Abstract
Dissection of cell signaling requires tools that can mimic spatiotemporal dynamics of individual pathways in living cells. Optogenetic methods enable manipulation of signaling processes with precise timing and local control. In this review, we describe recent optogenetic approaches for regulation of cell signaling, highlight their advantages and limitations, and discuss examples of their application.
Keywords: optogenetics, signaling
Introduction
In cells, information is relayed in the form of complex and dynamic signaling events that translate into various cellular functions (29). One protein can mediate different signaling outputs depending on its binding partners (25). Although binding partners are critical in determining signaling outcomes, temporal activity dynamics can also dictate function (7, 37, 52). Furthermore, signaling events are compartmentalized and occur at specific subcellular locations (26). Therefore, a thorough investigation of signaling molecules requires the ability to target them to specific binding partners and mimic their complex spatiotemporal dynamics. This challenging task is greatly aided by innovative tools designed to interrogate cell signaling with physiologically relevant dynamics. Although generally useful, gene silencing or knockout, as well as diffusible activators and inhibitors, act on cells globally and irreversibly. These applications lack the intricate control needed to parse out complex signaling events. To develop tools that overcome these limitations, cell biologists adapted optogenetic approaches from neuroscience and further developed them to manipulate cellular signaling.
Optogenetics in cell biology involves the genetic modification of proteins to couple their functional output with an optical input (59). This offers an unprecedented flexibility for the temporal and spatial regulation of protein activity using light. Importantly, low-intensity light is minimally invasive to living cells and is less likely to cause off-target effects. Optogenetics was first employed in neuroscience, when scientists exploited rhodopsin photoreceptors to regulate neuronal function by light, enabling precise control over the firing of individual neurons and characterization of their distinct functions (4, 15). Inspired by these approaches, cell biology witnessed an emergence of a variety of optogenetic tools to study cell signaling. In this review, we will address recent advances in optogenetic tool development and their applications to complex cell signaling pathways.
Types of Photoreceptors
The diverse applicability of optogenetic tools stems from the wide variety of well-described photoreceptors. Photoreceptors are proteins that respond to specific wavelengths of light, commonly referred to as the photoactive range, to induce a specific conformational change in the protein. They span a variety of species, structures, mechanisms of activation, and photoactive ranges, allowing researchers to select the photoreceptor that best suits their needs. Although an in-depth description of these photoreceptors is beyond the scope of this review, several points should be highlighted. There are multiple modes of action following stimulation with different wavelengths of light: homo- or heterodimerization, oligomerization, intramolecular conformational changes, dissociation, DNA binding, or photocleavage. A detailed description of these photoreceptors, along with related literature, can be found at OptoBase.org and within the cited reviews (1, 2, 16, 30, 32, 39, 48). Table 1 details several commonly used photoreceptors.
Table 1.
Commonly used photoreceptors
| Photoreceptor | Wavelength | Mode of Action | Engineered Modifications |
|---|---|---|---|
| UVR8 | 300 nm | Homodimer, heterodimer (COP1) | N/A |
| AsLOV2 | 450 nm | Intramolecular conformational change, heterodimer | iLID, LOVTRAP, TULIP |
| VVD | 450 nm | Homodimer, heterodimer (magnets) | Magnets |
| CRY2 | 450 nm | Homooligomer, heterodimer (CIB1, CIBN) | |
| pdDronpa1 | 500 nm 400 nm (reversion) |
Homodimer, dissociation | Point mutations: M40I, V60A |
| Opsin (BeCyclOp) | 530 nm | cGMP production | |
| PhyB/PIF | 660 nm 740 nm (reversion) |
Heterodimer |
Utilization of Photoreceptor Dimerization
One aspect that determines the function of signaling molecules is their association with specific protein complexes within certain subcellular compartments. However, dissecting the role of protein localization and interactions in signaling has proven difficult by using classical molecular biology and pharmacological approaches. Optogenetics provides a modular toolbox to reconstruct and study these interactions at subcellular levels (30). Dimerizing photoreceptors are useful in regulating subcellular localization, protein-protein interaction, transcription, and protein activation, as we will discuss below.
Regulation of Subcellular Localization
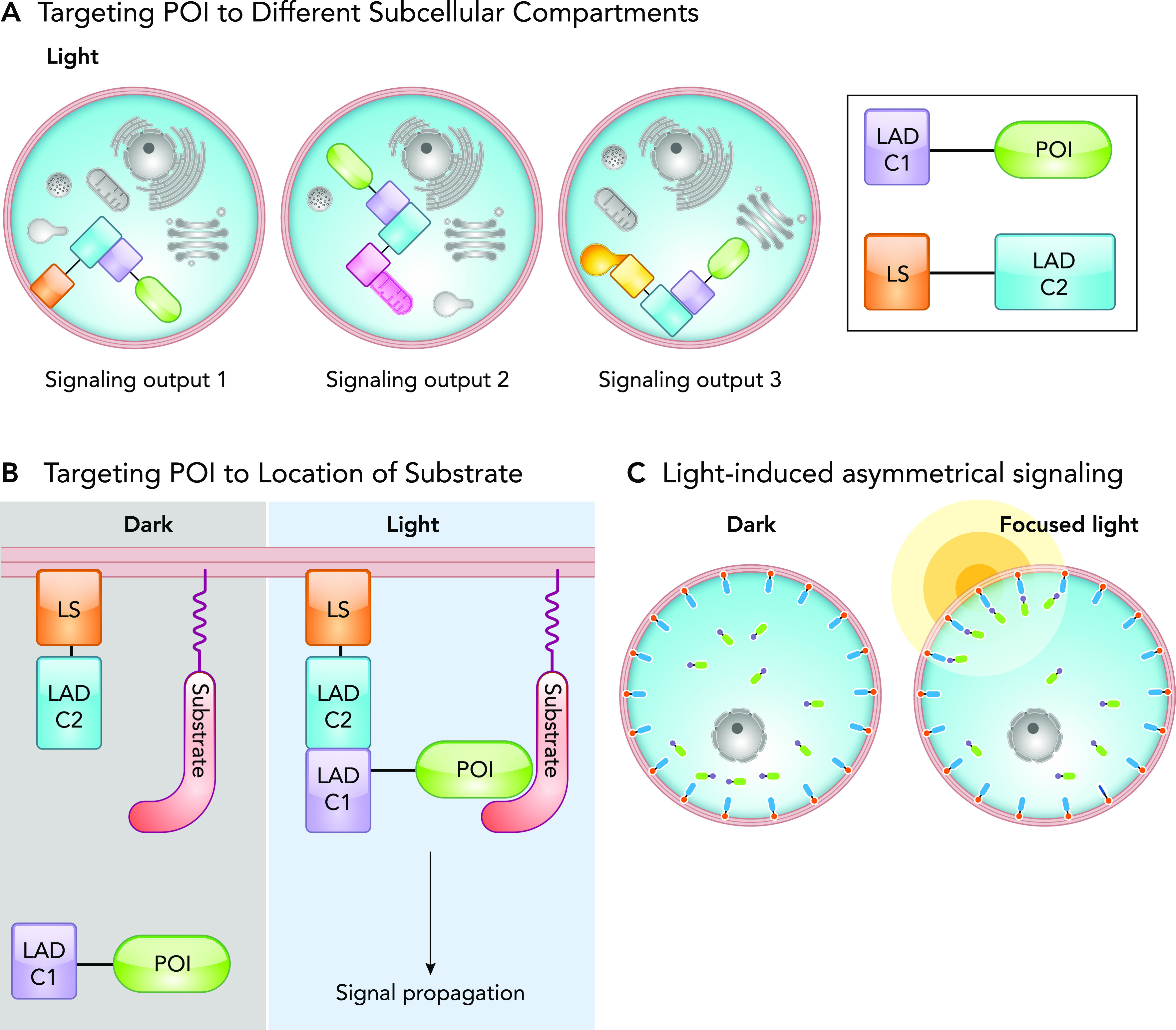
Targeting a protein of interest (POI) to different cellular compartments allows researchers to investigate its specific local signaling. Cry2-CIBN system is the most widely used photoreceptor for subcellular targeting applications. However, recent research demonstrated that other dimer pairs, such as iLID or VVD magnets, offer improved dimerization and dissociation kinetics (1). As a general design, a light-controlled system consists of two protein components that homo- or heterodimerize on illumination. A POI is fused to one light activated dimer component (LADC1) of the system, whereas the other component (LADC2) is localized to the targeted subcellular compartment via a localization signal (LS) (2, 5, 21) (FIGURE 1A). The POI is diffuse throughout the cell in the dark, and in light the components will dimerize, thereby forcing translocation of POI to desired location in the cell. This general approach can be used to 1) localize the POI to different subcellular compartments, 2) localize the POI to the site of its substrate or interacting partner, and 3) asymmetrically target the POI to one side of the cell using focused light (FIGURE 1, A–C).
FIGURE 1.
Regulation of protein localization by light
A: schematic representation of light-induced localization of a protein of interest (POI) to different subcellular compartments. Varying the localization signal (LS) on the LAD component 2 (LADC2) allows targeting of the POI to different organelles. B: schematic representation of an optogenetic system in which a POI is targeted to a location containing its substrate. POI is fused to one component of a light-activated dimer (LADC1), whereas the other LAD component (LADC2) is targeted to a specific location by a localization signal (LS). The “light” panel demonstrates the light-induced dimerization of the LAD components resulting in POI translocation to its substrate and subsequent signal propagation. C: schematic representation of targeting a POI to a portion of the cell using focused light. Light-induced dimerization of the LAD domains only occurs at the illuminated region, resulting in asymmetric localization of the POI.
Targeting POI to different subcellular compartments.
Proteins can exhibit different signaling based on their subcellular localization. Most techniques used for the interrogation of signaling act globally on cells, making it impossible to study signaling events in different cellular compartments. Using optogenetics methods, one can distinctly interrogate signaling pathways initiated by a POI at any subcellular compartment. This is achieved by tagging one LAD component with a LS of an organelle of interest, for example, using CAAX motif for plasma membrane localization. The second LAD component is fused to the POI, allowing for targeting of the POI to an organelle of interest through light-induced dimerization of the LAD components (FIGURE 1A). This targeting approach can be applied to investigate different signaling impacts of an active protein at different locations, such as the study conducted with protein kinase A (PKA). By targeting the catalytically active PKA subunit to the outer mitochondrial membrane and plasma membrane, researchers defined compartment-specific PKA signaling (46). The applications of this technique were expanded with the development of OpEn-Tag (optogenetic endomembrane targeting), a customizable toolbox for targeting POI to various compartments within the cell (39). This two-component system contains a variety of endomembrane-targeting peptides fused to an mCherry-labeled CBIN and a POI fused to CRY2. To demonstrate the applicability of the OpEn-Tag system, the researchers selectively targeted AKT1 to the plasma membrane, and inner and outer mitochondrial membranes. In doing so, they determined the plasma membrane has a significant role in AKT1 activation (39).
Targeting POI to the location of a signaling partner or substrate.
A POI can be targeted to a region containing its substrate or signaling partners to induce signaling. In the dark, this active POI, fused to LADC1, is diffuse throughout the cell and unable to induce signaling. Stimulation with light targets the POI to the site where the substrate resides (FIGURE 1B). This provides direct control over the initiation of signaling pathways and thus an elegant approach to study their dynamics.
This technique was applied to regulate phospholipid signaling and metabolism at the plasma membrane through specific targeting of an inositol phosphatase, phosphoinositide 3-kinase (PI3K), or protein kinase AKT (72). Idevall-Hegran et al. used CRY2-CIBN system to target inositol-5-phosphatse to the plasma membrane (23). Inositol-5-phosphatse was fused to CRY2 (LADC1), whereas CIBN (LADC2) had a plasma membrane localization signal. The plasma membrane is rich in PI(4,5)P2, an inositol-5-phosphatase substrate. Therefore, targeting the phosphatase to the plasma membrane to act on its substrate regulates phospholipid signaling with tight spatiotemporal control (23). A similar targeting approach also has been used to target guanine nucleotide exchange factors (GEFs) to the plasma membrane to activate their specific membrane localized GTPases (34, 60, 63, 79). Toettcher et al., using the Phy/Pif system, targeted SOS to the plasma membrane to induce Ras activation and investigate the role of Ras/ERK dynamics in cell fate determination (60). Meanwhile, other groups have used the blue light dimer system iLID/SspB to target specific GEFs for Rac1, CDC42, and RhoA GTPases to the plasma membrane (79).
Light induced asymmetric signaling.
Some cellular functions require asymmetric signaling activity, especially for polarized cellular behaviors such as migration. This can be mimicked by utilizing a similar approach as those discussed above; the POI is fused to LADC1 while LADC2 is tagged with a specific localization signal. The application of light results in the translocation of the POI to the membrane of interest. Spatial regulation is further enhanced by using a focused beam of light or a gradient of light; researchers can illuminate one side of the cell to stimulate asymmetrical signaling, thereby inducing polarized cellular behaviors (13, 26, 47, 74) (FIGURE 1C). O’Neil and Gautam employed this strategy to dissect the role of G-protein signaling gradients in cells. By using a membrane localized CIBN, a negative regulator of G-protein signaling (RGS) fused to CRY2 was targeted to a membrane following blue light illumination. By applying focused light to only one side of the cell, the targeted inhibition of G-protein signaling was limited to the illuminated side of the cell. Combining this with global chemical activation of G-protein signaling, the cell developed polarized signaling with the dark side actively signaling, whereas the lit side was inhibited. This mimicked a G-protein signaling gradient and specified its role in immune cell migration (47). In another example, Grazanio et al. employed local recruitment of PI3K to define the role of sustained PIP3 signaling at the leading edge of a migrating neutrophil (17). Using localized illumination, they targeted the PIF-tagged PI3K to a membrane-bound PhyB on one side of the cell, generating a highly polarized and sustained PIP3 production. They demonstrated that PIP3 signaling is sufficient to induce cell polarization and migration. Importantly, this study found that sustained PIP3 signaling induces only a transient Rac activation through a mechanism where actin assembly and ArhGAP15 signaling trigger a negative feedback loop downregulating Rac1.
Protein-Protein Interactions
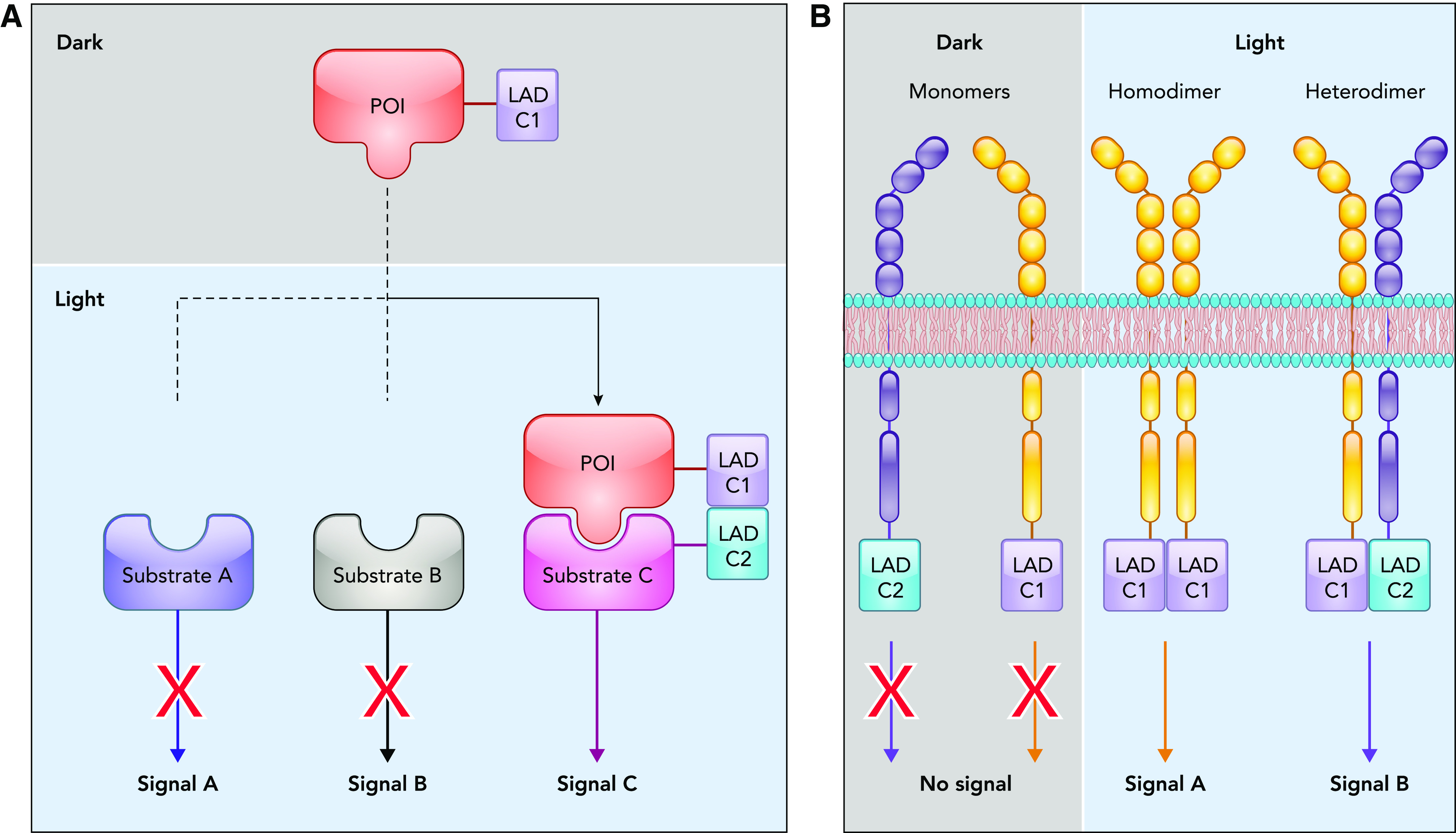
Protein-protein interactions are critical in regulating and propagating signals within cells. One signaling molecule can induce different cellular responses depending on the identity of its binding partner. Although point mutations can alter protein-protein interactions, this approach lacks temporal regulation and often prevents the formation of multiple signaling complexes. Light-induced dimerization or oligomerization of LAD components fused to a POI enables dynamic regulation of protein-protein interactions as well as specific protein clustering. Two types of protein-protein interactions have been successfully regulated by using optogenetics: 1) interaction of a POI with a specific downstream target and 2) dimerization or oligomerization of the POI to induce activation (FIGURE 2).
FIGURE 2.
Light-mediated regulation of protein interactions
A: schematic representation of light-induced dimerization of a POI with a specific effector to dissect different signaling pathways. B: schematic representation of light-induced dimerization employed for regulation of receptor tyrosine kinase homo- and heterodimerization.
Light-induced dimerization to regulate protein interactions with downstream effectors.
Signaling molecules often interact with multiple partners, leading to activation of multiple downstream signaling pathways. Due to this multidirectional complexity of signaling, dissection of individual pathways is challenging. Using optogenetics, it is possible to activate a single downstream pathway of a POI without affecting the others. This is achieved by fusing the POI to LADC1 and fusing a specific signaling partner to LADC2. In response to light, the POI will bind and signal through that particular signaling partner, eliciting the downstream effects specific to that pathway (FIGURE 2A). This was applied to interrogate the role of Cdc42 interaction with a specific effector, WASP, in actin assembly. Using the heterodimerizing LAD pair PhyB and Pif3 fused to Cdc42 and WASP, respectively, Cdc42 and WASP interaction was induced following red-light exposure. This study determined that Cdc42-WASP interaction was sufficient to activate the Arp2/3 complex and induce actin assembly (33).
Light induced homo- and heterodimerization of kinases.
Although optogenetic regulation of protein-protein interaction has been used for a variety of signaling molecules, it has been particularly beneficial in studying receptor tyrosine kinases (RTKs) and other kinase dimers. Different subtypes of RTKs often can homo- or heterodimerize on the interaction with specific ligands. This dimerization results in the trans-phosphorylation and activation of the RTK. Each combination of dimers can mediate different signaling outcomes (FIGURE 2B). Optogenetics has allowed researchers to control dimerization of RTKs independently of ligand binding and thus interrogate specific signaling outputs of unique dimer pairs with spatial and temporal control (FIGURE 2B). This approach was used to induce homodimerization and activation of fibroblast growth factor receptor, epidermal growth factor receptor, and c-MET receptor (6, 8, 9, 18). An additional level of regulation was achieved using the homo-oligomerization property of CRY2 to cluster active RTK dimers into physiologically relevant signaling hubs (8). This enhanced RTK signaling while avoiding artifacts resulting from overexpression or hypersensitivity to the ligand. These oligomerization approaches are not limited to RTKs and have been applied to RhoA, LRP6, and Rab GTPases to enhance their signaling pathways beyond what can be achieved with ligand stimulation (8, 41). Light-regulated dimerization was also employed to interrogate function of non-receptor kinases (9, 68). Optogenetic control of b-Raf and c-Raf homo- and heterodimers demonstrated that the effect of clinically approved Raf inhibitors on signaling depends on the type of the dimer pairs (9).
Split-Protein Reassembly
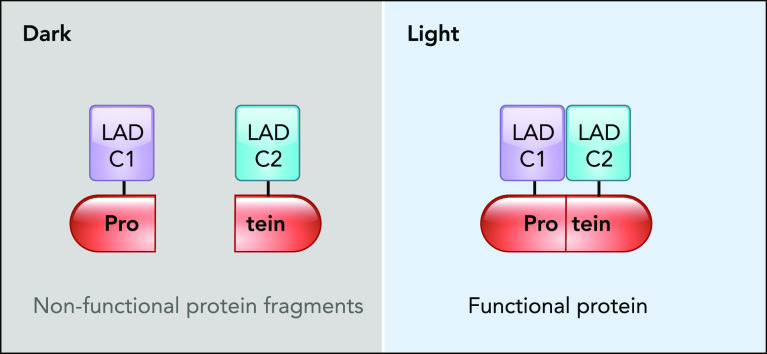
The fundamental realization that fragments of a protein can reassemble to form a functional protein (53, 61, 64) led to the development of split reporters for studying protein-protein interaction and tools for regulating protein activity (35, 49, 67, 69). Importantly, non-covalent reconstruction of inactive protein fragments using photo-dimers has been successful in modulating protein function (54). Magnets and Cry2-CIB are commonly used heterodimer systems in engineering optogenetic split-protein reassembly (31, 50, 51, 66). As a general strategy, a protein is split into nonfunctional halves, each fused to a different LAD component. Once exposed to light, the dimer is formed, and the protein fragments are brought together, leading to the reassembly of a functional protein (FIGURE 3). For instance, CRY2-CIB dimerization system was implemented for light-regulated genetic manipulation through engineering DNA-editing enzymes. The Tucker laboratory created split Cre recombinase by using CRY2-CIB and demonstrated its in vivo functionality in rodent brains (38, 58). Sato group also developed a photoactivatable split Cre recombinase (PA-Cre) by using magnets dimerization system (27). PA-Cre was capable of genetically recombining DNA in a mouse organ in response to external LED light. The same group developed a photoactivatable split Cas9 (paCas9) (42) and later engineered the recently emerged RNA-guided DNA nuclease, Cpf1 (43). Also, split-protein reassembly by magnets system was used to develop a novel optogenetically activated intracellular antibody (optobody) (28, 75). Intracellular antibodies (intrabodies) are small antibody fragments that bind and neutralize endogenous proteins. Using optobodies, one can control the affinity of intrabodies in living cells in a spatiotemporal manner. This provides means to manipulate and define the role of endogenous proteins, as was demonstrated by inhibiting endogenous gelsolin and beta-2 adrenergic receptor (75).
FIGURE 3.
Schematic of light-regulated split-protein reassembly
In response to light, the split fragments reassemble into a functional protein following LADC1 and LADC2 dimerization.
The red/far-red PhyB-Pif dimer system was implemented to engineer optogenetic split transcription factors (TFs) (40, 55). Typically, a TF is split into its DNA binding domain (DBD) and activation domain (AD), each fused with one LAD component. Upon light irradiation, the DBD and AD are brought together through the light-induced dimer system, and subsequently gene expression is initiated (55). The DBD can be customized to target any gene of interest (31, 50, 51); the AD can also be replaced with a repression domain to induce silencing of a target gene (31).
Although optogenetic split-protein reassembly has been proven to work, this approach presents some challenges. Split proteins can spontaneously reassemble, depending on the selected split site, resulting in undesired basal activity of the protein in the dark state. Fragments could also fail to fully reassemble, thus preventing effective activation. Therefore, finding the optimal splitting site of a POI is subject to extensive trial and error. Recently, the Dokholyan group developed a server to predict and rank potential splitting sites for regulating a POI with light or cell-permeable ligands (spell.dokhlab.org) (11), to aid in developing such tools.
Allosteric Regulation
Allosteric regulation of enzymes is desirable for studying catalytic effects of proteins whose dimerization is not a prerequisite for their activation. Proteins engineered to be regulated allosterically retain their native subcellular localization, intermolecular interactions, and access to their substrates. Moreover, once an enzyme is engineered to be allosterically regulated, the approach can be generally applied to other enzymes that share similar structure (22).
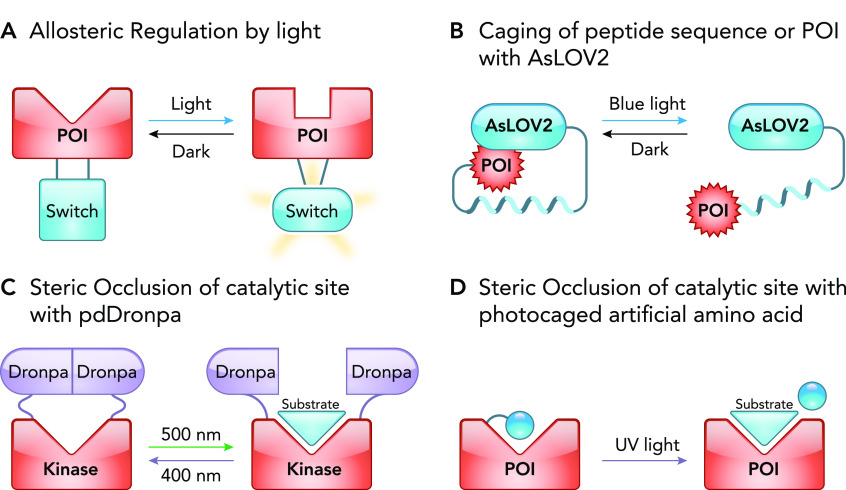
Photoreceptors often undergo significant conformational change upon illumination with light. These changes have been harnessed by protein engineers to achieve allosteric regulation of a protein. As a general design, a photoreceptor is inserted into a POI at a position where it induces reorganization of the POI’s functional core upon illumination with light (FIGURE 4A). Development of such photo-regulatable chimera is guided by an available structure of the POI and can be complemented by molecular dynamics simulations that predict potential structural changes inflicted by the inserted photoreceptor (12). Common insertion sites are often selected in the loops within the POI that are distant, yet structurally coupled to critical function residues (10, 12). However, attachment of photoreceptor at the end of a POI can also result in allosteric regulation (70). Illumination of the engineered protein with light can result in either activation or inactivation of the POI (FIGURE 4A), depending on the photoreceptor and the design of the construct. Below, we discuss several examples where this strategy was successfully employed.
FIGURE 4.
Light-mediated regulation of proteins using allosteric and steric occlusion mechanisms
A: a light-sensitive allosteric switch regulates the structure of the catalytic site of a POI. B: schematic representation of photocaged signaling peptide or POI with AsLOV2. By fusing the POI to the Jα helix, AsLOV2 sterically occludes the POI in the dark. In the light, the Jα dissociates and becomes disordered, thus exposing and uncaging the POI. C: using the reversible dimerization property of pdDronpa, the active site of the POI is occluded by the pdDronpa dimer but exposed when pdDronpa dissociates. D: sterically occluding the active site by incorporating a photosensitive artificially caged amino acid near the substrate-binding pocket of a POI. Following exposure to light, the artificial amino acid is cleaved, resulting in the uncaging of the active site.
An elegant study by Hahn group used AsLOV2 domain to create an allosteric light switch to reversibly inhibit protein activity in response to blue light (12). AsLOV2 was inserted in a previously characterized allosteric loop in Src kinase (24). Since the NH2 and COOH termini of AsLOV2 are just 10 Å apart in the dark, they hypothesized that inserting AsLOV2 in the allosteric loop of a constitutively active Src should not perturb its structure and hence its function. However, when the NH2 and COOH termini of AsLOV2 separate in response to blue light, it introduces disorder in the coupled catalytic domain, resulting in the deactivation of Src. This generated a photo-inhibitable Src, or PI-Src (12). To demonstrate its generalizability, they applied the method to GTPases and GEFs to produce PI-GTPase and PI-GEF, respectively. AsLOV2 domain was employed to engineer a photoactivatable Escherichia coli trp repressor (trpR) (57) and a photoinhibitable protein tyrosine phosphatase 1B (20). Photoactivatable proteins also have been generated by using the allosteric regulation approach. A photoactivatable bacteriophage T7 RNA polymerase was developed by using VVD photoreceptor as an allosteric switch (19). Addition of AsLOV2 at the NH2-terminus of a constitutively active Rac1 was also successfully used to achieve light-mediated regulation of Rac1 at the subcellular level. Interestingly, the initial design was to sterically block Rac1 interaction with its effectors in the dark and unblock it in the light (71). However, a new study reveals that photoactivatable Rac1is actually regulating Rac1 activity through an allosteric mechanism (70). This highlights a need for continued investigation into the mechanisms of these tools to better inform future tool development.
Regulation by Steric Interference
Steric interference, when implemented in optogenetics, is referred to as photo-caging. This approach uses a light-sensitive group to cage a peptide or a region of interest in a protein, thereby sterically occluding it and thus blocking its function. The function is then recovered by uncaging the region in response to a specific wavelength of light (FIGURE 4, B–D). These photo-caging tools most commonly utilize AsLOV2 photoreceptor. We will also discuss pdDronpa1, a switch domain engineered from the green fluorescent protein DronpaN145 to photocage catalytic sites. Furthermore, in addition to using photosensory protein domains, photo-caging was made possible by using light sensitive caged artificial amino acids.
LOV2 domain from Avena sativa phototropin 1 (AsLOV2) is extensively utilized in photocaging due to the reversible structural change it undergoes in response to blue light, as revealed by NMR studies. AsLOV2 structure is characterized by a COOH-terminal helix (Jα helix) docked onto a compact core in the dark. In response to blue light, the Jα helix undocks and becomes exposed. Protein engineers exploited this feature to cage peptides by incorporating them in the Jα helix. In the dark, the peptide is masked, reducing the affinity to its binding partners. Blue light unmasks the peptide, enabling binding (FIGURE 4B). This strategy was applied by two groups to generate light-inducible nuclear localization signal (LINus) and light-activated nuclear shuttle (LANS) (44, 76). Both tools function to translocate proteins from the cytosol to the nucleus by incorporating a nuclear localization signal (NLS) in the Jα helix. Similarly, this strategy was used to engineer a light-inducible nuclear export system (LEXY) by incorporating a nuclear export signal (NES) in the Jα helix of AsLOV2 (45). Since transcriptional factors are generally sequestered in the cytosol in their inactive state and shuttled to the nucleus when active, light-regulated caging and uncaging of the NLS or NES motifs provides an effective method to regulate transcription within the cell (44, 76, 77). To read more about optogenetic transcription systems, we refer the reader to another review article that covers this topic in depth (14). Peptide caging using AsLOV2 is not limited to the regulation of protein localization. Studies also used AsLOV2 to photocage inhibitory peptides to manipulate the activity of endogenous kinases (PKA and MLCK) by light (73). AsLOV2 was also used for light-dependent colocalization (36). By masking ipaA and SsrA peptides (using LOV-ipaA and LOV-SsrA) from their binding partners (vinclulin and SspB), it was possible to induce their colocalization by using light (36). This method was also used to regulate protein degradation signal by adding a degron to the COOH terminus of AsLOV2 (3, 62), allowing for light-induced degradation of the POI simply by fusing it with the degron containing LOV domain.
Using light to control steric hindrance of a catalytic site has been employed for regulation of enzymatic activity. Lin group applied this approach to create photoswitchable kinases by using the engineered photo-dimer pdDronpa, which dissociates in blue light and reassembles in violet light. pdDronpa is incorporated into the kinase such that the dimer encircles the catalytic pocket, sterically occluding the substrate binding when dimerized in blue light (78). In violet light, the dimer dissociates, allowing the substrate to access the kinase catalytic pocket and subsequently be phosphorylated (FIGURE 4C). This process is reversible and therefore provides temporal control over activity (78). A similar strategy was later applied by Klaus Hahn’s group to develop a photocage system named Z-lock (56). Here, LOV and Zdk photodimer system, instead of pdDronpa, was utilized to cage the catalytic site of cofilin and αTAT. This system is smaller in size relative to pdDronpa, facilitating engineering. Also, LOV and Zdk affinity is adjustable, making Z-lock a tunable system (56).
Alternatively, a catalytic domain can be sterically blocked by using a photo-caged amino acid. Recently, a strategy named “computationally aided and genetically encoded proximal decaging” (CAGE-prox) was developed (65). This approach incorporates an artificially caged amino acid in a position proximal to the catalytic region. This is sufficient to block the activity of the POI until it is restored by uncaging the amino acid by using UV light (FIGURE 4D). This approach has the advantage of using a small caged amino acid instead of bulky photo-domains; however, it is an irreversible tool and lacks subcellular regulation capabilities.
Future Directions
Light is considered to be minimally invasive to biological samples, such as living cells and tissues. However, at high intensities, light can become phototoxic. Therefore, there is a continuous effort in the field to develop optogenetic tools with high sensitivity that can respond to low light intensities. Highly sensitive optogenetic systems, or those capable of responding to two-photon stimulation, are especially advantageous when they are applied in dense tissue samples or in in vivo applications where light scattering and reduced illumination depth make optogenetic regulation challenging. Since red light penetrates tissue deeper than blue light and is less cytotoxic, we expect to see the development of more red-shifted optogenetic tools that should advance in vivo applications of optogenetics and allow independent regulation of multiple pathways when combined with the tools operating in the blue spectrum. There is also room for improvement in the kinetics of these tools, not only in how fast they respond to turning the light on but also in how quickly they turn off. Finally, most optogenetic tools are exogenous systems, and their efficient expression in primary cell lines is challenging. However, with the new genome-editing techniques being developed, it is becoming more feasible to incorporate these tools in the genome of cells and organisms.
Acknowledgments
We are grateful to the National Institutes of Health for the funding (R21 CA-223915 to A.V.K., 5T32 HL-007829-26 to J.F.). All figures were generated using biorender.com.
No conflicts of interest, financial or otherwise, are declared by the author(s).
M.S. and J.F. prepared figures; M.S. and J.F. drafted manuscript; M.S., J.F., and A.V.K. edited and revised manuscript; A.V.K. approved final version of manuscript.
References
- 1.Benedetti L, Barentine AES, Messa M, Wheeler H, Bewersdorf J, De Camilli P. Light-activated protein interaction with high spatial subcellular confinement. Proc Natl Acad Sci USA 115: E2238–E2245, 2018. doi: 10.1073/pnas.1713845115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer HM, Naumann S, Weber W, Radziwill G. Optogenetic control of signaling in mammalian cells. Biotechnol J 10: 273–283, 2015. doi: 10.1002/biot.201400077. [DOI] [PubMed] [Google Scholar]
- 3.Bonger KM, Rakhit R, Payumo AY, Chen JK, Wandless TJ. General method for regulating protein stability with light. ACS Chem Biol 9: 111–115, 2014. doi: 10.1021/cb400755b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyden ES. Optogenetics and the future of neuroscience. Nat Neurosci 18: 1200–1201, 2015. doi: 10.1038/nn.4094. An erratum for this article is available at . [DOI] [PubMed] [Google Scholar]
- 5.Buckley CE, Moore RE, Reade A, Goldberg AR, Weiner OD, Clarke JDW. Reversible optogenetic control of subcellular protein localization in a live vertebrate embryo. Dev Cell 36: 117–126, 2016. doi: 10.1016/j.devcel.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV. Optogenetic protein clustering and signaling activation in mammalian cells. Nat Methods 10: 249–252, 2013. doi: 10.1038/nmeth.2360. [DOI] [PubMed] [Google Scholar]
- 7.Bugaj LJ, Sabnis AJ, Mitchell A, Garbarino JE, Toettcher JE, Bivona TG, Lim WA. Cancer mutations and targeted drugs can disrupt dynamic signal encoding by the Ras-Erk pathway. Science 361: eaao3048, 2018. doi: 10.1126/science.aao3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugaj LJ, Spelke DP, Mesuda CK, Varedi M, Kane RS, Schaffer DV. Regulation of endogenous transmembrane receptors through optogenetic Cry2 clustering. Nat Commun 6: 6898, 2015. doi: 10.1038/ncomms7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatelle CV, Hövermann D, Müller A, Wagner HJ, Weber W, Radziwill G. Optogenetically controlled RAF to characterize BRAF and CRAF protein kinase inhibitors. Sci Rep 6: 23713, 2016. doi: 10.1038/srep23713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagliyan O, Dokholyan NV, Hahn KM. Engineering proteins for allosteric control by light or ligands. Nat Protoc 14: 1863–1883, 2019. doi: 10.1038/s41596-019-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagliyan O, Krokhotin A, Ozkan-Dagliyan I, Deiters A, Der CJ, Hahn KM, Dokholyan NV. Computational design of chemogenetic and optogenetic split proteins. Nat Commun 9: 4042, 2018. doi: 10.1038/s41467-018-06531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagliyan O, Tarnawski M, Chu P-H, Shirvanyants D, Schlichting I, Dokholyan NV, Hahn KM. Engineering extrinsic disorder to control protein activity in living cells. Science 354: 1441–1444, 2016. doi: 10.1126/science.aah3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Beco S, Vaidžiulytė K, Manzi J, Dalier F, di Federico F, Cornilleau G, Dahan M, Coppey M. Optogenetic dissection of Rac1 and Cdc42 gradient shaping. Nat Commun 9: 4816, 2018. doi: 10.1038/s41467-018-07286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mena L, Rizk P, Rincon-Limas DE. Bringing light to transcription: the optogenetics repertoire. Front Genet 9: 518, 2018. doi: 10.3389/fgene.2018.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci 18: 1213–1225, 2015. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goglia AG, Toettcher JE. A bright future: optogenetics to dissect the spatiotemporal control of cell behavior. Curr Opin Chem Biol 48: 106–113, 2019. doi: 10.1016/j.cbpa.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graziano BR, Gong D, Anderson KE, Pipathsouk A, Goldberg AR, Weiner OD. A module for Rac temporal signal integration revealed with optogenetics. J Cell Biol 216: 2515–2531, 2017. doi: 10.1083/jcb.201604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grusch M, Schelch K, Riedler R, Reichhart E, Differ C, Berger W, Inglés-Prieto Á, Janovjak H. Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J 33: 1713–1726, 2014. doi: 10.15252/embj.201387695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han T, Chen Q, Liu H. Engineered photoactivatable genetic switches based on the bacterium phage T7 RNA polymerase. ACS Synth Biol 6: 357–366, 2017. doi: 10.1021/acssynbio.6b00248. [DOI] [PubMed] [Google Scholar]
- 20.Hongdusit A, Zwart PH, Sankaran B, Fox JM. Minimally disruptive optical control of protein tyrosine phosphatase 1B. Nat Commun 11: 788, 2020. doi: 10.1038/s41467-020-14567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hörner M, Chatelle C, Mühlhäuser WWD, Stocker DR, Coats M, Weber W, Radziwill G. Optogenetic control of focal adhesion kinase signaling. Cell Signal 42: 176–183, 2018. doi: 10.1016/j.cellsig.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell 109: 275–282, 2002. doi: 10.1016/S0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 23.Idevall-Hagren O, Dickson EJ, Hille B, Toomre DK, De Camilli P. Optogenetic control of phosphoinositide metabolism. Proc Natl Acad Sci USA 109: E2316–E2323, 2012. doi: 10.1073/pnas.1211305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karginov AV, Ding F, Kota P, Dokholyan NV, Hahn KM. Engineered allosteric activation of kinases in living cells. Nat Biotechnol 28: 743–747, 2010. doi: 10.1038/nbt.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karginov AV, Tsygankov D, Berginski M, Chu PH, Trudeau ED, Yi JJ, Gomez S, Elston TC, Hahn KM. Dissecting motility signaling through activation of specific Src-effector complexes. Nat Chem Biol 10: 286–290, 2014. doi: 10.1038/nchembio.1477. . A correction for this article is available at . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karunarathne WKA, O’Neill PR, Gautam N. Subcellular optogenetics - controlling signaling and single-cell behavior. J Cell Sci 128: 15–25, 2015. doi: 10.1242/jcs.154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawano F, Okazaki R, Yazawa M, Sato M. A photoactivatable Cre-loxP recombination system for optogenetic genome engineering. Nat Chem Biol 12: 1059–1064, 2016. doi: 10.1038/nchembio.2205. [DOI] [PubMed] [Google Scholar]
- 28.Kawano F, Suzuki H, Furuya A, Sato M. Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat Commun 6: 6256, 2015. doi: 10.1038/ncomms7256. [DOI] [PubMed] [Google Scholar]
- 29.Kholodenko BN. Cell-signalling dynamics in time and space. Nat Rev Mol Cell Biol 7: 165–176, 2006. doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klewer L, Wu Y-W. Light-induced dimerization approaches to control cellular processes. Chemistry 25: 12452–12463, 2019. doi: 10.1002/chem.201900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konermann S, Brigham MD, Trevino A, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA, Church GM, Zhang F. Optical control of mammalian endogenous transcription and epigenetic states. Nature 500: 472–476, 2013. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HN, Mehta S, Zhang J. Recent advances in the use of genetically encodable optical tools to elicit and monitor signaling events. Curr Opin Cell Biol 63: 114–124, 2020. doi: 10.1016/j.ceb.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung DW, Otomo C, Chory J, Rosen MK. Genetically encoded photoswitching of actin assembly through the Cdc42-WASP-Arp2/3 complex pathway. Proc Natl Acad Sci USA 105: 12797–12802, 2008. doi: 10.1073/pnas.0801232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461: 997–1001, 2009. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci USA 101: 12288–12293, 2004. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lungu OI, Hallett RA, Choi EJ, Aiken MJ, Hahn KM, Kuhlman B. Designing photoswitchable peptides using the AsLOV2 domain. Chem Biol 19: 507–517, 2012. doi: 10.1016/j.chembiol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185, 1995. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 38.Meador K, Wysoczynski CL, Norris AJ, Aoto J, Bruchas MR, Tucker CL. Achieving tight control of a photoactivatable Cre recombinase gene switch: new design strategies and functional characterization in mammalian cells and rodent. Nucleic Acids Res 47: e97, 2019. doi: 10.1093/nar/gkz585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mühlhäuser WWD, Weber W, Radziwill G. OpEn-Tag-A customizable optogenetic toolbox to dissect subcellular signaling. ACS Synth Biol 8: 1679–1684, 2019. doi: 10.1021/acssynbio.9b00059. [DOI] [PubMed] [Google Scholar]
- 40.Müller K, Engesser R, Metzger S, Schulz S, Kämpf MM, Busacker M, Steinberg T, Tomakidi P, Ehrbar M, Nagy F, Timmer J, Zubriggen MD, Weber W. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res 41: e77, 2013. doi: 10.1093/nar/gkt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen MK, Kim CY, Kim JM, Park BO, Lee S, Park H, Heo WD. Optogenetic oligomerization of Rab GTPases regulates intracellular membrane trafficking. Nat Chem Biol 12: 431–436, 2016. doi: 10.1038/nchembio.2064. [DOI] [PubMed] [Google Scholar]
- 42.Nihongaki Y, Kawano F, Nakajima T, Sato M. Photoactivatable CRISPR-Cas9 for optogenetic genome editing. Nat Biotechnol 33: 755–760, 2015. doi: 10.1038/nbt.3245. [DOI] [PubMed] [Google Scholar]
- 43.Nihongaki Y, Otabe T, Ueda Y, Sato M. A split CRISPR-Cpf1 platform for inducible genome editing and gene activation. Nat Chem Biol 15: 882–888, 2019. doi: 10.1038/s41589-019-0338-y. [DOI] [PubMed] [Google Scholar]
- 44.Niopek D, Benzinger D, Roensch J, Draebing T, Wehler P, Eils R, Di Ventura B. Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nat Commun 5: 4404, 2014. doi: 10.1038/ncomms5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niopek D, Wehler P, Roensch J, Eils R, Di Ventura B. Optogenetic control of nuclear protein export. Nat Commun 7: 10624, 2016. doi: 10.1038/ncomms10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Banion CP, Priestman MA, Hughes RM, Herring LE, Capuzzi SJ, Lawrence DS. Design and profiling of a subcellular targeted optogenetic cAMP-dependent protein kinase. Cell Chem Biol 25: 100–109.e8, 2018. doi: 10.1016/j.chembiol.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Neill PR, Gautam N. Subcellular optogenetic inhibition of G proteins generates signaling gradients and cell migration. Mol Biol Cell 25: 2305–2314, 2014. doi: 10.1091/mbc.e14-04-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pathak GP, Vrana JD, Tucker CL. Optogenetic control of cell function using engineered photoreceptors. Biol Cell 105: 59–72, 2013. doi: 10.1111/boc.201200056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelletier JN, Campbell-Valois FX, Michnick SW. Oligomerization domain-directed reassembly of active dihydrofolate reductase from rationally designed fragments. Proc Natl Acad Sci USA 95: 12141–12146, 1998. doi: 10.1073/pnas.95.21.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polstein LR, Gersbach CA. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat Chem Biol 11: 198–200, 2015. doi: 10.1038/nchembio.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polstein LR, Gersbach CA. Light-inducible spatiotemporal control of gene activation by customizable zinc finger transcription factors. J Am Chem Soc 134: 16480–16483, 2012. doi: 10.1021/ja3065667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauch N, Rukhlenko OS, Kolch W, Kholodenko BN. MAPK kinase signalling dynamics regulate cell fate decisions and drug resistance. Curr Opin Struct Biol 41: 151–158, 2016. doi: 10.1016/j.sbi.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 53.Richards FM. On the enzymic activity of subtilisin-modified ribonuclease. Proc Natl Acad Sci USA 44: 162–166, 1958. doi: 10.1073/pnas.44.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shekhawat SS, Ghosh I. Split-protein systems: beyond binary protein-protein interactions. Curr Opin Chem Biol 15: 789–797, 2011. doi: 10.1016/j.cbpa.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu-Sato S, Huq E, Tepperman JM, Quail PH. A light-switchable gene promoter system. Nat Biotechnol 20: 1041–1044, 2002. doi: 10.1038/nbt734. [DOI] [PubMed] [Google Scholar]
- 56.Stone OJ, Pankow N, Liu B, Sharma VP, Eddy RJ, Wang H, Putz AT, Teets FD, Kuhlman B, Condeelis JS, Hahn KM. Optogenetic control of cofilin and αTAT in living cells using Z-lock. Nat Chem Biol 15: 1183–1190, 2019. doi: 10.1038/s41589-019-0405-4. . A correction for this article is available at . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strickland D, Moffat K, Sosnick TR. Light-activated DNA binding in a designed allosteric protein. Proc Natl Acad Sci USA 105: 10709–10714, 2008. doi: 10.1073/pnas.0709610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taslimi A, Zoltowski B, Miranda JG, Pathak GP, Hughes RM, Tucker CL. Optimized second-generation CRY2-CIB dimerizers and photoactivatable Cre recombinase. Nat Chem Biol 12: 425–430, 2016. doi: 10.1038/nchembio.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toettcher JE, Voigt CA, Weiner OD, Lim WA. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nat Methods 8: 35–38, 2011. doi: 10.1038/nmeth.f.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toettcher JE, Weiner OD, Lim WA. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155: 1422–1434, 2013. doi: 10.1016/j.cell.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ullmann A, Jacob F, Monod J. Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. J Mol Biol 24: 339–343, 1967. doi: 10.1016/0022-2836(67)90341-5. [DOI] [PubMed] [Google Scholar]
- 62.Usherenko S, Stibbe H, Muscò M, Essen L-O, Kostina EA, Taxis C. Photo-sensitive degron variants for tuning protein stability by light. BMC Syst Biol 8: 128, 2014. doi: 10.1186/s12918-014-0128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valon L, Marín-Llauradó A, Wyatt T, Charras G, Trepat X. Optogenetic control of cellular forces and mechanotransduction. Nat Commun 8: 14396, 2017. doi: 10.1038/ncomms14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villarejo M, Zamenhof PJ, Zabin I. Beta-galactosidase. In vivo -complementation. J Biol Chem 247: 2212–2216, 1972. [PubMed] [Google Scholar]
- 65.Wang J, Liu Y, Liu Y, Zheng S, Wang X, Zhao J, Yang F, Zhang G, Wang C, Chen PR. Time-resolved protein activation by proximal decaging in living systems. Nature 569: 509–513, 2019. doi: 10.1038/s41586-019-1188-1. [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Chen X, Yang Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods 9: 266–269, 2012. doi: 10.1038/nmeth.1892. [DOI] [PubMed] [Google Scholar]
- 67.Wehrman T, Kleaveland B, Her JH, Balint RF, Blau HM. Protein-protein interactions monitored in mammalian cells via complementation of beta -lactamase enzyme fragments. Proc Natl Acad Sci USA 99: 3469–3474, 2002. doi: 10.1073/pnas.062043699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wend S, Wagner HJ, Müller K, Zurbriggen MD, Weber W, Radziwill G. Optogenetic control of protein kinase activity in mammalian cells. ACS Synth Biol 3: 280–285, 2014. doi: 10.1021/sb400090s. [DOI] [PubMed] [Google Scholar]
- 69.Wilson CG, Magliery TJ, Regan L. Detecting protein-protein interactions with GFP-fragment reassembly. Nat Methods 1: 255–262, 2004. doi: 10.1038/nmeth1204-255. [DOI] [PubMed] [Google Scholar]
- 70.Winkler A, Barends TR, Udvarhelyi A, Lenherr-Frey D, Lomb L, Menzel A, Schlichting I. Structural details of light activation of the LOV2-based photoswitch PA-Rac1. ACS Chem Biol 10: 502–509, 2015. doi: 10.1021/cb500744m. [DOI] [PubMed] [Google Scholar]
- 71.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461: 104–108, 2009. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu Y, Nan D, Fan J, Bogan JS, Toomre D. Optogenetic activation reveals distinct roles of PI P3 and Akt in adipocyte insulin action. J Cell Sci 129: 2085, 2016. doi: 10.1242/jcs.174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi JJ, Wang H, Vilela M, Danuser G, Hahn KM. Manipulation of endogenous kinase activity in living cells using photoswitchable inhibitory peptides. ACS Synth Biol 3: 788–795, 2014. doi: 10.1021/sb5001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell 18: 226–236, 2010. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu D, Lee H, Hong J, Jung H, Jo Y, Oh B-H, Park BO, Heo WD. Optogenetic activation of intracellular antibodies for direct modulation of endogenous proteins. Nat Methods 16: 1095–1100, 2019. doi: 10.1038/s41592-019-0592-7. [DOI] [PubMed] [Google Scholar]
- 76.Yumerefendi H, Dickinson DJ, Wang H, Zimmerman SP, Bear JE, Goldstein B, Hahn K, Kuhlman B. Control of protein activity and cell fate specification via light-mediated nuclear translocation. PLoS One 10: e0128443, 2015. doi: 10.1371/journal.pone.0128443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yumerefendi H, Lerner AM, Zimmerman SP, Hahn K, Bear JE, Strahl BD, Kuhlman B. Light-induced nuclear export reveals rapid dynamics of epigenetic modifications. Nat Chem Biol 12: 399–401, 2016. doi: 10.1038/nchembio.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou XX, Fan LZ, Li P, Shen K, Lin MZ. Optical control of cell signaling by single-chain photoswitchable kinases. Science 355: 836–842, 2017. doi: 10.1126/science.aah3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmerman SP, Asokan SB, Kuhlman B, Bear JE. Cells lay their own tracks - optogenetic Cdc42 activation stimulates fibronectin deposition supporting directed migration. J Cell Sci 130: 2971–2983, 2017. doi: 10.1242/jcs.205948. [DOI] [PMC free article] [PubMed] [Google Scholar]