Summary
Background
Kidney function assessment by estimated glomerular filtration rate (eGFR) equations, such as the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation, is important to determine dosing and eligibility for anticancer drugs. Inclusion of race in eGFR equations calculates a higher eGFR at a given serum creatinine concentration for Black patients versus non-Black patients. We aimed to characterise the effect of removing race from the CKD-EPI equation on dosing and eligibility of anticancer drugs with kidney function cutoffs.
Methods
We did a retrospective analysis of patients enrolled in phase 1 studies sponsored by the Cancer Therapy Evaluation Program between January, 1995, and October, 2010. eGFR based on creatinine (eGFRCr) was calculated by the CKD-EPI equation and a version of the CKD-EPI equation without the race term (CKD-EPIwithout race). Estimated creatinine clearance (eClCr) was calculated by the Cockcroft-Gault equation. Dosing simulations based on each assessment of kidney function were done for ten anticancer drugs with kidney function cutoffs for dosing (oxaliplatin, capecitabine, etoposide, topotecan, fludarabine, and bleomycin) or eligibility (cisplatin, pemetrexed, bendamustine, and mitomycin) based on labelling approved by the US Food and Drug Administration or consensus guidelines. The absolute proportion of patients eligible or in each renal dosing range was calculated for each drug. Eligibility and dosing discordance rates were also calculated.
Findings
Demographics and laboratory values from 340 Black patients (172 men and 168 women) were used. Median age was 57 years (IQR 47–64), median bodyweight was 78·1 kg (67·0–89·8), median body surface area was 1·91 m2 (1·77–2·09), and median serum creatinine concentration was 0·9 mg/dL (0·8–1·1). Median eGFRCr or eClCr was 103 mL/min (85–122) calculated by CKD-EPI, 89 mL/min (73–105) by CKD-EPIwithout race, and 90 mL/min (72–120) by Cockcroft-Gault. Black patients were recommended to receive dose reductions or were rendered ineligible to receive drug more frequently when using CKD-EPIwithout race than when using CKD-EPI, but at a similar rate as when using Cockcroft-Gault. The number of patients ineligible for therapy or recommended to receive any renal dose adjustment when CKD-EPIwithout race versus CKD-EPI was used increased by 72% (from 25 of 340 to 43 of 340 patients) for cisplatin, by 120% (from five to 11) for pemetrexed, by 67% (from three to five) for bendamustine, by 150% (from ten to 25) for capecitabine, by 150% (from ten to 25) for etoposide, by 67% (from three to five) for topotecan, by 61% (from 74 to 119) for fludarabine, and by 163% (from eight to 21) for bleomycin. Up to 18% of patients had discordant recommendations using CKD-EPIwithout race versus CKD-EPI.
Interpretation
Removing race from the CKD-EPI equation will calculate a lower eGFR for Black patients and exclude more patients from receiving anticancer therapy, which could lead to undertreatment of Black patients with cancer and adversely affect their outcomes.
Funding
National Institutes of Health.
Introduction
Accurate assessment of kidney function is crucial in oncology for informing decisions regarding anticancer drug dosing and eligibility.1 Glomerular filtration rate (GFR) is widely accepted as the most accurate measure of kidney function and is typically estimated using serum creatinine-based formulae. The Cockcroft-Gault equation has been used in clinical oncology to assess kidney function for several decades.2,3 However, the accuracy of estimates of kidney function based on Cockcroft-Gault is limited by the equation’s use of estimated creatinine clearance (eClCr) as a surrogate for GFR, derivation from a small and non-diverse study population (249 patients from a Canadian Veterans’ Hospital), and the inability to be re-expressed with standardised serum creatinine values. The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) study equation is a more precise formula for estimated GFR (eGFR) and is currently recommended for the assessment of kidney function in cancer patients by the Kidney Disease Improving Global Outcomes (KDIGO) guideline group.4,5
The CKD-EPI equation includes a race term that calculates a 15·9% higher eGFR for Black patients than for non-Black patients, reflecting the higher measured GFR observed in Black versus non-Black patients of a similar age, the same sex, and similar serum creatinine concentration.5 Over the past year, inclusion of race in eGFR equations has been questioned, prompting discussion of the consequences of both the inclusion and exclusion of race.6–9 A joint National Kidney Foundation–American Society of Nephrology task force on reassessing the inclusion of race in diagnosing kidney diseases has been convened and is critically evaluating the issue.6
Black patients in the USA are disproportionately affected by cancer, as evidenced by disparities in both incidence and outcomes. For example, 460·4 new cancer cases per 100 000 population and 186·4 cancer-related deaths per 100 000 population are observed in Black patients per year, compared with 448·4 new cases per 100 000 and 158·2 deaths per 100 000 in the total population.10 As such, Black patients are particularly susceptible to undertreated disease, and optimal anticancer drug use is paramount. The method of kidney function assessment, and specifically whether to include or exclude race from eGFR equations, has important implications for anticancer drug eligibility and dosing in Black patients. In the current analysis, we assess the effects of including or excluding race in CKD-EPI-derived estimates of kidney function on patient dosing and eligibility recommendations for ten clinically utilised anticancer agents with kidney function cutoffs. We also compare the results with Cockcroft-Gault-based recommendations, because Cockcroft-Gault remains widely used in oncology practice to inform drug dosing and eligibility.
Methods
Study design and data sources
The dataset used in this study was extracted from the National Cancer Institute Theradex database and has been described previously.3 It includes patients enrolled in single-agent, adult phase 1 studies sponsored by the Cancer Therapy Evaluation Program between 1979 and October, 2010, and was not filtered on the basis of cancer type or other clinical characteristics. All patients provided written informed consent, and all trials were approved by institutional review boards. Eligibility criteria were trial-specific; they ensured that enrolled patients were suitable for participation in phase 1 trials and typically included criteria such as serum creatinine concentration of 1·5 mg/dL or lower or eClCr of 60 mL/min or higher, and Eastern Cooperative Oncology Group performance status of 0–1. The dataset included demographic information, physical measurements, and pretreatment serum creatinine values. Race recorded in the dataset originated from patient records, which was self-reported and entered into the chart by clinical staff. Race was available for patients from 1995 onwards and therefore only patient data from January, 1995, to October, 2010, were included in the current analysis. Patients were excluded from the analysis if race, units of bodyweight measurement, or height data were not available or if height was recorded as less than 100 cm.
Estimation of kidney function
Kidney function was estimated by eGFR based on creatinine (eGFRCr), calculated by the 2009 CKD-EPI equation with and without race (CKD-EPI and CKD-EPIwithout race), and eClCr calculated by the Cockcroft-Gault equation.2,5 The CKD-EPI equation is:
The CKD-EPIwithout race equation removes the race term from this equation:
For both CKD-EPI equations with and without race, κ is 0·7 for female patients and 0·9 for male patients, and α is −0·329 for female patients and −0·411 for male patients. The Cockcroft-Gault equation is:
In all three equations, SCr is serum creatinine concentration in mg/dL and age is in years, and in the Cockcroft-Gault equation, bodyweight is in kg. Although the CKD-EPI equation is currently recommended for use in patients with cancer,4 we included Cockcroft-Gault as a comparator because it is still widely used in oncology practice. Actual bodyweight was used to calculate the Cockcroft-Gault equation. Body surface area-indexed eGFR (ie, eGFR reported in mL/min per 1·73 m2, the standard CKD-EPI output) was converted to absolute or de-indexed eGFR (ie, eGFR reported in mL/min) by multiplying eGFR by patient body surface area divided by 1·73 m2. Patients were classified by chronic kidney disease stage for each estimate of kidney function, as per KDIGO guidelines: stage 1 (eGFRCr or eClCr ≥90 mL/min), stage 2 (eGFRCr or eClCr 60–89 mL/min), stage 3a (eGFRCr or eClCr 45–59 mL/min), stage 3b (eGFRCr or eClCr 30–44 mL/min), stage 4 (eGFRCr or eClCr 15–29 mL/min), or stage 5 (eGFRCr or eClCr <15 mL/min).11
Drug dosing simulations
A simulation study was done to compare dosing and eligibility recommendations for several anticancer drugs based on eGFRCr calculated by CKD-EPI and CKD-EPIwithout race and eClCr calculated by Cockcroft-Gault. Drugs with kidney function-based eligibility recommendations and their respective cutoffs included: cisplatin (<60 mL/min), pemetrexed (<45 mL/min), bendamustine (<40 mL/min), and mitomycin (<30 mL/min). Drugs with renal dosage adjustment recommendations and the corresponding number of dosing ranges included: oxaliplatin (two), capecitabine (three), etoposide (three), topotecan (three), fludarabine (four), and bleomycin (six). With the exception of cisplatin, for which eligibility was based on consensus guidelines,12 renal dosing and eligibility recommendations were based on package inserts retrieved from Drugs@FDA.
The absolute proportion of patients eligible for or in each renal dosing range for each drug was calculated. Eligibility discordance (ie, eligible to receive drug with CKD-EPI, but ineligible with CKD-EPIwithout race; or eligible to receive drug by either Cockcroft-Gault or CKD-EPIwithout race, but not both) and dosing discordance (ie, recommended to receive a lower dose with CKD-EPIwithout race vs CKD-EPI, or recommended to receive a different dose with CKD-EPIwithout race vs Cockcroft-Gault) rates were calculated. Subgroup analyses were done for the following clinically relevant groups: bodyweight (<60 kg, 60–90 kg, and >90 kg), body surface area (≤1·6 m2, >1·6 m2 and <1·9 m2, and ≥1·9 m2), age (<40 years, 40–60 years, and >60 years), and sex (male and female).
Statistical analysis
Data were expressed using standard descriptive statistics (means, SD, and ranges or medians and IQRs, as appropriate). Body surface area and kidney function calculations were done in Excel (Microsoft Office 2019). Dosing simulations and descriptive statistical analyses were done in Stata (version 16.1).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
For this retrospective analysis, the original dataset included 4118 patients enrolled from January, 1995, to October, 2010. After removing patients without race data (n=1), without kg as the units for bodyweight data (n=136), without height data (n=36), or height of less than 100 cm (n=14), 3931 patients remained. Of these, 340 (9%) were Black patients (172 male and 168 female), who had a median age of 57 years (IQR 47–64), height of 170·0 cm (162·6–177·4), bodyweight of 78·1 kg (67·0–89·8), body surface area of 1·91 m2 (1·77–2·09), and serum creatinine concentration of 0·9 mg/dL (0·8–1·1).
Median eGFRCr calculated by CKD-EPI was 103 mL/min (IQR 85 to 122) and calculated by CKD-EPIwithout race was 89 mL/min (73 to 105; figure 1A), corresponding to a change in eGFRCr of ‒14 mL/min (‒17 to ‒12) with the exclusion of race (figure 1B). Median eClCr calculated by Cockcroft-Gault was 90 mL/min (72 to 120; appendix p 1). The proportions of patients with eGFR or eClCr of less than 90 mL/min and of less than 60 mL/min were 110 (32%) of 340 and 25 (7%) of 340 for CKD-EPI, 174 (51%) of 340 and 43 (13%) of 340 for CKD-EPIwithout race, and 166 (49%) of 340 and 43 (13%) of 340 for Cockcroft-Gault. 90 (26%) patients were reclassified to a more severe chronic kidney disease stage when CKD-EPIwithout race was used versus CKD-EPI. 34 (10%) patients were reclassified into a more severe chronic kidney disease stage and 27 (8%) patients were reclassified into a less severe chronic kidney disease stage when CKD-EPIwithout race was used versus Cockcroft-Gault (figure 1C).
Figure 1: Effect of removing race from the CKD-EPI equation on eGFR for Black patients (n=340).
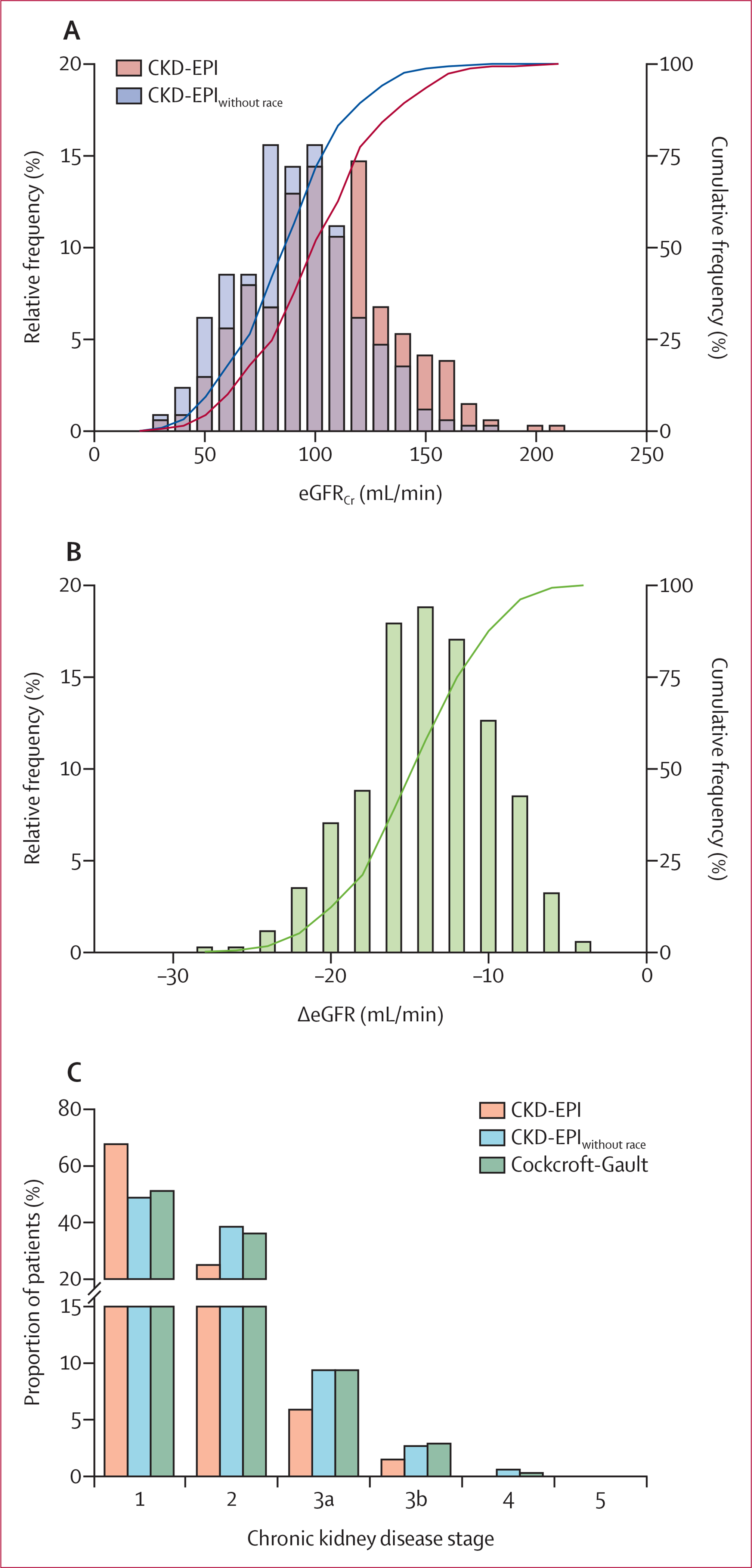
eGFRCr estimated by CKD-EPI and CKD-EPIwithout race (A) and difference in eGFRCr when calculated by CKD-EPIwithout race versus CKD-EPI (B), shown as frequency (bars) and cumulative frequency (lines) of the patient sample. (C) Proportion of patients in each stage of chronic kidney disease, based on eGFRCr estimated by CKD-EPI and CKD-EPIwithout race, and on estimated creatinine clearance calculated by Cockcroft-Gault. Chronic kidney disease stages were defined as per Kidney Disease Improving Global Outcomes guidelines. CKD-EPI=Chronic Kidney Disease-Epidemiology Collaboration. CKD-EPIwithout race=CKD-EPI equation with the race term removed. eGFR=estimated glomerular filtration rate. eGFRCr=eGFR based on creatinine. ΔeGFR=change in eGFR.
Use of CKD-EPIwithout race and Cockcroft-Gault led to exclusion of patients from therapy or recommended dose reduction at similar rates, and these rates were higher than when CKD-EPI was used. The proportion of patients ineligible to receive a drug ranged from 25 (7%) of 340 to 43 (13%) of 340 for cisplatin, from five (1%) to 11 (3%) for pemetrexed, from three (1%) to five (1%) for bendamustine, and from none to two (1%) for mitomycin, depending on how kidney function was calculated (table 1, figure 2). The number of patients ineligible for therapy when CKD-EPIwithout race versus CKD-EPI was used increased by 72% (from 25 of 340 to 43 of 340) for cisplatin, 120% (from five of 340 to 11 of 340) for pemetrexed, and 67% (from three of 340 to five of 340) for bendamustine. Eligibility discordance between CKD-EPI and CKD-EPIwithout race ranged from two (1%) of 340 patients to 18 (5%) of 340 patients, and between Cockcroft-Gault and CKD-EPIwithout race ranged from one (<1%) patient to 18 (5%) patients (table 2).
Table 1:
Renal eligibility and dosing recommendations for anticancer drugs when using CKD-EPI, CKD-EPIwithout race, and Cockcroft-Gault to estimate eGFRCr or eClCr in Black patients (n=340)
| CKD-EPI | CKD-EPIwithout race | Cockcroft-Gault | |
|---|---|---|---|
|
| |||
| Drugs with renal eligibility cutoffs | |||
| Cisplatin | |||
| Eligible (≥60 mL/min) | 315 (93%) | 297 (87%) | 297(87%) |
| Ineligible (<60 mL/min) | 25 (7%) | 43 (13%) | 43 (13%) |
| Pemetrexed | |||
| Eligible (≥45 mL/min) | 335 (99%) | 329 (97%) | 329 (97%) |
| Ineligible (<45 mL/min) | 5 (1%) | 11 (3%) | 11 (3%) |
| Bendamustine | |||
| Eligible (≥40 mL/min) | 337 (99%) | 335 (99%) | 336 (99%) |
| Ineligible (<40 mL/min) | 3 (1%) | 5 (1%) | 4 (1%) |
| Mitomycin | |||
| Eligible (≥30 mL/min) | 340 (100%) | 338 (99%) | 339 (100%) |
| Ineligible (<30 mL/min) | 0 | 2 (1%) | 1 (<1%) |
| Drugs with renal dosing recommendations | |||
| Oxaliplatin | |||
| 100% (≥30 mL/min) | 340 (100%) | 338 (99%) | 339 (100%) |
| 75% (<30 mL/min) | 0 | 2 (1%) | 1 (<1%) |
| Capecitabine | |||
| 100% (>50 mL/min) | 330 (97%) | 315 (93%) | 317 (93%) |
| 75% (30–50 mL/min) | 10 (3%) | 23 (7%) | 22 (7%) |
| Ineligible (<30 mL/min) | 0 | 2 (1%) | 1 (<1%) |
| Etoposide | |||
| 100% (≥50 mL/min) | 330 (97%) | 315(93%) | 317 (93%) |
| 75% (15–50 mL/min) | 10 (3%) | 25 (7%) | 23 (7%) |
| Ineligible (<15 mL/min) | 0 | 0 | 0 |
| Topotecan | |||
| 100% (≥40 mL/min) | 337 (99%) | 335 (99%) | 336 (99%) |
| 50% (20–39 mL/min) | 3 (1%) | 5 (1%) | 4 (1%) |
| Ineligible (<10 mL/min) | 0 | 0 | 0 |
| Fludarabine | |||
| 100% (≥80 mL/min) | 266 (78%) | 221 (65%) | 223 (66%) |
| 80% (50–79 mL/min) | 66 (19%) | 98 (29%) | 95 (28%) |
| 60% (30–49 mL/min) | 8 (2%) | 19 (6%) | 21 (6%) |
| Ineligible (<30 mL/min) | 0 | 2 (1%) | 1 (<1%) |
| Bleomycin | |||
| 100% (≥50 mL/min) | 332 (98%) | 319 (94%) | 318 (94%) |
| 70% (40–50 mL/min) | 5 (1%) | 16 (5%) | 18 (5%) |
| 60% (30–40 mL/min) | 3 (1%) | 3 (1%) | 3 (1%) |
| 55% (20–30 mL/min) | 0 | 2 (1%) | 1 (<1%) |
| 45% (10–20 mL/min) | 0 | 0 | 0 |
| 40% (5–10 mL/min) | 0 | 0 | 0 |
Data are n (%). Cutoffs are eGFRCr values for CKD-EPI and CKD-EPIwithout race, or eCLCr values for Cockcroft-Gault. CKD-EPI=Chronic Kidney Disease-Epidemiology Collaboration. CKD-EPIwithout race=CKD-EPI equation with the race term removed. eGFR=estimated glomerular filtration rate. eGFRCr=eGFR based on creatinine. eClCr=estimated creatinine clearance.
Figure 2: Effect of removing race from the CKD-EPI equation on drug eligibility cutoffs.
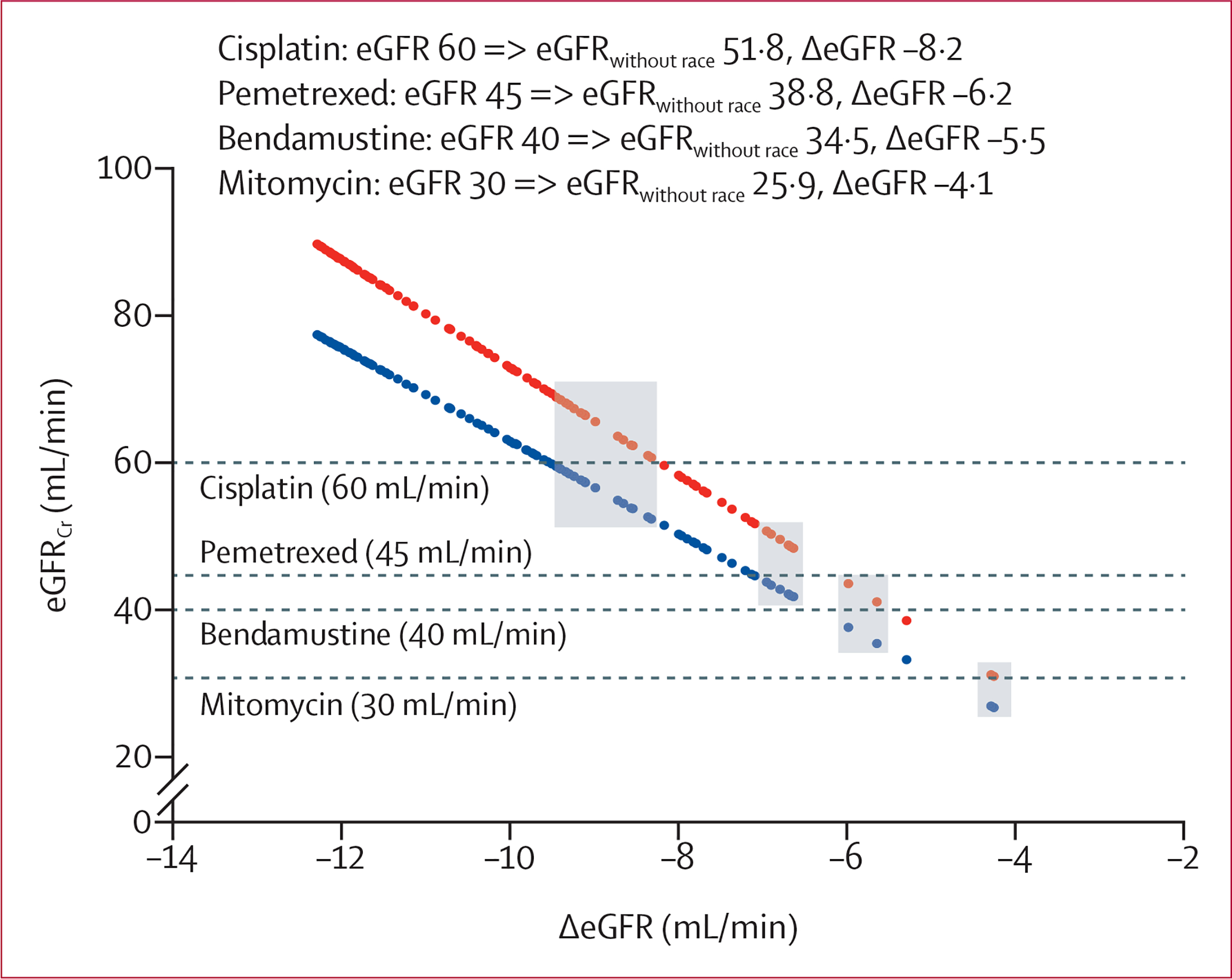
eGFRCr was calculated by CKD-EPI and CKD-EPIwithout race and rounded to the nearest hundredth for patients with eGFR <90 mL/min calculated by CKD-EPI (n=111) . Values were indexed to change in eGFR (ΔeGFR) such that each vertical pair of points corresponds to the eGFRs calculated by CKD-EPI (red) and CKD-EPIwithout race (blue) of a single patient. Dashed horizontal lines represent kidney function eligibility cutoffs for each drug. Patients highlighted in grey were deemed eligible for therapy when eGFR was calculated with CKD-EPI and ineligible when eGFR was calculated with CKD-EPIwithout race (ie, eligibility discordance). The inset depicts the minimum eGFR for eligibility when calculated by CKD-EPI and the corresponding eGFR calculated by CKD-EPIwithout race and ΔeGFR. CKD-EPI=Chronic Kidney Disease-Epidemiology Collaboration. CKD-EPIwithout race=CKD-EPI equation with the race term removed. eGFR=estimated glomerular filtration rate. eGFRCr=eGFR based on creatinine. ΔeGFR=change in eGFR.
Table 2:
Discordance in renal eligibility and dosing recommendations when using CKD-EPI or Cockcroft-Gault versus CKD-EPIwithout race to estimate kidney function in Black patients (n=340)
| CKD-EPI vs CKD-EPIwithout race |
Cockcroft-Gault vs CKD-EPIwithout race |
|||
|---|---|---|---|---|
| Total discordance* | Total discordance† | Eligible with Cockcroft-Gault only or higher dose with Cockcroft-Gault | Eligible with CKD-EPIwithout race only or higher dose with CKD-EPIwithout race | |
|
| ||||
| Drugs with renal eligibility cutoffs | ||||
| Cisplatin | 18 (5%) | 18 (5%) | 9 (3%) | 9 (3%) |
| Pemetrexed | 6 (2%) | 6 (2%) | 3 (1%) | 3 (1%) |
| Bendamustine | 2 (1%) | 3 (1%) | 2 (1%) | 1 (<1%) |
| Mitomycin | 2 (1%) | 1 (<1%) | 1 (<1%) | 0 |
| Drugs with renal dosing recommendations | ||||
| Oxaliplatin | 2 (1%) | 1 (<1%) | 1 (<1%) | 0 |
| Capecitabine | 17 (5%) | 11 (3%) | 7 (2%) | 4 (1%) |
| Etoposide | 15 (4%) | 10 (3%) | 6 (2%) | 4 (1%) |
| Topotecan | 2 (1%) | 3 (1%) | 2 (1%) | 1 (<1%) |
| Fludarabine | 60 (18%) | 50 (15%) | 26 (8%) | 24 (7%) |
| Bleomycin | 17 (5%) | 16 (5%) | 8 (2%) | 8 (2%) |
Data are n (%). CKD-EPI=Chronic Kidney Disease-Epidemiology Collaboration. CKD-EPIwithout race=CKD-EPI equation with the race term removed.
Total discordance for CKD-EPI versus CKD-EPIwithout race was defined as patients who were eligible to receive the drug with CKD-EPI but ineligible with CKD-EPIwithout race (ie, eligibility discordance) or recommended to receive a lower dose with CKD-EPIwithout race versus CKD-EPI (ie, dosing discordance).
Total discordance for Cockcroft-Gault versus CKD-EPIwithout race was defined as patients who were eligible to receive drug by either Cockcroft-Gault or CKD-EPIwithout race but not both (ie, eligibility discordance) or recommended to receive a different dose with CKD-EPIwithout race versus CG (ie, dosing discordance); because there is no consistent directionality between estimated glomerular filtration rate based on creatinine calculated by CKD-EPIwithout race and estimated creatinine clearance calculated by Cockcroft-Gault, the proportion of patients who were eligible for a drug or for a higher dose of drug by each kidney function-estimating equation is provided.
The proportion of patients recommended to receive any renal dose reduction (ie, recommended to not receive the full dose) ranged from none to two (1%) of 340 for oxaliplatin, ten (3%) of 340 to 25 (7%) of 340 for capecitabine, ten (3%) to 25 (7%) for etoposide, three (1%) to five (1%) for topotecan, 74 (22%) to 119 (35%) for fludarabine, and eight (2%) to 21 (6%) for bleomycin, depending on how kidney function was calculated (table 1). The number of patients recommended to receive any renal dose adjustment when CKD-EPIwithout race versus CKD-EPI was used increased from 0 of 340 patients to 2 of 340 patients for oxaliplatin, and by 150% for capecitabine (from ten to 25 patients), by 150% for etoposide (from ten to 25 patients), by 67% for topotecan (from three to five patients), by 61% for fludarabine (from 74 to 119 patients), and by 163% for bleomycin (from eight to 21 patients). Dosing discordance between CKD-EPI and CKD-EPIwithout race ranged from two (1%) of 340 patients to 60 (18%) of 340 patients, and between Cockcroft-Gault and CKD-EPIwithout race ranged from one (<1%) of 340 patients to 50 (15%) of 340 patients (table 2).
Subgroup analyses were done by bodyweight, body surface area, age, and sex (appendix pp 2–5). CKD-EPIwithout race was more likely to recommend ineligibility and dose reduction for patients with higher bodyweight, larger body surface area, and younger age compared with both CKD-EPI and Cockcroft-Gault. Higher rates of eligibility and dose discordance were observed for CKD-EPIwithout race versus CKD-EPI for patients with lower weight, smaller body surface area, older age, and female sex (appendix pp 2–5).
Discussion
This retrospective analysis of National Cancer Institute phase 1 clinical trial participant data shows that removing the race term from the CKD-EPI equation will increase the number of Black patients with cancer who are deemed ineligible for therapy or require a dose reduction for anticancer drugs.
Quantitative assessment of kidney function is a crucial consideration when prescribing a patient’s anticancer therapy regimen (ie, first-line versus second-line therapy, dose reduction, or omission of drugs from the regimen). The CKD-EPI study equation is regarded as the current best approach for estimating GFR in patients with cancer.4 However, including race (a social construct) as a surrogate for serum creatinine homoeostasis (a biological process) in the CKD-EPI equation has come under scrutiny.6–9 A joint National Kidney Foundation–American Society of Nephrology task force on reassessing the inclusion of race in diagnosing kidney diseases is critically evaluating the issue.6 Leaders of the two groups have asserted that “race modifiers should not be included in equations to estimate kidney function”, and that “current race-based equations should be replaced by a substitute that is accurate, representative, unbiased, and provides a standardised approach to diagnosing kidney diseases”.13 However, the timeline for implementation of new race-agnostic equations is unclear, and some institutions have already removed the race term from existing GFR-estimating equations.14 To our knowledge, this is the first study to evaluate the potential effects of removing race from the CKD-EPI equation on pharmacotherapeutic decisions for patients with cancer.
Removing the race term from the CKD-EPI equation almost doubled the proportion (from 7% to 13%) of patients in our cohort with an eGFR of less than 60 mL/min, a clinically relevant cutoff below which many drugs, including anticancer drugs, begin to have recommendations for renal dose adjustments and eligibility. The proportion of patients who were ineligible to receive a drug or who were recommended to receive a dose reduction increased by between 61% and 163%. Additionally, up to 5% of patients had discordant recommendations for drug eligibility and up to 18% had discordant recommendations for drug dosing if CKD-EPIwithout race versus CKD-EPI was used to estimate GFR. Drugs that had higher kidney function cutoffs (ie, ≥50 mL/min; fludarabine, cisplatin, capecitabine, bleomycin, and etoposide) had higher rates of eligibility and dosing discordance than did drugs with lower cutoffs. This finding reflects the direct proportionality between a patient’s eGFR and the absolute reduction in eGFR that results from removing the race factor from the CKD-EPI equation. Black patients whose kidney function exceeds, but is close to, a numerically higher renal dose adjustment cutoff value when eGFR is calculated by CKD-EPI will be more affected by the removal of race from the CKD-EPI equation. These patients are more likely to have corresponding eGFR values based on CKD-EPIwithout race that fall below the dose adjustment cutoff and thus will be more likely to receive a discordant recommendation.
Unlike the CKD-EPI equation, the Cockcroft-Gault equation was developed in a likely predominantly White population and does not include race as a covariate. Arguably, the Cockcroft-Gault equation converts the higher serum creatinine in Black patients at equal kidney function into negatively biased kidney function estimates, resulting in Black patients being inappropriately underdosed or deemed ineligible for cancer therapeutics. The Cockcroft-Gault equation has previously been shown to underestimate creatinine clearance by 16–35% in Black patients.15 In our cohort, the proportion of patients deemed ineligible for therapy or requiring a dose reduction by eGFRCr calculated with CKD-EPIwithout race was similar to that by eClCr calculated with Cockcroft-Gault, probably reflecting, in part, the absence of race adjustment in both kidney function estimation equations. Furthermore, Cockcroft-Gault-derived kidney function cutoffs for traditional anticancer drugs were often established before racial categorisation was common,3 and if reported, the study populations had extremely low non-White representation (eg, for cisplatin, non-White participants comprised 2%,16 3%,17 or 0%18 in previous studies; for pemetrexed, they comprised 0%19 or 13%;20 for bendamustine, 6%;21 for oxaliplatin, 18%22). The CKD-EPI equation would be expected to correct for the racial bias inherent in many anticancer drug cutoffs by its inclusion of race as a covariate, which adjusts for the higher serum creatinine in Black patients at a given eGFR that was not accounted for in studies supporting the Cockcroft-Gault-derived cutoffs. Thus, excluding the race term from the CKD-EPI equation erases an important distinction between CKD-EPI-derived and Cockcroft-Gault-derived estimates of GFR.
Removal of race from the CKD-EPI equation was previously reported to potentially improve care for many Black patients with kidney disease by increasing referrals to nephrology specialists, expanding Medicare coverage, and increasing transplant list eligibility.23 Here, we report that removing race from the CKD-EPI equation will have the effect of excluding more Black patients with cancer from receiving full doses of potentially life-saving anticancer drugs. Anticancer drug ineligibility or dose reduction can be associated with worse survival; for example, therapy dose reductions have been shown to increase mortality in ovarian cancer,24 and carboplatin dose reductions of only 10% can double 5-year relapse rates in patients with seminoma tumours.25 Therefore, removing race from GFR-estimating equations could lead to disease undertreatment, potentially worsening cancer survival outcomes in Black patients. However, we acknowledge that removal of race could more accurately reflect GFR in individual Black patients, which could have important clinical implications in these patients, because racial disparities in anticancer drug toxicity have been reported. For example, some,26 but not all,27 studies suggest that Black patients are at increased risk of cisplatin-associated nephrotoxicity, although whether this increased risk is related to kidney function or other patient factors remains unclear. Removal of race from the CKD-EPI equation could potentially prevent overdosing and reduce the rate of anticancer drug toxicity.
The ultimate goal of renal eligibility and dosing recommendations for anticancer drugs should be to achieve optimal outcomes by balancing anticancer drug efficacy and toxicity. Until race-agnostic equations can be validated and implemented, a pragmatic and patient-centred approach to anticancer drug eligibility and dosing should be used by clinicians when presented with discrepant recommendations depending on the method of kidney function assessment used. Such an approach should not be directed unilaterally by GFR-estimating equation results, but instead be guided by clinical judgment, individualised patient care, and a thorough understanding of the limitations of the various GFR-estimating equations.28
Alternatives to the 2009 CKD-EPI equation that do not incorporate race into their estimates of GFR have been developed, including the 2012 CKD-EPI equation based on cystatin C (CKD-EPIcystatin C) and several equations published in 2020 that use panels of filtration markers.29,30 Additionally, equations that de-emphasise race, such as the 2012 CKD-EPI equation based on creatinine and cystatin C (CKD-EPICr-cystatin C), have been published.29 These equations use alternative filtration markers, such as cystatin, β-trace protein, and β2-microglobulin in place of, or in conjunction with, serum creatinine, thereby attenuating the need to account for non-GFR determinants of serum creatinine and subsequently decreasing the emphasis on race or removing race from calculations. Although these equations are promising, they are not without limitations. For example, they have not been validated in patients with cancer, and analytical standards have not yet been developed and validated for β-trace protein and β2-microglobulin to reduce interlaboratory variability. Additionally, cystatin C-based equations are not yet recommended for use in patients with cancer.4 However, these equations do offer promise for race-agnostic methods of assessing kidney function.
Our analysis has some limitations. First, our cohort included patients enrolled in phase 1 oncology studies and thus did not necessarily reflect the prevalence of kidney impairment in the general population of individuals with cancer. For example, 7% of patients had an eGFR of less than 60 mL/min and 32% had an eGFR of less than 90 mL/min, as calculated by CKD-EPI, compared with approximately 25% and 50%, respectively, reported for all patients with solid tumour.31,32 Therefore, the absolute proportion of patients in our study who were deemed ineligible for a drug or required a dose reduction probably underestimates the true effect size that would be observed in clinical practice. Additionally, we did not have measured GFRs to assess the accuracy or precision of the kidney function estimates evaluated in our patient population. The CKD-EPI equation is considered the current best approach for estimating GFR in cancer patients, and is less biased in Black patients with the race term included;4,33 however, to our knowledge, the accuracy and precision of the CKD-EPI equation with and without race has not been assessed specifically in Black patients with cancer. A National Cancer Institute-sponsored trial (NRG-GY022, ClinicalTrials.gov number NCT03997370) is assessing the performance of Cockcroft-Gault and CKD-EPI in predicting measured GFR and evaluating which demographic factors (including race) are important to consider in these predictions. Lastly, our study is limited to assessing the effects of removing the race factor from CKD-EPI on Black patients with cancer only. Although CKD-EPI estimates for other races might also improve by application of a race factor, the effect on cancer therapy dosing remains to be defined.33
In summary, removal of race from the CKD-EPI equation results in estimates of GFR that will change care for Black patients with cancer by excluding more patients from receiving full doses of potentially life-saving therapy, which could adversely affect survival outcomes. Exclusion of the race term from the CKD-EPI equation could paradoxically worsen care for Black patients with cancer. Although guidance on implementation of new, race-agnostic methods of kidney function assessment is forthcoming, the timeline for widespread clinical implementation is unclear and omission of race from the CKD-EPI equation in the interim could negatively affect care for Black patients with cancer.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, without any language or date restrictions, for published literature using terms including “GFR estimation AND race”, “CKD-EPI AND race”, and “kidney function estimation AND oncology”. Glomerular filtration rate (GFR)-estimating equations are used in oncology to inform renal drug dosing and eligibility. Although the Cockcroft-Gault equation remains widely used in clinical oncology practice, the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation is recommended for use for patients with cancer by the Kidney Disease Improving Global Outcomes guideline group. The CKD-EPI equation includes a race term, which presumably reflects differences in non-GFR determinants of serum creatinine that differ by race and accounts for higher measured GFR at a given age, sex, and serum creatinine concentration observed in Black versus non-Black patients during equation development and validation. By contrast, the Cockcroft-Gault equation was developed in a predominantly White cohort and does not include race. Inclusion of a race term in GFR-estimating equations has recently been questioned, prompting discussion regarding the implications of removing it on the care of Black patients, including an increase in the diagnosis of chronic kidney disease and more patients considered ineligible to receive drugs with renal cutoff criteria. To our knowledge, the effects of removing the race term from GFR-estimating equations on cancer pharmacotherapy have not yet been evaluated.
Added value of this study
This study uses a clinically relevant dataset from 15 years of National Cancer Institute phase 1 clinical trials to evaluate the effects of removing the race term from the CKD-EPI equation on anticancer drug dosing and eligibility. The study shows that removing the race term from GFR-estimating equations will calculate a lower estimated GFR for Black patients with cancer and will reduce the proportion of Black patients eligible to receive anticancer drugs. Up to 18% of Black patients with cancer in this study cohort would have received discordant drug dosing or eligibility recommendations. This finding underscores the crucial impact that the choice of GFR-estimating equation, including whether or not to include race in the calculation, has on drug eligibility and dosing in Black patients with cancer.
Implications of all the available evidence
Black patients experience disparities in both cancer incidence and mortality, and are therefore especially susceptible to disease undertreatment. Removal of race from GFR-estimating equations could lead to higher rates of anticancer drug exclusion, dose reduction, and disease undertreatment in Black patients with cancer, and thus could adversely affect survival outcomes. Although race-agnostic GFR-estimating equations have been developed, the timeline for widespread implementation in clinical oncology practice is unclear and could take many years. This study underscores the need for careful clinical judgement and a patient-centred approach in interpreting and comparing kidney function estimates from different GFR-estimating equations, including a thorough understanding of the limitations of each equation, especially when selecting and dosing anticancer drugs with a narrow therapeutic index.
Acknowledgments
This study was supported in part by National Institutes of Health (NIH) grants UM1CA186690, P30CA47904, and U24CA247643, and contract NO2-CM37106. SPI is employed by the NIH. JHB is in receipt of NIH grants.
Declaration of interests
MAC reports personal fees from AstraZeneca, outside the submitted work. JHB has received expert witness fees from Pfizer, and through his institute has received research support from AbbVie and Spectrum Pharmaceuticals, outside the submitted work; his spouse holds GlaxoSmithKline stocks. TDN reports personal fees from MediBeacon and CytoSorbents, and royalties from McGraw-Hill Education, outside the submitted work. SPI declares no competing interests.
Footnotes
Data sharing
Data are available from the corresponding author on reasonable request. All requests for raw and analysed data will be reviewed by the corresponding author to verify whether the request is subject to confidentiality obligations.
For the Drugs@FDA database see https://www.accessdata.fda.gov/scripts/cder/daf
Contributor Information
Morgan A Casal, Department of Pharmacy and Therapeutics, School of Pharmacy, University of Pittsburgh, Pittsburgh, PA, USA.
S Percy Ivy, Investigational Drug Branch, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD, USA.
Jan H Beumer, Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, Pittsburgh, PA, USA; Hematology/Oncology Division, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Cancer Therapeutics Program, UPMC Hillman Cancer Center, Pittsburgh, PA, USA.
Thomas D Nolin, Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, Pittsburgh, PA, USA; Renal-Electrolyte Division, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
References
- 1.Casal MA, Nolin TD, Beumer JH. Estimation of kidney function in oncology: implications for anticancer drug selection and dosing. Clin J Am Soc Nephrol 2019; 14: 587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron J 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 3.Beumer JH, Ding F, Tawbi H, et al. Effect of renal dysfunction on toxicity in three decades of cancer therapy evaluation program-sponsored single-agent phase I studies. J Clin Oncol 2016; 34: 110–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porta C, Bamias A, Danesh FR, et al. KDIGO Controversies Conference on onco-nephrology: understanding kidney impairment and solid-organ malignancies, and managing kidney cancer. Kidney Int 2020; 98: 1108–19. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado C, Baweja M, Burrows NR, et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN task force. Am J Kidney Dis 2021; 1: 103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt IM, Waikar SS. Separate and unequal: race-based algorithms and implications for nephrology. J Am Soc Nephrol 2021; 32: 529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med 2020; 383: 874–82. [DOI] [PubMed] [Google Scholar]
- 9.Grubbs V Precision in GFR reporting: let’s stop playing the race card. Clin J Am Soc Nephrol 2020; 15: 1201–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2012; 3: 1–150. [Google Scholar]
- 12.Galsky MD, Hahn NM, Rosenberg J, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol 2011; 12: 211–14. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation. Removing race from estimates of kidney function. March9, 2021. https://www.kidney.org/news/removing-race-estimates-kidney-function (accessed April 24, 2021).
- 14.Lucas A, Wyatt CM, Inker LA. Removing race from GFR estimates: balancing potential benefits and unintended consequences. Kidney Int 2021; 1: 11–13. [DOI] [PubMed] [Google Scholar]
- 15.Goldwasser P, Aboul-Magd A, Maru M. Race and creatinine excretion in chronic renal insufficiency. Am J Kidney Dis 1997; 30: 16–22. [DOI] [PubMed] [Google Scholar]
- 16.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068–77. [DOI] [PubMed] [Google Scholar]
- 17.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006; 107: 506–13. [DOI] [PubMed] [Google Scholar]
- 18.Cho KS, Joung JY, Seo HK, et al. Renal safety and efficacy of cisplatin-based chemotherapy in patients with a solitary kidney after nephroureterectomy for urothelial carcinoma of the upper urinary tract. Cancer Chemother Pharmacol 2011; 67: 769–74. [DOI] [PubMed] [Google Scholar]
- 19.Ando Y, Hayashi T, Ujita M, et al. Effect of renal function on pemetrexed-induced haematotoxicity. Cancer Chemother Pharmacol 2016; 78: 183–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latz JE, Chaudhary A, Ghosh A, Johnson RD. Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol 2006; 57: 401–11. [DOI] [PubMed] [Google Scholar]
- 21.Owen JS, Melhem M, Passarell JA, D’Andrea D, Darwish M, Kahl B. Bendamustine pharmacokinetic profile and exposure-response relationships in patients with indolent non-Hodgkin’s lymphoma. Cancer Chemother Pharmacol 2010; 66: 1039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takimoto CH, Remick SC, Sharma S, et al. Dose-escalating and pharmacological study of oxaliplatin in adult cancer patients with impaired renal function: a National Cancer Institute Organ Dysfunction Working Group study. J Clin Oncol 2003; 21: 2664–72. [DOI] [PubMed] [Google Scholar]
- 23.Diao JA, Wu GJ, Taylor HA, et al. Clinical implications of removing race from estimates of kidney function. JAMA 2021; 325: 184–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denduluri N, Lyman GH, Wang Y, et al. Chemotherapy dose intensity and overall survival among patients with advanced breast or ovarian cancer. Clin Breast Cancer 2018; 18: 380–86. [DOI] [PubMed] [Google Scholar]
- 25.Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol 2011; 29: 957–62. [DOI] [PubMed] [Google Scholar]
- 26.Bhat ZY, Cadnapaphornchai P, Ginsburg K, et al. Understanding the risk factors and long-term consequences of cisplatin-associated acute kidney injury: an observational cohort study. PLoS One 2015; 10: e0142225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motwani SS, McMahon GM, Humphreys BD, Partridge AH, Waikar SS, Curhan GC. Development and validation of a risk prediction model for acute kidney injury after the first course of cisplatin. J Clin Oncol 2018; 36: 682–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson JQ, Nolin TD. Pragmatic use of kidney function estimates for drug dosing: the tide is turning. Adv Chronic Kidney Dis 2018; 25: 14–20. [DOI] [PubMed] [Google Scholar]
- 29.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inker LA, Couture SJ, Tighiouart H, et al. A new panel-estimated GFR, including β2-microglobulin and β-trace protein and not including race, developed in a diverse population. Am J Kidney Dis 2021; 77: 673–83.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janus N, Launay-Vacher V, Byloos E, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer 2010; 103: 1815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer 2007; 110: 1376–84. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol 2020; 15: 1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


