Supplemental Digital Content is Available in the Text.
Key Words: COVID-19, socioeconomic factors, HIV/AIDS, social inequalities, alcohol drinking, SARS-CoV-2
Background:
Limited empirical evidence exists about the extent to which the current HIV epidemic intersects with COVID-19 infections at the area/geographic level. Moreover, little is known about how demographic, social, economic, behavioral, and clinical determinants are jointly associated with these infectious diseases.
Setting:
Contiguous US counties (N = 3108).
Methods:
We conducted a cross-sectional analysis and investigated the joint association between new HIV infection prevalence in 2018 and COVID-19 infections (January 22, 2020 and October 7, 2020) and explore the contribution of factors such as income inequality, binge drinking, and socioeconomic deprivation. We used Bayesian multivariate spatial models to estimate the cross-disease correlations between these diseases and identified hotspots, which we defined as a county with a posterior probability greater than 80% of being in the top decile of that disease.
Results:
New HIV infection prevalence and COVID-19 infection moderately and significantly intersect [spatial correlation = 0.37, 95% credible interval (CrI) = 0.36–0.37]. Seventy-five counties, mostly in the south, were at elevated burden for HIV and COVID-19 infections. Higher income inequality was positively associated with both COVID-19 (relative risk 1.05, 95% CrI = 1.03–1.07) and HIV infection (relative risk = 1.12, 95% CrI = 1.09–1.15).
Conclusions:
We found that there is a considerable intersection between the current distribution of HIV burden with COVID-19 infections at the area level. We identified areas that federal funding and vaccination campaigns should prioritize for prevention and care efforts.
INTRODUCTION
Addressing the intersecting epidemics of COVID-19 and HIV infection has emerged as a global priority. The preponderance of studies thus far has focused on the biological or psychological effects and implications of COVID-19 infections for individual-level biomedical prevention among people with HIV (PWH).1 Some studies have reported excess risk for COVID-19 infection and mortality in the presence of an HIV infection among adults.2 However, these results have been mixed. Some findings indicate that PWH who had interruptions in taking antiretroviral therapy as prescribed are more vulnerable to COVID-19 infection because their immunity will have been compromised because of an uncontrolled HIV viral load.3 However, some studies show no difference in COVID-19 severity between the HIV-positive and HIV-negative population4,5 or PWH who have their HIV suppressed to undetectable levels have similar risk of COVID-19 infection and severity as those who are not HIV-positive.3
Beyond biological plausibility, the susceptibility to COVID-19 infection among PWH is related to distributions of social determinants, including higher rates of housing insecurity and congregate living and employment interruptions, which affected people's availability to access treatment and afford their medications. COVID-19 also affected population health through shifting demand on the health systems where hospital beds, diagnostic screening capacities, and other policies diverted funding away from diseases such as cancer and HIV to COVID-19.6
At the individual level, COVID-19 infection and HIV infection are influenced by similar risk behaviors such as substance use,7 which compromises one's immunological state.8
To date, however, there is less published empirical evidence about how the HIV epidemic intersects with the COVID-19 pandemic at the area level. Moreover, it is unknown how demographic, social, economic, behavioral, and clinical determinants underlie the joint distribution of these diseases. When one considers geography, we know that the burden of COVID-19 infection and mortality is concentrated among communities with a high proportion of people of color.9,10 Social epidemiology studies have shown that higher risk of infection and poorer prognosis of disease burden (whether COVID-19 or HIV infection) cannot be adequately explained by compositional attributes such as biological differences or risk behaviors of individuals residing in those areas.11–14 Rather, higher burden of disease and disparities across COVID-19 and HIV infection are shaped by the inequitable distribution of socioeconomic resources15–18 and other vulnerability-related determinants19,20 such as environmental health hazards, violence, poorer access to care, and other trauma-causing exposures.6,21,22
In this analysis, we quantified the extent of coclustering between these 2 diseases at the area level. We then quantify the extent that demographic, social, economic, and environmental factors are related to both. The vast disparities and persistence of the COVID-19 pandemic and HIV epidemic necessitates ecological analysis like these that potentially inform COVID-19 vaccination distribution and identify regions of greatest need for Ending the HIV Epidemic interventions.23
METHODS
Study Region and COVID-19 and HIV Infection Data
We obtained the count of COVID-19 infection between January 2020 and October 7, 2020, for every county in the contiguous United States, retrieved from the Johns Hopkins University Coronavirus Resource Center dashboard (https://coronavirus.jhu.edu). County-level prevalent HIV infection counts in 2018 were obtained from AIDSVu—an online platform that collects national HIV surveillance data from several jurisdictions (https://aidsvu.org). The 2018 counts were the most recent publicly available HIV data at the county level. As geographic patterns of new HIV infections have been relatively constant between 2014 and 2018, we assumed that new infections would follow similar distributions in 2020.24 In the case when the numerator count is between 2 and 4 or when the total population of a county is less than 100, the value was suppressed to protect personal privacy. The corresponding total population for each county was also collected in the data sets. County and state boundary files were downloaded from the US Census Bureau. We restricted our analysis to N = 3108 contiguous counties, which had no missing data on the study variables (99% of 3143 counties).
County-Level Covariates
County-level covariates considered to account for potential confounding25 included percent of the population who were Black/African American, percent who were Hispanic/Latino, proportion of the population between the ages of 35–64 years, and population density per square mile, retrieved from the 2018 American Community Survey 5-year estimates through Social Explorer online database (https://socialexplorer.com). Percent of uninsured adults younger than 65 years were retrieved from the US Census Bureau through the 2018 Small Area Health Insurance Estimates program. Chlamydia infection rate per 100,000 general population in 2017 was retrieved from the Centers for Disease Control and Prevention through the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Atlas. Chlamydia was chosen to represent overall burden of sexually transmitted infections (STIs) in a county because it is the most common notifiable condition and comprises the largest proportion of reportable STIs in the United States.26
Other County-Level Determinants
Other county-level determinants considered as potential explanatory variables were socioeconomic deprivation, created from a principal components index (Cronbach alpha = 0.83) included percent of population younger than 25 years with less than a high school diploma, median household income, percent of population 16 years and older unemployed, and percent of people aged 18 to 64 years living in poverty. All variables were from the 2018 American Community Survey 5-year estimates through Social Explorer. Percentage of adults with reported binge drinking in 2017 (5 or more drinks per occasion for men and 4 or more drinks per occasion for women) were retrieved from the Behavioral Risk Factor Surveillance System (BRFSS) through PolicyMap online database. Income inequality was measured with the Gini coefficient and was retrieved from the 2010 Decennial Census through Social Explorer.
Statistical Analysis
We used a Bayesian multivariate spatial model to conduct a cross-sectional analysis of county-level COVID-19 and HIV infection. The model was specified as Yik ∼ Poisson (mik × Pik) and ,
where Pik and mik are the total population and the underlying kth infection rate (k = 1, COVID-19; k = 2, HIV) at the ith county (i = 1, 2,…, 3108). The parameter αk represents the average COVID-19 (k = 1) or HIV (k = 2) infection rate over the whole nation; Xim is the mth covariate at the ith county with corresponding coefficient βm. All the 9 county-level covariates and determinants were included in the statistical model in one step/block, and sik is the spatial random-effects term, indicating spatial variations of the infection rate (k = 1, COVID-19; k = 2, HIV) that are not captured by the covariates and determinants. A Poisson distribution truncated to the range 2 and 4 was used to model the suppressed HIV infection values in the data set. The multivariate Leroux prior,27 a conditional autoregressive prior with a spatial correlation parameter, was assigned to sik. This joint modeling of multiple spatial random effects allows the modeling of the mix of spatial clustering and spatial heterogeneity in the diseases and calculating the cross-disease correlations, thus improving model fit. The inverse of the 2 × 2 variance matrix of sik was assigned a Wishart prior distribution. The model was implemented using the package ‟R2WinBUGS” in R version 3.5.1. Final covariates and determinants in the multivariate model are based on those statistically significantly correlated in bivariate tests with each disease. To check the robustness of our statistical model, we considered a model that accounted for potential higher-level clustering of counties within the 9 census regions. Further information about the models and results is provided in the Appendix, Supplemental Digital Content, http://links.lww.com/QAI/B693.
Spatial Pattern Uncertainty
To quantify the uncertainty associated with the estimated infection rates of COVID-19 infection or HIV infection for each county, we generated 10,000 samples from the posterior distribution of mik. For each sample, we calculated the rank (among the total 3108 counties) of each county regarding its COVID-19 and HIV infection rates. If more than 80% of the samples indicate that a county ranks among the top decile for a specific disease, that county is classified as a hotspot of the disease. In other words, the posterior probability of a county ranking in the top decile for a disease is greater than 0.8 is strong evidence that the county has the highest decile of COVID-19 and HIV infection rates.28
The ecological study was not considered human subjects research by the IRB.
RESULTS
HIV Infection and COVID-19 Infection Concentrate Geographically
We identified spatial clustering independently for both COVID-19 infection in 22, 2020 and HIV infection in 2018, with the former having a stronger autocorrelation (ie, spatial clustering pattern). The spatial autocorrelation parameters (ie, posterior mean) between COVID-19 infection and HIV infection are 0.89 [95% credible interval (CrI): 0.80–0.98] and 0.69 (95% CrI: 0.57–0.82), respectively. A spatial correlation parameter approaching 1 indicates strong spatial clustering that neighboring counties have similar levels of COVID-19 or HIV infection. This spatial clustering pattern can be seen for HIV infection in 2018 (ie, top decile; Figs. 1A, 1C.). Compared with the maps of posterior means of each disease (Figs. 1B, 1D), posterior probability maps accounting for the uncertainty associated with the point estimate thus are more reliable approaches to assess whether a county has truly elevated COVID-19 or HIV infection.
FIGURE 1.
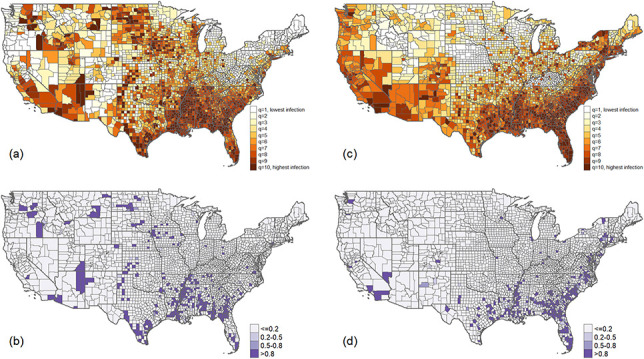
(A) Estimated COVID-19 infection rate as of October 7, 2020, adjusting for covariates and determinants. (B) The posterior probability of a county with COVID-19 infection rate in the top decile (ie, hotspot). (C) The estimated HIV infection rate in 2018 adjusting for covariates. (D) The posterior probability of a county with HIV infection rate in the top decile (ie, hotspot).
Strong Overlap Between COVID-19 Infection and HIV Infection
Adjusting for covariates and other determinants, the spatial correlation between COVID-19 and HIV infection is 0·37 (95% CrI: 0.36–0.37). This statistically significant positive correlation suggests that a county with a high rate of HIV infection is moderately likely to also have high COVID-19 infection.
Several Areas Share Intersecting Burden of COVID-19 Infection and HIV Infection
Figure 2 shows a clear geographic patterning of coclustering between COVID-19 and HIV infection. We found at least 75 counties that should be prioritized for the COVID-19 vaccination given the dual threat to the ongoing HIV epidemic. The intersecting burden of HIV and COVID-19 primarily was in the southern census region. Except for New York (1), most coexisting COVID-19 and HIV infection hotspots were across counties in Virginia (3), Tennessee (3), Arkansas (3), Alabama (5), South Carolina (5), Texas (6), Mississippi (8), Louisiana (10), Georgia (14), and Florida (17). A complete enumeration of counties in each category of hotspot (eg, HIV cluster, COVID-19 cluster, and HIV and COVID-19 cocluster) is provided in Appendix Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B693.
FIGURE 2.
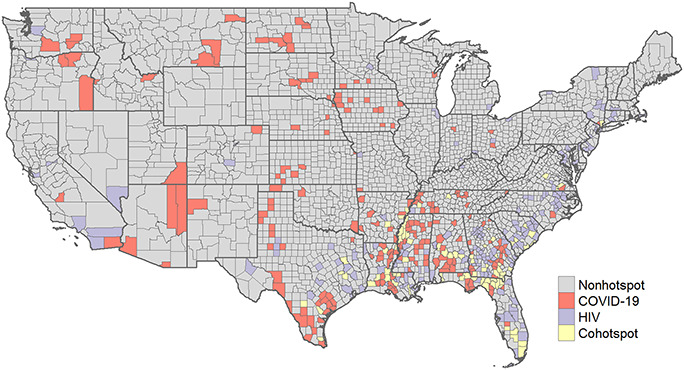
Coexisting hotspots between COVID-19 and HIV infection are located primarily in the southern census region of the country.
Multivariate Association of Demographic, Social, Economic, Behavioral, and Clinical Determinants With COVID-19 and HIV Infection
The results from the robustness check showed that adding the census region clustering did not significantly improve the model fit. The Deviance Information Criterion difference between the old model and the newly fitted model is smaller than 2 (49,650.35 vs 49,648.99), suggesting that the models fitted the data equally well. In fact, the same 75 counties were identified as cohotspots of COVID-19 and HIV infections (which is also an indication of the robustness of the simple model without census region-level random effects). Details of the model and results are available in the Appendix Table 2, Supplemental Digital Content, http://links.lww.com/QAI/B693. We report the results of the most parsimonious model with no higher order clustering.
Table 1 reports the associations from the multivariable model where the variables were regressed on both COVID-19 and HIV infection rates. We identify that income inequality, prevalence of binge drinking, and STIs (as assessed by chlamydia infection rate) were positively and significantly associated with both diseases. The strength of associations was similar in magnitude given that the point estimates for both diseases were within the credible intervals of each other. For instance, a one-point SD increase in binge drinking rate was associated with a 12% higher COVID-19 infection rate [relative risk (RR) = 1.12; 95% CrI 1.08–1.15] and a 10% higher HIV infection rate (RR = 1.10, 95% CrI 1.06–1.15). Population density was negatively associated with COVID-19 infection here but positively associated with HIV infection.
TABLE 1.
Multivariate Results From Bayesian Spatial Poisson Regression Analysis for Factors Associated With COVID-19 and HIV Infection
| Distribution of Study Variables | COVID-19 Infection | HIV Infection | |
| Mean (SD) | Relative Risk and (95% CrI) | Relative Risk and (95% CrI) | |
| % Black | 9.14% (14.59%) | 1.123 (1.08–1.165) | 1.583 (1.515–1.651) |
| % Hispanic | 9.3% (13.86%) | 1.332 (1.279–1.372) | 1.187 (1.138–1.236) |
| % Aged 35 to 64 years | 38.66% (2.98%) | 0.957 (0.938–0.976) | 1.106 (1.077–1.136) |
| Socioeconomic deprivation* | −1.85 (0.46) | 1.022 (0.996–1.051) | 1.066 (1.026–1.107) |
| Population density | 272.94 (1812.99) | 0.970 (0.946–0.997) | 1.053 (1.028–1.073) |
| Chlamydia rate | 391.85 (277.08) | 1.063 (1.042–1.085) | 1.161 (1.126–1.199) |
| % Uninsured younger than 65 years | 14.19% (6.14%) | 1.105 (1.066–1.137) | 1.042 (0.992–1.089) |
| Gini coefficient | 0.43 (0.04) | 1.046 (1.028–1.066) | 1.117 (1.090–1.148) |
| % Binge drinking | 16.95% (2.98%) | 1.118 (1.085–1.150) | 1.104 (1.060–1.147) |
A principal component index of percent of population younger than 25 years with less than a high school diploma, median household income, percent of population 16 years and older unemployed, and percent of people aged 18 to 64 years living in poverty.
A one-point SD increase in chlamydia rate was associated with a 6% increase in COVID-19 infection rate (RR = 1.06, 95% CrI 1.04–1.11) and 16% increase in HIV infection rate (RR = 1.16, 95% CrI 1.13–1.20). Income inequality had similar size of effects as chlamydia where a one-unit standardized increase in the Gini coefficient corresponded to a 5% increase in COVID-19 (RR = 1.05, 95% CrI 1.03–1.07) and 12% increase in HIV infection (RR = 1.12, 95% CrI 1.09–1.15).
In total, the 9 covariates and determinants (regardless of statistical significance) explained 38.4% (95% CrI: 34.8%–42.2%) and 80.3% (95% CrI: 70.6%–93.5%) variances in COVID-19 and HIV infection, respectively.
DISCUSSION
To the best of our knowledge, this is the first national study to quantify the extent to which the HIV epidemic, using most recent publicly available data, intersects with COVID-19 infection in 2020 at the area level. We documented a sizeable overlap in disease burden (approximately 37%) between COVID-19 infection and HIV infection even after accounting for demographic, social, economic, and behavioral county-level characteristics. Funding is scarce in this economic crisis. What might our results mean for prevention and policy? First, we used a robust statistical model to identify at least 75 areas that could be prioritized for the COVID-19 vaccination given the raging dual threat of COVID-19 spread and the pre-existing HIV epidemic. The coexisting hotspots were particularly pronounced in areas from the southern census region. For example, among the 75 counties identified as coexisting hotspots, 17 were from Florida, 14 from Georgia, 10 from Louisiana, and 8 from Mississippi. Strikingly, although almost all cohotspots are within states prioritized by the Ending the HIV Epidemic initiative, among those counties, only 2 (Broward and Miami-Dade, Florida) are currently prioritized for funding.29
We also identified some determinants that potentially drive the joint distribution of these 2 infectious diseases. Income inequality was positively associated with COVID-19 and HIV infection, which is consistent with published reports.30,31 One novel finding we contribute to this topic is the link between alcohol use and COVID-19 infection (and HIV infection) at the population level, which remains a major public health concern.32 To date, much of the literature documenting associations between alcohol and COVID-19 are among individuals. Limited per capita on alcohol sales data suggests that consumption increased during the COVID-19 pandemic.33 We found that areas with higher binge drinking prevalence had higher COVID-19 infection rates. The findings that chlamydia prevalence is jointly associated with higher COVID-19 and HIV infection rates have also not been previously described. It is well known that at the individual and area level, the presence of STI increases risk for sexual transmission of HIV.34 Currently, we show that area-level STI burden is associated with COVID-19 infection rates. Together, binge drinking, chlamydia infection rate, income inequality, and other covariates accounted for 38% of COVID-19 infection burden and 80% of HIV infection burden across these areas. Therefore, interventions that address a combination of one or more of those variables might result in substantial public health benefits.
More broadly, beyond the practical relevance for HIV prevention, this study has important implications for how we understand syndemic effects on population health and the policy recommendations.19 Specifically, few studies have theorized about, let alone investigate, the potential synergistic effects of these 2 epidemics at the area level.35 With the introduction of a COVID-19 vaccine, it is possible that we see the virus controlled in a few years. However, there is no vaccine for HIV—an epidemic that has been here for almost 40 years. The current study underscores a need to conduct joint disease analysis to inform how we respond to HIV care. The COVID-19 pandemic may have synergistic effects with one or more epidemics (eg, the opioid crisis and the financial crisis) that are likely to influence the ongoing HIV epidemic.
One primary limitation of some previous studies is that they examined 1 disease outcome only and so cannot precisely and jointly identify coexisting risks or predictors associated with both disease outcomes. Findings from our study are enhanced by the large number of counties that give us sufficient power to estimate the joint association between COVID-19 infection and HIV infection with reliability and precision. Next, we applied the Bayesian multivariate spatial model, which is a robust statistical technique. A Poisson distribution truncated to a range is used to model the suppressed values rather than discarding them from the analysis. This approach uses more available information for statistical inferences and thus strengthens our findings. Furthermore, specifying a multivariate Leroux prior to the spatial random effects in the model allows ‟borrowing” information not only from neighboring counties for a specific disease (ie, COVID-19 and HIV infection) but also between the 2 diseases. This specification significantly improves model fit (more details in the Appendix, Supplemental Digital Content, http://links.lww.com/QAI/B693) and again enhances our conclusions. Our findings showed that higher Black racial concentration and higher socioeconomic deprivation are independently and positively correlated with both COVID-19 infection (although borderline nonsignificant in our study) and HIV infection. These findings are also consistent with theory and other empirical studies.6,15,16,36
Our study strengths and findings should be considered in the context of the following limitations: We used crude rates of both diseases and at different times (diagnosed HIV infection in 2018 and diagnosed COVID-19 infection in 2020). However, geographic patterns of HIV have been relatively stable and so the 2018 patterns are assumed to be similar in 2020. Nevertheless, some of the covariates such as chlamydia infection, if measured in 2020 might have less of an impact on COVID-19 because social contact was reduced and health care screenings for other sexually transmitted diseases during COVID-19 declined.37
We are aware that there is an age patterning in demographic and epidemiologic profiles that may influence the extent of COVID-19 and HIV infection disparities.38 Age-adjusted estimates for COVID-19 are still scarce and not systematically available at the county level because there is no uniform mandate and public health agencies report their data differently. Although age-adjusted estimates of HIV infection are available, to strengthen the comparability of our models, we applied crude rates to both diseases. The extent of bias is somewhat mitigated by controlling for age group, which is 1 procedure used in other studies.25 We included a selection of covariates that had high biological plausibility and empirically related to both COVID-19 infection and HIV infection. In all epidemiologic studies, it is impossible to identify or include every factor so there remains residual confounding unaccounted for by our models.
Considering omitted variables, we do not anticipate that their omission and potential bias would have substantially undermined the utility of our findings, especially given that we included a spatial random-effects term in our statistical models that could serve as surrogates of unincorporated covariates. In fact, several of the covariates and determinants in our model were robust and in total and explained a sizeable proportion of variance for both diseases. Although our Bayesian multivariate spatial model is robust, the conditional autoregressive prior (ie, Leroux) used in our study assumes that neighboring counties are equally influential, which could be too restrictive. Locally adaptive spatial weight matrices could be tested in future studies. We used US data, but these analyses can be replicated with COVID-19 and HIV data in other countries and at smaller geographic levels (eg, ZIP codes and census tracts).
Although we adjusted for several covariates, the study was not designed to identify factors that fully explain the spatial clustering between COVID-19 infection and HIV infection. Future studies should be conducted to explain this coclustering by including structural conditions that characterize the health care environment, such as number of free or low-cost clinics or health care facilities, health literacy, and transportation barriers. Other determinants should include indicators of the political landscape and determinants that affect the distribution of resources to diagnose and treat both COVID-19 and HIV.24,39 Although ours and other studies40 indicate that Black racial composition is a strong predictor of both COVID-19 infection and HIV infection, future work should examine formal measures of racial residential segregation as a fundamental cause of disparities41 in these outcomes. Finally, although we primarily examined structural and biological determinants, future work should include determinants that characterize the social environment and relationships among people such as social cohesion and social capital, which are associated with COVID-19 infections and HIV diagnosis at the ecological level.42–44
Our findings suggest that there is considerable intersection between the current distribution of HIV infection burden with COVID-19 infections at the area level. We identified areas that federal funding and vaccination campaigns should prioritize for prevention and care efforts. We also recommend that public health departments develop surveillance systems to capture syndemic effects of COVID-19, HIV infection, and other epidemics on population health. Our findings raise several questions for future research. While the COVID-19 pandemic is the current focus, it is well known that the opioid overdose crisis is raging in the United States, Europe, and globally.45 Future research should use syndemics theory applied to the ecological level35 to investigate potential synergistic impacts of multiple “converging crises”46 such as HIV infection, opioid overdose mortality, unemployment, and racial justice movements and their impacts on COVID-19 mortality rates.
Footnotes
Y.R. is currently receiving a Grant (#K01MH111374) from the National institute of Mental Health (NIMH). H.L. is currently receiving a Grant (Data Science Seed Grant Initiative) from the University of Oregon. The remaining authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Jones DL, Morgan KE, Martinez PC, et al. COVID-19 rurden and risk among people with HIV. J Acquir Immune Defic Syndr. 2021;87:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J, Xie N, Hu X, et al. Epidemiological, virological and serological reatures of coronavirus disease 2019 (COVID-19) cases in people living with Human Immunodeficiency Virus in Wuhan: a population-based cohort study. Clin Infect Dis. 2020;17:ciaa1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachdev D, Mara E, Hsu L, et al. COVID-19 susceptibility and outcomes among people living with HIV in San Francisco. J Acquir Immune Defic Syndr. 2021;86:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karmen-Tuohy S, Carlucci PM, Zervou FN, et al. Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;85:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millett GA. New pathogen, same disparities: why COVID-19 and HIV remain prevalent in U.S. communities of colour and implications for ending the HIV epidemic. J Int AIDS Soc. 2020;23:e25639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang QQ, Kaelber DC, Xu R, et al. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams EC, Hahn JA, Saitz R, et al. Alcohol use and Human Immunodeficiency Virus (HIV) infection: current knowledge, implications, and future directions. Alcohol Clin Exp Res. 2016;40:2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on Black communities. Ann Epidemiol. 2020;47:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linley L, Prejean J, An Q, et al. Racial/ethnic disparities in HIV diagnoses among persons aged 50 years and older in 37 US States, 2005–2008. Am J Public Health. 2012;102:1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380:341–348. [DOI] [PubMed] [Google Scholar]
- 12.Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. J Am Med Assoc. 2020;24:2466–2467. [Google Scholar]
- 13.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;39:887–903. [DOI] [PubMed] [Google Scholar]
- 14.Krieger N, Waterman PD, Chen JT, et al. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures—the public health disparities geocoding project (US). Public Health Rep. 2003;118:240–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmot M, Allen J. COVID-19: exposing and amplifying inequalities. J Epidemiol Community Health. 2020;74:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clouston SA, Natale G, Link BG. Socioeconomic inequalities in the spread of coronavirus-19 in the United States: a examination of the emergence of social inequalities. Soc Sci Med. 2021;268:113554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris R. Exploring the neighbourhood-level correlates of Covid-19 deaths in London using a difference across spatial boundaries method. Health Place. 2020;66:102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce JB, Harrington K, McCabe ME, et al. Racial/ethnic minority and neighborhood disadvantage leads to disproportionate mortality burden and years of potential life lost due to COVID-19 in Chicago, Illinois. Health Place. 2021;68:102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam N, Lacey B, Shabnam S, et al. Social inequality and the syndemic of chronic disease and COVID-19: county-level analysis in the USA. J Epidemiol Community Health. 2021;5:215626. [DOI] [PubMed] [Google Scholar]
- 20.Snyder BF, Parks V. Spatial variation in socio-ecological vulnerability to Covid-19 in the contiguous United States. Health Place. 2020;66:102471. [DOI] [PubMed] [Google Scholar]
- 21.Daras K, Alexiou A, Rose TC, et al. How does vulnerability to COVID-19 vary between communities in England? Developing a small area vulnerability index (SAVI). J Epidemiol Community Health. 2021;4:215227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicodemo C, Barzin S, Cavalli N, et al. Measuring geographical disparities in England at the time of COVID-19: results using a composite indicator of population vulnerability. BMJ Open. 2020;10:e039749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore M, Boily MC, Mitchell KM, et al. Identifying regions of greatest need for Ending the HIV Epidemic: a plan for America. J Acquir Immune Defic Syndr. 2020;85:395–398. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan PS, Satcher Johnson A, Pembleton ES, et al. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet. 2021;397:1095–1106. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Waterman P, Krieger N. COVID-19 and the Unequal Surge in Mortality Rates in Massachusetts, by City/Town and ZIP Code Measures of Poverty, Household Crowding, Race/Ethnicity, and Racialized Economic Segregation. Harvard Center for Population and Development Studies Working Paper Series. Cambridge, MA: Harvard University; 2020. [Google Scholar]
- 26.National Academies of Sciences E, and Medicine. Sexually Transmitted Infections: Adopting a Sexual Health Paradigm. 2021. Available at: https://www.nap.edu/catalog/25955/sexually-transmitted-infections-adopting-a-sexual-health-paradigm. Accessed May, 2021. [PubMed] [Google Scholar]
- 27.Leroux BG, Lei X, Breslow N. Estimation of disease rates in small areas: a new mixed model for spatial dependence. In: Halloran ME, Berry D, eds. New York, NY: Springer-Verlag; 1999:179–191. [Google Scholar]
- 28.Richardson S, Thomson A, Best N, et al. Interpreting posterior relative risk estimates in disease-mapping studies. Environ Health Perspect. 2004;112:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV Epidemic: a plan for the United States ending the HIV epidemic. JAMA. 2019;321:844–845. [DOI] [PubMed] [Google Scholar]
- 30.Oronce CIA, Scannell CA, Kawachi I, et al. Association between state-level income inequality and COVID-19 cases and mortality in the USA. J Gen Intern Med. 2020;35:2791–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buot ML, Docena JP, Ratemo BK, et al. Beyond race and place: distal sociological determinants of HIV disparities. PLoS One. 2014;9:e91711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clay JM, Parker MO. Alcohol use and misuse during the COVID-19 pandemic: a potential public health crisis? Lancet Public Health. 2020;5:e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute on Alcohol Abuse and Alcoholism. Surveillance Report COVID-19: Alcohol Sales During the COVID-19 Pandemic. Surveillance Reports 2020. 2020. Available at: https://pubs.niaaa.nih.gov/publications/surveillance-covid-19/COVSALES.htm. Accessed January 19, 2020. [Google Scholar]
- 34.Ward H, Rönn M. The contribution of STIs to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai AC, Mendenhall E, Trostle JA, et al. Co-occurring epidemics, syndemics, and population health. Lancet. 2017;389:978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ransome Y, Kawachi I, Braunstein S, et al. Structural inequalities drive late HIV diagnosis: the role of black racial concentration, income inequality, socioeconomic deprivation, and HIV testing. Health Place. 2016;42:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latini A, Magri F, Donà MG, et al. Is COVID-19 affecting the epidemiology of STIs? The experience of syphilis in Rome. Sex Transm Infect. 2021;97:78. [DOI] [PubMed] [Google Scholar]
- 38.Gross CP, Essien UR, Pasha S, et al. Racial and ehnic disparities in population-level Covid-19 mortality. J Gen Intern Med. 2020;35:3097–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kavanagh NM, Goel RR, Venkataramani AS. County-level socioeconomic and political predictors of distancing for COVID-19. Am J Prev Med. 2021;61:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millett GA, Honermann B, Jones A, et al. White counties stand apart: the primacy of residential segregation in COVID-19 and HIV diagnoses. AIDS Patient Care STDS. 2020;34:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ransome Y, Ojikutu O, Buchanan M, et al. Neighborhood social cohesion and inequalities in COVID-19 diagnosis rates by area-level Black/African American racial composition. J Urban Health. 2021;98:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ransome Y, Galea S, Pabayo R, et al. Social capital is associated with late HIV diagnosis: an ecological analysis. J Acquir Immune Defic Synd. 2016;73:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makridis CA, Wu C. How social capital helps communities weather the COVID-19 pandemic. PLoS One. 2021;16:e0245135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394:1560–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez PJ, Neely AH. Fundamentally uncaring: the differential multi-scalar impacts of COVID-19 in the U.S. Soc Sci Med. 2021;272:113707. [DOI] [PMC free article] [PubMed] [Google Scholar]


