Abstract
Background
A large number of patients around the world are recovering from coronavirus disease 2019 (COVID-19); many of them report persistence of symptoms. The aim of this study was to test pulmonary, cardiovascular, and peripheral responses to exercise in patients recovering from COVID-19.
Methods
Patients who recovered from COVID-19 were prospectively evaluated using a combined anatomic and functional assessment. All patients underwent clinical examination, laboratory tests, and combined stress echocardiography and cardiopulmonary exercise testing. Left ventricular volumes, ejection fraction, stroke volume, heart rate, E/e′ ratio, right ventricular function, oxygen consumption (Vo2), lung volumes, ventilatory efficiency, oxygen saturation, and muscle oxygen extraction were measured in all effort stages and compared with values in historical control subjects.
Results
A total of 71 patients were assessed 90.6 ± 26 days after the onset of COVID-19 symptoms. Only 23 (33%) were asymptomatic. The most common symptoms were fatigue (34%), muscle weakness or pain (27%), and dyspnea (22%). Vo2 was lower among post-COVID-19 patients compared with control subjects (P = .03, group-by-time interaction P = .007). Reduction in peak Vo2 was due to a combination of chronotropic incompetence (75% of post-COVID-19 patients vs 8% of control subjects, P < .0001) and an insufficient increase in stroke volume during exercise (P = .0007, group-by-time interaction P = .03). Stroke volume limitation was mostly explained by diminished increase in left ventricular end-diastolic volume (P = .10, group-by-time interaction P = .03) and insufficient increase in ejection fraction (P = .01, group-by-time interaction P = .01). Post-COVID-19 patients had higher peripheral oxygen extraction (P = .004) and did not have significantly different respiratory and gas exchange parameters compared with control subjects.
Conclusions
Patients recovering from COVID-19 have symptoms associated with objective reduction in peak Vo2. The mechanism of this reduction is complex and mainly involves a combination of attenuated heart rate and stroke volume reserve.
Keywords: COVID-19, Long COVID, Post-COVID, Cardiopulmonary exercise test, Stress echocardiography
Abbreviations: COVID-19, Coronavirus disease 2019; CPET, Cardiopulmonary exercise testing; HR, Heart rate; LV, Left ventricular; LVEDV, Left ventricular end-diastolic volume; LVEF, Left ventricular ejection fraction; LVESV, Left ventricular end-systolic volume; RER, Respiratory exchange ratio; RV, Right ventricular; SV, Stroke volume; TAPSE, Tricuspid annular plane systolic excursion; Vco2, Carbon dioxide production; VE, Minute ventilation; Vo2, Oxygen consumption
The majority of publications concerning cardiovascular manifestations of coronavirus disease 2019 (COVID-19) deal with the acute infection.1, 2, 3 In contrast, less is known about the long-term cardiovascular consequences of COVID-19. Researchers have described a high incidence of fatigue, dyspnea, muscle pain, chest pain, and neuropsychiatric symptoms among patients recovered from COVID-19.4, 5, 6 Other researchers in Germany, China, the United States, and the United Kingdom have described the extent of cardiac injury in recovered patients.7, 8, 9, 10, 11 However, these studies involved only cardiac imaging performed at rest.7, 8, 9, 10, 11 Impaired exercise capacity in patients recovering from COVID-19 may be multifactorial. Apart from ventilatory or gas exchange abnormalities secondary to residual pulmonary disease, attenuated increase in stroke volume (SV) could be the result of left ventricular (LV) dysfunction due to myocarditis or cytokine dysregulation,12, 13, 14 right ventricular (RV) dysfunction induced by pulmonary hypertension, and diastolic dysfunction.1 , 15 Other possible causes include chronotropic incompetence and reduced peripheral oxygen extraction due to direct viral damage or deconditioning secondary to muscle disuse. None of these previous studies included any form of objective functional assessment. Given the large number of patients recuperating from COVID-19, functional studies to help delineate the cause of post-COVID-19 symptoms are needed.
Cardiopulmonary exercise testing (CPET) is the gold standard for the assessment of integrative exercise responses involving the pulmonary, cardiovascular, and skeletal muscle systems.16 , 17 CPET objectively categorizes physical effort during predefined activity levels (rest, anaerobic threshold, and peak exercise), providing factual assessment of pulmonary and cardiac function, as well as estimations of peripheral vascular resistance during all exercise stages. Furthermore, as COVID-19 severe enough to require medical attention manifests with lung injury18 and often has cardiac complications,1 we also performed echocardiography at rest. Our CPET protocol also included echocardiography performed during anaerobic threshold and maximal exercise.19 , 20 We previously showed that these combined examinations allow accurate discrimination of different causes of effort intolerance, by recognizing ventilatory limitation, abnormal gas exchange, limited SV reserve, chronotropic incompetence, and/or reduced peripheral muscular oxygen extraction.19 , 20 Two previous small studies of patients recovered from COVID-19 who underwent CPET showed mostly ventilatory and presumed muscular limitations to exercise.21 , 22 A study of pulmonary function and 6-min walking testing in patients recovering from acute COVID-19 showed worse diffusion capacity and lung volumes and shorter walking distances in patients recovering from severe acute disease compared with mild disease.23 We aimed to test whether pulmonary, cardiovascular, and peripheral responses to exercise in patients recovered from COVID-19 are abnormal and correlate with residual symptoms.
Methods
Study Protocol
We prospectively evaluated individuals recovering from COVID-19 in a dedicated outpatient clinic. All patients had well-established diagnoses of COVID-19, survived the acute event, and met the World Health Organization criteria for discontinuation of quarantine. The inclusion criteria were all patients with COVID-19 who were evaluated in the emergency department at the Tel Aviv Medical Center, including those requiring hospitalization and those subsequently treated as outpatients, ranging from mild to critical acute disease according to the National Institutes of Health definitions (mild: signs and symptoms not including shortness of breath or dyspnea on exertion, without abnormal imaging findings; moderate: evidence of lower respiratory disease during clinical assessment or imaging, with oxygen saturation ≥ 94% on room air; severe: oxygen saturation < 94% on room air, respiratory rate > 30 breaths/min, partial pressure of oxygen/fraction of inspired oxygen < 300 mm Hg, or lung infiltrates > 50%; critical: acute respiratory distress syndrome, septic shock, and multiorgan involvement). The only exclusion criteria were inability to provide informed consent and refusal to participate in the study. All patients underwent comprehensive medical assessment with detailed history, physical examination, and blood tests. We specifically inquired about symptoms related to effort intolerance. Dyspnea was defined as the subjective experience of breathing discomfort comprising qualitatively distinct sensations varying in intensity. We also performed a comprehensive cardiopulmonary evaluation that included (1) rest echocardiography, (2) lung spirometry, and (3) combined CPET and exercise echocardiography to objectively discriminate among the mechanisms responsible for different symptoms. The study was approved by the institutional review board (0574-16-TLV). All patients provided informed consent. Patients recovered from COVID-19 were compared with 35 control subjects. The control subjects were selected using a frequency-matching approach from a pool of 76 patients who performed combined CPET and stress echocardiography, using the same protocol, demonstrating normal peak oxygen consumption (Vo 2) at our institution. The predefined baseline matching parameters were age (within 5 years), height (within 10 cm), weight (within 10 kg), exact gender, and prevalence of ischemic heart disease, diabetes, and hypertension. The selection process produced groups with comparable determinants of peak Vo 2 and balanced comorbidities. The groups were different in size, as there were no set couples of matched patients. Subanalyses included comparing patients recovered from COVID-19 who had persistent symptoms (fatigue, dyspnea, or muscle weakness or pain) with patients who were free of these symptoms and comparing patients recovered from COVID-19 according to their acute disease severity. The CPET and echocardiographic results of patients recovered from COVID-19 were also compared with well-characterized reference values.16 , 17 , 24
Rest Echocardiography
Echocardiography was performed, as recommended,24, 25, 26 by cardiologists with expertise in echocardiographic acquisition and interpretation and using the same equipment for all patients (CX 50; Philips Medical Systems, Best, the Netherlands).
Exercise Protocol
A symptom-limited graded-ramp bicycle exercise test was performed in the semisupine position on a tilting, dedicated, microprocessor-controlled eddy current brake stress echocardiographic cycle ergometer (Ergoselect 1000 L; CareFusion, Houten, the Netherlands; Supplemental Methods). Forced vital capacity and forced expiratory volume in 1 sec were measured before exercise. Maximal voluntary ventilation was calculated by multiplying forced expiratory volume in 1 sec by 35.17 We estimated expected peak Vo 2 on the basis of age, height, and weight, with an equation pertaining to bicycle testing.16 , 17 , 27 Breath-by-breath minute ventilation (VE), carbon dioxide production (Vco 2), their ratio (VE/Vco 2, representing ventilatory efficiency), and Vo 2 were measured using a Medical Graphics metabolic cart (ZAN, nSpire Health, Longmont, CO). The oxygen pulse was calculated as Vo 2/heart rate (HR).28 Anaerobic threshold was determined manually using the modified V-slope method.17 Arterial blood oxygen saturation was measured using noninvasive pulse oximetry. The intensity of dyspnea or fatigue was assessed using the modified Borg scale. The exercise-effort quality was assessed by respiratory exchange ratio (RER) at peak exercise. A 12-lead electrocardiogram was monitored continuously, and HR and blood pressure were measured at rest and every minute during exercise. HR reserve was calculated as the change in HR from rest to peak exercise, divided by the difference between age-predicted maximal HR and resting HR. Chronotropic incompetence was defined as failure to achieve ≥80% of HR reserve during exercise.29 In patients treated with β-blockers, chronotropic incompetence was defined as failure to achieve >62% of HR reserve.30
Stress Echocardiographic Testing
Echocardiographic images were obtained concurrently with breath-by-breath gas exchange measurements at rest, immediately after reaching a stable RER of ≥1.00 (anaerobic threshold), and at peak effort by a single cardiologist (Y.S.).19 , 20 Data collected at each time period included LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV ejection fraction (LVEF), SV, peak E-wave velocity, e′ in the septal and lateral mitral annulus, tricuspid annular plane systolic excursion (TAPSE), and RV S′. LV diameters and volumes and LVEF were measured as recommended using two-dimensional echocardiography.25 Forward SV was calculated from LV outflow tract Doppler with subsequent calculation of cardiac output and index.
A-Vo 2 difference, which represents oxygen extraction by the muscles, was calculated by using the Fick equation at each activity level.31 , 32 As there are no reference values for LVEDV, LVESV, and A-Vo 2 difference in response to exercise, we selected the cutoffs for these parameters on the basis of our previous work and corroborated the cutoffs in our control cohort.33 Cutoff values for RV stress echocardiographic parameters were derived from the recommendations of the European Association of Cardiovascular Imaging.34
All test results were analyzed by an experienced physician who was unaware of participant group or symptomatology (Y.T.) (Supplemental Methods).
Interobserver and Intraobserver Variability
Interobserver variability for LVEDV and LVESV was determined by a second independent blinded observer who measured these variables in 15 randomly selected patients. Intraobserver variability was determined by having the observer who measured the data in all patients remeasure the echocardiographic variables in 15 patients 1 week later. Interobserver and intraobserver variability was assessed using the intraclass correlation coefficient and the within-subject coefficient of variation (calculated as the ratio of the SD of the measurement difference to the mean value of all measurements). An intraclass correlation coefficient > 0.85 was used as a measure of good variability and positive reproducibility for the measurements.
Statistical Analysis
Descriptive results are expressed as mean ± SD for continuous variables and as percentages for categorical variables. The distributions of continuous variables were tested to ensure that normality assumptions were fulfilled. Group comparisons used analysis of variance, the Wilcoxon test, the Fisher exact test, or the χ2 test, as appropriate. To compare sample means with known reference values, a one-sample t test was used. For comparison with the control group, or between subgroups, and to avoid bias incurred by multiple comparisons, we used repeated-measures linear-model analysis to define the within-group effect for each parameter over time, the between-group differences over time, and the group-by-time interactions. To analyze independent determinants of exercise tolerance, the primary end point was peak Vo 2. Stepwise multivariate linear regression models were constructed (with peak Vo 2 as the dependent variable and the different clinical, CPET, echocardiographic, or combined CPET and exercise echocardiographic variables as independent variables). To correct for possible overfitting of the model considering that there were only 71 subjects, we grouped the variables into clinical, laboratory, LV, RV, respiratory, and peripheral parameters. The first step was to select for each group all the variables with P values < .05 in a univariable analysis. In the second step, to detect multicollinearity, we used correlation factor analyses to determine if any pairs of predictor variables were highly correlated (correlation coefficient > 0.7) and therefore likely to result in multicollinearity. In the third step, in each subgroup, the variable with the lowest P value was chosen to be included in the analysis. Because of their known associations with peak Vo 2, we forced age and gender into all analyses. The final parameters included age, gender, troponin, HR, SV, TAPSE, and A-Vo 2 difference. All computations were performed using JMP for Windows version 9.0 (SAS Institute, Cary, NC).
Results
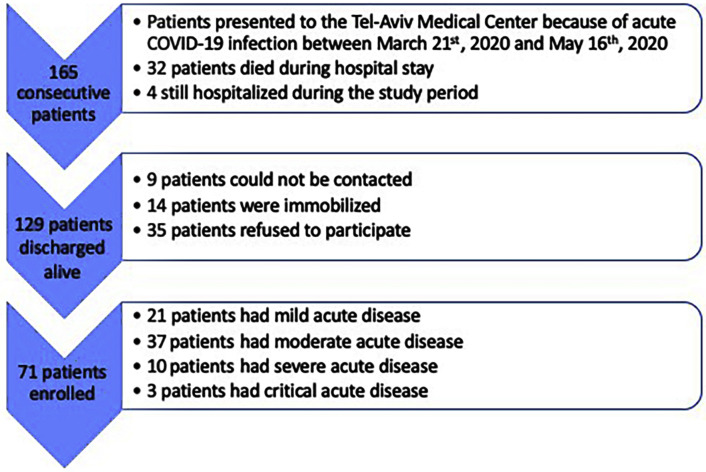
We identified 165 consecutive adult patients who presented to the Tel Aviv Medical Center (between March 21, 2020, and May 16, 2020) for acute COVID-19 (Figure 1 ). Among these patients, 32 died during the hospitalization and four were still hospitalized. From the remaining 129 patients discharged alive, nine patients could not be contacted, 14 patients were immobilized and debilitated at baseline, and 35 patients refused to attend the post-COVID-19 clinic. All 71 patients who recovered from COVID-19 and were prospectively evaluated between June 15 and August 1, 2020, at our clinic and agreed to participate in the present study represent the study group (mean age, 52.6 ± 16 years [range: 18–80 years]; 47 men [66%]). During the acute phase, 21 patients (30%) had mild disease, 37 (52%) had moderate disease, 10 (14%) had severe disease, and three (4%) had critical disease (two required prolonged mechanical ventilation and one required venoarterial extracorporeal membrane oxygenation). Troponin was elevated in 22 patients (31%) during the acute infection. In 10 patients, it was >4 times higher than the upper limit of normal, in three patients 3 to 4 times higher, in two patients 2 to 3 times higher, and in the remaining patients only marginally elevated. Troponin remained elevated in only two patients (3%) at the time of follow-up (P < .0001). In both patients, troponin was only marginally elevated (less than twice the upper limit of normal). Before acute infection, 50 patients (71%) exercised at least once a week, while 21 (29%) did not perform any type of routine exercise. Patients were assessed a mean of 90.6 ± 26 days after the onset of their first COVID-19 symptoms. At the time of evaluation, none of the patients had fever or any signs or symptoms of acute illness. However, only 23 (33%) were completely free of symptoms, while 48 (67%) had one or more of the following symptoms: fatigue (34%), muscle weakness or pain (27%), dyspnea (22%), chest pain (18%), anxiety (17%), and anosmia (12%). The mean time after acute illness did not differ between patients with and those without symptoms (88.8 ± 20 vs 88.6 ± 24 days, P = .98). Symptomatic status was independent of time after acute illness (hazard ratio: 1.00; 95% CI: 0.96–1.04; P = .98). Baseline characteristics of the study cohort are presented in Table 1 . Baseline characteristics of patients who did not undergo the post-COVID-19 evaluation are shown in Supplemental Table 1. These patients were older and had higher rates of diabetes and heart failure.
Figure 1.
Flowchart of study design.
Table 1.
Patient characteristics
| Variable | Post-COVID-19 |
Control |
P |
|---|---|---|---|
| (n = 71) | (n = 35) | ||
| Clinical characteristics | |||
| Age, y | 52.6 ± 16 | 53.6 ± 16 | NS |
| Sex, male | 47 (66) | 24 (68) | NS |
| Height, m | 1.71 ± 0.1 | 1.70 ± 0.1 | NS |
| Weight, kg | 82 ± 21 | 79.6 ± 15 | NS |
| Body mass index, kg/m2 | 27.5 ± 6 | 27.4 ± 5 | NS |
| Body mass index ≥ 35 kg/m2 | 4 (5.5) | 3 (8.5) | NS |
| Hypertension | 30 (43) | 16 (46) | NS |
| Diabetes | 9 (13) | 5 (14) | NS |
| Heart failure | 1 (1.5) | 0 (0) | NS |
| Ischemic heart disease | 3 (4) | 1 (3) | NS |
| Lung disease, any | 11 (15) | 2 (6) | .15 |
| Chronic obstructive pulmonary disease | 6 (8) | 1 (3) | NS |
| Smoking | 8 (11) | 1 (3) | .14 |
| Bronchodilator inhalers | 8 (11) | 1 (3) | .14 |
| β-blockers | 8 (11) | 5 (15) | NS |
| Furosemide | 0 (0) | 1 (3) | NS |
| Angiotensin-converting enzyme inhibitor | 10 (14) | 5 (14) | NS |
| Symptoms and signs | |||
| Fatigue | 24 (34) | 9 (26) | NS |
| Muscle weakness/pain | 19 (27) | 0 (0) | NA |
| Dyspnea | 16 (22) | 26 (74) | <.0001 |
| Chest pain | 13 (18) | 0 (0) | NA |
| Memory loss | 12 (17) | 0 (0) | NA |
| Anxiety | 12 (17) | 0 (0) | NA |
| Anosmia | 9 (12) | 0 (0) | NA |
| Any symptom | 48 (67) | 29 (83) | NS |
| Lung crackles | 1 (1) | 0 (0) | NA |
| Leg edema | 6 (9) | 0 (0) | NA |
| Laboratory evaluation | |||
| Hemoglobin, g/dL | 13.9 ± 1.3 | 13.9 ± 1.3 | NS |
| White blood cells, 103/μL | 7.5 ± 1.9 | NA | NA |
| Lymphocytes, 103/μL | 2.2 ± 0.8 | NA | NA |
| Platelets, 103/μL | 231 ± 74 | NA | NA |
| Glucose, mg/dL | 98 ± 37 | NA | NA |
| Troponin I, ng/L | 11.4 ± 20 | NA | NA |
| Brain natriuretic peptide, pg/mL | 24.3 ± 19 | NA | NA |
| d-dimer, mg/L | 1.3 ± 0.7 | NA | NA |
| C-reactive protein, mg/L | 5.1 ± 13 | NA | NA |
| Fibrinogen, mg/dL | 318 ± 78 | NA | NA |
| Ferritin, ng/mL | 135 ± 167 | NA | NA |
| Blood urea nitrogen, mg/dL | 17.9 ± 6 | NA | NA |
| Creatinine, mg/dL | 0.9 ± 0.2 | 1.01 ± 0.2 | NS |
| Bilirubin, mg/dL | 0.6 ± 0.4 | NA | NA |
| Aspartate aminotransferase, U/L | 29.1 ± 29 | NA | NA |
| Alanine transaminase, U/L | 29.3 ± 41 | NA | NA |
| Albumin, g/L | 44.7 ± 3 | NA | NA |
| Right ventricle | |||
| Pulmonary acceleration time, msec | 117 ± 24 | NA | NA |
| Right atrial pressure, mm Hg | 6.0 ± 2 | 5.1 ± 1 | .002 |
| RV end-diastolic area, cm2 | 19.5 ± 5.5 | 20.5 ± 6.5 | NS |
| RV end-systolic area, cm2 | 11.3 ± 3.7 | 11.3 ± 3.5 | NS |
| RV fractional area change, % | 46.7 ± 8 | 44.3 ± 9 | NS |
| TAPSE, cm | 2.3 ± 0.4 | NA | NA |
| RV S′, cm/sec | 10.4 ± 2 | NA | NA |
| Respiratory evaluation | |||
| FVC, L | 3.9 ± 1.4 | 3.7 ± 1.2 | .06 |
| FVC, % predicted | 103.7 ± 31 | 94.9 ± 15 | .06 |
| FEV1, L | 3.2 ± 1.0 | 3.3 ± 1.1 | NS |
| FEV1, % predicted | 100.8 ± 23 | 98.8 ± 17.4 | NS |
| FEV1 < 70% predicted | 3 (4) | 0 (0) | NS |
| FEV1/FVC ratio | 79.6 ± 10 | 85.5 ± 9 | .006 |
| FEV1/FVC, % predicted | 99.0 ± 12 | 109.5 ± 11.1 | .002 |
| CPET parameters | |||
| Anaerobic threshold, L/min | 0.97 ± 0.27 | 1.06 ± 0.44 | .32 |
| Anaerobic threshold, mL/min/kg | 12.3 ± 3.6 | 15.4 ± 5.7 | .02 |
| Oxygen uptake efficiency slope, mL/min | 1,690 ± 787 | NA | NA |
| Oxygen uptake efficiency slope < 85% expected | 34 (48) | NA | NA |
| VE/Vo2 at anaerobic threshold | 24.9 ± 4 | 27.7 ± 5 | .01 |
| Minimal oxygen saturation, % | 97.8 ± 1.5 | 98.1 ± 1.3 | NS |
| Breathing reserve < 15 | 3 (4) | 0 (0) | NS |
| Peak breathing frequency ≥ 50 breaths/min | 5 (7) | 1 (3) | NS |
| Abnormal VE/Vco2 at anaerobic threshold | 4 (5) | 1 (3) | NS |
| Abnormal oxygen saturation | 6 (8) | 0 (0) | .09 |
| Chronotropic incompetence | 53 (75) | 3 (8) | <.0001 |
| Duration of exercise, min | 10.2 (8.2–12.3) | 8.0 (6.2–17.9) | NS |
| RER | 1.15 ± 0.07 | 1.16 ± 0.15 | .87 |
Data are expressed as mean ± SD, number (percentage), or median (interquartile range).
FEV1, Forced expiratory volume in 1 sec; FVC, forced vital capacity; NA, not available.
The 35 historical control patients were matched for main clinical characteristics and comorbidities (age, gender, weight, height, hypertension, diabetes). Baseline characteristics and echocardiographic and CPET parameters of the study cohort compared with control subjects are shown in Table 1.
Combined CPET and Stress Echocardiography
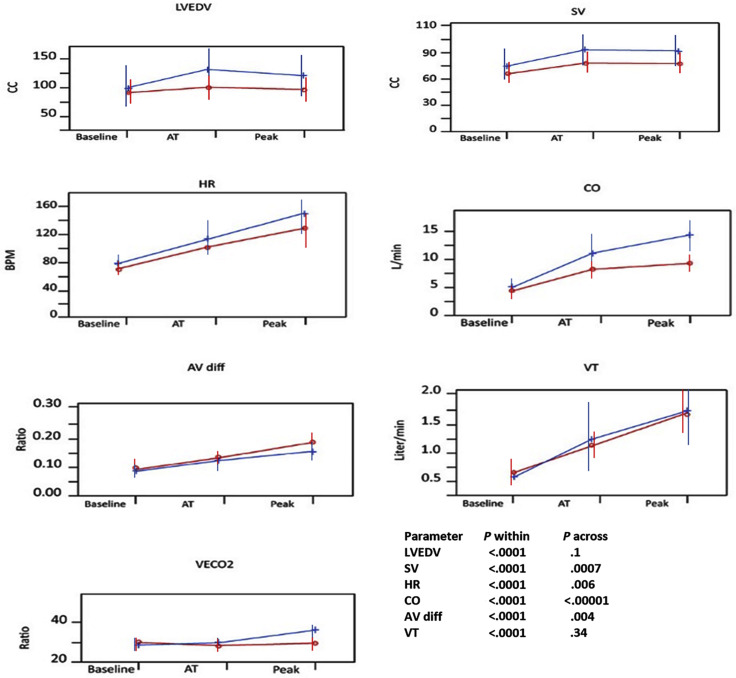
The results of CPET and stress echocardiography during different stages of exertion of the study cohort compared with control subjects are shown in Table 1, Table 2 , and Figure 2 . Post-COVID-19 patients reached a lower anaerobic threshold and lower peak Vo 2, with lower HR and oxygen pulse, compared with control subjects. They also had lower LVEDV, LVEF, SV, and cardiac output in all stages of exertion. A-Vo 2 difference was higher among the post-COVID-19 group. There was no significant difference in breathing rate, tidal volume, or VE/Vco 2 ratio. Importantly, 53 post-COVID-19 patients (75%), including eight patients treated with β-blockers, demonstrated obvious chronotropic incompetence, in contrast to three patients (8%) in the control group. RER in patients with and those without chronotropic incompetence was similar (1.16 ± 0.05 vs 1.15 ± 0.06, P = .23). The results of combined CPET and stress echocardiography for the study cohort compared with normal reference values are shown in Supplemental Table 2.
Table 2.
Combined CPET and stress echocardiographic parameters during different stages of exertion compared with historical control subjects
| Measurement | Baseline | Anaerobic threshold | Maximal effort | P value, between groups | P value, within groups | P value, group-by-time interaction |
|---|---|---|---|---|---|---|
| Vo2 | ||||||
| Vo2, L/min | ||||||
| COVID-19 recovery | 0.4 ± 0.13 | 0.97 ± 0.27 | 1.6 ± 0.5 | .03 | <.0001 | .007 |
| Control | 0.4 ± 0.1 | 1.01 ± 0.4 | 2.24 ± 0.9 | |||
| Oxygen pulse, mL/beat | ||||||
| COVID-19 recovery | 5.5 ± 1.7 | 9.6 ± 2.5 | 12.4 ± 4.1 | .60 | <.0001 | .03 |
| Control | 5.1 ± 1.5 | 9.3 ± 2.7 | 14.6 ± 5.3 | |||
| HR, beats/min | ||||||
| COVID-19 recovery | 70.1 ± 11 | 101.8 ± 14 | 135.9 ± 23 | .006 | <.0001 | .87 |
| Control | 78.4 ± 10 | 112.0 ± 20 | 150.3 ± 21 | |||
| Left ventricle | ||||||
| LVEDV, mL | ||||||
| COVID-19 recovery | 93.8 ± 23 | 111.0 ± 27 | 107.9 ± 26 | .10 | <.0001 | .03 |
| Control | 112.7 ± 43 | 131.5 ± 44 | 124.1 ± 41 | |||
| LVESV, mL | ||||||
| COVID-19 recovery | 35.0 ± 18 | 36.9 ± 28 | 31.2 ± 20 | .82 | .99 | .07 |
| Control | 38.1 ± 25 | 30.1 ± 19 | 31.0 ± 24 | |||
| LVEF, % | ||||||
| COVID-19 recovery | 64.7 ± 14 | 66.9 ± 20 | 71.8 ± 16 | .01 | .01 | .01 |
| Control | 65.3 ± 12 | 78.2 ± 21 | 73.4 ± 21 | |||
| Hemodynamics | ||||||
| SV, mL | ||||||
| COVID-19 recovery | 60.6 ± 13 | 75.6 ± 17 | 72.9 ± 16 | .0007 | <.0001 | .03 |
| Control | 74.4 ± 20 | 98.4 ± 22 | 92.9 ± 2 | |||
| Cardiac output, L/min | ||||||
| COVID-19 recovery | 4.4 ± 1.0 | 7.8 ± 1.9 | 9.8 ± 2.7 | <.0001 | <.0001 | .005 |
| Control | 5.8 ± 1.3 | 11.6 ± 3.9 | 14.0 ± 4.2 | |||
| E-wave velocity, cm/sec | ||||||
| COVID-19 recovery | 62.9 ± 14 | 80.4 ± 16 | 90.8 ± 19 | .06 | <.0001 | .0002 |
| Control | 59.2 ± 14 | 97.5 ± 28 | 121.5 ± 21 | |||
| e′ septal, cm/sec | ||||||
| COVID-19 recovery | 7.6 ± 2.4 | 10.1 ± 2.6 | 11.8 ± 3.5 | .16 | <.0001 | .01 |
| Control | 7.3 ± 3.3 | 12.2 ± 6.5 | 12.0 ± 3.5 | |||
| E/e′ septal ratio | ||||||
| COVID-19 recovery | 8.7 ± 3.0 | 8.4 ± 2.4 | 8.2 ± 2.6 | .48 | .23 | .27 |
| Control | 9.3 ± 3.9 | 8.9 ± 2.7 | 8.0 ± 2.5 | |||
| Ventilation and gas exchange | ||||||
| Breathing rate, breaths/min | ||||||
| COVID-19 recovery | 20.7 ± 5.3 | 25.4 ± 5.0 | 37.1 ± 7.2 | .90 | <.0001 | .90 |
| Control | 20.3 ± 3.9 | 25.4 ± 6.2 | 36.7 ± 9.2 | |||
| Tidal volume, L | ||||||
| COVID-19 recovery | 0.65 ± 0.16 | 1.1 ± 0.3 | 1.7 ± 0.5 | .34 | <.0001 | .04 |
| Control | 0.58 ± 0.08 | 1.3 ± 0.7 | 1.9 ± 0.8 | |||
| VE/Vco2 ratio | ||||||
| COVID-19 recovery | 30.0 ± 9 | 27.6 ± 3 | 29.8 ± 3 | .46 | .02 | .10 |
| Control | 28.9 ± 4 | 29.6 ± 5 | 30.7 ± 3 | |||
| Peripheral extraction | ||||||
| A-Vo2 difference, L/L | ||||||
| COVID-19 recovery | 0.09 ± 0.03 | 0.13 ± 0.03 | 0.18 ± 0.05 | .004 | <.0001 | .20 |
| Control | 0.08 ± 0.03 | 0.11 ± 0.03 | 0.13 ± 0.04 |
Data are expressed as mean ± SD.
Figure 2.
Baseline, anaerobic threshold (AT), and maximal CPET and stress echocardiography in patients recovering from COVID-19 (red line) and historical control subjects (blue line) for LVEDV, SV, HR, cardiac output (CO), arteriovenous oxygen (A-Vo2) difference (AV diff), tidal volume (VT), and VE/Vco2 ratio (VECO2).
Recovery From Severe or Critical versus Mild or Moderate COVID-19
In comparison with patients who had mild or moderate disease during the acute infection, patients with histories of severe or critical acute disease had higher LVEDVs, lower LVEFs, and poorer gas exchange parameters (worse saturation response and higher VE/Vco 2 and VE/Vo 2 ratios at anaerobic threshold; Supplemental Table 3).
Subgroup Analysis on the Basis of Symptoms
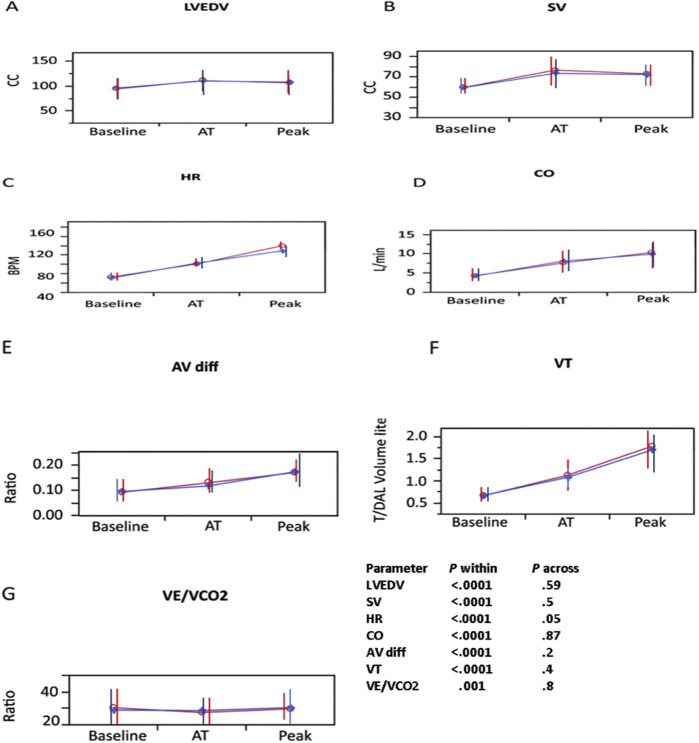
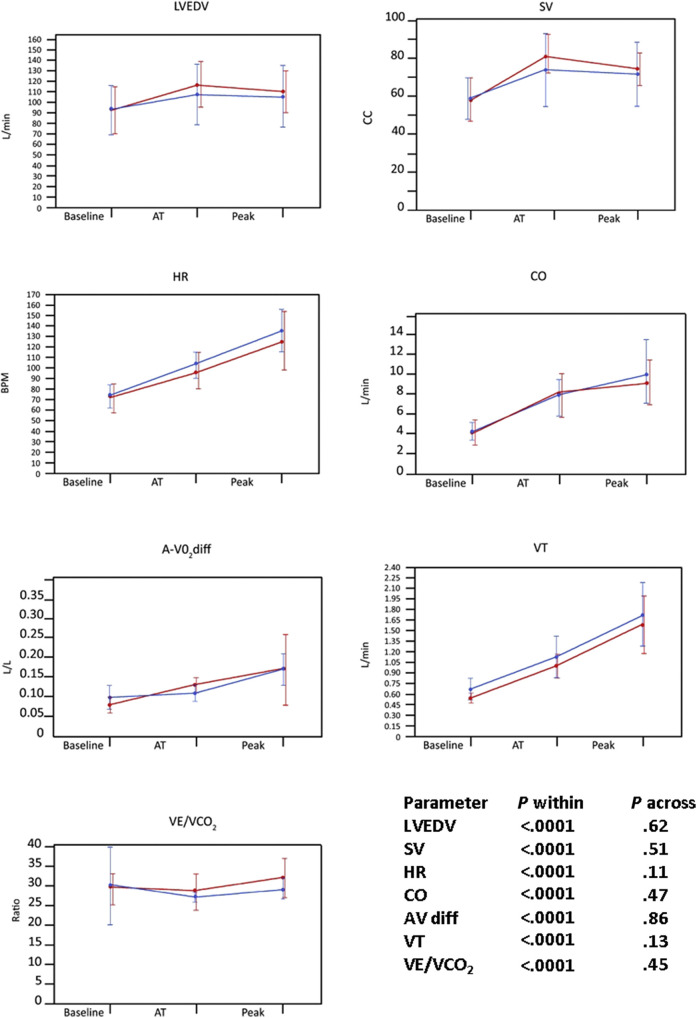
Patients who reported ongoing symptoms were compared with asymptomatic patients (Table 3 ). Patients who were symptomatic had smaller left ventricles and higher LVEFs. There was no difference in peak Vo 2, HR, SV, cardiac output, E/e′ ratio, tidal volume, VE/Vco 2 ratio, or A-Vo 2 difference. Supplemental Figure 1, Supplemental Figure 2, Supplemental Figure 3 depict the differences between patients with specific symptoms and those without these symptoms. Patients with dyspnea (Supplemental Figure 1) had attenuated changes in SV, HR, cardiac output, and lung tidal volume and increased VE/Vco 2 ratio (decreased ventilatory efficiency). Patients with fatigue (Supplemental Figure 2) had attenuated changes in HR but otherwise were not different from patients without fatigue in any of the combined CPET and exercise echocardiographic parameters. Patients with muscle weakness or pain (Supplemental Figure 3) did not differ from patients without these symptoms.
Table 3.
Combined CPET and stress echocardiographic parameters during different stages of exertion comparing symptomatic and asymptomatic patients who recovered from COVID-19
| Measurement | Baseline | Anaerobic threshold | Maximal effort | P value, between groups | P value, within groups | P value, group-by-time interaction |
|---|---|---|---|---|---|---|
| Vo2 | ||||||
| Vo2, L/min | ||||||
| Asymptomatic | 0.38 ± 0.13 | 0.99 ± 0.26 | 1.7 ± 0.5 | .46 | <.0001 | .54 |
| Symptomatic | 0.40 ± 0.13 | 0.92 ± 0.22 | 1.5 ± 0.5 | |||
| Oxygen pulse, mL/beat | ||||||
| Asymptomatic | 5.1 ± 1.9 | 10.0 ± 2.6 | 12.5 ± 3.5 | .76 | <.0001 | .65 |
| Symptomatic | 5.1 ± 1.5 | 9.2 ± 2.4 | 12.2 ± 4.5 | |||
| HR, beats/min | ||||||
| Asymptomatic | 70.9 ± 11 | 99.7 ± 12 | 136.5 ± 19 | .77 | <.0001 | .84 |
| Symptomatic | 73.8 ± 13 | 101.7 ± 14 | 133.1 ± 22 | |||
| Left ventricle | ||||||
| LVEDV, mL | ||||||
| Asymptomatic | 98.1 ± 26 | 114.7 ± 29 | 112.1 ± 28 | .05 | <.0001 | .88 |
| Symptomatic | 90.6 ± 23 | 107.8 ± 27 | 104.0 ± 26 | |||
| LVESV, mL | ||||||
| Asymptomatic | 37.2 ± 23 | 43.2 ± 29 | 35.9 ± 25 | .03 | .001 | .68 |
| Symptomatic | 32.9 ± 19 | 33.6 ± 28 | 28.3 ± 24 | |||
| LVEF, % | ||||||
| Asymptomatic | 64.5 ± 15 | 63.2 ± 20 | 69.7 ± 17 | .008 | .0008 | .82 |
| Symptomatic | 65.8 ± 14 | 68.5 ± 28 | 75.2 ± 23 | |||
| Hemodynamics | ||||||
| E-wave velocity, cm/sec | ||||||
| Asymptomatic | 59.6 ± 13 | 81.0 ± 14 | 94.3 ± 12 | .69 | <.0001 | .27 |
| Symptomatic | 63.6 ± 14 | 80.6 ± 18 | 89.3 ± 22 | |||
| e′ septal, cm/sec | ||||||
| Asymptomatic | 7.7 ± 2 | 10.2 ± 2 | 12.3 ± 4 | .27 | <.0001 | .17 |
| Symptomatic | 7.3 ± 2 | 10.2 ± 3 | 11.6 ± 3 | |||
| E/e′ septal ratio | ||||||
| Asymptomatic | 8.2 ± 3 | 8.5 ± 2 | 8.5 ± 3 | .33 | .56 | .37 |
| Symptomatic | 9.2 ± 3 | 8.5 ± 3 | 8.1 ± 3 | |||
| SV, mL | ||||||
| Asymptomatic | 60.1 ± 12 | 76.5 ± 17 | 73.9 ± 15 | .56 | <.0001 | .88 |
| Symptomatic | 57.7 ± 11 | 75.5 ± 18 | 71.7 ± 16 | |||
| Cardiac output, L/min | ||||||
| Asymptomatic | 4.4 ± 1.1 | 7.7 ± 1.7 | 10.0 ± 2 | .88 | <.0001 | .83 |
| Symptomatic | 4.2 ± 0.9 | 7.8 ± 2.0 | 9.5 ± 3 | |||
| Ventilation and gas exchange | ||||||
| Breathing rate, breaths/min | ||||||
| Asymptomatic | 20.1 ± 4 | 24.5 ± 5 | 35.0 ± 7 | .57 | <.0001 | .33 |
| Symptomatic | 20.8 ± 6 | 25.3 ± 5 | 37.7 ± 7 | |||
| Tidal volume, L | ||||||
| Asymptomatic | 0.64 ± 0.17 | 1.12 ± 0.3 | 1.76 ± 0.5 | .83 | <.0001 | .73 |
| Symptomatic | 0.66 ± 0.15 | 1.10 ± 0.3 | 1.65 ± 0.4 | |||
| VE/Vco2 ratio | ||||||
| Asymptomatic | 33.0 ± 4 | 26.7 ± 2.3 | 28.9 ± 2.7 | .73 | <.0001 | .07 |
| Symptomatic | 28.9 ± 4 | 28.4 ± 4.2 | 30.5 ± 4.0 | |||
| Peripheral extraction | ||||||
| A-Vo2 difference, L/L | ||||||
| Asymptomatic | 0.09 ± 0.02 | 0.13 ± 0.03 | 0.17 ± 0.03 | .91 | <.0001 | .28 |
| Symptomatic | 0.09 ± 0.03 | 0.12 ± 0.02 | 0.18 ± 0.06 |
Data are expressed as mean ± SD.
Supplemental Figure 1.
Baseline, anaerobic threshold (AT), and maximal CPET and stress echocardiography in patients recovering from COVID-19 with dyspnea (blue line) or without dyspnea (red line) for LVEDV, SV, HR, cardiac output (CO), A-Vo2 difference (AV diff), tidal volume (VT), and VE/Vco2 ratio. Note that patients with dyspnea had lower LVEDV, SV, HR, cardiac output, and VT and marginally higher VE/Vco2 ratio.
Supplemental Figure 2.
Baseline, anaerobic threshold (AT), and maximal CPET and stress echocardiography in patients recovering from COVID-19 with fatigue (blue line) or without fatigue (red line) for (A) LVEDV, (B) SV, (C) HR, (D) cardiac output (CO), (E) A-Vo2difference (AV diff), (F) tidal volume (VT), and (G) VE/Vco2 ratio. Note that patients with fatigue had lower HR, but otherwise there were no significant differences in all other parameters compared with patients without fatigue.
Supplemental Figure 3.
Baseline, anaerobic threshold (AT), and maximal CPET and stress echocardiography in patients recovering from COVID-19 with muscle weakness or pain (blue line) and without muscle weakness or pain (red line) for LVEDV, SV, HR, cardiac output (CO), A-Vo2 difference (AV diff), tidal volume (VT), and VE/Vco2 ratio. Note that patients with muscle weakness or pain did not have significantly different CPET parameters compared with patients without muscle weakness or pain.
Univariate and Multivariate Analyses
Clinical parameters associated with reduced peak Vo 2 were older age, male sex, lower weight, lower hemoglobin, and higher troponin and creatinine at the time of presentation to the post–acute COVID-19 care clinic. The only rest echocardiographic or respiratory parameters associated with reduced peak Vo 2 were those related to smaller LV size (LVEDV or LV diameter), larger RV size (RV end-diastolic area), poorer RV function (lower TAPSE), or low lung volume (forced expiratory volume in 1 sec, forced vital capacity). Stepwise multivariable analyses of predictors of peak Vo 2 are shown in Supplemental Table 4.
Interobserver and Intraobserver Variability
Comparison of inter- and intraobserver variability for LVEDV and LVESV showed good agreement between measurements (Supplemental Table 5).
Discussion
This is the first study to evaluate patients recovered from COVID-19 with combined stress echocardiography and CPET. Assessing patients in separate stages of effort, including anaerobic threshold, is more relevant to everyday activity than peak exercise alone. Our study represents an attempt to objectively explain the functional impairment reported by many patients recovering from COVID-19. Our major findings are as follows: (1) two thirds of the patients reported at least one residual symptom, similar to previous publications4, 5, 6; (2) abnormally low peak Vo 2 is common among patients recovering from COVID-19; (3) the mechanism of the reduction is a combination of chronotropic incompetence and attenuated SV reserve and is different according to specific symptoms; (4) severe gas exchange abnormalities and very limited breathing reserve are rather rare causes of effort intolerance; and (5) chronotropic incompetence, limited SV reserve, limited RV systolic reserve, and peripheral factors (low peak A-Vo 2 difference) each contributes independently to the reduced exercise capacity in patients recovering from COVID-19.
Chronotropic Incompetence
In patients recovering from COVID-19, peak-exercise HR was reduced and contributed to their limited exercise capacity. Interestingly, chronotropic incompetence was equally present in patients recovering from mild or moderate and severe or critical acute disease. Furthermore, chronotropic incompetence was specifically more common among patients reporting dyspnea and/or fatigue compared with patients free of these symptoms and was a strong independent predictor of peak Vo 2. Moreover, we found that chronotropic incompetence was not the consequence of reduced motivation during CPET (as demonstrated by RER). On the basis of the available data, it is not possible to determine why patients recovering from COVID-19 have chronotropic incompetence, so further research is necessary in this area.
Insufficient Increase in SV
Normally, SV increases during exercise until the anaerobic threshold, secondary to increases in LVEDV and contractility, both resulting from heightened adrenergic tone. Then, SV plateaus because of increased tachycardia and shortened filling time of the left ventricle.20 Insufficient increase in SV during the initial stages of stress seen in post-COVID-19 patients compared with control subjects may be the result of either insufficient increase in LVEDV or a blunted increase in contractility. Although we cannot determine the exact mechanism responsible for this limitation, several explanations exist: (1) Reduced LV contractile reserve (Video 1; available at www.onlinejase.com). Acute COVID-19 can induce endothelialitis,35 which may cause microvascular dysfunction resulting in LV contractile abnormalities. Microvascular dysfunction usually manifests with reduced maximal Vo 2, preserved anaerobic threshold, ST-segment abnormalities, and low peak oxygen pulse with failure of Vo 2 and oxygen pulse to increase appropriately at the last phase of exercise, associated with decreasing SV and increasing A-Vo 2 difference.28 (2) Diastolic dysfunction with poor LV compliance and increased LV filling pressure. In our cohort, only one patient had an increase in E/e′ ratio above 14 suggestive of increased LV filling pressure. (3) Reduced RV systolic reserve resulting in poor LV filling (Video 2; available at www.onlinejase.com). This was the case in eight patients in our cohort. RV dysfunction is well described among hospitalized patients with acute COVID-191 , 36 and may persist into recovery. (4) Severe dehydration or bleeding. None of the studied patients had clinical or laboratory parameters to suggest either factor. (5) Insufficient recruitment of blood from the splanchnic vascular compartment, which has been historically termed “vasoregulatory asthenia”33 , 34 , 37 (Video 3; available at www.onlinejase.com). We cannot exclude this etiology in our cohort. Autonomic dysregulation was not directly assessed in our study, but interestingly it can result in both chronotropic incompetence and abnormal blood flow distribution. It has been described in post-COVID-19 patients and may explain these two main abnormalities.38, 39, 40
Peripheral Factors
Peak A-Vo 2 difference is an independent predictor of reduced exercise capacity. Surprisingly, patients recovering from COVID-19 had higher peak A-Vo 2 difference than control subjects. As patients recovering from COVID-19 had reduced peak Vo 2 compared with control subjects, the increase in peak A-Vo 2 difference suggests that patients recovering from COVID-19 attempt to compensate for reduced cardiac output by increasing peripheral muscle oxygen extraction, resulting in increased peak A-Vo 2 difference, and that reduced peak Vo 2 is not merely the result of long-lasting deconditioning. This contrasts with the conclusions of a prior study of 10 post-COVID-19 patients with persistent dyspnea who underwent CPET.22 In that study, eight patients were found to have abnormally low peak Vo 2 (<85% of predicted), but only one had a cardiac limitation and two had ventilatory limitations. The authors concluded that metabolic limitation and muscular deficiency were the likely etiology in the rest of the patients. We show that a cardiac limitation is more likely than a peripheral muscular limitation.
Ventilatory and Gas Exchange Abnormalities
Acute COVID-19 causes respiratory illness ranging from mild upper respiratory disease to severe pneumonia. Surprisingly, we found that during the recovery phase, baseline respiratory parameters were normal in the majority of patients, and limited breathing reserve was the limiting factor for effort in only three patients (4%). Severe abnormal gas exchange was also rare. Nevertheless, as opposed to hemodynamic parameters, ventilatory and gas exchange abnormalities were more common in patients recovering from severe or critical compared with mild or moderate acute disease. During exercise, elevated breathing rate, increased VE/Vco 2, and low oxygen saturation were notable only in patients recovering from severe or critical acute infection, probably reflecting residual pulmonary damage. Similar findings were found in a study of 28 post-COVID-19 patients who underwent CPET, in which eight patients were found to have elevated VE/Vco 2, reflecting an exercise ventilatory inefficiency and related to lower HR recovery.21 In a study of 57 post-COVID-19 patients who underwent pulmonary function and 6-min walk tests, the patients were found to have impaired diffusion capacity and lower lung volumes. Those with histories of severe infection, compared with those with nonsevere infection, had a higher incidence of these abnormalities, as well as shorter 6-min walk distances.23
Subgroup Analysis on the Basis of Symptoms
We show how patients with specific symptoms compared with those free of those symptoms. Patients with dyspnea were found to have various cardiac and respiratory abnormalities in response to exercise, while patients presenting with fatigue presented mostly with attenuated HR response. Patients with muscle weakness or pain did not have significantly different CPET parameters compared with those without these symptoms. As a whole, symptomatic patients did not have significant differences in peak Vo 2 and other CPET parameters compared with asymptomatic patients. This could be explained by the fact that many survivors of COVID-19 continue to report different psychological and emotional difficulties,41 , 42 which can also present with physical symptoms, without objective limitations.
Study Limitations
The present analysis was performed at a single point in time, about 3 months following COVID-19 acute infection, and it is unknown whether the abnormalities identified will translate into long-term abnormalities. We report that peak HR is blunted in patients recovered from COVID-19, but we cannot exclude the possibility that the continued effects of β-blockers or mild ventilatory limitations may have influenced the blunted HR response. Nevertheless, the results were unchanged when adjustments were performed for long-term β-blocker use, and very low breathing reserve was a very rare cause of effort limitation in our cohort. Our imaging protocol was performed in the semisupine position, which generates a somewhat different hemodynamic response than the more commonly used treadmill exercise. SV and cardiac output measurements may have been underestimated or overestimated because of the technical challenge of acquiring echocardiographic images during exercise. However, this technique has been used successfully and validated against radionuclide angiography and Fick SV with reported excellent day-to-day reproducibility and intraobserver and interobserver variability.31 A-Vo 2 difference was not independently measured but was calculated using the Fick equation as Vo 2/cardiac output.31 , 32 The number of patients who had severe or critical acute disease was small, resulting in a lack of power to detect clinically significant differences in peak Vo 2 compared with patients with mild or moderate acute disease. It is possible that a larger cohort of such patients would yield a stronger association between acute disease severity and different exercise abnormalities. Selection bias was inherent to this study. First, we included only patients who required emergency department evaluation during acute COVID-19. Second, 20% of patients died during their acute illness. Last, among patients who were discharged alive, a considerable number of patients could not perform CPET because of immobilization or did not agree to participate. We present a cohort of limited size. As matching our cohort to historical data may introduce potential unknown confounders, the differences in CPET and stress echocardiographic parameters between patients recovering from COVID-19 and the control group should be interpreted cautiously.
Conclusion
Most patients recovering from COVID-19 report ongoing symptoms and exhibit significant objective reduction in effort capacity. The mechanism of this reduction is complex and mainly involves a combination of attenuated HR and SV reserve.
Footnotes
Drs. Szekely and Lichter contributed equally to this work.
This work was supported by a research grant from Novartis Israel.
Conflicts of interest: None.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.echo.2021.08.022.
Supplementary data
Four-chamber view of a patient with abnormal LV systolic reserve at peak stress. Note the significant decrease in ejection fraction during stress.
Four-chamber view of a patient with abnormal RV reserve at peak stress. Note the dilatation of the right ventricle with bowing of the interventricular septum to the left, decreasing LVEDV during stress.
Four-chamber view at peak stress of a patient with possible “vasoregulatory asthenia.” Note that LV and RV contraction are normal, but both ventricles are small, resulting in attenuated increase in SV. The same echocardiographic appearance may be seen with dehydration or bleeding; however, no patients had clinical or laboratory evidence of dehydration or bleeding.
Supplemental Methods
Rest Echocardiography
Measurements of mitral inflow included the peak early filling (E-wave) and late diastolic filling (A-wave) velocities and E/A ratio. Early diastolic mitral septal and lateral annular velocities (e′) were measured in the apical four-chamber view.1 Left atrial volume was calculated using the biplane area-length method at end-systole. From four-chamber views encompassing the entire right ventricle, end-systolic and end-diastolic RV areas and the tricuspid annulus were measured. Apart from qualitative grading, RV function was evaluated by TAPSE, systolic tricuspid lateral annular velocity (RV S′), and fractional area change.2 , 3 Hemodynamic right-sided assessment included measurements of the pulmonic flow acceleration time, to assess pulmonary vascular resistance and assessment of right atrial pressure using the inferior vena cava method.4
Exercise Protocol
A symptom-limited graded-ramp bicycle exercise test was performed in the semisupine position on a tilting dedicated microprocessor-controlled eddy current brake stress echocardiographic cycle ergometer (Ergoselect 1000 L). Forced vital capacity and forced expiratory volume in 1 sec were measured before exercise. Maximal voluntary ventilation was calculated by multiplying forced expiratory volume in 1 sec by 35.5 We estimated expected peak Vo 2 on the basis of age, height, and weight.5 , 6 We then calculated the workload necessary to reach the patient's estimated peak Vo 2 in 8 to 12 min. The protocol included 3 min of unloaded pedaling, symptom-limited ramp-graded exercise, and 2 min of recovery. Breath-by-breath VE, Vco 2, their ratio (VE/Vco 2), and Vo 2 were measured using a Medical Graphics metabolic cart (ZAN). Calibration was done before each test. Peak Vo 2 was the highest averaged 30-sec Vo 2 during exercise and was expressed as absolute peak Vo 2 and the ratio of measured peak Vo 2 to expected Vo 2 by age, sex, and height.5 , 6 The oxygen pulse was calculated as Vo 2/HR. VE/Vco 2 at anaerobic threshold was defined as the lowest immediately after anaerobic threshold and before the onset of ventilatory compensation for exercise-induced lactic acidosis and was expressed as absolute nadir VE/Vco 2. Anaerobic threshold was determined manually using the modified V-slope method.5 Vco 2 was plotted against Vo 2. A line parallel to the line of identity was drawn through Vco 2 versus Vo 2 points during the incremental phase of the exercise test. The point at which Vco 2 departed from the line (began to increase more rapidly than Vo 2) was taken as the V-slope anaerobic threshold. Arterial blood oxygen saturation was measured using noninvasive pulse oximetry. Quality of exercise effort was assessed by respiratory RER (ratio of Vco 2 to Vo 2) at peak exercise. A 12-lead electrocardiogram was monitored continuously, and HR and blood pressure were measured at rest and every minute during exercise. β-Blockers were left unchanged.
Exercise Echocardiographic Testing
Echocardiographic images were obtained concurrently with breath-by-breath gas exchange measurements, at rest, immediately after reaching a stable RER of ≥1.00 (anaerobic threshold) and at peak effort by a single cardiologist (Y.S.).7 , 8 Data collected at each time period included LVEDV, LVESV, LVEF, SV, peak E velocity, e′ in the septal and lateral mitral annulus, TAPSE, and RV S′. LVEDV, LVESV, and LVEF were calculated on the basis of the Simpson apical four-chamber view.7 , 8 Adequate images for evaluation of LV volumes were defined as a well-aligned left ventricle without apical foreshortening and with the ventricular endocardial contours well visualized for tracing. LV SV was calculated by multiplying LV outflow tract area at rest by the LV outflow tract velocity-time integral measured using pulsed-wave Doppler during each activity levels. E/e′ ratio was calculated at all effort stages to assess LV filling pressure. During sinus tachycardia, whenever merging of mitral E and A velocities or e′ and a′ occurred, whether to a single waveform (complete merging), or whether E and A waves could still be identified (incomplete merging), peak E-wave velocity, e′, and E/e′ ratio were measured using the methods of Nagueh et al. 9 and Sohn et al. 10 A-Vo 2 difference was calculated by using the Fick equation as Vo 2/echocardiography-calculated cardiac output, at each activity level.11 , 12 All exercise echocardiograms were analyzed by an experienced physician trained in quantitative analyses who was unaware of participant group or condition (Y.T.).
Supplemental Results
Comparison with Reference Values
ΔLVEDV, ΔLVEF, and ΔSV were attenuated in 49%, 30%, and 30% of patients recovering from COVID-19, compared with normal values.5 , 6 , 13 The insufficient increase in HR and SV resulted in a cardiac output response to exercise that was lower than normal in 69% of the post-COVID-19 patients. The attenuated cardiac output response resulted in abnormal peak Vo 2 in 59% of patients recovering from COVID-19 compared with normal reference values expected for age and sex (Supplemental Table 2).
Appendix
Supplemental Table 1.
Characteristics of patients who were not assessed at the post-acute COVID-19 clinic
| Variable | Study group |
Patients who were not assessed at the post–acute COVID-19 clinic |
P |
|---|---|---|---|
| (n = 71) | (n = 58) | ||
| Clinical characteristics | |||
| Age, y | 52.6 ± 16 | 63.5 ± 19 | .02 |
| Sex, male | 47 (66) | 32 (55) | .20 |
| Height, m | 1.71 ± 0.1 | 1.67 ± 0.1 | .04 |
| Weight, kg | 82 ± 21 | 73.9 ± 15 | .03 |
| Hypertension | 30 (43) | 33 (57) | .11 |
| Diabetes | 9 (13) | 21 (36) | .002 |
| Heart failure | 1 (1.5) | 6 (10) | .04 |
| Ischemic heart disease | 3 (4) | 6 (14) | .17 |
| Lung disease | 11 (15) | 12 (21) | .49 |
| Smoking | 8 (11) | 11 (19) | .31 |
| β-blockers | 8 (11) | 14 (24) | .32 |
| Furosemide | 0 (0) | 5 (9) | .01 |
| Angiotensin-converting enzyme inhibitor | 10 (14) | 10 (17) | .86 |
Data are expressed as mean ± SD or as number (percentage).
Supplemental Table 2.
Combined CPET and stress echocardiographic parameters during different stages of exertion compared with normal values
| Measurement | Baseline | AT | Maximal effort | P for trend | Threshold | Deviating n (%) |
|---|---|---|---|---|---|---|
| Vo2 | ||||||
| Vo2, L/min | 0.4 ± 0.13 | 0.97 ± 0.27 | 1.6 ± 0.5 | <.0001 | Peak < 85% expected | 42 (59) |
| Vo2/kg, mL/min/kg | 5.0 ± 1.7 | 12.3 ± 3.6 | 21.1 ± 6.1 | <.0001 | Peak < 85% expected | 49 (69) |
| Vo2/BMI, mL/min/(kg/m2) | 15.9 ± 4.8 | 35.8 ± 8.7 | 60.1 ± 17.2 | <.0001 | Peak < 85% expected | 50 (70) |
| Oxygen pulse, mL/beat | 5.5 ± 1.7 | 9.6 ± 2.5 | 12.4 ± 4.1 | <.0001 | Peak < 85% expected | 13 (18) |
| HR, beats/min | 70.1 ± 11 | 101.8 ± 14 | 135.9 ± 23 | <.0001 | <85% expected | 53 (75) |
| Left ventricle | ||||||
| LVEDV, mL | 93.8 ± 23 | 111.0 ± 27 | 107.9 ± 26 | <.0001 | Maximum < 110 mL | 35 (49) |
| LVESV, mL | 35.0 ± 18 | 36.9 ± 28 | 31.2 ± 20 | <.0001 | Maximum < 20 mL | 16 (23) |
| LVEF, % | 64.7 ± 14 | 66.9 ± 20 | 71.8 ± 16 | .001 | Peak < 63% | 21 (30) |
| Right ventricle | ||||||
| RV S′, cm/sec | 10.4 ± 2.0 | 12.8 ± 2.7 | 14.1 ± 3.2 | <.0001 | Peak < 10.5 cm/sec | 8 (11) |
| TAPSE, cm | 2.3 ± 0.4 | 2.5 ± 0.4 | 2.6 ± 0.4 | <.0001 | Peak < 1.9 cm | 2 (3) |
| Hemodynamics | ||||||
| E-wave velocity, cm/sec | 62.9 ± 14 | 80.4 ± 16 | 90.8 ± 19 | <.0001 | 0.91–1.1 | 0 (0) |
| e′ septal, cm/sec | 7.6 ± 2.4 | 10.1 ± 2.6 | 11.8 ± 3.5 | <.0001 | 12.4–14.1 | 37 (52) |
| e′ lateral, cm/sec | 9.2 ± 2.9 | 12.3 ± 3.0 | 13.9 ± 3.7 | <.0001 | 16.1–18.1 | 53 (75) |
| E/e′ septal ratio | 8.7 ± 3.0 | 8.4 ± 2.4 | 8.2 ± 2.6 | .31 | ≥15 | 3 (4) |
| E/e′ lateral ratio | 7.3 ± 2.5 | 6.9 ± 1.9 | 6.8 ± 1.9 | .65 | ≥14 | 0 (0) |
| E/e′ average ratio | 7.9 ± 2.6 | 7.5 ± 1.9 | 7.4 ± 1.9 | .16 | ≥14 | 1 (1.5) |
| SV, mL | 60.6 ± 13 | 75.6 ± 17 | 72.9 ± 16 | <.0001 | ΔSV < 20% | 21 (30) |
| Cardiac output, L/min | 4.4 ± 1.0 | 7.8 ± 1.9 | 9.8 ± 2.7 | <.0001 | Peak < 12.1 L/min | 49 (69) |
| Ventilation | ||||||
| Breathing rate, breaths/min | 20.7 ± 5.3 | 25.4 ± 5.0 | 37.1 ± 7.2 | <.0001 | Peak > 50 | 5 (7) |
| Tidal volume, L | 0.65 ± 0.16 | 1.1 ± 0.3 | 1.7 ± 0.5 | <.0001 | Peak < 85% expected | 26 (37) |
| Breathing reserve, % | 75.6 ± 7 | 63.4 ± 10 | 35.3 ± 13 | <.0001 | Peak < 15% | 3 (4) |
| Gas exchange | ||||||
| Oxygen saturation, % | 97.8 ± 1.5 | 97.7 ± 1.8 | 97.6 ± 2.0 | .58 | <95% | 6 (8) |
| VE/Vco2 ratio | 30.0 ± 9 | 27.6 ± 3 | 29.8 ± 3 | .03 | AT > 34 | 4 (6) |
| VE/Vo2 ratio | 26.5 ± 8 | 24.9 ± 4 | 34.5 ± 5 | <.0001 | AT > 34 | 2 (3) |
| Peripheral extraction | ||||||
| A-Vo2 difference, L/L | 0.09 ± 0.03 | 0.13 ± 0.03 | 0.18 ± 0.05 | <.0001 | Peak < 0.1 | 2 (3) |
Data are expressed as mean ± SD.
AT, Anaerobic threshold; BMI, body mass index.
Supplemental Table 3.
Combined CPET and stress echocardiographic parameters during different stages of exertion according to acute disease severity
| Measurement | Baseline | Anaerobic threshold | Maximal effort | P value, between groups | P value, within groups | P value, group-by-time interaction |
|---|---|---|---|---|---|---|
| Vo2 | ||||||
| Vo2, L/min | ||||||
| Mild/moderate disease | 0.40 ± 0.13 | 0.98 ± 0.3 | 1.7 ± 0.5 | .32 | <.0001 | .35 |
| Severe/critical disease | 0.42 ± 0.15 | 0.89 ± 0.2 | 1.5 ± 0.5 | |||
| Vo2/kg, mL/min/kg | ||||||
| Mild/moderate disease | 5.0 ± 1.8 | 12.6 ± 3.7 | 21.7 ± 6.2 | .28 | <.0001 | .26 |
| Severe/critical disease | 5.1 ± 1.3 | 11.0 ± 2.8 | 18.3 ± 4.9 | |||
| Vo2/BMI, mL/min/(kg/m2) | ||||||
| Mild/moderate disease | 16.6 ± 4.9 | 37.4 ± 8.1 | 63.9 ± 15.3 | .02 | <.0001 | .12 |
| Severe/critical disease | 13.9 ± 3.9 | 30.7 ± 9.0 | 49.0 ± 17.9 | |||
| Oxygen pulse, mL/beat | ||||||
| Mild/moderate disease | 5.5 ± 1.6 | 9.7 ± 2.6 | 12.6 ± 4.3 | .63 | <.0001 | .82 |
| Severe/critical disease | 5.5 ± 1.8 | 9.3 ± 2.3 | 11.6 ± 3.2 | |||
| HR, beats/min | ||||||
| Mild/moderate disease | 72.8 ± 13 | 103.0 ± 13 | 137.3 ± 24 | .17 | <.0001 | .54 |
| Severe/critical disease | 76.0 ± 11 | 95.5 ± 14 | 129.3 ± 16 | |||
| Left ventricle | ||||||
| LVEDV, mL | ||||||
| Mild/moderate disease | 92.0 ± 20 | 108.4 ± 24 | 104.5 ± 24 | .04 | <.0001 | .33 |
| Severe/critical disease | 102.4 ± 34 | 128.9 ± 32 | 126.4 ± 32 | |||
| LVESV, mL | ||||||
| Mild/moderate disease | 33.2 ± 18 | 34.0 ± 15 | 29.6 ± 13 | .12 | .0002 | .65 |
| Severe/critical disease | 43.4 ± 25 | 55.9 ± 29 | 46.0 ± 24 | |||
| LVEF, % | ||||||
| Mild/moderate disease | 65.5 ± 14 | 68.8 ± 19 | 73.2 ± 16 | .07 | .001 | .79 |
| Severe/critical disease | 59.3 ± 12 | 57.7 ± 18 | 64.6 ± 14 | |||
| Right ventricle | ||||||
| RV S′, cm/sec | ||||||
| Mild/moderate disease | 10.5 ± 2 | 12.9 ± 3 | 14.3 ± 3 | .27 | <.0001 | .20 |
| Severe/critical disease | 10.2 ± 2 | 12.3 ± 2 | 13.3 ± 2 | |||
| TAPSE, cm | ||||||
| Mild/moderate disease | 2.3 ± 0.4 | 2.5 ± 0.4 | 2.7 ± 0.4 | .22 | <.0001 | .94 |
| Severe/critical disease | 2.2 ± 0.4 | 2.4 ± 0.3 | 2.5 ± 0.3 | |||
| Hemodynamics | ||||||
| E-wave velocity, cm/sec | ||||||
| Mild/moderate disease | 63.7 ± 14 | 79.7 ± 16 | 91.6 ± 20 | .47 | <.0001 | .07 |
| Severe/critical disease | 59.2 ± 11 | 73.4 ± 17 | 86.3 ± 13 | |||
| e′ septal, cm/sec | ||||||
| Mild/moderate disease | 7.7 ± 2 | 10.3 ± 3 | 11.9 ± 4 | .65 | <.0001 | .68 |
| Severe/critical disease | 6.5 ± 2 | 9.5 ± 2 | 11.4 ± 3 | |||
| e′ lateral, cm/sec | ||||||
| Mild/moderate disease | 9.4 ± 3 | 12.5 ± 3 | 14.3 ± 4 | .04 | <.0001 | .41 |
| Severe/critical disease | 7.9 ± 2 | 11.4 ± 2 | 12.0 ± 2 | |||
| E/e′ septal ratio | ||||||
| Mild/moderate disease | 8.7 ± 3 | 8.4 ± 2 | 8.2 ± 3 | .64 | .47 | .67 |
| Severe/critical disease | 8.9 ± 4 | 8.9 ± 2 | 8.0 ± 2 | |||
| E/e′ lateral ratio | ||||||
| Mild/moderate disease | 7.2 ± 2 | 6.8 ± 2 | 6.7 ± 2 | .77 | .98 | .50 |
| Severe/critical disease | 7.5 ± 2 | 7.5 ± 2 | 7.4 ± 2 | |||
| E/e′ average ratio | ||||||
| Mild/moderate disease | 7.9 ± 2 | 7.4 ± 2 | 7.3 ± 2 | .92 | .58 | .49 |
| Severe/critical disease | 8.2 ± 3 | 8.1 ± 2 | 7.6 ± 2 | |||
| SV, mL | ||||||
| Mild/moderate disease | 58.7 ± 11 | 75.0 ± 18 | 72.0 ± 17 | .48 | <.0001 | .54 |
| Severe/critical disease | 59.1 ± 11 | 78.1 ± 15 | 77.5 ± 13 | |||
| Cardiac output, L/min | ||||||
| Mild/moderate disease | 4.2 ± 1.0 | 7.8 ± 1.9 | 9.8 ± 2.7 | .86 | <.0001 | .63 |
| Severe/critical disease | 4.5 ± 1.1 | 7.6 ± 1.9 | 10.1 ± 2.5 | |||
| Ventilation | ||||||
| Breathing rate, breaths/min | ||||||
| Mild/moderate disease | 20.0 ± 5 | 25.5 ± 4 | 36.3 ± 7 | .03 | <.0001 | .04 |
| Severe/critical disease | 24.0 ± 6 | 24.5 ± 7 | 41.7 ± 5 | |||
| Tidal volume, L | ||||||
| Mild/moderate disease | 0.65 ± 0.16 | 1.1 ± 0.3 | 1.7 ± 0.5 | .95 | <.0001 | .63 |
| Severe/critical disease | 0.64 ± 0.16 | 1.15 ± 0.4 | 1.6 ± 0.4 | |||
| Breathing reserve, % | ||||||
| Mild/moderate disease | 75.8 ± 7 | 63.8 ± 11 | 36.4 ± 11 | .12 | <.0001 | .008 |
| Severe/critical disease | 74.2 ± 4 | 62.3 ± 10 | 30.8 ± 12 | |||
| Gas exchange | ||||||
| Oxygen saturation, % | ||||||
| Mild/moderate disease | 98.0 ± 1 | 97.8 ± 2 | 97.8 ± 2 | .04 | .27 | .30 |
| Severe/critical disease | 97.0 ± 2 | 97.1 ± 2 | 96.4 ± 3 | |||
| VE/Vco2 ratio | ||||||
| Mild/moderate disease | 29.7 ± 9 | 27.3 ± 3 | 29.0 ± 3 | .04 | <.0001 | .05 |
| Severe/critical disease | 31.2 ± 3 | 29.6 ± 4 | 33.2 ± 4 | |||
| VE/Vo2 ratio | ||||||
| Mild/moderate disease | 26.1 ± 5 | 24.5 ± 4 | 33.3 ± 5 | .01 | <.0001 | .04 |
| Severe/critical disease | 28.0 ± 3 | 26.9 ± 4 | 39.8 ± 5 | |||
| Peripheral extraction | ||||||
| A-Vo2 difference, L/L | ||||||
| Mild/moderate disease | 0.09 ± 0.03 | 0.13 ± 0.03 | 0.18 ± 0.05 | .19 | <.0001 | .39 |
| Severe/critical disease | 0.09 ± 0.03 | 0.12 ± 0.03 | 0.16 ± 0.05 |
Data are expressed as mean ± SD.
BMI, Body mass index.
Supplemental Table 4.
Multivariable analysis: prediction of peak Vo2
| Variable | Rest |
Peak exercise |
|---|---|---|
| Coefficient ± SE | Coefficient ± SE | |
| Age | −0.01 ± 0.02 (P = .0003) | NS |
| Gender, male | 0.22 ± 0.15 (P = .008) | 0.08 ± 0.05 (P = .02) |
| Troponin I | NS | NS |
| SV | 0.02 ± 0.01 (P = .0003) | 0.02 ± 0.001 (P < .0001) |
| TAPSE | 0.27 ± 0.2 (P = .02) | 0.12 ± 0.03 (P = .03) |
| HR | −0.01 ± 0.02 (P = .0009) | 0.01 ± 0.001 (P < .0001) |
| A-Vo2 difference | 6.6 ± 3.1 (P = .009) | 7.0 ± 1.2 (P = .0005) |
| P | <.0001 | <.0001 |
| R2 | 0.67 | 0.91 |
Supplemental Table 5.
Inter- and intraobserver agreement
| Mean difference, mL | Intraclass correlation coefficient | Within-subject coefficient of variation, % | |
|---|---|---|---|
| Intraobserver agreement | |||
| LVEDV | 0.8 | 0.93 (P = .65) | 1.8 |
| LVESV | 1.5 | 0.89 (P = .82) | 6.2 |
| Interobserver agreement | |||
| LVEDV | 0.7 | 0.89 (P = .81) | 2.5 |
| LVESV | 1.0 | 0.85 (P = .83) | 7.5 |
References
- 1.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I., et al. Spectrum of cardiac manifestations in COVID-19. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rey J.R., Caro-Codón J., Rosillo S.O., Iniesta Á.M., Castrejón-Castrejón S., Marco-Clement I., et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22:2205–2215. doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra V., Flanders S.A., O'Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajpal S., Tong M.S., Borchers J., Zareba K.M., Obarski T.P., Simonetti O.P., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotecha T., Knight D.S., Razvi Y., Kumar K., Vimalesvaran K., Thornton G., et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moody W.E., Liu B., Mahmoud-Elsayed H.M., Senior J., Lalla S.S., Khan-Kheil A.M., et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiogr. 2021;34:562–566. doi: 10.1016/j.echo.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T.J. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churchill T.W., Bertrand P.B., Bernard S., Namasivayam M., Churchill J., Crousillat D., et al. Echocardiographic features of COVID-19 illness and association with cardiac biomarkers. J Am Soc Echocardiogr. 2020;33:1053–1054. doi: 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sud K., Vogel B., Bohra C., Garg V., Talebi S., Lerakis S., et al. Echocardiographic findings in patients with COVID-19 with significant myocardial injury. J Am Soc Echocardiogr. 2020;33:1054–1055. doi: 10.1016/j.echo.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S.S., Liu Q., Raikhelkar J., Fried J., Elias P., Poterucha T.J., et al. Indications for and findings on transthoracic echocardiography in COVID-19. J Am Soc Echocardiogr. 2020;33:1278–1284. doi: 10.1016/j.echo.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balady G.J., Arena R., Sietsema K., Myers J., Coke L., Fletcher G.F., et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 17.Guazzi M., Adams V., Conraads V., Halle M., Mezzani A., Vanhees L., et al. EACPR/AHA scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichter Y., Topilsky Y., Taieb P., Banai A., Hochstadt A., Merdler I., et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020;46:1873–1883. doi: 10.1007/s00134-020-06212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufer-Perl M., Gura Y., Shimiaie J., Sherez J., Pressman G.S., Aviram G., et al. Mechanisms of effort intolerance in patients with rheumatic mitral stenosis: combined echocardiography and cardiopulmonary stress protocol. JACC Cardiovasc Imaging. 2017;10:622–633. doi: 10.1016/j.jcmg.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Shimiaie J., Sherez J., Aviram G., Megidish R., Viskin S., Halkin A., et al. Determinants of effort intolerance in patients with heart failure: combined echocardiography and cardiopulmonary stress protocol. JACC Heart Fail. 2015;3:803–814. doi: 10.1016/j.jchf.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Dorelli G., Braggio M., Gabbiani D., Busti F., Caminati M., Senna G., et al. Importance of cardiopulmonary exercise testing amongst subjects recovering from COVID-19. Diagnostics (Basel) 2021;11:507. doi: 10.3390/diagnostics11030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohr A., Dannerbeck L., Lange T.J., Pfeifer M., Blaas S., Salzberger B., et al. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidiscip Respir Med. 2021;16:732. doi: 10.4081/mrm.2021.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell C., Rahko P.S., Blauwet L.A., Canaday B., Finstuen J.A., Foster M.C., et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., III, Dokainish H., Edvardsen T., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 27.Inbar O., Oren A., Scheinowitz M., Rotstein A., Dlin R., Casaburi R. Normal cardiopulmonary responses during incremental exercise in 20- to 70-yr-old men. Med Sci Sports Exerc. 1994;26:538–546. [PubMed] [Google Scholar]
- 28.Wasserman K., Hansen J.E., Sue D.Y., Stringer W.W., Sietsema K.E., Sun X.-G., et al. Principles of exercise testing and interpretation: including pathophysiology and clinical applications. 5th ed. Wolters Kluwer; Amsterdam, the Netherlands: 2011. [Google Scholar]
- 29.Brubaker P.H., Kitzman D.W. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan M.N., Pothier C.E., Lauer M.S. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol) Am J Cardiol. 2005;96:1328–1333. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 31.Haykowsky M.J., Brubaker P.H., Stewart K.P., Morgan T.M., Eggebeen J., Kitzman D.W. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitzman D.W., Brubaker P.H., Morgan T.M., Stewart K.P., Little W.C. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rozenbaum Z., Khoury S., Aviram G., Gura Y., Sherez J., Man A., et al. Discriminating circulatory problems from deconditioning: echocardiographic and cardiopulmonary exercise test analysis. Chest. 2017;151:431–440. doi: 10.1016/j.chest.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Lancellotti P., Pellikka P.A., Budts W., Chaudhry F.A., Donal E., Dulgheru R., et al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17:1191–1229. doi: 10.1093/ehjci/jew190. [DOI] [PubMed] [Google Scholar]
- 35.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szekely Y., Lichter Y., Hochstadt A., Taieb P., Banai A., Sapir O., et al. The predictive role of combined cardiac and lung ultrasound in coronavirus disease 2019. J Am Soc Echocardiogr. 2021;34:642–652. doi: 10.1016/j.echo.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmgren A. Vasoregulatory asthenia. Can Med Assoc J. 1967;96:904–907. [PMC free article] [PubMed] [Google Scholar]
- 38.Shouman K., Vanichkachorn G., Cheshire W.P., Suarez M.D., Shelly S., Lamotte G.J., et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31:385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson M., Ståhlberg M., Runold M., Nygren-Bonnier M., Nilsson J., Olshansky B., et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dani M., Dirksen A., Taraborrelli P., Torocastro M., Panagopoulos D., Sutton R., et al. Autonomic dysfunction in “long COVID”: rationale, physiology and management strategies. Clin Med (Lond) 2021;21:e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellan M., Soddu D., Balbo P.E., Baricich A., Zeppegno P., Avanzi G.C., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4:e2036142. doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplemental References
- 1.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., III, Dokainish H., Edvardsen T., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 2.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Topilsky Y., Khanna A.D., Oh J.K., Nishimura R.A., Enriquez-Sarano M., Jeon Y.B., et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929–1939. doi: 10.1161/CIRCULATIONAHA.110.991018. [DOI] [PubMed] [Google Scholar]
- 4.Kitabatake A., Inoue M., Asao M., Masuyama T., Tanouchi J., Morita T., et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–309. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 5.Guazzi M., Adams V., Conraads V., Halle M., Mezzani A., Vanhees L., et al. EACPR/AHA scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balady G.J., Arena R., Sietsema K., Myers J., Coke L., Fletcher G.F., et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 7.Laufer-Perl M., Gura Y., Shimiaie J., Sherez J., Pressman G.S., Aviram G., et al. Mechanisms of effort intolerance in patients with rheumatic mitral stenosis: combined echocardiography and cardiopulmonary stress protocol. JACC Cardiovasc Imaging. 2017;10:622–633. doi: 10.1016/j.jcmg.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Shimiaie J., Sherez J., Aviram G., Megidish R., Viskin S., Halkin A., et al. Determinants of effort intolerance in patients with heart failure: combined echocardiography and cardiopulmonary stress protocol. JACC Heart Fail. 2015;3:803–814. doi: 10.1016/j.jchf.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Nagueh S.F., Mikati I., Kopelen H.A., Middleton K.J., Quiñones M.A., Zoghbi W.A. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 10.Sohn D.W., Kim Y.J., Kim H.C., Chun H.G., Park Y.B., Choi Y.S. Evaluation of left ventricular diastolic function when mitral E and A waves are completely fused: role of assessing mitral annulus velocity. J Am Soc Echocardiogr. 1999;12:203–208. doi: 10.1016/s0894-7317(99)70136-7. [DOI] [PubMed] [Google Scholar]
- 11.Haykowsky M.J., Brubaker P.H., Stewart K.P., Morgan T.M., Eggebeen J., Kitzman D.W. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzman D.W., Brubaker P.H., Morgan T.M., Stewart K.P., Little W.C. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozenbaum Z., Khoury S., Aviram G., Gura Y., Sherez J., Man A., et al. Discriminating circulatory problems from deconditioning: echocardiographic and cardiopulmonary exercise test analysis. Chest. 2017;151:431–440. doi: 10.1016/j.chest.2016.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Four-chamber view of a patient with abnormal LV systolic reserve at peak stress. Note the significant decrease in ejection fraction during stress.
Four-chamber view of a patient with abnormal RV reserve at peak stress. Note the dilatation of the right ventricle with bowing of the interventricular septum to the left, decreasing LVEDV during stress.
Four-chamber view at peak stress of a patient with possible “vasoregulatory asthenia.” Note that LV and RV contraction are normal, but both ventricles are small, resulting in attenuated increase in SV. The same echocardiographic appearance may be seen with dehydration or bleeding; however, no patients had clinical or laboratory evidence of dehydration or bleeding.