To the Editor:
Clonal hematopoiesis of indeterminate potential (CHIP) is a premalignant precursor state to Myelodysplastic Syndrome (MDS). It is characterized by expansion of hematopoietic clones harboring MDS-initiating driver mutations without clinically evident cytopenia or dysplasia [1], and is associated with a 40% increased risk of cardiovascular disease (CVD), especially myocardial infarction (MI), independent of traditional risk factors [2, 3]. It is unclear if this risk persists after evolution into MDS and the relationship between CHIP mutations and CVD among MDS patients is an emerging area of research [4]. These patients experience high rates of CVD and cardiovascular mortality constitutes the most common non-disease-related cause of death in MDS [5, 6]. However, studies exploring cardiovascular risk in older adults with cancer largely exclude MDS [7] and a true estimate of the magnitude of MDS as a risk factor for CVD in the elderly remains unknown.
We performed a matched cohort study of MDS patients and non-cancer controls in a heterogenous, nationally representative sample using the Surveillance, Epidemiology, and End Results (SEER) cancer registry linked to Medicare administrative claims. The SEER-Medicare database represents 28% of the United States population from 20 disparate geographic regions and includes sociodemographic factors, clinical procedures, treatments and diagnoses from inpatient and outpatient settings in adults over 65 years, constituting a valuable resource for cancer research in the elderly [8]. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. The MDS cohort included Medicare beneficiaries diagnosed with MDS between 2004 and 2014. Exclusion criteria included lack of continuous coverage, enrollment in plans not captured by administrative claims, diagnosis obtained solely from death certificates or autopsy, and documented ischemic stroke (CVA) or MI preceding MDS diagnosis. The control cohort was obtained from a 5% sample of randomly selected non-cancer Medicare beneficiaries residing in SEER regions during the study period.
Comorbidity burden was estimated using the Charlson comorbidity index (CCI), a weighted score of 18 preexisting medical conditions validated to measure comorbidity from administrative data [9]. To effectively calculate pre-diagnosis CCI using at least 1 year of claims after Medicare eligibility, only patients diagnosed at 66 years or older were included. MDS was identified using International Classification for Diseases in Oncology morphology codes [10] and grouped into low, intermediate and high-risk groups based on MDS histology classification [5]. Transfusion dependence for red blood cells (RBC) or platelets was defined as two or more transfusions episodes within 60 days of diagnosis. The primary outcome was incidence of CVD, a composite of CVA and MI. Secondary outcomes included incidence of CVA and MI, survival after CVD, and MDS-related factors associated with increased CVD (Supplement Table).
Propensity matching using the nearest neighbor method based on demographic and geographic factors was used to pair MDS cases with non-cancer controls. MDS diagnosis date served as pseudo-diagnosis date for controls. We used cumulative incidence functions to assess the risk of incident CVD, Cox proportional hazards models to evaluate MDS as a risk factor for CVD, adjusting for age, gender, race, cardiovascular comorbidities, and geographic region, Log–log plots to test violation of the proportional hazard assumption, and Kaplan-Meier with log-rank test for survival analysis.
We identified 13,972 patients with incident diagnosis of MDS, mostly males (55.2%), and non-hispanic whites (82.1%) from urban areas (82.5%). Median age at diagnosis was 82 years (IQR, 78–87). Most had intermediate-risk disease (68.8%). Median pre-diagnosis CCI score was 0 (IQR, 0–2). A total of 9047 MDS patients were paired 1:1 with a propensity matched non-cancer control accounting for age, gender, race, and residence (Table 1).
Table 1.
Baseline characteristics of the matched cohort.
| Characteristic | MDS patients (N= 9,047) | Non-cancer controls (N= 9047) |
|---|---|---|
|
| ||
| Age, median (IQR) | 82 (67–78) | 79 (66–77) |
| Gender | ||
| Male | 4970 (55) | 3291 (36.5) |
| Female | 4077 (45) | 5756 (63.5) |
| Race | ||
| White | 8038 (88.8) | 7921 (87.6) |
| Black | 437 (4.8) | 485 (5.4) |
| Hispanic | 273 (3) | 282 (3.1) |
| Asian | 132 (1.5) | 186 (2.1) |
| Other | 167 (1.9) | 173 (1.8) |
| Residence | ||
| Metropolitan | 7430 (82.1) | 738 (80) |
| Urban | 1407 (15.5) | 1575 (17.4) |
| Rural | 210 (2.4) | 234 (2.6) |
| Comorbidity index | ||
| CCI ≤ 1 | 6255 (70.2) | 7051 (77.9) |
| CCI > 1 | 2692 (29.8) | 1996 (22.1) |
| MDS riska | ||
| Low | 2534 (18.1) | – |
| Intermediate | 9590 (68.8) | – |
| High | 1848 (13.1) | – |
| Transfusion dependencea | ||
| Red Blood cells | 4060 (29) | – |
| Platelets | 848 (6.1) | – |
Data are represented as N (%) unless otherwise specified.
CCI Charlson Comorbidity Index, IQR interquartile range, MDS myelodysplastic syndromes.
MDS risk and transfusion dependence numbers presented for full MDS cohort (N= 13,972)
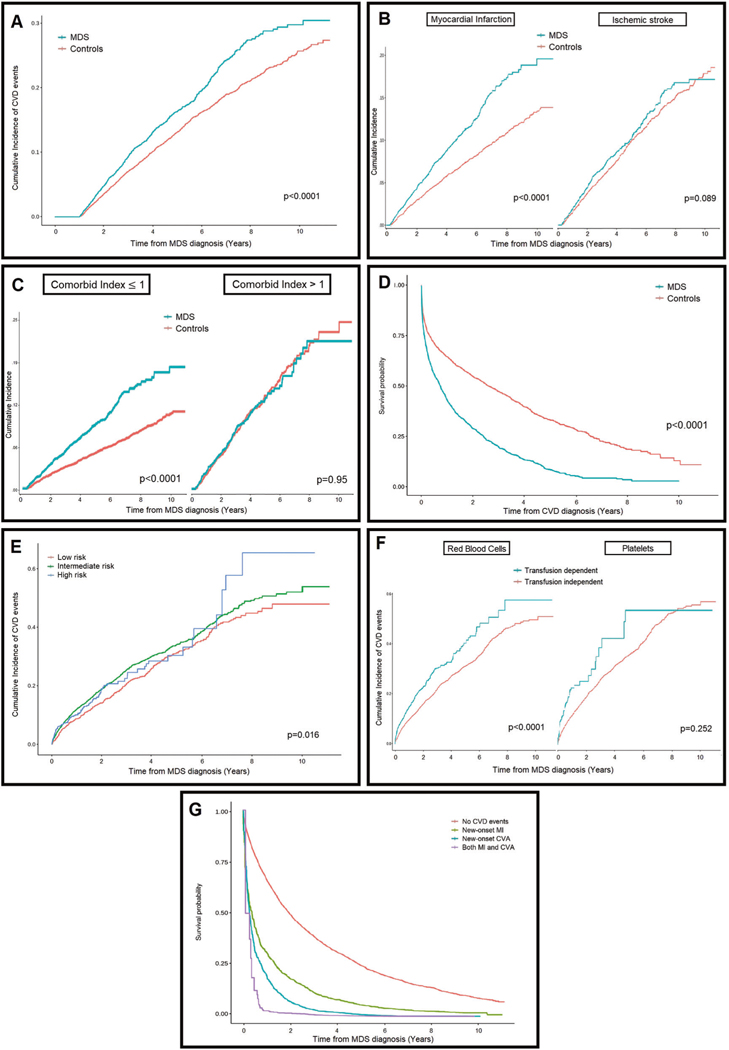
The 5-year cumulative incidence of CVD was 17% among MDS patients vs. Thirteen percent among non-cancer controls (p < 0.001, Fig. 1a). In multivariate regression analysis adjusted for age, gender, and comorbidities, MDS was associated with increased risk of CVD compared to controls (HR, 1.2; 95% CI, 1.2–1.3). In multivariate secondary outcome analysis, MDS was significantly associated with increased risk of MI (HR, 1.4; 95% CI, 1.2–1.5) but not CVA (HR, 1.01; 95% CI, 0.89–1.15) (Fig. 1b). The effect of MDS on MI was more notable in patients with lower comorbidity burden (CCI scores ≤ 1), among whom the 5-year incidence of MI doubled compared to controls (10% vs. 5%, p < 0.001, Fig. 1c). To account for anemia as etiologic factor of MI, the multivariate analysis was repeated after excluding RBC transfusion dependent MDS patients and their matched controls. In this subset, MDS still significantly influenced the incidence of MI (HR 1.3; 95% CI, 1.2–1.4).
Fig. 1. Myelodysplastic Syndrome (MDS) and risk of Cardiovascular Disease (CVD).
a Risk of incident CVD in patients with MDS and matched non-cancer controls. b Risk of incident acute ischemic stroke and myocardial infarction in MDS patients compared with noncancer controls. c The effect of a diagnosis of MDS on the risk of newonset MI is more pronounced in otherwise relatively healthy patients with none or one single coexistent comorbid condition. d Survival analysis after an incident cardiovascular event in patients with MDS compared to matched controls. e In MDS patients, higher histologic risk category was associated with increased incidence of cardiovascular events. f Impact of transfusion dependence on incidence of CVD in MDS patients. g Survival analysis of patients with MDS, stratified by incident cardiovascular events.
Survival following a CVD event was significantly shorter in MDS patients compared to controls (1-year survival 44.5% vs. 66%, p < 0.001, Fig. 1d). In multivariate regression adjusted for age, gender, and comorbidities, MDS was associated with increased mortality risk after CVD events compared to controls with similar events (HR, 2.1; 95% CI, 1.9–2.4), and this effect was significant in MDS patients with both MI (HR, 2.4; 95% CI, 2–2.9) and CVA (HR, 1.7; 95% CI, 1.5–1.9).
In adjusted multivariate analysis of MDS patients, higher comorbidity with CCI >1 (HR, 1.4; 95% CI, 1.2–1.5), higher-risk MDS category (HR, 1.1; 95% CI, 1.01–1.2, Fig. 1e), and RBC transfusion dependence (HR 1.3; 95% CI, 1.2–1.5) were associated with increased risk of CVD; however, platelet transfusion dependence was not significantly associated (Fig. 1f). Survival following MDS diagnosis was significantly shorter in patients who develop CVD events, with 1-year survival rates of 65%, 30%, and 19% for those with no CVD, incident MI, and incident CVA, respectively (Fig. 1g). MDS factors, particularly higher-risk morphology (HR, 1.6; 95% CI, 1.5–1.7) and transfusion dependence for RBC or platelets (HR, 1.9; 95% CI, 1.8–2.0) were also associated with higher mortality.
Consequently, in this nationally representative, heterogeneous SEER-Medicare cohort, MDS constituted an independent risk factor for CVD in older adults and incident CVD events were associated with higher mortality in MDS patients compared to that in controls. The MDS effect was with and without MDS. MDS factors associated with largely attributed to increased risk of MI, with no percei- increased risk of CVD were higher-risk bone marrow vable difference in CVA incidence between older adults morphology and RBC transfusion dependence.
Prior studies show that CVD is the most common disease-unrelated cause of death in MDS. The mortality rate from cardiovascular causes is higher in MDS compared to the general population [5] and has not declined over time despite advances in MDS therapy [11]. Our study suggests that MDS is a risk factor for CVD and provides evidence that higher-risk MDS categories and transfusion dependence influence the risk for CVD in MDS patients. While these factors have been shown to influence the risk of leukemia and mortality in MDS, their role in CVD has not been previously reported [12].
With recently approved treatments and improving supportive care, patients with MDS can have prolonged clinical courses during which few disease-specific interventions offer a survival benefit [13]. Our results show that an MDS diagnosis confers a significant risk for MI, even in otherwise relatively healthy older adults with minimal cardiovascular comorbidities. The increased risk of MI compared to controls persisted after excluding RBC transfusion dependent patients, suggesting it cannot be fully explained by anemia. Although current expert consensus is to avoid screening for CHIP, our study provides a rationale to promote better cardiovascular screening and preventive strategies in patients with an MDS diagnosis, with the goal of improving survival. Though the underlying mechanism accounting for increased cardiovascular risk in MDS is unknown, we speculate that accelerated atherosclerosis driven by MDS-defining somatic mutations as TET2, DMT3A, and JAK2V617F or altered endothelial inflammatory response from these mutations may likely play a role [14].
We used validated methods for registry and Medicare claim analysis [8] but our findings should be interpreted in the context of the study limitations. First, our data relied on administrative claims and lacked information about CHIP mutations, cellular counts and cytogenetics, with MDS risk stratification based solely on histology. Second, surveillance bias is possible, with closer follow-up of MDS patients leading to higher detection of CVD. Third, generalizability may be hampered by including patients 66 years or older, though our population represents the median age at MDS diagnosis in the United States [15]. Fourth, we used an analytical approach to isolate the effect of MDS in the risk for CVD but acknowledge the inherent limitations of a propensity score matched control cohort, including potential unmeasured confounders. Despite these limitations, SEER-Medicare has been proven useful for population research in MDS patients, and this study offers important insight into a significant non-cancer related effect.
In conclusion, MDS was independently associated with higher risk of CVD, particularly MI. The risk was particularly pronounced in those with few traditional cardiovascular risk factors, and among those with higher-risk MDS and RBC transfusion dependence. Incident CVD was associated with higher mortality among MDS patients compared to non-cancer controls with similar events. Thus, we suggest that MDS is an important and yet underappreciated independent risk factor for CVD. Further research to elucidate the mechanisms of CVD in MDS and define optimal screening and preventive strategies is strongly warranted. We hope this ultimately leads to multidisciplinary clinical management, appropriate counselling for cardiovascular risk and early interventions in MDS patients as it could greatly impact their survival.
Supplementary Material
Acknowledgements
AS is supported by the National Cancer Institute Paul Calabresi Career Development Award (5K12CA132783–10) & The Leukemia Lymphoma Society. SBM is supported by the National Institutes of Health (K23NS105948) & the Leon Levy Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all the data in this study and independently made the decision to submit for publication. No medical writers or editors were involved in the creation of this manuscript. The authors wish to acknowledge the help of the SEER-Medicare staff.
Footnotes
Financial Disclosure AS has received consultancy fees from Guidepoint consultation.
Compliance with ethical standards
Conflict of interest AS has received consultancy fees from Guidepoint consultation. The remaining authors declare that they have no conflict of interest.
Supplementary information The online version of this article (https://doi.org/10.1038/s41375-019-0673-8) contains supplementary material, which is available to authorized users.
References
- 1.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;74:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naqvi K, Sasaki K, Montalban-Bravo G, Alfonso Pierola A, Yilmaz M, Short N, et al. Clonal hematopoiesis of indeterminate potential-associated mutations and risk of comorbidities in patients with myelodysplastic syndrome. Cancer. 2019. 10.1002/cncr.32056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner AM, Blonquist TM, Hobbs GS, Amrein PC, Neuberg DS, Steensma DP, et al. Risk and timing of cardiovascular death among patients with myelodysplastic syndromes. Blood Adv. 2017;1:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dayyani F, Conley AP, Strom SS, Stevenson W, Cortes JE, Borthakur G, et al. Cause of death in patients with lower-risk myelodysplastic syndrome. Cancer 2010;116:2174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 9.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. International classification of diseases for oncology (ICD-O). 3rd ed. 2013. http://www.who.int/iris/handle/10665/96612.
- 11.Polednak AP. Trend (1999–2009) in U.S. death rates from myelodysplastic syndromes: utility of multiple causes of death in surveillance. Cancer Epidemiol. 2013;37:569–74. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;28:2847–52. [DOI] [PubMed] [Google Scholar]
- 13.Zeidan AM, Wang R, Davidoff AJ, Ma S, Zhao Y, Gore SD, et al. Disease-related costs of care and survival among Medicare-enrolled patients with myelodysplastic syndromes. Cancer 2016;122:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sano S, Wang Y, Walsh K. Clonal hematopoiesis and its impact on cardiovascular disease. Circ J 2018;83:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer 2007;109:1536–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.