Abstract
Metagenomic analyses have revealed microbial dysbiosis in the gut of patients with colorectal cancer (CRC). The gut microbiota influences CRC via a variety of mechanisms, including microbial-derived factors such as metabolites or genotoxins. Pathogenic drivers and opportunistic passenger bacteria may underlie direct effect of the gut microbiota on carcinogenesis. We posit that metabolites generated by gut microbiota can influence CRC through a multitude of epigenetic or genetic effects on malignant transformation. A closer look at the cross talks between the commensals, epithelial cells, immune regulators etc., needs to be established with more substantiated studies. The recurrence of chemoresistant disease following therapy undoubtedly provides the impetus for morbidity and mortality; yet, the role of gut microbiome in drug resistance remains to be fully investigated. We review the current literature on microbial dysbiosis during CRC and discuss the mechanistic basis of CRC-associated bacteria in tumor initiation, progression and drug resistance.
Keywords: Gut Microbiome, Colorectal Cancer, Tumor Microbiome, Chemoresistance, FMT
Colorectal Cancer and Gut Microbiome
One of the leading causes of mortality and morbidity due to cancer, across the globe is colorectal cancer (CRC). It is the third most prevalent form of cancer and fourth most common cause of cancer deaths worldwide. The vast majority of CRC cases are sporadic (~70%) and non-inherited. Inherited predisposition syndromes including Lynch syndrome or familial adenomatous polyposis (FAP) account for about 5% of the new CRC diagnosis. 20–30% of the cases have a familial disposition with no associated or known germline mutation [1]. The disease originates from the epithelial cells that accumulate mutations and acquire malignant properties over time. In addition to genetic factors, factors such as diet, smoking, obesity, diabetes, alcohol consumption, and exposure to carcinogens increase the risk for developing sporadic CRC.
Human microbiome are complex ecosystems involving bacteria, viruses, archaea, or eukaryotes that are co-evolving in an environment subject to various selective pressures, such as antibiotic administration, diet and/or lifestyle. An optimal “balance” in the microbial population (termed “eubiosis”), is essential for the sustenance as well as maintenance of good health in humans. In the gut, such a complex bacterial community obtains energy from indigestible dietary components, provides accessory growth factors, protects against colonization by pathogens, provides post-natal maturation of mucosal structures and educates the immune system. The colon and ileocecal valve that exhibit the highest bacterial density along the gastrointestinal tract point towards an important role for the microbiota in CRC. While efforts are underway to specifically link microbial dysbiosis to consensus molecular subtypes (CMS), several bacterial species with causal and/or complicit roles in CRC have been identified [2*]. Studies have also revealed that during malignant transformation, loss of gut vascular barrier can facilitate dissemination of intestinal bacteria to the liver [3**] thereby suggesting that targeting tumor microbiome can be a viable strategy to target cancer progression and metastasis. It can therefore be gleaned from recent explosion of information on tumor microbiome that components of CRC including progression, treatment and even resistance to chemotherapy [4] and clinical outcomes can all be impacted by bacterial dysbiosis [5**].
Association vs. Causation – possible mechanisms implicating gut microbiome in CRC
Despite a plethora of studies linking changes in gut microbiome to CRC, the scientific community is still debating as to whether gut microbes should be considered causal, co-varying, or a necessary but not sufficient agent in CRC development. In 2011, Sears and Pardoll [6] proposed an ‘alpha-bug’ hypothesis wherein, species such as Bacteroides fragilis exert a central pro-oncogenic, enterotoxigenic role, thereby contributing to the onset of CRC. Later, Tjalsma H et al. [7] provided further credence to this concept by proposing the driver-passenger model in 2012 wherein, driver bacteria (e.g., B. fragilis) lead to a multistep development of colorectal tumorigenesis including inflammation, increased cellular proliferation, and/or the production of genotoxins. CRC with hypermutable microsatellite instability (MSI)-high and CpG island methylator phenotype (CIMP)-high phenotypes, as well as CRC with BRAF mutation, may alter the potential pathogenic influence of intestinal microbiota along the proximal-distal axis. To support this, it was shown by Dejea et al. that proximal but not distal colons consistently exhibit biofilm [8*] and interestingly, these biofilms invade the colonic crypts [9] consisting mainly of known CRC-associated bacteria [10]. Finally, Purcell et al. [11], showed that these bacteria are associated with the consensus molecular subtype (CMS) 1, which is called MSI immune subtype, marked by MSI, CIMP-high, BRAF mutations, and immune cell infiltration [11]. Thus, to evaluate their distinct features, it is important to consider microbiomes from proximal and distal colon cancer separately. Studies have also suggested different set of risk factors for these consensus molecular subtypes. Along these lines, smoking has been linked to proximal and rectal cancer but not distal colon cancer, while physical activity was inversely correlated to proximal and distal colon cancer, but not rectal [12–14]. Height and gender are also reported to impact the type of cancer, for example females are more prone to the proximal colon than distal cancer, compared to men, while greater height is correlated with higher risk for distal colon and rectal cancer, respectively [15]. Chronic inflammation resulting in polyps at the bowel lining has been linked with most of the CRC cases [16].
Propagation of chronic inflammation by genotoxins [17*] and altered signalling pathways [18] have been suggested as probable mechanisms of carcinogenesis in liu of gut dysbiosis. Other mechanisms include release of metabolites with context-dependent tumorigenic effects (Figure 1). For example, the production of secondary bile acids may alter immune function and influence tumour growth in the context of hepatobiliary cancer [19,20]; or levels of circulating oestrogens may be altered in the context of breast cancer [21, 22]. Studies have also shown that gut microbiota can promote colon cancer either through 8-oxoguanine-induced lesions in mismatch repair-deficient colons [23] or colibactin-induced DNA adduct formation [24]. In an elegant study, Kadosh et al. [25**] recently demonstrated that the gut microbiome switches mutant p53 from tumour-suppressive into an oncogenic protein by promoting the Tcf4-chromatin interaction and the hyperactivation of Wnt, thus conferring a malignant phenotype to the organoids and throughout the gut (Figure 1). Beyond the resident microbiota of the gut, existence of microbiota within the tumour microenvironment (TME) has been observed in tumours proximal and distal from the resident microbiota. The explanation for the existence of intratumoral bacteria however, is unclear although some studies suggest the role of altered mucosal integrity of microbiota-resident systems such as the GI tract and preferential homing of bacteria for the TME due to the rich vasculature and nutrients [26, 27]. Nevertheless, tumour microbiota may have an important role in the context of cancer treatment, including resistance to chemotherapy [28] and altered clinical outcomes [29].
Figure 1. Influence of gut microbiome on cellular transformation and metastasis.
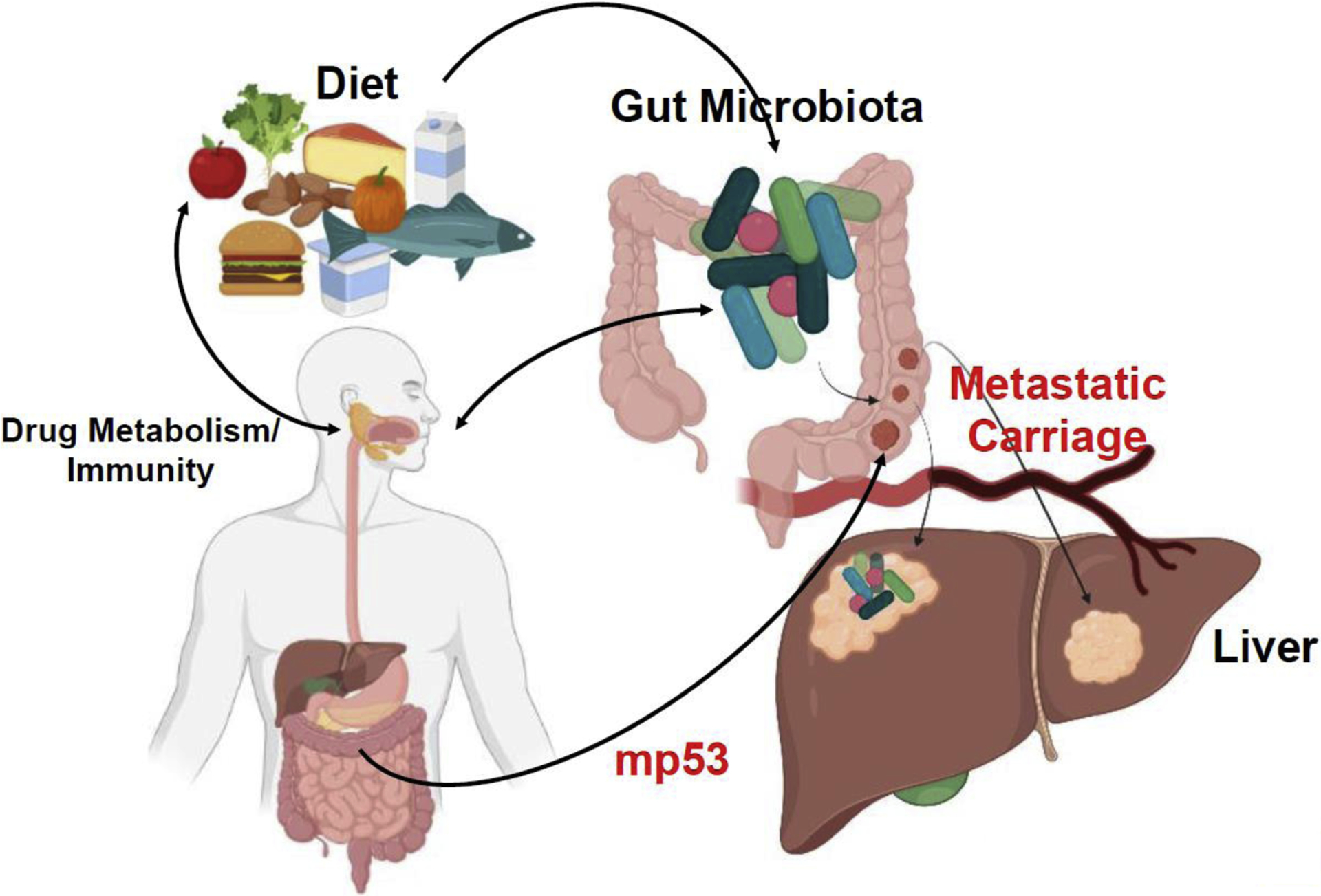
In a prototypic example, certain components of a Western diet can influence gut microbiota and generation of metabolites through altered metabolism and an exaggerated immune response. A combination of these changes may lead to cellular transformation and neoplasia. The onset of a malignant disease can be further influenced by disruption of gut vascular barrier leading to metastatic spread. The gut microbiome has also been recently shown to promote switching of mutant p53 (mp53) from tumor-suppressive into an oncogenic protein to confer a malignant phenotype throughout the gut.
Evidence towards a functional role of the gut microbiota in tumour development
In CRC, alterations in both tumor microbiome and those of the adjacent mucosa have been suggested as important determinants of disease progression [3**]. A combination of intrinsic as well as environmental factors including medication, diet, smoking etc., have been shown to affect the gut microbiome wherein, changes in species such as Fusobacterium nucleatum, Escherichia coli, and Bacteroides fragilis are directly implicated in disease occurrence in preclinical studies [3**]. Here, we briefly review the potential mechanisms through which these bacteria can influence CRC development and progression.
A. Fusobacterium nucleatum:
F. nucleatum has been linked to human diseases including periodontal diseases, pregnancy disorders, rheumatoid arthritis, respiratory tract infections and cancer. Fusobacterium appears to be an important component of the TME in CRC. A study in Chinese population with CRC revealed the enrichment of F. nucleatum in colon tissues [30, 31]. F. nucleatum mediates carcinogenesis through virulence factors that upregulate the expression of inflammatory genes and downregulate acquired immunity mediated by T cells [32]. FadA antigen, from F. nucleatum binds to the extracellular domain of E-cadherin to stimulate tumor proliferation through the Wnt signaling pathway [33]. Rubinstein and team [33] used FadA knock-out mutants to prove the effect of adhesion, and the β-catenin signaling cascades. Another mechanism involves suppression of anti-tumor immune response by expansion of FOXP3 non-Treg cells [34]. F. nucleatum was also correlated to the enhanced expression of another cell-adhesion protein, Fap2, a lactose-binding inhibitor that defends tumors from host immune attack, in the CRC patients [35].
B. Enterotoxigenic Bacteroides fragilis (ETBF):
ETBF produces toxin B. fragilis toxin (BFT), an established risk factor for CRC, and is more closely related to advanced stage of CRC [36]. BFT induces cancer proliferation by triggering pathways like nuclear Wnt/β-catenin and NF-κB signaling during CRC induced colitis and colonic tumors in ApcMin/+ mice [37**]. BFT-mediated pathogenesis also increases gut permeability, which enhances translocation of microbial products [37**]. Research team led by Purcell et al. [11], advocates the use of ETBF as a potential marker for early diagnosis of CRC.
C. Escherichia coli:
Compared to healthy individuals, E. coli was present in abundance in CRC patients. Prevalence of this strain is involved in the initiation and development of CRC [38]. The colon cancer associated E. coli takes over the regular p38 MAPK signaling pathway, which regulates autophagy [38]. Another E coli (pks+) strain with the polyketide synthase gene complex, mediates carcinogenesis by production of colibactin that promotes activation of senescence-associated secretory phenotype in malignant or premalignant epithelial cells that is associated with secretion of various growth factors, cytokines, chemokines, and enzymes [39, 40]. Some of these factors could be protective by contributing to immune-mediated tumor control, but prolonged senescence-associated secretory phenotype could cause immunosuppression [41]. In addition, enzymes secreted by senescent tumor cells, such as matrix metalloproteinases, could promote tumor invasion and metastasis [42*]. Factors like COX-2/PGE2 from E. coli secretome create a conducive TME for self-propagation. E. coli invades the intestinal epithelial cells through adhesin Afa and Eae, to activate similar pathways which further enhance proliferative activity [43**].
D. Streptococcus gallolyticus:
S. gallolyticus triggers carcinogenesis through invasion in premalignant lesions and uncontrolled proliferation [44]. Increased cell proliferation was observed on introduction of S. gallolyticus to cell line [45], which was attributed to increase in nuclear β-catenin. The increased cell proliferation and elevated β-catenin signaling was observed in in vitro cultured cells, in xenografts, and in colonic crypt cells in the mouse model. Another study posited over expression of COX2 and inflammation coupled with prevention of apoptosis [43]. Recruitment of tumor-infiltrating immune cells was reported, on exposure to S. gallolyticus, leading to suppression of immune system and inducing neoplasia [46].
E. Campylobacter species.
Some invasive Campylobacter species have been suggested to contribute to tumor progression by inducing a pro-inflammatory response driven by IL-18 [47].
Microbial Effects on Drug Metabolism and Chemoresistance
The growing relevance of the gut microbiota to various human disease may also directly impinge on the efficacy of chemotherapeutics. Array of anti-cancer drugs and combinations, primarily targeting the cellular signaling pathways involved in tumor survival and growth, have demonstrated increased survival in CRC patients. 5-fluorouracil (5-FU)- remains the mainstay of therapy for patients with CRC. 5-FU is a pyrimidine analog used as anti-neoplastic agent to induce cell death [48]. Platinum, leucovorin, oxaliplatin, irinotecan or monoclonal antibodies (as monotherapy or combinatorial therapy) have received wide application for CRC patients [49, 50]. In 1996, irinotecan (CPT-11), a semi-synthetic camptothecin derivative that selectively inhibits topoisomerase I (Topo I), was approved by the FDA for CRC treatment. CPT-11 forms a topoisomerase-inhibitor-DNA complex hampering the DNA function. Cyclophosphamide mediates its antitumor activity by stimulating the antitumor immune response, and controls tumor growth by inducing immunogenic cell death, destroying immunosuppressive T cells, or promoting Th1 and Th17 cells [51**]. Diaminocyclohexane (DACH) together with its platinum compound causes DNA to be less prone to the repair mechanisms, thus detrimental to the tumor cells. Capecitabine is the oral anti-metabolite first-line therapy for patients with metastatic CRC. Bevacizumab is a humanized mAb against vascular endothelial growth factor receptor (VEGFR-A) [52, 53]. In combination with FOLFOX, FOLFIRI, XELOX, and XELXIRI, bevacizumab has been found to increase the tumor control rate [53]. With the increased usage however, patients develop resistance with nearly half of them resistant to 5-FU-based chemotherapies [54]. TP converts capecitabine to 5-FU and is the driver of its chemoresistance. Oxaliplatin chemoresistance is caused by multiple mechanisms including nucleotide excision repair and WBSCR22 protein [55].
The gut microbiome, through its ability to metabolize drugs can have implications on drug activity/efficacy based on increased or decreased toxicity. Gut bacteria harbor enzymes and pump out other molecules that can influence how medications are activated or broken down. A growing body of evidence supports the impact of gut microbiota on development of chemoresistance to some of the most frequently used anticancer drugs like oxaliplatin, cyclophosphamide, irinotecan, cyclophosphamide anthracyclines etc., through mechanisms such as microbial translocation, immunomodulation, metabolism, enzymatic degradation and reduced ecological diversity [54] (Figure 1). It is suggested that antibiotic treatment might induce dysbiosis which further leads to prevalence of Staphylococcus and Clostridium coupled with depletion of Bacteroides and Lactobacillus, as observed in CRC patients displaying chemoresistance [55]. A recent study discovered that subcutaneous tumors fail to respond to immunotherapy and platinum chemotherapy after antibiotic treatment, whereas another study reported that the effect of Cyclophosphamide on the anti-tumor immune response relies on the presence of a healthy gut microbiota [51**]. Thus, both studies clearly suggest that microbial disturbance through the use of antibiotics severely compromises efficacy of both immunostimulatory (CpG, Cyclophosphamide) and platinum-based chemotherapeutics. F. nucleatum adopts the strategy of increased autophagy to develop chemoresistance against 5-FU and oxaliplatin, by targeting the immune receptors TLR-4 & MYD88 and specific mRNAs [4**]. Patients harboring F. nucleatum in abundance are more susceptible to disease relapse due to chemotherapy failure. E. coli and Clostridium species are known to metabolize Irinotecan increasing its toxicity and thus the drug efficacy is reduced, eventually leading to chemoresistance. In an elegant study, Geller et al. [26] reported that gemcitabine resistance could be caused by intra-tumoral bacteria thereby suggesting that gemcitabine-ciprofloxacin therapy is a viable option to enhance chemotherapeutic efficacy.
Microbe-based approaches to counter drug resistance
A new frontier targeting bacterial dysbiosis has emerged in our efforts to prevent or treat CRC. The aim is to achieve the “optimally balanced flora” to alter the community which further influences the metabolite production and modulatory functions. Fecal microbiota transplantation (FMT) for recurrent C. difficile infection for example, has been utilized to improve bacterial, viral, fungal or archaeal diversity into the host receiving the transplant. Additionally, several clinical trials based on FMT are underway for a myriad of diseases ranging from inflammatory bowel diseases to cancer to psychiatric conditions. FMT however, is not a one-size-fits-all strategy and is subject to safety concerns particularly in the setting of current pandemic. Beyond undefined fecal microbiota transplants, targeted formulations such as probiotics with live microorganisms are being developed as therapeutic agents with defined clinical benefit. These next generation probiotics include microorganisms that have been modified genetically but are still within the regulatory guidelines. Probiotics not only produce anti-inflammatory factors to extend the immune stimulating function, but also secrete antioxidant, anti-cancer compounds, and short chain fatty acids to improve intestinal barrier function. In the context of CRC, Lactobacillus casei variety rhamnosus (Lcr35) prevented FOLFOX-induced intestinal mucositis in CRC-bearing mice [57].
More recently, scientists have turned to compounds produced by microorganisms, released from food components or microbial constituents, including non-viable cells - also called postbiotics that have a potential to promote health and well-being when administered in adequate amounts. Along these lines, heat-inactivated Akkermansia muciniphila was observed to alleviate features of metabolic syndrome in overweight and obese subjects [58]. Similarly, diet, pre- and postbiotics are equally relevant in either improving the gut microbiota or reversing the microbial dysbiosis. Indeed, diet in addition to medication, can shift the the microbial profile within 24 hours and can have long term effects as well. Prebiotics or dietary fibers when combined with live beneficial microbes have the ability to alter the patient’s microbiota into favorable microbiome akin to FMT [59]. In near future, targeted removal of early-stage carcinogenic members of the gut microbial community, by employing bacteriophages are proposed to reduce the risk factors for CRC [59]. Moreover, profiling the oral microbiome may offer an attractive screening method to detect CRC [60, 61].
Conclusion.
Based on elegant studies focusing on gut microbiome, it can be inferred that gut microbiome can impact CRC and other cancers at distant sites through immune system modulation. Microbial species have been identified in many tissue types with the advent of bacterial RNA sequencing including breast, lung, ovary, pancreas, melanoma, bone, and brain tumors etc., [62, 63**]. The hypoxic tumor microenvironment can facilitate the growth of anaerobic and facultative anaerobic bacteria such as Clostridia [64] while necrotic areas of the tumor can release chemotactic compounds to promote bacterial invasion [64]. The leaky vasculature of cancerous tissues with either absence or low abundance of immune cells, may allow bacteria to enter and grow [64] (Figure 1). Along this line, intra-tumoral administration of non-pathogenic bacteria has been suggested as a mechanism of direct drug or therapeutic delivery [65, 66]. At the same however, intra-tumoral bacteria can also drive resistance to anti-cancer drugs [28]. Thus, a holistic approach is needed to develop mechanistic insights into how the microbiome can be manipulated to augment microbe-based therapies in cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- [1].Jasperson KW, Tuohy TM, Neklason DW, Burt RW., 2010. Hereditary and familial colon cancer. Gastroenterology, 138(6): 2044–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2] *.Purcell RV, Visnovska M, Biggs PJ, Schmeier S, Frizelle FA., 2017. Distinct gut microbiome patterns associate with consensus molecular subtypes of colorectal cancer. Scientific Report, 7(1): 11590. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this elegant article, the authors discuss recent insights into individual bacterial species associated with consensus molecular subtypes.
- [3] **.Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, Gandini S, Lizier M, Braga D, Asnicar F, Segata N, Klaver C, Brescia P, Rossi E, Anselmo A, Guglietta S, Maroli A, Spaggiari P, Tarazona N, Cervantes A, Marsoni S, Lazzari L, Jodice MG, Luise C, Erreni M, Pece S, Di Fiore PP, Viale G, Spinelli A, Pozzi C, Penna G, Rescigno M. 2021. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell, 39(5):708–724. [DOI] [PubMed] [Google Scholar]; In this elegant article, the authors discuss how impairment in gut vascular barrier disruption can be a prelude to liver metastasis.
- [4] **.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY, 2017. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell, 170(3): 548–563. [DOI] [PMC free article] [PubMed] [Google Scholar]; This elegant article describes the contribution of gut microbiota to chemoresistance in patients with colorectal cancer and provide a mechanism of how Fusobacterium nucleatum promotes chemoresistance by modulating autophagy.
- [5] **.Wargo JA, 2020. Modulating gut microbes. Science, 369(6509), pp.1302–1303. [DOI] [PubMed] [Google Scholar]; By focusing on recent discoveries, the author beautifully laid out the how strategies such as FMT, live biotherapeutics, dietary interventions as well as prebiotics can help in precision medicine.
- [6].Sears CL and Pardoll DM, 2011. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. Journal of Infectious Diseases, 203(3), pp.306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tjalsma H and Boleij A, 2012. Intestinal Host-Microbiome Interactions. Colorectal Cancer Biology-From Genes to Tumor. Shanghai: InTech, pp.391–410. [Google Scholar]
- [8] *.Dejea CM, Wick EC, Hechenbleikner EM, White JR, Welch JLM, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M and Stein E, 2014. Microbiota organization is a distinct feature of proximal colorectal cancers. Proceedings of the National Academy of Sciences, 111(51), pp.18321–18326. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this elegant article, the authors describe presence of invasive polymicrobial bacterial biofilms predominantly on right-sided tumors and suggests that Colon mucosal biofilm detection may predict increased risk for development of sporadic CRC.
- [9].Raskov H, Bjarnsholt T, Alamili M, Kragh KN and Gögenur I, 2018. Interaction between microbiota and immune system in colorectal cancer. Ugeskrift for laeger, 180(45). [PubMed] [Google Scholar]
- [10].Ternes D, Karta J, Tsenkova M, Wilmes P, Haan S and Letellier E, 2020. Microbiome in colorectal cancer: how to get from meta-omics to mechanism? Trends in Microbiology, 28(5), pp.401–423. [DOI] [PubMed] [Google Scholar]
- [11].Purcell RV, Pearson J, Aitchison A, Dixon L, Frizelle FA and Keenan JI, 2017. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PloS one, 12(2), p.e0171602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi REM and Corcione F, 2016. Worldwide burden of colorectal cancer: a review. Updates in surgery, 68(1), pp.7–11. [DOI] [PubMed] [Google Scholar]
- [13].Murphy C, Rettedal E, Lehouritis P, Devoy C and Tangney M, 2017. Intratumoural production of TNFα by bacteria mediates cancer therapy. PloS one, 12(6), p.e0180034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arnold CE, Isaiah A, Pilla R, Lidbury J, Coverdale JS, Callaway TR, Lawhon SD, Steiner J and Suchodolski JS, 2020. The cecal and fecal microbiomes and metabolomes of horses before and after metronidazole administration. Plos one, 15(5), p.e0232905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shin A, Joo J, Bak J, Yang HR, Kim J, Park S and Nam BH, 2011. Site-specific risk factors for colorectal cancer in a Korean population. PloS one, 6(8), p.e23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tanaka T, 2009. Colorectal carcinogenesis: Review of human and experimental animal studies. Journal of carcinogenesis, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17] *.Greten FR and Grivennikov SI, 2019. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity, 51(1), pp.27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an elegant overview of origins of inflammation in tumours and the mechanisms whereby inflammation drives tumour initiation, growth, progression, and metastasis.
- [18].Terzić J, Grivennikov S, Karin E and Karin M, 2010. Inflammation and colon cancer. Gastroenterology, 138(6), pp.2101–2114. [DOI] [PubMed] [Google Scholar]
- [19].Ocvirk S and O’Keefe SJ, 2017. Influence of bile acids on colorectal cancer risk: potential mechanisms mediated by diet-gut microbiota interactions. Current nutrition reports, 6(4), pp.315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jia W, Xie G and Jia W, 2018. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nature reviews Gastroenterology & hepatology, 15(2), p.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Samavat H and Kurzer MS, 2015. Estrogen metabolism and breast cancer. Cancer letters, 356(2), pp.231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Elliott MJ, Ennis M, Pritchard KI, Townsley C, Warr D, Elser C, Amir E, Bedard PL, Rao L, Stambolic V and Sridhar S, 2020. Association between BMI, vitamin D, and estrogen levels in postmenopausal women using adjuvant letrozole: a prospective study. NPJ breast cancer, 6(1), pp.1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Irrazabal T, Thakur BK, Kang M, Malaise Y, Streutker C, Wong EOY, Copeland J, Gryfe R, Guttman DS, Navarre WW, Martin A. 2020. Limiting oxidative DNA damage reduces microbe-induced colitis-associated colorectal cancer Nat Commun., 11(1):1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, Brennan CA, Chun E, Ngo L, Samson LD, Engelward BP, Garrett WS, Balbo S, Balskus EP. 2019. The human gut bacterial genotoxin colibactin alkylates DNA Science, 363(6428): eaar7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25] **.Kadosh E, Snir-Alkalay I, Venkatachalam A, May S, Lasry A, Elyada E, Zinger A, Shaham M, Vaalani G, Mernberger M and Stiewe T, 2020. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature, 586(7827), pp.133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this elegant study, the authors demonstrate that cancer mutations have substantial plasticity that can be influenced by gut microbiome through modulation of Wnt signalling thereby conferring a malignant phenotype to the organoids and throughout the gut.
- [26].Tirandaz H and Mohammadi E, 2013. Efficient tumor targeting by anaerobic butyrate-producing bacteria. Medical hypotheses, 80(5), pp.675–678. [DOI] [PubMed] [Google Scholar]
- [27].Khaja ASS, Toor SM, El Salhat H, Faour I, Haq NU, Ali BR and Elkord E, 2017. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget, 8(20), p.33159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K and Thaiss CA, 2017. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science, 357(6356), pp.1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heshiki Y, Vazquez-Uribe R, Li J, Ni Y, Quainoo S, Imamovic L, Li J, Sørensen M, Chow BK, Weiss GJ and Xu A, 2020. Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome, 8(1), pp.1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li YY, Ge QX, Cao J, Zhou YJ, Du YL, Shen B, Wan YJY and Nie YQ, 2016. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World journal of gastroenterology, 22(11), p.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M and Kostic AD, 2016. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut, 65(12), pp.1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, Kostic AD, Giannakis M, Watanabe H, Bullman S, Milner DA, Harris CC, Giovannucci E, Garraway LA, Freeman GJ, Dranoff G, Chan AT, Garrett WS, Huttenhower C, Fuchs CS, Ogino S. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol., 1(5):653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33] **.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW, 2013. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe, 14(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]; Based on recent studies reporting overabundance of Fusobacterium nucleatum (Fn) in colorectal carcinoma tissues, the authors elegantly expand on these findings by putting forth a mechanism through which Fn adheres to, invades and induces oncogenic and inflammatory responses to stimulate growth of CRC cells through its unique FadA adhesin.
- [34].Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S. 2016. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med., 22(6):679–684. [DOI] [PubMed] [Google Scholar]
- [35].Abed J, Emgård JE, Zamir G, Faroja M, Almogy G, Grenov A, Sol A, Naor R, Pikarsky E, Atlan KA, Mellul A, Chaushu S, Manson AL, Earl AM, Ou N, Brennan CA, Garrett WS, Bachrach G. 2016. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe, 20(2):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC and Platz EA, 2015. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clinical Infectious Diseases, 60(2), pp.208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37] **.Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, Dejea CM, Fathi P, Chen J, Finard BB, Tam AJ, McAllister F, Fan H, Wu X, Ganguly S, Lebid A, Metz P, Van Meerbeke SW, Huso DL, Wick EC, Pardoll DM, Wan F, Wu S, Sears CL, Housseau F. 2018. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe, 23(2):203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this elegant article, the authors dwell on the role of BFT in cellular transformation and colon cancer in pre-clinical models.
- [38].Raisch J, Rolhion N, Dubois A, Darfeuille-Michaud A, Bringer MA. 2015. Intracellular colon cancer-associated Escherichia coli promote protumoral activities of human macrophages by inducing sustained COX-2 expression. Lab Invest., 95(3):296–307. [DOI] [PubMed] [Google Scholar]
- [39].Iyadorai T, Mariappan V, Vellasamy KM, Wanyiri JW, Roslani AC, Lee GK, Sears C and Vadivelu J, 2020. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PloS one, 15(1), p.e0228217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lucas C, Salesse L, Hoang MHT, Bonnet M, Sauvanet P, Larabi A, Godfraind C, Gagnière J, Pezet D, Rosenstiel P, Barnich N, Bonnet R, Dalmasso G, Nguyen HTT. 2020. Autophagy of Intestinal Epithelial Cells Inhibits Colorectal Carcinogenesis Induced by Colibactin-Producing Escherichia coli in Apc Min/+ Mice. Gastroenterology, 158(5):1373–1388. [DOI] [PubMed] [Google Scholar]
- [41].Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Déchelotte P, Bonnet M, Pezet D, Wodrich H, Darfeuille-Michaud A, Bonnet R. 2014. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut, 63(12):1932–1942. [DOI] [PubMed] [Google Scholar]
- [42] *.Lasry A, Zinger A and Ben-Neriah Y, 2016. Inflammatory networks underlying colorectal cancer. Nature Immunology, 17(3), p.230. [DOI] [PubMed] [Google Scholar]; In this elegant review, the authors explore various inflammatory processes underlying the development and progression of colorectal cancer and discuss anti-inflammatory means for its prevention and treatment.
- [43] **.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science, 338(6103):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this elegant article, the authors provide evidence that colitis promotes tumorigenesis by altering microbial composition.
- [44].Abdulamir AS, Hafidh RR and Bakar FA, 2011. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. Journal of Experimental & Clinical Cancer Research, 30(1), pp.1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kumar R, Herold JL, Schady D, Davis J, Kopetz S, Martinez-Moczygemba M, Murray BE, Han F, Li Y, Callaway E and Chapkin RS, 2017. Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development. PLoS pathogens, 13(7), p.e1006440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang Y, Weng Y, Gan H, Zhao X, Zhi F 2018Streptococcus gallolyticus conspires myeloid cells to promote tumorigenesis of inflammatory bowel disease. Biochem. Biophys. Res. Commun, 506, 907–911. [DOI] [PubMed] [Google Scholar]
- [47].Kaakoush NO, Castaño-Rodríguez N, Mitchell HM and Man SM, 2015. Global epidemiology of Campylobacter infection. Clinical microbiology reviews, 28(3), pp.687–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Van der Jeught K, Xu HC, Li YJ, Lu XB and Ji G, 2018. Drug resistance and new therapies in colorectal cancer. World Journal of Gastroenterology, 24(34), p.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Maindrault-Goebel F, Tournigand C, André T, Carola E, Mabro M, Artru P, Louvet C, de Gramont A and French Oncology Research Group, 2004. Oxaliplatin reintroduction in patients previously treated with leucovorin, fluorouracil and oxaliplatin for metastatic colorectal cancer. Annals of Oncology, 15(8), pp.1210–1214. [DOI] [PubMed] [Google Scholar]
- [50].Fornaro L, Vasile E, Masi G, Loupakis F, Baldi GG, Allegrini G, Salvatore L, Cremolini C, Cupini S, Cortesi E and Tuzi A, 2012. Outcome of second-line treatment after first-line chemotherapy with the GONO FOLFOXIRI regimen. Clinical colorectal cancer, 11(1), pp.71–76. [DOI] [PubMed] [Google Scholar]
- [51] **.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ and Schlitzer A, 2013. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science, 342(6161), pp.971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study elegantly posited that the effect of Cyclophosphamide on the anti-tumour immune response relies on the presence of a healthy gut microbiota.
- [52].Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E and Ciardiello F, 2009. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nature Reviews Clinical Oncology, 6(9), p.519. [DOI] [PubMed] [Google Scholar]
- [53].Ruan WC, Che YP, Ding L, Li HF. 2018. Efficacy and Toxicity of Addition of Bevacizumab to Chemotherapy in Patients with Metastatic Colorectal Cancer. Comb Chem High Throughput Screen, 21(10):718–724. [DOI] [PubMed] [Google Scholar]
- [54].Zhou J, Kang Y, Chen L, Wang H, Liu J, Zeng S, Yu L. 2020The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front Pharmacol., 11:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yan D, Tu L, Yuan H, Fang J, Cheng L, Zheng X, Wang X. 2017. WBSCR22 confers oxaliplatin resistance in human colorectal cancer. Sci Rep., 7(1):15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Garajová I, Balsano R, Wang H, Leonardi F, Giovannetti E, Deng D, Peters GJ, 2021. The role of the microbiome in drug resistance in gastrointestinal cancers. Expert Rev Anticancer Ther., 21(2): 165–176. [DOI] [PubMed] [Google Scholar]
- [57].Chang CW, Liu CY, Lee HC, Huang YH, Li LH, Chiau JSC, Wang TE, Chu CH, Shih SC, Tsai TH and Chen YJ, 2018. Lactobacillus casei variety rhamnosus probiotic preventively attenuates 5-Fluorouracil/Oxaliplatin-induced intestinal injury in a syngeneic colorectal cancer model. Frontiers in microbiology, 9, p.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM and de Barsy M, 2019. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nature medicine, 25(7), pp.1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pandey KR, Naik SR and Vakil BV, 2015. Probiotics, prebiotics and synbiotics-a review. Journal of food science and technology, 52(12), pp.7577–7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Xu S, Xiang C, Wu J, Teng Y, Wu Z, Wang R, Lu B, Zhan Z, Wu H and Zhang J, 2020. Tongue Coating Bacteria as a Potential Stable Biomarker for Gastric Cancer Independent of Lifestyle. Digestive Diseases and Sciences, pp.1–17. [DOI] [PubMed] [Google Scholar]
- [61].Pandey K, Pandey R, Padhye S, Anant S and Umar S, 2020. Role of Oral Microbiome in Disease Predictions-Current Advances. Biomedical Journal of Scientific & Technical Research, 30(4), pp.23624–23630. [Google Scholar]
- [62].Mendonça LA, dos Santos Ferreira R, de Cássia Avellaneda Guimarães R, de Castro AP, Franco OL, Matias R and Carvalho CM, 2018. The complex puzzle of interactions among functional food, gut microbiota, and colorectal cancer. Frontiers in oncology, 8, p.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63] **.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E and Meltser A, 2020. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science, 368(6494), pp.973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]; Employing state of the art techniques, the authors performed a comprehensive analysis of the tumor microbiome wherein, they studied >1500 tumors across seven cancer types, including breast, lung, ovary, pancreas, melanoma, bone, and brain tumors and found a distinct microbiome (mostly intracellular) within each tumour type.
- [64].Geller LT, Straussman R. 2017. Intratumoral bacteria may elicit chemoresistance by metabolizing anticancer agents. Mol Cell Oncol., 5(1):e1405139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ai L, Tian H, Chen Z, Chen H, Xu J and Fang JY, 2017. Systematic evaluation of supervised classifiers for fecal microbiota-based prediction of colorectal cancer. Oncotarget, 8(6), p.9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cronin M, Morrissey D, Rajendran S, El Mashad SM, Van Sinderen D, O’sullivan GC and Tangney M, 2010. Orally administered bifidobacteria as vehicles for delivery of agents to systemic tumors. Molecular Therapy, 18(7), pp.1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]


