ABSTRACT
Lactobacillus crispatus is a well-established probiotic with antimicrobial activity against pathogens across several niches of the human body generally attributed to the production of bacteriostatic molecules, including hydrogen peroxide and lactic acid. Here, we show that the cell-free supernatants of clinical isolates of L. crispatus harbor robust bactericidal activity. We further identify phenyl-lactic acid as a bactericidal compound with properties and a susceptibility range nearly identical to that of the cell-free supernatant. As such, we hypothesize that phenyl-lactic acid is a key active ingredient in L. crispatus supernatant.
IMPORTANCE Although Lactobacillus crispatus is an established commensal microbe frequently used in probiotics, its protective role in the bladder microbiome has not been clarified. We report here that some urinary isolates of L. crispatus exhibit bactericidal activity, primarily due to its ability to excrete phenyl-lactic acid into its environment. Both cell-free supernatants of L. crispatus isolates and phenyl-lactic acid exhibit bactericidal activity against a wide range of pathogens, including several that are resistant to multiple antibiotics.
KEYWORDS: Lactobacillus, microbiome, phenyl-lactic acid, crispatus, probiotics
INTRODUCTION
Lactobacilli have been well-established as commensal microbiota in multiple niches of the human body, including the gut, vagina, and, more recently, the bladder (1, 2). Lactobacilli are commonly seen as probiotics, live microbes that can be administered safely to improve recipient health outcomes. Multiple species, including Lactobacillus jensenii and Lactobacillus gasseri, have inhibitory activity against invading pathogens (3–6). Associated with “healthy” asymptomatic genitourinary tracts, Lactobacillus crispatus is believed to harbor the most robust protective ability among the lactobacilli (7–11).
The specific mechanisms underlying the protective activity of lactobacilli have only been partially explored, with the greatest emphasis attributed to the production of antimicrobials, such as hydrogen peroxide and organic acids, especially lactic acid (5, 12–14). Lactobacilli produce other antimicrobials, including proteinaceous products, such as psoriasin (6, 15). These proteinaceous antimicrobials are active against a limited range of uropathogens, but their potency against antimicrobial-resistant (AMR) pathogens remains untested. This gap in knowledge coincides with the rise of AMR uropathogens, an increasingly serious issue both in the bladder and beyond (16). Thus, we investigated Lactobacillus crispatus to better understand its protective nature in hopes of combating uropathogens.
In this study, we present findings that the cell-free supernatant (CFS) of a bladder isolate of Lactobacillus crispatus has robust bactericidal activity and identify phenyl-lactic acid (PLA) as a principle active component of the CFS. We characterize the properties of both CFS and purified PLA, demonstrating that pure PLA recapitulates the activity of the CFS. Finally, we show that they are active against a wide range of microbes, including AMR uropathogens and fungi, whereas they affect urinary lactobacilli in a bacteriostatic, rather than bactericidal, fashion.
RESULTS
Recognition that L. crispatus CFS exhibits novel bactericidal activity against Escherichia coli.
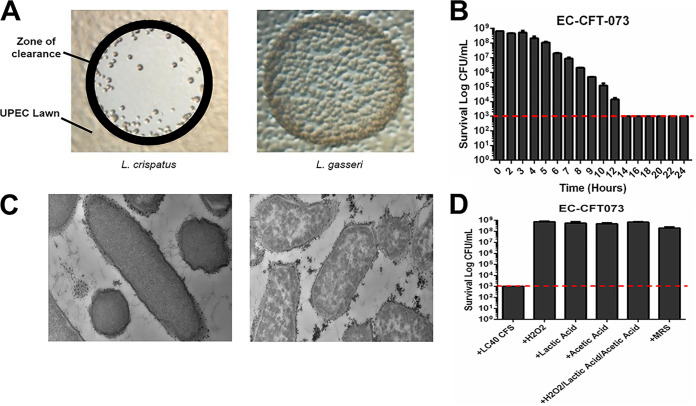
Over the course of multiple studies, we observed that L. crispatus and E. coli rarely cooccurred in transurethral catheter urine samples obtained from the bladders of adult women (cooccurrence correlation = −0.08), despite each species being otherwise very common (17). Based on this observation, we hypothesized that there may be an antagonistic relationship between these two microbial species. To test this hypothesis, we spotted cultures of L. crispatus urinary isolates onto a lawn of E. coli K-12 cells and found that many L. crispatus isolates produced a clear zone, whereas urinary isolates of other Lactobacillus species did not (Fig. 1A). We determined that the bactericidal activity was extracellular, as CFS of several L. crispatus urinary isolates reduced the CFU per milliliter of E. coli by many orders of magnitude (Fig. 1B) with a dramatic loss of cellular integrity (Fig. 1C). In contrast to previous research, the bactericidal activity was not due to lactic acid, acetic acid, hydrogen peroxide, or a combination of all three (Fig. 1D; see also Fig. S1 in the supplemental material).
FIG 1.
L. crispatus has robustly bactericidal CFS. (A) Lawns of UPEC strain NU14, grown overnight on TSA plates, were spotted with 10 μl of 48-h cultures of L. crispatus or L. gasseri and then photographed to compare zones of clearance. (B) CFS collected from 48-h L. crispatus culture was incubated in an equal volume of an overnight culture of UPEC strain CFT073 (normalized prior to an OD600 of 1.0 in tryptic soy broth); this mixture was incubated for the listed x axis time periods (e.g., 2 h, CFS and UPEC incubated together for 2 h), plated onto TSA plates, and incubated at 37°C/aerobic conditions for 24 h, and the CFU per milliliter was enumerated. The dotted line represents the limit of detection (1,000 CFU/ml) for the bactericidal activity assay. (C) Shown are 13,000× transmission electron microscopy (TEM) analyses comparing CFT073 E. coli that has been incubated with either MRS medium control (left) or L. crispatus CFS (right) for 24 h. (D) Physiological concentrations of common antimicrobials were quantified and added separately or in combination to CFT073 E. coli, and the impact on viability was assessed at 24 h compared to that of L. crispatus CFS and an MRS medium control. The dotted line represents the limit of detection (1,000 CFU/ml) for the bactericidal activity assay.
Identification of phenyl-lactic acid in CFS by analytical chemistry.
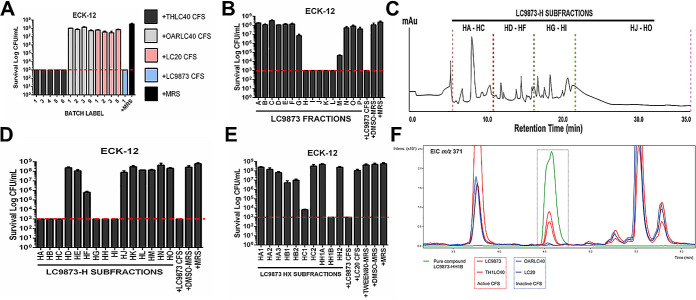
To identify the bactericidal component of CFS, we explored multiple independent cultures of bladder isolates of L. crispatus that either had bactericidal activity or lacked bactericidal activity. We collected CFS from these independent cultures and verified that (as expected based on prior culture) bactericidal activity was consistent across independent cultures of an isolate but that bactericidal activity varied between isolates (Fig. 2A). Given the variability among the isolates, we pursued comparative liquid chromatography-mass spectrometry (LC-MS)-based metabolomics of active and inactive CFS samples.
FIG 2.
Analytical chemistry of the bactericidal CFS of L. crispatus. (A) Prior to analytical chemistry, the bactericidal activities of multiple CFS batches from different L. crispatus isolates were tested against E. coli K-12. The dotted line represents the limit of detection (1,000 CFU/ml) for the bactericidal activity assay. (B) After ethyl acetate (EtOAc) treatment and chromatography of CFS of L. crispatus isolate LC9873, the resultant A to P fractions were tested for killing activity against E. coli K-12. The dotted line represents the limit of detection (1,000 CFU/ml) for the bactericidal activity assay. (C) The chemical diversity of LC9873-H subfractions generated via HPLC represented via chromatogram. (D) Following subfractionation via HPLC, LC9873-H subfractions were tested for killing against E. coli K-12, where they varied in bactericidal activity. The dotted line represents the limit of detection (1,000 CFU/ml) for the bactericidal activity assay. (E) Upon further LC9873-H fractionation and killing activity testing, “subfraction subsets” were identified and analyzed further. The dotted line represents the limit of detection (1,000 CFU/ml) for the bactericidal activity assay. (F) Comparative LC-MS of a specific LC9873 subfraction (HH1B) compared to that of CFS with strong killing (LC9873, THLC40) and weak killing (OARLC40, LC20). One specific spectrum feature corresponding to a compound with an m/z ratio of 371 (boxed) was present at very high concentrations in subfraction HH1B; it also was present at high concentrations in the strong bactericidal “active” CFS (red spectra, LC9873 and TH-LC40) relative to the weak killing “inactive” CFS (blue spectra, OARLC40 and LC20). This molecule was identified as a candidate bactericidal agent and the subfraction analyzed further.
Metabolomic profiles of two active and two inactive CFS samples led to the identification of several ions that were unique to active lactobacilli supernatants. In particular, six pseudomolecular ions at m/z 309, 371, 553, 659, 1,223, and 1,373 were unique to active lactobacilli, and one (m/z 371) was present in much higher concentrations in active versus inactive supernatants. To confirm that the observed bioactivity was possibly due to these compounds, we selected one isolate, LC9873, for further analysis and targeted metabolite isolation. To that end, LC-MS in conjunction with bioassay-guided isolation of LC9873-derived CFS was carried out.
Large-scale fermentation of LC9873 provided sufficient biomass to carry out bioactivity-guided fractionation efforts with the LC9873-derived CFS. CFS samples were extracted with ethyl acetate (EtOAc) followed by fractionation using Sephadex LH-20 size exclusion chromatography. We then tested fractions in a bactericidal activity assay against E. coli. Fractions A to P proved highly variable in activity against E. coli K-12, with fractions H to L demonstrating optimum activity on par with the intact nonfractionated CFS (Fig. 2B). High-pressure liquid chromatography (HPLC) fractionation of fraction H afforded subfractions HA to HO (Fig. 2C). Notably, the chromatogram revealed the presence in fraction H of several small molecules, though a clearly dominant species was evident within fractions HA to HC and HG to HI. Importantly, these fractions retained a high degree of activity against E. coli K-12 (Fig. 2D). Fractions HG to HI had similar LC profiles (dominant molecule with m/z ratio 371), and we combined them into fraction HH. After further subfractionation, we found multiple “subfraction subsets,” with LC9873-HH1B having the most robust killing activity (Fig. 2E). After we identified a specific LC-MS feature with m/z ratio 371 in high concentration in active supernatant samples and LC9873-HH1B subfraction relative to inactive supernatant samples (Fig. 2F), we performed targeted purification of the m/z 371 molecule via semiprep HPLC.
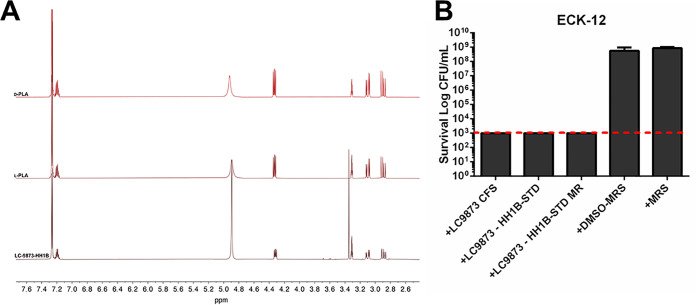
Through follow-up extraction ion chromatography (EIC) and comparative 1H nuclear magnetic resonance (NMR) analysis (Fig. 3A), we found that the properties of m/z 371 closely aligned with that of phenyl-lactic acid (PLA, m/z 371.2 [2M+K]+). PLA is an organic acid known to be secreted by lactobacilli, other Gram-positive microbes, and fungi, though it has been primarily studied in the food science field (18–20). It has been reported as a molecule with modest bacteriostatic and fungicidal activity, most effective in combating food spoilage (18, 20, 21). However, it has not been investigated in the context of bladder isolates from the urogenital microbiome.
FIG 3.
Phenyl-lactic acid (PLA) is a robust killing agent in the CFS of L. crispatus. (A) H-NMR spectroscopy comparing the profiles of LC9873-HH1B subfraction, PLA-L, and PLA-D. The peaks at δH 3.34 represent trace amounts of MeOH in the HH1B subfraction. The peak at δH 4.89 corresponds to water. (B) A chemical standard identical to LC9873-HH1B subfraction (and its methylated analogue MR) were solubilized in a 10% DMSO-90% MRS mixture and tested for killing activity against E. coli K-12. The dotted line represents the limit of detection (1,000 CFU/ml) for the bactericidal activity assay.
We solubilized a chemical analogue of m/z 371/phenyl-lactic acid (Sigma-Aldrich; 113069) and a methylated derivative (Sigma-Aldrich; 68193) in 10% dimethyl sulfoxide (DMSO)/90% MRS (de Man, Rogosa, and Sharpe) solution and tested for bactericidal activity against E. coli, against which it had robust activity (Fig. 3B). We conclude that PLA is a bactericidal component of L. crispatus CFS.
Characterization of properties and sensitivities of CFS and PLA.
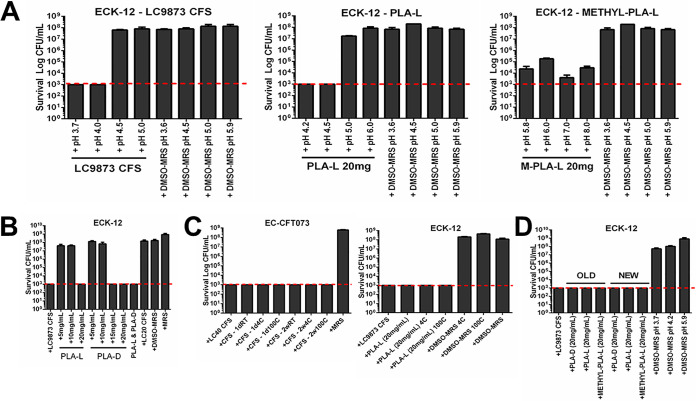
To determine how much of the bactericidal activity of CFS was due to PLA, we compared the following four major characterizations: pH sensitivity, concentration dependence, thermal stability, and temporal stability.
Because the pH of highly active CFS was consistently near pH 4, we assessed the pH sensitivity of LC9873 CFS (starting pH 3.7) and solutions of the L-enantiomer of PLA (PLA-L) at 20 mg/ml (starting pH 4.2) and a methylated PLA-L analogue at 20 mg/ml (starting pH 5.8). Through careful pH modification by dropwise addition of 1 M KOH or 1 M HCl, we modified the pH of each of the above solutions to investigate whether bactericidal activity was lost at higher pH. We found that LC9873 CFS and PLA-L retained bactericidal activity up to pH 4.0 and pH 4.5, respectively (Fig. 4, left and middle). Though bactericidal activity for CFS was lost at higher pH (7.0), reversion to a low pH (3.7) restores bactericidal activity (see Fig. S2 in the supplemental material). We hypothesized that bactericidal activity required protonation of PLA; we tested this hypothesis using a form of PLA-L with a methyl ester group at the protonation site (Fig. 4, right). We found that methylated PLA-L retained killing activity even at pH 8.0, which supported our hypothesis, though we note that the magnitude of activity was less than that of either LC9873 CFS or PLA-L (Fig. 4A, compare right to left and middle).
FIG 4.
PLA and CFS are highly similar in properties and sensitivities. (A) LC9873 CFS (starting pH 3.7), PLA-L (starting pH 4.2), and methyl-PLA-L (starting pH 5.8) were pH modified and tested for bactericidal activity against E. coli K-12, relative to pH-matched medium controls. MRS (de Man, Rogosa, and Sharpe) liquid medium is the medium control; it results in no reduction in E. coli survival. The dotted line represents the limit of detection (1,000 CFU/ml) for the bactericidal activity assay; this is also the case for subpanels B to D. (B) PLA-L and PLA-D solutions of various concentrations were made by dissolving purified stocks in 10% DMSO-90% MRS mixture and tested for killing against E. coli K-12. (C) LC9873 CFS (left) and PLA-L (right) were incubated for 1 h at either 4°C or 100°C and then stored on benchtop for 24 h or 2 weeks before being tested for killing activity against E. coli CFT073 or E. coli K-12. (D) PLA-L was stored on benchtop for 2 weeks (OLD) or 24 h (NEW) before being tested for killing activity against E. coli K-12.
To test concentration-dependent killing, we generated solutions of PLA at a range of concentrations from pure stocks solubilized in 10% DMSO-90% MRS. The concentration of PLA in CFS has been reported to vary significantly by species and even by strain, although 20 mg/ml has been found consistently in other species (18). Therefore, we assessed a range of concentrations, observing that PLA-L and PLA-D lacked activity at 10 mg/ml (Fig. 4B). This strongly suggests a concentration threshold that must be met for PLA to cause death, which aligns with the observation that dilution of L. crispatus CFS abrogates bactericidal activity (see Fig. S3 in the supplemental material).
To assess thermal stability, we incubated samples of each at 4°C, room temperature, or 100°C for 1 h before testing each sample for bactericidal activity against E. coli. Across all temperature conditions, both CFS and PLA retained robust killing activity (Fig. 4C). We conclude that both L. crispatus CFS and PLA are quite stable.
To test temporal stability, we incubated CFS and PLA solutions at room temperature on the benchtop for 14 days before testing alongside fresh CFS/PLA solutions. We found that extended incubation did not affect the bactericidal activity of either CFS or PLA, as both retained strong activity even after 14 days (Fig. 4D).
Overall, CFS and PLA exhibited extremely similar properties, suggesting that PLA suffices for most of the bactericidal activity of the CFS.
Determination of antimicrobial susceptibility range across physiologically relevant microbial categories.
We investigated the range of susceptibility across five major microbial categories as follows: Gram-positive uropathogens, Gram-negative uropathogens, antimicrobial-resistant (AMR) uropathogens, fungi, and two Lactobacillus species. To consistently assess susceptibility, we employed our standard bactericidal activity assay, though modified to accommodate differential growth condition preferences between microbes (e.g., longer incubation times and incubation in 5% CO2 versus incubation under ambient aerobic conditions).
The results of our susceptibility assessments are summarized in Table 1. We found that the Gram-positive uropathogens Corynebacterium amycolatum, Enterococcus faecalis, and group B Streptococcus are all highly susceptible to both L. crispatus CFS and PLA, with greater than 5-log-fold reductions in detectable CFU per milliliter relative to incubation with medium controls. We found similar patterns with the Gram-negative uropathogens Klebsiella pneumoniae and Pseudomonas aeruginosa with 5-log-fold or greater reductions in CFU per milliliter.
TABLE 1.
Species used in this study
| Species | Strain | Alias | Origin |
|---|---|---|---|
| Candida albicans | CA9885 | UMB9885 | Clinical isolate |
| Corynebacterium amycolatum | CA9950 | UMB9950 | Clinical isolate |
| Enterococcus faecalis | EF35 | N/A | AMR clinical isolate |
| Enterococcus faecalis | EF8812 | UMB8812 | Clinical isolate |
| Escherichia coli | ECK-12 | N/A | Domesticated lab strain |
| Escherichia coli | CFT073 | N/A | Clinical isolate |
| Escherichia coli | EC9875 | UMB9875 | Clinical isolate |
| Klebsiella pneumoniae | KP9987 | UMB9987 | Clinical isolate |
| Lactobacillus crispatus | LC9873 | UMB9873 | Clinical isolate |
| Lactobacillus crispatus | LC20 | UMB20 | Clinical isolate |
| Lactobacillus crispatus | LC40 | UMB40 | Clinical isolate |
| Lactobacillus gasseri | LG4205 | UMB4205 | Clinical isolate |
| Pseudomonas aeruginosa | PA9972 | UMB9972 | Clinical isolate |
| Pseudomonas aeruginosa | PA012 | N/A | AMR clinical isolate |
| Staphylococcus aureus | SA9671 | UMB9671 | Clinical isolate |
| Streptococcus agalactiae | SA9968 | UMB9968 | Clinical isolate |
Multiple AMR strains of E. faecalis and P. aeruginosa also were highly susceptible to L. crispatus CFS, more so than well-established antibiotics. AMR E. faecalis was comparatively less susceptible to PLA than CFS (and insensitive to methylated PLA) but still showed a 4-log-fold reduction in CFU per milliliter relative to pH-matched medium control. Finally, the fungal pathogen Candida albicans was highly susceptible to both L. crispatus CFS and PLA.
In contrast, CFS did not kill tested L. gasseri or L. crispatus isolates but rather inhibited their growth. This suggests that Lactobacillus species might possess some anti-PLA defense mechanism.
DISCUSSION
Here, we showed that CFS obtained from urinary isolates of L. crispatus can possess bactericidal activity, that the major bactericidal component is PLA, and that both CFS and PLA can kill a broad spectrum of bacterial species but not the tested Lactobacillus isolates.
Previously, PLA was reported to possess microcidal activity, primarily against fungal species in the context of combating food spoilage (21) but also E. coli and Listeria monocytogenes (22). It was also reported that other Lactobacillus species can produce PLA, but the tested species typically grow in plant environments (20). Others have reported that the organic acid PLA is pH sensitive and heat stable (23, 24). We corroborated these findings and found that PLA concentration is critical for its activity.
PLA was recently reported to possess bactericidal activity against the pathogens E. coli and L. monocytogenes (22). We have extended its range of susceptibility, finding that PLA’s activity extends across Gram-negative, Gram-positive, AMR, and fungal uropathogens. This suggests that PLA could be used to combat AMR pathogens, which are of increasing global concern (16).
Interestingly, we found that both CFS and PLA do not kill Lactobacillus species but rather only inhibit growth. This further highlights the potential for PLA as an antimicrobial agent, capable of killing harmful pathogens while leaving resident lactobacilli largely unharmed.
In conclusion, we have found that some human urinary isolates of L. crispatus produce PLA, a heat stable, pH-sensitive organic acid with robust bactericidal activity. We also found that this bactericidal activity extends to multiple classes of uropathogens, including several AMR strains.
MATERIALS AND METHODS
Strain selection and growth.
Clinical isolates of L. crispatus were selected from the Wolfe lab collection (Table 2) and struck onto Columbia nalidixic agar (CNA) plates. The plates were incubated at 37°C in 5% CO2 for 48 h, and bacterial growth was transferred to test tubes with de Man, Rogosa, and Sharpe (MRS) liquid medium. The liquid cultures were then incubated statically at 37°C in 5% CO2 for 48 h before supernatant collection and filtration as described below.
TABLE 2.
CFS and PLA susceptibility of the species used in this studya
| Species | Category | CFS susceptibility | PLA susceptibility |
|---|---|---|---|
| Corynebacterium amycolatum | Gram-positive uropathogen | +++ | +++ |
| Enterococcus faecalis | Gram-positive uropathogen | +++ | + |
| Staphylococcus aureus | Gram-positive uropathogen | +++ | +++ |
| Streptococcus agalactiae | Gram-positive uropathogen | +++ | +++ |
| Klebsiella pneumoniae | Gram-negative uropathogen | +++ | +++ |
| Pseudomonas aeruginosa | Gram-negative uropathogen | +++ | +++ |
| Enterococcus faecalis | AMR uropathogen strain | +++ | + |
| Pseudomonas aeruginosa | AMR uropathogen strain | +++ | +++ |
| Candida albicans | Fungal uropathogen | +++ | +++ |
| Lactobacillus gasseri | Commensal microbe | − | − |
| Lactobacillus crispatus | Commensal microbe | − | − |
+++, highly susceptible, with CFU below the level of detection; +, susceptible, with CFU above the level of detection; −, not susceptible, with CFU equivalent to medium control.
Clinical isolates of various uropathogens (E. coli, Klebsiella pneumoniae, Staphylococcus aureus, Enterococcus faecalis, and Candida albicans) were selected from the Wolfe lab collection (Table 2) and were struck onto sheep’s blood agar plates (BAP). The plates were incubated at 37°C in ambient aerobic conditions for 24 to 48 h, and bacterial growth was transferred to test tubes with tryptic soy broth (TSB) medium. The liquid cultures were then incubated in a shaking incubator at 37°C either in ambient aerobic conditions or in 5% CO2 for 24 h. AMR strains of Pseudomonas aeruginosa were kindly provided by the lab of Jonathan Allen and were cultured as described above.
Lawn spotting assay.
NU14, a uropathogenic strain of E. coli, was struck from frozen isolates onto BAP and incubated overnight at 37°C under ambient aerobic conditions before being transferred to TSB medium and incubated with aeration (225 rpm) at 37°C overnight. Liquid cultures were diluted to an optical density at 600 nm (OD600) of 1.0 in TSB liquid medium and then centrifuged at 16,000 × g for 2 min. The pellet was resuspended in 100 μl to produce a 10× concentrated culture; 50 μl was mixed with 700 μl fresh TSB medium and plated onto tryptic soy agar (TSA) plates and allowed to dry.
Lactobacillus species strains were struck from frozen isolates onto CNA plates and incubated at 37°C in 5% CO2 for 48 h; they were then transferred to MRS liquid medium and incubated at 37°C in 5% CO2 for 48 h. A 10× concentrated culture was made in the manner described above, except that we centrifuged at 1,500 × g, which we found preserves the viability of lactobacilli, which we found to be sensitive to larger relative centrifugal forces. Five microliters of the lactobacilli culture was spotted onto the dry uropathogenic Escherichia coli (UPEC) lawn, and the TSA plates were incubated for 24 h at 37°C in 5% CO2 before being imaged by light microscopy.
CFS collection and sterilization.
Forty-eight-hour liquid cultures of L. crispatus were centrifuged for 10 min at 1,500 × g. Using a serological pipette, the supernatant was collected and passed through a 0.22-μm filter for filter sterilization into a sterile 50-ml conical tube. The CFS was then stored covered at room temperature for future analysis.
Bactericidal activity assay.
For the bactericidal activity assay, a “target” species was grown as described above. The culture was then normalized to an OD600 of 0.1 in TSB medium in separate wells of a 96-well plate and incubated with an equal volume of L. crispatus CFS. Depending on the target species, the plate was then incubated at 37°C either in ambient aerobic conditions or in 5% CO2 for 24 to 72 h, either aerated (225 rpm) or held static.
After this incubation, the mixture of target culture and CFS was serially diluted 1:10 in 1× phosphate-buffered saline (PBS) from 10−1 to 10−8. Each dilution of each treatment condition was then plated in triplicate onto solid medium, which varied by target species; uropathogens were plated onto brain heart infusion (BHI) plates, and lactobacilli were plated onto MRS and CNA plates. The plates were incubated for 24 to 72 h either in aerobic conditions or in 5% CO2, and the CFU per milliliter was enumerated.
Antimicrobial concentration quantification.
Hydrogen peroxide (H2O2) in CFS with robust bactericidal activity was quantified via titration with potassium permanganate (KMNO4). KMNO4 (diluted in water and sulfuric acid) was added to CFS until the solution remained pink. The volume of KMNO4 solution required to reach this equivalence point was used to determine concentration, using the following equation: % H2O2 (by weight) = (ml KMnO4 × N × 0.01701 × 1,000)/(grams of H2O2 sample used). N is the normality of the standardized potassium permanganate. The concentrations of organic acids (lactic and acetic acid) in CFS were determined using commercially available Boehringer Mannheim kits (Boehringer Mannheim, Germany) utilizing UV-visible (UV-Vis) spectroscopy (25, 26).
Transmission electron microscopy.
CFT073, a uropathogenic strain of E. coli was struck from frozen isolates onto BAP and incubated overnight at 37°C under ambient aerobic conditions before being transferred to TSB medium and aerated (225 rpm) at 37°C under ambient aerobic conditions overnight. Liquid cultures were diluted to an OD600 of 1.0 in TSB medium before being aerated (225 rpm) at 37°C under ambient aerobic conditions with an equal volume of CFS prepared from the L. crispatus isolate LC40 or an MRS medium control. After 24 h, samples were submitted to the Loyola Microscopy Core (Loyola University Chicago) for epoxy resin infiltration, resin polymerization, sectioning, staining, and viewing.
CFS/PLA modification and characterization.
For pH sensitivity assessment, CFS collected from various L. crispatus isolates were checked for starting pH via pH meter. Then, 1 M HCl or 1 M NaOH was added dropwise (with the pH continuously checked via pH meter) until the desired pH was reached ±0.1 for pH meter natural error. Modified CFS were tested for bactericidal activity against E. coli via the bactericidal activity assay. For the pH reversion experiments, the supernatant was initially brought to a desired pH and incubated for 1 h at room temperature. The supernatant was then brought to a different pH and tested immediately for bactericidal activity against E. coli via the bactericidal activity assay. pH-modified MRS was used as a negative control. Untreated CFS with known bactericidal activity was used as a positive control.
For the thermal stability assessment, CFS from L. crispatus isolates were either held at 100°C for 1 h in a heat block before being stored at 25°C for specified times or stored at 4°C for specified times. Treated CFS was tested for bactericidal activity against E. coli via the bactericidal activity assay. Untreated MRS medium was used as a negative control. Untreated CFS with known bactericidal activity was used as a positive control.
For the temporal stability assessment, CFS from L. crispatus isolates was stored for 14 days (OLD) at room temperature on a benchtop in a covered container. After 14 days, CFS was collected from “fresh” 48-h L. crispatus cultures that had been started 2 days prior (FRESH). Both OLD and FRESH CFS were tested on the same day for bactericidal activity against E. coli via the killing activity assay. Untreated MRS medium was used as a negative control. Untreated supernatant with known bactericidal activity was used as a positive control.
For solutions of purified PLA, the pH, thermal, and temporal stability experiments were performed identically to those described for CFS. Because the purified PLA solutions included DMSO (used to solubilize the PLA), a solution of 10% DMSO-90% MRS was used as the negative control.
For concentration-dependent assessments of PLA activity, two experiments were performed. First, different concentrations of PLA were measured and solubilized into 10% DMSO-90% MRS solution and tested for bactericidal activity against E. coli via the bactericidal activity assay, with 10% DMSO-90% MRS medium as a negative control. Second, mixtures were generated containing varying amounts of either PLA enantiomer (PLA-L/PLA-D) in combination and tested for bactericidal activity against E. coli via the bactericidal activity assay, with 10% DMSO-90% MRS medium as a negative control.
LC-MS.
Ultra-high-performance liquid chromatography-high-resolution mass spectrometry (UHPLC-HRMS) was acquired using a Bruker maXis 4G electrospray ionization quadrupole time of flight (ESI-QTOF) mass spectrometer coupled with a Waters Acquity ultraperformance liquid chromatography (UPLC) system operated by Bruker HyStar software and a C18 column (Phenomenex; Kinetex 2.6 μm, 2.1 mm × 100 mm). Two active CFS samples (from L. crispatus isolates TH-LC40 and LC9873) and two inactive CFS samples (from LC20 and OAR-LC40) were subjected for LC-MS analysis. To prepare the samples for LC-MS, each supernatant sample (1.0 ml for each of the four isolates) was directly applied to an SPE column followed by washing with 1.0 ml of 10% aqueous MeOH (twice). Elution from SPE was achieved using 90% aqueous MeOH (1.0 ml), and the subsequent eluent was analyzed by LC-MS.
Fermentation and CFS extraction for fractionation.
L. crispatus isolate LC9873 was struck onto 15 CNA plates. The plates were incubated at 37°C in 5% CO2 for 48 h. A total of 1.0 ml MRS medium was added onto each plate to resuspend the cells. Aliquots (0.1 ml) of the cell suspension were transferred into 150 conical tubes (50 ml) with 40 ml MRS cultures. The liquid cultures (6.0 liters) were then incubated statically at 37°C in 5% CO2 for 72 h. The obtained liquid cultures of L. crispatus were centrifuged at 1,500 × g for 10 min. The supernatant was then subjected to liquid-liquid partitioning using ethyl acetate (EtOAc/CFS = 1:1). The obtained EtOAc portion was evaporated to dryness and stored at 4°C for further use.
CFS HPLC fractionation and compound purification.
The EtOAc-soluble partition (2.1 g) was dissolved in 5.0 ml of methanol (MeOH) and fractionated by Sephadex LH-20 column chromatography (column size, 500 × 40 mm, MeOH, 20 ml for each fraction). A total of 16 fractions were obtained. An active fraction (fraction H) was subjected to preparative reversed-phase high performance liquid chramotography (Prep-RP-HPLC) isolation (10%/90% to 100%/0% MeOH/H2O [with 0.1% acetic acid], 20 min, 20 ml/min) using a Phenomenex Gemini C18 column (250 × 30 mm). Fraction H was divided into 15 subfractions (subfractions HA to HO). Subfractions HG to HI showed the same HPLC profile; thus, HG and HI were combined into subfraction HH. Four active subfractions (HA to HC and HH) were further separated by semiprep HPLC (10%/90% to 100%/0% MeOH/H2O [with 0.1% acetic acid], 20 min, 4.0 ml/min for HA and HB; 20%/80% to 100%/0% MeOH/H2O [with 0.1% acetic acid], 20 min, 4.0 ml/min for HC and HH). The final active elutes (HC1 and HH1B) were combined and evaporated to dry for further use.
Structural elucidation of active elutes.
The structures of active elutes were elucidated based on high-resolution electrospray ionization mass spectrometry (HRESIMS) analysis, 1H NMR spectra analyses and 1H NMR spectra comparison with those of commercially available standards. HRESIMS data were acquired with a Bruker maXis 4G ESI-QTOF mass spectrometer. NMR spectra for protons and carbon were obtained in CD3OD (δH, 3.34 ppm; δC, 49.0 ppm, respectively) with a Bruker Avance 600 III MHz spectrometer.
ACKNOWLEDGMENTS
We thank Catherine Putonti for her advice and assistance. We thank Karen Visick, Francis Alonzo, and Jonathan Allen for providing both advice and several strains and reagents used in the above experiments.
We acknowledge our funding source as follows: NIH R01 DK104718 awarded to A.J.W. and NIH U19AI1427720 awarded to T.S.B.
Footnotes
Supplemental material is available online only.
Contributor Information
Alan J. Wolfe, Email: awolfe@luc.edu.
George O'Toole, Geisel School of Medicine at Dartmouth.
REFERENCES
- 1.Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, Kliethermes S, Schreckenberger PC, Brubaker L, Gai X, Wolfe AJ. 2014. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio 5:e01283-14. 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108:4680–4687. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atassi F, Servin AL. 2010. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol Lett 304:29–38. 10.1111/j.1574-6968.2009.01887.x. [DOI] [PubMed] [Google Scholar]
- 4.Atassi F, Pho Viet Ahn DL, Lievin-Le Moal V. 2019. Diverse expression of antimicrobial activities against bacterial vaginosis and urinary tract infection pathogens by cervicovaginal microbiota strains of Lactobacillus gasseri and Lactobacillus crispatus. Front Microbiol 10:2900. 10.3389/fmicb.2019.02900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hütt P, Lapp E, Štšepetova J, Smidt I, Taelma H, Borovkova N, Oopkaup H, Ahelik A, Rööp T, Hoidmets D, Samuel K, Salumets A, Mändar R. 2016. Characterisation of probiotic properties in human vaginal lactobacilli strains. Microb Ecol Heal Dis 27:30484. 10.3402/mehd.v27.30484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalyoussef S, Nieves E, Dinerman E, Carpenter C, Shankar V, Oh J, Burd B, Angeletti RH, Buckheit KW, Fredricks DN, Madan RP, Keller MJ, Herold BC. 2012. Lactobacillus proteins are associated with the bactericidal activity against E. coli of female genital tract secretions. PLoS One 7:e49506. 10.1371/journal.pone.0049506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceccarani C, Foschi C, Parolin C, D’Antuono A, Gaspari V, Consolandi C, Laghi L, Camboni T, Vitali B, Severgnini M, Marangoni A. 2019. Diversity of vaginal microbiome and metabolome during genital infections. Sci Rep 9:14095. 10.1038/s41598-019-50410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Hong Z, Wang W, Gu L, Gao H, Qiu L, Di W. 2019. Association between the vaginal microbiome and high-risk human papillomavirus infection in pregnant Chinese women. BMC Infect Dis 19:677. 10.1186/s12879-019-4279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghartey JP, Smith BC, Chen Z, Buckley N, Lo Y, Ratner AJ, Herold BC, Burk RD. 2014. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PLoS One 9:e96659. 10.1371/journal.pone.0096659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. 2009. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 9:116. 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler DSC, Silvestroni A, Stapleton AE. 2016. Cytoprotective effect of Lactobacillus crispatus CTV-05 against uropathogenic E. coli. Pathogens 5:27. 10.3390/pathogens5010027. [DOI] [Google Scholar]
- 12.Valore EV, Park CH, Igreti SL, Ganz T. 2002. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol 187:561–568. 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 13.Cadieux PA, Burton JP, Devillard E, Reid G. 2009. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J Physiol Pharmacol 60:13–18. [PubMed] [Google Scholar]
- 14.Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. 2001. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 185:375–379. 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- 15.Mildner M, Stichenwirth M, Abtin A, Eckhart L, Sam C, Gläser R, Schröder J-M, Gmeiner R, Mlitz V, Pammer J, Geusau A, Tschachler E. 2010. Psoriasin (S100A7) is a major Escherichia coli-cidal factor of the female genital tract. Mucosal Immunol 3:602–609. 10.1038/mi.2010.37. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 17.Price TK. 2015. The contribution of the female urinary microbiota to lower urinary tract symptoms. MSc Thesis, Loyola University, Chicago, IL. [Google Scholar]
- 18.Dieuleveux V, Van Der Pyl D, Chataud J, Gueguen M. 1998. Purification and characterization of anti-Listeria compounds produced by Geotrichum candidum. Appl Environ Microbiol 64:800–803. 10.1128/AEM.64.2.800-803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohhira I, Kuwaki S, Morita H, Suzuki T, Tomita S, Hisamatsu S, Sonoki S, Shinoda S. 2004. Identification of 3-phenyllactic acid as a possible antibacterial substance produced by Enterococcus faecalis TH 10. Biocontrol Sci 9:77–81. 10.4265/bio.9.77. [DOI] [Google Scholar]
- 20.Ström K, Sjögren J, Broberg A, Schnürer J. 2002. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl Environ Microbiol 68:4322–4327. 10.1128/AEM.68.9.4322-4327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svanström Å, Boveri S, Boström E, Melin P. 2013. The lactic acid bacteria metabolite phenyllactic acid inhibits both radial growth and sporulation of filamentous fungi. BMC Res Notes 6:464. 10.1186/1756-0500-6-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ning Y, Yan A, Yang K, Wang Z, Li X, Jia Y. 2017. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem 228:533–540. 10.1016/j.foodchem.2017.01.112. [DOI] [PubMed] [Google Scholar]
- 23.Lavermicocca P, Valerio F, Visconti A. 2003. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl Environ Microbiol 69:634–640. 10.1128/AEM.69.1.634-640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita T, Nguyen HD, Ito T, Zhou S, Osada L, Tateyama S, Kaneko T, Takaya N. 2013. Microbial monomers custom-synthesized to build true bio-derived aromatic polymers. Appl Microbiol Biotechnol 97:8887–8894. 10.1007/s00253-013-5078-4. [DOI] [PubMed] [Google Scholar]
- 25.Food and Feed Analysis. 2020. UV-method of determination of acetic acid in foodstuffs and other materials. Article number 10148261035. Boehringer Mannheim/R-Biopharm, Darmstadt, Germany. [Google Scholar]
- 26.Food and Feed Analysis. 2020. UV-method for the determination of L-lactic acid in foodstuffs and other materials. Article number 10139084035, Boehringer Mannheim/R-Biopharm, Darmstadt, Germany. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4. Download JB.00360-21-s0001.pdf, PDF file, 0.6 MB (610.5KB, pdf)