1. Introduction
A vaccination program is considered the most important public health tool in overcoming an infectious disease, with a key impact on morbidity and mortality at the global level. Therefore, the Coronavirus disease-2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) drove the need to rapidly test and approve new effective vaccines. The main objectives in the clinical development program were focused on protecting from symptomatic disease, reducing the risk of becoming seriously ill, and preventing infection by stopping the transmission. In this editorial, I review evidence of the safety, efficacy, and effectiveness of the COVID-19 vaccines authorized by the European regulatory authority until June 2021.
Clinical trials data: efficacy and safety
The European Medicines Agency (EMA) to confirm that a vaccine is safe, provides adequate protection, and is of suitable quality, recommends approval after a thorough evaluation. According to the clinical requirements for COVID-19 vaccines for obtaining a conditional marketing authorization in the European Union (EU), at least one well-designed large-scale phase III efficacy trial should be conducted, with the primary endpoint defined as laboratory-confirmed COVID-19 disease of any severity in study participants seronegative for SARS-CoV-2 at baseline; the point estimate of vaccine efficacy was set at least 50% with a lower bound of the 95% confidence interval >20% (preferably >30%), and it was recommended to assess vaccine efficacy against severe disease, hospitalization, and death as secondary endpoints [1]. Moreover, the pivotal trial should include individuals with pre-existing comorbidities and aged ≥65 years. A review of at least 6 weeks post-vaccination safety data should be available [1]; however, a median follow-up duration of 2 months after completion of the full vaccination regimen was considered sufficient by EMA to provide adequate information in the Dossier to assess a vaccine’s benefit-risk profile.
EMA, using the rolling review regulatory tool to speed up the assessment process during a public health emergency, granted four conditional approvals for COVID-19 vaccines that met a positive benefit-risk balance: BNT162b2 (Comirnaty), mRNA-1273 (COVID-19 Vaccine Moderna), ChAdOx1-S (Vaxzevria), and Ad26.COV2-S (COVID-19 Vaccine Janssen) (Table 1). Both BNT162b2 and mRNA-1273 are based on nucleoside-modified mRNA encoding for the full-length SARS-CoV-2 spike (S) glycoprotein encapsulated in lipid nanoparticles, while ChAdOx1-S and Ad26.COV2-S are monovalent, recombinant, replication-incompetent chimpanzee and type 26 adenovirus vectors, respectively, encoding the S glycoprotein [2–5].
Table 1.
COVID-19 vaccines approved in the European Union
| Generic name | Brand name | Marketing authorization holder | Composition | Posology | Approved indication |
| BNT162b2 | Comirnaty | BioNTech Manufacturing GmbH | 30 µg of mRNA (nucleoside modified), embedded in lipid nanoparticles | 2 doses (0.3 mL each) 3 weeks apart | ≥12 years |
| mRNA-1273 | COVID-19 Vaccine Moderna | Moderna Biotech Spain | 100 µg of mRNA (nucleoside modified), embedded in lipid nanoparticles | 2 doses (0.5 mL each) 4 weeks apart | ≥18 years |
| ChAdOx1-S | Vaxzevria | AstraZeneca AB | 2.5 x 108 infectious units of ChAdOx1-S (recombinant) | 2 doses (0.5 mL each) 4–12 weeks apart | ≥18 years |
| Ad26.COV2-S | COVID-19 Vaccine Janssen | Janssen-Cilag International NV | 8.92 log10 infectious units of Ad26.COV2-S (recombinant) | 1 dose (0.5 mL) | ≥18 years |
mRNA: single-strained, 5ʹ-capped messenger RNA produced using a cell-free in vitro transcription from corresponding DNA templates, encoding the viral spike protein of SARS-CoV-2 [2,3]. ChAdOx-S: Chimpanzee Adenovirus encoding the SARS-CoV-2 Spike glycoprotein, produced in genetically modified human embryonic kidney 293 cells and by recombinant DNA technology [4]. Ad26.COV2-S: Adenovirus type 26 encoding the SARS-CoV-2 Spike, produced in the PER.C6 TetR cell line and by recombinant DNA technology [5].
BNT162b2 was approved in December 2020 for individuals ≥16 years old, but at the end of May 2021 the marketing authorization was extended to children aged 12–15 years.
All vaccines are administered intramuscularly.
The efficacy and safety of COVID-19 vaccines were evaluated by Phase 3 randomized controlled trials, whose primary endpoint was the symptomatic infection. Among 34,922 participants evaluated at 7 days after the second dose, BNT162b2 was effective at 95.0% [95% Confidence Interval (CI) 90.0–97.9] in preventing symptomatic COVID-19, and the protective effect was consistent among age groups [2] (Figure 1A); while the efficacy of a single dose was 52% (95% CI 30–68). Similarly, mRNA-1273 efficacy, among 28,207 participants evaluated after 14 days post-dose 2, was 94.1% [95% CI 89.3–96.8], higher in those aged 18–64 years compared to ≥65 years old [3]. For ChAdOx1-S vaccine, the initial intent was to implement a one dose only immunization schedule, but following a review of immunogenicity data a second dose was introduced. However, due to delays that occurred in clinical trial material availability for second dose vaccinations, the interval between doses 1 and 2 ranged from 4 to 26 weeks. Data suggested that a longer interval could give better results. ChAdOx1-S efficacy was 59.5% (95% CI 45.8–69.7) in 10,448 participants (enrolled in two different trials conducted in UK and Brazil) receiving two shots with a dosing interval ranging from 4 to 12 weeks [4]; evidence showed protection starts from approximately 3 weeks after the first dose of vaccine, estimated to be about 70%, with significant differences between UK (40%) and Brazil (80%); of note, an increasing trend in efficacy was found across the dosing interval from 53.3% (95% CI −3.2–78.9) to 78.8% (95% CI 37.6–92.8) after 4 and 12 weeks respectively, probably due to the decrease of anti-vector antibodies. Most enrolled subjects were 18–55 years old, and those aged ≥65 years were only approximately 7% (about 600 participants), therefore disease protection was not estimated in the elderly. After at least 2 weeks from vaccination, Ad26.COV2-S vaccine, evaluated on 39,321 participants, showed an efficacy equal to 66.9% (95% CI 59.0–73.4), slightly higher in older adults (82.4%, 95% CI 63.9–92.4); moreover, higher efficacy was detected in the United States (74.4%, 95% CI 65.0–81.6) as compared to Brazil (66.2%, 95% CI 51.0–77.1) and South Africa (52.0%, 95% CI 30.3–67.4), likely due to virus variants [5].
Figure 1.
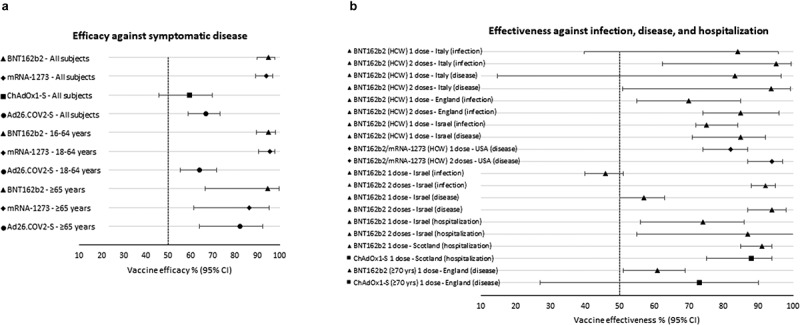
Efficacy (Panel A) and effectiveness (Panel B) of COVID-19 vaccines [2–10,12,13]
For the mRNA-based vaccines the most frequent adverse reactions were injection site pain, fatigue, headache, myalgia and chills, arthralgia, pyrexia; uncommon events were lymphadenopathy and hypersensitivity; rare events were acute peripheral facial paralysis and anaphylaxis. For ChAdOx1-S a common disorder was thrombocytopenia, and an imbalance in the frequency of adverse events reported as nervous system disorders was observed between vaccinated and controls; moreover, one case of transverse myelitis was considered related to the vaccine due to the timing and lack of alternative causes, further, there was a single case of multiple sclerosis whose causality to the vaccine could not be excluded. With Ad26.COV2-S an imbalance between vaccine and placebo groups was observed for embolic and thrombotic events (deep vein thrombosis, pulmonary embolism, sinus venous thrombosis), tinnitus, and urticaria.
Post-marketing experience: effectiveness
After marketing authorization, several studies were conducted to assess vaccines’ effectiveness (VE). Indeed, while efficacy can be defined as the performance of an intervention under ideal and controlled circumstances (i.e. clinical trial), effectiveness refers to its performance in the real-world setting.
Vaccination campaigns in EU initially targeted healthcare workers (HCW) who were immunized with BNT162b2 vaccine. In a retrospective cohort study conducted in an Italian province on 6,423 HCW, VE in preventing both asymptomatic and symptomatic SARS-CoV-2 infections was 84.1% (95% CI 39.7–95.8) at 14–21 days after the administration of the first dose, and 95.1% (95% CI 62.4–99.4) at least 7 days after the second dose, whereas VE against the symptomatic disease was 83.3% (95% CI 14.8–96.7) and 93.7% (95% CI 50.8–99.2) respectively [6] (Figure 1B). In a prospective cohort study conducted in England on 23,324 HCW, a single dose of the vaccine showed VE against infections of 70% (95% CI 55–85) 21 days after the first dose, which increased to 85% (95% CI 74–96) 7 days after two doses [7]. An early rate reduction of SARS-CoV-2 infection and COVID-19 was also assessed during the national immunization campaign in Israel on 9,109 HCW; VE against SARS-CoV-2 infection was 75% (95% CI 72–84), and increased to 85% (95% CI 71–92) for symptomatic COVID-19 for days 15–28 after the first dose [8]. Finally, results from a test-negative case-control study conducted among HCW at 33 sites in the United States indicated that VE against the symptomatic disease of a single dose (measured 14 days after the first dose through 6 days after the second dose) was 82% (95% CI 74–87) that increased to 94% (95% CI 87–97) at ≥7 days after the second dose [9].
A large study conducted in Israel (596,618 vaccinated persons matched to unvaccinated controls) estimated a VE at 14–20 days after the first dose and ≥7 days after the second equal to 46% (95% CI, 40–51) and 92% (95% CI 88–95) for SARS-CoV-2 infection, 57% (95% CI 50–63) and 94% (95% CI 87–98) for symptomatic COVID-19, 74% (95% CI 56–86) and 87% (95% CI 55–100) for hospitalization [10]. Another study in Israel following the nationwide vaccination campaign confirmed a VE after the second dose of 97.0% (95%CI 96.7, 97.2) against symptomatic COVID-19, and 97.2% (95%CI 96.8, 97.5) against hospitalization [11]. Data from mass COVID-19 vaccination in Scotland showed a VE of 91% (95%CI 85, 94) and 88% (95%CI 75, 94) in reducing hospital admissions at 28–34 days post the first dose for BNT162b2 and ChAdOx1-S, respectively [12]. According to a recent study in England, BNT162b2 and ChAdOx1-S vaccines were both effective at preventing older adults aged ≥70 years from symptomatic disease [13]. Specifically, BNT162b2 reached a VE of 61% (95% CI 51–69) from 28–34 days after vaccination and then stabilized, while ChAdOx1-S from a VE of 60% (95% CI 41–73) increased to 73% (95% CI 27–90) from day 35 onwards; reduced risk of emergency hospital admission was observed after one dose of BNT162b2 (43%, 95% CI 33–52) and ChAdOx1-S (37%, 95% CI 3–59).
All four vaccines were effective against the Alfa (B.1.1.7) variant, however, a decrease in neutralizing activity for Beta (501Y.V2) and Gamma (P.1) variants emerged. Specifically, mRNA-based and Ad26.COV2-S vaccines maintained even if reduced their efficacy [5,14], while ChAdOx1-S during a trial conducted in South Africa (which was stopped) showed minimal protection against the variant identified in that country. Finally, recent data suggest that protective immunity by the mRNA vaccines is likely retained against the Delta (B.1.617.1) variant [15].
Pharmacovigilance
A well-established pharmacovigilance system of the EU regulatory network (composed by national competent authorities of each member state) allows to monitor post-marketing safety, and to prepare a periodic report on the vaccines’ side effects to ensure that all new information collected are promptly reviewed and any emerging new information shared at Europen level [16].
A combination of thrombosis and thrombocytopenia, in some cases accompanied by bleeding, has been observed very rarely following vaccination with ChAdOx1-S and Ad26.COV2-S [4,5,17,18]. This includes severe cases presenting as venous thrombosis, comprising unusual sites such as cerebral venous sinus thrombosis, splanchnic vein thrombosis, as well as arterial thrombosis, concomitant with thrombocytopenia. Fatal outcomes have been reported. These cases occurred within the first three weeks following vaccination, and mostly in women under 60 years of age, with a notification rate of 1/100,000 doses. Analyzing some cases of severe venous thromboembolism in unusual sites and concomitant thrombocytopenia, a common denominator was a high level of antibodies to PF4–polyanion complexes, suggesting that vaccination with ChAdOx1 can result in the rare development of immune thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4, which clinically mimics autoimmune heparin-induced thrombocytopenia [19,20]. As a result of the safety signal and subsequent evaluation, several EU countries have changed their recommendations on the use of ChAdOx1, administering the vaccine only to certain age groups, in most cases the elderly ≥60 years old; in addition, some countries currently administer the second dose with an mRNA vaccine [21]. Pharmacovigilance Risk Assessment Committee (PRAC) has also identified capillary leak syndrome (a serious condition that causes fluid leakage from small blood vessels) as a side effect of ChAdOx1-S vaccine (mostly occurred in women) and concluded that this is a contraindication for people who have previously had this syndrome; moreover, PRAC assessed cases of Guillain-Barré syndrome (an immune system disorder that causes nerve inflammation), and of acute disseminated encephalomyelitis (an autoimmune disease characterized by sudden, widespread inflammation in the brain and spinal cord) and encephalitis reported as suspected side effects after vaccination with ChAdOx1-S [17]. PRAC is also continuing its assessment of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the membrane around the heart) reported in a small number of people following vaccination with COVID-19 vaccines. This assessment follows case reports of myocarditis/pericarditis (mainly males under 30 years of age) after vaccination with BNT162b2 and mRNA-1273 [22,23]. Moreover, reports of facial swelling in persons who have received dermatological fillers after mRNA-based vaccines were recorded.
The Italian Medicines Agency has published the fifth COVID-19 Vaccine Surveillance Report for the four vaccines used in the current vaccination campaign [24]. Over the period from December 2020 to May 2021, 66,258 reports were recorded out of 32,429,611 administered doses with a notification rate of 204 per 100,000 doses: 90% of notifications were related to non-serious events (mostly flu-like symptoms) and 10% (21 per 100,000 doses) to serious events, regardless the possible causal role of vaccination. Specifically, the notification rate (per 100,000 doses) was 36 for ChAdOx1-S, 18 for BNT162b2, 13 for mRNA-1273, and 5 for Ad26.COV2-S. BNT162b2 was the most used (69%) in the vaccination campaign in Italy, followed by ChAdOx1-S (21%), mRNA-1273 (9%), and Ad26.COV2-S (1%).
Concluding remarks
Data coming from vaccination campaigns confirmed that authorized vaccines are effective in preventing infection, disease, and hospitalization, supporting the results of phase III trials, although some differences emerged between mRNA- and vector-based vaccines. BNT162b2 and mRNA-1273 showed higher efficacy in randomized controlled trials with a better safety profile in the post-marketing, proving suitable for all age groups as compared to ChAdOx1-S and Ad26.COV2-S. As expected, after the first dose lower protection from COVID-19 was detected for BNT162b2 as compared to ChAdOx1-S that in the clinical development foresaw a single shot as Ad26.COV2-S; however, during the trial a second dose for longer-term protection was added. It is of note that mRNA-based platform is more flexible to adapt the antigen to new emerging virus variants in a short time, while vector-based vaccines may have low efficacy for the pre-existing anti-vector immune responses in case of repeated vaccinations are needed. However, mRNA- compared to vector-based vaccines have higher costs and require the availability of cold chain capacity; moreover, two close injections are required to be fully effective against the disease and its complications.
In the absence of definitive correlates of protective immunity, the presence of neutralizing antibodies against SARS-CoV-2 provides the best current indication for protection. Although the available evidence is still limited, results from observational studies of VE against symptomatic and asymptomatic infection, viral load, and duration of viral shedding are suggestive of substantial protection against infection, and consequently of relevant effect against transmission [25].
In conclusion, real-world VE data are key for guiding evolving COVID-19 vaccine policy, and further information and monitoring will be essential to make conclusions about the duration of protection.
Funding Statement
This paper was not funded.
Declaration of Interests
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer has disclosed that they have received personal consulting fees from Sanofi Pasteur outside the work reviewed in this manuscript.
Author contribution
SB conceived the review article, interpreted the relevant literature, and wrote the manuscript.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.European Medicines Agency . EMA considerations on COVID-19 vaccine approval (EMA/592928/2020), 16November2020. Available from:https://www.ema.europa.eu/en/ema-considerations-covid-19-vaccine-approval [Last accessed 28 June 2021] [Google Scholar]; •• This is a very important guideline on clinical aspects of the development of COVID-19 vaccines since it covers recommendations and scientific considerations related to requirements for marketing authorization and post-approval follow-up for safety and efficacy.
- 2.Annex 1, Summary of product characteristics (Comirnaty) . [Last accessed 28 June 2021]. Available fromhttps://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf [Google Scholar]
- 3.Annex 1, Summary of product characteristics (COVID-19 Vaccine Moderna) . Available from:https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-moderna-epar-product-information_en.pdf [Last accessed 28 June 2021]
- 4.Annex 1, Summary of product characteristics (Vaxzevria) . Available from: https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf[Last accessed 28 June2021]
- 5.Annex 1, Summary of product characteristics (COVID-19 Vaccine Janssen) . Available from:https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf [Last accessed 28 June 2021]
- 6.Fabiani M, Ramigni M, Gobbetto V, et al.Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy.27December2020to 24 March 2021. Euro Surveill 2021.26172100420. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is an important article that evaluates the effectiveness of BNT162b2 vaccine in preventing both asymptomatic and symptomatic infections among healthcare workers.
- 7.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;S0140-6736(21):00790–X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amit S, Regev-Yochay G, Afek A, et al. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel — 33 U.S. Sites .January–March2021. Morbidity and Mortality Weekly Report. Early Release. 70; May14. Available from:https://www.cdc.gov/mmwr/volumes/70/wr/mm7020e2.htm?s_cid=mm7020e2_w [Last accessed 28 June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagan N, Barda N, Kepten E, et al., BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 384(15): 1412–1423. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is an important article that evaluates the effectiveness of BNT162b2 vaccine in a national vaccination campaign.
- 11.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;S0140–6736(21):00947–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasileiou E, Simpson CR, Shi T, et al.Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study.Lancet. 2021;397(10285):1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is an important article that evaluates the effectiveness of BNT162b2 and ChAdOx1-S after one dose of vaccine against hospitalizations in a national campaign.
- 13.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdool Karim SS, de Oliveira T, New SARS.. CoV-2 Variants - Clinical, Public Health, and Vaccine Implications. N Engl J Med. 2021;384(19):1866–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edara VV, Lai L, Sahoo MK, et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv preprint. Available from:https://www.biorxiv.org/content/10.1101/2021.05.09.443299v1.full.pdf [Last accessed 28 June 2021] [DOI] [PMC free article] [PubMed]
- 16.European Medicines Agency . Pharmacovigilance Plan of the EU Regulatory Network for COVID-19 Vaccines (EMA/333964/2020). Available from:https://www.ema.europa.eu/en/documents/other/pharmacovigilance-plan-eu-regulatory-network-covid-19-vaccines_en.pdf [Last accessed 28 June 2021]
- 17.European Medicines Agency . COVID-19 vaccine safety update, 18 June 2021. Vaxzevria. Available from:https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-p reviously-covid-19-vaccine-astrazeneca-18-june-2021_en.pdf [Last accessed 28 June 2021]
- 18.European Medicines Agency . COVID-19 vaccine safety update, 18 June 2021. COVID-19 Vaccine Janssen. Available from:https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-covid-19-vaccine-janssen-18-june-2021_en.pdf [Last accessed 28 June 2021]
- 19.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(22):2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Centre for Disease Prevention and Control . Overview of EU/EEA country recommendations on COVID-19 vaccination with Vaxzevria, and a scoping review of evidence to guide decision-making. 18 May 2021. Stockholm: ECDC; 2021. Available from:https://www.ecdc.europa.eu/en/publications-data/overview-eueea-country-recommendations-covid-19-vaccination-vaxzevria-and-scoping [Last accessed 28 Jun]2021
- 22.European Medicines Agency . COVID-19 vaccine safety update, 18 June 2021. Comirnaty. Available from:https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-comirnaty-18-june-2021_en.pdf [Last accessed 28 June 2021]
- 23.European Medicines Agency . COVID-19 vaccine safety update, 18 June 2021. COVID-19 Vaccine Moderna. Available from:https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-spikevax-previously-covid-19-vaccine-moderna-18-june-2021_en.pdf [Last accessed 2021 June 28]
- 24.Agenzia Italiana del Farmaco . Rapporto sulla sorveglianza dei vaccini COVID-19 (5). [Italian Medicines Agency. Fourth AIFA Report on COVID-19 Vaccine Surveillance]. 27/12/2020 – 26/05/2021. Available from: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_vaccini_COVID-19_5.pdf [Last accessed 2021 June 28]
- 25.Leshem E, Lopman BA. Population immunity and vaccine protection against infection. Lancet. 2021;397(10286):1685–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]


