Abstract
Introduction
Declining cognition in later life is associated with loss of independence and quality of life. This decline in cognition may potentially be reduced or reversed through engaging in cognitively stimulating activities. This study examined the potential for university attendance in later life to enhance cognitive function in older adults.
Methods
Cognitively unimpaired adults (n = 485, 69% female, median age 60 years) were given the opportunity to undertake free university study. Repeated neurocognitive assessment was performed over 7 years.
Results
Participants in the university education group (n = 383) improved z = .02 SD (.01, .03) per year of the study compared to controls (P = .001; averaged across a battery of cognitive tests). The largest improvements were observed on tests of language and verbal learning, memory, and episodic memory.
Discussion
Later‐life university study was associated with improved cognitive trajectories. Later‐life education may preserve cognitive function, specifically for functions associated with communication, social interaction, and maintaining independence.
Keywords: age‐related cognitive decline, cognitive aging, cognitive reserve, dementia prevention, lifespan cognitive reserve
1. BACKGROUND
“Living well” into the later years of life is dependent not only on physical and mental health, but also on cognitive health. Social engagement and independence are closely tied to a capacity to communicate, make decisions, understand new information, remember past events, and form new memories. As populations age, cognitive functions display age‐related declines across the domains of attention, concentration, and processing speed, and the ability to remember events or details. These functions are important to maintain health and well‐being in later life.1, 2
There is a well‐established evidence base for the association between early life education, career attainment, and cognitively stimulating leisure activities with preserved later‐life cognitive function3, 4 and delayed or reduced cognitive symptoms of dementia.5, 6 The Lancet International Commission on Dementia Prevention and Care concluded that low educational attainment accounted for 7% of the population attributable fraction of global dementia risk.7 A proposed mechanism for the protective effect of education is cognitive reserve (CR)5—the compensatory ability of the brain to maintain function despite advancing brain pathology. Education and other cognitively stimulating experiences are thought to build a reserve of cognitive capacity.
The direct relationship between early life education and later‐life cognitive function may be confounded by intelligence quotient (IQ; people who perform well on cognitive tests tend to stay in school longer), and barriers to access including socio‐economic disadvantage and socio‐cultural factors.8 The same advantages that are associated with higher education are also associated with other health outcomes.9 Early life education may advance life‐long cognitively stimulating work and leisure activity, thereby contributing to the preservation of cognitive function, reserve against the insults of pathology, and brain maintenance through neuroplasticity.10 These effects may be cumulative or interdependent, so it is unclear what benefit education confers independently. Later‐life education, relatively free of expectations around work or career advancement, socio‐economic opportunity, and provided with fewer barriers to entry (through subsidized fees and relaxed entry criteria), may provide a conduit to further understanding of potential benefits of education in preserving cognitive function in later life.
Interventions aimed at assessing the benefit of later‐life cognitively stimulating activities such as brain‐training and exercises specific to building targeted cognitive functions have provided mixed evidence of their effectiveness in slowing cognitive decline and delaying the cognitive symptoms of dementia. Studies fall broadly into two categories—single‐domain cognitive training interventions and multi‐domain trials that may include cardiovascular, dietary, hypertension, or other interventions aimed at improving modifiable risk factors.
Single‐domain cognitive training interventions to date have had mixed results, but have been of shorter‐duration (5 to 6 weeks),11, 12 and initial post‐intervention gains have not been sustained at follow‐up.13 Multidomain trials have been of longer duration (up to 2 years)14 but have focused largely on older cohorts.15 It is difficult to attribute a benefit specifically to cognitive training or education in multidomain trial designs because the cognitive training occurs concurrently with other interventions.
The Tasmanian Healthy Brain Project (THBP) is an ongoing longitudinal intervention study of cognitively healthy individuals over 50 with a self‐selected intervention group who participated in university education. The aim of the present study was to evaluate differences in cognitive trajectories between participants in intervention and comparison groups on cognitive test scores across the domains of language processing, executive function, episodic memory, and working memory. This study extends earlier published work from the THBP,12, 16, 17, 18 covering a longer duration (7 years compared to 3 years). Because cognitive benefits of education may be age‐ and dose‐dependent, we investigated whether the putative benefits of later‐life education diminished for older participants, and whether benefits increase in proportion to academic load.
RESEARCH IN CONTEXT
Systematic Review: An extensive search for research literature on cognitive intervention studies for age‐related cognitive decline and dementia prevention identified two broad classes of research: single‐domain cognitive interventions, which were of short duration (5 to 6 weeks) and multi‐domain (eg, incorporating dietary interventions) with longer follow‐up (2 to 3 years). Both types had mixed results. It remains unclear if later‐life education slows age‐related cognitive decline.
Interpretation: We found that later‐life education slowed cognitive decline and improved cognitive function, especially verbal memory, verbal fluency, and episodic memory. Older participants benefited most from the intervention. This study is a world‐first longitudinal intervention study examining the effects of later‐life university education in an aging cohort.
Future Directions: These findings suggest that later‐life education improves cognitive trajectories in adults aged 50 and older. Randomized controlled trials may be justified to confirm the causal effect of education on cognitive outcomes and incidence of dementia.
HIGHLIGHTS
Cognitively unimpaired adults 50 and older were given the opportunity to undertake fee‐waived university‐level study. Participants in later‐life education and comparison groups were followed with repeated neurocognitive assessment over 7 years.
Participants in the later‐life education group had improved cognitive trajectories relative to controls on tests of language and verbal learning, verbal memory, and episodic memory.
Older participants benefited from later‐life education at least as much as their younger peers.
Later‐life education may slow age‐related cognitive decline, particularly for functions associated with communication, social interaction, and maintaining independence.
2. METHODS
2.1. Study design and participants
The Tasmanian Healthy Brain Project (THBP) is a non‐randomized prospective, longitudinal cohort study investigating the effect of university‐level education on age‐related cognitive decline and dementia risk in adults ages 50 to 79 at baseline entry into the study. The design and methods of the THBP have been described in detail previously.19, 20 Participants were unpaid volunteers recruited through print, television advertising, radio, and community information presentations. Participants were screened to exclude conditions independently associated with cognitive impairment. Baseline assessments beginning in 2011 were completed on 566 participants. To date, 156 participants (27.6%) have withdrawn from the THBP. The majority of withdrawing participants report factors unrelated to the study: 22% relocated, 13% unable to recontact, 9% too busy, 10% medical diagnosis, 6% deceased, 3% work commitments, 2% family issues, 28% provided no reason, and 7% found the assessments too stressful. Medical diagnoses were predominantly cancer and neurological disorders. No participants stated that dementia diagnosis was their reason for withdrawal. Of the 566 enrollments, 438 participants chose to join the intervention group and undertake university study in a course of their choosing. For the purposes of this study, which is to assess the association between later‐life education and cognitive trajectories rather than an assessment of fee‐waived education as an intervention, we included any participant in the intervention group who completed at least one unit of study. We excluded participants in post‐graduate courses because (1) it was not possible to determine an equivalent academic load and (2) there was a high likelihood of recent (but not necessarily post‐baseline or over 50) university‐level study. Furthermore, following scrutiny of university records, some participants in the comparison group had undertaken university study after the study began and were excluded from this analysis. These criteria resulted in exclusion of n = 55 participants in the intervention group and n = 26 participants in the comparison group (Table 1).
TABLE 1.
Participant characteristics at baseline, including full‐scale intelligence quotient (IQ) estimated using Wechsler Test of Adult Reading (WTAR‐FSIQ), early life education, and a socio‐behavioral proxy of cognitive reserve which is a composite of early life education, WTAR‐FSIQ, and items from the lifetime experience questionnaire including career attainment and cognitively stimulating leisure activities
| Comparison (n = 102) | Intervention (n = 383) | |
|---|---|---|
| Age | ||
| Mean (SD) | 63.2 (6.7) | 59.6 (6.6) |
| Median [Q1, Q3] | 64.0 [58.0, 68.0] | 59.0 [54.0, 64.0] |
| Gender | ||
| Female | 66 (64.7%) | 269 (70.2%) |
| Male | 36 (35.3%) | 114 (29.8%) |
| Academic course load (% of 1‐year FTE load)a | ||
| Mean (SD) | ‐ | 145 (123) |
| Median [Q1, Q3] | ‐ | 113 [50, 206] |
| WTAR‐FSIQb | ||
| Mean (SD) | 112 (4.9) | 112 (5.7) |
| Median [Q1, Q3] | 114 [109, 116] | 114 [110, 116] |
| Early life education (years) | ||
| Mean (SD) | 11.0 (1.2) | 11.3 (1.0) |
| Median [Q1, Q3] | 12.0 [10.0, 12.0] | 12.0 [10.0, 12.0] |
| Prior cognitive reserve (z) | ||
| Mean (SD) | –0.17 (1.05) | 0.04 (0.96) |
| Median [Q1, Q3] | 0.02 [–0·76, 0·57] | 0.05 [–0.48 0.73] |
aCumulative from baseline to 2019; FTE: full‐time or equivalent . bWechsler Test of Adult Reading, estimated full‐scale intelligence quotient and memory.
2.2. Procedures
Assessments were completed annually on a battery of cognitive tests (outlined in Table 2) for the first 36 months, and then every 2 years up to 7 years from baseline (six assessments in total have been included in this study). Tests were conducted using pen and paper or computer, as appropriate, under the supervision of trained research assistants in Hobart and Launceston, Tasmania, and verified by a clinical neuropsychologist (MJS) who provided feedback to participants at each assessment phase.
TABLE 2.
Sub‐set of THBP cognitive test battery19 assessed in this study
| Label | Instrument | Cognitive function/s assessed |
|---|---|---|
| BNT | Boston Naming Test | Verbal confrontation naming; language fluency |
| COWAT | Controlled Oral Word Association Test | Letter verbal fluency; verbal executive function |
| LM I & II | Logical Memory I & II | Immediate and delayed recall of verbal prose passages; verbal episodic memory |
| PAL ftm | Paired Associates Learning (first trial memory score) | Immediate recall of visual information; visual episodic memory |
| PAL te6 | Paired Associates Learning (total errors, six shapes) | Recall of visual information on six shapes trial; visual episodic memory recall |
| RAVLT rcl | Rey Auditory Verbal Learning Test (recall) | Immediate recall of verbal word lists; verbal episodic memory recall |
| RAVLT tot | Rey Auditory Verbal Learning Test (total) | Learning of verbal word lists; verbal episodic learning capacity |
| RCFT | Rey Complex Figure Test | Immediate recall of complex geometric design; visual episodic memory recall |
| RVP‐A | Rapid Visual Information Processing (A) | Visual sustained attention and signal detection sensitivity; visual executive function |
| SSP length | Spatial span (length) | Visual immediate memory span; visual short‐term memory capacity |
| STROOP C time | Stroop color (time) | Verbal information processing speed and impulse control; executive function |
| SWM be | Spatial working memory | Visual working memory capacity |
| TMT‐B | Trail Making Test (B) | Visuo‐motor information processing speed; executive function |
| WAIS comp | Wechsler Adult Intelligence Scale, 3rd edition (comprehension subtest) | Capacity to use language to express ideas and understand verbal communication; language capacity |
| WAIS ds | Wechsler Adult Intelligence Scale, 3rd edition (digit span subtest) | Verbal immediate memory span; verbal short‐term memory capacity |
| WAIS lns | Wechsler Adult Intelligence Scale, 3rd edition (letter‐number sequence subtest) | Verbal working memory capacity |
| WAIS voc | Wechsler Adult Intelligence Scale, 3rd edition (vocabulary subtest) | Word recognition and capacity to define words; language capacity |
Written informed consent was obtained from all participants at each assessment. Approval was granted by the University of Tasmania Human Research Ethics Committee (H11070/H18265) in accordance with the National Statement on Ethical Conduct in Human Research (National Health and Medical Research Council of Australia).
2.3. Outcome measures
A subset of cognitive tests from the full THBP battery was chosen for this study based on sensitivity to early cognitive decline across a range of functional cognitive domains. These are outlined in Table 2. Raw test scores (ie, not adjusted using age and gender normatives) were standardized to z‐scores using the formula , where is the ith score for cognitive test instrument t and , and are the mean and standard deviation for cognitive test instrument t. This puts scores from each test instrument on a comparable scale. Trail‐making (TMT) and Stroop tests were first ‐transformed and reversed, and paired associates learning (PAL te6) scores were transformed using the formula to improve the normality of residuals and correct sign inconsistencies across instruments.
2.4. Covariates
The benefit of later‐life education is potentially confounded by early life education and other cognitively stimulating activities, so a socio‐behavioral proxy of prior (baseline) CR was included as a covariate. The Wechsler Test of Adult Reading (WTAR‐FSIQ)21 and the Lifetime Experience Questionnaire (LEQ)10 were administered at baseline. The LEQ assesses participant educational and occupational attainment, along with cognitively stimulating and neuroprotective leisure activities. Weighted scores for items loading on a principal component across LEQ and WTAR‐FSIQ were summed using the formula below, following a previously described protocol,20
There is evidence that CR moderates the steepness of cognitive aging trajectories (although this is contested: see22 and23) and retest practice effects,24 so we included the prior CR variable as a time‐varying covariate.
Total university study completed before the sixth assessment was ascertained from academic records. Each unit of undergraduate study was measured as a proportion of 1 year full‐time or equivalent (FTE) study load, where a typical unit is weighted at 12.5% of a 1‐year FTE study load.
2.5. Statistical methods
All data‐handling and statistical analysis were conducted in the R (v3.6) statistical computing environment.25 Five participants had incomplete surveys at baseline such that prior CR scores could not be computed. CR for these participants was estimated using single imputation with years of education and WTAR‐FSIQ as linear predictors.
Conditional likelihoods were estimated using the lme4 26 and glmmTMB27 packages for linear mixed‐effects models. Time was modeled as a continuous variable (“Time”) in years since baseline. Intervention group membership was encoded as a dummy variable (“group”). Data were in long‐form, with a single column "Score" storing the z‐scores for each test, and another column “test” denoting the cognitive test instrument. For models estimating trajectories conditional on cognitive test instrument (“test”), by‐participant random coefficients were fitted for each instrument. Random slopes were not included in these models due to convergence issues. The formula for the adjusted model presented in Table S1 and Figure 1 is,
The formula for the (adjusted) by‐participant random intercept and slope models presented in Table 4 and Figure 2 is,
This model estimated by‐participant slopes (which capture individual differences in practice effects) and population‐level differences in these slopes between university study and comparison groups, adjusted for age and CR at baseline.
Note that the short‐hand notation for an interaction of the form A * B * C expands to all main effects and lower‐order interactions by convention in R, and the random effects structure is given in parentheses. The assumptions of linearity, homogeneity of variance, and normality of residuals were assessed using standard graphical methods, and were judged to be acceptable. Reproducible R code is provided at28 and all estimated parameters are reported in Table 4 and the Supplementary Tables.
TABLE 4.
Unadjusted and adjusted (for age and prior CR) linear mixed‐effects models assessing differences in trajectories of intervention group relative to the comparison group
| Score (z) | Score (z) | |||||
|---|---|---|---|---|---|---|
| Predictors | Estimates | 95% CI | P | Estimates | 95% CI | P |
| (Intercept) | –0.239 | –0.338, –0.141 | <.001 | –0.199 | –0.288, –0.109 | <.001 |
| Time (years) | 0.013 | 0.002, 0.023 | .016 | 0.044 | 0.032, 0.056 | <.001 |
| Group | 0.170 | 0.059, 0.281 | .003 | 0.035 | –0.068, 0.138 | .508 |
| Time × group | 0.021 | 0.009, 0.033 | .001 | 0.020 | 0.008, 0.032 | .001 |
| Age (years) | –0.208 | –0.251, –0.165 | <.001 | |||
| Prior CR (z) | 0.143 | 0.100, 0.185 | <.001 | |||
| Prior CR x time | 0.002 | –0.004, 0.007 | .499 | |||
| Random effects | ||||||
| σ2 | 0.73 | 0.73 | ||||
| τ00 | 0.24 Participant | 0.19 Participant | ||||
| τ11 | 0.00 Participant·Time | 0.00 Participant·Time | ||||
| ρ01 | 0.53 Participant | 0.29 Participant | ||||
| ICC | 0.26 | 0.22 | ||||
| N | 485 Participant | 485 Participant | ||||
| Observations | 37291 | 37291 | ||||
| Marginal R2/conditional R2 | 0.012/0.272 | 0.061/0.265 | ||||
To assess group differences in cognitive performance at entry into the study, Bayesian regression analysis was used to compare group means of cognitive tests scores, adjusted for age and CR (these covariates were also included in the primary analysis). Weakly informative student‐t priors were specified, and Bayes factors were computed with bridge‐sampling using the brms29 package in R. Unlike p‐values, Bayes factors allow a conclusion in favor of the null hypothesis (“no difference in cognitive test scores between groups at baseline”) to be drawn if supported by evidence. A concern is whether participants with lower test scores were more likely to drop out depending on whether they were in the university study group or comparison group. To test this we fitted a logistic regression model to estimate the expected probability of remaining in the study at year 5 conditional on the interaction between test scores (we used RAVLT scores) and intervention group.
3. RESULTS
3.1. Participants
Over 7 years (including up to six assessments per participant) of the project to 2019, this study included 2084 assessments and 37291 test scores (Table 3). At the time of data analysis, only 149 assessments had been completed for the 7th year assessments, which are ongoing, and delayed due to coronavirus disease 2019 (COVID‐19) restrictions. To the best of our knowledge, these restrictions did not discriminate against any part of our cohort, since they applied to all participants. The most commonly cited reason for withdrawing from the study was moving from Tasmania. Comparison group participants were significantly more likely to withdraw, with 112 leaving from the intervention group and 44 from the comparison group (χ2 = 6.5, P = .01); however, the proportion at each assessment varied little (Table 3). Nine participants were known to be deceased.
TABLE 3.
Participant characteristics at each assessment (years since baseline)
| Years since baseline | ||||||
|---|---|---|---|---|---|---|
| 0 (n = 485) | 1 (n = 388) | 2 (n = 393) | 3 (n = 361) | 5 (n = 308) | 7 (n = 149a) | |
| Age (years) | ||||||
| Mean (SD) | 60.3 (6.8) | 61.7 (6.8) | 62.7 (6.6) | 63.5 (6.7) | 65.2 (6.6) | 68.4 (6.5) |
| Age group | ||||||
| 50‐59 years | 233 (48.0%) | 157 (40.5%) | 135 (34.4%) | 117 (32.4%) | 77 (25.0%) | 20 (13.4%) |
| 60‐69 years | 205 (42.3%) | 179 (46.1%) | 195 (49.6%) | 170 (47.1%) | 144 (46.8%) | 55 (36.9%) |
| 70‐79 years | 47 (9.7%) | 52 (13.4%) | 61 (15.5%) | 72 (19.9%) | 81 (26.3%) | 69 (46.3%) |
| 80+ years | 0 (0%) | 0 (0%) | 2 (0.5%) | 2 (0.6%) | 6 (1.9%) | 5 (3.4%) |
| Gender | ||||||
| Female | 335 (69.1%) | 262 (67.5%) | 265 (67.4%) | 243 (67.3%) | 208 (67.5%) | 108 (72.5%) |
| Male | 150 (30.9%) | 126 (32.5%) | 128 (32.6%) | 118 (32.7%) | 100 (32.5%) | 41 (27.5%) |
| Group | ||||||
| Comparison | 102 (21.0%) | 82 (21.1%) | 85 (21.6%) | 76 (21.1%) | 63 (20.5%) | 40 (26.8%) |
| Intervention | 383 (79.0%) | 306 (78.9%) | 308 (78.4%) | 285 (78.9%) | 245 (79.5%) | 109 (73.2%) |
| Prior cognitive reserve (z) | ||||||
| Mean (SD) | –0.003 (0.977) | 0.019 (0.978) | 0.055 (0.961) | 0.017 (0.952) | –0.034 (0.949) | 0.093 (0.826) |
Assessments completed at time of analysis.
Participants in the intervention group completed a median (IQR) of 112.5% (50, 206) of 1‐year full‐time equivalent (FTE) study load (9 units). There were no discernable differences in groups on WTAR‐FSIQ (mean IQ 112 for both groups), or early life education (median 12 years in both groups). At baseline, comparison group participants were older, with mean age of 63.2 years (SD 6.7 years) compared to mean age of 59.6 years (SD 6.6 years) for the intervention group, and had slightly lower prior CR z‐scores (−.17 SD compared to .04 SD). Bayesian regression analysis (adjusted for age and prior CR) showed that there were no differences in mean cognitive test scores between groups at entry into the study (β = .05, 95% confidence interval (CI) .06, .16), , which is strong evidence in favor of the null). Neither group was significantly more likely to remain in the study at year 5 depending on Rey Auditory Verbal Learning Test (RAVLT) scores at baseline (P = .87).
3.2. Average of all cognitive tests
Aggregated over all cognitive test instruments, there was a significant time x group interaction after adjusting for age, prior CR, and a prior CR × time interaction (P = .001). Participants in the intervention group had a z = .02 SD [95% CI .01,−.03] per year increase in standardized scores relative to comparison group (Table 4). These estimates were identical to three significant digits in an unadjusted analysis (Table 4). After accounting for the group‐level (fixed) effects of time and the group × time interaction, and by‐participant (random) intercepts, there was little individual variation in slopes.
3.3. Group differences on specific cognitive tests
There was a significant time × group × test interaction (P = .016), suggesting that relative to the comparison group, intervention group trajectories differed across tests. Analysis of deviance statistics for the adjusted and unadjusted models, and a detailed table of coefficients and their CIs are reported in Table S1. Post hoc analysis using estimated marginal mean trends showed that the significantly different group trajectories were for Boston Naming Test (BNT) ( = −.043 SD [standard error (SE) .017], P = .009), RAVLT recall ( = −.041 SD [SE .016], P = .012), RAVLT total ( = −.056 SD [SE .016], P < .001), Wechsler Adult Intelligence Scale (WAIS) comprehension ( = −.038 SD [SE .016], P = .022), and spatial span (SSP) length ( = .040 SD [SE .017], P = .017). All differences favored the intervention group, with the exception of SSP length. Broadly, the greatest differences were observed on those tests that displayed the weakest practice effects over repeated tests (illustrated in Figure 1, and Table S2, which shows the estimated marginal mean time‐trends and post hoc contrasts).
FIGURE 1.
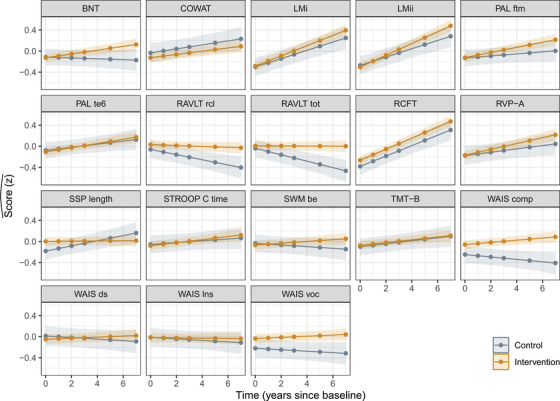
Estimated cognitive trajectories (with 95% confidence intervals [CIs]) over years since baseline for intervention group participants (those who undertook university study) and the comparison group (those who did not), holding age at entry into study and cognitive reserve (CR) at their respective means. The greatest group differences appear in tests where comparison group participants did not appear to benefit from re‐test practice effects: tests of verbal memory, vocabulary, and comprehension
3.4. Age
Participant age had a significant effect on cognitive trajectories (P < .001), with a 1 SD (≈7 year) increase in age‐reducing standardized cognitive test slopes by z = −.027 SD [95% CI −.037, −.017]. The estimated annualized difference between intervention and comparison group participants was z = .009 SD [− .002, −.02] per year of participant age, but this was not statistically significant (P = .122; Figure 2, Table S3).
FIGURE 2.
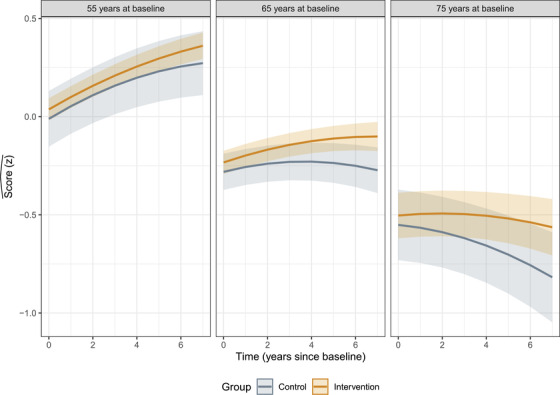
Estimated mean cognitive trajectories (with 95% confidence intervals [CIs]) over the years since baseline for intervention group participants (those who undertook university study) and the comparison group (those who did not), holding prior cognitive reserve (CR) as the mean value. Panels show expected trajectories for participants at 55, 65, and 75 years of age at entry into the study. Re‐test practice effects are most evident in the panel showing expected trajectories for someone entering the study at age 55. Older participants benefited from re‐test practice effects the least and appeared to gain the most from later‐life university education. The model decomposes age and re‐test time (in years since first assessment) as separate effects, so estimated trajectories are quadratic
3.5. Academic load
Aggregated over all test instruments, the dose‐dependent effect of academic load was estimated to be z = .004 SD [95% CI −.0004, .0094] per year, but this was not a statistically significant interaction (P = .07). Cognitive trajectories on different test instruments did not vary significantly with academic load. There was a significant main effect of prior CR (z = .121 SD [.033, −.209], P < .001), but the effect of CR on cognitive trajectories was not statistically significant (z = .004 SD [−.006, .013], P = .125) and did not appear to benefit comparison or intervention groups differentially (z = .001 SD [−.010, .013], P = .821).
4. DISCUSSION
The current study shows that later‐life university study improved cognitive trajectories when compared with participants who did not undertake university study. The benefits of university study were greatest in tests on the domains of verbal memory, verbal episodic memory, and language processing. These tests also had the weakest practice effects (evidenced by estimated marginal time‐trends for the comparison group). Older participants benefited the least from practice effects and the most from the intervention, particularly on verbal learning, memory, naming, and comprehension tests. Notably, tests that relate to executive function and visuo‐structural memory showed no benefit relative to university study.
This was a purposeful longitudinal study by design, focused on a real‐world intervention and formal tertiary‐level education, and follows a substantial number of studies indicating low levels of educational attainment early in life as a known risk‐factor for dementia.30 With comprehensive neuropsychological data at the mid‐point of the study, the current results indicate that engagement in education by older adults provides a protective benefit for specific areas of cognitive function.
Previous assessments of cognitive trajectories in the THBP have used factor‐derived composite scores of cognitive function.16, 17 For this analysis, we chose to report standardized scores on test instruments used in the THBP assessment battery. Shared and un‐shared test variance was partitioned through the model structure to estimate conditional likelihoods for each group and the characteristic trajectories of each test, giving a detailed and directly comparable picture of expected cognitive trajectories on often‐used cognitive tests. Substantial variation in trajectories on cognitive test instruments was demonstrated, along with variation in the estimated difference between intervention and comparison groups on these trajectories. There is established evidence that age‐related cognitive change is not universal across all cognitive domains.1 The domains on which the intervention group performed most strongly—verbal fluency and episodic memory—are domains that have been found previously to be most vulnerable to age‐related decline.15 For tests on other domains, there was little difference in trajectories between intervention and comparison groups, with both groups appearing to benefit from familiarity with the tests. These re‐test practice effects did not appear to be moderated by prior CR, which appeared to benefit only the level of age‐adjusted cognitive test performance, rather than the slope (similar to other findings for early life education as a proxy of CR; see23 and22 for reviews). A plausible interpretation is that later‐life education has a preservation or compensatory effect, rather than augmenting that which was not lost. Further work could explore potential differences between early and later‐life education in building CR, and how that relates to biological processes of development and aging.
The cognitive tests in the battery were not designed to be administered repeatedly over time, although, to minimize practice effects, some of these instruments included alternative forms. Although we anticipated that the relatively long inter‐trial intervals (12 and 24 months) would minimize practice effects, it is apparent that some tests (eg., logical memory, Rey complex figure) had substantial practice effects. These were also the tests where the least apparent benefit of later‐life education was observed. For statistical adjustment, we assumed that these practice trajectories were linear, but power‐law or non‐linear location‐scale models31 might be more appropriate as participants start to hit the practice effect ceiling.
Adults in mid‐life and post‐retirement form a growing cohort who are engaging in university‐level education32 around the world. This has included retirement complexes established on college/university grounds, with the capacity for older adults to engage in education on an audit basis. With respect to the current study results, it is unknown what dimension of university study is important (eg, lectures, tutorial or practical sessions, assessments, social interaction), or whether the sum of these experiences provides the benefit. Structured multi‐modal interventions have been the focus of many randomized‐controlled trials (FINGER and Disability and Multidomain Alzheimer Preventive Trial), resulting in no33 or small effect size14 improvements in cognitive function, with none yet showing mitigation of cognitive decline. These studies have been limited in duration, with subjects the relatively passive recipients of intervention. The key limitation of the current study is the lack of randomization to "treatment." This was a pragmatic design, balancing practical and ethical considerations against internal validity and generalizability. This raises questions about whether participants who opted into the intervention arm of the trial had greater capacity to undertake or benefit from later‐life education. Although our groups did not differ at baseline on mean cognitive test scores, or on other measures such as anxiety, depression, and CR,16 this does not rule out the existence of other unmeasured resources and capacities that differed between groups. For example, there is a growing body of evidence that perceived control and self‐efficacy are associated with cognitive test performance.34 It is plausible that participants with higher baseline self‐efficacy who chose to participate in university education and differences in cognitive test performance over time were correlated with this unmeasured selection bias.
The study is limited to an individual island community, with most Tasmanians being of predominantly European ancestry. Generalizability of findings to other populations would require further study. Conversely, this also likely supports the case‐control feature of the study, as both comparison and intervention groups would have more uniform life experiences. As a turn‐key intervention that is amenable to inclusion in public health measures relevant to aging‐related cognitive decline, accessibility is an important factor. Participants in the THBP had subsidized course fees, and Australian course fees are relatively low for domestic students. University course fees vary substantially around the world, to being mostly free or low‐cost in many European countries to costly in high‐prestige US tertiary institutions. Although university study also usually requires access to additional resources, such as location close to a provider and computer/internet access, the COVID‐19 pandemic has also meant that most university providers have developed comprehensive online offerings, which may improve access to a broader range of potential participants.
We have previously demonstrated that age alone had no impact on academic achievement (grade point average) in this cohort.35 Despite flatter cognitive trajectories overall, older participants appeared to obtain relatively more benefit from later‐life university study than younger participants. Although these estimates were not statistically significant, they suggest that increasing age does not necessarily diminish the benefits conferred from later‐life education.
There has been no qualitative analysis of participant experiences in the THBP; however, anecdotal reports from participants suggest that many have enjoyed being able to study without career expectations or the pressures that normally accompany higher education. The freedom to choose an area of study may be an important factor, both for subjective well‐being, and to encourage ongoing compliance and motivation to engage with the intervention over a duration of years. In addition, social aspects of the on‐campus study experience may contribute to subjective well‐being and cognitive health, albeit we have previously shown no differences in social networks between intervention and comparison groups.16 Collectively, the current study supports the value of a complex, real‐world intervention in the form of engagement in university study to attenuate decline in specific cognitive domains. The long‐term goal of the THBP over 15 years is to collect extended longitudinal data from a single cohort to examine whether additional education later in life is associated also with reduced risk of dementia. The long‐term nature of the THBP, as well as the relatively high retention rate of subjects, will determine if there is a subsequent mitigation of risk for significant cognitive decline and dementia.
AUTHOR CONTRIBUTIONS
Aidan D. Bindoff conducted statistical analysis, wrote the first draft, and finalized the final draft. Mathew J. Summers designed the study, recruited participants, conducted neuropsychological assessments, and contributed to interpretation. Edward Hill contributed to data collection and literature review. Jane Alty contributed to interpretation. James C. Vickers designed the study, recruited participants, and contributed to interpretation. All authors contributed to the writing and review of the article.
CONFLICTS OF INTEREST
Mathew J. Summers and James C. Vickers were awarded National Health and Medical Research Council (NHMRC) grant funding to their institutions for the current work. Aidan D. Bindoff, Mathew J. Summers, Edward Hill, Jane Alty, and James C. Vickers were awarded NHMRC grant funding to their institutions outside of the current work. Mathew J. Summers was awarded European Commission Horizon 2020 grant funding to his institution outside of the current work. James C. Vickers was awarded Medical Research Futures Fund, FightMND, and Royal Hobart Hospital Research Foundation grant funding to his institution outside of the current work. James C. Vickers has also served as an unpaid board member to Glenview Community Services and the Dementia Australia Research Foundation. Jane Alty was paid royalties for medical textbooks, and honoraria, accommodation and speaking fees paid to herself within the last 36 months, unrelated to the current work.
Supporting information
Supporting material
ACKNOWLEDGMENTS
We wish to acknowledge the J.O. and J.R. Wicking Trust (Equity Trustees) and the National Health and Medical Research Council (2011‐2020, Australia) for funding this ongoing study. The J.O. and J.R. Wicking Trust (Equity Trustees) provided funding for database support and salary for ADB. The National Health and Medical Research Council (2011‐2020, Australia) provided funding to support participant recruitment, data collection, analysis. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Bindoff AD, Summers MJ, Hill E, Alty J, Vickers JC. Studying at university in later life slows cognitive decline: A long‐term prospective study. Alzheimer's Dement. 2021;7:e12207. 10.1002/trc2.12207
DATA AVAILABILITY STATEMENT
De‐identified data analyses and study protocols may be shared by contacting the corresponding author. Reproducible statistical analysis code examples are available at https://github.com/ABindoff/thbp_7years.
REFERENCES
- 1.Ritchie SJ, Tucker‐Drob EM, Cox SR, et al. Predictors of ageing‐related decline across multiple cognitive functions. Intelligence. 2016;59:115‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisiacchi PS, Borella E, Bergamaschi S, Carretti B, Mondini S. Interplay between memory and executive functions in normal and pathological aging. J Clin Exp Neuropsychol. 2008;30:723‐733. [DOI] [PubMed] [Google Scholar]
- 3.Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans: results from the AHEAD sample. Res Aging. 2007;29:73‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewster PW, Melrose RJ, Marquine MJ, et al. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. 2014;28:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271:1004‐1010. [PubMed] [Google Scholar]
- 6.Yu J‐T, Xu W, Tan C‐C, et al. Evidence‐based prevention of Alzheimer's disease: systematic review and meta‐analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:1201‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18:223‐254. [DOI] [PubMed] [Google Scholar]
- 9.Cutler DM, Lleras‐Muney A, Vogl T. Socioeconomic Status and Health: Dimensions and Mechanisms. National Bureau of Economic Research; 2008. [Google Scholar]
- 10.Valenzuela MJ, Sachdev P. Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ). Psychol Med. 2007;37:1015‐1025. [DOI] [PubMed] [Google Scholar]
- 11.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PloS One. 2013;8:e61624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelinski EM, Spina LM, Yaffe K, et al. Improvement in memory with plasticity‐based adaptive cognitive training: results of the 3‐month follow‐up. J Am Geriatr Soc. 2011;59:258‐265. [DOI] [PubMed] [Google Scholar]
- 14.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255‐2263. [DOI] [PubMed] [Google Scholar]
- 15.Bäckman L, Wahlin Å, Small BJ, Herlitz A, Winblad B, Fratiglioni L. Cognitive functioning in aging and dementia: the Kungsholmen Project. Aging Neuropsychol Cogn. 2004;11:212‐244. [Google Scholar]
- 16.Thow ME, Summers MJ, Saunders NL, Summers JJ, Ritchie K, Vickers JC. Further education improves cognitive reserve and triggers improvement in selective cognitive functions in older adults: the Tasmanian Healthy Brain Project. Alzheimer's Dement. 2018;10:22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward DD, Summers MJ, Valenzuela M, et al. Associations of later‐life education, the BDNF Val66Met polymorphism and cognitive change in older adults. J Prev Alzheimer's Dis. 2020;7:37‐42. [DOI] [PubMed] [Google Scholar]
- 18.Ward DD, Andel R, Saunders NL, et al. The BDNF Val66Met polymorphism moderates the effect of cognitive reserve on 36‐month cognitive change in healthy older adults. Alzheimer's Dement. 2017;3:323‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summers MJ, Saunders NL, Valenzuela MJ, et al. The Tasmanian Healthy Brain Project (THBP): a prospective longitudinal examination of the effect of university‐level education in older adults in preventing age‐related cognitive decline and reducing the risk of dementia. Int Psychogeriatr. 2013;25:1145‐1155. [DOI] [PubMed] [Google Scholar]
- 20.Ward DD, Summers MJ, Saunders NL, Vickers JC. Modeling cognitive reserve in healthy middle‐aged and older adults: the Tasmanian Healthy Brain Project. Int Psychogeriatr. 2015;27:579‐589. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale—Third edition. San Antonio, TX: The; 1997. [Google Scholar]
- 22.Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Relationship between education and age‐related cognitive decline: a review of recent research. Psychogeriatrics. 2015;15:154‐162. [DOI] [PubMed] [Google Scholar]
- 23.Seblova D, Berggren R, Lövdén M. Education and age‐related decline in cognitive performance: systematic review and meta‐analysis of longitudinal cohort studies. Ageing Res Rev. 2020;58:101005. [DOI] [PubMed] [Google Scholar]
- 24.Rapport LJ, Brines DB, Theisen ME, Axelrod BN. Full scale IQ as mediator of practice effects: the rich get richer. Clin Neuropsychol. 1997;11:375‐380. [Google Scholar]
- 25.R Core Team . R: A Language and Environment for Statistical Computing. 3.6. ed: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 26.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed‐effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 27.Brooks ME, Kristensen K, van Benthem KJ, et al. glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. R J. 2017;9:378‐400. [Google Scholar]
- 28.Bindoff AD. 2021. https://github.com/ABindoff/thbp_7years
- 29.Bürkner P‐C. Advanced Bayesian multilevel modeling with the R package brms. R J. 2018;10. [Google Scholar]
- 30.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 31.Williams DR, Zimprich DR, Rast P. A Bayesian nonlinear mixed‐effects location scale model for learning. Behav Res Methods. 2019;51:1968‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isopahkala‐Bouret U. Benefits of higher education in mid‐life: a life course agency perspective. J Adult Continuing Educ. 2017;23:15‐31. [Google Scholar]
- 33.Andrieu S, Guyonnet S, Coley N, et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurol. 2017;16:377‐389. [DOI] [PubMed] [Google Scholar]
- 34.Windsor TD, Anstey KJ. A longitudinal investigation of perceived control and cognitive performance in young, midlife and older adults. Aging Neuropsychol Cogn. 2008;15:744‐763. [DOI] [PubMed] [Google Scholar]
- 35.Imlach A‐R, Ward DD, Stuart KE, et al. Age is no barrier: predictors of academic success in older learners. npj Sci Learn. 2017;2:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material
Data Availability Statement
De‐identified data analyses and study protocols may be shared by contacting the corresponding author. Reproducible statistical analysis code examples are available at https://github.com/ABindoff/thbp_7years.


