ABSTRACT
Purpose
We aimed to investigate the clinical performance of edoxaban for the treatment of pulmonary embolism (PE) in hospitalized COVID-19 patients.
Methods
We conducted a retrospective analysis selecting hospitalized patients with COVID-19 admitted to our Institution from 20 May 2020 to 20 November 2020 with computer tomography (CT) detected PE at admission, treated with edoxaban after initial parenteral therapy. Clinical outcomes were compared between patients with and without ARDS at admission and between those with and without CT confirmed PE resolution.
Results
50 patients were included. Mean follow-up was 42.5 ± 10 days. No baseline differences were found between patients with ARDS (30%) and those without ARDS at admission. Patients with PE resolution (84%) were younger (P = 0.03), had a shorter duration of fondaparinux therapy (9.9 ± 3.8 vs 15.8 ± 7.5 days; P = 0.0015) and length of hospitalization (36 ± 8 vs 46 ± 9 days: P = 0.0023) compared with those without PE resolution. 2 patients experienced major bleedings. At multivariate analysis the time to edoxaban switch was the only predictor of the PE resolution (HR: 0.92; 95% C.I. 0.86 to 0.99).
Conclusion
Edoxaban was an effective and safe treatment for acute PE in COVID-19 setting.
KEYWORDS: COVID-19, SARS-CoV-2, edoxaban, pulmonary embolism, ARDS, safety, efficacy
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly pathogenic human coronavirus recently recognized as the cause of the coronavirus disease 2019 (COVID-19), which spread rapidly form China to other countries, reaching devastating pandemic proportion [1]. Italy is the one of the hardest hit countries by COVID-19, with more than 540,000 laboratory-confirmed cases by 27 October 2020 [2]. Pulmonary embolism (PE) and acute distress respiratory syndrome (ARDS) are frequent encountered complications of COVID-19 and have been associated with significantly lower survival during hospitalization [3–6].
The optimal treatment of PE in the clinical contest of COVID-19 is still debated [7]. Even if non-vitamin K oral anticoagulant (NOACs) shares the same indication of parental anticoagulation with low molecular weight heparin (LMWH) or fondaparinux in general population [8], few clinical experiences on the NOACs use for the PE treatment have been reported in COVID-19 patients, with a recent case series describing a relatively high percentage of NOACs failure in COVID‐19 patients [9]. The aim of our study was to describe the real-world safety and effectiveness of edoxaban for PE treatment among COVID-19 patients.
2. Materials and methods
From 384 consecutive patients with laboratory confirmed COVID-19 admitted to our institution for acute dyspnea from 20 May 2020 to 20 November 2020, we retrospectively selected those with computer tomography (CT) detected PE at admission. All patients treated with edoxaban during hospitalization were included in the analysis. Baseline characteristics such as medical history, physical examination, laboratory evaluation, pharmacological therapy, chest X-Ray and CT features have been collected. Clinical outcomes as ARDS at admission or developed during hospitalization, resolution of PE at CT, length of hospitalization, overall bleedings and mortality were collected and analyzed. Baseline characteristics and clinical outcomes were compared between patients with ARDS at admission and patients without ARDS at admission as well as between patients with PE resolution and patients without PE resolution. ARDS diagnosis was defined according to the Berlin definition [10]. According to our hospital protocol, a follow-up CT scan evaluation performed at one and three months from the PE diagnosis or at hospital discharge, whichever came first. Complete PE resolution was defined as a CT pulmonary angiogram on follow-up that showed no evidence of PE in any vessel [11].
This study was conducted according to the Declaration of Helsinki and approved by the institutional ethics committee (ID-140220). The requirement for informed consent from individual patients was waived due to the observational retrospective design of the study.
2.1. Statistical analysis
The distribution of continuous data was tested with the Kolmogorov–Smirnov and the Shapiro-Wilk test. Normally distributed variables were expressed as mean ± standard deviation (SD), whereas non-normal distributed ones as median and interquartile range (IQR). Categorical variables were reported as numbers and percentages. Continuous variables were compared by using the Student t-test or the Mann-Whitney U test, when appropriate. Categorical variables were compared with chi-squared test, or Fisher exact test, when appropriate. The univariate and multivariate analysis for complete PE resolution was calculated using Cox proportional hazard model and presented as Hazard Ratio (HR) with their 95% confidence intervals (CI). All independent variables showing a P value <0.1 for the association with the outcome of interest at univariate analysis were tested in the multivariate model. PE resolution during follow-up was evaluated with the Kaplan-Meier method and compared with the log-rank test. For all test, a p value <0.05 was considered statistically significant. Analysis was performed by using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Among 384 COVID-19 patients admitted to our institution for acute dyspnea, 50 consecutive COVID-19 patients (13%) with PE diagnosed at admission were included in the present study. The mean age was 59.24 ± 15.00 years; 39 (78%) were males. No patients show hemodynamic instability and were all at intermediate risk according to the current guidelines [8]. The concomitant ARDS at admission was diagnosed in 15 patients (30%). COVID-19 patients with ARDS did not show significant difference in baseline clinical characteristics compared to those without ARDS. The Table 1 shows the baseline characteristics of study population. At PE diagnosis all patients started anticoagulant therapy with low molecular weight heparin (LMWH) (26%) or fondaparinux (74%), at therapeutic dosage according to body weight and renal function, and continued for a mean time of 15 ± 6 and 11 ± 5 days, respectively. No differences in the type and duration of parental anticoagulant therapy, both enoxaparin and fondaparinux, have been shown among patients with and without ARDS. All patients were switched to edoxaban therapy according to clinical decisions after a mean time of 13.0 ± 5.5 days. The mean duration of edoxaban treatment during the hospitalization was 25.3 ± 8.0 days. The median duration of hospitalization was 37.5 ± 9.0 days.
Table 1.
Clinical characteristics of the study population according to ARDS at admission
| Overall population (N = 50) |
Patients with ARDS (N = 15) |
Patients without ARDS (N = 35) |
P | |
|---|---|---|---|---|
| Males, n (%) | 39 (78%) | 11 (73%) | 28 (80%) | 0.59 |
| Age, mean± SD | 59.24 ± 15 | 55 ± 17.2 | 61.05 ± 12.5 | 0.17 |
| BMI>30, n (%) | 15 (30%) | 6 (40%) | 9 (26%) | 0.33 |
| Smoker, n (%) | 26 (52%) | 5 (33%) | 21 (60%) | 0.08 |
| Hypertension, n (%) | 31 (62%) | 7 (47%) | 24 (68.5%) | 0.15 |
| Diabetes Mellitus, n (%) | 13 (26%) | 4 (27%) | 9 (26%) | 0.94 |
| Cancer, n (%) | 3 (6%) | 0 | 3 (8.6%) | 0.25 |
| Atrial fibrillation, n (%) | 3 (6%) | 0 | 3 (8.6%) | 0.25 |
| *Hepatopathy, n (%) | 14 (28%) | 6 (40%) | 8 (23%) | 0.22 |
| Previous Stroke/TIA, n (%) | 5 (10%) | 1 (7%) | 4 (11.4%) | 0.64 |
| CKD, n (%) | 7 (14%) | 1 (7%) | 6 (17%) | 0.35 |
| CAD, n (%) | 10 (20%) | 2 (13.3%) | 8 (23%) | 0.44 |
| COPD, n (%) | 22 (44%) | 6 (40%) | 16 (46%) | 0.7 |
| PaO2/FiO2 ratio, median (IQ) | 197 (146.75–243.75) |
132 (116–149) |
224 (195–285) |
< 0.005 |
| D-Dimer (ng/mL), median (IQ) | 1875.5 (1290.25–2811.75) |
3215 (2342–4325) |
1513 (987–1934) |
0.09 |
| Enoxaparin Therapy, n (%) | 13 (26%) | 2 (13.3%) | 11 (31.4%) | 0.18 |
| Enoxaparin therapy duration (days), mean ± SD | 15 ± 6 | 17.5 ± 6.4 | 14.7 ± 5.9 | 0.55 |
| Fondaparinux Therapy, n (%) | 37 (74%) | 13 (87%) | 24 (68.5%) | 0.17 |
| Fondaparinux therapy duration (days), mean ± SD | 11 ± 5 | 13.4 ± 6.2 | 9.8 ± 4.1 | 0.04 |
| Hospitalization length (days), mean ± SD | 37.5 ± 9 | 39.3 ± 6.1 | 36.6 ± 9.5 | 0.32 |
| Edoxaban treatment length (days), mean ± SD | 25.3 ± 8 | 25.4 ± 7.1 | 25.3 ± 7.9 | 0.97 |
ARDS: acute respiratory distress syndrome; BMI: body mass index; CKD: chronic kidney disease; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; PaO2/FiO2: arterial pressure of oxygen/fraction of inspired oxygen; IQ: interquartile; *Hepatopathy defined as a raise in AST and ALT above the 99th percentile upper reference limit.
During the follow-up period (42.5 ± 10.0 days), 42 patients (84%) showed complete PE resolutions at CT and continued edoxaban therapy for at least 3 months. No patient showed at follow-up CT scan new emboli despite the anticoagulant treatment. Two major bleeding events were reported: one gastrointestinal bleeding due to colon polypsosis; one metrorragy not related to menstrual period in patient with newly diagnosed uterus cancer; both the events happened after the PE resolution and led to the early interruption of the anticoagulant treatment. No death was recorded.
The subgroup with complete PE resolution was younger (57.3 ± 14.0 vs 69.2 ± 9.0 years; P = 0.03) and showed more likely BMI> 30 (36% vs 0%; P = 0.04), shorter duration of fondaparinux therapy (9.9 ± 3.8 vs 15.8 ± 7.5 days; P = 0.0015) and length of hospitalization (35.9 ± 7.6 vs 45.7 ± 9.3: P = 0.0023) compared with those without PE resolution (Table 2). The rate of ARDS at admission was not statistically different between patients with and without PE resolutions (26.2% vs 50%; P = 0.1826). No significant differences in the type of parental anticoagulant therapies and edoxaban therapy were found between patients with and without PE resolution (Table 2).
Table 2.
Clinical characteristics of patients with and without PE resolution
| PE Resolution (N = 42) |
No resolution of PE (N = 8) |
P-value | |
|---|---|---|---|
| Males, n (%) | 33 (78.5%) | 6 (75%) | 0.83 |
| Age, mean ± SD | 57.3 ± 14.3 | 69.2 ± 8.8 | 0.03 |
| BMI>30 n (%) | 15 (36%) | 0 | 0.04 |
| Hypertension, n (%) | 26 (62%) | 5 (62.5%) | 0.99 |
| Smoker, n (%) | 22 (52.4%) | 4 (50%) | 0.9 |
| Diabetes Mellitus, n (%) | 10 (24%) | 3 (37.5%) | 0.43 |
| Cancer n (%) | 2 (4.8%) | 1 (12.5%) | 0.41 |
| Atrial fibrillation, n (%) | 2 (4.8%) | 1 (12.5%) | 0.41 |
| Hepatopathy n (%) | 12 (28.6%) | 2 (25%) | 0.84 |
| Previous Stroke, n (%) | 5 (12%) | 0 | 0.31 |
| CKD, n (%) | 7 (7%) | 0 | 0.44 |
| CAD, n (%) | 8 (19%) | 2 (25%) | 0.7 |
| COPD, n (%) | 20 (47.6%) | 2 (25%) | 0.24 |
| ARDS at admission | 11 (26.2%) | 4 (50%) | 0.18 |
| PaO2/FiO2 ratio, median (IQ) | 201 (159.75–267.75) |
142 (110–217) |
0.16 |
| D-Dimer (ng/mL), median (IQ) | 1811.5 (1290.25–2668.5) |
2952.5 (1254–11,059.75) |
0.2 |
| Enoxaparin Therapy, n (%) | 12 (28.6%) | 1 (12.5%) | 0.35 |
| Enoxaparin therapy duration (days), mean ± SD | 15.3 ± 6.0 | 13 | - |
| Fondaparinux Therapy, n (%) | 30 (71.4%) | 7 (87.5%) | 0.35 |
| Fondaparinux therapy duration (days), mean ± SD | 9.9 ± 3.8 | 15.8 ± 7.5 | 0.0015 |
| Azithromycin Therapy, n (%) | 35 (83.3%) | 6 (75%) | 0.58 |
| Corticosteroid, n (%) | 42 (100%) | 8 (100%) | - |
| Retroviral therapy, n (%) | 1 (2.4%) | 1 (12.5%) | 0.19 |
| Hospitalitazion length (days), mean ± SD | 35.9 ± 7.6 | 45.7 ± 9.3 | 0.0023 |
| Switch to Edoxaban (days), mean ± SD | 11.5 ± 5.1 | 15.5 ± 7 | 0.06 |
| Edoxaban treatment length (days), mean ± SD | 24.4 ± 6.7 | 30.2 ± 10.6 | 0.05 |
PE: pulmonary embolism; BMI: body mass index; CKD: chronic kidney disease; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; ARDS: acute respiratory distress syndrome; PaO2/FiO2: arterial pressure of oxygen/fraction of inspired oxygen; IQ: interquartile
According to the univariate Cox regression analysis, the age (HR: 0.97; 95% C.I. 0.95 to 0.99; p = 0.007), the length of hospitalization (HR: 0.96; 95% C.I. 0.93 to 0.99; p = 0.015) and the time to edoxaban switch (HR: 0.91; 95% C.I. 0.86 to 0.97; p = 0.022) were found to be dependent predictors of the complete PE resolution (Table 3). At multivariate Cox regression analysis only the time to edoxaban switch was confirmed to be an independent predictor (HR: 0.92; 95% C.I. 0.86 to 0.99; p = 0.02) of the complete PE resolution (Table 3). Figure 1 shows the Kaplan-Meier analysis of survival free from occurrence of PE resolution according to the ARDS diagnosis at admission (long rank P = 0.61).
Table 3.
Univariate and multivariate Cox regression models for PE resolution
| Clinical Variable | Univariate HR (95% CI) |
P value | Multivariate HR (95% CI) |
P value |
|---|---|---|---|---|
| Age | 0.97 (0.95–0.99) | 0.007 | 0.97 (0.95–1.01) | 0.067 |
| Hospitalization lenght (days) | 0.96 (0.93–0.99) | 0.015 | 1.01 (0.96–1.04) | 0.922 |
| Switch to edoxaban (days) | 0.91 (0.86–0.97) | 0.022 | 0.92 (0.86–0.98) | 0.017 |
PE: pulmonary embolism; HR: hazard ratio; CI: confidential interval.
Figure 1.
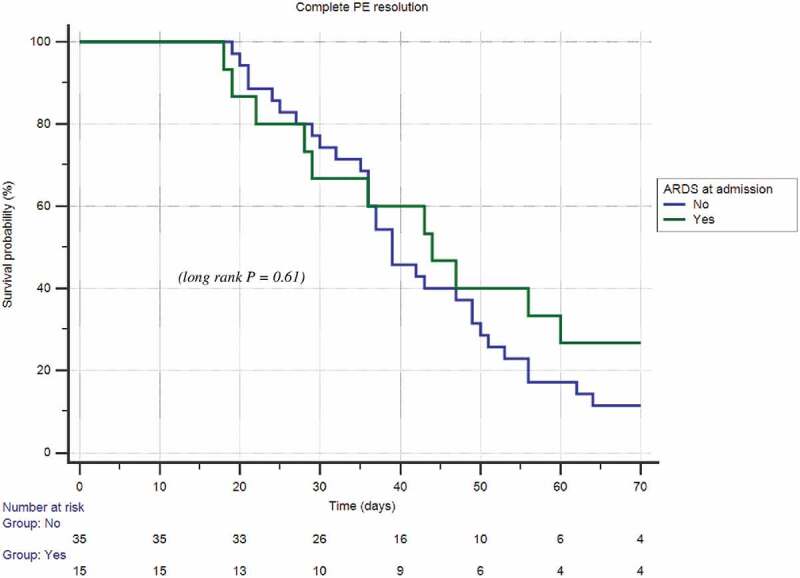
Kaplan-Meier curve of time to resolution of pulmonary embolism (PE) among patients with and without acute respiratory distress syndrome (ARDS)
4. Discussion
The main findings of the present study can be summarized as follows: 13% of COVID-19 patients admitted for acute dyspnea showed acute PE at admission and among them about 30% showed concomitant ARDS. Edoxaban, administrated after initial therapy with parenteral anticoagulants with LMWH/fondaparinux, was an effective and safe anticoagulant treatment for acute PE in COVID-19 setting. The earlier administration of edoxaban seems to be independently associated with PE resolution at CT.
Deep vein thrombosis (DVT) and PE are a major concern in COVID-19 patients. The relationship between SARS-CoV-2 infection, COVID-19 disease and coagulopathy has been well established and attributed to several factors such as increased vasoconstrictor angiotensin II [12] and sepsis-induced release of cytokines [13]. These factors could be responsible for platelet activation, endothelial dysfunction, and stasis with both arterial and venous thrombotic manifestations [14,15]. Indeed, up to 50% of COVID-19 patients showed abnormal and progressive D-dimer elevation and up to 40% experienced DVT and PE events [16], with a prevalence ranging between 12.9 and 19.5% [5,17]. Abnormal coagulation parameters and DVT are associated with poor prognosis in these patients [18–20]. In light of these considerations and supported by several clinical evidences [21–24], CHEST guidelines recommend parenteral over NOACs anticoagulation therapy for thromboprophylaxis and treatment of DVT in hospitalized acutely ill patients with COVID-19; moreover, the once-daily dosing regimen of low-molecular-weight heparins or fondaparinux should be preferred over unfractionated heparin to reduce personal protective equipment use and exposure of healthcare workers [7].
The major concerns in the use of NOACs among COVID-19 patients lie in drug interactions with antiviral agents [25] and their longer duration of action than heparins, which could result in an increased risk of bleeding in critically ill patients [7]; moreover, some case reports suggest the possibility that COVID‐19 may lead to higher rates of NOACs failure [9,26], probably due to lower anti-inflammatory effects compared to heparinoids [27]. According to the current guidelines [7], our study cohort started the parental anticoagulant therapy with fondaparinux or LWMH at PE diagnosis. Despite contrasting data about the fondaparinux safety among COVID-19 patients [28], its increasing use in our clinical practice was related to the once-daily administration and the more feasible dosing scheme compared with LMWH.
The choice of edoxaban for the VTE treatment among our study cohort was based on the once daily administration and the lack of pharmacokinetic interaction with the cytochrome P-450 system [29,30]. In the clinical setting of VTE treatment, it is already established that edoxaban should be administered after a minimum of 5 days of parenteral anticoagulation, therefore PE treatment with edoxaban was slightly influenced in outcomes by the in-hospital protocol of initiating therapy with LMWH or fondaparinux in COVID-19 patients with PE [8,31].
So far, little is still known about the clinical profile of NOACs for the treatment of VTE in COVID-19 patients. Edoxaban has demonstrated non-inferiority to standard therapy for the treatment of VTE in general population, preserving a high safety profile even in long term therapy, in frail patients and in severe clinical presentations [32]. Indeed, in the ‘Edoxaban versus Warfarin for the Treatment of Symptomatic Venous Thromboembolism (HOKUSAI-VTE)’ trial, edoxaban showed non inferiority compared to standard therapy even in severe PE (hazard ratio, 0.52; 95% CI, 0.28 to 0.98) [31]. Later, Brekelmans et al demonstrated edoxaban superiority in prevention of recurrent VTE in this subset of patient in a post-hoc analysis of the HOKUSAI-VTE trial [33].
The retrospective nature of the study, the small simple size and the absence of alternative anticoagulant treatment group represent the main limitations of the present analysis; however, to the best of our knowledge this is the first study directly exploring the clinical profile of edoxaban, following parental heparin anticoagulation, in COVID-19 patients with PE diagnosed at admission. Other limitations concern the lack of metrics to further classify illness severity in the PE subgroup and the lack of data about the concomitant deep venous thrombosis; however, the routine ultrasound screening for the detection of asymptomatic deep vein thrombosis (DVT) is not currently indicated [34].
5. Conclusion
Although our results need confirmation by prospective studies including a larger population, the combined anticoagulant strategy of LMWH/fondaparinux followed by edoxaban seems to be an effective and safe treatment for acute PE in the clinical contest of COVID-19.
Funding Statement
This paper was not funded.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Vincenzo Russo], [Valerio Langella] and [Antonio Asti]. The first draft of the manuscript was written by [Roberta Bottino] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of interest
The author(s) have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royaltiesThe authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Ethical approval was waived by the local Ethics Committee in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Consent to participate
Informed consent from individual patients was waived due to the observational retrospective design of this study.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Hui DS, I Azhar E, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus disease (COVID-19) situation reports [Internet]. [cited 2021 Feb 24]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 3.Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. [DOI] [PubMed] [Google Scholar]
- 4.Longhitano Y, Racca F, Zanza C, et al. Venous thromboembolism in critically ill patients affected by ARDS related to COVID-19 in Northern-West Italy. Eur Rev Med Pharmacol Sci. 2020;24:9154–9160. [DOI] [PubMed] [Google Scholar]
- 5.Scudiero F, Silverio A, Di Maio M, et al. Pulmonary embolism in COVID-19 patients: prevalence, predictors and clinical outcome. Thromb Res. 2021;198:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Current guideline for the management of anticoagulation for VTE and PE in COVID-19 patients.
- 8.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 9.Lewis P, Tharp JL.. Breakthrough venous thromboembolic events in five patients with COVID‐19 on direct oral anticoagulants. J Clin Pharm Ther. 2021;46(2):519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Case series focused on the use of NOACs in COVID-19 patients with VTE.
- 10.Ranieri VM, Rubenfeld GD, Thompson BT et al. ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 11.Stein PD, Yaekoub AY, Matta F, et al. Resolution of pulmonary embolism on CT pulmonary angiography. Am J Roentgenol. 2010;194(5):1263–1268. [DOI] [PubMed] [Google Scholar]
- 12.Miesbach W. Pathological role of angiotensin II in severe COVID-19. TH Open. 2020;4(2):e138–e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–241. [DOI] [PubMed] [Google Scholar]
- 15.Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17(2):283–294. [DOI] [PubMed] [Google Scholar]
- 16.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• State-of-the-Art review on the management of thrombotic or thromboembolic disease in COVID-19 patients.
- 17.Desai R, Gandhi Z, Singh S, et al. Prevalence of pulmonary embolism in COVID-19: a pooled analysis. SN Compr Clin Med. 2020;2(12):2722–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis), Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Micco P, Russo V, Carannante N, et al. Clotting factors in COVID-19: epidemiological association and prognostic values in different clinical presentations in an Italian Cohort. JCM. 2020;9(5):1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo V, Cardillo G, Viggiano GV, et al. Fondaparinux use in patients with COVID-19: a preliminary multicenter real-world experience. J Cardiovasc Pharmacol. 2020;76(4):369–371. [DOI] [PubMed] [Google Scholar]
- 22.Russo V, Cardillo G, Viggiano GV, et al. Thromboprofilaxys with Fondaparinux vs. Enoxaparin in hospitalized COVID-19 patients: a multicenter Italian observational study. Front Med. 2020;7:569567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold K, Xu Y, Sparkenbaugh EM, et al. Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced acute liver failure. Sci Transl Med. 2020;12(535):eaav8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poterucha TJ, Libby P, Goldhaber SZ. More than an anticoagulant: do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017;117(3):437–444. [DOI] [PubMed] [Google Scholar]
- 25.Russo V, Bottino R, Carbone A, et al. COVID-19 and heart: from clinical features to pharmacological implications. JCM. 2020;9(6):1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Tano G, Moschini L, Loffi M, et al. Late pulmonary embolism after COVID-19 pneumonia despite adequate Rivaroxaban treatment. Eur J Case Rep Intern Med. 2020;7:001790. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Case report on the use of Rivaroxaban in the setting of COVID-19 disease.
- 27.Cardillo G, Viggiano GV, Russo V, et al. Antithrombotic and anti-inflammatory effects of Fondaparinux and Enoxaparin in hospitalized COVID-19 patients: the Fondenoxavid study. JBM. 2021;12:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo V, Proietti R, Lodigiani C, et al. Fondaparinux and bleeding risk in COVID-19: unsolved question. Thromb Res. 2021;200:128–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corsini A, Ferri N, Proietti M, et al. Edoxaban and the issue of drug-drug interactions: from pharmacology to clinical practice. Drugs. 2020;80(11):1065–1083. [DOI] [PubMed] [Google Scholar]
- 30.Parasrampuria DA, Truitt KE. Pharmacokinetics and Pharmacodynamics of Edoxaban, a non-vitamin K antagonist oral anticoagulant that inhibits clotting factor Xa. Clin Pharmacokinet. 2016;55(6):641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Hokusai-VTE Investigators . Edoxaban versus Warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. [DOI] [PubMed] [Google Scholar]
- 32.Bottino R, Carbone A, Liccardo B, et al. Edoxaban (LIXIANA ®) in the treatment of venous thromboembolism. Future Cardiol. 2020. [Nov 24]. DOI: 10.2217/fca-2020-0139. [DOI] [PubMed] [Google Scholar]
- 33.Brekelmans MPA, Ageno W, Beenen LF, et al. Recurrent venous thromboembolism in patients with pulmonary embolism and right ventricular dysfunction: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol. 2016;3(9):e437–e445. [DOI] [PubMed] [Google Scholar]
- 34.Di Micco P, Russo V, Lodigiani C. Venous Thromboembolism and its association with COVID-19: still an open debate. Medicina (Kaunas). 2020;56(10):506. [DOI] [PMC free article] [PubMed] [Google Scholar]


