ABSTRACT
Introduction
Coronavirus disease 2019 (COVID-19) has had an enormous impact worldwide, and vaccination is believed to be the method that will control the pandemic. Several types of vaccines developed using different platforms have been authorized, but the immunogenicity and reactogenicity of heterologous prime–boost vaccination with different vaccines remain largely unclear.
Areas covered
Electronic databases including PubMed, Embase, medRxiv, Research Square, and SSRN were searched to investigate the immunogenicity and reactogenicity associated with heterologous vaccination.
As of 30 June 2021, four trials including 1,862 participants were identified. Heterologous administration of BNT162b2 (BNT) in ChAdOx1 (ChAd)-primed participants (ChAd/BNT) showed noninferior immunogenicity to homologous BNT administration (both prime and booster were BNT vaccines, BNT/BNT) with tolerable reactogenicity and higher T cell responses. Compared with homologous ChAdOX1 vaccination (ChAd/ChAd), heterologous ChAd/BNT was found to elicit higher immunogenicity (ChAd/BNT vs. ChAd/ChAd, antibody titer ratio: 9.2).
Expert opinion
Our systematic review found robust immunogenicity and tolerable reactogenicity of heterologous administration of a BNT162b2 boost in ChAdOx1-primed participants. An additional benefit of stronger T cellular immunity was also observed. Heterologous vaccination is a reasonable and feasible strategy to combat COVID-19. Further studies are warranted to confirm the benefits and identify the optimal combinations, doses, and intervals.
KEYWORDS: COVID-19, Sars-COV-2, vaccination, heterologous, boost, BNT162B2, chadox1, mRNA
1. Introduction
The pandemic coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in enormous health threats and impacts [1,2]. As of 30 June 2021, more than 180 million people had been infected, with approximately 4 million deaths [1]. Although various aggressive or moderate strategies have been applied to control the pandemic in many countries, such as lockdown of cities, border control, or widespread personal protective equipment use, the spread of COVID-19 is still rising in many areas [1,3,4]. Vaccination against SARS-CoV-2 was believed to be the key to controlling the pandemic, and many companies have invested in research and vaccine design since the beginning of the pandemic [5–7]. As of late June 2021, several vaccines produced with different platforms had been authorized for emergency use and administered to stop the pandemic [2]. As of late June 2021, approximately 23.8% of the world population had received at least one dose of a COVID-19 vaccine, and daily new cases slowed in countries with widespread vaccination [1]. Some countries lifted their lockdowns gradually, and the importance of COVID-19 vaccines as the critical tool in the pandemic was emphasized. Excellent vaccine effectiveness has been observed in real-world administrations, and increasing vaccine coverage is the next step in eliminating the pandemic [8].
Different platforms have been used to produce COVID-19 vaccines, including nucleic acid (DNA and RNA), viral vector, protein subunit, and whole-virus platforms. As of late June 2021, there were several approved vaccines available on the market, including BNT162b2 (BNT, messenger RNA platform, mRNA, by Pfizer/BioNTech, Germany), mRNA-1273 (mRNA platform, by Moderna, USA), ChAdOx1 (ChAd, AZD1222, adenovirus vectored platform, by AstraZeneca/Oxford, UK), Janssen/Ad26.COV 2.S (adenovirus vectored platform, by Johnson & Johnson, USA), Sinopharm (inactivated whole-virus vaccine, by Beijing Bio-Institute of Biological Products Co Ltd, China), and Sinovac-CoronaVac (inactivated whole-virus vaccine, by Beijing-based pharmaceutical company Sinovac, China), etc [2]. The premarket efficacy was good, and postmarketing surveillance also showed robust effectiveness [9]. According to the vaccine instructions and clinical trial design, two doses are required to ensure adequate protection for most of these vaccines, and the prime–boost interval ranged from 21 days to 3 months. The two doses should be the same (i.e. homologous vaccination), and the World Health Organization has not recommended heterologous vaccination at present [2].
Heterologous vaccination refers to the use of booster and priming vaccines developed with different platforms. Heterologous vaccination against COVID-19 should be considered under some circumstances. First, vaccine shortages and supply delays might occur. Although novel technology was introduced to manufacture these vaccines, the vaccine supply remains inadequate, and it will take time to produce enough vaccines for all global residents. A shortage of vaccines might result in delayed administration of the second dose. Timely second-dose administration remains a challenge in many countries, and only 1% of people in low-income countries have received full vaccination. The issue of vaccine equity and availability is a major concern. Furthermore, adenovirus-vector vaccine-related thromboembolic issues have attracted global attention and have changed vaccine policies in some areas [10]. For example, rare cases of blood clotting after ChAdOx1 (ChAd) vaccination have been reported, and since May 2021, the UK government has recommended that people younger than 40 years old seek an alternative vaccine. With this policy change, ChAd-primed people might receive different vaccines as a booster against COVID-19. Moreover, some patients may have serious adverse events after prime vaccination, such as anaphylaxis. In these cases, an alternative second vaccination should be recommended. Finally, the emerging variants of concern have drawn attention, and breakthrough infections have been reported [11]. Heterologous vaccination might provide better efficacy to combat COVID-19 variants [12,13].
As the situation has changed and supply might not meet demand, there is a need for heterologous vaccination with different COVID-19 vaccines. However, evidence supporting heterologous vaccination is scarce at present [14]. Some trials have been conducted to investigate the immunogenicity and reactogenicity of heterologous administration of COVID-19 [15]. Therefore, we conducted this systematic review to investigate the effects of heterologous COVID-19 vaccination.
2. Methods
2.1. Study design and literature search
Our study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and approved by the Institutional Review Board of the MacKay Memorial Hospital, Taipei, Taiwan (approval number, 20MMHIS140e) [16,17]. The ‘PICO’ (Patient, Intervention, Comparison, Outcome) used for the literature search of our study were as follows: patients were people with COVID-19 vaccination; intervention was heterologous vaccination; comparison was people with homologous vaccination; and outcomes were immunogenicity and reactogenicity. Therefore, we used ‘COVID-19 vaccine’ as a search term in electronic medical databases. We further used comprehensive keywords, such as ‘COVID-19,’ ‘COVID-2019,’ ‘severe acute respiratory syndrome coronavirus 2,’ ‘2019-nCoV,’ ‘vaccination,’ ‘BNT,’ “AstraZeneca“, ‘Moderna,’ ‘Janssen,’ ‘mRNA,’ ‘adenovirus vector,’ ‘heterologous,’ and ‘boost’ with Boolean operators and MeSH terms. Electronic medical databases including PubMed/Medline, EMBASE, and the Cochrane database were searched up to 30 June 2021. We also searched preprint medical databases including medRxiv, Research Square, and SSRN. No constraints were placed on language, year of publication, or participant characteristics to ensure a comprehensive search and identify the maximum number of potential articles. The search details and database websites are attached as supplementary file 1. Two authors performed a literature search independently, and disagreements were resolved through a discussion with the third author.
2.2. Study selection, data extraction, systematic review, and meta-analyses
Randomized controlled trials or cohort studies investigating heterologous COVID-19 vaccination were included. The exclusion criteria were as follows: duplicate publications from different databases, irrelevant articles (such as epidemiological studies or surveillance work), studies that did not evaluate immunogenicity or reactogenicity, simple case reports, animal studies, trials with the same platform or no comparison arm, editorials, and review articles. Primary outcomes were the immunogenicity of heterologous and homologous COVID-19 vaccination. Secondary outcomes were the reactogenicity of all vaccine combinations. Two authors appraised the selected articles independently and extracted the following data: name of the first author, study country, study type, participant population, participant age/gender, prime vaccine type, booster vaccine type, interval between prime and boost vaccine, immunogenicity profiles, clinical outcomes, reactogenicity, and author conclusion. For quality assessment, the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) for randomized controlled trials and the Newcastle–Ottawa Scale (NOS) for observational cohort studies were used [18,19]. Two authors assessed study quality independently based on the domains of selection, ascertainment, causality, and reporting. If disagreement occurred, a consensus was reached through a discussion with the third author.
Because the techniques used to measure COVID-19 antibodies and criteria for positivity vary in different laboratories at present, direct head-to-head antibody level comparison was inappropriate; we did not perform meta-analyses to compare the immunogenicity of different studies. We performed network meta-analyses using random-effect models to investigate reactogenicity, such as the incidence of fever after vaccination. We ranked the relative probabilities of the incidence of fever after vaccination and presented the rankings with forest plots and league tables.
2.3. Statistical analyses
Assuming that the true effect size was not the same, we used a random-effect regression model for meta-analyses. The Lu & Ades model for network meta-analysis, consisting of direct and indirect comparisons, was conducted to compare the effect sizes between studies [20]. The τ2 statistic was used to evaluate the heterogeneity among the included studies. Comparison-adjusted funnel plots and Egger tests were used to examine potential publication bias. We used the loop-specific approach and node-splitting models to investigate the potential inconsistency between the direct and indirect evidence within the network [21]. We ranked the relative probabilities for the incidence of fever after vaccination using the p score and the surface under the cumulative ranking curve, which reflected the percentage of incidence rates of each combination [22]. A p value less than 0.05 was considered statistically significant. MedCalc (MedCalc Software, Belgium) v18 and R software version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses.
3. Results
3.1. Flowchart of systematic review
As of 30 June 2021, 18,025 articles were identified in the medical research databases (Figure 1). A total of 10,067 duplicate articles in different databases were removed. We screened the title and abstract of the remaining articles and excluded articles with no heterologous vaccination, including basic trials, animal studies, editorial/review articles, and epidemiological and single-arm studies. Finally, 14 full-text articles were assessed for eligibility. The Sputnik V vaccine (or Gam-COVID-Vac, adenovirus vectored platform, by the Gamaleya Research Institute of Epidemiology and Microbiology, Russia) is also an adenovirus vectored vaccine produced using two recombinant replication-defective human adenoviruses: Ad26 and Ad5. The primary vaccination was Ad26-vectored, and the booster was Ad5-vectored. The immunogenicity of this heterologous administration was robust; however, it was not included in our review because it uses the same vaccine platform, and no comparison with vaccines produced using other platforms was conducted [23]. Two studies involving animal studies and one study with case reports were not included [24–26]. Two articles reported the results from the same trial (Com-COV) [15,27]. Finally, 4 trials from 5 articles were identified that fulfilled our inclusion and exclusion criteria (Table 1) [15,27–30]. One study was conducted in the UK, one in Spain, and two in Germany. Two were randomized controlled trials, and two were cohort studies. A total of 1862 participants with a female predominance (52.7%) were included, and the median ages in the studies were 30.5 to 57.8 years. The prime/boost interval ranged from 4 to 12 weeks. We used the following abbreviations for individual vaccines: ChAdOx1 (ChAd) and BNT162b2 (BNT).
Figure 1.
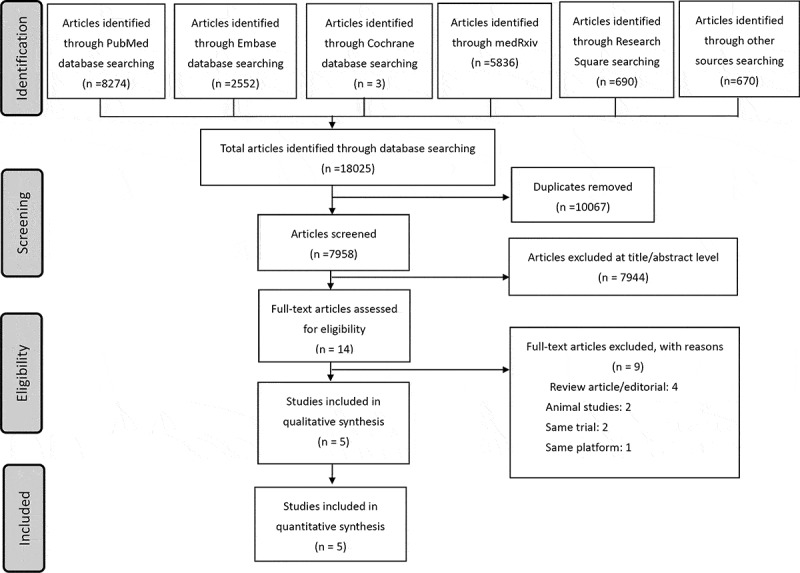
Flowchart of literature search and enrolled studies
Table 1.
Demographic characteristics of included studies investigating heterologous COVID-19 vaccination
| Study [ref] | Country | Design | Participants (N) | Age (years) | Male N (%) |
Comparisons | Interval of boost | Outcomes | Main results |
|---|---|---|---|---|---|---|---|---|---|
| CombiVacS[28] | Spain | Randomized controlled phase 2 trial | 676 | 43.98 | 294 (43) | ChAd; ChAd/BNT |
8–12 weeks (61%: 8–9 weeks 39%: 10–12 weeks) |
Spike protein Ab, RBD Ab, neutralizing Ab, cellular responses, reactogenicity | BNT162b2 given as a second dose in individuals prime vaccinated with ChAdOx1 induced a robust immune response, with an acceptable and manageable reactogenicity profile |
| Com-COV[15,27] | UK | Randomized, participant-blind trial | 830 | 57.8 | 450 (54.2) | ChAd/ChAd; ChAd/BNT; BNT/BNT; BNT/ChAd |
28 or 84 days | Spike protein Ab, neutralizing Ab, cellular Ab, reactogenicity | Despite the BNT/ChAd regimen not meeting non-inferiority criteria, the geometric mean concentrations of both heterologous schedules were higher than that of a licensed vaccine schedule (ChAd/ChAd) with proven efficacy. |
| David H[30] | Germany | Prospective cohort study | 330 | 35 | 127 (38.5) | BNT; BNT/BNT; ChAd; ChAd/BNT |
10–12 weeks | Spike protein Ab, neutralizing Ab, reactogenicity | Well tolerated and slightly more immunogenic compared to homologous BNT/BNT vaccination with three-week vaccine intervals |
| Rüdiger Groß[29] | Germany | Prospective cohort study | 26 | 30.5 | 10 (38.5) | ChAd/BNT; BNT/BNT |
8 weeks | Spike protein Ab, neutralizing Ab, neutralizing activity to variants, cellular responses, reactogenicity | This heterologous vaccination regimen is at least as immunogenic and protective as homologous vaccinations. |
Abbreviations: Ab, antibody; BNT, BNT162b2 COVID-19 vaccine; BNT/BNT: homologous vaccination with BNT as both prime and booster; BNT/ChAd: prime with BNT followed by a ChAdOx1 booster; ChAd, ChAdOx1 COVID-19 vaccine; ChAd/BNT: prime with ChAd followed by a BNT booster; ChAd/ChAd: homologous vaccination with ChAd as both prime and booster; RBD, receptor binding domain; Ref: reference.
Furthermore, we use a slash to indicate the prime and booster vaccines; for example, ChAd/BNT denotes prime with ChAd followed by a BNT booster, and BNT/BNT refers to homologous vaccination with BNT as both prime and booster. The antibody titers (spike protein and neutralizing antibody) and T cellular responses were investigated in all studies. One study determined the neutralizing activity for variants of concern [29]. Robust immunogenicity of ChAd/BNT heterologous vaccination was reported in all studies. The reactogenicity was tolerable across studies.
3.2. Comparison of immunogenicity
Six kinds of vaccine schedules were identified, and further comparisons of prime/boost combinations are summarized in Table 2, specifically, ChAd vs. ChAd/BNT, ChAd/ChAd vs. ChAd/BNT, BNT/BNT vs. ChAd/BNT, and BNT/BNT vs. BNT/ChAd. The immunogenicity profiles were summarized, including anti-receptor binding domain (RBD) antibody, anti-spike protein IgG, neutralizing antibody, and T cellular responses. The detected binding antibody titers were highest among participants with either homologous BNT/BNT or heterologous ChAd/BNT vaccination. However, stronger T cell immunity was observed in heterologous ChAd/BNT vaccination. Compared with single-dose ChAd vaccination, heterologous ChAd/BNT vaccination had superior immunogenicity (antibody titer ratios: 36.41 to 138). Compared with homologous ChAd/ChAd vaccination, heterologous ChAd/BNT also had a higher neutralizing antibody level (geometric mean titer ratio, GMR: 9.2). However, the heterologous BNT/ChAd vaccination was less potent and less immunogenic than homologous BNT/BNT (GMR: 0.51).
Table 2.
Comparison of different prime/boost combinations in included studies
| Comparison | Study | RBD Ab | Spike protein Ab | Neutralizing Ab | T cell response and others | Conclusion |
|---|---|---|---|---|---|---|
| ChAd/BNT vs ChAd | CombiVacS | Higher anti-RBD Ab titers (71.46→7756.68 BAU/ml); ChAd/BNT: ChAd ratio = 77.69. |
Higher anti-Spike protein Ab titers (98.4→3684.87 BAU/ml); ChAd/BNT: ChAd ratio = 36.41. |
ChAd: 41.81; ChAd/BNT: 1905.69; ChAd/BNT: ChAd ratio = 45.58. |
Higher T-cell response (INF – γ, ChAd: 122.67 pg/ml, ChAd/BNT: 521.22 pg/ml) Ratio = 4.25 |
ChAd/BNT> ChAd (Antibody ratio: 36.41–77.69) |
| David | Higher anti-RBD IgG in ChAd/BNT group (2.84 vs 1.28 S/Co) | Higher anti-SARS-CoV-2-S1 IgG in ChAd/BNT group (2.08 vs 0.52 S/Co) and higher reactive rates (100% vs 28.07%) | Higher reactive neutralizing Ab in ChAd/BNT group (100% vs 84.21%) | Higher T-cell response (INF – γ, ChAd: 1.2 AU, ChAd/BNT: 2.25 AU) Ratio = 1.88 |
ChAd/BNT> ChAd | |
| Rüdiger Groß | Median IgG titers increased 135-fold (63.9 U/mL→8815 U/mL) GMR: 138 |
Neutralizing correlated with Spike protein IgG titers. Median ACE2 neutralization increased after BNT booster (62%–>98%). | Variants of concern B.1.1.7, B.1.351 and B.1.617 are potently neutralized by sera of ChAd/BNT. | ChAd/BNT> ChAd (Antibody titer ratio: 138) | ||
| ChAd/BNT vs ChAd/ChAd |
Com-COV | ChAd/ChAd: 1392 ELU/ml ChAd/BNT: 12,906 ELU/ml GMR: 9.2 |
Pseudotype virus neutralizing Ab, NT50 ChAd/ChAd: 61 ChAd/BNT: 515 GMR: 8.5 |
Cellular responses (SFC/106) ChAd/BNT: 185 ChAd/ChAd: 50 GMR: 3.8 |
ChAd/BNT> ChAd/ChAd (Antibody titer ratio: 9.2) |
|
| ChAd/BNT vs BNT/BNT | Com-COV | ChAd/BNT:12906 ELU/ml BNT/BNT:14080 ELU/ml GMR: 0.92 |
Pseudotype virus neutralizing Ab, NT50 ChAd/BNT: 515 BNT/BNT: 574 GMR: 0.9 |
Cellular responses (SFC/106) ChAd/BNT: 185 BNT/BNT: 80 GMR: 2.31 |
ChAd/BNT ≥ BNT/BNT (comparable antibody titers and higher T cellular responses in ChAd/BNT) |
|
| David | ChAd/BNT: 100%, 5.37 S/Co BNT/BNT: 99.01%, 4.52 S/Co |
ChAd/BNT: 100% BNT/BNT: 99.01% |
Higher T cell activity in ChAd/BNT group (2.25 vs 1.67 AU) Ratio: 1.35 |
ChAd/BNT ≥ BNT/BNT (comparable antibody titers and higher T cellular responses in ChAd/BNT) |
||
| BNT/ChAd vs BNT/BNT | Com-COV | BNT/ChAd: 7133 ELU/ml BNT/BNT: 14,080 ELU/ml GMR: 0.51 |
Pseudotype virus neutralizing Ab, NT50 BNT/ChAd: 383 BNT/BNT: 574 GMR: 0.67 |
Cellular responses (SFC/106) BNT/ChAd: 99 BNT/BNT: 80 GMR: 1.24 |
BNT/ChAd < BNT/BNT (Antibody titer ratio: 0.51) |
Abbreviations: Ab, antibody; Ab, antibody; BNT, BNT162b2 COVID-19 vaccine; BNT/BNT: homologous vaccination with BNT as both prime and booster; BNT/ChAd: prime with BNT followed by a ChAdOx1 booster; ChAd, ChAdOx1 COVID-19 vaccine; ChAd/BNT: prime with ChAd followed by a BNT booster; ChAd/ChAd: homologous vaccination with ChAd as both prime and booster; GMR, geometric mean titer ratio; IgG: immunoglobulin G; INF – γ, interferon – γ; NT50: half-maximal neutralizing titer; RBD, receptor binding domain.
3.3. Comparison of reactogenicity
Reactogenicity was more common in people who received heterologous vaccination. Serious adverse events were reported in 4 participants but were not related to the vaccination [27]. Fatigue and myalgia, chills, and feverishness were common discomforts after vaccination. We further conducted a network meta-analysis to compare the incidence of objective fever after each vaccine combination (Figure 2). Individuals primed with ChAd had the highest rate of fever. We ranked the p score in descending order as ChAd, BNT/ChAd, ChAd/BNT, BNT/BNT, BNT, and ChAd/ChAd. Compared with homologous vaccination, heterologous boost induced higher rates of fever. The net graph, direct evidence estimates, Egger’s test, node-splitting forest plot, and league table were conducted to demonstrate the consistency of evidence (data not shown). We further summarized the immunogenicity and reactogenicity in plain language in Table 3.
Figure 2.
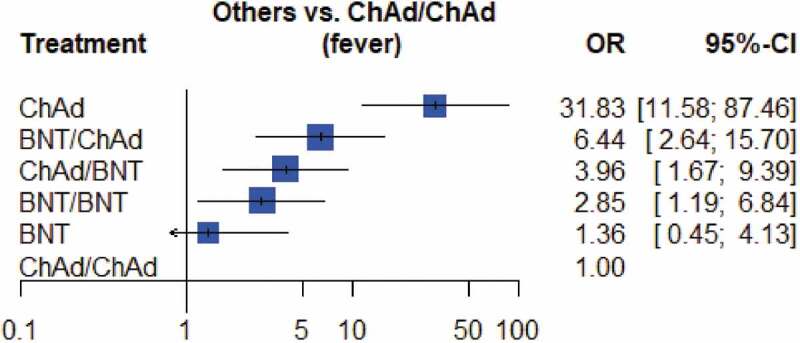
Forest plot of the incidence of fever after different COVID-19 vaccination
Table 3.
Summary of immunogenicity and reactogenicity of different vaccine combinations using star rating
| Vaccine | ChAd | ChAd/ChAd | BNT | BNT/BNT | ChAd/BNT | BNT/ChAd |
|---|---|---|---|---|---|---|
| Immunogenicity | ||||||
| Neutralizing Ab | ★ | ★★★ | ★ | ★★★★★ | ★★★★★ | ★★★★ |
| Binding Ab | ★ | ★★★ | ★ | ★★★★★ | ★★★★★ | ★★★★ |
| T cell responses | ★★★ | ★★★ | ★ | ★★★ | ★★★★★ | ★★★★ |
| Immunogenicity against delta variant# | ★ | ★★★ | ★ | ★★★ | ★★★★ | ? |
| Reactogenicity | ★★★ | ★ | ★ | ★★ | ★★ | ★★ |
*Abbreviations: Ab, antibody; Ab, antibody; BNT, BNT162b2 COVID-19 vaccine; BNT/BNT: homologous vaccination with BNT as both prime and booster; BNT/ChAd: prime with BNT followed by a ChAdOx1 booster; ChAd, ChAdOx1 COVID-19 vaccine; ChAd/BNT: prime with ChAd followed by a BNT booster; ChAd/ChAd: homologous vaccination with ChAd as both prime and booster.
**Star rating: 1 star denotes least effective; 5 stars denotes most effective.
#Effectiveness is estimated by present evidences and may change after more available studies.
4. Discussion
Our systematic review showed robust immunogenicity of heterologous vaccination with ChAd prime followed by BNT boost. The anti-spike protein and neutralizing antibody titers of ChAd/BNT were noninferior to homologous BNT/BNT vaccination; higher T cellular immunity was observed in the heterologous ChAd/BNT vaccination. Compared with single-dose ChAd and homologous ChAd/ChAd vaccination, heterologous ChAd/BNT had significantly higher immunogenicity. Heterologous vaccination with BNT prime followed by ChAd boost (BNT/ChAd) showed weaker immunogenicity than homologous BNT/BNT vaccination. Reactogenicity was common in all combinations and tolerable. The promising immunogenicity elicited by heterologous ChAd/BNT vaccination provides evidence for the feasibility of this additional vaccine strategy.
Heterologous vaccination has been applied in previous studies. Using a recombinant adenovirus 26 and 5 vector-based heterologous prime–boost COVID-19 vaccine, Sputnik V showed 91.6% efficacy in a phase 3 trial [23]. Combined spike/RBD immunizations in mice and macaques elicited potent immunogenicity [26]. Further animal studies with vaccines developed using different platforms (adenovirus vectors and mRNA vaccines) showed robust immunogenicity and indicated a potential role for heterologous administration [25]. Ostadgavahi et al. applied heterologous vaccinations with ChAd/BNT to 2 subjects and observed robust humoral immune responses in anti-spike, RBD, and neutralizing antibodies [24]. Our systematic review also demonstrated the promising efficacy of heterologous vaccination.
The immunologic benefits of heterologous vaccination were observed in our study, but the underlying mechanisms remain largely unclear. Studies investigating the complicated immune responses of COVID-19 infection and vaccine immunogenicity are imperative. A plentiful and complex cytokine cascade is observed in patients with COVID-19, and immune-mediated responses play a critical role in the pathophysiology of COVID-19 [31]. Also, significant changes in cytokine expression are reported after vaccination, and vaccine-evoked protection involves both humoral and cellular immunity [32,33]. Neutralizing antibodies are believed to be crucial in protection against COVID-19, and neutralizing levels correlate with clinical protection [34]. However, nonneutralizing antibodies also exhibit important effects via Fc (fragment crystallizable)-mediated effector functions, including antibody-dependent phagocytosis, cellular cytotoxicity and natural killer cell activation [33]. A robust cytotoxic CD8 + T cell response and a TH1 cell-biased CD4 + T cell effector response correlates with clinical protection, and innate immunity is also involved in immunity against COVID-19 [33]. Another hint is that good effectiveness after the first dose of vaccine is noted, but the neutralizing antibody level does not increase sharply. This observation indicates that other immunologic mechanisms are responsible for the protection after the first dose. Currently available vaccines are effective against COVID-19, but their underlying immune mechanisms seem to be different. For messenger RNA (mRNA) vaccines, extremely high neutralizing and binding antibody titers are elicited after vaccination, but the CD8 + T cell responses are relatively low [33,35].
In contrast, adenovirus-vectored vaccines elicit lower neutralizing and binding antibody levels but produce polyclonal antibodies after vaccination [36]. Protective immunity may be achieved by mediating other antibody-dependent effector mechanisms, including antibody-dependent complement deposition and monocyte-mediated and neutrophil-mediated phagocytosis [33]. Furthermore, potent T cell responses with the production of TNF and IFNγ from CD4 + T cells peaked at 14 days after a single dose of ChAd vaccine. These observations indicate that vaccines produced using different platforms elicit vaccine protection through different pathways. Thus, heterologous vaccination may elicit the immunological benefits of both platforms; similar to what occurs in infected people who receive a vaccination after recovery. Postinfection serum-neutralizing capacity is elevated approximately 1000-fold in convalescent individuals who receive mRNA vaccines, and the interaction of natural immunity and vaccine-generated immunity elicits more robust protection [37,38]. The concept of ‘hybrid vigor immunity’ is derived from plants [39]. When different plant lines breed together, the hybrid line produces a stronger plant. The benefits of heterologous vaccination may be attributed to similar interactions between different immune-mediated pathways, and our review provides more clinical evidence of these benefits.
Some other plausible mechanisms may contribute together to the observed immunological benefits of heterologous vaccination. First, differences in innate immunity after prime and booster vaccination and the potential role of trained innate cells may partially explain this phenomenon [40]. When we administered a prime vaccine, primary innate cells (dendritic cells, monocytes, and granulocytes) will activate antigen-specific naïve B and T cells. When the booster is administered, the free innate cells and trained innate cells will trigger different humoral immune responses and enhance antibody production. However, preexisting trained innate cells and antibodies to the same vaccine and adjuvant tend to impair antigen presentation in individuals who receive homologous boosters. The generation of appropriate inflammatory signals for T cells and homologous vaccination appears to be relatively less efficient at enhancing cellular immunity.
On the other hand, when an unrelated heterologous vaccine is administered, trained innate cells, hematopoietic stem and progenitor cells, and resident memory T cells may produce subsequent robust responses of naïve cells via epigenetic reprogramming. A second explanation may be the circumvention of vector immunity. ChAd priming elicits excellent T cellular responses; however, improved immunity after booster administration with a longer interval between homologous ChAd vaccinations has been reported. Evoked immunity against adenovirus vectors after priming is believed to be responsible for this phenomenon. A heterologous booster with an adenovirus vector-free vaccine may evade the interference of adenovirus vector immunity. Finally, the spike protein of SARS-CoV-2 is immunogenic, and the spike confirmation of BNT is different from that of ChAd. The BNT-specific two-proline mutation of the spike protein may further elicit stronger neutralizing responses [7,41]. However, the underlying mechanisms are under exploration. Based on our systematic review, the order of prime boost also matters, and heterologous vaccination with BNT/ChAd shows weaker immunogenicity than homologous BNT/BNT vaccination. Further studies are required to clarify the underlying mechanisms and harness the optimal vaccines, order, interval, and number of vaccinations.
The interval between prime and boost may affect the efficacy of COVID-19 vaccines. Homologous ChAd/ChAd vaccination showed higher efficacy in participants with prime–boost intervals of 12 weeks or more (12 weeks: 81.3%, less than 6 weeks: 55.1%) [42]. A recent study found higher antibody titers in participants with longer prime–boost intervals (median antibody titers for 8–12, 15–25, and 44–46 weeks were 923, 1860, and 3738 EU, respectively) [43]. The intervals of the included studies varied from 4 to 12 weeks, and the efficacy might be affected by the interval. Compared with the CombivacS trial, a shorter interval of the subsequent booster was found in the study of Rüdiger Groß (8 weeks vs. 8–12 weeks). A more significant increase in the antibody titer ratio was reported in a study with a shorter interval (antibody titer ratio, 138 vs. 36.41–77.69, Table 2). The conflicting data may result from differences in mean participant age (30.5 vs. 43.98 years, Table 1). The complex immunologic responses were affected by many confounding factors. However, the benefits of ChAd/BNT were consistent across studies; thus, heterologous ChAd/BNT administration is a feasible and efficacious strategy.
Furthermore, a third dose might be beneficial in evoking stronger and longer immunity [43]. Moreover, both mRNA-1273 (Moderna) and BNT were developed using the same platform, and a recent study showed a similar booster benefit with mRNA-1273 after ChAd priming [44]. At present, we do not know whether the immunogenicity and reactogenicity are the same when using the adenovirus-vectored Janssen COVID-19 vaccine as the prime vaccine. Additionally, there are outstanding COVID-19 vaccines produced with other platforms, such as protein subunits [6,45]. The effects of heterologous combinations involving protein subunit vaccines require further investigation.
Our review demonstrated excellent immunogenicity in participants with heterologous immunizations. However, there remain concerns regarding real-world effectiveness. Immunobridging correlating immunogenic efficacy with clinical effectiveness is an important issue. Khoury et al. found that neutralizing antibody levels were highly predictive of protection from symptomatic SARS-CoV-2 infection [34]. Although further studies are required to establish the correlation and conversion of immunogenic efficacy and clinical effectiveness, strong immunogenicity induced by heterologous vaccination provides evidence of a feasible vaccination strategy.
Furthermore, although there was some consistency between different measurement methods, the diagnostic protocols, measurements, and laboratory equipment used varied in different labs and trials. It might be less accurate to compare the immunogenicity of different studies directly [9]. Therefore, we did not conduct a meta-analysis to compare the immunogenicity of different vaccine combinations. Apparent differences in antibody titers in individual trials were observed; thus, the benefits of heterologous ChAd/BNT were accentuated (Table 2). We used a network meta-analysis to investigate the incidence of objective fever and found an increased likelihood of fever in participants who received heterologous boosters. However, there were no vaccine-related serious adverse events in the enrolled trials, and the adverse events associated with heterologous vaccination were tolerable. The benefits of heterologous vaccination outweigh the risks, and heterologous vaccination is a feasible and reasonable strategy.
The emergence of variants of concern has received global attention and might interfere with vaccination protection [13,32,46,47]. Heterologous vaccination might provide an opportunity for better protection against variants [41]. Rüdiger Groß et al. showed potent neutralization of B.1.1.7, B1.351, and B.1.617 by sera of all participants with heterologous ChAd/BNT immunization [29]. A recent study investigated the effects of heterologous ChAd/BNT vaccination on the B.1.1.7, B.1.351, and P.1 variants of concern [48]. Compared with homologous ChAd/ChAd vaccination, heterologous vaccination generated significantly higher frequencies of spike-specific CD4+ and CD8 + T cells and high titers of neutralizing antibodies against variants. Furthermore, prolonged intervals between homologous ChAd/ChAd vaccinations might lead to better efficacy against variants [42]. A complete 2-dose vaccination offers better immunity against the delta variant [46]. These strategies may enhance vaccine-generated protection to combat emerging COVID-19 variants.
Our study was subject to some limitations. First, the combination and prime/boost interval of enrolled patients varied in individual studies. The median ages of the participants and measurements of immunogenicity were also different. Therefore, direct comparisons were unavailable for some combinations. Second, several ongoing trials are investigating this crucial issue, including NCT04993560, NCT04988048, NCT04983537, NCT04962906, NCT04927936, NCT04907331, NCT04889209, NCT04776317, etc., and their outcomes will contribute to a better understanding of heterologous vaccination. Finally, there are concerns regarding the correlation between laboratory efficacy and real-world effectiveness. All current vaccines on the market are effective against hospitalization, severe complications, and mortality due to COVID-19. In areas with fluctuating vaccine supply, the best vaccine is the one that is available.
5. Conclusion
In conclusion, our systematic review showed comparable immunogenicity of heterologous ChAd/BNT vaccination and homologous BNT/BNT vaccination. Furthermore, ChAd/BNT elicited the strongest T cellular responses and was effective against variants of concern (B.1.1.7, B.1.351, and B.1.617). Although adverse events were more common in heterologous vaccinations, they were tolerable, and there were no serious adverse events. Heterologous vaccination with ChAd/BNT is a reasonable and feasible strategy to combat COVID-19.
Acknowledgments
We thank everyone’s efforts to combat COVID-19.
Funding Statement
This research received no external funding.
Article highlights
COVID-19 vaccines produced using different platforms have been widely administered, but challenges have arisen, including vaccine supply shortages, perceived serious but very rare adverse events after the first dose, the much-publicized thromboembolic effects, and the emergence of variants of concern. Heterologous prime–boost vaccination refers to a scheme in which the booster vaccination and prime vaccination utilize different platforms; heterologous vaccination might be an alternative strategy, but the immunogenicity and reactogenicity remain largely unclear.
Our systematic review identified four trials with 1862 participants, and we found robust immunogenicity of heterologous administration of BNT162b2 (BNT) in ChAdOx1(ChAd)-primed participants (ChAd/BNT).
Homologous BNT162b2 vaccinations and heterologous ChAd/BNT had the highest antibody titers.
Heterologous ChAd/BNT had the highest T cellular responses.
Higher neutralizing activities against variants of concern B.1.1.7, B.1.351, and B.1.617 were observed in heterologous ChAd/BNT vaccinations.
Although adverse events were more commonly reported in the BNT-boosted participants, reactogenicity was tolerable in all combinations.
Heterologous vaccination was a feasible and reasonable strategy. Further studies are encouraged to confirm the clinical effectiveness and identify the optimal combinations, doses, and intervals.
Declaration of interest
The authors declare no conflict of interest.
Reviewer disclosures
A reviewer has declared they are an employee of EpiVax, Inc., a biotech company that is developing a COVID-19 vaccine. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Authors’ contributions
NCC, HC, and CYL involved in conceptualization; YKT, YNH, YLT, SLW, LC, DTNH, FYH, and CYL performed literature search and screen. YNH, YLT, and CYL performed quality assessment and analysis; YKT and CYL were responsible for methodology and software; NCC wrote the first draft. All authors have read and agreed to the published version of the manuscript.
Ethical Approval and Consent to participate
This study was approved by the Institutional Review Board of the MacKay Memorial Hospital, Taipei, Taiwan (approval number, 20MMHIS140e).
Availability of supporting data
The datasets used for the analysis in the present study are available from the corresponding author on reasonable request.
References
- 1.Our World in Data. Coronavirus Pandemic (COVID-19). (2021) [cited 2021 June 30] Available from:https://ourworldindata.org/covid-vaccinations.
- 2.WHO . Coronavirus disease (COVID-19): vaccines. (2021) [cited 2021 June 30]. Available from:https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines?adgroupsurvey={adgroupsurvey}&gclid=CjwKCAjw_o-HBhAsEiwANqYhp_ZinKdR5N3rlvVfpDQU_0xaeUGWru7XUg-RVhFvrLPo1iM19w4lzBoCHzQQAvD_BwE.
- 3.Chen CC, Tseng CY, Choi WM, et al. taiwan government-guided strategies contributed to combating and controlling COVID-19 pandemic. Front Public Health. 2020;8:547423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu NC, Chi H, Tai YL, et al. Impact of wearing masks, hand hygiene, and social distancing on influenza, enterovirus, and all-cause pneumonia during the coronavirus pandemic: retrospective national epidemiological surveillance study. J Med Internet Res. 2020;22(8):e21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath PT, Galiza EP, Baxter DN, et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med. 2021. DOI: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald I, Murray SM, Reynolds CJ, et al. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bian L, Gao F, Zhang J, et al. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20(4):365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 Variants. N Engl J Med. 2021;384(23):2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause PR, Fleming TR, Longini IM, et al. SARS-CoV-2 Variants and Vaccines. N Engl J Med. 2021;385(2):179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledford H.Could mixing COVID vaccines boost immune response? Nature. 2021;590(7846):375–376. [DOI] [PubMed] [Google Scholar]
- 15.Shaw RH, Stuart A, Greenland M, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. [DOI] [PubMed] [Google Scholar]
- 17.Chi H, Chiu NC, Tai YL, et al. Clinical features of neonates born to mothers with coronavirus disease-2019: a systematic review of 105 neonates. J Microbiol Immunol Infect. 2021;54(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343(d5928):d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (Ottawa Hospital Research Institute, 2021) Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 20.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. [DOI] [PubMed] [Google Scholar]
- 21.Yu-Kang T. Node-Splitting Generalized Linear Mixed Models for Evaluation of Inconsistency in network meta-analysis. Value Health. 2016;19(8):957–963. [DOI] [PubMed] [Google Scholar]
- 22.Chaimani A, Salanti G, Leucht S, et al. Common pitfalls and mistakes in the set-up, analysis and interpretation of results in network meta-analysis: what clinicians should look for in a published article. Evid Based Ment Health. 2017;20(3):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostadgavahi AT, Booth R, Sisson G, et al. Heterologous immunization with Covishield and Pfizer vaccines against SARS-CoV-2 elicits a robust humoral immune response. J Infect Dev Ctries. 2021;15(5):653–656. [DOI] [PubMed] [Google Scholar]
- 25.Spencer AJ, McKay PF, Belij-Rammerstorfer S, et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat Commun. 2021;12(1):2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan HX, Juno JA, Lee WS, et al. Immunogenicity of prime-boost protein subunit vaccine strategies against SARS-CoV-2 in mice and macaques. Nat Commun. 2021;12(1):1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Shaw RH, Stuart AS, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a Single-Blind, randomised, non-inferiority trial. Lancet. 2021. DOI: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groß R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity. medRxiv. 2021. DOI: 10.1101/2021.05.30.21257971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021. DOI: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edara VV, Hudson WH, Xie X, et al. Neutralizing Antibodies Against SARS-CoV-2 Variants After Infection and Vaccination. JAMA. 2021;325(18):1896–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. [DOI] [PubMed] [Google Scholar]
- 35.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett JR, Belij-Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279–288. [DOI] [PubMed] [Google Scholar]
- 37.Stamatatos L, Czartoski J, Wan Y-H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds CJ, Pade C, Gibbons JM, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372(6549):1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crotty S. Hybrid immunity. Science. 2021;372(6549):1392. [Google Scholar]
- 40.Palgen J-L, Feraoun Y, Dzangué-Tchoupou G, et al. Optimize prime/boost vaccine strategies: trained immunity as a new player in the game. Front Immunol. 2021;12(554). DOI: 10.3389/fimmu.2021.612747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Zhu L, Huang W, et al. Potent RBD-specific neutralizing rabbit monoclonal antibodies recognize emerging SARS-CoV-2 variants elicited by DNA prime-protein boost vaccination. Emerg Microbes Infect. 2021;10(1):1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flaxman A, Marchevsky N, Jenkin D, et al. Tolerability and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 (AZD1222). SSRN preprint, (2021). DOI: 10.2139/ssrn.3873839 Available from:https://ssrn.com/abstract=3873839 [DOI]
- 44.Normark J, Vikström L, Gwon Y-D, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N Engl J Med. 2021. DOI: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo TY, Lin MY, Coffman RL, et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci Rep. 2020;10(1):20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. [DOI] [PubMed] [Google Scholar]
- 48.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021. DOI: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for the analysis in the present study are available from the corresponding author on reasonable request.


