding affinity between the entire CoV2-S and the ACE2 compared to that between its isolated RBD domain and the ACE2.14,24,25 Similar results were also observed by Peng and co-workers based on replica-exchange molecular dynamics simulations.25
Figure 4.

(A) One-dimensional free energy profile for the S-proteins of the SARS-CoV-2 (blue) and SARS-CoV (red) along the reaction coordinate RMSD. (B,C) Two-dimensional free energy profile for the S-proteins of the SARS-CoV-2 (B) and SARS-CoV (C) along the reaction coordinates RMSD and RBD bending angle. Colored lines are PaCS-MD trajectories for the successful down-to-up transition events. Green points represent the snapshots extracted from the last 60 ns of the six conventional MD simulation trajectories at the up-state plotted with a time interval of 5 ns. The dashed line represents the threshold value of the RBD angle (52.2°), above which the RBD domain will be open enough to access the receptor ACE2 as proposed in ref (25).
Hydrogen-Bond Network and Interchain Interactions
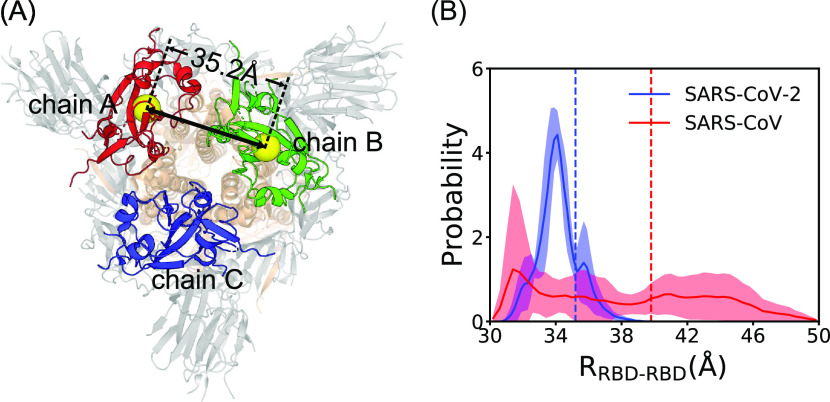
To better understand the molecular mechanism for the above-observed differences in the activation pathways and free energy landscapes between the CoV2-S and CoV-S, we also performed conventional MD simulations with a length of 100 ns around the down-state and up-state, respectively, and calculated the distributions of inter-RBD distances (Figure 5). Six independent MD simulations were conducted for each case. The snapshots of the first 40 ns were omitted in the calculation of the distributions. The time series of inter-RBD distances are shown in Figure S3. One can see that the inter-RBD distances of the CoV-S have a much wider distribution compared to that of the CoV2-S. The average distances are ∼3.4 and 3.7 nm, respectively, for the above two proteins. The much tighter packing in the interfaces of the neighboring RBDs for CoV2-S suggests that the interfacial interactions of the RBD are stronger in the down-state of the CoV2-S, which is in line with the higher free energy barrier encountered in the activation process. Such tighter interactions also reduce the positional fluctuations of the RBDs in the down-state (Figure S4A), whereas the intra-domain fluctuations of the RBDs are minor for the two S-proteins, suggesting that the conformational fluctuations mainly arise from the domain-wise motions (Figure S4B). In comparison, both proteins show dramatic fluctuations at the up-state due to the breaking up of the interfacial interactions. We also plotted the snapshots from the conventional MD simulations at the up-state on the two-dimensional free energy profiles (Figure 4B,C). One can see that the snapshots from the conventional MD trajectories can cover well the free energy basin of the up-state, suggesting the consistency between the free energy calculation and the conventional MD simulations. Similar results can be observed when the Amber ff14SB and charmm36 force fields were used, as demonstrated by the time series of the RMSD and RBD-angle trajectories in Figure S7.
Figure 5.
(A) Top view of the three-dimensional structure of the S-protein of the SARS-CoV-2 at down-state. The distance between two RBDs is 35.2 Å. (B) Distributions of the inter-RBD distances at the down-state for the S-proteins of the SARS-CoV-2 (blue) and SARS-CoV (red). Dashed lines represent the inter-RBD distances in the native structures. The errors were estimated based on the standard deviation from six independent MD simulations.
Further analysis of the trajectories from PaCS-MD showed that the number of hydrogen bonds between the RBD and the neighboring chains is different. For example, at the down-state, the residues of chain A (of which the RBD involves down-to-up transition) form a larger number of hydrogen bonds with the residues of the other two chains (chains B and C) (Figures 6 and 7). As a result, more hydrogen bonds need to be broken during the down-to-up transition of the CoV2-S compared to that of the CoV-S (Figures 6A and 7A, dash squares). Particularly, the RBD of chain A forms at least five hydrogen bonds with the RBD of chain C (Figure 6A, black dash square; Figure 6D,E), which involve the residue pairs R403(A)-A372(C), R403(A)-F374(C), T500(A)-N439(C), N501(A)-N437(C), and N501(A)-N440(C). Here (X) represents the chain identity. Similarly, four hydrogen bonds, including G381(A)-R983(C), K386(A)-L981(C), K386(A)-L984(C), and D428(A)-R983(C), are formed between the S2 of chain C and the RBD of chain A. All these hydrogen bonds need to be broken simultaneously during the early stage of the down-to-up transition (Figure 6E). Such simultaneous breaking up of several hydrogen bonds often renders a high energy barrier, which can well explain the much higher free energy barrier observed in the down-to-up transition of the CoV2-S (Figure 4). In comparison, the interfacial hydrogen bonds are rarely observed between the RBDs of the CoV-S (Figure 7A). The simultaneous ruptures of multiple hydrogen bonds involved in the down-to-up transition of the CoV2-S were also observed by Gur et al. based on steered MD simulations and free energy calculations.35 The sequence alignment analysis showed that the difference of the hydrogen bonds involved in the down-to-up conformational transition between the CoV2-S and CoV-S is not strongly correlated with the sequence variations at the local sites of these hydrogen bonds (Figure S6). For several hydrogen bonds showing different behaviors in the down-to-up transitions of the CoV2-S and CoV-S, the donor or acceptor residues are conserved. Such results may suggest that the effects of sequence variation on the conformational dynamics of the two proteins involve long-range structural coupling, in addition to the local structural perturbations.
Figure 6.

Hydrogen-bond network and interchain interactions of the S-protein of SARS-CoV-2. (A) Probabilities of contact formation between the residues of chain A and the residues of chains B and C at different stages of the down-to-up transitions. The structures from PaCS-MD around the RMSD values of 4.5, 11.0, 15.0, and 18.0 Å (from left to right panels) were used to calculate the probabilities of the contact formation according to the free energy landscape shown in Figure 4A. The squares with different colors represent the contacts formed between the RBD of chain A and different regions of chains B and C as indicated by the labels. (B, C) Structural illustrations for the interfacial hydrogen bonds formed between the RBD of chain A and the S2 of chain C (B), and the donor–acceptor distances for the corresponding hydrogen bonds as a function of PaCS-MD cycles (C). The representative structures with RMSD values of 4.5 and 18.0 Å (corresponding to the two free energy minima in the free energy landscape) sampled by PaCS-MD were used to illustrate the structural features in the down- and up-states, respectively, according to the free energy landscape shown in Figure 4A. (D, E) Same as (B, C) but for the interfacial hydrogen bonds formed between the RBD of chain A and the RBD of chain C. (F, G) Same as (B, C) but for the interfacial hydrogen bonds formed between the RBD of chain A and the NTD of chain C.
Figure 7.

Hydrogen-bond network and interchain interactions of the S-protein of SARS-CoV. (A) Probabilities of contact formation between the residues of chain A and the residues of chains B and C at different stages of the down-to-up transitions. The structures from PaCS-MD around the RMSD values of 2.5, 10.0, 15.0, and 27.0 Å (from left to right panels) were used to calculate the probabilities of contact formation. (B, C) Structural illustrations for the interfacial hydrogen bonds formed between the RBD of chain A and the S2 of chain C (B), and the donor–acceptor distances for the corresponding hydrogen bonds as a function of PaCS-MD cycles (C). (D, E) Same as (B, C) but for the interfacial hydrogen bonds formed between the RBD of chain A and the NTD of chain C.
To demonstrate that the reaction coordinate RMSD used in the PaCS-MD simulations and umbrella sampling simulations is appropriate for describing the down-to-up conformational change, we plotted the two-dimensional free energy profile of the CoV2-S along the reaction coordinates RMSD and the formed hydrogen-bond number based on the umbrella sampling results by reweighting analysis (Figure S8). The hydrogen bonds shown in Figure 6C,E,G were used to calculate the number of formed hydrogen bonds. The result showed that the sampled conformational space with small RMSD values (down-state) tends to have a larger number of formed hydrogen bonds. Similarly, the sampled conformational space with large RMSD values (up-state) tends to have a smaller number of formed hydrogen bonds. Such results suggest that the RMSD value is a reasonable reaction coordinate, which can well capture the difference of the structural features between the up- and down-states.
Among the RBD residues involved in hydrogen bonds, the N501 is subject to mutagenesis (N501Y) involved in the SARS-CoV-2 variants B.1.1.7 and B.1.351.36 A recent computational study showed that the N501Y mutation can improve the direct interactions between the RBD and ACE2.37,38 As shown in the above results, the N501 of chain A forms hydrogen bonds with the N437 and N440 of chain C at the down-state of the CoV2-S, which plays important role in the stabilization of the down-state. Therefore, the N501Y mutation may affect the activation dynamics of the S-protein and contribute to the increased infectivity by altering the N501-mediated hydrogen bonds.
For both S-proteins, the RBD of chain A forms hydrogen bonds with the S2 subunit and the NTD domain of chain C at the down-state, and most of these hydrogen bonds are broken at the up-state. Compared to the CoV-S, the number of hydrogen bonds is much larger for the CoV2-S (Figures 6A and 7A), which can also contribute to its biased conformational equilibrium to the down-state and the higher activation barrier height.
Conclusions
In this work, we investigated the activation pathways and free energy landscapes of the S-proteins of SARS-CoV-2 and its closely related counterpart SARS-CoV by using MD simulations. Both the activation kinetics and free energy landscapes showed that the down-to-up transitions of the SARS-CoV-2 S-protein are more difficult than that of the SARS-CoV S-protein, whereas the reversal processes are comparable. Furthermore, the two S-proteins present different conformational transition pathways. Such differences mainly resulted from the tighter interchain packing and the additional hydrogen bonds in the SARS-CoV-2 S-protein. Previous simulations and experiments have shown that the ACE2 binds with the RBD of CoV2-S with a higher binding affinity. However, a similar or even weaker binding affinity was observed for the entire SARS-CoV-2 S-protein. Our results demonstrated that such differences in the binding affinity between the RBD domain and the entire protein mainly arise from the difference in the conformational transitions. Although the RBD of the CoV2-S binds to the ACE2 much more tightly, the biased conformational equilibrium to the down-state tends to decrease the stability of the activated state, which in turn decreases the overall affinity between the RBD and the entire S-protein of SARS-CoV-2. Such results are in line with the previous proposal by Shang and co-workers24 that the compensation of the RBD-ACE2 affinity and the biased conformational equilibrium toward the down-state are relevant to the high infectivity of the SARS-CoV-2. The increased biases of the conformational equilibrium toward the down-state destabilize the up-state and the exposure time, which may shield the SARS-CoV2-S protein from the immune response.24 Meanwhile, the decreased affinity due to conformational biases can be counteracted by the increased affinity between the RBD and the ACE2, which may lead to the enhanced overall infectivity of the SARS-CoV-2. In addition to characterizing the down-to-up transition dynamics, it is also crucially important to investigate the possible ways to regulate such conformational transition dynamics. Interestingly, a recent simulation work identified a transient pocket involved in the RBD opening. The binding of a ligand to the pocket tends to allosterically shift the conformational equilibrium toward the up-state, demonstrating a possible way to control the conformational dynamics of the RBD.39
In this work, the glycans were not included in the simulations. Previous studies showed that the glycans of the CoV2-S can affect the viral entry by shielding the RBD to evade host immune response27 by affecting the conformational equilibrium and the activation dynamics of the CoV2-S,26,27 or by regulating the spike protein proteolysis.40 It is interesting in future studies to investigate how the glycan effects can contribute to the difference in the activation dynamics between the CoV2-S and CoV-S.
Materials and Methods
MD Simulations
In all of the MD simulations, the atomic coordinates for the down- and up- states of the CoV2-S (CoV-S) were taken from the protein data bank with the entries of 6vxx and 6vyb (6acc and 6acd), respectively.14,41 The missing residues in the PDB structures were reconstructed by Modeller.42 The initial structure was solvated in a TIP3P water box.43 The Amber ff99SB force field was used for the protein system.44 Na+ and Cl– were added to neutralize the system and simulate the salt concentration of 150 mM. The final system contains 431 831 atoms for the CoV2-S and 407918 atoms for the CoV-S. The solvated systems were first minimized for 50 000 steps by steepest descent. Then, the minimized structures were heated to 310 K during the period of 1.0 ns in the NVT ensemble, with the heavy atoms of the proteins being restrained by a harmonic potential. The heated systems were further relaxed for 1.0 ns in the NPT ensemble at 310 K and 1.0 atm, which was followed by another 1.0 ns simulations without applying restraints. The relaxed structures were then used as the initial structures in the subsequent PaCS-MD simulations and conventional MD simulations. Software PyMol was used for the structural visualizations.45
Sampling Activation Pathways by Parallel Cascade Selection Molecular Dynamics
The down-to-up conformational transitions of the S-proteins involve a high free energy barrier, and therefore, the related timescale for sampling such rare events is far beyond the capability of conventional MD simulations.46,47 Although one can speed up the sampling of conformational transition events by introducing biasing potential toward the target state, it tends to distort the kinetics and leads to unphysical transition pathways.48 As a solution, Harada and Kitao developed a parallel cascade selection molecular dynamics (PaCS-MD) method based on a genetic-type algorithm, which can generate conformational transition pathways without applying biasing potential.28 With PaCS-MD, one can sample transition pathways for protein systems involving a high free energy barrier but without sampling unphysically high-energy regions in the conformational space.
The PaCS-MD simulations consist of the following steps: (1) starting from the three-dimensional structure of the down-conformation, we conducted 10 independent short MD simulations of 100.0ps, and the snapshots were saved with an interval of 1.0 ps; (2) from these sampled structures, we picked up 10 structures closest to the three-dimensional structure of the up-conformation measured by the root mean square deviation (RMSD) of the RBD domain relative to the open structure; and (3) we then conducted 10 independent short MD simulations starting from the chosen structures. By repeating steps (2) and (3), we can sample the down-to-up transition pathways after a number of cycles. We conducted five independent PaCS-MD simulations for the down-to-up transitions. Similarly, we performed the PaCS-MD simulations for the up-to-down transitions. In calculating the RMSD of the RBD domain, we first aligned the structure by superimposing the β sheets of the NTD and the helices of the S2 subunit in chain C. Then, we calculated the RMSD of the RBD of chain A with the target conformation being used as a reference structure. Therefore, the RMSD value not only measures the intra-domain structural change of the RBD but also measures the collective motions of the whole RBD domain.
Free Energy Calculations by Umbrella Sampling
Umbrella sampling is a powerful method for free energy calculations.49,50 However, because the sampling along the direction perpendicular to the used reaction coordinates is often limited, the initial structures for the simulations at each umbrella window should be close to the path of the conformational transition. The PaCS-MD described above can meet such a requirement. Therefore the combination of the PaCD-MD and the umbrella sampling can be an effective strategy to calculate the free energy landscapes in the conformational space along the transition pathways.30 In this work, the conformations sampled by PaCS-MD simulations were used as initial structures of the umbrella sampling simulations. Similar to the PaCS-MD, we used the RMSD of the RBD as the reaction coordinate for the umbrella sampling, with the down-state conformation being used as a reference structure. The spring constant of the harmonic potential applied to each umbrella window was set as 1000 kJ/mol/nm2. The simulations were conducted by combining Gromacs201851 and PLUMED.52 The sampled conformations at each umbrella window were reweighed by PyMBAR to construct the free energy landscapes.53 The centers of the umbrella windows along the reaction coordinates were set as 0.0, 0.2, 0.4, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.2, 1.3, 1.4, 1.6, 1.7, 1.8, 2.0, 2.2, and 2.4 nm for the simulations of the CoV2-S. Similarly, the centers of the umbrella windows along the reaction coordinates were set as 0.0, 0.2, 0.4, 0.5, 0.6, 0.7, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, 3.0, 3.2, 3.4, and 3.6 nm for the simulations of the CoV-S. For the umbrella windows with the centers of RMSD less than 1.6 nm (1.4 nm) in the simulations of CoV2-S (CoV-S), the initial structures were taken from the down-to-up transition pathways sampled by PaCS-MD simulations, whereas the initial structures for the umbrella windows with the centers of RMSD larger than 1.3 nm (1.2 nm) were taken from the up-to-down transition pathways. The umbrella windows with RMSDs between 1.3 nm (1.2 nm) and 1.6 nm (1.4 nm) were conducted using both sets of initial structures to minimize the errors due to reweighting.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (11974173, 11934008, and 11874197) and the HPC center of Nanjing University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03384.
MD trajectories; results of sequence alignment; free energy profiles; and additional structural and dynamics information (Figures S1–S8) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zhou P.; Yang X.; Wang X.; Hu B.; Zhang L.; Zhang W.; Si H.-R.; Zhu Y.; Li B.; Huang C.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N.; Zhang D.; Wang W.; Li X.; Yang B.; Song J.; Zhao X.; Huang B.; Shi W.; Lu R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C.; Alsafi Z.; O’Neill N.; Khan M.; Kerwan A.; Al-Jabir A.; Iosifidis C.; Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.-Y.; Li J.-L.; Yang X.-L.; Chmura A. A.; Zhu G.; Epstein J. H.; Mazet J. K.; Hu B.; Zhang W.; et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M. A.; Veesler D.. Complementary Strategies to Understand Virus Structure and Function. In Advances in Virus Research; Academic Press, 2019; Vol. 105, pp 93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Wang F.; Shen C.; Peng W.; Li D.; Zhao C.; Li Z.; Li S.; Bi Y.; Yang Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R.; Shan C.; Duan X.; Chen Z.; Liu P.; Song J.; Song T.; Bi X.; Han C.; Wu L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Freitas F. C.; Ferreira P. H. B.; Favaro D. C.; de Oliveira R. J. Shedding Light on the Inhibitory Mechanisms of SARS-CoV-1/CoV-2 Spike Proteins by ACE2-Designed Peptides. J. Chem. Inf. Model. 2021, 61, 1226–1243. 10.1021/acs.jcim.0c01320. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Wang Y.; Zhu Y.; Liu C.; Gu C.; Xu S.; Wang Y.; Zhou Y.; Wang Y.; Han W.; et al. Development and structural basis of a two-MAb cocktail for treating SARS-CoV-2 infections. Nat. Commun. 2021, 12, 264 10.1038/s41467-020-20465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B. J.; van der Zee R.; de Haan C. A. M.; Rottier P. J. M. The Coronavirus Spike Protein Is a Class I Virus Fusion Protein: Structural and Functional Characterization of the Fusion Core Complex. J. Virol. 2003, 77, 8801–8811. 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M.; KleineWeber H.; Schroeder S.; Krüger N.; Herrler T.; Erichsen S.; Schiergens T. S.; Herrler G.; Wu N.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialo F.; Daniele A.; Amato F.; Pastore L.; Matera M. G.; Cazzola M.; Castaldo G.; Bianco A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. 10.1007/s00408-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A. C.; Park Y.; Tortorici M. A.; Wall A.; McGuire A. T.; Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. 10.1016/j.cell.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D.; Wang N.; Corbett K. S.; Goldsmith J. A.; Hsieh C.; Abiona O.; Graham B. S.; McLellan J. S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R.; Edwards R. J.; Mansouri K.; Janowska K.; Stalls V.; Gobeil S. M. C.; Kopp M.; Li D.; Parks R.; Hsu A. L.; et al. Controlling the SARS-CoV-2 spike glycoprotein conformation. Nat. Struct. Mol. Biol. 2020, 27, 925–933. 10.1038/s41594-020-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. G.; Lin T.; Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.; Tao H.; Yan Y.; Huang S.-Y.; Xiao Y. Molecular Mechanism of Evolution and Human Infection with SARS-CoV-2. Viruses 2020, 12, 428 10.3390/v12040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari P.; Li N.; Shin M.; Steinmetz N. F.; Twarock R.; Podgornik R.; Ching W. Intra- and intermolecular atomic-scale interactions in the receptor binding domain of SARS-CoV-2 spike protein: implication for ACE2 receptor binding. Phys. Chem. Chem. Phys. 2020, 22, 18272–18283. 10.1039/D0CP03145C. [DOI] [PubMed] [Google Scholar]
- Amin M.; Sorour M. K.; Kasry A. Comparing the Binding Interactions in the Receptor Binding Domains of SARS-CoV-2 and SARS-CoV. J. Phys. Chem. Lett. 2020, 11, 4897–4900. 10.1021/acs.jpclett.0c01064. [DOI] [PubMed] [Google Scholar]
- Nguyen H. L.; Lan P. D.; Thai N. Q.; Nissley D. A.; O’Brien E. P.; Li M. S. Does SARS-CoV-2 Bind to Human ACE2 More Strongly Than Does SARS-CoV. J. Phys. Chem. B 2020, 124, 7336–7347. 10.1021/acs.jpcb.0c04511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinello A.; Saltalamacchia A.; Magistrato A. Is the Rigidity of SARS-CoV-2 Spike Receptor-Binding Motif the Hallmark for Its Enhanced Infectivity? Insights from All-Atom Simulations. J. Phys. Chem. Lett. 2020, 11, 4785–4790. 10.1021/acs.jpclett.0c01148. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Liu M.; Gao J. Enhanced receptor binding of SARS-CoV-2 through networks of hydrogen-bonding and hydrophobic interactions. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 13967–13974. 10.1073/pnas.2008209117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J.; Wan Y.; Luo C.; Ye G.; Geng Q.; Auerbach A.; Li F. Cell Entry Mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 11727–11734. 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C.; Zhu Z.; Shi Y.; Wang X.; Mu K.; Yang Y.; Zhang X.; Xu Z.; Zhu W. Computational Insights into the Conformational Accessibility and Binding Strength of SARS-CoV-2 Spike Protein to Human Angiotensin-Converting Enzyme 2. J. Phys. Chem. Lett. 2020, 11, 10482–10488. 10.1021/acs.jpclett.0c02958. [DOI] [PubMed] [Google Scholar]
- Sztain T.; Ahn S.-H.; Bogetti A. T.; Casalino L.; Goldsmith J. A.; Seitz E.; McCool R. S.; Kearns F. L.; Acosta-Reyes F.. et al. A Glycan Gate Controls Opening of the SARS-CoV-2 Spike Protein. Nat. Chem. 2021, 10.1038/s41557-021-00758-3 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino L.; Gaieb Z.; Goldsmith J. A.; Hjorth C. K.; Dommer A. C.; Harbison A. M.; Fogarty C. A.; Barros E. P.; Taylor B. C.; McLellan J. S.; et al. Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Cent. Sci. 2020, 6, 1722–1734. 10.1021/acscentsci.0c01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada R.; Kitao A. Parallel cascade selection molecular dynamics (PaCS-MD) to generate conformational transition pathway. J. Chem. Phys. 2013, 139, 035103 10.1063/1.4813023. [DOI] [PubMed] [Google Scholar]
- Zhou T.; Tsybovsky Y.; Gorman J.; Rapp M.; Cerutti G.; Chuang G.; Katsamba P. S.; Sampson J. M.; Schön A.; Bimela J.; et al. Cryo-EM Structures of SARS-CoV-2 Spike without and with ACE2 Reveal a pH-Dependent Switch to Mediate Endosomal Positioning of Receptor-Binding Domains. Cell Host Microbe 2020, 28, 867–879.e5. 10.1016/j.chom.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J.; Li J.; Lu J.; Li W.; Wang W. Role of substrate-product frustration on enzyme functional dynamics. Phys. Rev. E 2019, 100, 052409 10.1103/PhysRevE.100.052409. [DOI] [PubMed] [Google Scholar]
- Harada R. Simple, yet Efficient Conformational Sampling Methods for Reproducing/Predicting Biologically Rare Events of Proteins. Bull. Chem. Soc. Jpn. 2018, 91, 1436–1450. 10.1246/bcsj.20180170. [DOI] [Google Scholar]
- Ye C.; Ding C.; Ma R.; Wang J.; Zhang Z. Electrostatic interactions determine entrance/release order of substrates in the catalytic cycle of adenylate kinase. Proteins: Struct., Funct., Bioinf. 2019, 87, 337–347. 10.1002/prot.25655. [DOI] [PubMed] [Google Scholar]
- Zhu Q.; Yuan Y.; Ma J.; Dong H. A Data-Driven Accelerated Sampling Method for Searching Functional States of Proteins. Adv. Theory Simul. 2019, 2, 1800171 10.1002/adts.201800171. [DOI] [Google Scholar]
- Yuan Y.; Zhu Q.; Song R.; Ma J.; Dong H. A Two-Ended Data-Driven Accelerated Sampling Method for Exploring the Transition Pathways between Two Known States of Protein. J. Chem. Theory Comput. 2020, 16, 4631–4640. 10.1021/acs.jctc.9b01184. [DOI] [PubMed] [Google Scholar]
- Gur M.; Taka E.; Yilmaz S. Z.; Kilinc C.; Aktas U.; Golcuk M. Conformational transition of SARS-CoV-2 spike glycoprotein between its closed and open states. J. Chem. Phys. 2020, 153, 075101 10.1063/5.0011141. [DOI] [PubMed] [Google Scholar]
- Volz E.; Mishra S.; Chand M.; Barrett J. C.; Johnson R.; Geidelberg L.; Hinsley W. R.; Laydon D. J.; Dabrera G.; O’Toole Á.; et al. Transmission of SARS-CoV-2 Lineage B. 1.1. 7 in England: Insights from linking epidemiological and genetic data. MedRxiv 2021, 2020–12. 10.1101/2020.12.30.20249034. [DOI] [Google Scholar]
- Luan B.; Huynh T. Insights into SARS-CoV-2’s Mutations for Evading Human Antibodies: Sacrifice and Survival. J. Med. Chem. 2021, 10.1021/acs.jmedchem.1c00311. [DOI] [PubMed] [Google Scholar]
- Luan B.; Wang H.; Huynh T. Enhanced binding of the N501Y-mutated SARS-CoV-2 spike protein to the human ACE2 receptor: insights from molecular dynamics simulations. FEBS letters 2021, 595, 1454–1461. 10.1002/1873-3468.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon L.; Belfon K.; Raguette L.; Wang Y.; Corbo C.; Stepanenko D.; Cuomo A.; Guerra J.; Budhan S.; Varghese S.. et al. Free Energy Landscapes for RBD Opening in SARS-CoV-2 Spike Glycoprotein Simulations Suggest Key Interactions and a Potentially Druggable Allosteric Pocket. 2020. 10.26434/chemrxiv.13502646.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.; Hughes T. A.; Kelkar A.; Yu X.; Cheng K.; Park S.; Huang W.; Lovell J. F.; Neelamegham S. Inhibition of SARS-CoV-2 viral entry upon blocking N-and O-glycan elaboration. Elife 2020, 9, e61552 10.7554/eLife.61552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.; Gui M.; Wang X.; Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018, 14, e1007236 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fuentes N.; Fiser A.. Introduction to Protein Structure Prediction; John Wiley & Sons, Ltd,: 2010; Chapter 13, pp. 279–298. [Google Scholar]
- Jorgensen W. L.; Chandrasekhar J.; Madura J. D.; Impey R. W.; Klein M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. 10.1063/1.445869. [DOI] [Google Scholar]
- Hornak V.; Abel R.; Okur A.; Strockbine B.; Roitberg A.; Simmerling C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 2006, 65, 712–725. 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 1.8.
- Bernardi R. C.; Melo M. C.; Schulten K. Enhanced sampling techniques in molecular dynamics simulations of biological systems. Biochim. Biophys. Acta, Gen. Subj. 2015, 1850, 872–877. 10.1016/j.bbagen.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.; Li W.; Wang W. Modeling hydrogen exchange of proteins by a multiscale method. Chin. Phys. B 2021, 30, 078701 10.1088/1674-1056/abe377. [DOI] [Google Scholar]
- Zhu W.; Zhang J.; Wang J.; Li W.; Wang W. Enhanced sampling method with coarse graining of conformational space. Phys. Rev. E 2021, 103, 032404 10.1103/PhysRevE.103.032404. [DOI] [PubMed] [Google Scholar]
- Lemkul J. A.; Bevan D. R. Assessing the Stability of Alzheimer’s Amyloid Protofibrils Using Molecular Dynamics. J. Phys. Chem. B 2010, 114, 1652–1660. 10.1021/jp9110794. [DOI] [PubMed] [Google Scholar]
- Wang J.; Shao Q.; Xu Z.; Liu Y.; Yang Z.; Cossins B. P.; Jiang H.; Chen K.; Shi J.; Zhu W. Exploring Transition Pathway and Free-Energy Profile of Large-Scale Protein Conformational Change by Combining Normal Mode Analysis and Umbrella Sampling Molecular Dynamics. J. Phys. Chem. B 2014, 118, 134–143. 10.1021/jp4105129. [DOI] [PubMed] [Google Scholar]
- Abraham M. J.; Murtola T.; Schulz R.; Páll S.; Smith J. C.; Hess B.; Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- Bonomi M.; Bussi G.; Camilloni C.; Tribello G. A.; Banáš P.; Barducci A.; Bernetti M.; Bolhuis P. G.; Bottaro S.; Branduardi D.; et al. Promoting transparency and reproducibility in enhanced molecular simulations. Nat. Methods 2019, 16, 670–673. 10.1038/s41592-019-0506-8. [DOI] [PubMed] [Google Scholar]
- Shirts M. R.; Chodera J. D. Statistically optimal analysis of samples from multiple equilibrium states. J. Chem. Phys. 2008, 129, 124105 10.1063/1.2978177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.