Protein kinases that regulate the activity of specific transcription factors in response to extracellular stimuli not only are the subject of intense research but also are being chased as potential targets for development of new drugs for treatment of various human diseases. One such protein kinase is IKK, the IκB kinase that activates nuclear factor κB (NF-κB) through phosphorylation of IκB inhibitory proteins. In this review, we summarize the discovery of IKK and recent knowledge about its composition, regulation, and physiological functions.
NF-κB transcription factors regulate the expression of a large number of genes that are necessary for proper functioning of the immune system and are key mediators of inflammatory responses to pathogens (2, 4, 5). NF-κB is also associated with cellular transformation and oncogenesis, and one of its most important, but lately discovered functions, is the activation of an antiapoptotic gene expression program (6, 29, 36, 38, 39, 42). As a transcription factor that orchestrates the inflammatory response, NF-κB is rapidly activated, independently of new protein synthesis, in response to signals produced during infection (e.g., bacterial endotoxins and viral double-stranded RNA) (for a review, see reference 3). NF-κB activation is also a transient response; this is of importance because many of the genes that are activated by NF-κB encode potentially toxic products such as tumor necrosis factor (TNF). The key to NF-κB regulation is the inhibitory κB (IκB) proteins which retain NF-κB in the cytoplasm (reviewed in reference 37). In response to diverse stimuli, IκBs are rapidly degraded and the freed NF-κB dimers translocate to the nucleus. Several years ago, it was established that the critical event which triggers the polyubiquitination and degradation of IκBs via the 26S proteasome is their stimulus-dependent phosphorylation at two serine residues (residues 32 and 36 in IκBα) that are located within their conserved N-terminal regulatory region (1, 10–12, 18, 20, 34, 40). The protein kinase that phosphorylates these regulatory sites remained elusive, and without detailed knowledge about its molecular identity, there was little progress towards a full understanding of the signaling pathways that control NF-κB activity. The initial hunt for such a protein kinase yielded many false candidates, such as protein kinase C, casein kinase II, and ribosomal S6 kinase (pp90rsk) (reviewed in reference 43). Although most of these kinases phosphorylate IκB proteins in the test tube on different serine, threonine, or tyrosine residues, none of them was found to phosphorylate the two regulatory sites that trigger the degradation of IκBs in a stimulus-dependent manner.
A large-molecular-mass (700-kDa) protein kinase activity that phosphorylates IκBα on S32 and S36 in a ubiquitin-dependent manner was also detected in extracts of nonstimulated HeLa cells (13, 25). However, this activity was not reported to be stimulus dependent, and to date, its components and molecular identity are unknown.
A careful consideration of IκB phosphorylation indicated that the physiological IκB kinase had to fulfill several criteria. Its activity should be stimulated by inducers of NF-κB with kinetics that are consistent with those of NF-κB activation, and it should phosphorylate both S32 and S36 in the N terminus of IκBα and both S19 and S23 in the N terminus of IκBβ. In addition, since substitution of threonines for these serines results in IκB mutants that are resistant to degradation, the physiological IκB kinase should be serine specific (18).
IDENTIFICATION OF IKK AS THE PHYSIOLOGICAL IκB KINASE
To isolate a kinase that meets these requirements, DiDonato et al. (19) employed a biochemical approach to purify a 900-kDa protein kinase complex from extracts of TNF-treated HeLa cells. In preliminary experiments, this activity was found to phosphorylate IκBα at the proper serines and to discriminate against the mutant with threonine substitutions (19). Most importantly, this kinase activity was found to be rapidly stimulated by proinflammatory cytokines with the proper kinetics (19). A large-scale purification of this 900-kDa protein kinase complex, named IKK for IκB kinase, resulted in identification of two polypeptides, of 85 and 87 kDa, that coeluted with IκB kinase activity on several gel chromatography and affinity columns (19). Microsequencing and cDNA cloning identified these polypeptides as two closely related protein kinases, IKKα (IKK1) and IKKβ (IKK2) (19, 47). A similar approach undertaken by Mercurio and coworkers yielded identical results (31). At the same time, a two-hybrid screening conducted by Regenier et al. (32) resulted in the isolation of IKKα (previously identified as a protein kinase with unknown function named CHUK [16]) as a protein that interacts in yeast cells with another protein kinase called the NF-κB-inducing kinase (NIK). NIK is so called due to its ability to potently stimulate NF-κB activity in transiently transfected cells (30). Both IKKα and IKKβ were initially found to be cytokine-responsive IκB kinases whose kinetics of activation match those of IκBα phosphorylation (19, 47). In addition, expression of an antisense IKKα RNA or a kinase-defective mutant of IKKβ inhibits activation of NF-κB by proinflammatory cytokines, thus providing further support for the IKK complex being the long-sought-after IκB kinase (19, 31, 41, 47).
COMPOSITION OF THE IKK COMPLEX
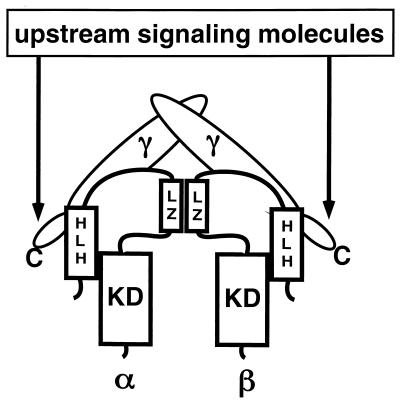
The IKK complex contains two catalytic subunits, IKKα and IKKβ, of 745 and 756 amino acids, respectively. In addition to a kinase domain at their N termini, IKKα and IKKβ contain protein interaction motifs, a leucine zipper (LZ), and a helix-loop-helix (HLH) motif at their C-terminal portions. The kinase domains are 64% identical, while the C-terminal LZ and HLH motifs exhibit 44% identity. IKKα and IKKβ can form homodimers and heterodimers (or tetramers) in vitro, and purified recombinant forms of each can directly phosphorylate IκBα and IκBβ at the proper sites (26, 46).
In addition, the IKK complex contains at least one regulatory subunit, IKKγ/NEMO, which was identified by two different and independent approaches. Using a monoclonal antibody to the IKKα subunit, the IKK complex was purified to near homogeneity from two human cell lines (33). This complex, which we refer to as the core complex because its isolation involved a stringent wash with 3 M urea (which may have removed loosely attached subunits), contains equimolar amounts of IKKα and IKKβ and two additional polypeptides, of 50 and 52 kDa (33). Microsequencing and molecular cloning revealed that these polypeptides, IKKγ1 and IKKγ2, represent differentially processed forms of the same protein (33). Complementation cloning of cDNAs whose products restore NF-κB activation in two cell lines that are completely defective in NF-κB activation resulted in isolation of the mouse homolog of IKKγ, named NEMO (NF-κB essential factor) (44). Expression of IKKγ/NEMO in these cell lines restores the ability to activate IKK and NF-κB in response to TNF alpha (TNF-α), interleukin 1 (IL-1), double-stranded RNA, and human T-cell leukemia type 1 infection (44). These studies provided the evidence that IKK is indeed the physiological IκB kinase necessary for NF-κB activation by all of these stimuli. The requirement of IKKγ for NF-κB activation was also demonstrated by an antisense RNA approach whereby cell lines made to express lower levels of IKKγ exhibited a considerable decrease in IκBα phosphorylation, degradation, and NF-κB activation (33). IKKγ/NEMO is a 419-amino-acid-long, glutamine-rich protein that lacks a known catalytic domain but contains several coiled-coil protein interaction motifs, including an LZ, next to its C terminus (33, 44). A C-terminally truncated version of IKKγ lacking the LZ can still bind IKKα-IKKβ heterodimers, but once expressed, it prevents IKK activation by a number of stimuli, including TNF and IL-1 (33). Most importantly, the IKK complex assembled around the C-terminally truncated IKKγ is refractory to activation by a variety of different stimuli (33). These results not only confirm the requirement of IKKγ for IKK activation but also suggest a specific function whereby it connects the IKK complex to upstream activators. Most likely, these connections occur via protein-protein interactions mediated by the C-terminal LZ (Fig. 1).
FIG. 1.
Composition of the IKK core complex and its connection to upstream signaling molecules. Two IKKγs are associated with a heterodimer of IKKα-IKKβ. The interaction of IKKα and IKKβ is mediated through their LZs. The C terminus of IKKγ links the IKK core to the upstream signaling molecules. KD, kinase domain.
Highly purified recombinant IKKγ by itself can form up to tetramers, but it appears to bind IKKα-IKKβ as a dimer (33). Recombinant IKKγ interacts directly with recombinant IKKβ but not with recombinant IKKα (33). Since IKKα and IKKβ are mostly present as heterodimers (or tetramers), it is possible that IKKγ also interacts with IKKα once bound to IKKβ. A preferential interaction of IKKγ with IKKβ would allow for differential regulation of the two catalytic subunits, such that upstream activators that interact with the C terminus of IKKγ would activate the IKK complex via its IKKβ subunit. If this is indeed the case, it is possible that IKKα would interact with a different regulatory subunit, similar to IKKγ, that connects the IKK complex to a distinct set of upstream activators. Such an arrangement may allow for differential regulation of IKKα and IKKβ catalytic activities.
As mentioned above, recombinant IKKα and IKKβ form homodimers and heterodimers whose apparent molecular masses, determined by gel filtration, are 230 kDa (46). Addition of purified recombinant IKKγ to IKKβ results in the formation of a large, 900-kDa complex (48). Interestingly, in cells lacking IKKγ/NEMO, the IKK complex migrates as a small, 200- to 300-kDa complex and expression of IKKγ/NEMO in these cells restores formation of the large, 900-kDa, IKK complex (44). The exact stoichiometry of the large IKK complex is yet to be determined, but these experiments suggest it may be composed solely of IKKα, -β, and -γ.
Using techniques originally developed for isolation of the c-Jun kinase (22), Cohen et al. (15) chromatographed extracts of IL-1-stimulated 293 cells on a glutathione S-transferase–IκBα substrate affinity column and identified an IκB kinase complex similar in size to IKK. In addition to IKKα, IKKβ, NIK, IκBα, and NF-κB/RelA, this complex contains a 150-kDa protein that is named IKAP (IKK complex-associated protein) (15). IKAP is suggested to function as a scaffold protein due to its ability to assemble IKKα, IKKβ, NIK, and NF-κB:IκB (15). It should be noted, however, that it is not clear whether the complex found by Cohen and coworkers was purified to homogeneity and whether the reported polypeptides are its only components. For instance, Cohen and coworkers did not examine whether IKKγ, which is tightly associated with IKKα-IKKβ, is part of this complex. Interestingly, the interaction of IKAP with IKKα-IKKβ appears to be transient such that cell stimulation with IL-1 or TNF results in dissociation of the IKAP-IKKα-IKKβ complex (15). This may be the reason why a polypeptide corresponding in size to IKAP was not part of the highly purified IKK complex described by Rothwarf et al., which was isolated from extracts of TNF-stimulated cells (33). It also remains to be determined whether IKAP is required for IKK activation at all and, if so, whether it is involved in responses to all NF-κB-activating stimuli or to only a subset of them. It is possible that while IKKγ, which is a stoichiometric component of IKK, is required for IKK activation by all stimuli, proteins like IKAP, which are substoichiometric components, may connect IKK to a specific set of upstream activators.
REGULATION OF IKK ACTIVITY
NF-κB is activated by diverse stimuli, including ligands that act through cell surface receptors (e.g., TNF and IL-1), viral RNA and specific viral transactivator proteins, and UV and gamma rays (3, 4). Recently, UV activation was shown to activate NF-κB through a mechanism that does not depend on either IKK activation or IκB N-terminal phosphorylation (8, 27), but all other NF-κB inducers seem to operate via IKK. Therefore the IKK complex must be able to receive and respond to all of these signals. Indeed, it is already known that the activity of IKK is stimulated by TNF-α, IL-1, 12-O-tetradecanoate-13-acetate, lipopolysaccharide, the Tax protein of human T-cell leukemia type 1, double-stranded RNA, shear stress, and ionizing radiation (9, 14, 35, 44, 45, 47). The mechanisms by which these highly diverse stimuli activate IKK are, however, poorly understood. Structure prediction programs suggest the presence of numerous docking sites for interacting proteins on IKKα, IKKβ, IKKγ, and IKAP, but the search for signaling molecules that directly dock to these sites is in its infancy. Mitogen-activated protein kinase/ERK kinase kinases (MAP3Ks), such as NIK and MEK kinase 1 (MEKK1), activate IKK when overexpressed (for a review, see reference 24). NIK may potentially interact with IKK through IKAP (15). However, there is little evidence to date that NIK or MEKK1 is a physiological IKK activator. In the case of IKKγ (but not IKAP), genetic and biochemical data clearly show that IKKγ is essential for IKK activation in response to at least six different stimuli (33, 44), but it is not known with which upstream activators IKKγ interacts. One would expect that at least some of the upstream activators would be found to directly interact with IKKγ, most probably through its C-terminal LZ.
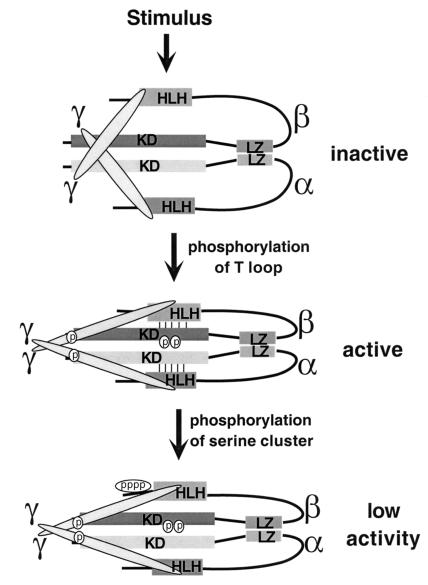
One of the central questions regarding regulation of IKK activity is, what are the specific roles of the individual subunits in its activation? IKK is activated by phosphorylation, since its treatment with protein phosphatase 2A results in its inactivation (19). Cell stimulation with TNF enhances the phosphorylation of all three IKK subunits (17). However, the bulk increase in IKK phosphorylation occurs with considerably slower kinetics than the increase in kinase activity. Phosphopeptide mapping of IKKβ (the subunit whose phosphorylation accounts for IKK activation) indicates that phosphorylation occurs at serine residues located in two regions: S177 and S181 in the T loop, and a cluster of 15 serines located between the HLH motif and the C terminus. Conversion of the T-loop serines of IKKβ to alanines prevents IKK activation, while substitution of alanine for serine in the equivalent sites in IKKα has no effect whatsoever on IKK activation by TNF, MEKK1, or NIK (17, 24). These experiments indicate that the IKKβ subunit is responsible for receiving the signals generated by cell stimulation with either TNF or IL-1. It is not yet clear which signals, if any, activate IKK via the IKKα subunit. To what extent phosphorylation of the activation sites at the T loop is due to the action of an upstream kinase and to what extent it is due to autophosphorylation are also not clear. Production of active recombinant IKKβ in insect cells also requires the phosphorylation of S177 and S181 within the T loop (17). Although it is possible that insect cells may contain an IKK-activating kinase, these results are more consistent with activation via autophosphorylation. It is possible that a small portion of IKK is activated initially via phosphorylation of IKKβ by an upstream kinase, which jump-starts the complex. A small number of activated IKK molecules then activate the rest by autophosphorylation.
Since prolonged NF-κB activation can lead to various inflammatory disorders and even death, due to excessive cytokine production, it is important not only to rapidly activate IKK and NF-κB in response to infection but also to quickly terminate their activities once the inflammatory stimulus has disappeared. While the T loop sites of IKKβ play a positive regulatory role, phosphorylation of the C-terminal serine cluster has a negative regulatory function (Fig. 2). Conversion of at least 10 of these serines to alanines results in a mutant form of IKKβ whose basal IκB kinase activity is severalfold higher than that of the wild-type form and whose activation lasts twice as long (17). Since these serines are autophosphorylated, the autophosphorylation of IKKβ appears to be an important negative autoregulatory mechanism preventing prolonged IKK activation.
FIG. 2.
Hypothetical scheme explaining the regulation of IKK by phosphorylation of the T loop and the C-terminal serine cluster of IKKβ. KD, kinase domain. See text for description of model.
A possible mechanism explaining how autophosphorylation negatively regulates IKK activity is suggested by the following findings. Mutations within the HLH motifs, which are located immediately next to the C-terminal serine cluster, abolish the kinase activity of purified recombinant IKKα and IKKβ (46). These results suggest that the HLH motif is required for full IKK activation through an intramolecular interaction with the kinase domain. Indeed, coexpression of a C-terminal fragment that includes the HLH motif with a deletion mutant of IKKβ lacking the C-terminal portion restores kinase activity to wild-type levels (17). Thus, it is likely that the C-terminal portion of IKKβ (and presumably IKKα), which contains the HLH motif, interacts with the kinase domain and that this interaction is required for full activity in addition to phosphorylation of the T loop. The close proximity of the C-terminal serine cluster to the HLH motif raises the possibility that its phosphorylation weakens the interaction between the HLH motif and the kinase domain, causing the kinase to reach a lower activity state (Fig. 2).
From data gathered so far, one can conclude that at least two events are required for full IKK activity. One is the interaction of the HLH motif with the kinase domain and the other is the phosphorylation of specific sites in the T loop of IKKβ. However, the order in which these two events occur and whether one depends on the other are not clear. Another mechanism that is essential for IKK function is the homo- or heterodimerization of IKKα and IKKβ. LZ mutations that disrupt dimerization also abolish kinase activity altogether, including autophosphorylation (46). These results may suggest that dimerization is required for transphosphorylation of the kinase domains. However, the fact that wild-type IKKβ heterodimerized with catalytically inactive IKKα can be fully activated (46) suggests otherwise. The exact mechanisms by which dimerization affects kinase activity is likely to be made more clear once the three-dimensional structure is solved.
Although the role of IKKγ in transducing the activating signals to the IKKα/β is established, its mechanistic details are not clear. Since the interaction of the HLH motif with the kinase domain is essential for kinase activity, IKKγ could stabilize this interaction. However, since purified IKKβ is very active in the absence of IKKγ and this activity is not further enhanced by binding to IKKγ, a structural role for IKKγ is unlikely. As discussed above, a more likely mechanism involves the recruitment of upstream activators (kinases) that act on IKKβ through interaction with the C-terminal LZ of IKKγ.
GENETIC ANALYSIS OF IKK FUNCTION
The experiments discussed above strongly suggest that IKKβ is far more important than IKKα for activation of the IKK complex in response to proinflammatory stimuli. As a genetic test of these findings and also to elucidate the biological functions of IKKα (if it is not involved in proinflammatory signaling), mouse mutants that lack either IKKα or IKKβ were generated. The results of these gene targeting experiments confirm the results of the biochemical experiments described above: IKKα is not required for activation of IKK in response to proinflammatory stimuli, whereas IKKβ is absolutely essential for this response. Most interestingly, however, these experiments indicate a new role for the IKKα in controlling the proliferation and differentiation of epidermal keratinocytes as well as affecting (directly or indirectly) other developmental decisions, including skeletal patterning (23, 33a). IKKα-deficient mice are born alive but die within 30 min. The mutant mice exhibit a plethora of developmental defects, the most striking of which are their taut, thick skin, highly rudimentary limbs and tail, and shorter head. It turns out, however, that IKKα−/− mice do have limbs and a tail whose proximal elements are almost normal, but they are hidden under their thickened skin. The distal elements of the limbs are maldeveloped due to a defect in interdigital apoptosis (23, 33a).
Histochemical and microscopic examination of IKKα−/− skin reveals marked hyperproliferation of the epidermal layer and an almost complete absence of differentiation. Due to the absence of fully keratinized cells, the mutant skin appears to be rather sticky, which causes the limbs and tail to be “glued” to the body instead of developing as well-separated outgrowths (23). Despite these marked alterations in morphology, the activation of IKK by TNF, IL-1, or lipopolysaccharide in the fibroblasts and liver of IKKα−/− mice seems to be normal (23).
The loss of IKKβ results in an expected phenotype, which confirms its importance for IKK activation by TNF and other proinflammatory stimuli. IKKβ−/− mouse embryos die on day 12 to 13 of gestation due to massive liver apoptosis (28). This phenotype is essentially identical to that of p65(RelA)-deficient mice (7), except that IKKβ−/− mice die a day or two earlier. This is probably due to the more severe decrease in NF-κB activity in IKKβ−/− cells than in RelA−/− cells. Indeed, protein kinase and mobility shift assays indicate that IKKβ−/− cells are completely defective in activation of IKK and NF-κB in response to TNF or IL-1 (28a).
In summary, despite the extensive sequence similarity between IKKα and IKKβ and their tight association in most cell types (33, 47), these two protein kinases play different regulatory and functional roles (Fig. 3). IKKβ is essential for IKK activation by proinflammatory cytokines and for IκB phosphorylation. Yet, IKKβ does not have an essential role in embryonic development. The hepatic apoptosis in IKKβ−/− embryos is simply due to a defect in NF-κB activation, which is required for protecting the liver from TNF-induced apoptosis (21). By contrast, IKKα is dispensable for IKK activation or IκB phosphorylation in response to proinflammatory stimuli but plays an essential role in epidermal development. The signals that activate IKKα during keratinocyte differentiation and its relevant substrates remain to be identified.
FIG. 3.
Summary of defects observed in mice lacking IKKα or IKKβ.
About 3 years have passed since the initial purification of the IKK complex. We have learned quite a bit during this period, but there are still many open questions. Given the rapid pace of progress so far, it is likely that many of these questions will be answered in the near future. It is also likely that, with time, we will learn about new substrates and functions of IKK that extend well beyond the realm of NF-κB.
ACKNOWLEDGMENT
E. Z. was supported in part by the Leukemia Society of America.
REFERENCES
- 1.Alkalay I, Yaron A, Hatzubai A, Jung S, Avraham A, Gerlitz O, Pashut-Lavon I, Ben-Neriah Y. In vivo stimulation of IκB phosphorylation is not sufficient to activate NF-κB. Mol Cell Biol. 1995;15:1294–1301. doi: 10.1128/mcb.15.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Baltimore D. NF-κB—ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin A S. The NF-κB and IκB proteins—new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 5.Barnes P J, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 7.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component in NF-κB. Nature. 1995;376:167–169. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 8.Bender K, Gottlicher M, Whiteside S, Rahmsdorf H J, Herrlich P. Sequential DNA damage-independent and -dependent activation of NF-κB by UV. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhullar I S, Li Y S, Miao H, Zandi E, Kim M, Shyy J Y, Chien S. Fluid shear stress activation of IκB kinase is integrin-dependent. J Biol Chem. 1998;273:30544–30549. doi: 10.1074/jbc.273.46.30544. [DOI] [PubMed] [Google Scholar]
- 10.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκBα proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1491. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκB to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 14.Chu Z L, DiDonato J A, Hawiger J, Ballard D W. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IκB kinases containing IKKα and IKKβ. J Biol Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 15.Cohen L, Henzel W J, Baeuerle P A. IKAP is a scaffold protein of the IκB kinase complex. Nature. 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 16.Connelly M A, Marcu K B. CHUK, a new member of the helix-loop-helix and leucine zipper families of interacting proteins, contains a serine-threonine kinase domain. Cell Mol Biol Res. 1995;41:537–549. [PubMed] [Google Scholar]
- 17.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 18.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 20.DiDonato J A, Mercurio F, Karin M. Phosphorylation of IκBα precedes but is not sufficient for its dissociation from NF-κB. Mol Cell Biol. 1995;15:1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doi T S, Marino M W, Takahashi T, Yoshida T, Sakakura T, Old L J, Obata Y. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc Natl Acad Sci USA. 1999;96:2994–2999. doi: 10.1073/pnas.96.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibi M, Lin A N, Smeal T, Minden A, Karin M. Identification of an oncoprotein-responsive and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Baus V, Delhase M, Zhang P, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of the IκB kinase. Science. 1999;284:318–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 24.Karin M, Delhase M. JNK or IKK, AP-1 or NF-κB, which are the targets for MEK kinase 1 action? Proc Natl Acad Sci USA. 1998;95:9067–9069. doi: 10.1073/pnas.95.16.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee F S, Peters R T, Dang L C, Maniatis T. MEKK1 activates both IκB kinase α and IκB kinase β. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Van Antwerp D, Mercurio F, Lee K-F, Verma I M. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 28a.Li, Z., et al. Unpublished data.
- 29.Liu Z G, Hsu H L, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions—Jnk activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 30.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 31.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 32.Regnier C H, Yeong Song H, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 33.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 33a.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 34.Traenckner E B M, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκBα on serines 32 and 36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlik M, Good L, Xiao G, Harhaj E W, Zandi E, Karin M, Sun S C. NF-κB-inducing kinase and IκB kinase participate in human T-cell leukemia virus I Tax-mediated NF-κB activation. J Biol Chem. 1998;273:21132–21136. doi: 10.1074/jbc.273.33.21132. [DOI] [PubMed] [Google Scholar]
- 36.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 37.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 38.Wang C Y, Mayo M W, Baldwin A S. TNF- and cancer therapy-induced apoptosis—potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 39.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 40.Whiteside S T, Ernst M K, LeBail O, Laurent-Winter C, Rice N, Israël A. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol Cell Biol. 1995;15:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 42.Wu M X, Ao Z, Prasad K V, Wu R, Schlossman S F. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- 43.Wulczyn F G, Krappmann D, Scheidereit C. The NF-κB/Rel and IκB gene families: mediators of immune response and inflammation. J Mol Med. 1996;74:749–769. doi: 10.1007/s001090050078. [DOI] [PubMed] [Google Scholar]
- 44.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 45.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-1 Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 46.Zandi E, Chen Y, Karin M. Direct phosphorylation of IκB by IKKα and IKKβ: discrimination between free and NF-κB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 47.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 48.Zandi, E., and M. Karin. Unpublished data.