Abstract
An organism’s ability to learn about and respond to stimuli in its environment is crucial for survival, which can involve learning simple associations such as learning what stimuli predict danger. However, individuals must also be able to use contextual information to adapt to changing environmental demands. While the circuitry that supports fear conditioning has been extensively studied, the circuitry that allows individuals to regulate fear under different circumstance is less well understood. A view of ventromedial prefrontal cortex (vmPFC) function has emerged wherein the prelimbic region of the vmPFC supports fear expression, while the infralimbic region supports fear inhibition. However, despite a rich literature exploring the role of these regions in appetitive learning and memory suggesting a more nuanced function, there has been little integration of this literature with studies of the vmPFC in fear learning. In this review, we argue that the function of the vmPFC in fear learning is not restricted to fear inhibition versus expression per se. Instead, the vmPFC uses contextual information to guide behavior, particularly in situations of ambiguity or conflict.
Introduction
An organism’s ability to appropriately learn about and respond to stimuli in its environment is crucial for survival. This can involve learning simple associations such as learning what stimuli predict danger. However, more sophisticated processes such as the ability to adapt behavior to changing environmental demands are equally important. The prefrontal cortex (PFC) is known to support a number of executive functions that enable such flexible processes (Kesner & Churchwell, 2011). The PFC can be divided into a number of regions, each serving distinct roles. The ventromedial region of the rodent PFC (vmPFC), consisting of the prelimbic (PL) and infralimbic (IL) cortices, is known to play an important role in decision-making, attention and behavioral flexibility (Clark et al., 2004; Delatour & Gisquet-Verrier, 2000).
Over the past several decades, the vmPFC has also emerged as playing a key role in the regulation of fear learning and memory. Fear learning is often studied in the laboratory using Pavlovian fear conditioning. In this procedure an initially neutral conditional stimulus (CS), such as a tone or context, is paired with an aversive unconditional stimulus (US), such as mild footshock. Following development of this association, the subject will show a conditional fear response (CR) when presented with the CS.
Considerable attention has also been devoted to understanding the mechanisms of fear inhibition. This is typically studied through a procedure known as fear extinction, where following conditioning the CS is repeatedly presented on its own until it no longer elicits a conditional fear response. Importantly, fear extinction is not believed to involve the erasure of the original CS-US association, as would be predicted by classical models of associative learning (Rescorla & Wagner, 1972). Instead, extinction involves the development of a competing, inhibitory CS-no US association that competes with the original learning (Bouton, 2002). Contextual cues act to select which of these associations are retrieved. This renders extinction highly context-dependent such that the extinguished fear response can return following a change in context, a phenomenon known as renewal (Bouton & Bolles, 1979; Bouton & King, 1983). Fear can also return following the passage of time, a phenomenon known as spontaneous recovery that is often interpreted as the passage of time itself producing a change in context (Bouton, 2002).
While the circuitry underlying fear acquisition is well-understood, the mechanisms of fear extinction are less well understood. Work from Quirk and colleagues has led to the development of a model where the PL and IL play opposing roles in the realm of fear learning: the PL is purported to be necessary for the expression of fear learning, while the IL is thought to be necessary for extinction learning (Sierra-Mercado et al., 2011).
Despite a rich literature exploring the role of the vmPFC in appetitive learning and memory, in which cues and responses are associated with rewards such as food or drug, there has been little integration of this literature with studies of the vmPFC in fear learning. In this review, we will offer a reconceptualization of the role of the vmPFC in fear learning in the context of the vmPFC’s broader role in executive functioning, synthesizing findings from both the appetitive and fear learning domains. We argue that the function of the vmPFC in fear learning is not restricted to fear inhibition versus expression. Instead, a more parsimonious view is that the vmPFC uses contextual information to guide behavior in situations of ambiguity. This review will focus on findings from the rodent literature, though this work does have important implications for human conditions including anxiety disorders and drug addiction. As a comprehensive review of all literature pertaining to vmPFC function is beyond the scope of this paper, we will discuss work from both appetitive and fear learning domains that has the strongest implications for the role of vmPFC function with respect to fear learning and extinction.
The vmPFC in the regulation of fear
The basic circuit underlying Pavlovian fear conditioning is well understood (Fendt & Fanselow, 1999). During fear conditioning, sensory information about the CS and US from the sensory cortex and thalamus converge on neurons within the basolateral amygdala (BLA), which is divided into the lateral (LA) and basal (BA) nuclei (Figure 1). Following conditioning, presentations of the CS allow the BLA to drive a fear response via its projections to the central amygdala (CeA), which in turn project to targets in the brainstem, midbrain and hypothalamus that produce specific fear responses, such as freezing. Located in between the BLA and CeA are clusters of GABAergic neurons known as the intercalated cells (ITCs). The ITCs receive excitatory inputs from the BLA and send inhibitory projections to the CeA, making them an attractive candidate for playing a role in fear inhibition (Likhtik et al., 2008; Paré & Smith, 1993).
Figure 1.
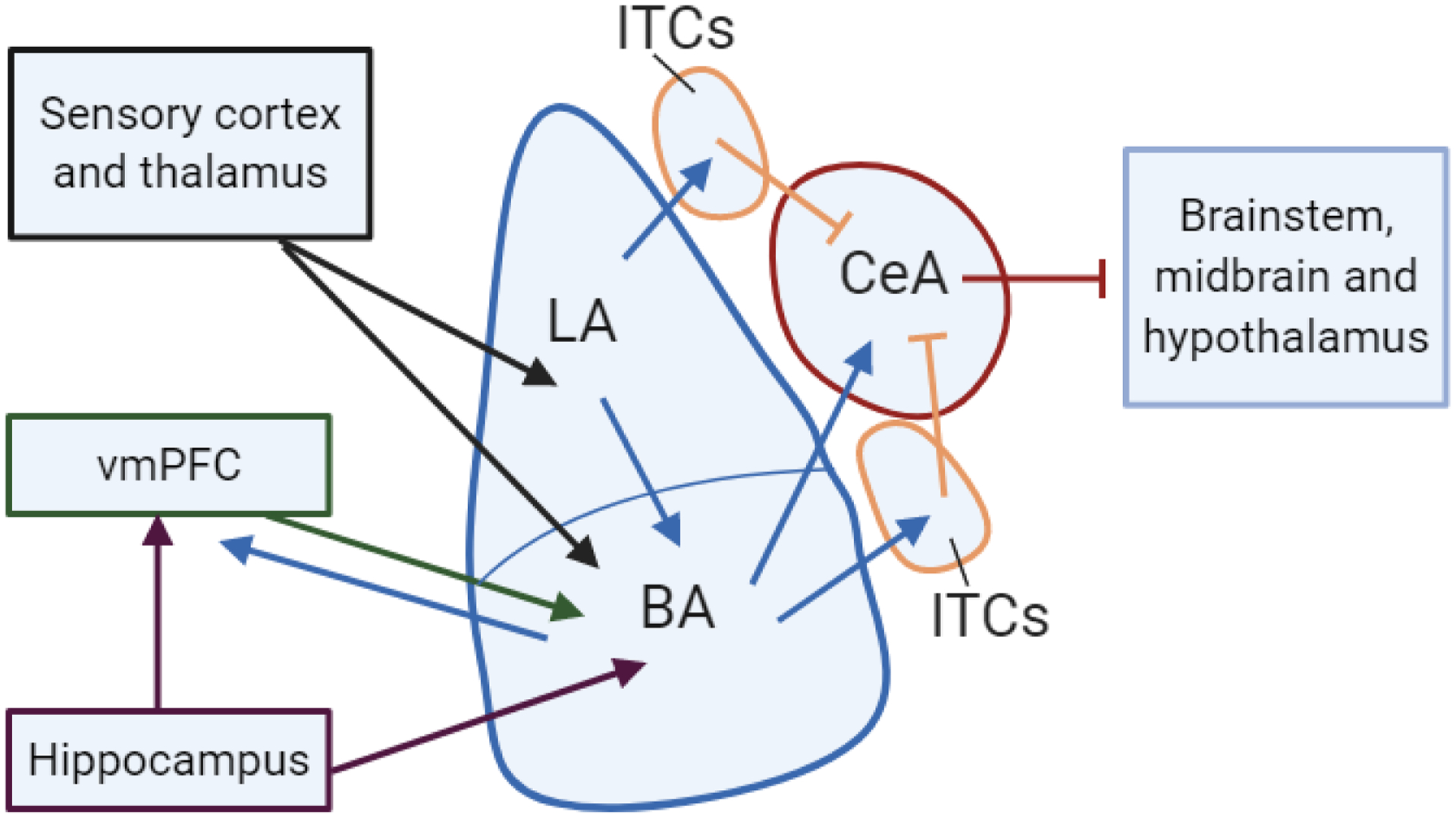
Fear learning circuitry. Diagram showing connectivity between the basolateral amygdala (BLA), hippocampus and ventromedial prefrontal cortex (vmPFC). Other abbreviations: lateral nucleus of BLA (LA), basal nucleus of BLA (BA), intercalated cells (ITCs), central amygdala (CeA).
Simple forms of Pavlovian fear conditioning, such as when a tone perfectly predicts a shock, can be learned by the thalamo-amygdala subcortical network without requiring prefrontal input (Romanski & LeDoux, 1992). However, as learning becomes more complex additional brain structures such the prefrontal cortex and hippocampus are recruited to modulate the subcortical circuits that support simple fear conditioning (Kim et al., 1993; Morgan et al., 1993). Fear extinction is one of the clearest examples of this phenomenon, in which a stimulus that had been reinforced is now presented without reinforcement and subjects must rely on contextual cues to resolve the now-ambiguous meaning of stimuli (Bouton, 2002).
The vmPFC is well-situated to regulate fear learning and memory, as both the PL and IL receive extensive projections from the hippocampus and BLA (Cenquizca & Swanson, 2007; Hoover & Vertes, 2007; Jay & Witter, 1991), and send projections back to the BLA (Hurley et al., 1991; Sesack et al., 1989; Vertes, 2004). While the vmPFC has few direct projections to the ITCs, the vmPFC is able to indirectly regulate the activity of the ITCs via the BLA (Pinard et al., 2012; Strobel et al., 2015). The PL and IL also have reciprocal connections, with PL-IL projections being more prominent while IL-PL projections are relatively sparse (Marek, Xu, et al., 2018). Over the past several decades, a model of vmPFC function in fear learning and extinction has emerged wherein the IL is necessary for fear extinction, while the PL enables fear expression (Sierra-Mercado et al., 2011). Each of these ideas will be discussed in greater detail below.
The role of the IL in fear extinction
One of the first studies to suggest a role for the IL in fear inhibition was from Morgan and LeDoux (1993), which indicated that vmPFC lesions impaired fear extinction. An extensive series of studies from Quirk and colleagues indicated that the extinction impairments observed were likely due to disruption of the IL specifically, as pre-training vmPFC lesions that included both PL and IL impaired retrieval of an extinguished fear response, while IL-sparing vmPFC lesions did not produce this effect (Quirk et al., 2000). This paved the way for further dissection of a fear extinction circuit, focusing on the IL as the hub for fear inhibition (Milad & Quirk, 2012).
Initial findings that pretraining IL lesions impaired extinction memory retrieval and that IL activity increased at the start of subsequent extinction sessions suggested that IL the may critical for the storage or retrieval of extinction (Milad & Quirk, 2002; Quirk et al., 2000). Importantly, the IL was not viewed as necessary for fear suppression per se, as IL lesions did not impact within-session extinction (Quirk et al., 2000). However, later studies utilizing more temporally and spatially precise techniques suggested that the role of the IL in fear extinction may be more nuanced. While both optogenetic and pharmacological inactivation of IL during or immediately after training impaired subsequent recall of extinction memory and IL activation during training enhanced extinction recall, optogenetic IL inactivation at test did not impair retrieval (Bukalo et al., 2015; Do-Monte et al., 2015). These findings, combined with evidence that BLA-ITC projections are strengthened by extinction training and that this strengthening is blocked by IL inactivation (Bloodgood et al., 2018), led to the current model of extinction in which the IL mediates the strength of BLA-ITC projections but is not required for the storage or retrieval of extinction memory (Do-Monte et al., 2015).
The role of the prelimbic cortex in fear expression
Reports that PL inactivation reduced fear during both training and test (Corcoran & Quirk, 2007), combined with evidence of correlations between PL activity and fear (Burgos-Robles et al., 2009), have led to the proposal that PL activity is necessary for fear expression. This is somewhat surprising given that numerous studies demonstrated that vmPFC lesions did not impair acquisition or expression of Pavlovian fear conditioning to a discrete tone or context CS (Lebrón et al., 2004; Morgan et al., 1993; Zelikowsky et al., 2013). However, it is possible that such pre-training lesions could allow for the development of compensatory mechanisms (Fanselow, 2010).
While both of these views of vmPFC function have considerable support, there has been relatively little integration of these models with the established role of the vmPFC in context-sensitive forms of learning and memory (Kesner & Churchwell, 2011; Kesner et al., 1996; Ragozzino et al., 1998). We will now reevaluate findings regarding the role of the vmPFC in fear learning and extinction in the broader context of contextual control of behavior in situations of uncertainty. Rather than promoting fear suppression versus inhibition per se, we argue that the vmPFC is recruited to navigate situations of conflict and uncertainty, including but not limited to fear extinction.
The vmPFC and contextual control of fear extinction
The vmPFC is well-situated to receive and use contextual information to navigate competing CS-US and CS-no US associations during fear extinction as it receives input from the hippocampus and BLA and projects backs to the BLA (Cenquizca & Swanson, 2007; Hoover & Vertes, 2007; Hurley et al., 1991; Jay & Witter, 1991; Sesack et al., 1989; Vertes, 2004). This suggests that during the formation and retrieval of the extinction memory, information about the context and emotional valence of stimuli is passed to the vmPFC, which in turn uses this information to modulate BLA activity. Appropriate responding following extinction therefore requires coordinated activity of the vmPFC, hippocampus and BLA (Bloodgood et al., 2018; Knapska et al., 2012; Orsini et al., 2011).
The vmPFC appears to be important for the context-appropriate retrieval of fear extinction memory. Presentations of the extinguished CS are associated with increased activation of IL neurons when presented in the extinction context, and increased activity in the PL when presented outside the extinction context (Knapska & Maren, 2009; Orsini et al., 2011). Interestingly, Orsini et al (2011) found a selective increase in the activity of BLA-projecting PL neurons during renewal compared to extinction retrieval but no differences in overall PL neuron activity, suggesting that the PL projections to other targets may have concurrently been mediating behaviors in addition to fear renewal. Optogenetic and pharmacological IL inactivation has been shown to impair the retrieval of extinction memory when the extinguished CS was presented in the extinction context (Kim et al., 2016; Marek, Jin, et al., 2018). Conversely, our laboratory has demonstrated that IL lesions increase fear renewal when the extinguished CS is presented outside of the extinction context, while PL lesions attenuated fear renewal (Zelikowsky et al., 2013).
Given the established role of the hippocampus in contextual learning and memory (Kim & Fanselow, 1992; Holland & Bouton, 1999; Fanselow, 2000), it is not surprising that hippocampal function is necessary for the contextually appropriate retrieval of extinction memory. Presentations of an extinguished CS have been shown to increase activity in both dorsal hippocampus (DH) and ventral hippocampus (VH) when presented in either the extinction or renewal context (Knapska & Maren, 2009). Pre-training and post-training DH lesions, as well as pharmacological DH inactivation, have been shown to impair the context-specificity of fear extinction by reducing renewal (Corcoran & Maren, 2001; Ji & Maren, 2005). The encoding of extinction memory also appears to depend on hippocampal function, as inactivation of DH and VH during extinction training has been shown to impair the retrieval of extinction memory (Corcoran et al., 2005; Sierra-Mercado et al., 2011).
The contextually appropriate retrieval of extinction memory further relies on interactions between the hippocampus, vmPFC and BLA. Projections from the VH to the BLA and vmPFC are necessary for fear renewal (Knapska et al., 2012; Orsini et al., 2011). A recent study demonstrated a particular role for VH projections targeting inhibitory interneurons in the vmPFC in regulating fear renewal, as chemogenetic activation of VH projections targeting inhibitory interneurons in the IL impaired extinction recall, while inhibition of these projections reduced fear renewal (Marek, Jin, et al., 2018). Plasticity within the BLA is also necessary for fear extinction, and following extinction some individual BLA neurons show increased responses to the CS after fear conditioning, while others show increased responses to CS presentation in the extinction context after extinction training (Falls et al., 1992; Herry et al., 2008; Hobin et al., 2003).
As discussed previously, the current model of an extinction circuit posits that the IL supports the development of BLA-ITC connections during extinction training but is not involved in the subsequent retrieval of extinction memory (Do-Monte et al., 2015). This appears to be at odds with evidence that an important function of the vmPFC is the context-appropriate retrieval of extinction memory (Kim et al., 2016; Marek, Jin, et al., 2018). One potential explanation is a difference in testing procedures. Marek et al (2018) found that IL inactivation did not impact freezing during the first few trials testing extinction memory retrieval, which appeared to be due to elevated freezing in the control subjects. This suggested that spontaneous recovery during early test trials could potentially mask the effects of IL manipulations during short test sessions as were used by Do-Monte et al (Do-Monte et al., 2015; Marek, Jin et al., 2018).
Another potential explanation is that context-specificity of extinction can be affected by differences in training procedures, such as where fear conditioning versus extinction training take place. In many of the studies conducted by Quirk and colleagues, extinction training and testing typically occur within the same context (Corcoran & Quirk, 2007; Sierra-Mercado et al., 2006; Sierra-Mercado et al., 2011). In contrast, Kim et al (2016) and Marek et al (2018) performed fear conditioning in one context (e.g. Context A) and extinction training in a different context (e.g. Context B). When fear conditioning and extinction occur in different contexts, the renewal of fear when the extinguished CS is presented in the original training context (known as ABA renewal) or in a novel context (known as ABC renewal) is generally quite robust (Bouton et al., 2011; Thomas et al., 2003). However, renewal has been shown to be less robust when training and extinction occur in the same context, a procedure known as AAB renewal ((Nakajima, Tanaka, Urushihara K, et al., 2000; Nakajima, Tanaka, Urushihara, et al., 2000; Thomas et al., 2003).
This suggests that conducting fear conditioning and extinction within the same context may reduce the context-specificity of extinction and promote use of different strategies for determining the now-ambiguous meaning of the CS. For example, it has been suggested that extinction training in original conditioning context may cause the CS to lose some of its excitatory associative value, in addition to promoting the development of a context-specific CS-no US association (Thomas et al., 2003). It is therefore plausible that the necessity of IL in the retrieval of extinction memory is influenced by contextual dependence of that memory. In support of this, IL inactivation has been shown to impair retrieval of context extinction memory (Laurent & Westbrook, 2009). Interestingly, IL lesions did not impair performance on a feature-negative discrimination procedure, in which a discrete stimulus (as opposed to a context) signaled the non-reinforcement of a tone stimulus (Meyer & Bucci, 2014).
In summary, the vmPFC appears to work in concert with the BLA and hippocampus to guide the context-appropriate expression of fear. While current models of vmPFC function posit that the IL is necessary for the acquisition, but not expression of fear extinction (Do-Monte et al, 2015), other findings suggest that the IL is indeed necessary for the retrieval of extinction memory (Kim et al., 2016; Marek, Jin, et al., 2018). This discrepancies could potentially be explained by differences in training or testing procedures.
The vmPFC and extinction of reward-seeking behavior
The vmPFC is also implicated in the extinction of reward-seeking behavior. This topic is often explored using self-administration procedures in which subjects are first trained to make an operant response such as a lever press to receive a drug reward, followed by extinction sessions where the response is no longer reinforced (Millan et al., 2011). At test, an extinguished response can return following presentation of the drug (reinstatement), presentation of a cue associated with the drug (cue-induced reinstatement) or change in context (context-induced reinstatement) (Crombag et al., 2002; de Wit & Stewart, 1983; Millan et al., 2011). As in fear extinction, pharmacological and optogenetic inhibition of IL activity immediately following extinction training impaired extinction of a lever press response that had previously been rewarded with cocaine (Gutman et al., 2017; LaLumiere et al., 2010). IL activity is also necessary for the retrieval of extinction memory, as pharmacological inhibition of IL caused a return of an extinguished lever press response while pharmacological and chemogenetic IL activation prevented cue-induced reinstatement (Augur et al., 2016; Peters et al., 2008).
Context-induced reinstatement has many similarities with fear renewal, and similarly depends on coordinated activity of the vmPFC and hippocampus. Context-induced reinstatement is associated with increased activity of PL neurons, while PL inactivation attenuates this reinstatement (Palombo et al., 2017; Trask et al., 2017; Willcocks & McNally, 2013). This effect appears to depend on hippocampal activity, as pharmacological inhibition of the ventral hippocampus and chemogenetic inhibition of VH-IL projections reduce context-induced reinstatement of cocaine and heroin seeking (Bossert & Stern, 2014; Lasseter et al., 2010; Wang et al., 2018).
A number of findings suggest that the IL also modulates the retrieval of extinction memory for appetitive Pavlovian conditioning, in which a CS is associated with a food reward US. IL lesions have been shown to increase spontaneous recovery and renewal of the extinguished CS without impacting within-session extinction or savings between extinction sessions (Rhodes & Killcross, 2007; Rhodes & Killcross, 2004). Conversely, optogenetic IL stimulation following extinction training has been shown to reduce renewal and spontaneous recovery (Villaruel et al., 2018).
The vmPFC and contextual control of behavior beyond extinction
While much of the work studying context control of fear learning has employed fear extinction, several studies from our laboratory utilizing other procedures suggest a more general role of the vmPFC in context-sensitive learning memory systems, rather than fear expression and inhibition specifically. A previous report from our laboratory demonstrated that IL lesions impaired the ability of animals to discriminate between a context in which a tone had been paired with a shock, and a novel context (Zelikowsky et al., 2013). This effect was not driven by just increased fear to the novel context, as would be expected if the only role of the IL was to inhibition fear, but rather by intermediate levels of fear to both contexts. In contrast, PL lesions had no impact on either fear to the trained context or generalization to a novel context. A more recent study found that IL lesions impaired the ability of stress to enhance fear to a context paired with an aversive acoustic startle stimulus, while leaving stress-enhanced fear to a tone CS intact (Pennington et al., 2017). In addition, while context fear conditioning is typically dependent on the dorsal hippocampus, the vmPFC can compensate for pre-training hippocampal damage (Zelikowsky et al., 2013). This ability does not depend on either the PL or IL specifically, but instead requires communication between the two regions as disconnection of PL and IL prevents compensation.
In addition, a study from our laboratory utilized cellular compartment analysis of temporal activity using fluorescence in situ hybridization (catFISH) to label neurons within the BLA, dorsal hippocampus and vmPFC during both context fear conditioning and during test of memory recall in subjects that did or did not form a context-fear association (Zelikowsky et al., 2014). BLA neurons only showed activation at both time points in the animals that formed a fear memory, while both groups showed similar reactivation of hippocampal neurons, indicating that these regions encoded emotional and spatial properties of the context, respectively. The pattern of reactivation in PL suggested that this region may integrate both spatial and emotional information, as a substantial number of PL neurons were reactivated in both groups although more were reactivated in the animals that formed a fear memory.
While the role of the vmPFC in regulating both fear and drug-seeking behavior is typically interpreted as serving a “stop-go” function, a number of studies in the appetitive domain utilizing a variety of tasks suggest that the PL and IL mediate contextually-appropriate behavior beyond this simple dichotomy. A recent study recorded from PL and IL neurons during the performance and extinction of a discriminative stimulus-driven sucrose seeking task (Moorman & Aston-Jones, 2015). In this task, one auditory stimulus indicated that a lever press would be reinforced, while a second stimulus indicated that a lever press would not be reinforced. While a “stop-go” view of vmPFC function would predict PL activity to be elevated during drug seeking and IL activity to be elevated during suppression of drug seeking, this is not what was observed. Instead, both PL and IL neurons both tended to be active when the correct response was made, i.e. firing when a lever response was made during reinforced trials and when the response was withheld during nonreinforced trials. Furthermore, during extinction both PL and IL neurons switched to signal the withholding of a response during previously-reinforced trials. These results suggest that the vmPFC plays a role in signaling the reinforcement contingencies currently in effect, enabling the appropriate execution or inhibition of behavior under changing circumstances.
Another task that has proven powerful for investigating the role of the vmPFC in the contextual control of behavior is the biconditional discrimination task developed by Killcross and colleagues (Haddon & Killcross, 2006; Marquis et al., 2007). In this task, rats were presented with a pair of visual stimuli (e.g. V1 and V2) in one context and a pair of auditory stimuli (e.g. A1 and A2) in the second context. Presentations of A1 and V1 indicated that pressing one of the two available levers would result in food reward, while A2 and V2 indicated that pressing the other lever would result in food reward. As a result, neither response was consistently reinforced or not reinforced, and subjects had to use these stimuli to determine which response would be reinforced. During the critical test, audiovisual compounds were presented in each context that signaled either reinforcement of the same lever press (e.g. A1V1) or of different lever presses (e.g. A1V2). These incongruent trial types required the animals to use contextual cues to determine which type of stimulus (visual or auditory) to attend to.
vmPFC lesions did not impair acquisition of the individual discriminations within each context, indicating that subjects were able to use non-contextual stimuli to navigate between conflicting responses (Haddon & Killcross, 2006). However, performance was impaired during incongruent trials, indicating that animals were not able to appropriately use contextual cues to guide which stimuli to attend to. This ability was shown to be dependent specifically on the PL, as PL inactivation reproduced this effect while IL inactivation did not (Marquis et al., 2007). Similar finding have been shown in a fear conditioning preparations, in which PL inactivation impaired the ability of subjects to use contextual cues to determine which of two auditory stimuli signaled footshock (M. Sharpe & S. Killcross, 2015), while PL lesions impaired the ability of mice to use contextual cues to determine whether a single auditory stimulus signaled footshock or not (Kim et al., 2013).
In summary, a number of findings suggest that vmPFC has a more sophisticated role than simply allowing the expression or inhibition of behavior. Work from our laboratory suggests that vmPFC manipulations may affect several aspects of contextual processing (Zelikowsky et al., 2013; Zelikowsky et al., 2014; Pennington et al., 2017). The vmPFC further appears to regulate the use of contextual information to guide reward-seeking behavior (Haddon & Killcross, 2006; Marquis et al., 2007; Moorman & Aston-Jones, 2015).
Opposing roles of PL and IL in controlling context specificity of behavior
At first glance, the results of extinction studies versus biconditional discrimination studies seem at odds-why do lesions of IL but not PL impair retrieval of extinction, while lesions of PL but not IL impair performance on a different task in which context is explicitly used to determine which set of associations to attend to?
An intriguing explanation has been offered by Killcross and colleagues, who have suggested that the PL and IL play opposing roles not in expression versus inhibition, but in regulating the context specificity of behavior. Specifically, they suggested that the PL enables the direction of attention towards relevant stimuli, facilitating the use of contextual cues to guide behavior, while the IL supports the ability of certain behaviors to persevere across multiple contexts (Haddon & Killcross, 2007; Marquis et al., 2007; Rhodes & Killcross, 2004; Roughley & Killcross, 2019). Within this view, IL lesions serve to enhance the context-specificity of extinction. This would result in a reduced ability of the test context to retrieve the extinction memory, producing the observed increases in spontaneous recovery and renewal, without affecting rates of within-session extinction. In contrast, because accurate performance on the biconditional discrimination task depends on the context specificity of behavior, PL lesions would be expected to produce the observed impairments.
In support of this view, IL lesions have been reported to enhance performance on tasks requiring use of spatial cues. Ashwell and Ito (2014) demonstrated that neither PL nor IL lesions impacted the ability of rats to associate visual stimuli presented in the arms of a radial arm maze with a food reward. However, differences emerged when subjects were required to use spatial cues to determine which stimulus presentations would be reinforced. IL-lesioned animals showed enhanced performance compared to PL-lesioned animals both during initial discrimination training and during reversal training when the locations of reward stimulus locations were reversed. Interestingly, the latter was driven by reduced responding to the non-reinforced stimulus. This decrease in preservative behaviors following IL lesions was interpreted as evidence that IL lesions caused behavior to become more sensitive to changing environmental contingencies (Ashwell & Ito, 2014).
However, just as we argue that a dichotomous view of the vmPFC as solely promoting fear expression versus inhibition is overly simplistic, its role may also be more nuanced than solely promoting context specificity versus generality of behavior. Recently, Riaz et al (2019) demonstrated that both PL- and IL-lesioned rats showed impaired ability to use contextual cues to determine which of two auditory stimuli would be reinforced. In both groups, this appeared to be due to both decreased responding to the reinforced stimulus and increased responding to the nonreinforced stimulus (Riaz et al., 2019). While this goes against an account of IL lesions promoting context-specificity of behavior, it nevertheless suggests that both regions are involved in using environmental cues to navigate situations of ambiguity or conflict.
Methodological considerations that may impact vmPFC recruitment
An attentional account of vmPFC function posits that a critical role of the PL is to guide attention towards relevant stimuli, facilitating the use of such information to guide behavior (Floresco et al., 2008; Sharpe & Killcross, 2014). It has therefore been suggested that PL lesions may not impact fear expression per se, but rather impairs the ability of animals to attend to aspects of the environment that are predictive of danger (M. J. Sharpe & S. Killcross, 2015). The role of the PL in attending to predictive stimuli and ignoring irrelevant behavior could depend on the degree of competition between the cues present during learning. In a Pavlovian fear conditioning situation in which a tone CS is paired with a shock US, both the tone and the context will compete to be associated with the shock (Anagnostaras et al., 2001). Competition between cues and context is affected by a variety of experimental parameters. For example, competition between cues and context can be reduced by relatively long inter-trial intervals (ITIs) or pre-exposure to the conditioning context, and enhanced by relatively short inter-trial intervals or pre-exposure to the CS. Sharpe and Killcross (2015) demonstrated that the effects of PL lesions depended on the degree of competition between discrete cues and context, as PL lesions reduced fear to a discrete CS when parameters favored competition between the CS and context (short ITIs and no pre-exposure to context). However, PL lesions had no effect when training parameters favored learning about the CS and not the context (long ITIs and context pre-exposure).
The possibility that the PL becomes particularly important when attending to multiple stimuli suggests that vmPFC recruitment during fear conditioning may be influenced by specific training procedures that employ high levels of competition between the CS and context or require subjects to navigate between competing responses. In several studies demonstrating the necessity of the PL for fear expression, subjects received nonreinforced CS presentations immediately prior to CS-US pairings (Burgos-Robles et al., 2009; Sierra-Mercado et al., 2006; Sierra-Mercado et al., 2011). While such procedures may be necessary for identification of CS-sensitive cells in in vivo electrophysiological recording experiments, they can also produce a phenomenon known as latent inhibition in which nonreinforced CS presentations impair subsequent acquisition of a CS-US presentations (Lubow et al., 1976). Similar to extinction, during CS pre-exposure subjects appear to learn a context-dependent, CS-no US association (Bouton, 1993; Westbrook et al., 2000). Additionally, latent inhibition decreases the salience of, or attention devoted to, the CS, necessitating greater vmPFC participation (McLaren & Mackintosh, 2000). Indeed, one theory of latent inhibition posits that context-CS associations acquired during pre-exposure result in priming CS representations later in training and testing and this priming reduces the surprise and salience of a CS when it is presented in the pre-exposure context (Vogel et al, 2019).
A second procedure that could introduce additional conflict is the use of conditioned suppression as a measure of fear, in which subjects can freely bar-press for food during testing and reductions in pressing during CS presentations are taken as an index of fear (Kamin et al., 1963). While such a measure has a long history of use, it does introduce additional conflict in that subjects must navigate between competing reward-seeking and defensive behaviors. A number of studies demonstrating the necessity of the PL in fear expression employ such procedures, which are further complicated by bar-press training and fear conditioning frequently occurring within the same context (Corcoran & Quirk, 2007; Sierra-Mercado et al., 2006; Sierra-Mercado et al., 2011). Such considerations imply that while the PL may play a role in fear expression, the extent of PL involvement may be contingent on task demands.
Implications for understanding human fear and anxiety
Understanding the mechanisms through which behavior is guided by changing environmental contingencies has a powerful impact for understanding a number of human conditions. Post-traumatic stress disorder (PTSD) is characterized by a dysregulation of fear responses, including exaggerated response to mild stressors that are reminiscent of the original trauma and impaired fear extinction (Bremner et al., 1995; Dykman et al., 1997; Jovanovic et al., 2012). When treating drug addiction, a major challenge is the relapse to drug use after periods of abstinence following exposure to environmental stimuli associated with drug use (Hunt et al., 1971). Accordingly, prefrontal cortex dysfunction is increasingly being looked at as a marker of susceptibility to PTSD and drug addiction (Goldstein & Volkow, 2011; Koenigs & Grafman, 2009). While reduced PFC activity is typically associated with worse outcomes (Koenigs & Grafman, 2009; Milad et al., 2005), the multifaceted role of the PFC in regulating behaviors such as fear learning indicates that its role in these conditions is equally complex.
Conclusion
In contrast to the view that the PL and IL play opposing roles in the expression versus inhibition of fear, we argue that the roles of the vmPFC in fear acquisition and extinction are much more complex. Research from the appetitive domain suggests that the vmPFC play a critical role in using contextual information to guide behavior, particularly in situations of ambiguity or conflict. Such findings have important implications for understanding the role of the vmPFC in the realm of fear learning. Moving forward, it will be valuable to further delineate the precise contributions of the vmPFC to different aspects of fear learning and memory.
Table 1.
Abbreviations and full names.
| Abbreviation | Full name |
|---|---|
| PFC | Prefrontal cortex |
| vmPFC | Ventromedial prefrontal cortex |
| PL | Prelimbic cortex |
| IL | Infralimbic cortex |
| BLA | Basolateral amygdala |
| LA | Lateral nucleus of basolateral amygdala |
| BA | Basal nuclues of basolateral amygdala |
| CeA | Central amygdala |
| ITCs | Intercalated cells |
| DH | Dorsal hippocampus |
| VH | Ventral hippocampus |
| catFISH | Cellular compartment analysis of temporal activity using fluorescence in situ hybridization |
| CS | Conditional stimulus |
| US | Unconditional stimulus |
| CR | Conditional response |
| ITI | Inter-trial interval |
| PTSD | Post-traumatic stress disorder |
Public Significance Statement:
This review discusses advancements made in our understanding of how the prefrontal cortex regulates fear learning and memory. Drawing on work from both appetitive and fear learning domains, we discuss how the prefrontal cortex guides behavior in situations of ambiguity or conflict.
Acknowledgments
This research was supported by NIH Grant RO1-MH62122 and the Staglin Center for Brain and Behavioral Health. The authors are solely responsible for the opinions expressed and were not influenced by the funding agencies.
References
- Anagnostaras SG, Gale GD, & Fanselow MS (2001). Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus, 11(1), 8–17. [DOI] [PubMed] [Google Scholar]
- Ashwell R, & Ito R (2014). Excitotoxic lesions of the infralimbic, but not prelimbic cortex facilitate reversal of appetitive discriminative context conditioning: the role of the infralimbic cortex in context generalization. Front Behav Neurosci, 8, 63. 10.3389/fnbeh.2014.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, & Peters J (2016). Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. Journal of Neuroscience, 36(39), 10174–10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood DW, Sugam JA, Holmes A, & Kash TL (2018, 03). Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl Psychiatry, 8(1), 60. 10.1038/s41398-018-0106-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, & Stern AL (2014, May). Role of ventral subiculum in context-induced reinstatement of heroin seeking in rats. Addict Biol, 19(3), 338–342. 10.1111/adb.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2002, November). Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry, 52(10), 976–986. [DOI] [PubMed] [Google Scholar]
- Bouton ME, & Bolles RC (1979, October). Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process, 5(4), 368–378. [DOI] [PubMed] [Google Scholar]
- Bouton ME, & King DA (1983, July). Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process, 9(3), 248–265. [PubMed] [Google Scholar]
- Bouton ME (1993). Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological bulletin, 114(1), 80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Vurbic D, & Winterbauer NE (2011). Renewal after the extinction of free operant behavior. Learning & Behavior, 39(1), 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1995, October). Functional neuroanatomical correlates of the effects of stress on memory. J Trauma Stress, 8(4), 527–553. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Pinard CR, Silverstein S, Brehm C, Hartley ND, Whittle N, Colacicco G, Busch E, Patel S, Singewald N, & Holmes A (2015, July). Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci Adv, 1(6). 10.1126/sciadv.1500251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, & Quirk GJ (2009, July). Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci, 29(26), 8474–8482. 10.1523/JNEUROSCI.0378-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, & Swanson LW (2007, November). Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev, 56(1), 1–26. 10.1016/j.brainresrev.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, & Robbins TW (2004, June). The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn, 55(1), 41–53. 10.1016/S0278-2626(03)00284-7 [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, & Maren S (2005, September). Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci, 25(39), 8978–8987. 10.1523/JNEUROSCI.2246-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, & Maren S (2001, March). Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci, 21(5), 1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, & Quirk GJ (2007, January). Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci, 27(4), 840–844. 10.1523/JNEUROSCI.5327-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, & Shaham Y (2002, December). Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology, 27(6), 1006–1015. 10.1016/S0893-133X(02)00356-1 [DOI] [PubMed] [Google Scholar]
- de Wit H, & Stewart J (1983). Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology, 79(1), 29–31. [DOI] [PubMed] [Google Scholar]
- Delatour B, & Gisquet-Verrier P (2000, April). Functional role of rat prelimbic-infralimbic cortices in spatial memory: evidence for their involvement in attention and behavioural flexibility. Behav Brain Res, 109(1), 113–128. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente K, Ramos-Medina L, & Quirk GJ (2015, February). Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci, 35(8), 3607–3615. 10.1523/JNEUROSCI.3137-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykman RA, Ackerman PT, & Newton JE (1997, 1997 Jan-Mar). Posttraumatic stress disorder: a sensitization reaction. Integr Physiol Behav Sci, 32(1), 9–18. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, & Davis M (1992, March). Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci, 12(3), 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS (2000). Contextual fear, gestalt memories, and the hippocampus. Behavioural brain research, 110(1–2), 73–81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (2010) From contextual fear to a dynamic view of memory systems. Trends in Cognitive Sciences, 14, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt A, & Fanselow MS (1999). The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience & Biobehavioral Reviews, 23(5), 734–760. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, & Tse MT (2008, June). Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res, 190(1), 85–96. 10.1016/j.bbr.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, & Volkow ND (2011, October). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci, 12(11), 652–669. 10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman AL, Nett KE, Cosme CV, Worth WR, Gupta SC, Wemmie JA, & LaLumiere RT (2017). Extinction of cocaine seeking requires a window of infralimbic pyramidal neuron activity after unreinforced lever presses. Journal of Neuroscience, 37(25), 6075–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon JE, & Killcross S (2006, March). Prefrontal cortex lesions disrupt the contextual control of response conflict. J Neurosci, 26(11), 2933–2940. 10.1523/JNEUROSCI.3243-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon JE, & Killcross S (2007, May). Contextual control of choice performance: behavioral, neurobiological, and neurochemical influences. Ann N Y Acad Sci, 1104, 250–269. 10.1196/annals.1390.000 [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, & Lüthi A (2008, July). Switching on and off fear by distinct neuronal circuits. Nature, 454(7204), 600–606. 10.1038/nature07166 [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, & Maren S (2003, September). Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci, 23(23), 8410–8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, & Bouton ME (1999, April). Hippocampus and context in classical conditioning. Curr Opin Neurobiol, 9(2), 195–202. 10.1016/s0959-4388(99)80027-0 [DOI] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2007, September). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct, 212(2), 149–179. 10.1007/s00429-007-0150-4 [DOI] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, & Branch LG (1971, October). Relapse rates in addiction programs. J Clin Psychol, 27(4), 455–456. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, & Saper CB (1991, June). Efferent projections of the infralimbic cortex of the rat. J Comp Neurol, 308(2), 249–276. 10.1002/cne.903080210 [DOI] [PubMed] [Google Scholar]
- Jay TM, & Witter MP (1991, November). Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol, 313(4), 574–586. 10.1002/cne.903130404 [DOI] [PubMed] [Google Scholar]
- Ji J, & Maren S (2005, 2005 May-Jun). Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem, 12(3), 270–276. 10.1101/lm.91705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, & Davis M (2012, February). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62(2), 695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin LJ, Brimer CJ, & Black AH (1963, June). Conditioned suppression as a monitor of fear of the CS in the course of avoidance training. J Comp Physiol Psychol, 56, 497–501. 10.1037/h0047966 [DOI] [PubMed] [Google Scholar]
- Kesner RP, & Churchwell JC (2011, October). An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem, 96(3), 417–431. 10.1016/j.nlm.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunt ME, Williams JM, & Long JM (1996, 1996 Mar-Apr). Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex, 6(2), 311–318. 10.1093/cercor/6.2.311 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kim N, Kim HT, & Choi JS (2013). The prelimbic cortex is critical for context-dependent fear expression. Front Behav Neurosci, 7, 73. 10.3389/fnbeh.2013.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Cho HY, Augustine GJ, & Han JH (2016, April). Selective Control of Fear Expression by Optogenetic Manipulation of Infralimbic Cortex after Extinction. Neuropsychopharmacology, 41(5), 1261–1273. 10.1038/npp.2015.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, & Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science, 256(5057), 675–677. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, & Fanselow MS (1993, December). Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci, 107(6), 1093–1098. [DOI] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, Pieprzyk M, Cymerman IA, Werka T, Sheng M, Maren S, Jaworski J, & Kaczmarek L (2012, October). Functional anatomy of neural circuits regulating fear and extinction. Proc Natl Acad Sci U S A, 109(42), 17093–17098. 10.1073/pnas.1202087109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, & Maren S (2009, August). Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem, 16(8), 486–493. 10.1101/lm.1463909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, & Grafman J (2009). Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. The Neuroscientist, 15(5), 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, & Kalivas PW (2010, April). The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem, 17(4), 168–175. 10.1101/lm.1576810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, & Fuchs RA (2010, December). Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience, 171(3), 830–839. 10.1016/j.neuroscience.2010.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, & Westbrook RF (2009, September). Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem, 16(9), 520–529. 10.1101/lm.1474609 [DOI] [PubMed] [Google Scholar]
- Lebrón K, Milad MR, & Quirk GJ (2004, 2004 Sep-Oct). Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem, 11(5), 544–548. 10.1101/lm.78604 [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, & Paré D (2008, July). Amygdala intercalated neurons are required for expression of fear extinction. Nature, 454(7204), 642–645. 10.1038/nature07167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE, Schnur P, & Rifkin B (1976). Latent inhibition and conditioned attention theory. Journal of Experimental Psychology: Animal Behavior Processes, 2(2), 163. [Google Scholar]
- Marek R, Jin J, Goode TD, Giustino TF, Wang Q, Acca GM, Holehonnur R, Ploski JE, Fitzgerald PJ, Lynagh T, Lynch JW, Maren S, & Sah P (2018, 03). Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat Neurosci, 21(3), 384–392. 10.1038/s41593-018-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Xu L, Sullivan RKP, & Sah P (2018, 05). Excitatory connections between the prelimbic and infralimbic medial prefrontal cortex show a role for the prelimbic cortex in fear extinction. Nat Neurosci, 21(5), 654–658. 10.1038/s41593-018-0137-x [DOI] [PubMed] [Google Scholar]
- Marquis JP, Killcross S, & Haddon JE (2007, January). Inactivation of the prelimbic, but not infralimbic, prefrontal cortex impairs the contextual control of response conflict in rats. Eur J Neurosci, 25(2), 559–566. 10.1111/j.1460-9568.2006.05295.x [DOI] [PubMed] [Google Scholar]
- McLaren IPL, & Mackintosh NJ (2000). An elemental model of associative learning: I. Latent inhibition and perceptual learning. Animal Learning & Behavior, 28(3), 211–246. [Google Scholar]
- Meyer HC, & Bucci DJ (2014). The contribution of medial prefrontal cortical regions to conditioned inhibition. Behavioral Neuroscience, 128(6), 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, & Rauch SL (2005, July). Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A, 102(30), 10706–10711. 10.1073/pnas.0502441102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2002, November). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature, 420(6911), 70–74. 10.1038/nature01138 [DOI] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol, 63, 129–151. 10.1146/annurev.psych.121208.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, & McNally GP (2011, March). Extinction of drug seeking. Behav Brain Res, 217(2), 454–462. 10.1016/j.bbr.2010.10.037 [DOI] [PubMed] [Google Scholar]
- Moorman DE, & Aston-Jones G (2015, July). Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc Natl Acad Sci U S A, 112(30), 9472–9477. 10.1073/pnas.1507611112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, & LeDoux JE (1993, November). Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett, 163(1), 109–113. 10.1016/0304-3940(93)90241-c [DOI] [PubMed] [Google Scholar]
- Nakajima S, Tanaka S, Urushihara K, & H31(4):416–31., I. (2000). Renewal of extinguished lever-press responses upon return to the training context. Learning and Motivation, 31(4), 416–431. [Google Scholar]
- Nakajima S, Tanaka S, Urushihara K, & Imada H (2000). Renewal of extinguished lever-press responses upon return to the training context. Learning and Motivation, 31(4), 416–431. [Google Scholar]
- Orsini CA, Kim JH, Knapska E, & Maren S (2011, November). Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci, 31(47), 17269–17277. 10.1523/JNEUROSCI.4095-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo P, Leao RM, Bianchi PC, de Oliveira PEC, Planeta CDS, & Cruz FC (2017). Inactivation of the Prelimbic Cortex Impairs the Context-Induced Reinstatement of Ethanol Seeking. Front Pharmacol, 8, 725. 10.3389/fphar.2017.00725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, & Smith Y (1993, December). The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience, 57(4), 1077–1090. 10.1016/0306-4522(93)90050-p [DOI] [PubMed] [Google Scholar]
- Pennington ZT, Anderson AS, & Fanselow MS (2017, 09). The ventromedial prefrontal cortex in a model of traumatic stress: fear inhibition or contextual processing? Learn Mem, 24(9), 400–406. 10.1101/lm.046110.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, & Kalivas PW (2008, June). Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci, 28(23), 6046–6053. 10.1523/JNEUROSCI.1045-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard CR, Mascagni F, & McDonald AJ (2012, March). Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience, 205, 112–124. 10.1016/j.neuroscience.2011.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, & Lebron K (2000, August). The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci, 20(16), 6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, & Kesner RP (1998, April). Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci, 112(2), 293–303. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, & Wagner AR (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Classical conditioning II: Current research and theory, 2, 64–99. [Google Scholar]
- Rhodes SE, & Killcross AS (2007, November). Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. Eur J Neurosci, 26(9), 2654–2660. 10.1111/j.1460-9568.2007.05855.x [DOI] [PubMed] [Google Scholar]
- Rhodes SE, & Killcross S (2004, 2004 Sep-Oct). Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem, 11(5), 611–616. 10.1101/lm.79704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz S, Puveendrakumaran P, Khan D, Yoon S, Hamel L, & Ito R (2019, March). Prelimbic and infralimbic cortical inactivations attenuate contextually driven discriminative responding for reward. Sci Rep, 9(1), 3982. 10.1038/s41598-019-40532-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, & LeDoux JE (1992, August). Bilateral destruction of neocortical and perirhinal projection targets of the acoustic thalamus does not disrupt auditory fear conditioning. Neurosci Lett, 142(2), 228–232. 10.1016/0304-3940(92)90379-l [DOI] [PubMed] [Google Scholar]
- Roughley S, & Killcross S (2019, February). Loss of Hierarchical Control by Occasion Setters Following Lesions of the Prelimbic and Infralimbic Medial Prefrontal Cortex in Rats. Brain Sci, 9(3). 10.3390/brainsci9030048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, & Bunney BS (1989, December). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol, 290(2), 213–242. 10.1002/cne.902900205 [DOI] [PubMed] [Google Scholar]
- Sharpe M, & Killcross S (2015, February). The prelimbic cortex uses contextual cues to modulate responding towards predictive stimuli during fear renewal. Neurobiol Learn Mem, 118, 20–29. 10.1016/j.nlm.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Sharpe MJ, & Killcross S (2014, April). The prelimbic cortex contributes to the down-regulation of attention toward redundant cues. Cereb Cortex, 24(4), 1066–1074. 10.1093/cercor/bhs393 [DOI] [PubMed] [Google Scholar]
- Sharpe MJ, & Killcross S (2015, June). The prelimbic cortex directs attention toward predictive cues during fear learning. Learn Mem, 22(6), 289–293. 10.1101/lm.038273.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Corcoran KA, Lebron-Milad K, & Quirk GJ (2006). Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. European Journal of Neuroscience, 24(6), 1751–1758. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, & Quirk GJ (2011, January). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology, 36(2), 529–538. 10.1038/npp.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel C, Marek R, Gooch HM, Sullivan RKP, & Sah P (2015, March). Prefrontal and Auditory Input to Intercalated Neurons of the Amygdala. Cell Rep, 10(9), 1435–1442. 10.1016/j.celrep.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Thomas BL, Larsen N, & Ayres JJ (2003). Role of context similarity in ABA, ABC, and AAB renewal paradigms: Implications for theories of renewal and for treating human phobias. Learning and Motivation, 34(4), 410–436. [Google Scholar]
- Trask S, Shipman ML, Green JT, & Bouton ME (2017). Inactivation of the prelimbic cortex attenuates context-dependent operant responding. Journal of Neuroscience, 37(9), 2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004, January). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51(1), 32–58. 10.1002/syn.10279 [DOI] [PubMed] [Google Scholar]
- Villaruel FR, Lacroix F, Sanio C, Sparks DW, Chapman CA, & Chaudhri N (2018, 12). Optogenetic Activation of the Infralimbic Cortex Suppresses the Return of Appetitive Pavlovian-Conditioned Responding Following Extinction. Cereb Cortex, 28(12), 4210–4221. 10.1093/cercor/bhx275 [DOI] [PubMed] [Google Scholar]
- Vogel EH, Ponce FP, & Wagner AR (2019). The development and present status of the SOP model of associative learning. Quarterly Journal of Experimental Psychology, 72(2), 346–374. [DOI] [PubMed] [Google Scholar]
- Wang N, Ge F, Cui C, Li Y, Sun X, Sun L, Wang X, Liu S, Zhang H, Liu Y, & Jia M (2018). Role of glutamatergic projections from the ventral CA1 to infralimbic cortex in context-induced reinstatement of heroin seeking. Neuropsychopharmacology, 43(6), 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook RF, Jones ML, Bailey GK, & Harris JA (2000). Contextual control over conditioned responding in a latent inhibition paradigm. Journal of Experimental Psychology: Animal Behavior Processes, 26(2), 157. [DOI] [PubMed] [Google Scholar]
- Willcocks AL, & McNally GP (2013, January). The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci, 37(2), 259–268. 10.1111/ejn.12031 [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, & Fanselow MS (2013, June). Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci U S A, 110(24), 9938–9943. 10.1073/pnas.1301691110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, & Fanselow MS (2014, June). Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci, 34(25), 8462–8466. 10.1523/JNEUROSCI.3624-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]


