Abstract
Background aims:
VSV-G pseudotyped lentiviral vectors (LVs) are widely used to reliably generate genetically modified, clinical grade T-cell products. However, the results of genetically modifying NK cells with VSV-G LVs have been variable. We explored if inhibition of the IKK-related protein kinases, TBK1/IKKε, key signaling molecules of the endosomal TLR4 pathway, which is activated by VSV-G, would enable the reliable transduction of NK cells by VSV-G LVs.
Methods:
We activated NK cells from PBMCs using standard procedures and transduced them with VSV-G LVs encoding a marker gene (YFP) or functional genes (CARs, costimulatory molecules) in the presence of three TBK1/IKKε inhibitors (MRT67307, BX-795, Amlexanox). NK cell transduction was evaluated by flowcytometry and/or western blot and the functionality of expressed CARs was evaluated in vitro.
Results:
Blocking TBK1/IKKε during transduction of NK cells enabled their efficient transduction by VSV-G LVs as judged by YFP expression of 40 to 50% with EC50s of 1.1 μM (MRT67307), 5 μM (BX-795), and 24.8 μM (Amlexanox). Focusing on MRT67307, we successfully generated NK cells expressing CD19-CARs or HER2-CARs with an inducible costimulatory molecule. CAR NK cells exhibited increased cytolytic activity and ability to produce cytokines in comparison to untreated controls, confirming CAR functionality.
Conclusions:
We demonstrate that inhibition of TBK1/IKKε enables the reliable generation of genetically modified NK cells using VSV-G LVs. Our protocol can be readily adapted to generate clinical grade NK cells and thus, has the potential to facilitate the clinical evaluation of genetically modified NK cell-based therapeutics in the future.
Keywords: Immunotherapy, Chimeric Antigen Receptor, Natural Killer, Cancer, Lentivirus, TBK1, IKKε, VSV-G
Introduction
Immunotherapy with genetically modified natural killer (NK) cells has garnered significant interest due to the recent encouraging clinical results of NK cells genetically modified to express CD19-specific chimeric antigen receptors (CARs) and interleukin (IL)15 for patients with non-Hodgkin lymphoma1,2. In this clinical study and other preclinical studies, NK cells were genetically modified with GALV- or RD114-pseudotyped retroviral vectors2.
In addition to recombinant retroviruses, lentiviruses (LVs) are attractive vectors to genetically modify immune cells for adoptive immunotherapy. However, in contrast to T cells, the transduction efficiency of current Vesicular Stomatitis Virus G (VSV-G) pseudotyped LVs for NK cells has been disappointing3. Several groups have explored strategies to improve the transduction efficiency of NK cells by VSV-G pseudotyped LVs (VSV-G LVs), including using protamine sulfate or upregulating the LDL receptor, the receptor required for LVs insertion4,5. In addition, one preclinical study demonstrated that inhibiting the antiviral pathway signaling through TBK1/IKKα/β/ε with a non-specific PDKI inhibitor, BX-795, resulted in increased transduction of NK cells6. More recently, several studies have highlighted that baboon retroviral envelope glycoprotein (BaEV) pseudotyped LVs allowed for efficient transduction of NK cells3,7. Unfortunately, high titer viral preparations of BaEV-LVs are difficult to produce with standard protocols8 and to our knowledge no clinical study is currently being conducted with BaEV-LV transduced cells.
As highlighted by one previous publication6, the inefficiency of VSV-G LVs might not to be due to limited transduction, but due to triggering of toll like receptor (TLR) 3 and/or cytosolic antiviral innate immune responses by VSV-G resulting in NK cell death. However, the successful use of BaEV-LV particles to transduce NK cells suggests that VSV-G might trigger TLRs via proteins rather than cytosolic viral RNA sensors. VSV-G activates TRL4 signaling pathways9,10, and TLR4 is expressed on the surface of, and intracellularly, in NK cells allowing for a diverse repertoire of cognate ligands11,12.
Here, we explored if specific and transient blockade of the IKK-related protein kinases, TBK1/IKKε, which are central players within the innate immune signaling pathways including endosomal TLR413,14, permits VSV-G LVs to effectively and efficiently transduce NK cells. We demonstrate that inhibition of TBK1/IKKε enables expression of functional transgene in primary human NK cells post-VSV-G LV transduction. Thus, our protocol will enable the generation of clinical grade NK cells that are transduced with genetically modified VSV-G LVs.
Methods
Cell lines
BV173, and A549 were obtained and grown as per American Type Culture Collection (ATCC, Manassas, VA, USA) instructions. K562 with modified membrane bound interleukin (IL)-15 and 4–1BB ligand15, feeder cells, were a generous gift from Dr. Dario Campana (National University of Singapore) and grown in IMDM media with 10% fetal bovine serum (FBS; Hyclone Laboratories, Chicago, IL, USA). CD19 BV173 cell line were generated with CRISPR/Cas9 technology using a published method16.
Generation of lentiviral vectors
The generation of the CD19-CAR.4–1BB.CD3δ LV was previously described17; this LV is also used in our current clinical study to generate clinical grade CD19-CAR T cells (NCT03573700). The same LV backbone, except that the insulators were removed from the self-inactivating 3′ partially deleted viral long terminal repeats, was used to generate the LV encoding HER2-CAR.CD3δ.2A.iMC. This expression cassette was subcloned from a previously published retroviral vector18 and is under control of the MND promoter (myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted). The cloned construct was verified by sequencing at the Hartwell Center at St. Jude Children’s Research Hospital (St. Jude). Purified VSV-G pseudotyped LV particles were produced by the St. Jude Vector Core Laboratory by using transient transfection, followed by fast protein liquid chromatography purification8. The St. Jude Vector Core Laboratory also provided VSV-G pseudotyped LV particles encoding YFP.
NK cell activation, expansion, and genetic modification
Human peripheral blood mononuclear cells (PBMCs) were obtained from whole blood of healthy donors under an IRB approved protocol at St. Jude, after informed consent was obtained in accordance with the Declaration of Helsinki. Cells were subjected to ACK Red Blood cell lysis and Ficcol Hypaque (Sigma-Aldrich, St. Louis, MO, USA) gradient separation. Cellular subtype analysis was performed with BD whole blood analysis kit on a BD Lyric flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA). Cells were aliquoted and Freezing Media with 10% DMSO at 1×107 cells per mL and stored in liquid nitrogen vapor phase until use. 150 Gray cesium-irradiated feeder cells were added to thawed PBMCs at a ratio of 5–10:1 feeder to NK cells as determined previously. Cells were grown in Stemcell Genix (20802–0500, Cellgenix, Portsmouth, MA, USA) growth media with 20% FBS and 10 Units/mL of IL-2, (Peprotech, Rocky Hill, NJ, USA). After 5–7 days cells were phenotyped and used for downstream experiments.
Genetically modified NK cells were generated as follows. 500,000 NK cells were seeded per well of a 24 well tissue culture plate in a volume of 1 mL of complete growth media. Inhibitors BX795, MRT67307, and Amlexanox (702675–74-9, 1190378–57-4, and 68302–57-8, Cayman Chemical, Ann Arbor, MI, USA) were added at indicated concentrations for 30 minutes and LVs were then added at indicated MOIs. NK cells were incubated for 24 hours and then media was removed and replaced with 2 mL of complete growth media. NK cells where then assessed for transgene expression 72 hours later.
Western Blot
Cell lysates were prepared with RIPA buffer with protease inhibitor cocktail. 20μg of total protein were loaded in 12% pre-cast gels (Biorad Laboratories, Hercules, CA, USA) gels were transferred with the iBlot2 system with run template P0(Applied Biosystems, Foster City, CA, USA). Antibodies used were anti-CD3δ (6B10.2, Santa Cruz Biotechnology, 1:1,000) with anti-Mouse HRP (NA931, Cytiva, Chicago, IL, USA; 1:10,000), Anti-GAPDH–HRP (GAPDH 71.1; catalog G9295, Sigma-Aldrich; 1:10,000), anti-HA(A190–108A, Bethyl Laboratories; 1:1000) with anti-Rabbit HRP (NA934V, Cytiva; 1:10,000) Were used for chemiluminescent detection digitally imaged on a Li-COR machine (Li-COR, Lincoln, NE, USA).
Flow cytometry
250,000 NK cells were collected and washed twice in DPBS. Surface HER2-CAR detection was determined via immunolabeling with anti-F(ab’)2 -AF647 (109–606-006, Jackson Labs, Bar Harbor, ME, USA; 1:100), CD19-CAR was detected with PE conjugated recombinant human CD19 protein (3309HP, Creative Biomart, Shirley, NY, USA; 1:100), or YFP were utilized for detection on a BD FACS Lyric machine and analyzed with FlowJo v10 (BD). Immunophenotyping was performed on a BD Symphony flow cytometer. Antibodies used are delineated in Supplemental Table 1.
IsoLight cytokine detection
Briefly, 500,000 NK cells were labeled with cell trace violet 1:1000 (ThermoFisher Scientific, Waltham, MA, USA) co-cultured at a 2:1 ratio with A549 targets for 4 hours in a 24 well plate. NK cells were removed and washed twice with PBS and resuspended in complete growth media without IL-2. These cells were loaded onto a single cell secretomic chip, IsoLight, that detects 32 distinct proteins19. Results were analyzed on IsoSpeak version 2.7.0.0 (IsoPlexis, Branford, CT, USA).
Cytotoxicity assays
A549: 10,000 A549 cells as targets for HER2-CAR NK cells, cocultures were set at 2:1, 1:1, and 0.5:1 effector to target (E:T) ratios for 4 hours in a 96 well plate. Cytotoxicity was quantified by a chromogenic MTS assay measured on a plate reader (Tecan, Männedorf, Switzerland) detecting remaining viable adherent tumor cells. Virally transduced, but MRT67307 untreated NK cells were used as controls (untreated). BV173: 50,000 BV173 cancer cells as targets for CD19-CAR NK cells, BV173 cancer cells were labeled with CFSE (ThermoFisher, 1:500) and co-cultures were set at indicated ratios in 200μL and after 4-hours of co-incubation, 3000 BD countbright beads were added to each well and assessed via flowcytometry for remaining CFSE positive BV173 cells. Samples were acquired until 100 beads were recorded. The percentage of remaining CFSE tumor cells compared to control ‘no NK cells’ wells was calculated according to the following equation: .
Results
We explored the feasibility of modifying NK cells with VSV-G LVs using molecules that transiently block TBK1/IKKε, downstream of the endosomal TLR4 pathway, which is activated by VSV-G9,10. An overview of this pathway and drug compounds used in our study are depicted in (Figure 1A). NK cells were generated from PBMCs by employing standard expansion techniques using irradiated K562 cells, expressing membrane bound IL-15 and 4–1BB ligand, in the presence of exogenous IL-2. NK cells were then transduced with VSV-G LVs encoding a yellow fluorescent protein (YFP) at an MOI of 10 on days 5 to 7 post initial activation and selective expansion. Concurrent with viral particle addition, increasing concentrations of inhibitors were added. After 24 hours, inhibitors and LV containing media was removed and replaced with fresh NK cell growth media. We decided to evaluate 3 compounds, MRT6730720, BX-79521, and Amlexanox22. All drugs block at least TBK1/IKKε (Figure 1A) with MRT67307, a derivative of BX-795, being more specific for TBK1/IKKε than BX-79520. Transduction efficacy was determined by FACS analysis 72 hours post transduction (Figure 1B). In the presence of all three compounds, NK cells expressed YFP in a dose dependent manner with an EC50 of 1.1 μM for MRT67307, 5 μM for BX-795, and 24.8 μM for Amlexanox (Figure 1C). Based on these results we used the compound with the lowest EC50, MRT67307, at a 2 μM concentration for all subsequent experiments going forward. At this concentration MRT67307 treatment had no effect on NK cell phenotype (Supplemental Figure 1).
Figure 1: Viral pathway inhibition allows for VSV-G LV transduction of NK cells.
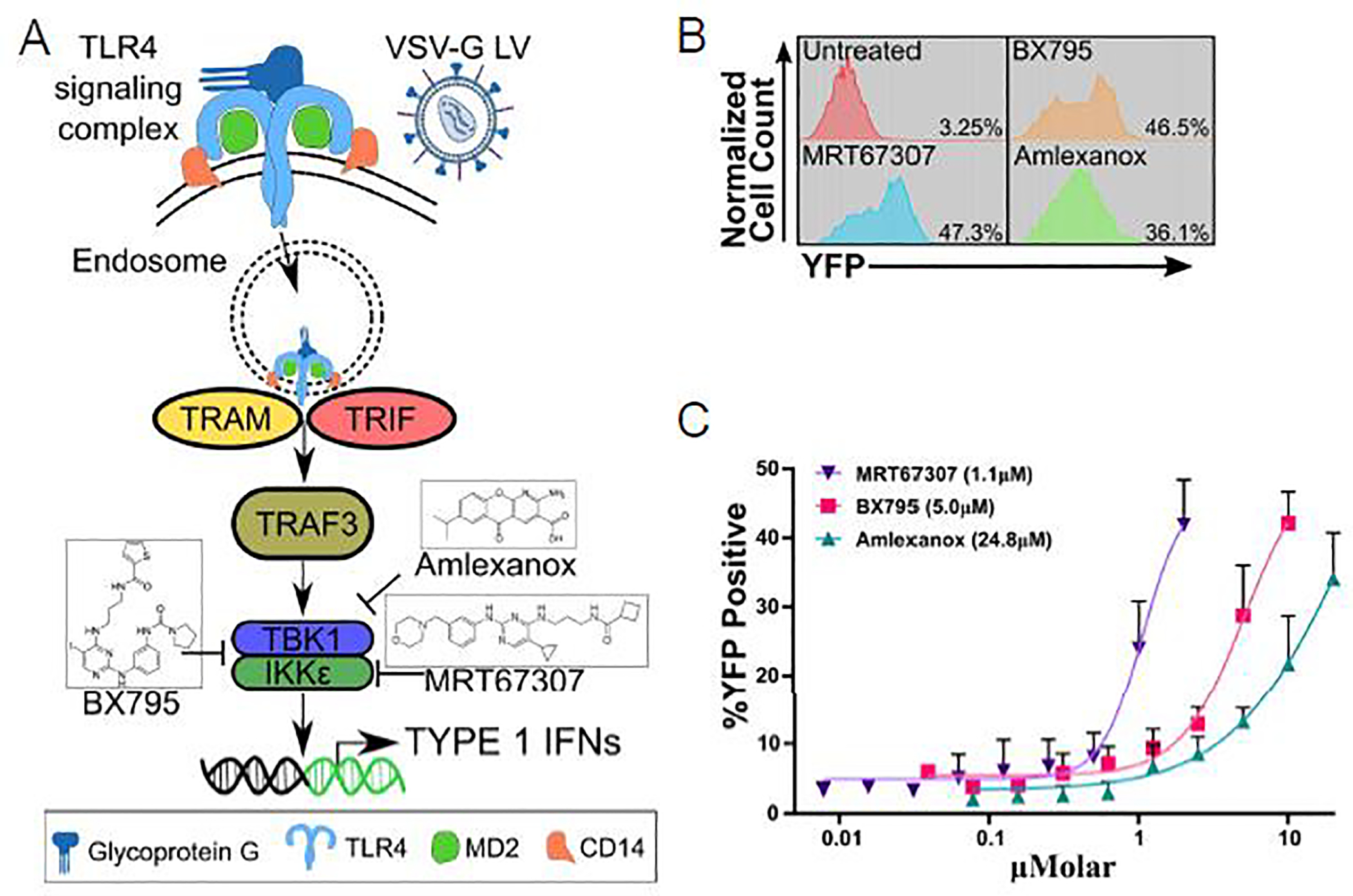
(A) Simplified endosomal TLR4 viral sensing pathway schematic in NK cells with indicated drug targeting. (B) Representative flow cytometry histogram plots of YFP expression in NK cells at highest used drugs concentrations (untreated: NK cells transduced in the absence of TBK1/IKKε inhibitors). (C) %YFP expression curves, N=3 mean±sem, in response to increasing drug concentrations; the half maximal effective concentration (EC50) for each compound is shown in parentheses.
Cationic additives such as polybrene, protamine sulfate, and LentiBOOST™ have been utilized to enhance transduction efficiencies of VSV-G LVs for other cell types29,30. We therefore determined the effect of LentiBOOST™ in the presence of TBK1/IKKε inhibition, and LentiBOOST™ did not further enhance transduction efficiencies (Supplemental Figure 2). Since LentiBOOST™ by itself did not enhance transduction efficiencies of NK cells, we confirmed our finding for a 2nd cationic additive (polybrene) (Supplemental Figure 2).
We next sought to explore the possibility of inserting larger constructs into NK cells using our developed protocol. We elected to use a CD19-CAR designed to target CD19+ malignancies with a 4–1BB.CD3δ signaling domain23 (Figure 2A). We employed a range of MOIs and observed similar transduction efficiencies as determined by FACS analysis for CAR expression (Figure 2B). To validate CD19-CAR functionality, we compared the cytolytic activity of CD19-CAR NK cells against the CD19+ leukemia cell line, BV173 (BV173.wt). Furthermore, to show target antigen specificity we used BV173 in which CD19 was knocked out by CRISPR/Cas9 gene editing (BV173.CD19KO). CD19-CAR NK cells were capable of targeting BV173 cells regardless of CD19 expression at high effector to target ratios during a 4-hour co-culture assay. However, expression of CD19-CAR in NK cells enabled them to kill CD19+ BV173 cells, BV173.wt, at a very low effector to target ratio (0.62 and 0.31) in contrast to BV173.CD19KO cells (Figure 2C).
Figure 2: CD19-CAR NK cells recognize and kill CD19-positive target cells.

(A) Scheme of CD19-CAR (GREEN: antigen recognition domain, GREY: hinge and transmembrane domain, ROSE: costimulatory domain, BLUE: signaling domain). (B) Representative flow cytometry histogram plot depiction CD19-CAR expression with indicated MOIs (untreated: NK cells transduced in the absence of MRT67307 at MOI of 30). (C) Flow cytometric based cytotoxicity assay of CD19-CAR NK cells targeting leukemia cell lines BV173 and BV173.CD19KO at indicated effector to target (E:T) ratios; N=4 donors, averaged technical triplicates per donor, mean±sem; unpaired two-tailed student’s t-test was used to determine significance, **p<0.01.
Expanding upon our previous findings, we sought to insert two genes, encoded by a single vector, into NK cells. The LV construct encoded a 1st generation HER2-CAR24, a 2A peptide, and an inducible MyD88/CD40 (iMC)18 costimulatory molecule (Figure 3A). This entire vector insert is approximately 2.8 kilobases in length. We analyzed surface CAR expression by flow cytometry and validated the expression of the full-length CAR and iMC via western blot. We achieved approximately 50% transduction of NK cells as judged by CAR cell surface expression (Figure 3B); in addition, western blot demonstrated CAR and iMC expression (Figure 3C).
Figure 3: Viral pathway inhibition allows insertions of two transgenes into NK cells.
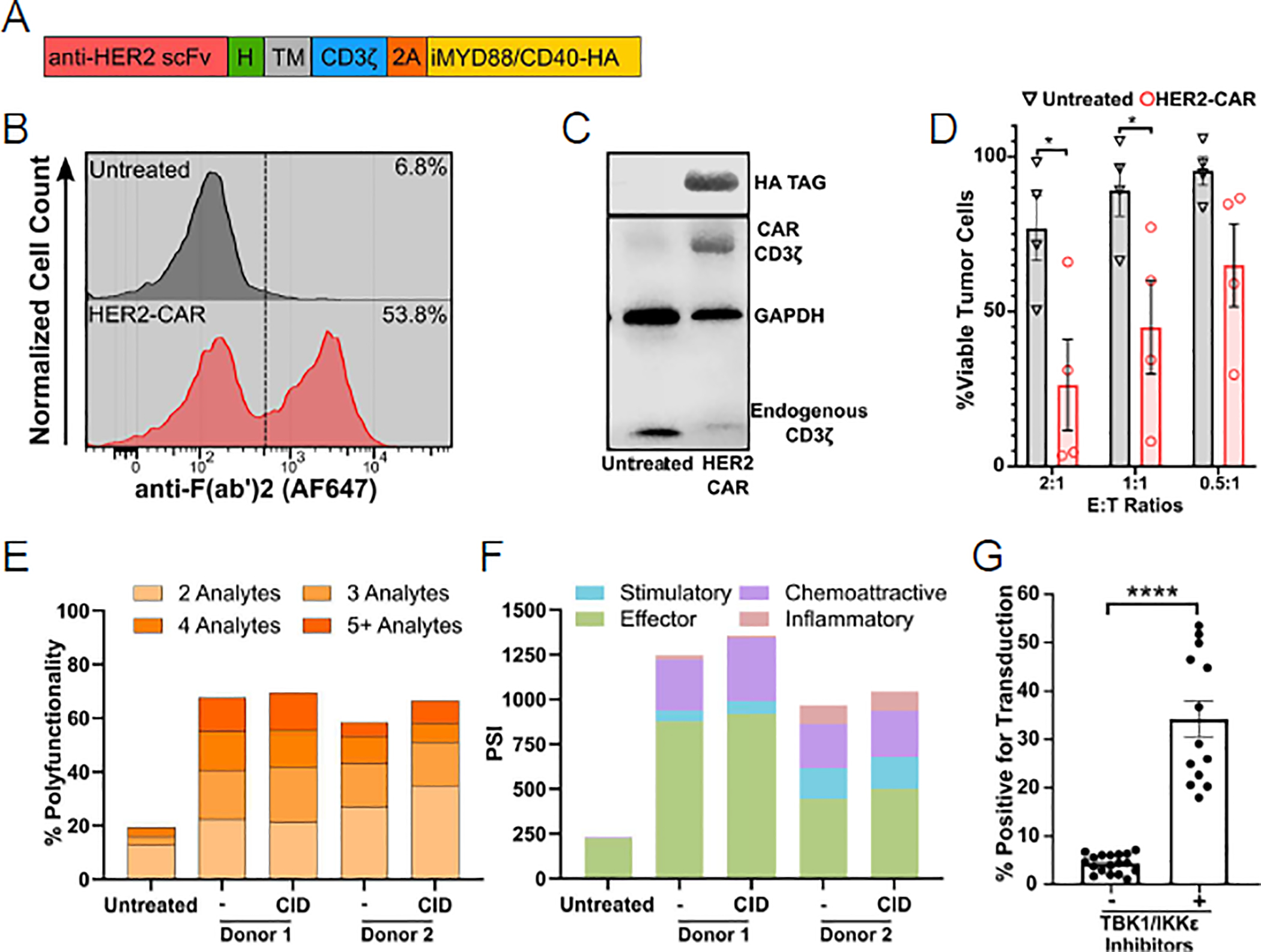
(A) Scheme of HER2-CAR.2A.iMC expression cassette (RED: antigen recognition domain, GREEN: hinge (H), GREY: transmembrane Domain (TM), BLUE: signaling domain, ORANGE: 2A sequence, GOLDENROD: iMC). (B) Representative flow cytometry plot demonstrating HER2-CAR expression detected with anti-F(ab’)2. (C) Western blot analysis of HER2-CAR NK cells demonstrating i) HER2-CAR.CD3δ and endogenous CD3δ (anti-CD3δ), and ii) iMC (anti-HA tag) expression. GAPDH was used a loading control. (D) 4-hour MTS cytotoxicity assay at an E:T ratio of 2:1, 1:1, and 0.5:1 with NK cells that were transduced with HER2-CAR LVs in the absence (untreated) or presence of MRT67307 (HER2-CAR) as effectors and A549 cells as targets, N=4 donors, averaged technical replicates per donor, mean±sem. Unpaired two-tailed student’s t-test was used to determine significance, *p<0.05. (E) Percentage of NK cells that produced 2, 3, 4, or 5+ effector molecules of a 32-analyte panel. Chemical Inducer of Dimerization: CID was used to initiate iMC signaling. (F) Polyfunctional Strength Index detailing effector, stimulatory, regulatory, chemo attractive, and inflammatory molecules; N=2 donors. (G) Aggregate data of NK cells transduced in the absence (−) (N=18) or presence (+) (BX-795 N=4, MRT67307 N=9) of TBK1/IKKε inhibitors; mean±sem; ****p<0.0001; Mann Whitney U-test.
Further, we assessed CAR functionality in a 4-hour cytotoxicity assay against the HER2+ A549 (lung cancer adenocarcinoma) cell line. At an E:T ratio of 2:1 and 1:1, HER2-CAR NK cells exhibited significant killing of A549 cells (Figure 3D). In addition, we analyzed the expression of cytolytic molecules, cytokines, and chemokines after activating NK cells for 4 hours with A549 cells using the IsoLight single cell secretomics instrument. On average, HER2-CAR NK cells secreted granzyme B, perforin, TNF-α, MIP1-β, MCP-1, RANTES, and IL-8 at exceptionally increased levels; in contrast, less than 13% of unmodified NK cells secreted Granzyme B and less than 3.5% secreted the other cytokines. MIP1-α and IFN-γ were nearly identical between all groups. Additionally, some HER2-CAR NK cells could produce upwards of 10 effector molecules simultaneously. However, the majority produced 5 effector molecules or less (Figure 3E,F). In contrast, unmodified NK cells were only capable of secreting on average 2 to 3 effector molecules at a far lower frequency. Activating iMC did not have a significant impact on effector molecule production in this 4-hour co-culture assay (Figure 3E,F). To demonstrate the reproducibility that TBK1/IKKε inhibition enhances VSV-G LV transduction at various MOIs (10 – 30), we compiled obtained transduction efficiencies from all our experiments (Figure 3G). The mean transduction efficiency was 34.22% (range: 18–53.5%), which was significantly greater (p<0.0001) than for NK cells that were transduced without TBK1/IKKε inhibition (mean: 4.39%, range: 1.12–7.19%).
Discussion
We have developed a method for NK cell transduction with VSV-G LVs that utilizes a transient antiviral pathway blockade. The distinct lack and failed attempts of VSV-G-based LV modification for NK cells highlights the additional requirements or alternate methods needed for successful transduction7. However, there are few reports that have used VSV-G LVs25–27 that encoded GFP, a relatively small transgene (~800 bp). The procedures were not always well defined, typically involved multiple rounds of transduction and sorting both of which increases the risk of contamination. Our protocol describes a method that utilizes a single round transduction enabling large gene insertions (~3,000 bp). Further, we have successfully used a low MOI (5) for robust expression of clinical grade CARs as compared to other reports using upwards of 150 MOI28. CAR NK cells showed enhanced cytolytic activity against antigen-positive target cells, demonstrating CAR functionality. Here, we did not HLA or KIR type donor NK cells and/or tumor cells. However, in future studies we are planning to confirm our findings in the autologous setting in which tumor and NK cells are HLA and KIR matched to account for enhanced killing due to a mismatched KIR/KIR-ligand pair in the allogeneic setting.
We selected the three compounds to demonstrate unequivocally that blocking TBK1/IKKε is critical for the observed benefit. Starting with a more broad spectrum inhibitor of PDK1, ULK1, and NF-kB activation, BX795, an increasingly specific inhibitor, MRT67307, and finally, Amlexlanox, an FDA approved drug32. LentiBOOST™ did not further enhance transduction efficiency, suggesting that viral binding to the cell surface is not limiting NK cell transduction by VSV-G LVs. One limitation to our study remains that MRT67307 may also inhibit ULK1, a major protein in the autophagy pathway. Autophagy is used to limit viral spread upon infection33. However, we believe that the main reason that all three drugs enable transgene expression in NK cells post VSV-G LV transduction is their ability to block TBK1/IKKε downstream of endosomal TLR4 signaling. Based on our findings, we speculate that other more ‘innate-like’ cell populations may benefit from antiviral pathway inhibition during VSV-G LV transduction including γδ T cells 34,35.
In conclusion, TBK1/IKKε inhibition enables the transductions of NK cells with VSV-G LVs. Our approach could readily be translated into clinical grade production of genetically modified NK cells, and thus has the potential to have a significant impact on the rapidly expanding NK cell therapy field.
Supplementary Material
Highlights.
VSV-G LVs cannot be used to transduce NK cells with standard protocols.
VSV-G activates TLR4; which activates innate anti-viral immune responses.
Inhibition of endosomal TLR4 signaling enables the transduction of NK cells with VSV-G LVs.
TLR4 inhibition should enable generation of clinical grade LV-transduced NK cells.
Acknowledgements
We would like to thank Abishek Vaidya and Jessica Wagner for technical assistance. The work was supported by the St. Jude Sumara Fellowship (PC), the American Lebanese Syrian Associated Charites (SG), and the National Institutes of Health (NIH)/National Cancer Institute (NCI) grant P30 CA021765. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest
SG has patent applications in the fields of T-cell and/or gene therapy for cancer. He has a research collaboration with TESSA Therapeutics, is a DSMB member of Immatics, and on the scientific advisory board of Tidal.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu E et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531, doi: 10.1038/leu.2017.226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu E et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med 382, 545–553, doi: 10.1056/NEJMoa1910607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bari R et al. A Distinct Subset of Highly Proliferative and Lentiviral Vector (LV)-Transducible NK Cells Define a Readily Engineered Subset for Adoptive Cellular Therapy. Front Immunol 10, 2001, doi: 10.3389/fimmu.2019.02001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amirache F et al. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood 123, 1422–1424, doi: 10.1182/blood-2013-11-540641 (2014). [DOI] [PubMed] [Google Scholar]
- 5.De Sanctis JB, Blanca I, Radzioch D & Bianco NE Expression and function of low-density lipoprotein receptors in CD3-CD16+CD56+ cells: effect of interleukin 2. Cell Immunol 167, 18–29, doi: 10.1006/cimm.1996.0003 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Sutlu T et al. Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: implications for gene therapy. Hum Gene Ther 23, 1090–1100, doi: 10.1089/hum.2012.080 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colamartino ABL et al. Efficient and Robust NK-Cell Transduction With Baboon Envelope Pseudotyped Lentivector. Front Immunol 10, 2873, doi: 10.3389/fimmu.2019.02873 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauler M et al. Production of Lentiviral Vectors Using Suspension Cells Grown in Serum-free Media. Mol Ther Methods Clin Dev 17, 58–68, doi: 10.1016/j.omtm.2019.11.011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgel P et al. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology 362, 304–313, doi: 10.1016/j.virol.2006.12.032 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Olejnik J, Hume AJ & Muhlberger E Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog 14, e1007390, doi: 10.1371/journal.ppat.1007390 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza-Fonseca-Guimaraes F et al. Toll-like receptors expression and interferon-γ production by NK cells in human sepsis. Crit Care 16, R206, doi: 10.1186/cc11838 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adib-Conquy M, Scott-Algara D, Cavaillon J-M & Souza-Fonseca-Guimaraes F TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunology & Cell Biology 92, 256–262, doi: https://doi.org/ 10.1038/icb.2013.99 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Amaya M, Keck F, Bailey C & Narayanan A The role of the IKK complex in viral infections. Pathog Dis 72, 32–44, doi: 10.1111/2049-632X.12210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kravchenko VV, Mathison JC, Schwamborn K, Mercurio F & Ulevitch RJ IKKi/IKKepsilon plays a key role in integrating signals induced by pro-inflammatory stimuli. J Biol Chem 278, 26612–26619, doi: 10.1074/jbc.M303001200 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Imai C, Iwamoto S & Campana D Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106, 376–383, doi: 10.1182/blood-2004-12-4797 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gundry MC et al. Highly Efficient Genome Editing of Murine and Human Hematopoietic Progenitor Cells by CRISPR/Cas9. Cell Rep 17, 1453–1461, doi: 10.1016/j.celrep.2016.09.092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan WK et al. Chimeric antigen receptor-redirected CD45RA-negative T cells have potent antileukemia and pathogen memory response without graft-versus-host activity. Leukemia 29, 387–395, doi: 10.1038/leu.2014.174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mata M et al. Inducible Activation of MyD88 and CD40 in CAR T Cells Results in Controllable and Potent Antitumor Activity in Preclinical Solid Tumor Models. Cancer Discov 7, 1306–1319, doi: 10.1158/2159-8290.CD-17-0263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, Paczkowski P, Mackay S, Ng C & Zhou J Single-Cell Multiplexed Proteomics on the IsoLight Resolves Cellular Functional Heterogeneity to Reveal Clinical Responses of Cancer Patients to Immunotherapies. Methods Mol Biol 2055, 413–431, doi: 10.1007/978-1-4939-9773-2_19 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Clark K et al. Novel cross-talk within the IKK family controls innate immunity. Biochem J 434, 93–104, doi: 10.1042/BJ20101701 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Clark K, Plater L, Peggie M & Cohen P Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem 284, 14136–14146, doi: 10.1074/jbc.M109.000414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reilly SM et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med 19, 313–321, doi: 10.1038/nm.3082 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velasquez MP et al. CD28 and 41BB Costimulation Enhances the Effector Function of CD19-Specific Engager T Cells. Cancer Immunol Res 5, 860–870, doi: 10.1158/2326-6066.CIR-17-0171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed N et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res 67, 5957–5964, doi: 10.1158/0008-5472.CAN-06-4309 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Micucci F et al. High-efficient lentiviral vector-mediated gene transfer into primary human NK cells. Exp Hematol 34, 1344–1352, doi: 10.1016/j.exphem.2006.06.001 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Gong Y et al. Rosuvastatin Enhances VSV-G Lentiviral Transduction of NK Cells via Upregulation of the Low-Density Lipoprotein Receptor. Mol Ther Methods Clin Dev 17, 634–646, doi: 10.1016/j.omtm.2020.03.017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran J & Kung SK Lentiviral vectors mediate stable and efficient gene delivery into primary murine natural killer cells. Mol Ther 15, 1331–1339, doi: 10.1038/sj.mt.6300184 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Boissel L et al. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma 53, 958–965, doi: 10.3109/10428194.2011.634048 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauber I et al. Improving Lentiviral Transduction of CD34(+) Hematopoietic Stem and Progenitor Cells. Hum Gene Ther Methods 29, 104–113, doi: 10.1089/hgtb.2017.085 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Simon B et al. Enhancing lentiviral transduction to generate melanoma-specific human T cells for cancer immunotherapy. J Immunol Methods 472, 55–64, doi: 10.1016/j.jim.2019.06.015 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Jamali A et al. Highly Efficient Generation of Transgenically Augmented CAR NK Cells Overexpressing CXCR4. Front Immunol 11, 2028, doi: 10.3389/fimmu.2020.02028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell J Amlexanox for the treatment of recurrent aphthous ulcers. Clin Drug Investig 25, 555–566, doi: 10.2165/00044011-200525090-00001 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Kudchodkar SB & Levine B Viruses and autophagy. Rev Med Virol 19, 359–378, doi: 10.1002/rmv.630 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher J & Anderson J Engineering Approaches in Human Gamma Delta T Cells for Cancer Immunotherapy. Front Immunol 9, 1409, doi: 10.3389/fimmu.2018.01409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vantourout P & Hayday A Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 13, 88–100, doi: 10.1038/nri3384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


