Abstract
Objective
The relationship between 25-hydroxyvitamin D3 (25(OH)D), the surrogate marker for vitamin D3, serum concentration and COVID-19 has come to the forefront as a potential pathway to improve COVID-19 outcomes. The current evidence remains unclear on the impact of vitamin D status on the severity and outcomes of COVID-19 infection. To explore possible association between low 25(OH)D levels and risk of developing severe COVID-19 (i.e. need for invasive mechanical ventilation, the length of hospital stay, total deaths). We also aimed to understand the relationship between vitamin D insufficiency and elevated inflammatory and cardiac biomarkers.
Methods
We conducted a comprehensive electronic literature search for any original research study published up to March 30, 2021. For the purpose of this review, low vitamin D status was defined as a range of serum total 25(OH)D levels of <10 to <30 ng/ml. Two independent investigators assessed study eligibility, synthesized evidence, analyzed, critically examined, and interpreted herein.
Results
Twenty-four observational studies containing 3637 participants were included in the meta-analysis. The mean age of the patients was 61.1 years old; 56% were male. Low vitamin D status was statistically associated with higher risk of death (RR, 1.60 (95% CI, 1.10–2.32), higher risk of developing severe COVID-19 pneumonia (RR: 1.50; 95% CI, 1.10–2.05). COVID-19 patients with low vitamin D levels had a greater prevalence of hypertension and cardiovascular diseases, abnormally high serum troponin and peak D-dimer levels, as well as elevated interleukin-6 and C-reactive protein than those with serum 25(OH)D levels ≥30 ng/ml.
Conclusions
In this meta-analysis, we found a potential increased risk of developing severe COVID-19 infection among patients with low vitamin D levels. There are plausible biological mechanisms supporting the role of vitamin D in COVID-19 severity. Randomized controlled trials are needed to test for potential beneficial effects of vitamin D in COVID-19 outcomes.
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic is undoubtedly one of the most unprecedented infectious diseases in the recent history. The first reported case of COVID-19 goes back to December 2019 with exponential proliferation of the cases within a very short time, as of April 7, 2021 there have been 131,837,512 cases and 2,862,664 deaths worldwide (1). COVID-19 is caused by a novel coronavirus, Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) and was first discovered in Wuhan, Hubei Province, China (2). Based on phylogenetic analysis, it is postulated that this SARS-like virus originated in bats and subsequently transmitted via an intermediate carrier into humans (3). SARS-CoV-2 differs from other coronaviruses of the past due to its very high rate of transmission and its relatively high mortality (2, 4–6). Although geographical variability exists, it is worth recognizing that SARS-CoV-2 appears to have a lower case-fatality rate compared to other coronaviruses (7, 8). COVID-19 has a variety of symptoms ranging from asymptomatic to severe outcomes such as respiratory failure and death (9). There are also strong association between COVID-19 infection and the presence of preexisting comorbidities such as hypertension, diabetes, obesity, chronic obstructive pulmonary disorder, cardiovascular disease, malignancy, human immunodeficiency virus, and renal disease. These conditions are not only associated with higher risk of infection but also increased risk of severe disease and mortality (10). Age has been found to be one of the most prominent risk factors for severity of COVID-19 where children specifically are less frequently and less severely affected than adults and geriatric patients (11).
Vitamin D3 is often recognized for its role in calcium and phosphorous homeostasis, however it is also a key hormone in myriad diverse biological processes (12). There are two main ways to acquire vitamin D3, either through synthesis within the body or by ingestion via food or dietary health supplements. The primary source of vitamin D in the body is the epidermis where 7-dehydrocholesterol transformed into vitamin D3 under ultraviolet B (UVB) light from sunlight (13). Vitamin D status has been linked to many factors including seasonal variation (increase in summer and decrease in winter) or latitude (greater in latitudes close to the equator) (14). Vitamin D3 is biologically inactive and requires further cytochrome P450 (CYP)-mediated hydroxylation for activity. Vitamin D3 is first converted to 25-hydroxyvitamin D3 (25(OH)D3) by CYP2R1 (25-hydroxylase) in hepatocytes (15). The second hydroxylation occurs in the kidney where 25(OH)D3 is converted by CYP27B1 to its biologically active form, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), also known as calcitriol (16). It is important to recognize that due to technological convenience and circulating levels, 25(OH)D3 is measured as the surrogate marker of vitamin D levels and activity in humans, whereas calcitriol is the most potent vitamin D derivative (13, 17).
Though calcitriol has multiple functions, it is now known as a cornerstone immunomodulatory hormone through its action at the vitamin D3 receptor (VDR). VDR is present in a multitude of different cell types throughout the body and is also present in many cells of the immune system such as neutrophils, T-lymphocytes, macrophages and dendritic cells (18). Calcitriol has multiple effects on both adaptive and innate immune system and consequently is a key regulator of inflammation. In the innate immune system, calcitriol has been shown to promote differentiating effects on monocytes and monocyte-derived cell lines, resulting in phenotypical features of macrophages (19). It also has the ability to improve the chemotactic and phagocytic capacity of macrophages (20). In the adaptive immune system, calcitriol targets both antigen-presenting cells (APCs), like dendritic cells, and T helper (Th) cells directly. In APCS, calcitriol inhibits the surface expression of MHC-II-complexed antigen, other co-stimulatory molecules and production of interleukin-12 (IL-12) and IL-23, which indirectly causes a shift in T cells to a Th2 phenotype (21). These key immunomodulatory and anti-inflammatory properties of calcitriol are potentially the reasons that it is widely researched for a variety of disease states and most recently for potential benefits in COVID-19.
The cases of COVID-19-related acute cardiac injury have become increasingly prevalent. The inflammatory response to COVID-19 leads to increased systemic inflammatory markers and potential abnormal function of vital organ systems, including pulmonary and cardiovascular. We have previously shown that there is significant association between elevated cardiac and inflammatory biomarkers and the severity of COVID-19 (22). To our knowledge, no meta-analysis has addressed the association between vitamin D levels with cardiac and inflammatory biomarkers. At the time of writing this article, there is very limited clinical trial data on vitamin D3 supplementation as a treatment for COVID-19. There are however some observational study reports showing the potential of vitamin D3 as a viable treatment option. The primary goal of this meta-analysis is to study the relationship between vitamin D3 serum levels and the risk of developing COVID-19 infection in terms of mortality, severity, and inflammatory markers.
Methods
Study design
This review was designed to answer the following clinical research question: For adults age over 18 years old diagnosed with COVID-19 pneumonia, does vitamin D status impact the severity and outcomes of COVID-19 infection? The meta-analysis was performed based on the principles of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (23, 24). The primary outcomes of the study are all-cause mortality and COVID-19 severity including total number of severe cases, hospital duration, and the need for mechanical ventilation. Secondary outcomes of this study are laboratory markers of cardiac and inflammatory markers.
The primary objectives of this review were to evaluate the association between vitamin D level and risk of developing severe outcomes of COVID-19 infection and death. Data on total mortality at any stage of the illness, total number of severe patients, the need of invasive mechanical ventilation, and total days of hospital stay was sought and analyzed. The secondary focus of this review was to understand the relationship between low vitamin D (levels <30 ng/ml) and the inflammatory biomarkers such as C-reactive protein (CRP) and IL-6. We aimed to understand the potential mechanism behind cardiac injury with significant elevation of troponin and D-dimer. These biomarkers were found to be the most common in all the included studies.
Literature search strategy
We performed a comprehensive electronic search for any articles published up to March 30th, 2021, in the following databases: MEDLINE, EMBASE (through Ovid), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Cochrane Database of Systematic Reviews. The combination of the following medical subheadings (MeSH) and key words was used for database searches: COVID-19 OR coronavirus disease 2019 OR SARS-CoV-2 OR severe acute respiratory syndrome coronavirus 2 OR 2019-nCOV OR 2019 novel coronavirus OR coronavirus AND vitamin D OR ergocalciferol OR cholecalciferol. Alternative spellings and abbreviations of the above key words were also considered. The search results were limited to articles that were published in English language.
Inclusion criteria
Study titles and abstracts were reviewed, and publications were selected based on the following criteria: studies had to have a control group (sufficient vitamin D level), original study with COVID-19 patients, adults aged 18 years or older, serum 25(OH)D levels were reported. Reported serum 25(OH)D levels from the control and low vitamin D groups were needed for inclusion in our analysis. For the purpose of this review, low vitamin D level was defined as a serum total 25(OH)D level in the range from <10 to <30 ng/ml. Two investigators (MBE and RH) selected eligible trials according to the inclusion criteria independently. Disagreements were resolved by discussion until consensus was achieved or was discussed with another expert (JMW and SD) until consensus was achieved.
Exclusion criteria
Studies that are not conducted in humans, and adults <18 years of age were excluded. Abstracts were excluded if they were commentary, letter to editors, reviews (expert opinion, narrative, systematic review, or an overview), mechanistic papers, conference posters, non-COVID-19 patients, or had no control groups. Upon reading the full text, we excluded studies from further consideration if the patients were on active treatments for COVID-19 including vitamin D supplementations, lab reporting issues or if there was no mention of total number of subjects in each group. Studies were also excluded if they were focused on COVID-19 outcomes other than those listed above. We did not anticipate that any participants will be evaluated for vitamin D levels prior to COVID-19 infection. Therefore, it is unlikely that studies will select patients with deficient vitamin D levels, which is the group of individuals most likely to be affected by COVID-19.
Data extraction
The review authors (MBE, RH) independently performed the data extraction in duplicate. Discrepancies in data extraction were resolved by rechecking the data, discussing the outcome, and reaching to a consensus between the review authors and experts.
Data analysis
We used Review Manager (RevMan. version 5.3. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) to perform data synthesis and meta-analysis. Studies were weighted according to their sample size and event rate to produce the final-pooled risks ratios (RR). We performed meta-analysis using the Mantel–Haenszel method for dichotomous outcomes RR with 95% CI using a random-effect model, and the inverse variance method for continuous outcomes as mean differences (MD) with 95% confidence interval (95% CI) using a random-effect model. For laboratory values, if only the median and interquartile range (IQR25, IQR75) were reported, then it was assumed that the median was equal to the mean and that the standard deviation (SD) was (Q75-Q25)/1.35.
Assessment of heterogeneity
The I2 statistic test was performed to assess in-between study heterogeneity (I2 of < 25%, 25–50%, 50–75%, and >75% indicating no, low, moderate, and high degree of heterogeneity, respectively). The statistical significance was set at 95% CI and p value < 0.05. Each laboratory parameter from the included studies was reported in different units, which were all converted into one common unit for the final analysis.
Results
Study characteristics
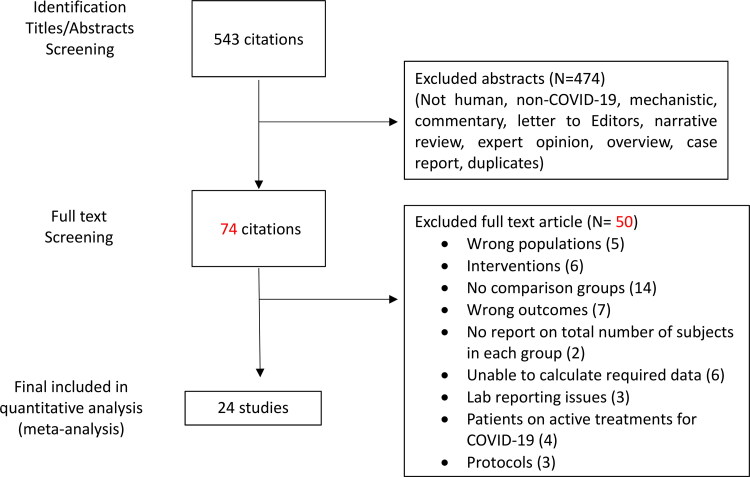
After screening of 543 citations, 474 studies were excluded, and a total of 24 observational studies were included in the final analysis (25–48). Sixteen studies were retrospective and eight studies were prospective in design. In total, 3637 patients were included. All were problematic with potential confounding as expected from non-randomized studies. Figure 1 shows the detailed selection process and Table 1 summarizes the characteristics of the included studies in this meta-analysis. The included studies represent broad geographic representation with mixed populations. Most studies were performed exclusively in hospital settings, mainly in Europe (UK, Italy, Germany, Belgium, Greece, and Spain), North America (USA), and Asia (China, Turkey, Iran, India, Pakistan). No studies were performed in Africa or in Australia.
Figure 1.
Flow chart of literature search and selection process.
Table 1.
Summary characteristics of included studies.
| First author country |
Total N | Age Years | % Male | Low Vitamin D* cutoff | Study design | Comorbidity % >10% | Ref. |
|---|---|---|---|---|---|---|---|
| Abrishami Iran |
73 | 55 | 64% | <25 ng/ml | Retrospective study | Hypertension (24.7%) Chronic kidney disease (21.9%) |
(25) |
| Adami Italy |
61 | 69.4 | 52.5% | <20 ng/ml | Retrospective observational study | Hypertension (59%) Cardiovascular diseases (27.8%) Diabetes. (18%) Cancer (18%) Chronic kidney disease (18%) Chronic obstructive pulmonary disease (18%) |
(26) |
| Angelidi USA |
144 | 66 | 44.4% | <30 ng/ml | Retrospective, observational, cohort | Hypertension (73.6%) Hyperlipidemia (54.9%) Diabetes. (43.8%) |
(27) |
| Anjum Pakistan |
140 | 42.5 | 58.6% | <10 ng/ml | Prospective cohort | NR | (28) |
| Baktash UK |
70 | 80 | 70% | <30 ng/ml | Prospective cohort study | Hypertension (48.5%) Diabetes (37.1%) |
(29) |
| Carpagnano Italy |
42 | 65 | 71% | <30 ng/ml | Retrospective, observational single center study | Total: 86% Hypertension (26%) |
(30) |
| Cereda Italy |
129 | 77 | 54% | <20 ng/ml | Prospective cohort | Hypertension (70.1%) Ischemic heart disease (40.9%) Diabetes. (30.7%) |
(31) |
| Charoenngam USA |
287 | 62 | 52.6% | <30 ng/ml | Retrospective chart review cross-sectional study | Hypertension (79.9%) Diabetes (56%) Hyperlipidemia (58.2%) Chronic kidney disease (37.6%) |
(32) |
| Demir Turkey |
227 | 46.3 | 44.66% | <30 ng/ml | Retrospective cohort study | NR | (33) |
| De Smet Belgium |
186 | 69 | 58.6% | <20 ng/ml | Retrospective observational trial | Coronary artery disease (61.5%) |
(34) |
| Gavioli USA |
437 | 67 | 48% | <30 ng/ml | Retrospective, observational cohort study | Hypertension (68%) Diabetes (45%) Coronary artery disease (30%) Malignancy (24%) |
(35) |
| Hernandez Spain |
197 | 61 | 62.4% | <20 ng/ml | Retrospective case-control study | Hypertension (38.6%) | (36) |
| Jevalikar India |
409 | 54 | 68.9% | <20 ng/ml | Prospective observational study | Diabetes (46.1%) Hypertension (40%) Hypothyroidism (14.9%) |
(37) |
| Karahan Turkey |
149 | 63.5 | 54.4% | <20 ng/ml | Retrospective observational study | Hypertension (57%) Diabetes (40.9%) Dyslipidemia (26.2%) Coronary artery disease (21.5%) Chronic kidney disease (19.5%) |
(38) |
| Luo China |
74 | 62.5 | 58.1% | <30 ng/ml | Retrospective cross-sectional study | Total 67.6% | (39) |
| Maghbooli Iran |
235 | 59 | 61.3% | <30 ng/ml | Retrospective cross-sectional study/analysis |
Hypertension (44.4%) Diabetes (36.6%) |
(40) |
| Orchard UK |
50 | 60 | 56% | <20.83 ng/ml | Prospective cohort | Hypertension (40%) Diabetes (28%) Asthma (10%) |
(41) |
| Radujkovic Germany |
185 | 60 | 51% | <12 ng/ml | Prospective cohort | Cardiovascular disease (31%) | (42) |
| Ricci Italy |
52 | 68.4 | 48 % | <10 ng/ml | Cohort | Hypertension (42.3%) Obesity (23%) |
(43) |
| Tehrani Iran |
205 | 59.7 | 69% | <30 ng/ml | Descriptive retrospective study | Hypertension (44.4%) Diabetes (35.1%) Ischemic heart disease (24.9%) Chronic kidney disease (23.4%) |
(44) |
| Vassiliou Greece |
30 | 65 | 80% | <15.2 ng/ml | Retrospective observational study | Hypertension (50%) Hyperlipidemia (30%) |
(45) |
| Ye China |
60 | 43 | 37% | <12 ng/ml | Case control study | Hypertension (10%) Renal failure (26.6%) Diabetes (8.3%) |
(46) |
| Kerget Turkey |
88 | 49.1 | 46.6% | <20 ng/ml | Prospective cohort | Hypertension (11.36%) Diabetes (9%) |
(47) |
| Jain India |
154 | 46 | 48.5% | < 20 ng/ml | Prospective observational study | Diabetes Hypertension |
(48) |
Low vitamin D = vitamin D levels ranges from <10 to <30 ng/ml, NR: not reported.
Most of the patients were older with a pooled mean age of 61.1 years, predominately male (56%), and had multiple comorbid conditions. Various comorbidities were reported with hypertension being the most frequent, followed by cardiovascular diseases and diabetes. There were significant associations reported between low levels of vitamin D and severity of infection with cardiovascular diseases including hypertension being the most common. The definitions for vitamin D deficient were heterogeneous across studies. Low levels of 25(OH)D ranged from <10 to <30 ng/ml in low vitamin D group compared to the rest as the control group.
All-cause mortality
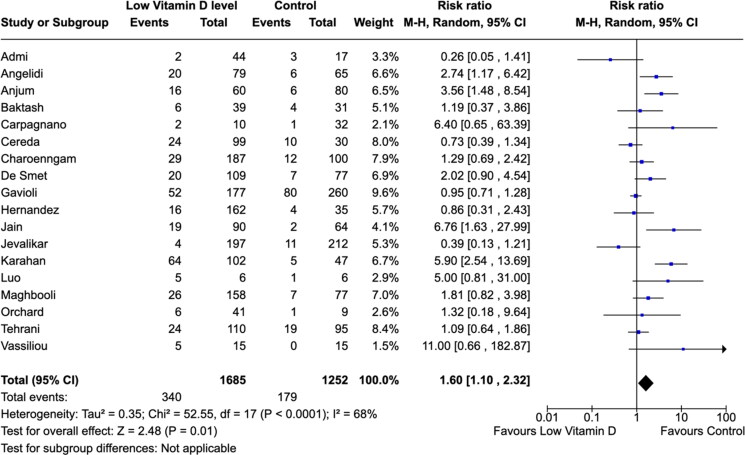
There is an association between low vitamin D levels and mortality. The meta-analysis of the 18 included studies indicated that low vitamin D level, compared with controls, was associated with higher risk of death (RR, 1.60 [95% CI,1.10–2.32]; P = 0.01; I2 = 68% (Figure 2). Data on total mortality was collected from a total of 2937 COVID-19 patients. Event rates were 340 per 1685 (20.17%) among COVID-19 patients with low vitamin D status versus 179 per 1252 (14.29%) in control group patients (Figure 2).
Figure 2.
Association between vitamin D levels and all-cause mortality.
COVID-19 severity
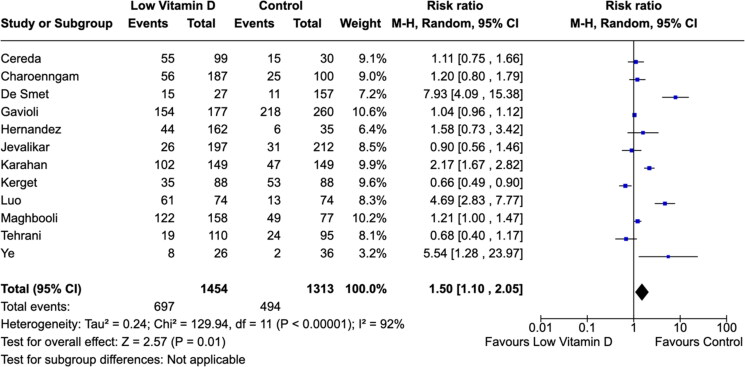
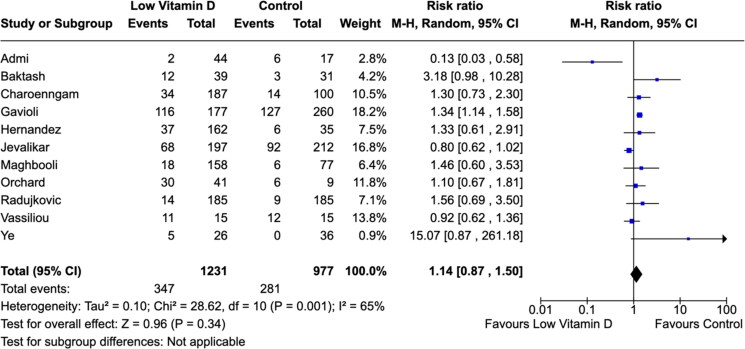
The definitions for severe COVID-19 were heterogeneous across studies. There were significant associations between low vitamin D level with severe COVID-19 cases (Figures 3 and 4). The meta-analysis suggested a considerable higher risk of severe COVID-19 infection in individuals with low vitamin D levels (RR: 1.50; 95% CI, 1.10–2.05) (Figure 3). Overall, 28.4% of COVID-19 patients required ICU admissions (628/2208). We found no difference in the need for mechanical ventilations in low vitamin D level ICU patients compared to control group patients (RR: 1.14; 95% CI, 0.87–1.50) (Figure 4).
Figure 3.
Association between vitamin D levels and COVID-19 severity.
Figure 4.
Association between vitamin D levels and need for mechanical ventilation.
Difference in biological markers and disease severity
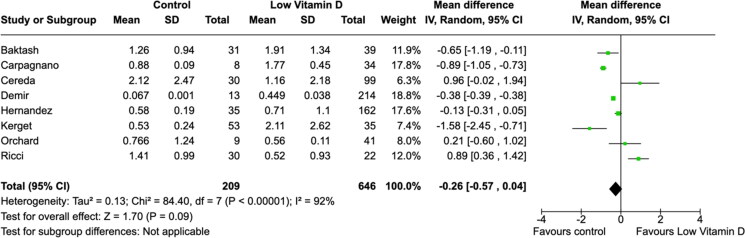
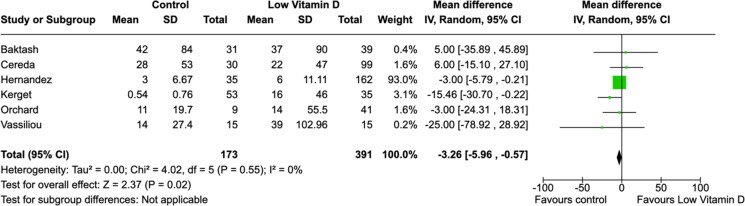
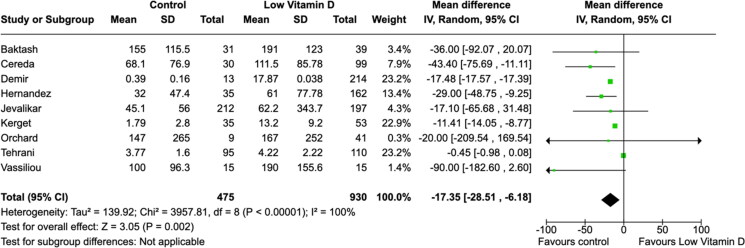
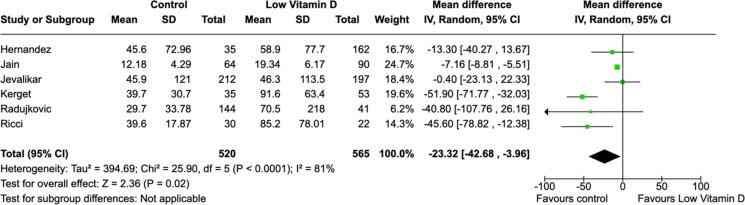
All laboratory parameters, both cardiac (troponin and D-dimer) and inflammatory (CRP and IL-6) biomarkers, were found to have significant mean differences between low vitamin D group and the control group (Figures 5–8). The cardiac biomarkers were abnormally high at all times. The difference in CRP and IL-6 levels between low vitamin D and control groups were statistically significant in all patients. Heterogeneity of the studies were very high for all biomarkers.
Figure 5.
Association between vitamin D and D-dimer (µg/ml) levels.
Figure 6.
Association between vitamin D and troponin (ng/L) levels.
Figure 7.
Association between vitamin D and C-reactive protein (mg/L) levels.
Figure 8.
Association between vitamin D and interleukin-6 (pg/mL) levels.
Discussion
This meta-analysis underlines the potential benefits of sufficient vitamin D levels and serves as a comprehensive summation of the currently available research on various COVID-19 outcomes in relation to measured serum vitamin D levels. Our work has described the association between vitamin D levels and COVID-19 markers representing severity of the disease including mortality, use of mechanical ventilation, and inflammatory parameters. The connection between vitamin D levels and COVID-19 is a prevalent topic of discussion to predict disease outcomes such as mortality and disease severity. In recent times, vitamin D has been highlighted as one of the key immunomodulatory hormones both in the innate and adaptive immune systems that support routine immune functions through VDR-mediated actions. In addition, the role of vitamin D in the inhibition of Renin Angiotensin System (RAS) links the importance of adequate vitamin D status as a protective factor during COVID-19 pandemic. These non-classical actions of regulating immune cell differentiation and proliferation by vitamin D have sparked research interest to help combat the ongoing COVID-19 pandemic (49).
We found a significant increase in all-cause mortality and COVID-19 severity among people with low vitamin D levels and the risk of death increases in patients with preexisting cardiovascular diseases and older adults. Sufficient vitamin D levels (levels >20–30 ng/ml) were found to decrease all-cause mortality and COVID-19 severity with p = 0.0001 and p < 0.00001, respectively. Cardiac biomarker (troponin and D-dimer) levels tended to be lower in the vitamin D sufficient COVID-19 patients. Biomarkers of inflammation (CRP and IL-6) were significantly higher in patients with low vitamin D levels. Our findings are consistent with other reviews. The meta-analysis reported by Bassatne et al. (2021) included studies up to December 18, 2020, and demonstrated a positive trend of increased mortality risk in patients with vitamin D levels <20 ng/ml (RR = 2.09, 0.92-4.77). The work included data only from seven studies for mortality outcome, with insufficient evidence provided with data only from two to three studies on ICU admission or invasive mechanical ventilation requirement (50). A systematic review and meta-analysis conducted by Pereira et al. (2020) included studies up to October 9, 2020, and found similar results in terms of mortality where vitamin D levels of less than 75 nmol/L (approximately 30 ng/ml) were found to be inversely related with mortality (OR = 1.82, 95% CI = 1.06–2.58; I2 = 59.0%), with a significant increased chance of hospitalization (OR = 1.81, 95% CI = 1.41–2.21; I2 = 0.0%) (51). Munshi et al. (2021), with studies analyzed up to June 8, 2020, found further supporting results in which patients with poor prognosis had significantly lower vitamin D serum levels compared to those with good prognosis. Poor prognosis was defined as severe presentation and ICU admission. The adjusted mean difference was −0.58 (95% CI = −0.83 to −0.34, p < 0.001) and −0.84 (95% CI = −1.32 to −0.36, p = 0.001) between ICU and floor admission, respectively (52).
The biological mechanisms linking vitamin D deficiency and excessive deaths are plausible. The exact mechanism of the beneficial effects of vitamin D sufficiency found in this meta-analysis is a topic for future research. However, it can be postulated that these are the results of anti-inflammatory actions of vitamin D (53). Mortality from COVID-19 is typically caused by severe acute respiratory syndrome, uncontrolled release of pro-inflammatory cytokines alongside unbalanced immune response, which eventually lead to cytokine storm and diffuse micro and macrovascular thrombosis, the occurrence of new disease states (e.g., myocarditis) or worsening of preexisting diseases (e.g., cardiovascular and kidney disease) (53, 54). Thus, association of low vitamin D levels and increased cardiovascular and inflammatory markers could translate into increased risk of cardiovascular morbidity and mortality (55).
Vitamin D may potentially reduce severity of respiratory tract infections. In the adaptive immune system vitamin D causes a shift away from Th-1 responses and toward Th-2 responses therefore decreasing the viral induction of inflammatory genes (56). Furthermore, vitamin D inhibits the development of pro-inflammatory Th-17 cells as well as modulates pro-inflammatory cytokines such as IL-1, IL-6 and IL-10 which are heavily involved in cytokine storms (57). These anti-inflammatory properties of vitamin D provide a link between COVID-19 mortality and severity and vitamin D levels (48, 54). According to a meta-analysis by Ji et al. (2020), elevated levels of white blood cells, CRP, erythrocyte sedimentation rate, IL-6, and IL-10 showed more severe COVID-19 disease and higher risk of death during follow up (58).
Due to the fast onset and evolving nature of the COVID-19 pandemic, there is a lack of randomized control trials on sufficient vitamin D levels and its potential benefits in COVID-19. The best available data at this time is strictly observational and lacks the robustness to prove a causative relationship between vitamin D serum levels and COVID-19 outcomes. We cannot exclude the possibility of residual confounding and did not aim to assess the quality of the included studies as a part of this review. The limitations of these associations are that the overall quality of evidence is low. Unmeasured differences in baseline comorbidities in combination with other potential confounding is the reason for high heterogeneity among the studies. It is important to point out that low RRs should be interpreted with caution, since RRs of observational studies <2 fall into the gray area of potential bias where usually confounding factors are difficult to control. In addition, the obtained confidence intervals have a very wide interval which indicates the need for larger sample size to better estimate the true value in the population. There is a lack of data availability for race or ethnicity in the included studies which precluded us from evaluating any potential effect of genetics or diet on their vitamin D status. Similarly, the contribution of seasonal variations on vitamin D levels in these studies remains unknown. Also, we aimed to evaluate the association between vitamin D levels and total days of hospital stay. However, there are no accurate data reported on this outcome. There was no difference in the average length of stay between vitamin D subgroups with 10.9 days in controls versus 14.4 days in low vitamin D patients. The results for this specific endpoint have been reported only in eleven included studies, which represent a small sample size with several associated issues described in details by Baktash et al. (29).
There are no clinically established data yet on the range of vitamin D levels recommended to protect against COVID-19. In general, in non-COVID-19 situations, there is consensus that sufficient vitamin D status is defined as >30 ng/ml (59–62). However, there has been ongoing debate regarding the definition of vitamin D deficiency as noted by different recommendations from various expert groups. The Institute of Medicine (United States) indicates that levels of <12 ng/ml are deficient, >20 ng/ml are sufficient and >50 ng/ml are potentially toxic (61, 62). In contrast, the Endocrine Society recognizes significantly higher levels for those categories: <20 ng/ml is deficient, and 21–29 ng/ml is insufficient (60, 62). Thus, there is disagreement on how to approach levels between 12 ng/ml and 30 ng/ml. Guidelines from certain agencies recommend a threshold value of 20 ng/ml, whereas others suggest a benefit for a higher threshold of 25(OH)D levels ≥30 ng/ml (60–62). For the purpose of this review, low vitamin D status was defined as a range of serum total 25(OH)D levels of <10 to <30 ng/ml. A sensitivity analyses were performed to evaluate the effect of each study’s different 25(OH)D cutoff values on the overall pooled effects, which did not significantly change these findings (Supplementary Materials). The results of this meta-analysis found a significant increase in all-cause mortality and COVID-19 severity among people with vitamin D levels <30 ng/ml, and the risk of death increases in preexisting cardiovascular diseases in older adults. The strength of the present study is that twenty-four studies were included and serves as an exhaustive list of the most current research in vitamin D levels and COVID-19. Based on our review, the clinical aim should be to raise vitamin D levels >30 ng/ml which will likely be the range of protection by vitamin D against respiratory infections. However, adequate supplementation is necessary to achieve >30 ng/ml 25(OH)D levels. It is important to point out that there are significant differences in the guidelines across the health agencies for daily recommended intake of vitamin D. The standard supplementation of 600 IU/day (Institute of Medicine, United States) will likely not achieve the concentrations needed to offer protection from COVID-19 infection. Recommendations for higher vitamin D supplementation (at least 20–25 micrograms or 800–1000 IU per day) for COVID-19 treatment were provided by the European and American Societies for Clinical Nutrition and Metabolism, European Food Safety Authority, National Institute for Health and Care Excellence, and Australian high-priority guidelines (63–65). People with different physiological factors, for example, obesity, might need greater intake of vitamin D to achieve >30 ng/ml 25(OH)D levels compared to healthy individuals (66). However, during pre-COVID-19 situations Endocrine Society of United States had recommended 1500-2000 IU/day intake to achieve sufficient levels of vitamin D (62). It is critical to recognize that the current vitamin D supplementation guidelines for COVID-19 are not evidence based and there is an urgent need for more information to guide clinical decision-making for COVID-19 patients.
To our knowledge, no meta-analysis has addressed the association between vitamin D levels with cardiac and inflammatory biomarkers. Herein, we show that vitamin D status may influence the severity of responses to COVID-19 infection. The evidence appears to be enough that clinicians and patients should be aware that severe COVID-19 cases may occur in people with vitamin D deficiency. Since vitamin D deficiency is modifiable, identifying individuals most susceptible to severe infection and treating them for vitamin D deficiency may represent a practical way to minimize COVID-19-associated fatality. If a causal link is established, VDR could be a potential therapeutic target. Vitamin D might be able to protect patients against developing severe form of disease. It has been hypothesized that vitamin D supplementation potentially would reduce the risks of cytokine storm, myocarditis, or cardiac injury via inhibition of RAS (48, 53–55, 58). Vitamin D supplements would present relatively safer (wide therapeutic window) and economic low risk intervention. Although currently there are 44 registered trials on supplementation of vitamin D in COVID-19 (https://clinicaltrials.gov/), currently there is no evidence that supplements reduce the risk of COVID-19 infection. Limited interventional trials have investigated the role of vitamin D supplementation for the treatment COVID-19 infection (67, 68). A Cochrane meta-analysis using a living systematic review approach, which has included evidence up to 11 March, 2021, identified three randomized clinical trials with 356 participants, of whom 183 received vitamin D (68). According to this analysis, there is currently insufficient evidence to determine the benefits and harms of vitamin D supplementation as a treatment of COVID-19. Future research should focus on well-designed studies with robust methods, which will likely improve our understanding of the role of vitamin D and its clinical benefits in COVID-19. Further randomized control studies are needed to demonstrate whether vitamin D might be effective in reducing all-cause mortality and COVID-19 morbidly.
Conclusion
Vitamin D status may play a significant role in developing severe COVID-19 infection. In this study low vitamin D status was associated with higher risk of all-cause mortality in COVID-19 positive patients. There is a plausible anti-inflammatory biological mechanism supporting the protective role of vitamin D in COVID-19 severity. Further research is needed in this area, in the form of randomized control trials, to determine whether there is a cause-effect relationship between low vitamin D status and COVID-19 outcomes.
Supplementary Material
Disclosure statement
The authors declare that they have no conflicts of interest.
References
- 1.World Health Organization (WHO) . WHO Coronavirus Disease (COVID-19) Dashboard. 2020. [cited 2020 October 14]; https://covid19.who.int/.
- 2.Naserghandi A, Allameh SF, Saffarpour R.. All about COVID-19 in brief. New Microbes New Infect. 2020;35:100678. doi: 10.1016/j.nmni.2020.100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R.. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–8. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G.. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123–38. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Rezza G, Brusaferro S.. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020; 323(18):1775–6. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 6.Bhowmik T, Tirtha SD, Iraganaboina NC, Eluru N.. A comprehensive analysis of COVID-19 transmission and mortality rates at the county level in the United States considering socio-demographics, health indicators, mobility trends and health care infrastructure attributes. PLoS One. 2021;16(4):e0249133. doi: 10.1371/journal.pone.0249133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lui GC-Y, Yip TC-F, Wong VW-S, Chow VC-Y, Ho TH-Y, Li TC-M, Tse Y-K, Chan HL-Y, Hui DS-C, Wong GL-H, et al. Significantly lower case-fatality ratio of coronavirus disease 2019 (COVID-19) than Severe Acute Respiratory Syndrome (SARS) in Hong Kong-a territory-wide cohort study. Clin Infect Dis. 2021;872(10):e466–e75. doi: 10.1093/cid/ciaa1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sreedharan J, Nair SC, Muttappallymyalil J, Gopakumar A, Eapen NT, Satish KP, Manda V.. Case fatality rates of COVID-19 across the globe: are the current draconian measures justified? Z Gesundh Wiss. 2021:1–9. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, Abosalif KOA, Ahmed Z, Younas S.. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833–9. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S.. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 12.Pike JW, Christakos S.. Biology and mechanisms of action of the vitamin D hormone. Endocrinol Metab Clin North Am. 2017;46(4):815–43. doi: 10.1016/j.ecl.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piotrowska A, Wierzbicka J, Żmijewski MA.. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. 2016;63(1):17–29. doi: 10.18388/abp.2015_1104. [DOI] [PubMed] [Google Scholar]
- 14.Bagri H, Dahri K, Legal M.. Hospital pharmacists’ perceptions and decision-making related to drug-drug interactions. Can J Hosp Pharm. 2019;72(4):288–94. [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW.. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA. 2004;101(20):7711–5. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S.. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277(5333):1827–30. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 17.Garg MK, Kalra S, Mahalle N.. Defining vitamin D deficiency, using surrogate markers. Indian J Endocrinol Metab. 2013;17(5):784–6. doi: 10.4103/2230-8210.117189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White JH.Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76(9):3837–43. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin MD, Xing N, Kumar R.. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–45. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Soruri A, Gieseler RK, Peters JH.. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38(6):535–40. doi: 10.1111/j.1365-3083.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 21.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C.. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Walker C, Deb S, Ling H, Wang Z.. Assessing the elevation of cardiac biomarkers and the severity of COVID-19 infection: a meta-analysis. J Pharm Pharm Sci. 2020;23:396–405. doi: 10.18433/jpps31501. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D.. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrishami A, Dalili N, Mohammadi Torbati P, Asgari R, Arab-Ahmadi M, Behnam B, Sanei-Taheri M.. Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: a retrospective study. Eur J Nutr. 2021;60(4):2249–57. doi: 10.1007/s00394-020-02411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adami G, Giollo A, Fassio A, Benini C, Bertoldo E, Bertoldo F, Orsolini G, Idolazzi L, Viapiana O, Giannini S, et al. Vitamin D and disease severity in coronavirus disease 19 (COVID-19). Reumatismo. 2021;72(4):189–96. doi: 10.4081/reumatismo.2020.1333. [DOI] [PubMed] [Google Scholar]
- 27.Angelidi AM, Belanger MJ, Lorinsky MK, Karamanis D, Chamorro-Pareja N, Ognibene J, Palaiodimos L, Mantzoros CS.. Vitamin D status is associated with in-hospital mortality and mechanical ventilation: a cohort of COVID-19 hospitalized patients. Mayo Clin Proc. 2021;96(4):875–886. doi: 10.1016/j.mayocp.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anjum S.Examine the association between severe Vitamin D deficiency and mortality in patients with Covid-19. Pak J Med Health Sci. 2020;14(3):1184–6. [Google Scholar]
- 29.Baktash V, Hosack T, Patel N, Shah S, Kandiah P, Van den Abbeele K, et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2021;97:442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, Mandal AKJ, Missouris CG.. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2021;44(4):765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cereda E, Bogliolo L, Klersy C, Lobascio F, Masi S, Crotti S, Bruno R, Corsico AG, Di Sabatino A, Perlini S, et al. Vitamin D 25OH deficiency in COVID-19 patients admitted to a tertiary referral hospital. Clin Nutr. 2021;40(4):2469–2472. doi: 10.1016/j.clnu.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charoenngam N, Shirvani A, Reddy N, Vodopivec DM, Apovian CM, Holick MF.. Association of vitamin D status with hospital morbidity and mortality in adult hospitalized patients with COVID-19. Endocr Pract. 2021;27(4):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demir M, Demir F, Aygun H.. Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease. J Med Virol. 2021;93(5):2992–9. doi: 10.1002/jmv.26832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA.. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am J Clin Pathol. 2021;155(3):381–8. Feb 11doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavioli EM, Miyashita H, Hassaneen O, Siau E.. An evaluation of serum 25-hydroxy vitamin D Levels in patients with COVID-19 in New York City. J Am Coll Nutr. 2021:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez JL, Nan D, Fernandez-Ayala M, Garcia-Unzueta M, Hernandez-Hernandez MA, Lopez-Hoyos M, Muñoz-Cacho P, Olmos JM, Gutiérrez-Cuadra M, Ruiz-Cubillán JJ, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2021;106(3):e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jevalikar G, Mithal A, Singh A, Sharma R, Farooqui KJ, Mahendru S, Dewan A, Budhiraja S.. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci Rep. 2021;11(1):6258. doi: 10.1038/s41598-021-85809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karahan S, Katkat F.. Impact of serum 25(OH) vitamin D level on mortality in patients with COVID-19 in Turkey. J Nutr Health Aging. 2021;25(2):189–96. doi: 10.1007/s12603-020-1479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo X, Liao Q, Shen Y, Li H, Cheng L.. Vitamin D deficiency is inversely associated with COVID-19 incidence and disease severity in Chinese people. J Nutr. 2021;151(1):98–103. doi: 10.1093/jn/nxaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maghbooli Z, Sahraian MA, Ebrahimi M, Pazoki M, Kafan S, Tabriz HM, Hadadi A, Montazeri M, Nasiri M, Shirvani A, et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One. 2020;15(9):e0239799. doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Orchard L, Baldry M, Nasim-Mohi M, Monck C, Saeed K, Grocott MPW, Ahilanandan D.. Vitamin D levels and intensive care unit outcomes of a cohort of critically ill COVID-19 patients. Clin Chem Lab Med. 2021;59(6):1155–1163. doi: 10.1515/cclm-2020-1567. [DOI] [PubMed] [Google Scholar]
- 42.Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U.. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020;12(9):2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricci A, Pagliuca A, D’Ascanio M, Innammorato M, De Vitis C, Mancini R, Giovagnoli S, Facchiano F, Sposato B, Anibaldi P, et al. Circulating vitamin D levels status and clinical prognostic indices in COVID-19 patients. Respir Res. 2021;22(1):76. doi: 10.1186/s12931-021-01666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tehrani S, Khabiri N, Moradi H, Mosavat MS, Khabiri SS.. Evaluation of vitamin D levels in COVID-19 patients referred to Labafinejad hospital in Tehran and its relationship with disease severity and mortality. Clin Nutr Espen. 2021;42:313–7. doi: 10.1016/j.clnesp.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vassiliou AG, Jahaj E, Pratikaki M, Orfanos SE, Dimopoulou I, Kotanidou A.. Low 25-hydroxyvitamin D levels on admission to the intensive care unit may predispose COVID-19 pneumonia patients to a higher 28-day mortality risk: a pilot study on a Greek ICU Cohort. Nutrients. 2020;12(12):3773. doi: 10.3390/nu12123773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye K, Tang F, Liao X, Shaw BA, Deng M, Huang G, Qin Z, Peng X, Xiao H, Chen C, et al. Does serum vitamin D level affect COVID-19 infection and its severity?-A case-control study. J Am Coll Nutr. 2020;13:1–8. [DOI] [PubMed] [Google Scholar]
- 47.Kerget B, Kerget F, Kızıltunç A, Koçak AO, Araz Ö, Yılmazel Uçar E, Akgün M.. Evaluation of the relationship of serum vitamin D levels in COVID-19 patients with clinical course and prognosis. Tuberk Toraks. 2020;68(3):227–35. doi: 10.5578/tt.70027. [DOI] [PubMed] [Google Scholar]
- 48.Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S.. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):20191. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA.. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci. 2018;19(9):2663. doi: 10.3390/ijms19092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G.. The link between COVID-19 and vitamin D (VIVID): a systematic review and meta-analysis. Metabolism. 2021;119:154753. Jundoi: 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pereira M.Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;1–9. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 52.Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, Youssef MR, Omar M, Attia AS, Fawzy MS, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2021;93(2):733–40. doi: 10.1002/jmv.26360. [DOI] [PubMed] [Google Scholar]
- 53.Mercola J, Grant WB, Wagner CL.. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients. 2020;12(11):3361. doi: 10.3390/nu12113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Las Heras N, Martin Gimenez VM, Ferder L, Manucha W, Lahera V.. Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID-19: therapeutic effects of vitamin D. Antioxidants (Basel). 2020;9(9):897. doi: 10.3390/antiox9090897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Lucena TMC, da Silva Santos AF, de Lima BR, de Albuquerque Borborema ME, de Azevedo Silva J.. Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab Syndr. 2020;14(4):597–600. doi: 10.1016/j.dsx.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beard JA, Bearden A, Striker R.. Vitamin D and the anti-viral state. J Clin Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide‑induced acute lung injury via regulation of the renin‑angiotensin system. Mol Med Rep. 2017;16(5):7432–8. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji P, Zhu J, Zhong Z, Li H, Pang J, Li B, Zhang J.. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine (Baltimore)). 2020;99(47):e23315. doi: 10.1097/MD.0000000000023315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giustina A, Bouillon R, Binkley N, Sempos C, Adler RA, Bollerslev J, Dawson-Hughes B, Ebeling PR, Feldman D, Heijboer A, et al. Controversies in vitamin D: a statement from the third international conference. JBMR Plus. 2020;4(12):e10417. doi: 10.1002/jbm4.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM.. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 61.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–52. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Institutes of Health (NIH). Office of Dietary Supplements. Vitamin D Fact Sheet for Health Professionals . 2021. [cited June 15, 2021]. https://ods.od.nih.gov/factsheets/Vitamin%20D-HealthProfessional/.
- 63.Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, Kiesswetter E, Maggio M, Raynaud-Simon A, Sieber CC, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 64.National Institute for Health and Care Excellence: Clinical Guidelines . COVID-19 rapid guideline: vitamin D. London; 2020. https://pubmed.ncbi.nlm.nih.gov/33378143/. [PubMed] [Google Scholar]
- 65.Griffin G, Hewison M, Hopkin J, Kenny RA, Quinton R, Rhodes J, Subramanian S, Thickett D.. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clin Med (Lond)). 2021;21(1):e48–e51. doi: 10.7861/clinmed.2020-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drincic A, Fuller E, Heaney RP, ArmasLA.. 25-Hydroxyvitamin D response to graded vitamin D3 supplementation among obese adults. J Clin Endocrinol Metab. 2013;98(12):4845–51. doi: 10.1210/jc.2012-4103. [DOI] [PubMed] [Google Scholar]
- 67.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, Quesada Gomez JM.. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf M-I, Benstoem C, Meybohm P, Becker M, Skoetz N, Stegemann M, et al. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;245:CD015043. doi: 10.1002/14651858.CD015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.