Coronavirus disease 2019 (COVID-19), an acute respiratory illness caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been a terrible worldwide pandemic since December 2019. As of late August 2021, over 210 million people have contracted COVID-19, with over 4.4 million deaths globally (see https://coronavirus.jhu.edu/map.html). Typical presentations of this infection include fever, cough, fatigue, pneumonia, and loss of taste or smell.1 Although relatively less common than respiratory symptoms, gastrointestinal symptoms including anorexia, diarrhea, vomiting, and abdominal discomfort occur in about 18% of COVID-19 patients.2 Detection of virus RNA in fecal samples in up to 50% of COVID-19 patients infers the importance of gastrointestinal tract infection.2 Gastrointestinal symptoms have also occurred before the appearance of respiratory conditions, thus supporting viral involvement in the gastrointestinal tract in SARS-CoV-2 infection.3
Current Knowledge of Interplay Between SARS-CoV-2 Infection and Gut Microbiota
Gut Dysbiosis and COVID-19 Severity
The human gastrointestinal tract harbors trillions of microorganisms that form an ecologic community known as gut microbiota, of which its alteration, termed “dysbiosis,” has been associated with various human diseases. Microbial diversity in fecal samples of patients with COVID-19 was found to be decreased and accompanied by enrichment of opportunistic pathogens including Clostridium hathewayi and Ruminococcus species.4, 5, 6 Data from 2 studies that used metagenomic sequencing showed that several beneficial commensals such as Faecalibacterium prausnitzii and Eubacterium rectale were depleted in fecal samples of COVID-19 cases.5 , 6 F prausnitzii, a major producer of short-chain fatty acids (SCFAs; crucial for maintaining intestinal homeostasis) in the gut with anti-inflammatory potential due to induction of interleukin-10 production,7 was found to be low in abundance in feces of COVID-19 patients and had an inverse correlation with disease severity. In contrast, C hathewayi and Clostridium ramosum, both known to be associated with bacteremia and inflammation, were positively correlated with COVID-19 severity. In addition, fecal samples with high SARS-CoV-2 infectivity had a higher abundance of opportunistic pathogenic bacteria (eg, Collinsella species, Morganella morganii) and lower abundance of SCFA-producing bacteria (eg, Parabacteroides merdae, Lachnspiraceae bacterium) compared with samples with low SARS-CoV-2 viral infectivity.8 Altogether these findings imply that gut dysbiosis with enrichment of pathogenic bacteria and depletion of beneficial commensals is closely related to disease severity in COVID-19.
Persistent Dysbiosis After SARS-CoV-2 Clearance
SARS-CoV-2 infection not only causes acute infection but also lingering symptoms after the acute episode. Over 80% of COVID-19 patients have persistent symptoms known as post–acute COVID-19 syndrome and/or developed multisystem inflammation after viral clearance.9 Multiple studies have reported a marked difference in gut microbiota between recovered patients and healthy adults, and dysbiosis can persist up to 30 days after disease resolution.5 , 6 Infectious bacteria such as Bifidobacterium dentium and Klebsiella pneumoniae were enriched in recovered patients, whereas Bacteroides species including Bacteroides dorei, Bacteroides thetaiotaomicron, and Bacteroides massiliensis as well as the anti-inflammatory F prausnitzii were depleted.4 , 6 , 8 Prolonged dysbiosis in COVID-19 patients despite viral clearance may contribute to persistent illness of which secondary invasion of bacterial pathogens and reduction of beneficial commensals may become paramount for complete disease resolution.
Mechanistic Link Between SARS-CoV-2 Infection and Gut Microbiota
In SARS-CoV-2 infection, pathogenesis begins with interactions between SARS-CoV-2 and the viral entry receptor, angiotensin-converting enzyme (ACE)-2. ACE-2, a membrane-bound protein highly expressed in gut enterocytes, works with its countering opponent, ACE, to maintain balance of the renin-angiotensin system (RAS; mediator of fluid and electrolyte balance). ACE-2 is also a key regulator of dietary amino acid homeostasis, microbial ecology, and innate immunity.10 ACE-2 can be hijacked as a receptor for SARS-CoV-2 to undergo replication for promoting viral infection.11, 12, 13, 14 This viral-mediated reduction of ACE-2 leads to accumulation of its ligand angiotensin II and RAS imbalance, resulting in enhanced intestinal permeability and leaky gut syndrome.15 With a disrupted gut barrier, bacteria and endotoxins (eg, lipopolysaccharides) can enter the systemic circulation and contribute to the exaggerated production of cytokines and eventually trigger endotoxemia and inflammation. Both F prausnitzii and E rectale were found to be negatively associated with the proinflammatory cytokines C-X-C motif chemokine ligand 10 (CXCL10) and tumor necrosis factor-α,5 thus implicating their anti-inflammatory potential against SARS-CoV-2 infection. Interestingly, new evidence reported that SARS-CoV-2 infection could reduce local inflammation in the gut, whereas hospitalized COVID-19 patients presenting with gastrointestinal symptoms benefited from significant reduction in disease severity.16 These findings therefore implicate the potential of the gastrointestinal tract and gut microbiota in attenuating symptoms of COVID-19.
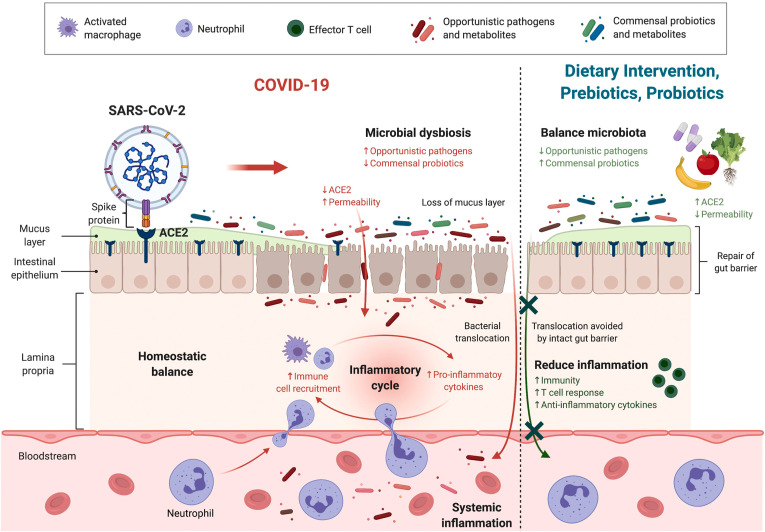
SARS-CoV-2 infection can also alter the profile of gut metabolites (intermediate or end products of microbial metabolism). For instance, an inducer of oxidative DNA damage and cytokine production guanosine and its derivatives 8-hydroxydeoxyguanosine were reported to be enriched in COVID-19 patients.8 Glycolysis and its intermediate product L-serine were found to be increased in patients’ samples, of which pathogenic bacteria could catabolize L-serine to confer a growth advantage against other bacterial competitors in the inflamed gut.17 Moreover, depletion of SCFA-producing bacteria, especially F prausnitzii, after viral infection led to decreased synthesis of anti-inflammatory SCFAs. Collectively, SARS-CoV-2 invades gut enterocytes through ACE-2 and causes alterations in gut microbiota and their metabolites, impaired barrier function, and bacterial translocation into the circulation, leading to aggravated systemic inflammation and multiple-organ involvement (Figure 1 ).
Figure 1.
Infection begins with the binding of SARS-CoV-2 to ACE-2 expressed in the membrane of intestinal epithelium cells. Enrichment of opportunistic pathogens (eg, C hathewayi) and depletion of commensal probiotics (eg, F prausnitzii) occur after viral infection. Reduction in functional ACE-2 leads to leaky gut syndrome with increased permeability of the gut barrier and an impaired mucous layer. Pathogens and harmful metabolites (eg, guanosine, L-serine) can enter the systemic circulation and contribute to inflammation with exaggerated production of proinflammatory cytokines (eg, CXCL10, tumor necrosis factor-α). Dietary intervention, prebiotics, and probiotics can restore the balance of gut microbiota and replenish the loss of ACE-2 on SARS-CoV-2 infection. These facilitate the repair of the gut barrier, thus reducing bacterial translocation and alleviating inflammation. The growth of probiotics and synthesis of beneficial metabolites (eg, SCFAs) can also be stimulated, thereby hastening recovery. (Figure was created with BioRender.com.)
Microbiota Modulation as Potential Therapeutics in COVID-19
Dietary Intervention
Dietary nutrients are a convenient and safe way to prevent disease and reduce disease severity. In SARS-CoV-2 infection, excessive production of proinflammatory cytokines and acute inflammation occurred; hence, intake of nutrients with anti-inflammatory and antioxidant effects may be beneficial in COVID-19 patients. These dietary components involve omega-3 polyunsaturated fatty acids, vitamins, zinc, plant-based polyphenols (eg, flavonoids and phenolic acids), polysaccharides, and a panoply of herbs from traditional Chinese medicine.18 Dietary fiber is protective against gut barrier disruption and can restrict bacterial translocation into the systemic circulation, whereas a high fat and protein diet are correlated with mucosal barrier dysfunction.19 A multicenter retrospective study with over 7300 subjects found that outcomes of patients with COVID-19 with pre-existing type 2 diabetes, who are known to have a high mortality rate, can be improved by well-controlled blood glucose.20 These data highlight the feasibility of a low glycemic index diet with green vegetables and fruits to improve outcome in hospitalized patients. Through appropriate dietary intervention, COVID-19 patients can potentially benefit from strengthened immunity and reduced inflammation and oxidative stress, thus alleviating disease severity and speeding recovery.
Prebiotics
Specific dietary components can serve as prebiotics (a group of dietary fibers including fructans and galactans that can only be digested by gut microbes but not the host) to stimulate an abundance of probiotics. For instance, plant-based fiber can promote the growth of the probiotics Lactobacillus and Bifidobacterium and reduce opportunistic pathogenic bacteria (eg, Clostridium).18 These prebiotic fibers are degraded by gut microbes to generate SCFAs (eg, acetate, propionate, and butyrate) as end products. SCFAs are immunomodulatory metabolites capable of enhancing effector activities of B cells and CD8+ T cells21 and producing anti-inflammatory cytokines.7 Dietary prebiotics serve as an effective means to stimulate SCFA synthesis through promoting growth of SCFA-producing bacteria. F prausnitzii is a key producer of SCFAs but is consistently depleted in COVID-19 patients.5 , 6 To restore its abundance, different nutrients derived from plant-based fiber can stimulate the growth of F prausnitzii,22 thereby rebalancing gut microbiota and dysregulated metabolites in the gut. There is a lack of dietary interventional studies in COVID-19, and it is likely that it may act as an adjunct to current therapeutics.
Probiotics
Substantial interest has emerged to develop therapeutic strategies against COVID-19 by modulating gut microbiota. Administration of probiotics, particularly Lactobacillus or Bifidobacterium, has long been associated with health benefits such as improvement of immunity and restoration of microbial balance. For example, commercially used probiotics Lactobacillus rhamnosus enhanced the T cell–mediated immune response in pneumococcal-infected mice23 and alleviated symptoms of acute respiratory infection in children.24 Although no published studies have reported the efficacy of probiotics for COVID-19 management, several clinical trials using a single strain or a cocktail of probiotics to reduce COVID-19 severity and/or improve treatment efficacy are in progress (Table 1 ). Results of an open-label pilot study (NCT04950803) showed that 4-week oral supplementation of a probiotic formula (S1M01; GenieBiome, Hong Kong; a probiotic blend of 3 Bifidobacterium) targeted to replenish bacteria species known to be depleted in COVID-19 subjects hastened recovery, enhanced immunity, and suppressed serum proinflammatory cytokines in hospitalized patients. Enrichment of beneficial bacteria in feces of patients receiving the formula were seen at 5 weeks after therapy that was not seen in the standard arm group.25
Table 1.
Ongoing Clinical Studies of Gut Microbiota Regarding COVID-19a
| Study type | Trial identification and phaseb | Main aim | Country | Estimated participants |
|---|---|---|---|---|
| Observational |
NCT04770649 Phase: N/A |
Influence of microbiota and its function on immune system and efficacy of COVID-19 vaccine | United States | 10,000 |
| Observational |
NCT04359836 Phase: N/A |
Change in faecal microbiota of COVID-19 patients | United States | 250 |
| Observational |
NCT04980560 Phase: N/A |
Microbiota difference among subjects receiving different COVID-19 vaccines and recovered patients | Hong Kong | 200 |
| Observational |
NCT04669938 Phase: N/A |
Microbiota of COVID-19 patients in ICU for outcome prediction of disease severity | France | 200 |
| Observational |
NCT04447144 Phase: N/A |
Influence of dietary habits on outcomes of COVID-19 patients | Egypt | 200 |
| Observational |
NCT04497402 Phase: N/A |
Sex difference in microbiota of COVID-19 patients | Italy | 88 |
| Interventional |
NCT04366089 Phase: II |
Efficacy of an ozone therapy-based intervention with a probiotic mixture (SivoMixxc) in COVID-19 patients and preventing the need for ICU hospitalization Dose: 500 g azithromycin + 2 × 109 CFU SivoMixxc daily for 21 days |
Italy | 152 |
| Interventional |
NCT04540406 Phase: II |
Efficacy of a botanical-based fixed-combination drug NBT-NM108 in early-stage suspected or confirmed symptomatic COVID-19 patients Dose: 4 times (30 g/sachet) daily for 28 days |
United States | 100 |
| Interventional |
NCT04941703 Phase: I and II |
Efficacy of combining magnesium citrate with probiotics in hospitalized COVID-19 patients Dose: 296 mL magnesium citrate + probiotics twice daily for 6 days or until discharge |
United States | 30 |
| Interventional |
NCT04884776 Phase: N/A |
Efficacy of a microbiome immunity formula to improve immune functions, reduce adverse events associated with COVID-19 vaccinations, and reduce hospitalization in vulnerable subjects (patients with underlying type 2 diabetes mellitus and the elderly) Dose: 1 × 109 CFU of 3 Bifidobacteria and 3 prebiotics twice daily for 12 weeks |
Hong Kong | 484 |
| Interventional |
NCT04950803 Phase: N/A |
Efficacy of an oral microbiome immunity formula to enhance immunity and reduce long-term complications in patients recovered from COVID-19 Dose: 1 × 109 CFU of 3 Bifidobacteria and 3 prebiotics daily for 3 months |
Hong Kong | 280 |
| Interventional |
NCT04730284 Phase: N/A |
Efficacy of a synbiotic formula in hospitalized COVID-19 patients Dose: 4 g tailored-made synbiotics daily for 28 days |
Hong Kong | 20 |
| Interventional |
NCT04420676 Phase: N/A |
Efficacy of a probiotic mixture (Omni-Biotic 10 AADd) to improve gastrointestinal symptoms of COVID-19 and disease severity Dose: Omni-Biotic 10 AAD (Institut AllergoSan, Graz, Austria) twice daily for 30 days |
Austria | 120 |
| Interventional |
NCT04813718 Phase: N/A |
Efficacy of a mixture of prebiotics and probiotics Omni-Biotic Pro Vi 5 (Institut AllergoSan, Graz, Austria) against post–COVID-19 syndrome Dose: N/A |
Austria | 20 |
| Interventional |
NCT04666116 Phase: N/A |
Efficacy of nutritional supplement by nasopharyngeal smear to decrease viral load in hospitalized COVID-19 patients Dose: Supplement of mixture of probiotics (Bifidobacterium longum, Bifidobacterium animalis ssp. lactis and Lactobacillus rhamnosus), vitamin D, zinc, and selenium |
Spain | 96 |
| Interventional |
NCT04922918 Phase: N/A |
Impact of probiotics on preventing SARS-CoV-2 infection in elderly living in a nursing home Dose: 1 × 109 CFU of Ligilactobacillus salivarius MP101 daily for 4 months |
Spain | 25 |
| Interventional |
NCT04621071 Phase: N/A |
Efficacy of probiotics to reduce duration and symptoms of COVID-19 patients with self-caring at home Dose: 1 × 109 CFU of 2 probiotic strains daily for 25 days or until hospitalized |
Canada | 84 |
| Interventional |
NCT04486482 Phase: N/A |
Efficacy of a glycan KB109 in COVID-19 patients in the outpatient setting Dose: KB109 (Kaleido Biosciences, Lexington, MA) for 35 days |
United States | 50 |
| Interventional |
NCT04847349 Phase: N/A |
Efficacy of a probiotic mixture (OL-1)e to boost immunity of unvaccinated patients with previous SARS-CoV-2 infection Dose: OL-1 daily for 21 days |
United States | 45 |
| Interventional |
NCT04734886 Phase: N/A |
Impact of probiotics on SARS-CoV-2 antibody response in healthy adults Dose: 1 × 108 CFU of Lactobacillus reuteri DSM 17938 + 10 μg vitamin D3 twice daily for 6 months |
Sweden | 400 |
CPU, colony-forming unit; ICU, intensive care unit; N/A, not applicable.
The search was conducted on clinicaltrials.gov in July 2021. Completed, not yet recruiting, suspended, or terminated trials were excluded.
US Food and Drug Administration definitions of clinical trial phases were used.
Probiotics in SivoMixx are Streptococcus thermophiles (DSM322245), Bifidobacterium lactis (DSM 32246), Bifidobacterium lactis (DSM 32247), Lactobacillus acidophilus (DSM 32241), Lactobacillus helveticus (DSM 32242), Lactobacillus paracasei (DSM 32243), Lactobacillus plantarum (DSM 32244), and Lactobacillus brevis (DSM 2796).
Probiotics in Omni-Biotic 10 AAD include Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Enterococcus faecium W54, Lactobacillus acidophilus W37, Lactobacillus acidophilus W55, Lactobacillus paracasei W20, Lactobacillus plantarum W1, Lactobacillus plantarum W62, Lactobacillus rhamnosus W71, and Lactobacillus salivarius W24.
Probiotics in OL-1 include Bifidobacterium lactis Bl-04, Bifidobacterium longum subsp. infantis Bi-26, Lactobacillus rhamnosus Lr-32, Lactobacillus paracasei Lpc-37, and Lactobacillus salivarius Ls-33.
Therapeutic approaches that target ACE-2 to restore RAS balance including ACE inhibitors or angiogenesis receptor blockers are rapidly being developed and tested in clinical trials.15 Gut microbes interacted with ACE-2, and microbial alterations in COVID-19 patients were shown to be correlated with ACE-2,6 suggesting the feasibility of combined pharmacologic agents with probiotics against COVID-19. Caution is needed, however, because these drugs can also upregulate ACE-2 expression and may enhance viral entry into host cells.26 To address this concern, soluble recombinant human ACE-2 has emerged that can act as a competitive interceptor by binding the viral spike protein to neutralize SARS-CoV-2 and also minimize organ injuries by rebalancing RAS and lowering circulatory concentration of angiotensin II.15 , 27 Interestingly, bioengineering the probiotic species Lactobacillus paracasei into a live vector for oral delivery of recombinant human ACE-2 showed positive outcomes in mice, and this approach may facilitate large-scale production of high-quality ACE-2 with sufficient bioavailability in the future.28
Previous studies have reported moderate efficacy of probiotics against acute respiratory infection, whereas probiotics as adjuvants may improve clinical outcomes.29 Several clinical studies are investigating the efficacy of probiotics in patients with COVID-19. Researchers in Austria are investigating the efficacy of a probiotic mixture to alleviate gastrointestinal symptoms in hospitalized COVID-19 patients (NCT04420676) and complications after discharge (NCT04813718). The combined use of prebiotics and probiotics in hospitalized COVID-19 patients is being studied by researchers in Spain (NCT04666116) and Hong Kong (NCT04730284). The use of probiotics as adjuvant therapy for COVID-19 management is also of interest. A phase II trial in Italy applied ozone therapy plus a probiotic mixture to COVID-19 patients to prevent deterioration, need for hospitalization, or intensive care unit admission (NCT04366089). Studies are also ongoing to investigate whether probiotics can lower the risk of SARS-CoV-2 infection by boosting immunity in uninfected elderly subjects (NCT04922918) and healthy individuals (NCT04734886). Clinical studies targeting gut microbiota as therapeutics in COVID-19 are summarized in Table 1.
Fecal Microbiota Transplantation
A case report on 2 subjects showed that fecal microbiota transplantation was safe in patients with recurrent Clostridioides difficile infection and coexisting COVID-1930 and might have a role in hastening recovery. A larger clinical trial to examine the role of fecal microbiota transplantation in COVID-19 is in progress (NCT04824222). However, the presence of SARS-CoV-2 in fecal samples in asymptomatic individuals has had a large impact on donor screening, and vigilant SARS-CoV-2 testing should be performed in all donors to ensure the safety of fecal microbiota transplantation during the COVID-19 pandemic.31
Future Directions
At the society level, the long-lasting COVID-19 pandemic has dramatically upended daily lives. Current pandemic control measures and practices will have substantial and potentially long-term effects on the human microbiota worldwide, given strict implementation of hygiene measures, physical separation, travel barriers, and other measures that influence overall microbial diversity and loss. Several studies reported increased rates of cesarean sections in COVID-19–positive women, and initial recommendations discouraged COVID-19–positive mothers from breastfeeding and participating in skin-to-skin care, all of which will impact early microbiota development. Infection control measures must be balanced with strategies that promote microbial diversity to impart optimal health outcomes and potentially modulate susceptibility of children to COVID-19.
At the individual level, the World Health Organization has proposed a shift from a Western-style diet with high-fat and high-sugar content to a well-balanced and diversified diet. This is especially crucial in elderly subjects and those with cardiovascular disease, type 2 diabetes, or other chronic diseases who are known to have poorer outcomes with SARS-CoV-2 infection. Intake of adequate nutrients is recommended to avoid malnutrition and maintain immune homeostasis in these high-risk individuals.32 A diversified diet can also beneficially impact gut microbiota with enrichment of probiotics (eg, Lactobacillus, Bifidobacterium, and Streptococcus thermophilus) and SCFAs.33 Whether modulating gut microbiota with dietary intervention can reduce susceptibility of SARS-CoV-2 infection and severity warrants prospective studies.
At the hospital level, the liberal use of antibiotics in COVID-19 patients could have a detrimental impact on gut microbiota. Up to three-fourths of COVID-19 patients received empirical antibiotics to prevent bacterial infection, although coinfection was identified in less than 5% of patients.34 Studies have reported no difference in outcomes with or without antibiotics in hospitalized COVID-19 patients.5 , 6 Gut microbiota in antibiotic-treated patients displayed further dissimilarity to microbiota of healthy individuals with greater depletion of beneficial commensals including F prausnitzii, E rectale, and L bacterium compared with patients treated without antibiotics. Hence, caution is needed when prescribing antibiotics because the unnecessary use of antibiotics can cause more severe gut dysbiosis and depletion of beneficial bacteria and increased antimicrobial resistance. In the latest World Health Organization guidance for COVID-19 clinical management (see https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1), antibiotic therapy or prophylaxis was not recommended in patients with mild or moderate COVID-19 unless there is clinical suspicion of bacterial infection. In addition, longer follow-up of patients with COVID-19 up to 12 months after recovery is needed to address questions related to the duration of dysbiosis postrecovery, the link between dysbiosis and post–acute COVID-19 syndrome, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health issues.
Although emerging evidence supports the importance of gut microbiota in COVID-19 pathogenesis and severity, data are mostly derived from descriptive and associative human studies. Extensive in-depth mechanistic and murine studies are required to decipher the causal relationship between gut microbiota and SARS-CoV-2 infection. However, although animal experiments are necessary for mechanistic investigation, a mouse model that can accurately mimic SARS-CoV-2 infection in humans has been poorly established. Although some preprint articles infected a transgenic mouse model with expression of human ACE-2 (namely K18-hACE2) with SARS-CoV-2 to assess drug efficacy35 or dysbiosis-associated bacteremia,36 to what extent these mice can replicate viral infection in humans requires further validation. Moreover, to date published clinical trial data supporting the use of probiotics in COVID-19 are scarce, and outstanding challenges include determination of an optimal strain(s), dosing regimen, and duration of intervention as well as selection of the appropriate clinical and mechanistic outcomes
At the global level, it is currently unclear how long immunity lasts from SARS-CoV-2 vaccines, and scientists around the world are racing to determine what level of neutralizing antibodies or immune marker is most closely related with COVID-19 vaccine’s effectiveness. The surge in cases caused by the delta variant worldwide has caused some countries to consider booster vaccines in at-risk groups despite the lack of evidence.37 In 2019 gut microbiota was shown to influence vaccine efficacy because a significant impairment of antibody response was observed in healthy subjects receiving antibiotics before H1N1 influenza vaccination.38 It is likely that the gut microbiome equally plays an important role in SARS-CoV-2 vaccine immune response and vaccine-related adverse effects. Preliminary results of an ongoing clinical study showed that subjects who took a probiotic formula for 2 months had higher serum SARS-CoV-2 IgG antibody levels and decreased proinflammatory cytokines (NCT04980560). A large population-based study in the United States is recruiting up to 10,000 participants to decipher the correlation between gut microbiota and efficacy of COVID-19 vaccine (NCT04770649).
Waning immunity and SARS-CoV-2 variants will likely be a long-term challenge. In light of this, every means to prolong immunity and reduce complications are needed. The central role of gut microbiota in immunity against SARS-CoV-2 infection and microbiota modulation to improve SARS-CoV-2 vaccine efficacy are low-hanging fruit that should be seriously considered.
Footnotes
Author names in bold designate shared co-first authorship.
Conflicts of interest This author discloses the following: Siew C. Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and Abbvie and as speaker for Ferring, Tillotts, Menarini, Janssen, Abbvie, and Takeda. She is serving as scientific cofounder of GenieBiome Limited and has received research grants from Olympus, Ferring, and Abbvie. The remaining authors disclose no conflicts.
Funding This study was supported by the National Key R&D Program of China (no. 2020YFA0509200/2020YFA0509203), RGC Theme-based Research Scheme Hong Kong (T21-705/20-N), Vice-Chancellor’s Discretionary Fund Chinese University of Hong Kong.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung K.S., Hung I.F.N., Chan P.P.Y., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotfis K., Skonieczna-Zydecka K. COVID-19: gastrointestinal symptoms and potential sources of SARS-CoV-2 transmission. Anaesthesiol Intensive Ther. 2020;52:171–172. doi: 10.5114/ait.2020.93867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren Z., Wang H., Cui G., et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut. 2021;70:1253–1265. doi: 10.1136/gutjnl-2020-323826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeoh Y.K., Zuo T., Lui G.C., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo T., Zhang F., Lui G.C.Y., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alameddine J., Godefroy E., Papargyris L., et al. Faecalibacterium prausnitzii skews human DC to prime IL10-producing T cells through TLR2/6/JNK signaling and IL-10, IL-27, CD39, and IDO-1 induction. Front Immunol. 2019;10:143. doi: 10.3389/fimmu.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo T., Liu Q., Zhang F., et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carfi A., Bernabei R., Landi F., et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto T., Perlot T., Rehman A., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang J., Ye G., Shi K., Wan Y., et al. Structural basis of receptor recognition by SARS-CoV–2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamers M.M., Beumer J., van der Vaart J., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J., Li C., Liu X., et al. Infection of bat and human intestinal organoids by SARS-CoV–2. Nat Med. 2020;26:1077–1083. doi: 10.1038/s41591-020-0912-6. [DOI] [PubMed] [Google Scholar]
- 14.Zang R., Gomez Castro M.F., McCune B.T., et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5:eabc3582. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penninger J.M., Grant M.B., Sung J.J.Y. The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Gastroenterology. 2021;160:39–46. doi: 10.1053/j.gastro.2020.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livanos A.E., Jha D., Cossarini F., Gonzalez-Reiche A.S., Tokuyama M., Aydillo T., Parigi T.L., et al. Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160:2435–2450. doi: 10.1053/j.gastro.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamoto S., Alteri C.J., Rodrigues M., et al. Dietary L-serine confers a competitive fitness advantage to Enterobacteriaceae in the inflamed gut. Nat Microbiol. 2020;5:116–125. doi: 10.1038/s41564-019-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iddir M., Brito A., Dingeo G., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020:12. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camilleri M., Lyle B.J., Madsen K.L., et al. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol Gastrointest Liver Physiol. 2019;317:G17–G39. doi: 10.1152/ajpgi.00063.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L., She Z.G., Cheng X., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trompette A., Gollwitzer E.S., Pattaroni C., et al. Dietary fiber confers protection against flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism. Immunity. 2018;48:992–1005. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Benus R.F., van der Werf T.S., Welling G.W., et al. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br J Nutr. 2010;104:693–700. doi: 10.1017/S0007114510001030. [DOI] [PubMed] [Google Scholar]
- 23.Barbieri N., Herrera M., Salva S., et al. Lactobacillus rhamnosus CRL1505 nasal administration improves recovery of T-cell mediated immunity against pneumococcal infection in malnourished mice. Benef Microbes. 2017;8:393–405. doi: 10.3920/BM2016.0152. [DOI] [PubMed] [Google Scholar]
- 24.Laursen R.P., Hojsak I. Probiotics for respiratory tract infections in children attending day care centers—a systematic review. Eur J Pediatr. 2018;177:979–994. doi: 10.1007/s00431-018-3167-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang LXZ, Mak JWY, Chan FKL, et al. SIM01 as a novel microbiome replacement therapy for COVID-19: an open-label pilot study. Asian Pacific Digestive Week 2021. Virtual, Asian Pacific Digestive Week Federation, August 19–22, 2021. 2021:PP–0464.
- 26.Sanders J.M., Monogue M.L., Jodlowski T.Z., et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 27.Zoufaly A., Poglitsch M., Aberle J.H., et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma A., Xu K., Du T., et al. Expression of human ACE2 in Lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol Ther Methods Clin Dev. 2019;14:161–170. doi: 10.1016/j.omtm.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suez J., Zmora N., Segal E., et al. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 30.Bilinski J., Winter K., Jasinski M., et al. Rapid resolution of COVID-19 after faecal microbiota transplantation. Gut. 2021 doi: 10.1136/gutjnl-2021-325010. gutjnl-2021-325010. [DOI] [PubMed] [Google Scholar]
- 31.Ianiro G., Mullish B.H., Kelly C.R., et al. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut. 2020;69:1555–1563. doi: 10.1136/gutjnl-2020-321829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkert D., Beck A.M., Cederholm T., et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38:10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 33.De Filippis F., Pellegrini N., Vannini L., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 34.Vaughn V.M., Gandhi T.N., Petty L.A., et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72:e533–e541. doi: 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarado D.M., Son J., Thackray L.B., et al. Mesalamine reduces intestinal ACE2 expression without modifying SARS-CoV-2 infection or disease severity in mice. bioRxiv. 2021 doi: 10.1093/ibd/izab274. 2021.07.23.453393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venzon M., Bernard-Raichon L., Klein J., Axelrad J.E., et al. Gut microbiome dysbiosis during COVID-19 is associated with increased risk for bacteremia and microbial translocation. Res Sq. 2021 rs.3.rs-726620. [Google Scholar]
- 37.Callaway E. COVID vaccine boosters: the most important questions. Nature. 2021;596:178–180. doi: 10.1038/d41586-021-02158-6. [DOI] [PubMed] [Google Scholar]
- 38.Hagan T., Cortese M., Rouphael N., et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178:1313–1328. doi: 10.1016/j.cell.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]