Abstract
Background
Young adult (YA) survivors of allogeneic hematopoietic cell transplant (HCT) are at risk for late psychosocial challenges, including inability to return to work post-HCT. However, work-related outcomes in this population remain understudied.
Objectives
To assess the post-HCT work status of survivors of allogeneic HCT who underwent HCT as YA and analyze the patient-, disease-, and HCT-related factors associated with their work status at 1-year post-HCT.
Study Design
Using the Center for International Blood and Marrow Transplant Research (CIBMTR) data, we described post-HCT work status (full-time, part-time work, unemployed, and medical disability) of YA HCT survivors (N=1365) who underwent HCT between 2008 and 2015. Percentages of work status categories were reported at four timepoints: 6-months, 1-, 2-, and 3-year post-HCT. Percentages of post-HCT work status categories at the 1-year timepoint were also described in relation to survivors’ pre-HCT work status categories. Factors associated with 1-year post-HCT work status (full-time or part-time work) were examined using logistic regression.
Results
From 6 months to 3 years post-HCT, the percentage of survivors working full-time and part-time increased from 18.3% to 50.7%, and from 6.9% to 10.5%, respectively. Of patients in full-time work pre-HCT, 50% were unemployed or on medical disability at 1-year post-HCT. Female sex (Odds ratio [OR] 0.55; 95% confidence interval [CI] 0.40–0.77), HCT-comorbidity index (HCT-CI) score ≥3 (OR 0.57; 95% CI 0.39–0.82), pre-HCT unemployment (OR 0.37; 95% CI 0.24–0.56), and medical disability (OR 0.44; 95% CI 0.28–0.70), development of grade 3–4 acute graft vs. host disease (OR 0.52; 95% CI 0.34–0.80), and relapse within one-year post-HCT (OR 0.34; 95% CI 0.21–0.56) were associated with lower likelihood of employment at 1-year post-HCT. Compared to myeloablative conditioning with total body irradiation (TBI), myeloablative conditioning without TBI (OR 1.71; 95% CI 1.16–2.53) was associated with higher likelihood of employment at 1-year post-HCT. Graduate school level education (OR 2.47; 95% CI 1.49–4.10) was also associated with higher likelihood of employment at 1-year post-HCT.
Conclusions
While the work status among YA HCT survivors continued to improve over time, a substantial subset became or remained unemployed or on medical disability. These findings underscore the need for effective return to work supportive interventions in this population.
Keywords: hematopoietic cell transplant, return to work, quality of life, young adult
Introduction:
Allogeneic hematopoietic cell transplantation (HCT) is commonly used as a curative therapy for young adults (YA; age 18–39 years) with malignant and non-malignant hematological conditions.1,2 Annually nearly 1,500 YA undergo allogeneic HCT, and its utilization has increased by 40% in the last decade.3 Survival rates after HCT have gradually improved due to several factors, such as better donor availability, improvements in human leukocyte antigen (HLA) typing techniques, and supportive care.3 However, many survivors continue to be at risk for treatment-related late morbidity and impairments in quality of life (QOL).4–6
From prior studies focusing on YA cancer survivors, it is known that they have a unique set of long-term psychosocial challenges, affecting their social relationships and functioning, emotional health, and educational, vocational, and financial status.7–9 Given the age range of YA, they face critical personal and professional milestones, including transitioning into adult roles at the time of their illness, which may impact their ability to resume age appropriate activities, such as attending work or school, and may eventually affect their educational or vocational progression.9 Particularly, YA cancer survivors have been noted to struggle to return to work secondary to treatment-related physical and cognitive dysfunction,10 and have higher unemployment rates compared to the general population.7,8,11 Patients undergoing allogeneic HCT are known to be at a higher risk of late morbidities and QOL impairments compared to survivors treated with non-HCT cancer directed therapy.4,5 While work-related challenges in HCT survivors transplanted as children12 or older adults13–18 are known, and return-to-work at 1-year post-HCT is considered an important indicator of survivors’ overall social and economic well-being,19 YA HCT survivors’ ability to return to work and factors affecting their post-HCT work status remain understudied.
To address this knowledge gap, we aimed to characterize the post-HCT work status of survivors of allogeneic HCT who underwent HCT as YA using data from the Center for International Blood and Marrow Transplant Research (CIBMTR). We also analyzed the patient-, disease-, and HCT-related factors associated with work status at 1-year post-HCT.
Materials and Methods:
Data source:
The CIBMTR is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin. Approximately 200 transplant centers in the United States prospectively contribute data on consecutive transplants to the CIBMTR. The clinical database contains records of more than 550,000 patients. Participating centers are required to report all transplants consecutively, with long-term follow-up. The CIBMTR ensures data quality through computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits. Patients and/or guardian(s) provide written informed consent for data submission and research participation. This study was conducted in accordance with the Declaration of Helsinki; the institutional review board of the NMDP approved this study.
Patient eligibility:
Young adults (age 18–39 years at time of HCT) who underwent allogeneic HCT for malignant or non-malignant conditions between January 1, 2008 and December 31, 2015 in the United States and were reported to the CIBMTR were included (N=3,008). Patients transplanted with all donor types/graft sources and conditioning regimens were included, with the exception of patients undergoing syngeneic HCT (N=28). Patients who did not survive at least one-year post-HCT or were lost to follow-up prior to that time-point were excluded (N=530). Additional exclusions were made, if the completeness of data was <80% (N=58), the post-HCT CIBMTR case report forms were missing (N=18), or the patients did not consent to research (N=36). Patients who were reported to be students prior to HCT (N=442) or had missing work status at all possible longitudinal timepoints post-HCT (n=531) were also excluded. Our final study population consisted of 1,365 patients.
Statistical analysis:
Descriptive statistics were presented for patient-, disease-, and transplant-related variables. Categorical and continuous variables were described using frequency and percentages and median and ranges, respectively. Survivors’ characteristics were compared between those with and without available post-HCT work status information using Chi-square and Wilcoxon rank sum tests (Supplemental Table S1).
CIBMTR’s database was used to determine survivors’ work status information. The specific question regarding work status on the post-HCT data forms was: “What is the recipient’s current or most recent work status during the reporting period?” with the following response options: full-time work, part-time work, unemployed, medical disability, or unknown. This question has previously been used in our study which assessed post-HCT work status of adult survivors of childhood allogeneic HCT.12 Percentages of work status categories (full-time work, part-time work, unemployed, retired, and medical disability) were reported at four timepoints: 6-months, 1-, 2-, and 3-year post-HCT. Percentages of post-HCT work status categories at the 1-year timepoint were also described in relation to survivors’ pre-HCT work status categories. Acute graft-vs-host disease (GVHD) was graded according to the Glucksberg grading criteria20 and chronic GVHD according to the National Institutes of Health chronic GVHD consensus criteria21 as reported to the CIBMTR.
A logistic regression model was created to study factors associated with survivors’ work status at the 1-year timepoint post-HCT, using both pre-HCT and post-HCT covariates. The primary outcome was work status at 1-year dichotomized as working (full-time or part-time work) vs. unemployed (unemployed or claiming medical disability). Upon review of survivors’ work status at 1-year, 487 survivors were noted have missing work status. To account for these survivors, a separate logistic regression model was created using multiple imputation. Results of both models are shown (Supplemental Table S2). Patient- (age at HCT, sex, race/ ethnicity, Karnofsky score before HCT, hematopoietic cell transplant comorbidity index [HCT-CI], pre-HCT marital status, pre-HCT work status, pre-HCT highest education grade), disease- (disease diagnosis), and HCT-related (composite graft source and donor type variable, conditioning regimen, year of transplant, acute and chronic graft-vs-host disease [GVHD], and disease relapse) variables were included in the model. Odds ratio (OR) and 95% confidence intervals (CI) were provided. A P-value <0.05 was considered statistically significant. SAS 9.4 (SAS Inc., Cary, NC) was used for all analyses.
Results:
Patient characteristics:
Characteristics of the study population are described in Table 1. Median age at transplant was 30.8 years (range 18–39). Fifty-six percent were males and nearly 90% of patients received HCT for a malignant disease. Acute myeloid leukemia (41%) was the most common primary diagnosis. Ten percent of patients received HCT for non-malignant disorders such as severe aplastic anemia, inherited abnormalities of erythrocyte differentiation or function, and primary immune deficiency. Myeloablative conditioning (MAC) with total body irradiation (TBI) was the most common conditioning regimen (43%). Among those with available pre-HCT work status data, 57% were either in full- or part-time work and 23% were reported as unemployed. Two thirds of the population with available education information had college level or lower education. The median follow-up was 5.1 years (1–10.1). Forty-one percent developed acute GVHD with 33% having severe (Grade 3–4) acute GVHD. Chronic GVHD occurred in 26% survivors. Disease relapse or progression before 1-year post-HCT was noted in 17% of patients with malignancy.
Table 1:
Characteristics of young adult (YA) patients (age 18–39) that underwent first allogeneic HCT from 2008–2015 and survived for at least 1 year, reported to CIBMTR (N=1365)
| Variable | N (%) |
|---|---|
| Number of centers | 149 |
| Median age at transplant (range) | 30.8 (18–39) |
| Age groups at transplant, n (%) | |
| 18–24 years | 266 (19) |
| 25–29 years | 349 (26) |
| 30–34 years | 364 (27) |
| 35–39 years | 386 (28) |
| Sex, n (%) | |
| Male | 767 (56) |
| Female | 598 (44) |
| Race, n (%) | |
| Caucasian/White | 1089 (80) |
| Other | 230 (17) |
| Unknown/ declined | 46 (3) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 214 (16) |
| Non-Hispanic or Non-Latino | 1131 (83) |
| Missing | 20 (1) |
| Karnofsky score, n (%) | |
| <90 | 392 (29) |
| 90–100 | 953 (70) |
| Missing | 20 (1) |
| HCT-CI index, n (%) | |
| 0 | 501 (37) |
| 1 | 184 (13) |
| 2 | 223 (16) |
| ≥3 | 421 (31) |
| Missing | 36 (3) |
| Pre-transplant highest education grade, n (%) | |
| High school or lower | 489 (36) |
| College | 207 (15) |
| Graduate school | 362 (26) |
| Missing | 307 (22) |
| Pre-transplant work status, n (%) | |
| Full-time | 591 (43) |
| Part-time | 60 (4) |
| Unemployed | 265 (19) |
| Medical disability | 220 (16) |
| Unknown | 229 (17) |
| Pre-transplant Marital Status, n (%) | |
| Single, never married/ Separated/ Divorced/ Widowed | 644 (47) |
| Married or living with a partner | 695 (51) |
| Missing | 26 (2) |
| Insurance status, n (%) | |
| None | 25 (2) |
| Government sponsored | 444 (32) |
| Private | 871 (64) |
| Employer sponsored disability insurance | 18 (1) |
| Missing | 7 (<1) |
| Disease type, n (%) | |
| AML | 566 (41) |
| ALL | 281 (21) |
| CML | 83 (6) |
| MDS | 99 (7) |
| NHL | 80 (6) |
| HL | 65 (5) |
| Other heme malignancies1 | 42 (3) |
| Severe aplastic anemia | 88 (6) |
| Inherited abnormalities of erythrocyte differentiation/ function | 35 (3) |
| SCID and other immune system disorders | 19 (1) |
| Other non-malignant disorders2 | 7 (<1) |
| Graft source/ Donor type, n (%) | |
| 8/8 Matched related donor BM | 65 (5) |
| 8/8 Matched related donor PBSC | 266 (19) |
| ≤ 7/8 Mis-matched related donor BM | 45 (3) |
| ≤ 7/8 Mis-matched related donor PBSC | 54 (4) |
| 8/8 Unrelated donor BM | 141 (10) |
| 8/8 Unrelated donor PBSC | 376 (27) |
| ≤ 7/8 Mis-matched unrelated donor BM | 20 (1) |
| ≤ 7/8 Mis-matched unrelated donor PBSC | 90 (7) |
| Cord blood | 255 (19) |
| Missing | 53 (4) |
| Conditioning regimen, n (%) | |
| Myeloablative w TBI | 581 (43) |
| Myeloablative w/o TBI | 422 (31) |
| Reduced intensity/ Non-myeloablative w TBI | 177 (13) |
| Reduced intensity/ Non-myeloablative w/o TBI | 181 (13) |
| Missing | 4 (<1) |
| ATG/ Alemtuzumab, n (%) | |
| ATG only | 333 (24) |
| Alemtuzumab only | 36 (3) |
| None | 994 (73) |
| Missing | 2 (<1) |
| GVHD prophylaxis, n (%) | |
| None | 27 (2) |
| TAC + MTX +/− Others (except MMF, Post-Cy) | 581 (43) |
| TAC + MMF +/− Others (except Post-Cy) | 238 (17) |
| TAC +/− Others (except MMF, MTX, Post-Cy) | 149 (11) |
| CSA + MMF +/− Others (except Post-Cy) | 151 (11) |
| CSA + MTX +/− Others (except MMF, Post-Cy) | 95 (7) |
| CSA +/− Others (except MTX, MMF, Post-Cy) | 16 (1) |
| Post-Cy +/− Others | 68 (5) |
| Others3 | 39 (3) |
| Missing | 1 (<1) |
| Year of transplant, n (%) | |
| 2008 | 268 (20) |
| 2009 | 219 (16) |
| 2010 | 175 (13) |
| 2011 | 96 (7) |
| 2012 | 86 (6) |
| 2013 | 158 (12) |
| 2014 | 211 (15) |
| 2015 | 152 (11) |
| Median follow-up of survivors (range), months | 60.6 (12–121) |
N, number; HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic cell transplantation – comorbidity index; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; SCID, severe combined immunodeficiency; BM, bone marrow; PBSC, peripheral blood stem cells; TBI, total body irradiation; ATG, antithymocyte globulin; GVHD, graft-versus-host disease; Tac, tacrolimus; MTX, methotrexate; MMF, mycophenolate mofetil; CSA, cyclosporine
Other heme malignancy (Acute undifferentiated leukemia- 3, Biphenotypic, bilineage or hybrid leukemia- 17, chronic lymphocytic leukemia- 9, Blastic plasmacytoid dendritic cell neoplasm- 1, plasma cell disorders- 11, Solid tumors-1)
Other non-malignant conditions (Inherited disorder of metabolism- 2, Histiocytic disorders- 4, Other- 1)
Other GVHD prophylaxis (Ex vivo T-cell depletion-9, CD34 selection-21, ATG +/− others- 2, Sirolimus +/− others- 4, Methotrexate- 2, MMF-1)
In comparing HCT survivors according to the availability of post-HCT work status data (Supplemental Table S1), there were no differences in age at HCT or sex noted. Compared to survivors with available information on post-HCT work status (n=1365), those with missing work status data (n=531) were significantly different in terms of patient race/ ethnicity (P<0.001), pre-HCT highest educational grade (P<0.001), pre-HCT work status (P<0.001), pre-HCT marital status (P<0.001), disease type (P=0.04), and year of HCT (P<0.001). The median follow-up of survivors with missing post-HCT work status was shorter compared to those with available work-status (3.5 years [1–9.9] vs. 5.1 years [1–10.1]).
Post-HCT work status:
Figure 1 describes the percentages of work status categories post-HCT. Percentages of survivors working full-time and part-time increased from the 6-month to 3-year post-HCT timepoint (full-time: 18.3% at 6 months to 50.7% at 3 years; part-time: 6.9% at 6 months to 10.5% at 3 years). Similarly, the rates of unemployment (6 months: 38.2%; 3 years: 18.3%) and medical disability (6 months: 36.6%; 3 years: 21%) decreased from the 6-month to 3-year post-HCT timepoint. When studied in relation to survivors’ pre-HCT work status, 50% of those working full-time pre-HCT had returned to either full-time (41%) or part-time work (9%) by one year after HCT (Figure 2). In contrast, of those reported as unemployed or claiming medical disability pre-HCT, 19% and 29% had returned to some form of work (either full-time or part-time) at the 1-year timepoint, respectively.
Figure 1.
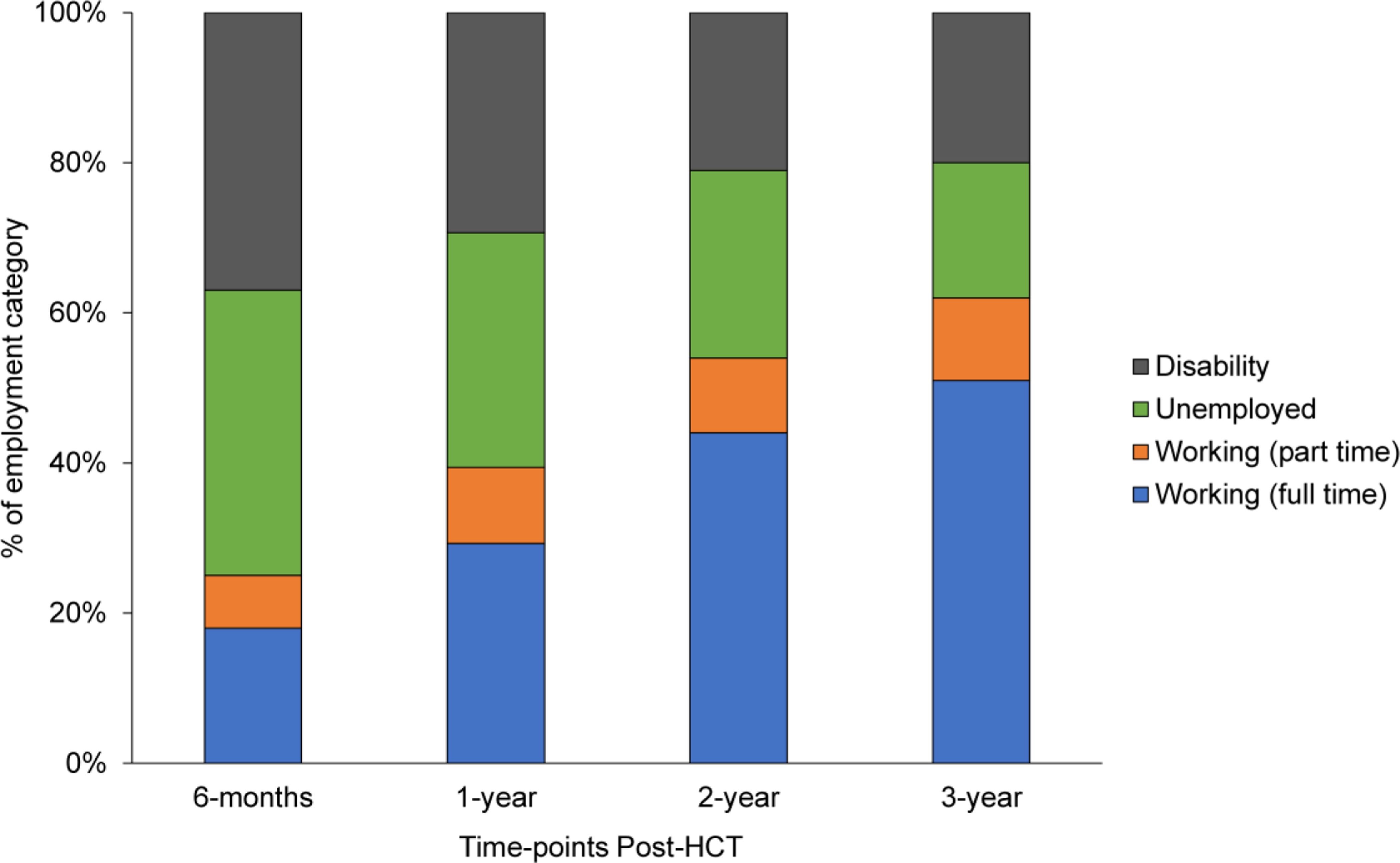
Work status of young adult (18–39 years) survivors of allogeneic hematopoietic cell transplant (HCT) at 6-months, 1-, 2-, and 3-year post-HCT
Figure 2.
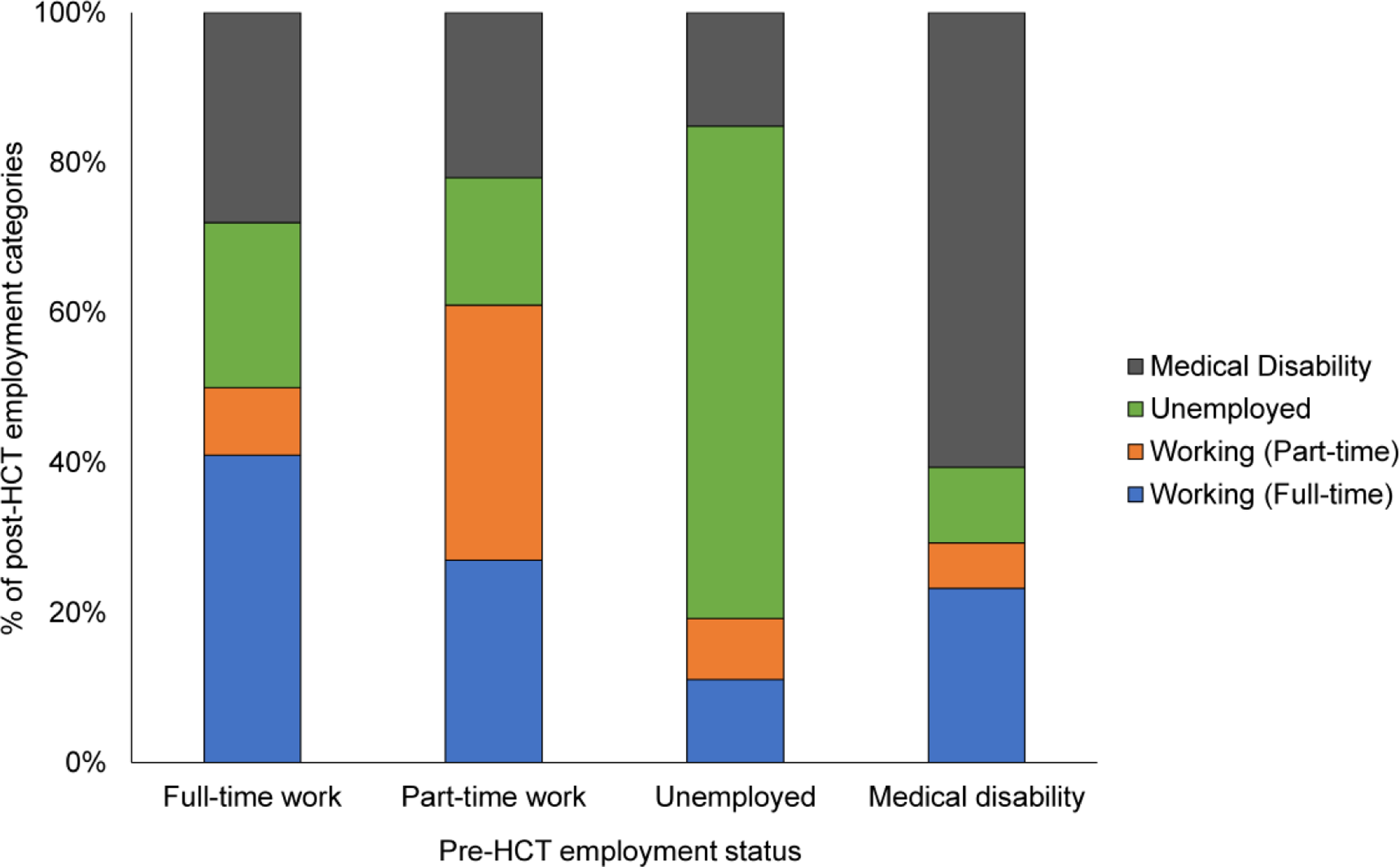
Work status of young adult (18–39 years) survivors of allogeneic hematopoietic cell transplant (HCT) at 1-year post-HCT by pre-HCT work status categories
Factors associated with work status at 1-year post-HCT:
A multivariable analysis (Table 2) assessing factors associated with post-HCT work status at the 1-year timepoint (in full- /part-time work vs. unemployed or claiming medical disability) showed that female sex, HCT-CI of 3 or more, pre-HCT unemployment or medical disability, acute GVHD, and relapse within 1-year post-HCT were significantly associated with being unemployed at one year after HCT. Conversely, graduate school educational level and myeloablative conditioning without TBI were associated with higher odds of being employed at 1-year post-HCT.
Table 2:
Multivariable logistic regression analysis of factors1 associated with work-status at 1-year post-HCT (full-time/ part-time work vs. unemployed)
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Sex | |||
| Male | 1 | ||
| Female | 0.55 | 0.40–0.77 | <0.001 |
| HCT Comorbidity Index | 0.016 | ||
| 0 | 1 | ||
| 1 | 0.72 | 0.47–1.12 | 0.147 |
| 2 | 0.66 | 0.43–1.01 | 0.055 |
| ≥3 | 0.57 | 0.39–0.82 | 0.002 |
| Pre-HCT Education | <0.001 | ||
| High school or lower | 1 | ||
| College | 1.09 | 0.65–1.80 | 0.743 |
| Graduate school | 2.47 | 1.49–4.10 | <0.001 |
| Pre-HCT Employment | <0.001 | ||
| Full-time work | 1 | ||
| Part-time work | 1.94 | 0.94–3.99 | 0.072 |
| Unemployed | 0.37 | 0.24–0.56 | <0.001 |
| Medical disability | 0.44 | 0.28–0.70 | <0.001 |
| Conditioning | <0.001 | ||
| Myeloablative with TBI | 1 | ||
| Myeloablative without TBI | 1.71 | 1.16–2.53 | 0.007 |
| Reduced Intensity/ Non-myeloablative with TBI | 1.58 | 0.95–2.62 | 0.08 |
| Reduced Intensity/ Non-myeloablative without TBI | 1.37 | 0.77–2.45 | 0.28 |
| Acute GVHD by 1-year | 0.017 | ||
| None | 1 | ||
| Grade 2 | 0.75 | 0.52–1.09 | 0.137 |
| Grade 3–4 | 0.52 | 0.34–0.80 | 0.003 |
| Relapse by 1-year | <0.001 | ||
| No | 1 | ||
| Yes | 0.34 | 0.21–0.56 | <0.001 |
| Non-malignant disease | 1.10 | 0.65–1.86 | 0.71 |
HCT, hematopoietic cell transplantation; OR, odds ratio; CI, confidence interval; TBI, total body irradiation; GVHD, graft-versus-host disease
Only info on patients with non-missing work status at 1 year was used (n=878; 1 patient who indicated to be retired at 1 year is removed from the analysis; n=2 patients with unknown conditioning are removed; remaining n=875)
Variables in the model: Age groups, sex, race, ethnicity, pre-HCT employment status, pre-HCT education, pre-HCT marital status, Karnofsky score, HCT-CI, disease type, graft source combined with donor type, TBI/ conditioning, year of transplant, acute GVHD by 1-year, chronic GVHD by 1-year, relapse by 1-year
Discussion:
Using a large nationally representative sample of YA HCT survivors, we showed that the percentage of survivors working full-time and part-time steadily increased post-HCT. At one-year post-HCT, only 39% were working full or part-time. By 3 years post-HCT, this rate had improved to 62% in full or part-time work. Of patients in full-time work pre-HCT, 50% were either unemployed or claimed medical disability status at 1-year post-HCT. This study also identified factors associated with survivors’ ability to return to work by one year after HCT. In particular, we found that female survivors, and those with pre-transplant comorbidities (HCT-CI ≥3), unemployment or medical disability, and lower educational attainment represented a vulnerable population, who were more likely to be unemployed at 1-year post-HCT. In addition, we found several modifiable risk factors for unemployment such as the use of TBI in a myeloablative conditioning regimen, grade 3–4 acute GVHD, and relapse.
Overall, we noted an increase in the proportion of YA HCT recipients returning to full-time or part-time work over time. This observation is consistent with prior studies focusing on YA with cancer. Parsons and colleagues reported work outcomes of YA cancer survivors and found that 72% of survivors working full-time or in school prior to diagnosis were back to work or school at 15 to 35 months post-diagnosis.7 Similarly, another study using the LIVESTRONG survey showed that 86% of YA breast cancer survivors were employed at nearly 3 years after diagnosis.22 Survivors’ ability to return to work is a reassuring observation and could potentially positively impact survivors’ social, emotional, and financial well-being.13,14,23 However, it could also be indicative of survivors’ rushed efforts to make up for lost time, go back to pre-diagnosis life, or alleviate the fear of falling behind.24 Efforts to transition back to work can be further challenged without an established professional network and persistent symptoms, such as fatigue and cognitive challenges, that may ultimately impact work performance.25,26 Therefore, further efforts are needed to explore survivors’ challenges even after returning to work.
Our study also noted that a substantial number of YA survivors were unemployed even at 3-year post-HCT. These unemployment estimates are higher than those reported among YA treated with non-HCT cancer directed therapy.7,8,10,11,27 These differences may be due to the higher intensity of treatment exposures, prolonged immune suppression, higher frequency of severe and life-threatening morbidities, and prolonged and recurrent hospitalizations requiring HCT survivors to be out of work for a prolonged period of time.4,5 Additionally, we found that a substantial proportion of survivors who were in full-time work prior to HCT were unable to return to work post-HCT. While the reason for their inability to return to work is unclear, it may be associated with the type of work they did (manual labor vs. desk job), their work demands, and employers’ willingness to provide a flexible work schedule (part-time vs. full-time).
While studying the factors associated with unemployment at 1-year post-HCT, patients who were unemployed or on medical disability prior to transplant were found to be more vulnerable. While the causes of unemployment were unavailable due to the CIBMTR dataset limitations, it is possible that survivors’ pre-HCT unemployment was due to illness/disability secondary to pre-HCT treatment-related toxicities. Another possible explanation for high pre-HCT unemployment and medical disability rates could be the disruption in YA patients’ school or college education while they undergo cancer-directed therapy.28 As education plays a major role in an individual’s ability to achieve employment, lower educational attainment could be associated with survivors unemployment prior to HCT, as it was associated with post-HCT work status in our study. Pre-HCT unemployment could also be due to survivors not being part of the labor force (e.g. students, homemakers); however, we excluded survivors who were reported to be students prior to HCT. None of the patients reported to be retired pre- or post-HCT.
Our study also revealed that female survivors had 50% lower odds of being employed compared to males. This finding is consistent with prior studies assessing work status in adult survivors of childhood cancer29 and older adult HCT survivors.14,16 While a clear explanation for this disparity is unknown, some studies have attributed worse health outcomes to female survivors compared to males. Specifically, Kirchhoff et al. found a differential impact of TBI on female survivors’ ability to return to work, which was not seen in male survivors.14 Syrjala and colleagues showed that female HCT survivors were significantly more likely to report depression, and treatment-related distress compared to males.30 The sex disparities in health outcomes are likely not age-dependent, since in our prior study assessing QOL among children and adolescent undergoing allogeneic HCT, we also found that female survivors were more likely to report worse QOL at 1-year post-HCT.31 Based on these findings, further work should be conducted to delineate factors affecting female health and employment outcomes.
Our study revealed several modifiable treatment-related factors associated with survivors’ work-status. Survivors who received MAC with TBI were less likely to be working at 1-year compared to those treated with myeloablative conditioning without TBI. In our previous study focusing on work outcomes of YA survivors of childhood HCT, we observed a similar impact of MAC with TBI on survivors’ work status post-HCT.12 This association may be explained by the myriad late effects associated with TBI, including neurocognitive dysfunction,32 and calls for development of less toxic preparative regimens while ensuring adequate disease control. Not unexpectedly, we also found that survivors who suffered from grade 3–4 acute GVHD or relapse were more likely to be out of work at 1-year post-HCT given those conditions are known to be associated with long-term morbidity and mortality after HCT. While donor and graft type were not found to be significantly associated, they have been shown to affect return to work status in a prior study. Lee et al. studied patient-reported outcomes among HCT survivors enrolled in the Blood and Marrow Transplant Clinical Trials Network 0201 study and found that survivors who received matched unrelated donor with a peripheral blood stem cell graft were significantly less likely to be working full-time or part-time at 5-years post-HCT compared to those who received a bone marrow graft.33 Factors such as pre-HCT marital status, disease diagnosis, or post-HCT chronic GVHD were not found to be associated with survivors’ work status.
While the use of the CIBMTR dataset allowed us to examine the understudied work outcomes in a large, nationally representative sample of YA HCT survivors, there are certain limitations to this approach which need to be acknowledged. We were unable to ascertain the causes or duration of unemployment, type of work, and changes in work status in between measurements in this population, as these outcomes are not captured through CIBMTR forms. Also, because of this limitation, we were unable to account for certain survivors who are not part of the labor force, such as those categorized as not seeking work. Lack of direct patient report did not allow us to study survivors’ self-reported health status, especially mental or physical function which could have impacted their return to work status. It is important to note that more than a third of the study eligible survivors had to be excluded due to missing work status at all possible timepoints post-HCT. Therefore, our study findings might be underrepresenting the true magnitude of unemployment among young adult HCT survivors. Additionally, we noted that several survivors had a missing work status at 1-year post-HCT. To overcome the limitation of patient exclusion, we performed a logistic regression analysis using multiple imputation and did not see a significant difference in results (Supplemental Table S2). Nevertheless, unavailability of work status is an important reminder for improving QOL measurement in clinical practice. Lastly, we could not account for the impact of external factors such as recession, seasonal employment variations, number of dependents, and availability of social support on survivors’ work status.
Despite these limitations, our study findings provide further insights into the factors affecting YA HCT survivors’ ability to return to work post-HCT and also emphasizes the need for further work in this direction. Return to work after completion of therapy is a complex process and strong support from caregivers, healthcare professionals, and employers is equally important. Therefore, additional efforts should be directed toward understanding the challenges, perceived barriers, and facilitators of the return to work process from the perspective of all stakeholders using both qualitative and quantitative research methodology. Since unemployment, underemployment, and medical disability have potential associations with poor QOL and insurance coverage, future work should also focus on understanding their relationship with survivors’ physical, social, and cognitive function, financial wellbeing, access to healthcare, and treatment adherence. Subsequently, informed by the findings of these studies, we envision development of scalable supportive care interventions adapted to the unique needs of YA HCT survivors with a goal to prevent, mitigate or ameliorate return to work challenges. These interventions should be directed towards at-risk population identified in our study (females, those with comorbidities, lower educational attainment, treated with TBI-based myeloablative conditioning, and who develop acute GVHD) and should be delivered earlier in their HCT course. These interventions should be multi-modal, including aggressive management of survivors’ chronic health conditions, pre- and post-HCT education/ training, and vocational rehabilitation, along with the development of effective communication strategies with employers to balance work expectations with survivors’ treatment-related complications. Additionally, given the impact of conditioning intensity, acute GVHD, and relapse on survivors’ ability to return to work, future interventions should also be directed toward development of reduced intensity and radiation free conditioning regimens and novel GVHD prophylaxis regimens. It will be critical to test the impact of novel treatment regimens on various QOL domains. Ultimately, we anticipate HCT centers to develop and implement standardized return to work guidelines and supportive care programs in order to ensure successful return to work, achieve better work productivity, and in turn, QOL in this at-risk population.
Supplementary Material
Highlights:
Nearly 40% of YA HCT survivors were out of work at 3-year post-HCT.
Of those in full-time work pre-HCT, 50% were not working at 1-year post-HCT.
MAC with TBI was associated with higher likelihood of unemployment at 1-year post-HCT.
Acknowledgements:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: Actinium Pharmaceuticals, Inc.; Adienne SA; Allovir, Inc.; Amgen, Inc.; Angiocrine Bioscience; Astellas Pharma US; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Incyte Corporation; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc.; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Merck Sharp & Dohme Corp.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Stemcyte; Takeda Pharma; Vor Biopharma; Xenikos BV.
Footnotes
Data Sharing:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Financial Disclosure Statement:
Dr. Sharma reports clinical trial salary support from Vertex Pharmaceuticals, CRISPR Therapeutics, Novartis paid to his institution, and personal consultancy fees from Spotlight Therapeutics, outside the submitted work.
Dr. Muffly reports grants from Servier, grants from Adaptive, personal fees from Amgen, personal fees from Pfizer, grants from Astellas, outside the submitted work.
Dr. Inamoto reports personal fees from Novartis, personal fees from Janssen, personal fees from Meiji Seika Pharma, outside the submitted work.
Dr. Ganguly reports personal fees from Seattle Genetics, personal fees from Kadmon, personal fees from Kite Pharma, personal fees from Astellas, personal fees from Quizartinib, personal fees from Sanofi, personal fees from BMS, outside the submitted work.
Dr. Bejanyan reports personal fees from Kiadis, outside the submitted work.
Dr. Schoemans reports personal fees and non-financial support from Incyte, personal fees from Janssen, grants, personal fees and non-financial support from Novartis, personal fees and non-financial support from Jazz Pharmaceuticals, personal fees from Takeda, non-financial support from Celgene, non-financial support from Abbvie, non-financial support from Gilead, non-financial support from EBMT (European Bone Marrow Transplantation Society), outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. Jama 2010; 303(16): 1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone marrow transplantation 2016; 51(6): 786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza A, Fretham C, Lee SJ, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 2020; 26(8): e177–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 2011; 118(5): 1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow EJ, Cushing-Haugen KL, Cheng GS, et al. Morbidity and Mortality Differences Between Hematopoietic Cell Transplantation Survivors and Other Cancer Survivors. J Clin Oncol 2017; 35(3): 306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yen HJ, Eissa HM, Bhatt NS, et al. Patient-reported outcomes in survivors of childhood hematologic malignancies with hematopoietic stem cell transplant. Blood 2020; 135(21): 1847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons HM, Harlan LC, Lynch CF, et al. Impact of cancer on work and education among adolescent and young adult cancer survivors. Journal of Clinical Oncology 2012; 30(19): 2393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy GP Jr., Yabroff KR, Ekwueme DU, et al. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Aff (Millwood) 2014; 33(6): 1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke L, Chung C, Grant M. Psychosocial care for adolescent and young adult hematopoietic cell transplant patients. J Psychosoc Oncol 2011; 29(4): 394–414. [PMC free article] [PubMed] [Google Scholar]
- 10.Stone DS, Ganz PA, Pavlish C, Robbins WA. Young adult cancer survivors and work: a systematic review. J Cancer Surviv 2017; 11(6): 765–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirchhoff AC, Lyles CR, Fluchel M, Wright J, Leisenring W. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer 2012; 118(23): 5964–72. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt NS, Brazauskas R, Tecca HR, et al. Post-transplantation employment status of adult survivors of childhood allogeneic hematopoietic cell transplant: A report from the Center for International Blood and Marrow Transplant Research (CIBMTR). Cancer 2019; 125(1): 144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bieri S, Roosnek E, Helg C, et al. Quality of life and social integration after allogeneic hematopoietic SCT. Bone Marrow Transplant 2008; 42(12): 819–27. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff AC, Leisenring W, Syrjala KL. Prospective predictors of return to work in the 5 years after hematopoietic cell transplantation. Journal of Cancer Survivorship 2010; 4(1): 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosher CE, DuHamel KN, Rini C, Corner G, Lam J, Redd WH. Quality of life concerns and depression among hematopoietic stem cell transplant survivors. Supportive Care in Cancer 2011; 19(9): 1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Socié G, Mary JY, Esperou H, et al. Health and functional status of adult recipients 1 year after allogeneic haematopoietic stem cell transplantation. British journal of haematology 2001; 113(1): 194–201. [DOI] [PubMed] [Google Scholar]
- 17.Winterling J, Johansson E, Wennman-Larsen A, Petersson LM, Ljungman P, Alexanderson K. Occupational status among adult survivors following allo-SCT. Bone marrow transplantation 2014; 49(6): 836–42. [DOI] [PubMed] [Google Scholar]
- 18.Tichelli A, Gerull S, Holbro A, et al. Inability to work and need for disability pension among long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant 2017; 52(10): 1436–42. [DOI] [PubMed] [Google Scholar]
- 19.Morrison EJ, Ehlers SL, Bronars CA, et al. Employment status as an indicator of recovery and function one year after hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation 2016; 22(9): 1690–5. [DOI] [PubMed] [Google Scholar]
- 20.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18(4): 295–304. [DOI] [PubMed] [Google Scholar]
- 21.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015; 21(3): 389–401 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketterl TG, Syrjala KL, Casillas J, et al. Lasting effects of cancer and its treatment on employment and finances in adolescent and young adult cancer survivors. Cancer 2019; 125(11): 1908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khera N, Chang Y-h, Hashmi S, et al. Financial burden in recipients of allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation 2014; 20(9): 1375–81. [DOI] [PubMed] [Google Scholar]
- 24.Brauer ER, Pieters HC, Ganz PA, Landier W, Pavlish C, Heilemann MV. “From Snail Mode to Rocket Ship Mode”: Adolescents and Young Adults’ Experiences of Returning to Work and School After Hematopoietic Cell Transplantation. J Adolesc Young Adult Oncol 2017; 6(4): 551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen KJ, Boisen KA, Midtgaard J, Elsbernd A, Larsen HB. Facing the Maze: Young Cancer Survivors’ Return to Education and Work-A Professional Expert Key Informant Study. J Adolesc Young Adult Oncol 2018; 7(4): 445–52. [DOI] [PubMed] [Google Scholar]
- 26.Butow P, Laidsaar-Powell R, Konings S, Lim CYS, Koczwara B. Return to work after a cancer diagnosis: a meta-review of reviews and a meta-synthesis of recent qualitative studies. J Cancer Surviv 2020; 14(2): 114–34. [DOI] [PubMed] [Google Scholar]
- 27.Leuteritz K, Friedrich M, Sender A, et al. Return to Work and Employment Situation of Young Adult Cancer Survivors: Results from the Adolescent and Young Adult-Leipzig Study. J Adolesc Young Adult Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 28.Vetsch J, Wakefield CE, McGill BC, et al. Educational and vocational goal disruption in adolescent and young adult cancer survivors. Psychooncology 2018; 27(2): 532–8. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhoff AC, Leisenring W, Krull KR, et al. Unemployment among adult survivors of childhood cancer: a report from the childhood cancer survivor study. Medical care 2010; 48(11): 1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. Jama 2004; 291(19): 2335–43. [DOI] [PubMed] [Google Scholar]
- 31.Bhatt NS, Brazauskas R, Tecca HR, et al. Female Sex is Associated With Poor Health-related Quality of Life in Children at 12 Months Post-Hematopoietic Cell Transplantation. J Pediatr Hematol Oncol 2019; 41(3): 233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly DL, Buchbinder D, Duarte RF, et al. Neurocognitive Dysfunction in Hematopoietic Cell Transplant Recipients: Expert Review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Complications and Quality of Life Working Party of the European Society for Blood and Marrow Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018; 24(2): 228–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ, Logan B, Westervelt P, et al. Comparison of Patient-Reported Outcomes in 5-Year Survivors Who Received Bone Marrow vs Peripheral Blood Unrelated Donor Transplantation: Long-term Follow-up of a Randomized Clinical Trial. JAMA Oncol 2016; 2(12): 1583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


