Abstract
The stable globin mRNAs provide an ideal system for studying the mechanism governing mammalian mRNA turnover. α-Globin mRNA stability is dictated by sequences in the 3′ untranslated region (3′UTR) which form a specific ribonucleoprotein complex (α-complex) whose presence correlates with mRNA stability. One of the major protein components within this complex is a family of two polycytidylate-binding proteins, αCP1 and αCP2. Using an in vitro-transcribed and polyadenylated α-globin 3′UTR, we have devised an in vitro mRNA decay assay which reproduces the α-complex-dependent mRNA stability observed in cells. Incubation of the RNA with erythroleukemia K562 cytosolic extract results in deadenylation with distinct intermediates containing a periodicity of approximately 30 nucleotides, which is consistent with the binding of poly(A)-binding protein (PABP) monomers. Disruption of the α-complex by sequestration of αCP1 and αCP2 enhances deadenylation and decay of the mRNA, while reconstitution of the α-complex stabilizes the mRNA. Similarly, PABP is also essential for the stability of mRNA in vitro, since rapid deadenylation resulted upon its depletion. An RNA-dependent interaction between αCP1 and αCP2 with PABP suggests that the α-complex can directly interact with PABP. Therefore, the α-complex is an mRNA stability complex in vitro which could function at least in part by interacting with PABP.
mRNA turnover is an important step in the regulation of eukaryotic gene expression. All mRNAs have an intrinsic half-life that contributes to their general level of expression. At one extreme, short-lived mRNAs are necessary to ensure transient expression at distinct stages, as demonstrated by the pattern of c-Myc protein expression (48). Conversely, long-lived mRNAs are generally associated with specialized differentiated cells that require the accumulation of distinct proteins, as typified by the accumulation of hemoglobin in erythrocytes (50). Most eukaryotic mRNAs contain elements at either terminus that contribute to their mRNA stability. The 5′ end contains a m7G cap structure, while the 3′ end contains a polyadenylate [poly(A)] tract. Both of these structures are involved in the stability of an mRNA by providing a layer of protection for the body of the mRNA (48, 51, 53). The m7G cap, along with the cap-binding proteins, protects the 5′ end from most 5′-3′ exoribonucleases (40, 57), while the poly(A) tract and the poly(A)-binding protein (PABP) protect the 3′ end from 3′-5′ exoribonucleases (48). In many instances, deadenylation and decapping precede decay of the mRNA (8, 11, 39, 55, 57).
The poly(A) tail functions as a ribonucleoprotein (RNP) complex with PABP, since PABP is essential to stabilize the 3′ end of an mRNA in mammalian cells (1, 17). The normally stable polyadenylated β-globin mRNA is destabilized in cytosolic extract depleted of PABP or in extract containing PABP sequestered by poly(A) competitor (1). Similarly, poly(A) competition causes a rapid deadenylation of exogenous polyadenylated simian virus 40 3′ untranslated region (3′UTR) (17). PABP is a highly conserved, abundant protein present in divergent organisms. A high degree of conservation exists in the amino-terminal region of the protein, which contains four RNP motif RNA-binding domains (RBDs); the carboxyl terminus is more divergent (22). Although all four RNP motifs are competent to bind RNA individually or in combination, the first two RNP motifs contain the highest affinity for poly(A) sequences and are the major contributors of the poly(A)-binding activity (7, 42).
Specific cis elements other than the m7G cap and poly(A) tail also contribute to mRNA stability. Many of these elements lie in the 3′UTR (12, 25). The most extensively studied element is the AU-rich element (ARE) found in the 3′UTRs of many proto-oncogenes and cytokines. The ARE appears to stimulate deadenylation and subsequent decay of an mRNA (9). ARE-binding proteins have been identified and implicated in both rapid mRNA decay (4) as well as mRNA stabilization (14, 46). However, the mechanism by which they function remains unclear. The protein coding region of an mRNA also contains cis elements associated with mRNA stability (2, 45, 56), as well as elements linked to nonsense-mediated mRNA decay in yeast (26) and mammals (42).
The globin mRNAs are among the most stable mRNAs characterized, with estimated half-lives ranging from 24 to 60 h (36, 49, 59). They therefore provide an ideal model system to study determinants of mRNA stability. The stability of α-globin mRNA is conferred by sequences in the 3′UTR. This was first evident from a natural occurring α-thalassemia mutation, Constant Spring, which contains a single base substitution at the termination codon which allows ribosomal entry into the 3′UTR and results in reduced mRNA levels (35). The ribosomal entry into the 3′UTR disrupts a specific RNP complex termed the α-complex which correlates with mRNA stability (60–62). The α-complex consists of up to six distinct proteins or protein families (28). Identities of four of these proteins, with apparent molecular masses of 58, 55, 50, and 28 kDa, and are currently unknown. One of the identified proteins is the AUF1/hnRNP D protein (28), which is implicated in the ARE-mediated turnover of c-myc mRNA (4, 63). A second identified protein family in the α-complex consists of the polycytidylate [poly(C)]-binding protein α-complex protein 1 (αCP1) and the highly homologous αCP2 (30; also referred to as PCBP in reference 34 and hnRNP E in reference 44). These proteins have been implicated in both mRNA stability and translational regulation (3, 18, 30, 44, 60). αCP1 and αCP2 are essential for the formation of the α-complex since sequestration of these proteins by the addition of poly(C) or poly(dC) competitor or depletion of the extract with poly(C)-agarose beads prevents assembly of the α-complex (29, 30, 60). Although the α-complex has been implicated in mRNA turnover, the mechanism by which it functions remains unknown. Use of transgenic mice expressing the human α-globin mRNA suggests that the α-complex may contribute to the deadenylation rate of the mRNA since the wild-type α-globin mRNA had a length different from that of a naturally occurring mutant mRNA (39).
In our efforts to determine the mechanism by which the α-complex might function to stabilize mRNA, we devised an in vitro mRNA decay system. Studies in higher eukaryotes have been hampered by the lack of assays which enable manipulation of the mRNA and protein components. Nevertheless, various in vitro systems have proven useful for certain mRNAs. Current systems to study mRNA turnover in vitro include the use of polysomal fractions, ribosomal salt wash fractions, or nuclear extracts (6, 17, 47); more recently, soluble cytosolic extract systems have also been developed (5, 16). We have expanded on these approaches and used polyadenylated mRNA with soluble cytosolic S130 extract to demonstrate an α-complex-dependent RNA stabilization in vitro by an interaction of the α-complex with PABP.
MATERIALS AND METHODS
Plasmid constructs.
Plasmids pGBD-αCP1, pGBD-αCP2 and pGBD-Ugly, which express the Gal4 DNA-binding domain fused to αCP1, αCP2, and the hnRNP U protein RBD, respectively, have previously been described (28). Plasmid pαmtΔ9-21 is the α-globin 3′UTR with a deletion of nucleotides 25 to 66, which corresponds to removal of codons 9 to 21 in the 3′UTR relative to the α-globin mRNA translation termination (nomenclature of Weiss and Liebhaber [62]). The 5′ and 3′ halves of the deletion were generated by cleaving the αH9 and αH21 mutations described by Weiss and Liebhaber (62) with HindIII. The resulting halves of the 3′UTR were ligated with a four-nucleotide linker at the HindIII site. The region was subsequently amplified by PCR to insert a T7 promoter at the 5′ end and five adenosine residues at the 3′ end and cloned into the pCR-Trap vector (GenHunter). The terminal five adenosine residues were added to improve the efficiency of the subsequent polyadenylation reaction. The His-tagged human PABP expression plasmid (pET28a-PABP) contained the PABP open reading frame from pET11-hPABP (19) inserted into the same sites of pET28a (Novagene). The bovine poly(A) polymerase (bPAP) expression plasmid bPAP-423 and the U1A expression plasmid pET-U1A are described by Gunderson et al. (23). PCR constructs were confirmed by sequencing.
Extract preparation.
Human erythroleukemia K562 cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum containing penicillin (100 U/ml) and streptomycin (100 μg/ml). Isolation of the S130 extract was carried out as previously described (28, 29). Briefly, cells were washed twice in phosphate-buffered saline (PBS) and resuspended in buffer A (10 mM Tris-HCl [pH 7.5], 1 mM potassium acetate, 1.5 mM magnesium acetate, 2 mM dithiothreitol [DTT]; 1.5 ml per 108 cells). Cells were lysed with 25 strokes of a type B Dounce homogenizer, and nuclei were removed by centrifugation for 10 min at 2,000 × g. The supernatant was layered over buffer A containing 30% (wt/vol) sucrose and centrifuged at 130,000 × g for 2 h. The supernatant was removed without disturbing the S130/sucrose interface, supplemented with glycerol to a final concentration of 5% (vol/vol), and frozen in aliquots at −70°C. The αCP proteins were purified from K562 cell S100 extract by SP-Sepharose, DEAE-Sephacel, and single-stranded DNA-cellulose chromatography as described by Kiledjian et al. (29, 30).
Poly(C)-depleted extracts were generated using poly(C)-agarose beads (Sigma). Three milligrams of K562 S130 extract was bound to 0.2 mg of poly(C) coupled to agarose beads for 20 min at 4°C in buffer A containing 300 mM KCl. Following a brief spin to pellet the beads, the supernatant containing unbound protein was incubated with an additional 0.2 mg of poly(C)-agarose beads. This was repeated once more, and the final supernatant was diluted with buffer A and concentrated with a Centricon 10 spin column. Poly(A) depletion was similarly carried out except that poly(A)-agarose beads were used in a buffer consisting of 10 mM Tris-HCl (pH 7.5), 8 mM EDTA, 500 mM KCl, 250 mM NaCl, and 5% glycerol.
Glutathione S-transferase (GST) fusion proteins were produced in Escherichia coli BL21 and purified on glutathione-Sepharose beads as instructed by the manufacturer (Pharmacia). Recombinant His-tagged bPAP was isolated from E. coli BL21(DE3) cells freshly transformed with bPAP expressing plasmid bPAP-423 grown to an optical density at 600 nm (OD600) of 0.5 and induced overnight with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at room temperature. Recombinant bPAP was purified on a nickel column as recommended by the manufacturer (Novagene). Recombinant His-tagged human PABP was expressed and purified similarly except bacterial growth was carried out at 37°C and induced with 0.2 mM IPTG for 12 h.
Western analysis.
Western blots were carried out with a total of 50 μg of S130 extract or depleted S130 extract. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5% polyacrylamide gel) and transferred to nitrocellulose as previously described (27). The αCP proteins were detected by a chicken anti-αCP which was generated against a GST-αCP1 fusion protein (24) and subsequently affinity purified with the HiTrap (Pharmacia) column conjugated to His-tagged αCP1 as recommended by the manufacturer. A 1:100 dilution of the primary antibody was used and visualized by enhanced chemiluminescence using a horseradish peroxidase-conjugated goat anti-chicken secondary antibody (1:7,000 dilution; Accurate Chemicals, Westbury, N.Y.). PABP was detected by antibody 10E10 (19) at a 1:1,000 dilution and visualized by enhanced chemiluminescence using horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:5,000 dilution; Cappel, West Chester, Pa.).
RNA production and polyadenylation.
The α-globin 3′UTR was PCR amplified with primers that introduce a T7 bacteriophage promoter at the 5′ end. The PCR product was phenol-chloroform extracted once, chloroform extracted twice, ethanol precipitated, and washed with 70% ethanol. RNA transcripts were generated with T7 polymerase (Promega) according to the manufacturer’s protocol, using 200 ng of template. Uniformly labeled riboprobes were generated similarly except that the reaction was carried out in the presence of [α-32P]UTP and m7G(5′)ppp(5′)G cap analog. Unincorporated nucleotides were remove with a Sephadex G-50 spin column (Pharmacia). 5′-end-labeled RNA was generated with vaccinia virus capping enzyme (54). Twenty picomoles of uncapped RNA was incubated with 20 U of capping enzyme for 1 h at 37°C in a 15-μl reaction mixture containing 50 mM Tris-HCl (pH 7.9), 1.25 mM MgCl2, 6 mM KCl, 2.5 mM DTT, 1 mM S-adenosylmethionine, 100 μg of bovine serum albumin per ml, and 90 μCi of [α-32P]GTP. The reaction was terminated with ULB (7 M urea, 2% SDS, 0.35 M NaCl, 10 mM EDTA, 10 mM Tris [pH 7.5]), ethanol precipitated with 20 μg of glycogen carrier, and resolved on an 8% denaturing polyacrylamide gel. The RNA was visualized and excised from the wet gel with autoradiographic guidance, and the RNA was eluted with a 45-min incubation at 65°C in elution buffer (20 mM Tris [pH 7.5], 0.5 M sodium acetate, 10 mM EDTA, 1% SDS). Residual acrylamide was removed by passage through a glass wool spin column, and the RNA was ethanol precipitated with 20 μg of glycogen carrier. A second ethanol precipitation was followed by a 70% ethanol wash to ensure that all of the SDS was removed.
Polyadenylation reactions were carried out as described by Gunderson et al. (23), with slight modifications. Approximately 20 pmol of α-globin 3′UTR riboprobe was adenylated under nonspecific polyadenylation conditions with purified His-tagged bPAP in a buffer consisting of 10 mM Tris (pH 7.5), 65 mM KCl, 0.75 mM MnCl2, 5 mM DTT, 0.14 mM EDTA, 11% glycerol, 0.12 mg of bovine serum albumin per ml, 0.05 mg of tRNA per ml, and 0.75 mM ATP. The reactions were carried out at 37°C. The amount of bPAP and the time of incubation were determined experimentally for each enzyme preparation and RNA sample. Usually, 1 to 3 μl of bPAP was required for a 15-min reaction to yield ∼90 adenylate residues. The reaction was terminated with ULB, and the polyadenylated RNA was gel purified as described above.
In vitro mRNA decay assays.
In vitro mRNA decay reactions were carried out with 0.1 pmol (103 cpm) of 5′ capped and polyadenylated α-globin 3′UTR with 60 μg of protein from K562 S130 extract in IVDA buffer (10 mM Tris [pH 7.5]), 100 mM potassium acetate, 2 mM magnesium acetate, 2 mM DTT, 10 mM creatine phosphate, 1 mM ATP, 0.4 mM GTP, 0.1 mM spermine) for the indicated times at 37°C. Reactions were stopped with the addition of 150 μl of ULB spiked with a 32P-labeled oligonucleotide which was used as an internal control for RNA extractions and precipitation. Following a phenol-chloroform extraction, the RNA was ethanol precipitated with 20 μg of glycogen, resuspended in 80% formamide dye, and resolved on an 8% polyacrylamide–7 M urea gel. The dried gel was exposed to Kodak BioMax film. Quantitations were carried out on a Molecular Dynamics PhosphorImager using the ImageQuant software.
Yeast two-hybrid interaction assays.
Saccharomyces cerevisiae Hf7c cells transformed with a plasmid expressing a Gal4 DNA-binding domain fusion protein and a plasmid expressing a Gal4 transcription activation domain fusion protein were grown overnight at 30°C in synthetic medium lacking tryptophan and leucine. Tenfold dilutions corresponding to 10 μl of a 0.25-OD600 culture or 10 μl of a 1:10 or 1:100 dilution of these cells were spotted onto Trp− Leu− His− synthetic medium plates to determine the presence of protein-protein interactions. An interaction between the DNA-binding domain and the transcriptional activation domain of the fusion proteins results in the production of the HIS3 gene product and enables the cells to grow on minimal medium lacking histidine. Similarly, cells were spotted on Trp− Leu− synthetic medium plates to determine the density of cells grown without selective pressure for protein-protein interactions. β-Galactosidase assays were carried out with yeast extract as described elsewhere (13). The human HeLa cDNA library, plasmids pGBT9 and pGAD424, and S. cerevisiae Hf7c were obtained from Clontech Inc. The library screening was carried out as suggested by the manufacturer with approximately 6 × 106 total transformants seeded. Of the original 62 positive growth colonies analyzed, 5 were determined to be authentic interactions; one of these was PABP.
In vitro translation and protein-protein interactions.
In vitro translation and protein-protein interaction assays were carried out as described by Kiledjian et al. (28, 29). Bacterial extract containing 3 μg of full-length GST-αCP1, GST-αCP2, or GST domain alone was bound to 25 μl of glutathione-Sepharose beads in PBS containing 0.5% Triton X-100, 3 μg of leupeptin per ml, and 0.5% aprotinin for 15 min at 4°C, washed four times in the same buffer, and then washed in RNA-binding buffer (10 mM Tris [pH 7.0], 150 mM KCl, 0.5 mM DTT). GST fusion proteins coupled to glutathione-Sepharose beads were incubated with an equivalent of 1.5 × 105 cpm of trichloroacetic acid-precipitable counts of [35S]methionine-labeled in vitro-translated PABP or U1A protein to detect protein-protein interactions. Incubations were carried out for 1 h at 4°C on a nutator in RNA-binding buffer with 3 μg of leupeptin per ml and 0.5% aprotinin. The beads were subsequently washed five times in the PBS containing 1% Triton X-100 and once with PBS. The dried beads were resuspended in SDS-PAGE sample buffer, boiled for 3 min to elute proteins, and resolved on a SDS-PAGE (12.5% gel) followed by autoradiography. Where indicated, 20 pmol of oligo(dC) was incubated with the GST fusion protein bound to the GST-Sepharose beads. Binding of oligo(dC) to the fusion protein was carried out on a nutator for 5 min at room temperature prior to addition of the in vitro-translated PABP or U1A protein. When RNase was included in the interaction reactions, either 40 ng of RNase A and 4 U of RNase T1 or 1 μg RNase A and 150 U of RNase T1 were used as indicated.
RESULTS
Establishment of an in vitro mRNA decay assay.
To begin addressing the mechanism by which the α-complex influences mRNA turnover, we established an in vitro mRNA decay assay. We used the α-globin 3′UTR RNA and cytosolic S130 extract as the protein source. The choice of these reagents was based on the observation that the stability of α-globin is conferred by the 3′UTR and the α-complex proteins are present in the cytoplasmic fraction. To establish conditions which recapitulate the in vivo α-complex-dependent RNA stability in vitro, we compared the stability of the wild type α-globin 3′UTR to that of a mutant 3′UTR. The mutant 3′UTR was generated by a deletion of the region implicated in vivo to be essential for α-globin mRNA stability (62). The deletion removes 39 nucleotides of the 3′UTR sequence and is unable to bind the α-complex (data not shown). This same region was also demonstrated to be the binding site for the α-complex in vitro (24).
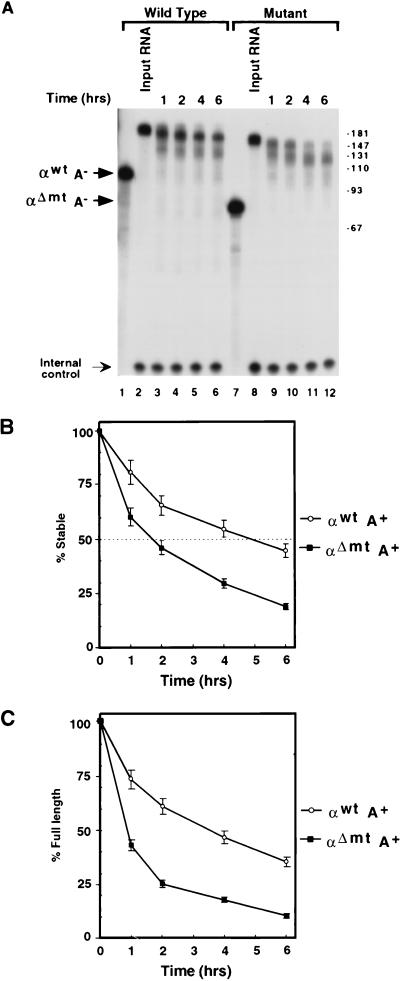
In an effort to closely mimic a natural mRNA, the in vitro-transcribed 3′UTR was capped at the 5′ end and polyadenylated at the endogenous poly(A) addition site at the 3′ end (see Materials and Methods). The capping reaction was carried out with [α-32P]GTP to label the 5′ end of the RNA. Polyadenylated 3′UTR containing approximately 80 to 120 adenylate residues was gel purified and incubated with K562 cytosolic S130 extract (Fig. 1A). Incubation of the polyadenylated α-globin wild-type 3′UTR (αwtA+) or the polyadenylated deletion mutant 3′UTR (αΔmtA+) with S130 extract for up to 6 h reveals three interesting observations. First, αwtA+ was considerably more stable than αΔmtA+ (Fig. 1A; compare lanes 2 to 6 with lanes 8 to 12). With these assay conditions, the half-lives of αwtA+ and αΔmtA+ are approximately 5 and 1.5 h, respectively (Fig. 1B). Similar results were obtained with uniformly labeled RNAs (data not shown). The differences in stability are consistent with the in vivo α-complex-dependent stability reported by Weiss and Liebhaber (62) for RNAs that can and cannot form the α-complex (60). Second, decay of the RNA did not yield a smear; rather, it resulted in discrete bands. Since the RNA used in this experiment is labeled at the 5′ end, the resulting intermediates are a result of 3′-to-5′ decay products. The observed bands are predominantly longer than the 3′UTR, indicating that they are within the poly(A) tail. These bands were uniformly spaced at approximately 30 nucleotides (also shown in Fig. 2 and 4). The size is consistent with the length of a single PABP-binding site (19, 52) and most likely corresponds to monomers of PABP being removed progressively from the 3′ end of the poly(A) tail (see below). Differential stability and phased deadenylation were also observed when mouse erythroleukemia cell extract was used (data not shown). These data are consistent with those obtained for reticulocytes expressing endogenous mouse globin (20, 21) and transgenic mice expressing human α-globin (39). In both cases, phased poly(A) tail lengths were observed. Third, the fully adenylated form of the αwtA+ (Fig. 1A, top band, lanes 3 to 6) persisted longer than did the fully adenylated form of αΔmtA+ (Fig. 1A, top band, lanes 9 to 12). The persistence of the fully adenylated form of αwtA+ compared to αΔmtA+ is plotted in Fig. 1C. This result demonstrates that the two RNAs deadenylate at different rates and the initial deadenylation rate is higher for the mutant RNA. These data are consistent with observations by Morales et al. (39). Therefore, the overall differential stability observed between the wild-type and mutant α-globin mRNAs which are unable to form the α-complex is conserved in this in vitro assay system which uses the 3′UTR only. The data further suggest that the α-complex is involved in mRNA stability in vitro.
FIG. 1.
Differential stability of α-globin mRNA in an in vitro mRNA decay assay. In vitro mRNA decay reactions were carried out with 5′-end-labeled αwtA+ or αΔmtA+. (A) Decay reactions were carried out in the presence of K562 S130 extract at 37°C. Positions of migration of the unadenylated 3′UTRs are labeled “αwtA−” and “αΔmtA−” in lanes 1 and 7, respectively, and the input adenylated 3′UTRs are shown in lanes 2 and 8. Incubation times ranged from 1 h to 6 h as indicated. Reactions were stopped with ULB; RNA was isolated and resolved on an 8% polyacrylamide–7 M urea gel and visualized by autoradiography (see Materials and Methods). The bands designated “Internal control” are derived from a labeled oligonucleotide which was included in ULB to normalize for RNA extractions and gel loading. Positions of DNA size markers in nucleotides are indicated on the right. (B) Quantitation of the decay of αwtA+ and αΔmtA+ RNAs. The half-life is indicated with a dotted line at 50% RNA remaining. The half-life of the wild-type αwtA+ is approximately 5 h; that of the mutant αΔmtA+ is approximately 1.5 h. The values were derived from an average of three independent experiments; standard deviations are shown for each time point. (C) Quantitation demonstrating the amount of full-length adenylated αwtA+ and αΔmtA+ RNAs remaining. These data provide a comparison for the initial deadenylation rate of the fully adenylated RNAs. The values were derived from an average of three independent experiments; standard deviations are shown.
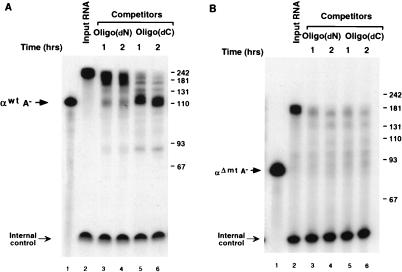
FIG. 2.
Deadenylation of wild-type α-globin 3′UTR is sensitive to oligo(dC) competition. (A) An in vitro mRNA decay reaction was carried out with uniformly labeled αwtA+ in the presence of 28 pmol of oligo(dN) or oligo(dC) at 37°C for 1 h (lanes 3 and 5) or 2 h (lanes 4 and 6). A trace amount of poly(A) competitor (0.1 pmol) was also included to partially sequester soluble poly(A)-binding activity in the extract (5, 16). Migration of the unadenylated RNA (αwtA−) is shown. Termination of the reactions, RNA analysis, gel conditions, internal control, and markers are as described in the legend to Fig. 1. (B) The uniformly labeled and polyadenylated deletion mutant αΔmtA+ was used in an in vitro decay reaction in the presence of oligo(dN) or oligo(dC) competitor as describe above except that poly(A) was omitted. Migration of the unadenylated αΔmtA− is indicated.
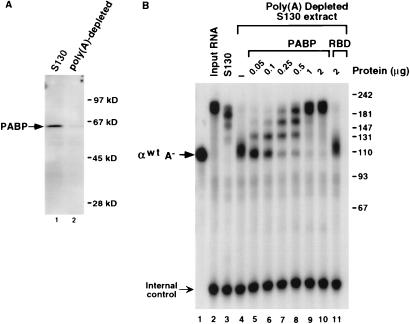
FIG. 4.
PABP contributes to the stabilization of polyadenylated mRNA. (A) Western analysis detecting PABP in K562 S130 extract (lane 1) and poly(A)-depleted S130 extract (lane 2), using the PABP-specific monoclonal antibody 10E10. Positions of migration of PABP and molecular weight markers are shown. (B) A 2-h in vitro mRNA decay reaction using 5′-end-labeled αwtA+ was carried out with poly(A)-depleted K562 S130 extract. Complete S130 extract was used in lane 3; poly(A)-depleted extract was used in lanes 4 through 11. The amount of recombinant PABP or RBD added is indicated. “αwtA−” denotes migration of the unadenylated αwt RNA. Resolution of the RNA and molecular size markers (indicated in nucleotides) are as described in the legend to Fig. 1.
To determine whether the α-complex contributes to the stability of the α-globin 3′UTR, we used competitor oligonucleotides that are capable of disrupting formation of the α-complex by sequestering the poly(C)-binding proteins αCP1 and αCP2. Since these proteins are necessary to assemble the α-complex, addition of poly(C) (30, 60), poly(dC) (29), or a thioated oligonucleotide containing 16 cytidylate bases [oligo(dC)] (data not shown) disrupts formation of the α-complex on the α-globin 3′UTR. To circumvent concerns pertaining to the potential degradation of competitor RNA oligonucleotides, thioated deoxyoligonucleotides were used to disrupt the α-complex. Uniformly labeled αwtA+ was gel purified and incubated with K562 cytosolic S130 extract in an in vitro mRNA decay assay for up to 2 h. Incubation of αwtA+ with S130 extract was carried out in the presence of either nonspecific competitor consisting of a thioated deoxyoligonucleotide with random sequence (Fig. 2, lanes 3 and 4) or oligo(dC) to compete the α-complex (lanes 5 and 6). During the 2-h incubation in the presence of the nonspecific competitor, 60% of the mRNA remained in the intact polyadenylated form (lanes 3 and 4). In contrast, addition of the oligo(dC) competitor, which disrupts the α-complex, resulted in a greater decay of the RNA, with only 30% of the polyadenylated RNA retained and a more pronounced deadenylation (lanes 5 and 6). Incubation of the oligonucleotides alone with the RNA had no effect (data not shown). As expected, oligo(dC) did not have an effect on the decay or deadenylation of the deletion mutant, αΔmtA+, which does not form the α-complex (Fig. 2B). These data suggest that the α-complex is necessary for the overall stability of the α-globin mRNA and that it functions at least partially by hindering deadenylation.
The α-complex impedes deadenylation.
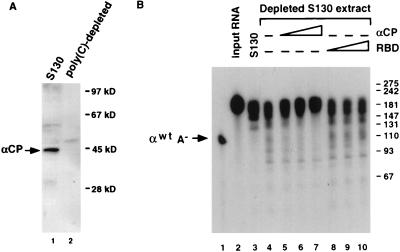
To more precisely ascertain whether the effect on deadenylation is a consequence of the α-complex, and in particular αCP1 and αCP2, rather than a result of using deletion mutations or oligonucleotide competitors, the mRNA decay reactions were repeated with poly(C)-depleted extract. Poly(C)-binding activity (which includes αCP1 and αCP2) was removed from the S130 extract by repeated incubations with poly(C)-agarose beads (see Materials and Methods). The extent of αCP depletion is demonstrated in Fig. 3A by Western analysis with an antibody specific to αCP. The resulting extract, which lacks the ability to form the α-complex (30), was used in the in vitro mRNA decay reactions. On average, approximately twofold less fully adenylated input αwtA+ was observed with poly(C)-depleted extract than with complete S130 extract (Fig. 3B, lanes 3 and 4). This result demonstrates that the deadenylation and decay observed with the addition of the oligo(dC) competitor in Fig. 2 was not due to a nonspecific effect of oligonucleotide addition to the reaction but due to the removal of the αCPs. Furthermore, the involvement of the αCPs in the stability was demonstrated by a brief preincubation of the depleted extract with an increasing amount of a purified fraction containing both αCP1 and αCP2 prior to addition of the RNA. A stabilization of approximately 85% of the input adenylated RNA was observed upon addition of the αCPs (Fig. 3B, lanes 5 to 7). No significant effect was observed upon addition of a nonspecific RNA-binding protein (the hnRNP U carboxyl-terminal RBD [27]) (lanes 8 to 10). These data demonstrate that the α-complex is involved in mRNA stability and the αCPs are integral to the α-complex-mediated stabilization of adenylated α-globin mRNA in vitro.
FIG. 3.
αCP1 and αCP2 can promote stabilization of mRNA. (A) Western analysis of K562 S130 extract (lane 1) or poly(C)-depleted S130 extract (lane 2), using αCP-specific antibodies. Positions of the αCP band and molecular size markers are indicated on the left and right, respectively. (B) An in vitro mRNA decay reaction was carried out for 30 min in the presence of K562 S130 extract depleted of poly(C)-binding activity. Complete S130 extract was used in lane 3; poly(C)-depleted extract was used in lanes 4 to 10. RNA decay reactions were carried out in the presence of purified αCP1 and αCP2 from K562 cells (lanes 5 to 7) or the hnRNP U protein RBD (lanes 8 to 10). The amount of protein added ranged from 25 ng (lanes 5 and 8) to 100 ng (lanes 7 and 10). The RNA was isolated following a 30-min incubation, resolved on an 8% polyacrylamide–7 M urea gel, and detected by autoradiography. The position of the unadenylated probe (αwtA−) is indicated; numbers on the right represent nucleotide size markers.
PABP is important in protecting the α-globin poly(A) tail.
The binding of PABP to the poly(A) tail appears essential for stabilizing the 3′ end of higher eukaryotic mRNAs (1, 17). The periodicity of the deadenylation products observed in Fig. 1 to 3 suggests a pausing of nuclease activity at PABP monomers and supports the role of PABP in this assay system. To directly determine if PABP contributes to the stability of the α-globin 3′UTR in this system, poly(A)-depleted extract was used with 5′-end-labeled αwtA+ in an in vitro mRNA decay reaction. Poly(A)-binding activity was removed with repeated incubation of the S130 extract with poly(A)-agarose beads, and the extent of depletion is shown by the Western blot in Fig. 4A with an antibody specific to PABP. Incubation of 5′-end-labeled αwtA+ with complete S130 extract for 2 h produced the characteristic deadenylated intermediates which retained approximately 75% of the input RNA as expected (Fig. 4B, lane 3). However, use of the poly(A)-depleted extract resulted in rapid deadenylation, with subsequent accumulation of the deadenylated RNA (lane 4). The rapid deadenylation was slowed, and a progressive stabilization of αwtA+ was observed upon addition of human recombinant His-tagged PABP to the depleted extract (lanes 5 to 10). There was a direct correlation between the increasing amount of PABP added to the depleted extract and the length of the stable poly(A) remaining. At the highest concentrations of PABP added, approximately 95% of the input RNA was retained after 2 h. No effect was detected with the addition of the hnRNP U RBD at the highest concentration (lane 11). Interestingly, PABP does not appear to be in excess since addition of PABP further stabilized the RNA compared to complete extract (compare lane 10 to lane 3). Furthermore, the length increase was consistent with the intermediates observed in Fig. 1 through 3, demonstrating that these intermediates are the result of PABP monomers bound to the poly(A) tail. Collectively, the above results demonstrate that both the α-complex and PABP contribute to protecting the 3′ end of the mRNA in this in vitro system.
αCP1 and αCP2 can interact with PABP.
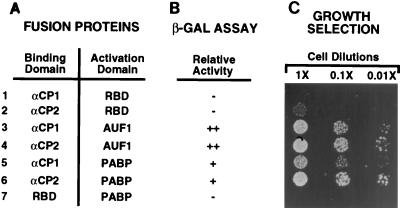
In a parallel effort to identify proteins which interact with the αCPs, we used the yeast two-hybrid strategy (15). The human αCP2 coding region fused to the Gal4 DNA-binding domain was used to screen a human cDNA library. Interestingly, one of the positive clones identified encoded PABP. The isolated clone contained a truncation of the first 198 amino acids. This truncation deletes the first two RNP motif RBDs and the amino-terminal 19 amino acids of the third RBD. The resulting protein therefore contains one intact RBD (the fourth RNP motif) and the entire carboxyl-terminal auxiliary domain which has been proposed to mediate protein-protein interactions (33). A representative example of the protein-protein interactions between the isolated N-terminal truncated PABP and the proteins αCP1 and αCP2 determined by both a β-galactosidase assay and growth selection, are shown in Fig. 5. Figure 5A lists the combination of proteins used, and the corresponding results are shown in Fig. 5B and C. Figure 5C shows a growth assay with a serial 10-fold dilution of cells. Cells containing fusion proteins that are able to interact are competent to grow under selective conditions (see Materials and Methods). Transformed cells harboring αCP1 fused to the DNA-binding domain were unable to interact with the nonspecific hnRNP U RBD (row 1). αCP2 contained a low level of background interaction, as detected by growth at the lower dilution (row 2). Both αCP1 and αCP2 proteins are able to interact with the AUF1 protein (also known as hnRNP D), as previously demonstrated (28). Both proteins are also able to efficiently interact with PABP, as demonstrated by selective growth at the higher dilutions (compare rows 1 and 5 and rows 2 and 6). The interaction with PABP appears specific since PABP was unable to interact with the hnRNP U RBD (row 7). Similar results were obtained in a β-galactosidase assay to detect protein-protein interactions (Fig. 5B). Use of the hnRNP U RBD control further suggests that the interaction between the αCPs and PABP does not occur through RNA tethering since the control is also an RNA-binding protein. Surprisingly, the interaction of αCP1 and αCP2 with full-length PABP was less efficient than with the isolated N-terminal truncated PABP in the yeast two-hybrid assay (data not shown). It is possible that inclusion of the N-terminal portion within the contents of a Gal4-PABP fusion protein interferes with the interaction in this system. Nevertheless, PABP does have the potential to specifically interact with both αCP1 and αCP2 in cells.
FIG. 5.
αCP1 and αCP2 can interact with PABP. (A) Combinations of test proteins (fusion protein containing the Gal4 DNA-binding domain [left column] and fusion protein containing the Gal4 transcription activation domain [right column]) used in the yeast two-hybrid analysis. αCP1, αCP2, AUF1, and PABP, test proteins fused to the indicated Gal4 domain; RBD, the hnRNP U protein RBD fused to the indicated Gal4 domain. (B) Relative extent of protein-protein interactions as determined by β-galactosidase (β-GAL) assays, ranging from (−) no interaction to (++) very strong interaction. (C) Tenfold serial dilutions of cells, harboring plasmids expressing the indicated fusion test proteins. Growth of the cells spotted results from protein-protein interactions occurring between the test proteins.
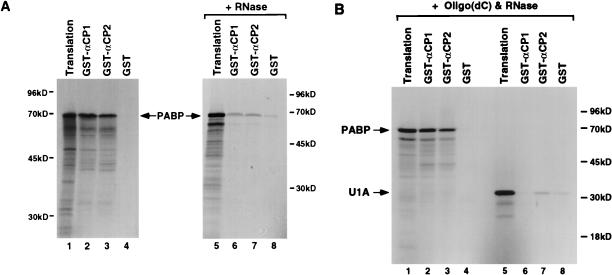
The interaction between αCP1 and αCP2 with full-length PABP can be detected in vitro in GST pull-down assays (Fig. 6A). PABP that was transcribed and translated in vitro in the presence of [35S]methionine can copurify with bacterially expressed GST-αCP1 and GST-αCP2 fusion proteins (lanes 2 and 3). The interaction of PABP with αCP1 and αCP2 is specific since an interaction was not detected with the GST domain alone (lane 4). However, this interaction was dependent on RNA since mild RNase A and T1 treatment significantly reduced the extent of the interaction between the αCPs and PABP (lanes 6 and 7). To rule out the possibility that the interaction was mediated through RNA tethering, the 16-base oligonucleotide oligo(dC) was used as a binding substrate for αCP1 or αCP2. An oligonucleotide of this length is sufficient to bind and sequester the αCPs (29) (Fig. 2). The fact that PABP does not bind poly(C) (7, 33, 43) and that the oligonucleotide is of a minimal size makes it unlikely that both an αCP and a PABP molecule are tethered simultaneously on the same oligonucleotide. Figure 6B demonstrates that the interaction of αCP1 or αCP2 with PABP was resistant to excessive RNase treatment when the αCP proteins were bound to oligo(dC) (lane 2 and 3). However, addition of oligo(dC) had no effect on the interaction of the αCPs with another RNA-binding protein, the U1 snRNP-specific U1A protein, where efficient interactions were not detected under similar conditions (lanes 5 to 8). These data support the premise that the interaction is nucleic acid dependent but not caused by the tethering of the proteins on the same RNA molecule. Furthermore, these data indicate that the α-complex could influence deadenylation through an interaction of either αCP1 or αCP2 with PABP.
FIG. 6.
The interaction of αCP1 and αCP2 with PABP is RNA dependent. (A) Bacterially expressed GST-αCP1, GST-αCP2, and GST alone bound to glutathione-Sepharose beads were incubated with [35S]methionine-labeled PABP without (lanes 1 to 4) or with RNases (40 ng of RNase A and 4 U of RNase T1; lanes 5 to 8). Copurified proteins were isolated following extensive washes and resolved by SDS-PAGE (12.5% gel) followed by autoradiography. An aliquot of the total translation product is shown in lanes 1 and 5, and the interacting protein is shown in the remaining lanes. (B) Interactions with PABP (lanes 2 to 4) or U1A (lanes 6 to 8) were carried out in the presence of 20 pmol of oligo(dC) with 1 μg of RNase A and 150 U RNase T1. On average, approximately 40% of the total input [35S]methionine-labeled PABP efficiently interacted with GST-αCP1 or GST-αCP2. Positions of migration of full-length PABP and U1A (arrows) and the molecular size markers are indicated.
DISCUSSION
Using an in vitro mRNA decay assay, we have demonstrated that the α-complex is necessary for the stability of the α-globin 3′UTR in vitro. These observations are consistent with the turnover of α-globin mRNA in cells. Wild-type α-globin mRNA is approximately threefold more stable than a mutant mRNA unable to form the α-complex (62). We can detect similar ratios of differential stability in vitro for wild-type and mutant α-globin 3′UTRs (Fig. 1). The influence of the α-complex on deadenylation has been suggested by Morales et al. (39) for transgenic mice expressing human α-globin genes. Poly(A) tail lengths for the expressed wild-type transgene were longer than those of a mutant transgene unable to form the α-complex, suggesting that the rate of deadenylation was faster for the mutant. Consistent with these results, the initial rate of deadenylation observed for the αwtA+ RNA was greater than the rate of deadenylation for the αwtA+ RNA in vitro (Fig. 1A and C). In addition, distinct size classes of poly(A) tail lengths have been observed for globin mRNAs in cells with shorter poly(A) tails accumulating over time (20, 21). These data suggest that deadenylation is an initial step in the turnover of globin mRNA. In fact, deadenylation has been demonstrated as the initial step in the turnover of human γ-globin mRNA (37). Our data indicate that α-globin turnover also precedes through a deadenylation pathway in vitro and that this pathway is influenced by the α-complex. Removal of the α-complex from the α-globin mRNA, by either deleting its binding site or sequestering the αCPs, resulted in accentuated deadenylation and mRNA turnover. The interaction detected between the two proteins αCP1 and αCP2 and PABP indicates that the influence on deadenylation is mediated through PABP possibly by influencing the activity or binding affinity of this protein. Such a mechanism could also account for the stability of α-globin mRNA in vivo.
The phased distribution of the globin poly(A) tail length (20, 21, 39) suggests that deadenylation of globin mRNA occurs in a nonprocessive fashion involving sequential removal of PABP molecules. Removal of each PABP molecule exposes a segment of the poly(A) tail to a deadenylase which appears to pause upon contact with the next PABP. We have been able to recapitulate this activity in vitro with a polyadenylated α-globin 3′UTR. Consistent with previous reports (1, 10, 17, 31, 32), the presence of PABP inhibits deadenylation. Removal of PABP with poly(A) depletion results in rapid mRNA deadenylation which can be reversed upon addition of PABP (Fig. 5). Although the deadenylase has not been identified, it appears to be poly(A) specific since full-length unadenylated 3′UTR can be detected, suggesting that upon removal of the poly(A) tail, the deadenylase does not (at least efficiently) degrade the body of the mRNA. The deadenylation activity also requires divalent cations due to its sensitivity to EDTA and is ATP independent (data not shown). The activity might be similar to the deadenylating nuclease described by Körner et al. which is poly(A) specific and cation dependent (31, 32).
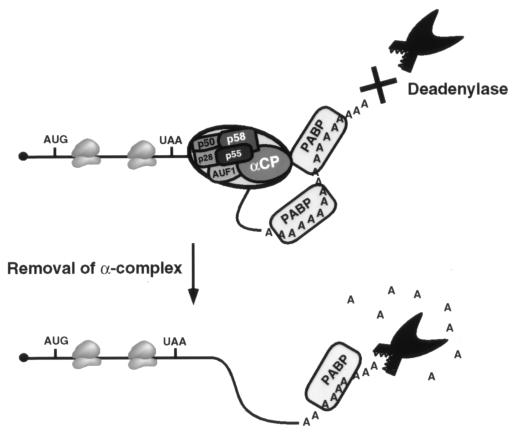
PABP appears to be the major contributor to α-globin mRNA stability with this in vitro system. Sequestration of PABP has a more dramatic effect on deadenylation (Fig. 4) than sequestration of the αCPs (Fig. 3). PABP most likely provides a default stability to the α-globin mRNA, and the α-complex seems to further augment the stabilizing ability of PABP. A model for the mechanism by which the α-complex influences deadenylation is presented in Fig. 7. The α-complex which binds to the α-globin 3′UTR can exert an influence on PABP bound to the poly(A) tail and slow deadenylation. This influence is most likely mediated through a direct interaction of αCP1 and αCP2 with PABP. It is unclear whether this interaction is with one or multiple PABPs. Upon removal of the α-complex, a default poly(A) tail-PABP complex that is less efficient at preventing the activity of a deadenylase remains. Like the interaction of yeast eIF4G with PABP, which requires RNA (58), interaction of the αCP proteins with PABP is RNA dependent. It appears that RNA binding of the αCPs enables a more efficient protein-protein interaction, perhaps by exposing an interaction domain. Such an interaction could serve to stabilize the binding of PABP to the poly(A) tail and inhibit deadenylation.
FIG. 7.
Model of the α-complex function during α-globin mRNA deadenylation. The α-globin mRNA is denoted by a line, the filled circle represents the 5′ cap, and the translation start (AUG) and stop (UAA) sites with the traversing ribosomes shown within the coding region. The α-complex indicated by the large oval on the 3′UTR includes the four unknown proteins p58, p55, p50, and p28 along with AUF1 and an αCP (28). The poly(A) tail is shown on the 3′ end bound by two PABPs. A potential interaction between the αCP in the α-complex and PABP on the poly(A) tail is shown. A putative deadenylase is indicated. The interaction between the α-complex and PABP is postulated to stabilize the poly(A) tail by rendering it resistant to deadenylation.
Although the overall conclusion from Fig. 1 through 3 is that the α-complex affects stability and deadenylation, slightly different intermediates and/or abundance of intermediates appear to accumulate with the different approaches used to sequester the α-complex. In particular, use of poly(C)-depleted extract resulted in decay intermediates within the 3′UTR (Fig. 3). The presence of these bands may reflect the removal of additional proteins during the poly(C) depletion which are involved in the turnover of α-globin mRNA. Alternatively, the α-complex may contribute to mRNA stability by a mechanism(s) in addition to deadenylation since stable unadenylated αwtA+ RNA is detected in Fig. 5 upon removal of PABP. The α-complex might affect the activity of an exoribonuclease or the activity of an endoribonuclease within the 3′UTR. Furthermore, our data do not exclude the possibility that factors in addition to the α-complex contribute to the stability of α-globin mRNA. Further studies are required to determine the potential contribution of the α-complex on RNases and the potential role of non-α-complex proteins in α-globin mRNA stability.
The PABP clone isolated in the two-hybrid screen as interacting with αCP2 contains an amino-terminal truncation of 198 amino acids. This clone contains the carboxyl two-thirds of the protein which includes part of the third and all of the fourth RNP motif and the entire C-terminal domain. Therefore, the interaction domain must be contained within this carboxyl two-thirds of the protein. PABP contains four RBDs which have all been demonstrated to bind RNA, yet the prominent poly(A)-binding activity of Xenopus and yeast PABP is dependent on the first two RNP motifs (7, 43). The fact that the first two RNP motifs are not necessary for the interaction of PABP with the α-complex indicates that the predominant poly(A)-binding activity and the α-complex interaction domains are contained in distinct regions of the protein. This is consistent with our model where PABP can interact with the α-complex while bound to the poly(A) tail (Fig. 7). Further mapping of the interaction domain will reveal the precise boundaries of these domains. An additional example of a KH domain protein interacting with PABP was recently reported. The yeast PBP2 protein, which shares approximately 25% identity with the αCPs, was isolated by its ability to interact with the yeast PABP in a two-hybrid screen (38). The significance of the PABP-PBP2 interaction and any potential role in mRNA turnover are not known.
The in vitro mRNA decay system described here could potentially be utilized with any mRNA whose turnover is regulated by soluble factors. It is a rapid and convenient assay system with ample versatility as a general functional assay for mRNA deadenylation and turnover. We are currently determining whether the α-complex can facilitate mRNA stability by means other than removal of the poly(A) tail in globin and other mRNAs to which it can bind. The use of this assay system should considerably expedite our ability to delineate the mechanism of regulated eukaryotic mRNA turnover.
ACKNOWLEDGMENTS
We thank K. Novick for excellent technical assistance, G. Dreyfuss and M. Siomi for providing the PABP clone and antibody, S. Gunderson for providing the bPAP expression plasmid bPAP-423 and the U1A expression plasmid pET-U1A, S. Liebhaber for plasmids pSV2A-α2H9 and pSV2A-α2H21, and S. Peltz for the vaccinia virus capping enzyme. We also thank S. Gunderson, J. Huibregtse, and S. Peltz for critical reading of the manuscript.
This work was supported by funds from Rutgers University and National Institutes of Health grant DK51611 to M.K. N.D. was supported by an American Heart Association predoctoral fellowship.
REFERENCES
- 1.Bernstein P, Peltz S W, Ross J. The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol Cell Biol. 1989;9:659–670. doi: 10.1128/mcb.9.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein P L, Herrick D J, Prokipcak R D, Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 1992;6:642–654. doi: 10.1101/gad.6.4.642. [DOI] [PubMed] [Google Scholar]
- 3.Blyn L B, Towner J S, Semler B L, Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991;11:2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer G. Characterization of c-myc 3′ to 5′ mRNA decay activities in an in vitro system. J Biol Chem. 1998;273:34770–34774. doi: 10.1074/jbc.273.52.34770. [DOI] [PubMed] [Google Scholar]
- 6.Brewer G, Ross J. Messenger RNA turnover in cell-free extracts. Methods Enzymol. 1990;181:202–209. doi: 10.1016/0076-6879(90)81122-b. [DOI] [PubMed] [Google Scholar]
- 7.Burd C G, Matunis E L, Dreyfuss G. The multiple RNA-binding domains of the mRNA poly(A)-binding protein have different RNA-binding activities. Mol Cell Biol. 1991;7:3419–3424. doi: 10.1128/mcb.11.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caponigro G, Parker R. Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol Rev. 1996;60:233–249. doi: 10.1128/mr.60.1.233-249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C-Y A, Shyu A-B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 10.Coller J M, Gray N K, Wickens M P. mRNA stabilization by poly(A) binding protein is independent of poly(A) and requires translation. Genes Dev. 1998;12:3226–3235. doi: 10.1101/gad.12.20.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couttet P, Fromont-Racine M, Steel D, Pictet R, Grange T. Messenger RNA deadenylation precedes decapping in mammalian cells. Proc Natl Acad Sci USA. 1997;94:5628–5633. doi: 10.1073/pnas.94.11.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decker C J, Parker R. Diversity of cytoplasmic functions for the 3′ untranslated region of eukaryotic transcripts. Curr Opin Cell Biol. 1995;7:386–392. doi: 10.1016/0955-0674(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 13.Dinman J D, Wickner R B. Translational maintenance of frame: mutants of Saccharomyces cerevisiae with altered −1 ribosomal frameshifting efficiencies. Genetics. 1994;136:75–86. doi: 10.1093/genetics/136.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan X H C, Steitz J A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–247. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 16.Ford L P, Watson J, Keene J D, Wilusz J. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes Dev. 1999;13:188–201. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford L P, Bagga P S, Wilusz J. The poly(A) tail inhibits the assembly of a 3′-to-5′ exonuclease in an in vitro RNA stability system. Mol Cell Biol. 1997;17:398–406. doi: 10.1128/mcb.17.1.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamarnik A V, Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 19.Görlach M, Burd C G, Dreyfuss G. The mRNA poly(A)-binding protein: localization, abundance, and RNA-binding specificity. Exp Cell Res. 1994;211:400–407. doi: 10.1006/excr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 20.Gorski J, Morrison M R, Merkel C G, Lingrel J B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974;86:363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- 21.Gorski J, Morrison M R, Merkel C G, Lingrel J B. Poly(A) size class distribution in globin mRNAs as a function of time. Nature. 1975;253:749–751. doi: 10.1038/253749a0. [DOI] [PubMed] [Google Scholar]
- 22.Grange T, de Sa C M, Oddos J, Pictet R. Human mRNA polyadenylate binding protein: evolutionary conservation of a nucleic acid binding motif. Nucleic Acids Res. 1987;15:4771–4787. doi: 10.1093/nar/15.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunderson S I, Beyer K, Martin G, Keller W, Boelens W C, Mattaj I. The human U1A snRNP protein regulates polyadenylation via a direct interaction with poly(A) polymerase. Cell. 1994;76:531–541. doi: 10.1016/0092-8674(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 24.Holcik M, Liebhaber S A. Four highly stable eukaryotic mRNAs assemble 3′ untranslated region RNA-protein complexes sharing cis and trans components. Proc Natl Acad Sci USA. 1997;94:2410–2414. doi: 10.1073/pnas.94.6.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson R J. Cytoplasmic regulation of mRNA function: the importance of the 3′ untranslated region. Cell. 1993;74:9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson A, Peltz S W. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 27.Kiledjian M, Dreyfuss G. Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. EMBO J. 1992;11:2655–2664. doi: 10.1002/j.1460-2075.1992.tb05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiledjian M, DeMaria C T, Brewer G, Novick K. Identification of AUF1/hnRNP-D as a component of the α-globin mRNA stability complex. Mol Cell Biol. 1997;17:4870–4876. doi: 10.1128/mcb.17.8.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiledjian M, Day N, Trifillis P. Purification and RNA-binding properties of the polycytidylate-binding proteins αCP1 and αCP2. Methods Companion Methods Enzymol. 1999;17:84–91. doi: 10.1006/meth.1998.0710. [DOI] [PubMed] [Google Scholar]
- 30.Kiledjian M, Wang X, Liebhaber S A. Identification of two KH domain proteins in the α-globin mRNP stability complex. EMBO J. 1995;14:4357–4364. doi: 10.1002/j.1460-2075.1995.tb00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Körner C G, Wahle E. Poly(A) tail shortening by a mammalian poly(A)-specific 3′-exoribonuclease. J Biol Chem. 1997;272:10448–10456. doi: 10.1074/jbc.272.16.10448. [DOI] [PubMed] [Google Scholar]
- 32.Körner C G, Wormington M, Muckenthaler M, Schneider S, Dehlin E, Wahle E. The deadenylating nuclease (DAN) is involved in poly(A) tail removal during the meiotic maturation of Xenopus oocytes. EMBO J. 1998;17:5427–5437. doi: 10.1093/emboj/17.18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kühn U, Pieler T. Xenopus poly(A) binding protein: functional domains in RNA binding and protein-protein interaction. J Mol Biol. 1996;256:20–30. doi: 10.1006/jmbi.1996.0065. [DOI] [PubMed] [Google Scholar]
- 34.Leffers H, Deigaard K, Celis J E. Characterization of two major cellular poly(rC)-binding human proteins, each containing three K-homologous (KH) domains. Eur J Biochem. 1995;230:447–453. [PubMed] [Google Scholar]
- 35.Liebhaber S A. Alpha thalassemia. Hemoglobin. 1989;13:685–731. doi: 10.3109/03630268908998845. [DOI] [PubMed] [Google Scholar]
- 36.Lodish H F, Small B. Different lifetimes of reticulocyte messenger RNA. Cell. 1976;7:59–65. doi: 10.1016/0092-8674(76)90255-5. [DOI] [PubMed] [Google Scholar]
- 37.Lumelsky N L. Decay of globin mRNA during megakaryocytic differentiation of erythroleukemia cell line is accomplished through shortening of the poly(A) tail and degradation from the 3′ end of the transcript. Biochim Biophys Acta. 1995;1261:265–271. doi: 10.1016/0167-4781(94)00249-3. [DOI] [PubMed] [Google Scholar]
- 38.Mangus D A, Amrani N, Jacobson A. Pbp1p, a factor interacting with Saccharomyces cerevisiae poly(A)-binding protein, regulates polyadenylation. Mol Cell Biol. 1998;18:7383–7396. doi: 10.1128/mcb.18.12.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales J, Russell J E, Liebhaber S A. Destabilization of human α-globin mRNA by translation anti-termination is controlled during erythroid differentiation and is paralleled by phased shortening of the poly(A) tail. J Biol Chem. 1997;272:6607–6613. doi: 10.1074/jbc.272.10.6607. [DOI] [PubMed] [Google Scholar]
- 40.Muhlrad D, Parker R. Premature translational termination triggers mRNA decapping. Nature. 1994;370:578–581. doi: 10.1038/370578a0. [DOI] [PubMed] [Google Scholar]
- 41.Muhlrad D, Decker C J, Parker R. Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′3′ digestion of the transcript. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- 42.Nagy E, Maquat L E. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci. 1998;23:198–199. doi: 10.1016/s0968-0004(98)01208-0. [DOI] [PubMed] [Google Scholar]
- 43.Nietfeld W, Mentzel H, Pieler T. The Xenopus laevis poly(A) binding protein is composed of multiple functionally independent RNA binding domains. EMBO J. 1990;9:3699–3705. doi: 10.1002/j.1460-2075.1990.tb07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostareck D H, Ostareck-Lederer A, Wilm M, Thiele B J, Mann M, Hentze M W. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3′ end. Cell. 1997;89:597–606. doi: 10.1016/s0092-8674(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 45.Parker R, Jacobson A. Translation and a 42-nucleotide segment within the coding region of the mRNA encoded by the MAT α1 gene are involved in promoting rapid mRNA decay in yeast. Proc Natl Acad Sci USA. 1990;87:2780–2784. doi: 10.1073/pnas.87.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng S S Y, Chen C Y A, Xu N H, Shyu A B. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross J. mRNA decay in cell-free systems. In: Belasco J, Brawerman G, editors. Control of mRNA stability. San Diego, Calif: Academic Press; 1993. pp. 417–448. [Google Scholar]
- 48.Ross J. mRNA Stability in mammalian cells. Microbiol Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross J, Sullivan T D. Half-lives of beta and gamma globin messenger RNAs and of protein synthetic capacity in cultured human reticulocytes. Blood. 1985;66:1149–1154. [PubMed] [Google Scholar]
- 50.Russell J E, Morales J, Liebhaber S A. The role of mRNA stability in the control of globin gene expression. Prog Nucleic Acid Res Mol Biol. 1997;57:249–287. doi: 10.1016/s0079-6603(08)60283-4. [DOI] [PubMed] [Google Scholar]
- 51.Sachs A B. Messenger RNA degradation in eukaryotes. Cell. 1993;74:413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- 52.Sachs A B, Davis R W, Kornberg R D. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987;7:3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shatkin A. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985;24:223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- 54.Shuman S. Catalytic activity of vaccinia mRNA capping enzyme subunits coexpressed in Escherichia coli. J Biol Chem. 1990;265:11960–11966. [PubMed] [Google Scholar]
- 55.Shyu A-B, Belasco J G, Greenberg M E. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- 56.Shyu A-B, Greenberg M E, Belasco J G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989;3:60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- 57.Stevens A. Eukaryotic nucleases and mRNA turnover. In: Belasco J, Brawerman G, editors. In Control of mRNA stability. San Diego, Calif: Academic Press; 1993. pp. 449–471. [Google Scholar]
- 58.Tarun S Z, Sachs A B. Association of yeast poly(A) tail binding protein with translation initiation in factor eIF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 59.Volloch V, Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981;23:509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Kiledjian M, Weiss I, Liebhaber S A. Detection and characterization of a 3′ untranslated region ribonucleoprotein complex associated with human α-globin mRNA. Mol Cell Biol. 1995;15:1769–1777. doi: 10.1128/mcb.15.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss I M, Liebhaber S A. Erythroid cell-specific determinants of alpha-globin mRNA stability. Mol Cell Biol. 1994;14:8123–8132. doi: 10.1128/mcb.14.12.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss I M, Liebhaber S A. Erythroid cell-specific mRNA stability elements in the α2-globin 3′ nontranslated region. Mol Cell Biol. 1995;15:2457–2465. doi: 10.1128/mcb.15.5.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W, Wagner B, Ehrenman K, Schaefer A W, DeMaria C T, Crater D, DeHaven K, Long L, Brewer G. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993;13:7652–7665. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]