ABSTRACT
Introduction
A number of anti-diabetic treatments have been favored during the continuing spread of the current SARS-CoV-2 pandemic. Glucagon like peptide-1 receptor agonists (GLP1-RAs) are a group of antidiabetic drugs, the glucose reducing effect of which is founded on augmenting glucose-dependent insulin secretion with concomitant reduction of glucagon secretion and delayed gastric emptying. Apart from their glucose lowering effects, GLP1-RAs also exert a plethora of pleiotropic activities in the form of anti-inflammatory, anti-thrombotic and anti-obesogenic properties, with beneficial cardiovascular and renal impact. All these make this class of drugs a preferred option for managing patients with type 2 diabetes (T2D), and potentially helpful in those with SARS-CoV2 infection.
Areas covered
In the present article we propose a hypothetical molecular mechanism by which GLP1-RAs may interact with SARS–CoV-2 activity.
Expert Opinion
The beneficial properties of GLP1-RAs may be of specific importance during COVID-19 infection for the most fragile patients with chronic comorbid conditions such as T2D, and those at higher cardiovascular and renal disease risk. Yet, further studies are needed to confirm our hypothesis and preliminary findings available in the literature.
KEYWORDS: SARS-COV-2, COVID-19, GLP-1RA, coronavirus, pandemic, diabetes, therapy
1. Introduction
Since the beginning of 2020, the global community has been facing a challenging pandemic caused by the coronavirus SARS-CoV-2 and its related disease COVID-19, with more than 180 million reported cases and about four million deaths as of 3 October 2020 [1]. The actual impact of COVID-19 is probably much larger than what has been assessed by official reports secondary to the reduced access of patients with acute and chronic diseases to health care facilities due to fear of contracting the virus [2]. Older individuals with chronic health conditions are more likely to develop severe symptoms and complications; for example, the presence of Type–2 diabetes (T2D) is associated with higher mortality and increased need for intensive care [3,4]. An effective and timely prevention strategy is, therefore, imperative [5].
Some international recommendations and shared practice on clinical experience and key learnings, for a better management of diabetic patients during the pandemic, are already available [6,7]. Other authors have also proposed potential mechanisms beyond the susceptibility for COVID-19 in patients with T2D [8]; indeed, the exposure to high glucose levels is a significant predictor of adverse outcome in COVID patients [9]. Such finding is not surprising since acute glucose variability can enhance oxidative stress, triggering the production of inflammatory cytokines and amplifying the inflammatory process [10]. Poor glycemic control has also been linked to negative changes in the innate-mediated and the cell-mediated adaptive immunity [11]. Finally, some authors have suggested that raised glucose levels may directly stimulate the replication of SARS CoV-2 virus [12,13].
In the present article we first discuss the close association between diabetes and COVID-19 hospitalization and mortality that has been reported in multiple cohorts across different countries, globally. Then, we discuss potential mechanisms by which GLP1-RAs may modulate SARS–CoV-2 activity, reducing viral entry and attenuating the infection. We indeed aim to provide a timely contribution useful for the medical and scientific community during this terrible pandemic.
2. Diabetes and COVID-19
Poor COVID-19 outcomes in patients with T2D has been shown since the beginning of the pandemic in many studies, highlighting immediately the need of tight control of diabetic patients, now more than ever, during this terrible pandemic [14,15]. Indeed, initial reports from Wuhan and from other regions in China have shown some divergent rates in the prevalence of T2D amongst COVID patients [16]. However, after the first smaller reports, findings from larger Chinese studies indicated more consistency, and in two multicenter reports, T2D was present in about 8% of subjects hospitalized for COVID [17,18]. Still from China, Zhu and colleagues [19] have also emphasized the importance of proper glycemic control for COVID outcome; in a retrospective multicenter study performed in patients with T2D and COVID-19, the authors reported that glycemic control was associated with significantly lower mortality.
Regarding early European reports, the first data were available from Italy, as the first hard-hit country in Europe: in Padua, Veneto, and in Milan, Lombardy, the prevalence of diabetes among hospitalized individuals with COVID-19 was 9% [20] and 15%, respectively [21]. Some other early reports were consistent with these findings, although higher prevalence rates of about 25% were reported in other European countries. like Spain [22], and the US [23]. In summary, making a comparative analysis of the prevalence of T2D in the above-mentioned large cohort studies conducted in different countries, the main early learnings were that diabetes represents a comorbidity very closely associated with COVID-19, its more severe forms that may also require hospitalization in intensive care units, and related mortality [24–26].
In consistent to that, Holman et al. [27] in a population‐based cohort study in England, have later shown a strong association between prior hyperglycemia and COVID-19 associated death after adjustment for other risk factors. In subjects with T2D, COVID-19 linked mortality was significantly higher in those with HbA1c ≥7.6% than in those with an HbA1c of 6.5‐7.0%). However, it should also be highlighted that low HbA1c was also associated with significantly increased COVID-19 mortality, which reinforces the clinical importance to avoid severe hypoglycemia. Another study from Italy has also shown that hyperglycemia at hospital admission is associated with the severity of COVID-19 prognosis [28]. Overall, these studies allude to a major role of T2D and the associated comorbidities/complications in conferring an increased susceptibility to develop COVID-19, and therefore require the adoption and implementation of international scientific guidelines would ensure use of appropriate drugs with proven benefit and safety [29].
3. GLP1-RAs in patients with T2D and SARS-CoV2 infection
GLP1-RAs, are incretin mimetics, whose anti-hyperglycemic effect is based on preserving pharmacologic levels of GLP1 which then increases glucose-dependent insulin secretion, decreases glucagon secretion, and delays gastric emptying. GLP1-RAs can be classified into long-acting (e.g. liraglutide, exenatide once weekly, dulaglutide, albiglutide, semaglutide) and short-acting (e.g. exenatide, lixisenatide) compounds [30]; furthermore, semaglutide is now also available as a pill, being the first oral GLP1-RA for the treatment of T2D [31];. GLP1-RAs have been shown to reduce HbA1c by approximately 0.8% to 1.6% [30,31] and some of these agents have increased homology to human GLP1 and therefore named as ‘GLP1-analogues’ (e.g. liraglutide, dulaglutide, albiglutide and semaglutide); the latter, beyond significant gluco-metabolic beneficial effects, have a favorable cardiovascular outcome, reducing cardiovascular events and mortality [32]. Yet, this may be not uniform among the class of GLP-1 RAs, since other agents have been discontinued due to adverse safety profiles [33,34].
GLP-1 RAs exert significant anti-inflammatory effects [35,36], which could theoretically blunt the exaggerated inflammatory response induced by pulmonary SARS-CoV2 infection, but not to reduce the infection per se. Given the GLP-1RAs beneficial role in patients with T2D at high risk for cardiovascular diseases, both diabetologists and cardiologists are now in agreement that represent first therapeutical choice for patients at such risk [37], which is of greater importance during COVID era [38]. Yet, there is still insufficient evidence to clarify the benefit of GLP-1RAs in patients with T2D and SARS-CoV2 infection, so far mainly limited to data obtained from retrospective analyses of few databases. In the US, the National COVID Cohort Collaborative (N3C) Consortium have performed the analysis of observational data from SARS-CoV-2-positive adults with a prescription for GLP1-RAs, sodium-glucose cotransporter 2 inhibitors (SGLT2i) inhibitors or dipeptidyl peptidase 4 inhibitors (DPP4i) within 24 months of positive SARS-CoV-2 PCR test [39]. Interestingly, the authors have very recently reported that premorbid GLP1-RAs and SGLT2i use, compared with DPP4i usage, was associated with lower odds of mortality and other adverse outcomes, such as emergency room visits and hospitalizations [39].
By contrast, an earlier retrospective study performed in Denmark have suggested that the use of incretin-based therapies in individuals with diabetes and SARS-CoV-2 was not associated with improved clinical outcomes [40]. Yet, this study suffers of a limited sample size. A neutral benefit with the use of GLP1-RAs has been also shown by Khunti et al in an observational nationwide study in England, where pre-COVID-19 prescription of glucose-lowering therapies and mortality risk of COVID-19 was analyzed in 2,85 million people with T2D. Metformin, SGLT2i, and sulfonylureas were associated with reduced risks of the COVID-19-related mortality, whereas insulin and DPP-4i were associated with increases in risk; neutral results were observed for GLP1-RAs and thiazolidinediones [41]. Other reports have shown a beneficial impact on COVID hospitalizations and mortality with the use of GLP1-RAs [42], and we cannot exclude that weight loss obtained with GLP1-RAs may be a protective mechanism in patients with T2D and COVID-19. In summary, available evidence suggest a beneficial impact of GLP1-RAs on hospitalizations and mortality in patients with T2D and COVID-19; yet, to date, no prospective studies investigating the association between GLP1-RAs use and COVID-19 outcome have been published.
4. Mechanisms by which GLP1-RAs may attenuate with SARS–CoV-2 infection
GLP1-RAs have been shown to exert several beneficial pleiotropic effects, beyond glycemic control [43,44]. One of them is the systemic anti-inflammatory effect, which is mediated as a consequence of the inhibitory effect on cytokine release due to their interference with the NF-kB signaling pathways [45]. This anti-inflammatory effect of GLP1 RA could theoretically blunt the exaggerated inflammatory response induced by pulmonary SARS-CoV-2 infection, but not reduce the infection per se.
The anti-inflammatory aspect of GLP1-RAs has been well demonstrated in animal model studies [46]. For example, the administration of liraglutide in lipopolysaccharide induced endotoxemia in animal models has been shown to improve survival and vascular dysfunction, along with salubrious actions on inflammatory and hemostatic parameters [47]. This pleiotropic activity of GLP1-RAs may be advantageous when it comes to the management of SARS–CoV-2 infection, as it may potentially reduce the severity of the viral infection (Figure 1). NF-κB is also the key and central mediator of the priming signal for NLR family pyrin domain containing 3 (NLRP3) inflammasome activation, and functions by stimulating the transcriptional expression of NLRP3 in response to multifarious Pattern Recognition Receptors (PRR) ligands and cytokines [48]. NLRP3 inflammasome is composed of NLRP3, the apoptosis-associated speck-like protein containing a CARD (ASC) and procaspase-1, as well as an essential regulatory protein, NIMA-related kinase 7 (NEK7) [49]. The NLRP3 gene is a direct target of NF-κB, which contains NF-κB-binding sites in its promoter region. Upon stimulation by NF-κB, the inflammasome receptors oligomerize and recruit pro-caspase 1 via ASC, thus stimulating pro-caspase 1 processing and conversion to active caspase 1. Activated caspase 1, then cleaves pro-IL-1b and pro-IL-18 into their mature forms [50]. Hence, NF-κB mediated secretion of these pro-inflammatory cytokines through NLRP3 priming attests to increase in the disease severity in SARS–CoV-2 infection. As GLP1-RAs interfere with the NF-κB signaling pathways, they could also attenuate NLRP3 mediated inflammation. However, this area requires further investigation.
Figure 1.
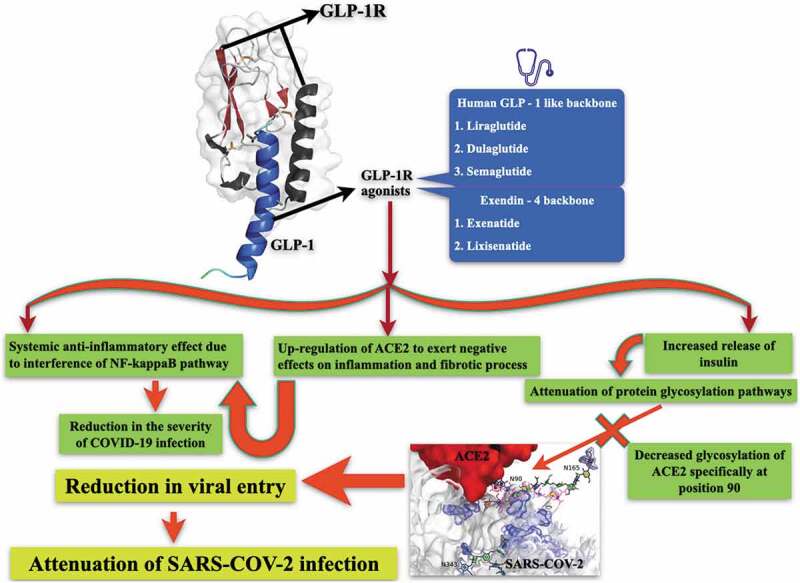
Potential mechanism by which GLP1-RAs may interract with SARS–CoV-2 activity
The structural coordinates of GLP-1 receptor complex (represented in the form of ribbon diagram) was downloaded from protein data bank (PDB ID: 5VEX). The details of the structure can be obtained from Song et al. Nature 2017;546:312–315. The concept of ACE2 and SARS-CoV-2 interaction was adopted with minor modifications from bioRxiv: the preprint server for biology 2020.06.25.172403. 24 July 2020, doi:10.1101/2020.06.25.172403.
SARS–CoV-2 infection through NF-κB mediated pathways induces a hyper-inflammatory state designated as ‘cytokine storm’ [51], involving significant systemic perturbations. These include iron dysregulation manifested as hyperferritinemia associated with disease severity [52]. Hyperferritinemia induces reactive oxygen species (ROS) production and promotes oxidative stress, which leads to mitochondrial damage [53]. Downstream to this mitochondrial damage, platelet issues and apoptosis may occur. The interaction of dysfunctional platelets with coagulation cascades aggravate clotting events and thrombus formation, the latter being one of the major causes of death in SARS–CoV-2 infection [54]. Since GLP1-RAs mitigate NF-κB signaling pathways, they could also induce mitochondrial protection leading to favorable anti-thrombotic effects. This possibility is supported through observations in ex vivo whole blood microfluidics model, where native GLP-1(7–36) has been shown to reduce entire blood thrombus formation at both venous and arterial flow shear rates, resulting in the formation of smaller and less contracted thrombi [55].
It is well known that GLP1-RAs stimulate the secretion of insulin from pancreatic beta-cells [56]. Insulin plays a key role in protein glycosylation [57], and glycosylation has been shown to play a pivotal role in SARS–CoV-2 infection. SARS-CoV-2 utilizes its highly glycosylated trimeric Spike protein to bind to the cell surface receptor ACE2 glycoprotein and facilitate host cell entry [58]. In fact, glycosylation of the ACE2 receptor at specific amino acid residues (such as at position 90) (Figure 1) is important for its binding to the SARS-CoV-2 Spike protein [58]. One of the key pathways for glycosylation is the hexosamine biosynthesis pathway [59]. This pathway functions in part as a glucose sensor and regulates cellular responses to insulin by controlling the levels of UDP-GlcNAc-mediated glycosylation of targets related to insulin activity [60]. The hexosamine biosynthesis pathway is highly responsive to glucose levels, and its flux is significantly increased in some tissues of patients with diabetes, leading to increased levels of UDP-GlcNAc and, thus, elevated glycosylation. Long-term exposure to high glucose increases O-GlcNAc modifications and, therefore, its biological effects; for example, in the context of diabetes, increases O-GlcNAc glycosylation of proteins, such as glycosylation of AKT, can lead to enhanced β-cell death [61]. Based on these observations, one can surmise that decreased insulin levels may increase glycosylation of the ACE2 receptor, augmenting its affinity for SARS-CoV-2 spike protein. Therefore, GLP1-RAs, apart from their favorable effect mediated through its impact on NF-κB signaling pathways during SARS–CoV-2 infection, may also impede virus entry through their modulatory effect on protein glycosylation, especially that of ACE2.
5. GLP1-RAs and ACE2 conundrum
It has been shown that GLP1 receptors contribute to airway remodeling [62]. Numerous clinical trials are currently underway to investigate the theoretical potential of GLP1-RAs for the improvement of chronic obstructive respiratory disease [63]. In animal studies, liraglutide has been associated with the upregulation of ACE2, abundantly expressed in alveolar epithelial cells, enterocytes and vessels upstream of the counter-regulatory RAS pathway, which exercises a negative effect on inflammatory and fibrotic processes [64]. Although still uncorroborated by well-defined translational substantiation, the GLP1-RAs-induced upregulation of ACE2 could ameliorate lung injury, antagonizing the reduction of ACE2 expression levels that are hallmarks of SARS-CoV-2 infection progression [65] and precluding the over-activated immune response critical for acute respiratory distress syndrome [66] (Figure 1). On the other hand, ACE2 enables virus entry into host target cells [67], raising speculation of augmented vulnerability to SARS-CoV-2 infection in the case of ACE2 overregulation, as an outcome of long-term treatment with ACE inhibitors and/or ANGII receptor blockers, GLP1-RAs or a combination of both in hypertensive patients with diabetes [68]. However, robust clinical/epidemiological studies which support the correlation between these medications, especially GLP1-RAs use, and SARS-CoV-2 infection severity, and adjusted for conceivable confounding variables such as sex, age and comorbidities possibly modulating the expression of ACE2, are unavailable. The subject of whether GLP1-RAs treatment-induced overregulation of ACE2 in target tissues would lead to an increased risk of SARS-CoV-2 infection, or their proposed protective activity (Figure 1) would predominate, is a conundrum yet to be solved.
6. Conclusions
We discussed in the present article about the importance of the anti-inflammatory properties and the beneficial metabolic and cardio-renal effects of GLP1-RAs, together with their safety. Further, we proposed potential mechanisms by which GLP1-RAs may modulate SARS–CoV-2 activity, albeit subject to confirmation in future studies. This is of particular importance due to the continuous spread of the virus and its related disease COVID-19, with increased number of cases, severe forms and complications, and related deaths.
7. Expert opinion
Our globe is facing a very difficult and challenging pandemic since the beginning of 2020, caused by the new coronavirus SARS-CoV-2 and its related disease COVID-19. All the continents have been seriously hit by the virus, starting from the town of Wuhan in China, and with billions of people worldwide following very strict social distancing regulations, as well as partial or full lockdowns. Currently, there is continuous spread of the virus and the actual impact of COVID-19 seems to be much larger than what assessed by official reports, for instance for the significant reduced access of patients with acute and chronic diseases to health care facilities due to fear of contracting the virus.
Older individuals with chronic health conditions are those more likely to develop severe symptoms and complications; for example, the presence of diabetes is associated with higher mortality and increased need for intensive care. A number of anti-diabetic treatments have been favored during the continuing spread of the current SARS-CoV-2 pandemic [69], and early use of GLP1-RAs has been advocated. Distinct and unique pharmacokinetic, metabolic and cardio-renal properties are present in the different GLP1-RAs approved for subcutaneous administration for the management of T2D and currently available in the markets, including twice-daily exenatide, once-daily liraglutide and lixisenatide, once-weekly exenatide, dulaglutide albiglutide and semaglutide, as well as semaglutide in the new daily oral formulation, the latest agent available.
Indeed, this class of novel anti-diabetic agents has beneficial effects on glucose metabolism, and some GLP1-RAs have also shown a beneficial cardiovascular and renal impact. This is of particular importance during COVID-19 infection for the most fragile patients with chronic conditions such as diabetes and those at higher cardiovascular and renal disease risk. In addition, some GLP1-RAs have significant anti-inflammatory properties that are also of importance during the COVID-19 era [70], since the latter has been shown to contribute to systemic inflammation that seems to underlie organ impairment, including myocardial injury [71].
Certain scientific recommendations issued during the COVID-19 pandemic have highlighted the safety of insulin therapy in patients with SARS-CoV-2 infection and diabetes, while exercising care when considering other oral/injectable therapies. On the other hand, it has also been suggested that patients with type 2 diabetes and SARS-CoV2 could reach better metabolic balance, decrease inflammation, and possibly avoid complications using a fixed combination with basal insulin and GLP1-RAs [6]. The latter provides good glycemic control while avoiding hypo and hyperglycemia and promoting an anti-inflammatory effect for patients with type 2 diabetes affected by SARS-CoV-2 infection [9].
In the present article we discussed on potential mechanisms by which GLP1-RAs may modulate SARS–CoV-2 activity, albeit subject to confirmation in future studies. Nevertheless, the beneficial anti-inflammatory properties as well as the metabolic and cardio-renal effects of GLP1-RAs, together with their safety, make this class of drugs as one of the best options for managing type 2 diabetes patients in the face of the COVID-19 pandemic.
Funding Statement
This paper was not funded.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have received speaking fees, consulting fees and research grants from Eli Lilly and Novo Nordisk; and research grants from AstraZeneca. A separate reviewer has disclosed that they have served as a consultant for Applied Therapeutics, Novo Nordisk, Pfizer and Sanofi; employee for Merck Research Laboratories (spouse) 2017-2020 and Janssen (spouse) May 2020 – present; research support (institutional contracts) for Applied Therapeutics/Medpace, Eli Lilly, Premier/Fractyl, Novo Nordisk, and Sanofi/Medpace. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
This article has been written independently, without any financial or professional help, and reflects only the authors’ opinion, without any role of the industry. The authors have given lectures, received honoraria and research support, and participated in conferences, advisory boards and clinical trials sponsored by several pharmaceutical companies, but they did not receive any financial or professional help with the preparation of the manuscript. A Sonmez is currently Secretary of the Turkish Society for Endocrinology and Metabolism. M Rizzo is full-time Professor of Internal Medicine at University of Palermo, Italy and currently Medical Director, Novo Nordisk Eastern Europe. AP Stoian is currently Vice President of Romanian National Diabetes Committee. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Article highlights
Diabetes represents a comorbidity very closely associated with COVID-19,
Hyperglycemia is associated with the severity of prognosis in patients with COVID-19.
Novel anti-diabetic drugs seem to have a greater role in the management of patients with T2D during COVID–19 era, due to their glyco-cardio-metabolic benefit.
We hypothesize that GLP1-RAs may modulate SARS–CoV-2 activity.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.World Health Organization . Coronavirus disease (COVID-19) pandemic. on 2021 Jul 3. Accessed athttps://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Rizzo M, Foresti L, Montano N.. Comparison of reported deaths from COVID-19 and increase in total mortality in Italy. JAMA Intern Med. 2020;180:1250–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al. COVID-19 Lombardy ICU network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoian AP, Banerjee Y, Rizvi AA, et al. Diabetes and the COVID-19 pandemic: how insights from recent experience might guide future management. Metab Syndr Relat Disord. 2020;18:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giorgino F, Bhana S, Czupryniak L, et al. Management of patients with diabetes and obesity in the COVID-19 era: experiences and learnings from South and East Europe, the Middle East, and Africa. Diabetes Res Clin Pract. 2021;172:108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longo M, Caruso P, Maiorino MI, et al. Treating type 2 diabetes in COVID-19 patients: the potential benefits of injective therapies. Cardiovasc Diabetol. 2020;19(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceriello A, Standl E, Catrinoiu D, et al. Diabetes : issues for the management of people with diabetes and COVID-19 in ICU. Cardiovasc Diabetol. 2020;19(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erener S. Diabetes, infection risk and COVID-19. Mol Metab. 2020;39:101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Codo AC, Davanzo GG, Monteiro LB, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 2020;32(3):437–446 e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S, Bae JH, Kwon HS, et al. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceriello A, Stoian AP, Rizzo M. COVID-19 and diabetes management: what should be considered? Diabetes Res Clin Pract. 2020;163:108151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw K. The impact of diabetes on COVID-19 infection. Pract Diab. 2020;37:79–81. [Google Scholar]
- 16.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan WJ, Ni ZY, Hu Y, et al. China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020Apr30;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(1068–1077.e3):1068–1077.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadini GP, Morieri ML, Longato E, et al. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizvi AA, Janez A, Al Mahmeed W, et al. Diabetes and COVID-19: a tale of two pandemics. J Cardiovasc Pharmacol. 2021Jun25;78(1):e1–e2. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantea Stoian A, Pricop-Jeckstadt M, Pana A, et al. Death by SARS-CoV 2: a Romanian COVID-19 multi-centre comorbidity study. Sci Rep. 2020;10:21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugliese G, Vitale M, Resi V, et al. Is diabetes mellitus a risk factor for COronaVIrus Disease 19 (COVID-19)? Acta Diabetol. 2020;57:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppelli A, Giannarelli R, Aragona M, et al. Pisa COVID-19 study group. hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the Pisa COVID-19 study. Diabetes Care. 2020;43:2345–2348. [DOI] [PubMed] [Google Scholar]
- 29.Banach M, Gaita D, Haluzik M; Cardio-Metabolic Academy Europe East. Adoption of the ADA/EASD guidelines in . 10 Eastern and Southern European countries: physician survey and good clinical practice recommendations from an international expert panel. Diabetes Res Clin Pract. 2021;172:108535. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo M, Rizvi AA, Spinas GA, et al. Glucose lowering and anti-atherogenic effects of incretin-based therapies: GLP-1 analogues and DPP4-inhibitors. Expert Opin Investig Drugs. 2009;18:1495–1503. [DOI] [PubMed] [Google Scholar]
- 31.Nauck MA, Quast DR, Wefers J, et al. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021Apr;46:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachinidis A, Nikolic D, Stoian AP, et al. Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metabolism. 2020;111:154343. [DOI] [PubMed] [Google Scholar]
- 33.Rosenstock J, Balas B, Charbonnel B, et al. T-emerge 2 study group. The fate of taspoglutide, a weekly GLP-1 receptor agonist, versus twice-daily exenatide for type 2 diabetes: the T-emerge 2 trial. Diabetes Care. 2013;36:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue X, Ren Z, Zhang A, et al. Efficacy and safety of once-weekly glucagon-like peptide-1 receptor agonists compared with exenatide and liraglutide in type 2 diabetes: a systemic review of randomised controlled trials. Int J Clin Pract. 2016;70:649–656. [DOI] [PubMed] [Google Scholar]
- 35.Rizzo M, Nikolic D, Patti AM, et al. GLP-1 receptor agonists and reduction of cardiometabolic risk: potential underlying mechanisms. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2814–2821. [DOI] [PubMed] [Google Scholar]
- 36.Yaribeygi H, Maleki M, Sathyapalan T, et al. Anti-inflammatory potentials of incretin-based therapies used in the management of diabetes. Life Sci. 2020;241:117152. [DOI] [PubMed] [Google Scholar]
- 37.Maranta F, Cianfanelli L, Rizzo M, et al. Filling the gap between guidelines and real world in the cardiovascular approach to the diabetic patients: the need for a call to action. Int J Cardiol. 2021;329:205–207. [DOI] [PubMed] [Google Scholar]
- 38.Stoian AP, Papanas N, Prazny M, et al. Incretin-Based Therapies Role in COVID-19 Era: evolving Insights. J Cardiovasc Pharmacol Ther. 2020Jul3;25(6):494–496. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Kahkoska AR, Abrahamsen TJ, Alexander GC, et al. N3C consortium. association between glucagon-like peptide 1 receptor agonist and sodium-glucose cotransporter 2 inhibitor use and COVID-19 Outcomes. Diabetes Care. 2021Jun16:dc210065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Israelsen SB, Pottegård A, Sandholdt H, et al. Comparable COVID-19 outcomes with current use of GLP-1 receptor agonists, DPP-4 inhibitors or SGLT-2 inhibitors among patients with diabetes who tested positive for SARS-CoV-2. Diabetes Obes Metab. 2021;23:1397–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khunti K, Knighton P, Zaccardi F, et al. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol. 2021;9:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyland JE, Raja-Khan NT, Bettermann K, et al. Diabetes, drug treatment and mortality in COVID-19: a multinational retrospective cohort study. SSRN Electron J. 2020. DOI: 10.2139/ssrn.3725612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nauck MA, Meier JJ, Cavender MA. Abd El Aziz M, DJ Drucker. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136(9):849–870. [DOI] [PubMed] [Google Scholar]
- 44.Verges B, Bonnard C, Renard E. Beyond glucose lowering: glucagon-like peptide-1 receptor agonists, body weight and the cardiovascular system. Diabetes Metab. 2011;37(6):477–488. [DOI] [PubMed] [Google Scholar]
- 45.Rowlands J, Heng J, Newsholme P, et al. Pleiotropic effects of GLP-1 and ##on. Front Endocrinol (Lausanne). 2018;9:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, Li S, Wang N, et al. Liraglutide, a glucagon-like peptide-1 receptor agonist, suppresses osteoclastogenesis through the inhibition of NF-kappaB and MAPK pathways via GLP-1R. Biomed Pharmacother. 2020;130:110523. [DOI] [PubMed] [Google Scholar]
- 47.Steven S, Hausding M, Kroller-Schon S, et al. Gliptin and GLP-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res Cardiol. 2015;110(2):6. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z, Wang X, Wang Y, et al. NLRP3 inflammasome activation regulated by NF-kappaB and DAPK contributed to paraquat-induced acute kidney injury. Immunol Res. 2017;65(3):687–698. [DOI] [PubMed] [Google Scholar]
- 49.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu T, Zhang L, Joo D, et al. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song P, Li W, Xie J, et al. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10(11):822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Saguner AM, An J, et al. Dysfunctional coagulation in COVID-19: from Cell to Bedside. Adv Ther. 2020;37(7):3033–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sternkopf M, Nagy M, Baaten C, et al. Native, intact glucagon-like peptide 1 Is a natural suppressor of thrombus growth under physiological flow conditions. Arterioscler Thromb Vasc Biol. 2020;40(3):e65–e77. [DOI] [PubMed] [Google Scholar]
- 56.Carlessi R, Chen Y, Rowlands J, et al. GLP-1 receptor signalling promotes beta-cell glucose metabolism via mTOR-dependent HIF-1alpha activation. Sci Rep. 2017;7(1):2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majumdar G, Wright J, Markowitz P, et al. Insulin stimulates and diabetes inhibits O-linked N-acetylglucosamine transferase and O-glycosylation of Sp1. Diabetes. 2004;53(12):3184–3192. [DOI] [PubMed] [Google Scholar]
- 58.Zhao P, Praissman JL, Grant OC, et al. Virus-Receptor Interactions of Glycosylated SARS-CoV-2 Spike and Human ACE2 Receptor. 2020. [DOI] [PMC free article] [PubMed]
- 59.de Queiroz RM, Oliveira IA, Piva B, et al. Dias WB: hexosamine Biosynthetic Pathway and Glycosylation Regulate Cell Migration in Melanoma Cells. Front Oncol. 2019;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akella NM, Ciraku L, Reginato MJ. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019;17(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma J, Hart GW. Protein O-GlcNAcylation in diabetes and diabetic complications. Expert Rev Proteomics. 2013;10(4):365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun YH, He L, Yan MY, et al. Overexpression of GLP-1 receptors suppresses proliferation and cytokine release by airway smooth muscle cells of patients with chronic obstructive pulmonary disease via activation of ABCA1. Mol Med Rep. 2017;16(1):929–936. [DOI] [PubMed] [Google Scholar]
- 63.Huang J, Yi H, Zhao C, et al. Glucagon-like peptide-1 receptor (GLP-1R) signaling ameliorates dysfunctional immunity in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3191–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pal R, Bhansali A:COVID-19. diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monda VM, Porcellati F, Strollo F, et al. ACE2 and SARS-CoV-2 infection: might GLP-1 receptor agonists play a role? Diabetes Ther. 2020;11(9):1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Florindo HF, Kleiner R, Vaskovich-Koubi D, et al. Immune-mediated approaches against COVID-19. Nat Nanotechnol. 2020;15(8):630–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gracia-Ramos AE. Is the ACE2 overexpression a risk factor for COVID-19 infection? Arch Med Res. 2020;51(4):345–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43(7):648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stoian AP, Catrinoiu D, Rizzo M, et al., COVID-19 and diabetes. Why it is a different story. Diabetes Metab Res Rev. 2020Jun27:e3379. Online ahead of print. DOI: 10.1002/dmrr.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stoian AP, Toth PP, Kempler P, et al., Gender differences in the battle against COVID-19: impact of genetics, comorbidities, inflammation and lifestyle on differences in outcomes. Int J Clin Pract. 2020Aug8:e13666. Online ahead of print. DOI: 10.1111/ijcp.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Babapoor-Farrokhran S, Gill D, Walker J, et al. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020Jul15;253:117723. [DOI] [PMC free article] [PubMed] [Google Scholar]


