Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a chronic lung condition characterised by persistent respiratory symptoms and limited lung airflow, dyspnoea and recurrent exacerbations. Suboptimal therapy or non‐adherence may result in limited effectiveness of pharmacological treatments and subsequently poor health outcomes.
Objectives
To determine the efficacy and safety of interventions intended to improve adherence to single or combined pharmacological treatments compared with usual care or interventions that are not intended to improve adherence in people with COPD.
Search methods
We identified randomised controlled trials (RCTs) from the Cochrane Airways Trials Register, CENTRAL, MEDLINE and Embase (search date 1 May 2020). We also searched web‐based clinical trial registers.
Selection criteria
RCTs included adults with COPD diagnosed by established criteria (e.g. Global Initiative for Obstructive Lung Disease). Interventions included change to pharmacological treatment regimens, adherence aids, education, behavioural or psychological interventions (e.g. cognitive behavioural therapy), communication or follow‐up by a health professional (e.g. telephone, text message or face‐to‐face), multi‐component interventions, and interventions to improve inhaler technique.
Data collection and analysis
We used standard Cochrane methodological procedures. Working in pairs, four review authors independently selected trials for inclusion, extracted data and assessed risk of bias. We assessed confidence in the evidence for each primary outcome using GRADE. Primary outcomes were adherence, quality of life and hospital service utilisation. Adherence measures included the Adherence among Patients with Chronic Disease questionnaire (APCD). Quality of life measures included the St George's Respiratory Questionnaire (SGRQ), COPD Assessment Test (CAT) and Clinical COPD Questionnaire (CCQ).
Main results
We included 14 trials (2191 participants) in the analysis with follow‐up ranging from six to 52 weeks. Age ranged from 54 to 75 years, and COPD severity ranged from mild to very severe. Trials were conducted in the USA, Spain, Germany, Japan, Jordan, Northern Ireland, Iran, South Korea, China and Belgium. Risk of bias was high due to lack of blinding. Evidence certainty was downgraded due to imprecision and small participant numbers.
Single component interventions
Six studies (55 to 212 participants) reported single component interventions including changes to pharmacological treatment (different roflumilast doses or different inhaler types), adherence aids (Bluetooth inhaler reminder device), educational (comprehensive verbal instruction), behavioural or psychological (motivational interview).
Change in dose of roflumilast may result in little to no difference in adherence (odds ratio (OR) 0.67, 95% confidence interval (CI) 0.22 to 1.99; studies = 1, participants = 55; low certainty). A Bluetooth inhaler reminder device did not improve adherence, but comprehensive verbal instruction from a health professional did improve mean adherence (prescription refills) (mean difference (MD) 1.00, 95% CI 0.46 to 1.54). Motivational interview improved mean adherence scores on the APCD scale (MD 22.22, 95% CI 8.42 to 36.02).
Use of a single inhaler compared to two separate inhalers may have little to no impact on quality of life (SGRQ; MD 0.80, 95% CI –3.12 to 4.72; very low certainty). A Bluetooth inhaler monitoring device may provide a small improvement in quality of life on the CCQ (MD 0.40, 95% CI 0.07 to 0.73; very low certainty).
Single inhaler use may have little to no impact on the number of people admitted to hospital compared to two separate inhalers (OR 1.47, 95% CI 0.75 to 2.90; very low certainty). Single component interventions may have little to no impact on the number of people expereincing adverse events (very low certainty evidence from studies of a change in pharmacotherapy or use of adherence aids). A change in pharmacotherapy may have little to no impact on exacerbations or deaths (very low certainty).
Multi‐component interventions
Eight studies (30 to 734 participants) reported multi‐component interventions including tailored care package that included adherence support as a key component or included inhaler technique as a component.
A multi‐component intervention may result in more people adhering to pharmacotherapy compared to control at 40.5 weeks (risk ratio (RR) 1.37, 95% CI 1.18 to 1.59; studies = 4, participants = 446; I2 = 0%; low certainty).
There may be little to no impact on quality of life (SGRQ, Chronic Respiratory Disease Questionnaire, CAT) (studies = 3; low to very low certainty).
Multi‐component interventions may help to reduce the number of people admitted to hospital for any cause (OR 0.37, 95% CI 0.22 to 0.63; studies = 2, participants = 877; low certainty), or COPD‐related hospitalisations (OR 0.15, 95% CI 0.07 to 0.34; studies = 2, participants = 220; moderate certainty). There may be a small benefit on people experiencing severe exacerbations.
There may be little to no effect on adverse events, serious adverse events or deaths, but events were infrequently reported and were rare (low to very low certainty).
Authors' conclusions
Single component interventions (e.g. education or motivational interviewing provided by a health professional) can help to improve adherence to pharmacotherapy (low to very low certainty). There were slight improvements in quality of life with a Bluetooth inhaler device, but evidence is from one study and very low certainty. Change to pharmacotherapy (e.g. single inhaler instead of two, or different doses of roflumilast) has little impact on hospitalisations or exacerbations (very low certainty). There is no difference in people experiencing adverse events (all‐cause or COPD‐related), or deaths (very low certainty).
Multi‐component interventions may improve adherence with education, motivational or behavioural components delivered by health professionals (low certainty). There is little to no impact on quality of life (low to very low certainty). They may help reduce the number of people admitted to hospital overall (specifically pharmacist‐led approaches) (low certainty), and fewer people may have COPD‐related hospital admissions (moderately certainty). There may be a small reduction in people experiencing severe exacerbations, but evidence is from one study (low certainty). Limited evidence found no difference in people experiencing adverse events, serious adverse events or deaths (low to very low certainty).
The evidence presented should be interpreted with caution. Larger studies with more intervention types, especially single interventions, are needed. It is unclear which specific COPD subgroups would benefit, therefore discussions between health professionals and patients may help to determine whether they will help to improve health outcomes.
Plain language summary
Which approaches help people with COPD to maintain taking medication as prescribed?
Review question
Which approaches help people maintain taking prescribed medication(s), improve quality of life and reduce hospital admissions in people with chronic obstructive pulmonary disease (COPD)?
Background
COPD is a lung condition that can cause long‐term breathing problems and includes symptoms such as shortness of breath. Medications do exist that can help but sometimes people do not take them as prescribed. Different approaches could help people to take their medication as prescribed and help improve symptoms or quality of life and reduce hospital admissions.
We wanted to find out if any approaches could help people with COPD take their medication(s) as prescribed.
Studies identified and selected
We searched databases to find the studies. Four people working in pairs looked at the lists of studies separately and agreed on which ones were included. The latest search for studies was conducted in May 2020.
Study characteristics
We included studies comparing simple ways to help improve medication use (e.g. different medication doses or single inhalers instead of two separate inhalers) to usual COPD care. We also included studies that tested combination approaches (e.g. information or training from nurses or pharmacists and monitoring medication use).
What were the key results?
We included 14 studies (2191 people) in our analysis. No studies were masked (people knew whether they were receiving an approach to help them with adherence or the alternative). Six studies used simple approaches (e.g. change to medication dosage, change to type of inhaler, or a Bluetooth inhaler reminder device), and eight studies used combined approaches (e.g. nurses or pharmacists giving advice or information about how to improve medication use).
We were uncertain about the effects of a different medication dose on people adhering to medication because it was no different to usual care (one study). Two separate studies found that a Bluetooth inhaler reminder device or a change in oral medication dose did not help to improve adherence. Health professionals involved in giving information may help to improve adherence (one study). This was measured by prescription refills. Motivating people to change their behaviour could help to improve adherence their medication (one study). This was measured by people completing a questionnaire about adherence to their medication.
One study found that a Bluetooth inhaler device might help to improve quality of life. There may be little to no difference between those using single inhalers in the number of people admitted to hospital compared to those using two separate inhalers (one study). We found no difference between single approaches and usual care on the number of people who had side effects.
Combined approaches may help to improve the number of people taking their medication, but we were not confident of this finding (four studies). They may have little to no impact on quality of life (three studies). Combined approaches could be beneficial in reducing the number of people with COPD admitted to hospital for any reason or because of COPD symptoms (two studies).
There was no difference between combined approaches and in the number of people who had adverse events.
Take home message
We could not say for certain that simple approaches are useful to help people with COPD to improve medication use because of very limited information. Combined approaches may help people to take their medication(s) as prescribed and can help to reduce the number of people admitted to hospital, however, more information is needed to help answer this question with confidence.
Summary of findings
Background
Description of the condition
The Global Burden of Disease study has shown that chronic obstructive pulmonary disease (COPD) causes an estimated three million deaths annually (GBD 2015 Incidence and Prevalence Collaborators), and affects an estimated 174 million people worldwide (GBD 2015 Chronic Respiratory Disease Collaborators; GBD 2015 Incidence and Prevalence Collaborators). COPD represents 2.6% of the entire global burden of disease (GBD 2015 Incidence and Prevalence Collaborators); it is also increasing, with more people suffering due to under‐recognition, under‐diagnosis, under‐treatment (Quaderi 2018), and non‐adherence (Humenberger 2018).
COPD is a progressive, chronic lung disease characterised by persistent respiratory symptoms and limited airflow due to airway or alveolar abnormalities (or both), resulting from significant exposure to noxious particles or gases. Causes include tobacco smoking and environmental factors such as exposure to biomass fuel and air pollution (WHO 2017; COPD Foundation 2018). COPD diagnosis is based on symptoms including dyspnoea (shortness of breath), cough or sputum production (or both) and is confirmed using spirometry demonstrating persistent airflow limitation, that is presence of postbronchodilator forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) less than 70% (GOLD 2021). Disease severity is associated with frequency of exacerbations or 'flare ups', and the presence of other comorbidities, such as cardiovascular disease, musculoskeletal impairment or diabetes (Vestbo 2013; GOLD 2021).
It has been estimated that only 50% of people in high‐income countries with chronic disease adhere to treatment recommendations (WHO 2003). Studies have shown that adherence rates in people with COPD range from 70% to 90%; however, this is unlikely to be reflected in clinical practice (Wiśniewski 2014). One study showed that only 34% of people with COPD completely adhered to medication (Brandstetter 2017).
Despite optimisation of treatments, some people with COPD continue to experience debilitating symptoms (e.g. increased exacerbations) that can impact on their functional status and quality of life, leading to hospitalisations and risk of mortality. Adherence to medication is one of the most important factors that enables successful treatment of COPD (Duncan 2015). Benefits to clinical outcomes are often limited due to people not taking medication as prescribed, which can also be costly and wasteful for health services (WHO 2003; Van Boven 2014; Chen 2017; Sulaiman 2017).
Description of the intervention
Pharmacological management of COPD often involves regular use of long‐acting bronchodilators: long‐acting beta2‐adrenoceptor agonists (LABAs) or long‐acting muscarinic receptor antagonists (LAMAs) or both, and for some people inhaled corticosteroids (ICS) will be added. These maintenance inhalers are intended to improve lung function, reduce the risk of exacerbations and improve quality of life. Most people will also be prescribed a short‐acting bronchodilator, such as a short‐acting beta2‐adrenoceptor agonist (SABA) or short‐acting muscarinic receptor antagonist (SAMA), to manage acute symptoms such as breathlessness (Ejiofor 2013; Vogelmeier 2017). Maintenance inhalers can be given as monotherapy, which can be 'stepped up' to dual or triple therapy if needed (e.g. ICS plus LABA, or ICS plus LABA plus LAMA) (Vogelmeier 2017). Other pharmacological treatments — such as oral glucocorticosteroids, phosphodiesterase‐4 (PDE4) inhibitors, mucolytic agents and prophylactic antibiotics — may also be prescribed for specific indications. Oral glucocorticoids, for example, may be used in the short term for acute exacerbations, while prophylactic antibiotics and mucolytics may have a benefit in preventing flare‐ups in people who experience frequent exacerbations (Walters 2014; Herath 2018; Poole 2019).
People's knowledge and understanding about medications as well as their physical and cognitive capabilities can affect medication adherence, that is the likelihood of medication being taken at the prescribed dose and frequency, and using the correct technique. Moreover, people's beliefs about medication can also influence their behaviours and, in turn, adherence. A report published by the World Health Organization (WHO) outlines three types of behaviour that can lead to non‐adherence to ICS therapy, as follows (WHO 2003).
Erratic non‐adherence: missing doses because of forgetting, changing schedules or busy lifestyles. People understand their prescribed regimen but compliance is difficult, or they have not prioritised treatment management.
Unwitting non‐adherence: non‐adherence due to not understanding the prescribed regimen, for example misunderstanding how many times an inhaler should be taken (leading to under‐use or over‐use), correct inhaler technique, how the treatment works or the importance of adhering to treatment.
Intelligent non‐adherence: people may alter or stop taking treatment deliberately because they feel better or that they no longer need to take their medication. They may stop taking medication because of short‐term (e.g. believing that they do not need to take their treatment) or long‐term effects, bad taste, complexity and interference in their daily routine, or thinking that there is more harm than benefit of continuing medication.
A range of interventions exist that can help people adhere to medication, for example, changes to medication (e.g. simplified drug regimens), and implementation of behavioural (e.g. cognitive behavioural therapy (CBT)) or educational interventions (e.g. discussion with a health professional or written information) (Bryant 2013; Blackstock 2016). Adherence aids such as Dosette boxes or multi‐compartment medication compliance aids (MCAs) could help those who have complex medication regimens (Furmedge 2018). Electronic inhaler monitoring devices or 'smart' inhalers may provide automated medication reminders for people at risk of forgetting to take their inhaler, or provide adherence feedback to the patient and healthcare professionals around which adherence discussions could take place. Other digital technologies aimed at aiding medication adherence are in development, such as mobile phone applications (Blakey 2018).
How the intervention might work
For an intervention to improve adherence to pharmacological treatment, its success is likely to be dependent on the type of intervention available, and type of non‐adherence that it is intended to target. A simple intervention, for example, could remind people to use their inhaler(s), while a complex intervention could provide education and support from a health professional to help change a person's behaviour towards their medication(s).
Adherence to medication for medical disorders has been investigated in a previous Cochrane Review (Nieuwlaat 2014). Of the 182 randomised controlled trials (RCTs) included, overall, the authors reported that effects of interventions and measures of adherence were varied from study to study. Only a few RCTs on integrated, tailored or multi‐component interventions that involved an individualised care plan, educational sessions about self‐management, visits by a health professional or team of health professionals, and regular telephone consultations (from a specialist case manager nurse, for example) showed improvement in adherence and clinical outcomes, but even these interventions would be difficult to apply in a usual practice setting (Nieuwlaat 2014).
Reasons for poor medication adherence in COPD are not well understood. Potentially these might include complex medication regimens which can lead to confusion and forgetting to take medication, or a busy, unpredictable lifestyle could lead to deviation in prescribed regimen (WHO 2003; Bryant 2013). Other contributing factors can include inadequate education or knowledge about the disease process or comorbidities, adverse effects of treatment, patient acceptability and preference, cost of medication, and belief that there are more disadvantages of taking treatment than advantages (Restrepo 2008; Normansell 2017). Poor medication adherence may be explained by the 'Ascertaining Barriers to Compliance (ABC)' taxonomy, which gives a description of the steps taken towards achieving medication adherence as prescribed (Vrijens 2016). The taxonomy is divided into three stages.
Initiation (when a person takes the first dose as prescribed).
Implementation (the extent to which a person's actual dosing corresponds to the prescribed regimen, from initiation until the last dose is taken).
Persistence (time elapsed from initiation to eventual treatment discontinuation).
Some reasons for poor adherence may act at several stages in the taxonomy and others predominantly affect a single stage. For example, not taking inhaled therapy (e.g. LABA, LAMA or ICS, combination of LABA and ICS as prescribed (under‐use or over‐use of inhalers)), or not using the correct inhaler technique may affect implementation with little impact on initiation or persistence (Vestbo 2009; Van Boven 2014). Poor adherence could also result from clinicians not giving the correct initiation instructions (BMJ 2012).
Sources of clinical diversity are variations of medical problems, treatment regimens and type of adherence intervention. There is currently no published evidence to determine which intervention would specifically help people with COPD to adhere to treatment (e.g. change in regimen, CBT or information from a health professional). The most appropriate type of intervention and its effectiveness will likely depend on the reason for a person's non‐adherence.
Why it is important to do this review
As part of a programme of work funded to look at issues important to people with COPD, Cochrane Airways has discussed medication adherence with a COPD patient advisory group. The group highlighted what helps them to take medication (e.g. correct information about how to use their inhaler, or alarms to remind them to take their tablets) or what prevents them from taking medications (e.g. health professionals not giving the correct information about how to use an inhaler, difficulty in taking tablets, adverse effects of inhalers, difficulty in taking too many medications or forgetting to take their medication). Some people in the patient advisory group said that they had become used to taking multiple medications, whereas some people said that they only took medication, such as a nebuliser, in an emergency. It was evident from the discussion that if one method of adherence worked for one person, it may not work for another person. We intend to investigate what helps people with COPD to adhere to pharmacological treatments and whether adherence improves other health outcomes (e.g. quality of life). We focused on investigating interventions to improve adherence to pharmacological treatments such as changes in treatment regimen, education or CBT. Interventions intended to help people to adhere to non‐pharmacological treatments for COPD such as smoking cessation, pulmonary rehabilitation, oxygen therapy and vaccinations were excluded from this review.
Objectives
To determine the efficacy and safety of interventions intended to improve adherence to single or combined pharmacological treatments compared with usual care or interventions that are not intended to improve adherence in people with COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs only (parallel, cluster‐randomised or cross‐over designs). We included studies reported in full text, those published as an abstract only and unpublished data. We included studies from community and hospital settings.
Types of participants
We included adults aged 35 years or over (NICE 2018) with a diagnosis of COPD according to established criteria, for example Global Initiative for Obstructive Lung Disease (GOLD) staging (GOLD 2021), European Respiratory Society (ERS), or American Thoracic Society (ATS) criteria (Qaseem 2011). We included adults with any comorbidities (e.g. diabetes), provided that the intervention was aimed at improving adherence to pharmacological treatment for COPD using at least one of the Types of interventions outlined below.
We excluded participants whose primary diagnosis was another respiratory condition such as asthma, bronchiectasis and cystic fibrosis. We included studies that included participants with 'asthma‐COPD overlap (ACO)' but we aimed to analyse outcomes in these participants separately if disaggregated data were available.
We also planned to include trials targeting health professionals, who then delivered the intervention to people with COPD.
Where trials included only a subset of participants (at least 50%) with a diagnosis of COPD, we included these participants, provided we were able to obtain disaggregated data from the trial authors.
Types of interventions
We included trials in which the aim of the intervention was primarily to help improve adherence to pharmacological therapy. We also planned to include studies in which the primary aim of the intervention was to improve inhaler technique, and reported this as a separate comparison.
We included the following intervention categories.
Changes to the pharmacological treatment itself, such as different medication formulations (e.g. using a combination inhaler or the same type of inhaler for all treatments rather than multiple different inhaler types, using tablets instead of inhalers or simplified drug regimens).
Adherence aids (e.g. Dosette box, alarm, reminder, keeping a list of all current medication).
Education (e.g. discussion with health professional, written information or pamphlets, access to health education information on the Internet, pictorial or audiovisual material).
Behavioural or psychological interventions (e.g. CBT, counselling).
Communication or follow‐up by health professional (e.g. by telephone, email, face‐to‐face, text‐messaging).
Multi‐component or tailored care package (provided that adherence was a key component of care or was one of the objectives of providing multi‐component intervention to the participant).
Interventions that aimed to improve inhaler technique (e.g. educational interventions, training tools; e.g. measured using a checklist).
We compared the above interventions separately with any intervention that was not intended to improve either adherence or inhaler technique (e.g. usual care, active control, placebo or sham intervention).
Types of outcome measures
Primary outcomes
Adherence to pharmacotherapya,b (all measures, as reported by trialists. We considered the following order of preference of measures: electronic monitors, canister weights, prescription refills, self‐report).
Quality of life (measured using a validated scale such as the St George's Respiratory Questionnaire (SGRQ) or COPD Assessment Test (CAT)).
Hospital service utilisation (e.g. hospital admissions going beyond the emergency department). Depending on the data available, we extracted the number of participants who required hospitalisation or the hospitalisation utilisation rate, or both (as defined by trialists).
Secondary outcomes
Exacerbationsc (e.g. number of people with one or more exacerbation).
Self‐efficacy (as a proxy for adherence, as reported by trialists).
All adverse events (AEs) (e.g. number of people with one or more AE).
Patient acceptability of intervention (as reported by trialists).
Inhaler technique (as reported by trialists, preferably using a validated scale or a validated videotaped scoring method (Rootmensen 2010)).
aOur understanding was that an observed improvement in adherence was likely to result in improved clinical outcomes. The National Heart, Lung, and Blood Institute states that attempts to improve adherence should be judged by their clinical benefits (NHLBI 1982), and that only measuring adherence does not determine whether patient outcomes are improved or not. Equally, measuring patient outcomes does not determine whether the effects are observed as a result of adherence. Therefore, we included adherence as our primary outcome, as well as quality of life and hospital service utilisation, since our aim was to assess whether improved adherence in turn improves clinical outcomes such as quality of life and hospital service utilisation. We also considered exacerbations, self‐efficacy and AEs as important outcomes.
bThe adherence process consists of three phases: initiation (starting medication), implementation (taking medication daily) and persistence (duration of taking medication) (Vrijens 2016; De Geest 2018). It was expected that studies would measure different stages in the adherence taxonomy. While we included all adherence intervention studies, we anticipated the majority would measure the implementation stage.
cModerate exacerbations are defined as worsening of respiratory status, requiring treatment with antibiotics or systemic corticosteroids (or both); severe exacerbations are defined as requiring hospitalisation.
We extracted data at all reported time points and for studies that reported outcomes at multiple time points we planned to analyse data in the following groupings by time from baseline: less than three months; three months or greater but less than six months; six months or greater but less than 12 months and 12 months or greater.
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from searches of the following databases and trial registries:
Cochrane Airways Trials Register (Cochrane Airways 2019), via the Cochrane Register of Studies, all years to 1 May 2020;
Cochrane Central Register of Controlled Trials (CENTRAL), via the Cochrane Register of Studies, 2020, Issue 4;
MEDLINE (OvidSP) all, 1946 to 1 May 2020;
Embase (OvidSP), 1974 to 1 May 2020;
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), all years to 1 May 2020;
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch), all years to 19 June 2019.
The database search strategies are listed in Appendix 1. The search strategies were developed by the Cochrane Airways Information Specialist, in collaboration with the review authors. The information specialist conducted the searches.
All databases and trials registries were searched from their inception with no restriction on language or type of publication. Handsearched conference abstracts and grey literature were identified through the Cochrane Airways Trials Register and the CENTRAL database.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for study information.
We searched PubMed for errata or retractions from included studies published in full text on 11 May 2021.
Data collection and analysis
Selection of studies
We used Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components:
known assessments, a service that matches records in the search results to records that have already been screened in Cochrane Crowd (Cochrane's citizen science platform where the Crowd help to identify and describe health evidence, crowd.cochrane.org) and labelled as 'RCT' or 'not an RCT';
the RCT classifier, a machine‐learning model that distinguishes RCTs from non‐RCTs and
Cochrane Crowd, if appropriate.
More detailed information about the Screen4Me components can be found in the following publications: McDonald 2017; Thomas 2017; Marshall 2018; Noel‐Storr 2018.
Following this initial assessment, four review authors (SJ, KP, RC, RF) independently, working in pairs, screened the titles and abstracts of the remaining search results independently and code them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports of all potentially eligible studies and four review authors (SJ, KP, RC, RF), working in pairs, independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. We resolved any disagreements through discussion (SJ, KP, RC, RF). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used an Excel data collection form for study characteristics and outcome data, which was piloted on at least one study in the review. Four review authors (SJ, KP, RC, MB), working in pairs, extracted the following study characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals and date of study, aim of the study (i.e. the primarily objective of the study was to improve adherence to pharmacotherapy).
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention (we used the classification list outlined in the Types of interventions section overall grouping of interventions. For multi‐component interventions, we extracted all information on components of the intervention), comparison, concomitant medications and excluded medications, duration of intervention, method of delivery and aim of the intervention (e.g. appropriate use of inhaler or to improve regularity of use).
Outcomes: primary and secondary outcomes specified and collected, and time points reported. For adherence, we recorded the type of non‐adherence that is being targeted in studies.
Notes: funding for studies and notable conflicts of interest of trial authors.
Four review authors (SJ, KP, RC, MB), working in pairs, independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by discussion (SJ, KP, RC, MB). One review author (SJ) transferred data into Review Manager 5 (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. One review author extracted study characteristics (MB), which was spot‐checked by a second review author (SJ) against the study report.
Assessment of risk of bias in included studies
Four review authors (SJ, KP, RC, MB) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We resolved any disagreements by discussion (SJ, KP, RC, MB). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We judged each potential source of bias as high, low or unclear, and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a participant‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table.
When considering treatment effects, we considered the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We analysed dichotomous data (e.g. people having an event or not having an event) as odds ratios (ORs) and continuous data as the mean difference (MD), where studies used the same scales, or standardised mean difference (SMD), where studies used different scales, with the 95% confidence intervals (CI). In a change to our protocol, we analysed one outcome as a risk ratio (RR) to allow incorporation of a study reporting RR and 95% CIs, rather than numbers of participants with an event. If rate ratios had been reported in a study, we planned to analyse them on this basis. If data from rating scales were combined in a meta‐analysis, we ensured they were entered with a consistent direction of effect (e.g. lower scores always indicated improvement).
We undertook meta‐analyses only where this was meaningful; that is, if the treatments, participants, outcomes (e.g. measure of adherence) and the underlying clinical question were similar enough for pooling to make sense. We described skewed data narratively (e.g. as medians and interquartile ranges for each group).
Where a study reported multiple trial arms, we included only the relevant arms. If two comparisons (e.g. treatment A versus placebo and treatment B versus placebo) were combined in the same meta‐analysis, we either combined the active arms or halved the control group to avoid double‐counting.
If adjusted analyses were available (analysis of variance (ANOVA) or analysis of covariance (ANCOVA)) we used these as a preference in our meta‐analyses. If both change‐from‐baseline and endpoint scores were available for continuous data, we used change‐from‐baseline unless there was low correlation between measurements in participants. If a study reported outcomes at multiple time points, we used the latest time point. Minimal important differences (MIDs) were reported where possible for continuous scales (e.g. SGRQ = 4 point change (Jones 2005); CCQ = 0.4 point change (Kocks 2006).
We did not include multi‐component or tailored care package interventions in meta‐analyses due to difficulty in determining the effects of a specific intervention on adherence to pharmacotherapy.
We used intention‐to‐treat (ITT) or 'full analysis set' analyses where they were reported (i.e. those where data had been imputed for participants who were randomly assigned but did not complete the study) instead of completer or per‐protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of people admitted to hospital, rather than number of admissions per person). We planned to meta‐analyse adjusted data from cluster‐RCTs to account for clustering, however, we found no trials of this design. For cross‐over RCTs, we planned to include data from the first part of the study in the meta‐analysis to exclude carryover effects.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtained missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis using the following criteria:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
If we identified substantial heterogeneity, we reported it and explored the possible causes using prespecified subgroup analysis. If we found considerably high unexplained heterogeneity, we did not pool the data, but we reported them narratively.
Assessment of reporting biases
We were unable to create funnel plots to explore possible small‐study and publication biases due to the limited number of studies included in meta‐analyses.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model where required.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses where there was significant heterogeneity:
COPD severity (moderate or severe);
cognitive impairment (cognitive impairment versus no cognitive impairment);
duration of intervention (less than 12 months versus 12 months or longer);
who the intervention was delivered to (participant or carer versus health professional); and
treatment comparison (e.g. usual care, active control, placebo or sham intervention).
We planned to include the following outcomes in subgroup analyses:
adherence;
health‐related quality of life;
hospital service utilisation; and
all AEs.
We performed the formal test for subgroup interactions in Review Manager 5 (Review Manager 2014).
Sensitivity analysis
We planned to carry out sensitivity analyses, removing the following from the primary outcome analyses:
subjective measures for adherence to therapy (e.g. self‐report);
trials that did not show improvement of adherence;
studies that recruited only people, or a subset of people, with ACO; and
studies at high risk of selection bias.
We also compared the results from a random‐effects model with those using a fixed‐effect model.
Summary of findings and assessment of the certainty of the evidence
We created separate summary of findings tables, one for each of the comparisons, using the following outcomes: adherence to pharmacotherapy, quality of life, hospital service utilisation, exacerbations and AEs. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence as it related to the studies that contributed data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), using GRADEpro GDT software. We justified all decisions to downgrade the certainty of the evidence using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
We conducted database searches in 2019 and 2020 (Figure 1, study flow diagram (Moher 2009)). From the searches, we retrieved 7405 records. After removing duplicates, 4896 records were further assessed, with 124 records being removed by Cochrane Crowd assessments and 1209 records removed by the RCT classifier. This left 3563 records for manual screening of titles and abstracts, after which we excluded 3454 records. We selected 109 articles for full‐text assessment. Of these, we excluded 43 trials plus 14 additional references with reasons. We included 52 articles after full‐text assessment. This included 16 trials plus an additional 11 references, 23 trials including mixed populations that we placed in Studies awaiting classification and two Ongoing studies. We did not include two studies in the analysis; Grandos‐Santiago 2020 reported data from baseline to discharge from hospital and NCT03379233 was terminated early due to devices malfunctioning. Therefore, we included 14 studies in the analysis.
1.
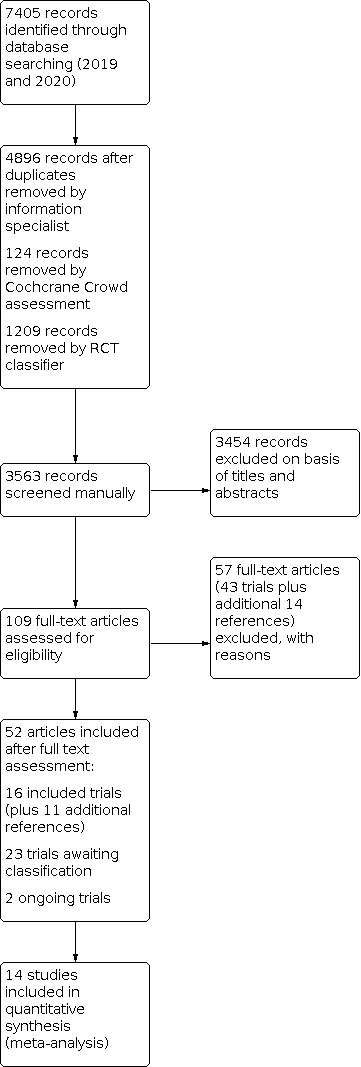
Study flow diagram.
Included studies
Details of the 16 included studies are described in the Characteristics of included studies table. NCT03379233 was terminated early due to devices malfunctioning and reported no results but its details are included below.
Setting, design, duration and funding
Nine studies were single‐centre (De Tullio 1987; Khdour 2009; Jarab 2012; Margolis 2013; Mochizuki 2013; Wei 2014; Naderloo 2018; Park 2019; To 2020), and seven studies were multi‐centre (Leiva‐Fernandez 2014; Hagedorn 2013; Tommelein 2014; Criner 2018; Grandos‐Santiago 2020; Thom 2018; NCT03379233) parallel‐assigned randomised trials.
Four single‐centre trials recruited participants from outpatient pulmonary or COPD clinics (De Tullio 1987; Khdour 2009; Jarab 2012; To 2020), a telephone‐based setting (Margolis 2013), medical centre (Park 2019), or regional hospital (Wei 2014). Multi‐centre trials recruited from regional hospitals (Grandos‐Santiago 2020), health centres (Leiva‐Fernandez 2014), community pharmacies (Tommelein 2014), or urban, county‐operated primary care clinics (Thom 2018). Setting was unclear in Mochizuki 2013 and NCT03379233.
Trials were conducted in the USA (De Tullio 1987; Margolis 2013; Criner 2018; Thom 2018), Spain (Leiva‐Fernandez 2014; Grandos‐Santiago 2020), Germany (Hagedorn 2013), Japan (Mochizuki 2013), Jordan (Jarab 2012), Northern Ireland (Khdour 2009), Iran (Naderloo 2018), South Korea (Park 2019), China (Wei 2014; To 2020), and Belgium (Tommelein 2014). NCT03379233 was conducted in Germany and the Netherlands.
Duration of studies ranged from six to 52 weeks.
Five trials did not report funding (De Tullio 1987; Khdour 2009; Wei 2014; Naderloo 2018; To 2020). Pharmaceutical companies funded Hagedorn 2013, Criner 2018, and NCT03379233 (AstraZeneca, GlaxoSmithKline and Novartis). Grandos‐Santiago 2020 received funding from Fundación Progreso y Salud (FPS), Boehringer Ingelheim España S.A. and Oximesa, Praxair; Alzaytoonah University of Jordan funded Jarab 2012; Carlos III Institute of Health (Instituto de Salud Carlos III), Health Research Fund Ministry of Science and Innovation funded Leiva‐Fernandez 2014; ASHP Research and Education Foundation Fostering Young Investigators Federal Services Junior Investigator Research Grant funded Margolis 2013; Asian Institute for Life Sciences and Asian Medical Centre funded Park 2019; Ghent University, Liège University and GlaxoSmithKline funded Tommelein 2014; and Patient‐Centered Outcomes Research Institute, NIH/NCRR Colorado CTSI funded Thom 2018. Mochizuki 2013 reported that they receive no funding.
Baseline participant characteristics
Baseline characteristics for each study are presented in the Characteristics of included studies table and Table 3. The mean age of participants across studies ranged from 54 to 75 years and COPD was diagnosed according to ATS or ERS guidelines, spirometry or by a hospital consultant. Six studies did not report how they diagnosed COPD (De Tullio 1987; Margolis 2013; Mochizuki 2013; Tommelein 2014; Wei 2014; Grandos‐Santiago 2020). Proportion of males across studies ranged from 39% (Jarab 2012) to 100% (To 2020).
1. Study baseline characteristics.
| Intervention group | Intervention | COPD severity | Concomitant medication | Exacerbations in the last 12 months prior to study enrolment | Hospitalisations in the last 12 months prior to study enrolment | Study ID |
| Single‐component interventions | Change to pharmacological treatment | GOLD stage III to IV | Anticholinergics, LABAs, ICS, LABA+ICS | Mean number of exacerbations: SFC: 2.2 (SD 0.5) and sal/FP: mean 2.3 (SD 0.6) | Number of people who had 1 hospitalisation: SFC: 19/107; sal/FP: 15/105 Number of people who had 2–4 hospitalisations: SFC: 4/107; sal/FP: 3/105 |
Hagedorn 2013 |
| Moderate to severe | NR | NR | NR | Mochizuki 2013 | ||
| Severe to very severe | LABA, LAMA, LABA+LAMA, ICS+LABA, ICS+LABA+LAMA | NR | NR | Park 2019 | ||
| Adherence aids | Moderate to severe | ICS/LABA | NR | NR | Criner 2018 | |
| Education | Mild to severe | NR | NR | NR | De Tullio 1987 | |
| Behavioural/psychological | Mild to very severe | NR | NR | NR | Naderloo 2018 | |
| Communication or follow‐up by HCP | No studies identified | |||||
| Multi‐component interventions | — | Moderate to severe | NR | NR | NR | Grandos‐Santiago 2020 |
| Moderate to severe | Median number of medications: 8 in each treatment group | NR | Mean hospital admissions (in the last 6 months): intervention: 52.0; control: 57.0 | Jarab 2012 | ||
| Mild to moderate | Anticholinergics, beta2‐adrenergics, ICS, xanthine | Mean exacerbations intervention: 0.92; control 0.82 | NR | Leiva‐Fernandez 2014 | ||
| Moderate to severe | Mean of 8 medications at baseline | NR | — | Khdour 2009 | ||
| NR | NR | NR | NR | Margolis 2013 | ||
| Moderate to very severe | LABA, LAAC, ICS, ICS+LABA (1 inhaler) | NR | NR | To 2020 | ||
| NR | SABA, LABA, SAAC, LAAC, SAAC+SABA, ICS, ICS+LABA, LAAC+LABA+ICS, theophylline, oral corticosteroids (mean number of COPD medications: 2.3) | 54% participants in each treatment group had ≥ 1 exacerbations | NR | Tommelein 2014 | ||
| Mild to severe | Anticholinergics, LABA, ICS, xanthines, carbocisteine | NR | NR | Wei 2014 | ||
| Moderate to severe | SABA, SAAC, LABA, LAMA, ICS | NR | NR | Thom 2018 | ||
COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Pulmonary Disease; HCP: healthcare professional; ICS: inhaled corticosteroid; LAAC: long‐acting anticholinergic; LABA: long‐acting beta2‐adrenoceptor agonist; LAMA: long‐acting muscarinic receptor antagonist; NR: not reported; SAAC: short‐acting anticholinergic; SABA: short‐acting beta2‐adrenoceptor agonist; SD: standard deviation; SFC: salmeterol–fluticasone combination; sal/FP: salmeterol/fluticasone propionate.
COPD severity ranged from mild to very severe across studies, and although at baseline some studies presented the different COPD severity groups, the outcome measures were reported for combined COPD severity groups.
The number and combinations of concomitant medications varied across studies, however, the types of medication included ICS, LABA, LAMA, long‐acting anticholinergic (LAAC), SABA, short‐acting anticholinergic (SAAC), carbocisteine, theophylline and xanthine. Six studies did not report concomitant medications (De Tullio 1987; Margolis 2013; Mochizuki 2013; Naderloo 2018; Grandos‐Santiago 2020; NCT03379233).
Only two studies reported exacerbations prior to study initiation (Leiva‐Fernandez 2014; Tommelein 2014). Leiva‐Fernandez 2014 reported slightly higher exacerbations per year in the intervention group (MD 0.92, 95% CI 0.67 to 1.17) compared to the control group (MD 0.82, 95% CI 0.59 to 1.05). Tommelein 2014 reported 54% of study participants in both intervention and control groups experiencing one or more exacerbation in the previous year.
Three studies reported hospitalisations (Khdour 2009; Jarab 2012; Hagedorn 2013). Most participants in Hagedorn 2013 had no hospitalisations in the previous year. Jarab 2012 reported hospital admissions in the last six months but there was no difference between treatment groups. Khdour 2009 reported a similar number of hospitalisations in both treatment groups in the previous year.
Description of interventions
All descriptions of interventions are presented in Table 4. Duration of trials ranged from six to 52 weeks.
2. Description of interventions.
| Study ID | Intervention | Intervention detail | Comparator detail | Duration |
| Single component interventions | Change to pharmacological treatment | Salmeterol xinafoate 50 µg and fluticasone propionate 500 µg inhalation powder at dosage of 1 inhalation twice daily (morning and evening) via single Diskus inhaler + individual existing therapy | Salmeterol xinafoate 50 µg and fluticasone propionate 500 µg inhalation powder at dosage of 1 inhalation twice daily (morning and evening) via 2 separate Diskus inhalers + individual existing therapy |
Hagedorn 2013 52 weeks |
| Change to pharmacological treatment | Once daily transdermal tulobuterol patch (2 mg, Hokunalin tape). After 12 weeks, participants received the control treatment | Twice daily salmeterol 50 µg. After 12 weeks, participants received the intervention |
Mochizuki 2013 12 weeks (cross‐over design) |
|
| Change to pharmacological treatment | Dose escalation: roflumilast 250 µg once daily for 4 weeks followed by roflumilast 500 µg for 12 weeks | Conventional dose group: roflumilast 500 µg for 12 weeks |
Park 2019 16 weeks |
|
| Adherence aids | 'BreatheMe' Bluetooth device monitoring daily Symbicort inhaler use and mobile phone application to provide support to people using Symbicort as part of COPD management therapy + current care. Audio‐visual daily reminders (beeps and flashes) on device to take Symbicort, and medication reminders from mobile phone application | Current care and medication usage monitoring device. Control group did not receive any reminders |
Criner 2018 26 weeks |
|
| Education | Comprehensive verbal instruction from pharmacist on importance to taking medication as prescribed. Discussion included how theophylline works and importance of maintaining blood levels to achieve therapeutic effect. Patients could ask questions about their current medication. Counselling session lasted 3–5 minutes | Control group did not receive counselling session, but were asked if they had any questions about their current medication |
De Tullio 1987 26 weeks |
|
| Behavioural/psychological intervention | MI group: 5 × 1‐to‐1 sessions held on 2 consecutive days. The first session was introductory. Session 2 focused on participants' feelings to help towards moving from extrinsic to intrinsic motivation for change. Session 3 was based on identification and resolving participants' uncertainties. Session 4 aimed to create and stimulate intrinsic desire to change and identify, clarify and acknowledge participants' values. Session 5 aimed to identify tempting situations and closing the programme. After the sessions, participants were given 2 sessions on medication, lifestyle and respiratory chest physiotherapy | Control group: 2 training sessions on medication use, lifestyle and respiratory chest physiotherapy; each session was 15–45 minutes |
Naderloo 2018 8.6 weeks |
|
| Communication or follow‐up by health professional | No studies found | |||
| Multi‐component interventions | Individualised shared decision‐making and patient engagement programme + standard treatment during hospitalisation period. The programme included pharmacological management, symptom control and healthy lifestyle promotion | Control group received standard treatment (medical and pharmacological care): systemic steroids, antibiotics, inhaled bronchodilators, oxygen therapy) |
Grandos‐Santiago 2020 (AECOPD population) From discharge to 13 weeks' follow‐up |
|
| Pharmaceutical care included structured patient education about COPD, management of symptoms and medication management, delivered by the clinical pharmacist in an outpatient clinic. Participants were given a booklet with information. The pharmacist used MI techniques to help to improve adherence to medication prescribed | Control group: no further information |
Jarab 2012 26 weeks |
||
| Clinical pharmacist‐led education on COPD, medication, importance of adherence, inhaler technique (written information) and COPD symptom management. The pharmacist demonstrated pursed lip technique, expectoration technique, and asked participants to carry out these techniques to understand if they fully understood how to perform them. MI technique aimed to increase self‐efficacy, and was used to advise participants on smoking. An individualised action plan was developed for each participant (for acute exacerbations, advice for GPs for antibiotic prescription and oral corticosteroid initiation. COPD education was re‐enforced by the clinical pharmacist at clinic visits and via telephone calls | Control group: usual hospital care from medical and nursing staff; no structured clinical pharmacist‐led programme was provided |
Khdour 2009 52 weeks |
||
| Group session and individual interventions: MIs were conducted to improve adherence (focus groups); participants were given information about their condition and their daily treatment with an aim to improve adherence from cognitive perspective; training on skills and development on inhaler technique based on SEPAR guidelines. Participants used placebo inhalers to practice techniques | Control group: NR |
Leiva‐Fernandez 2014 52 weeks |
||
| Tele‐pharmacy intervention to improve inhaler use: counselling intervention led by pharmacist who asked about participants' knowledge about medication; inhaler technique; and determining barriers to adherence through education, reminder techniques and MI. Follow‐up phone calls were made to the patients at 4 and 8 weeks after the tele‐pharmacy intervention | Control group: no further information |
Margolis 2013 (unpublished study) 26 weeks |
||
| Initial individual face‐to‐face session and 2 follow‐up sessions at 2 weeks after the face‐to‐face session. The programme consisted of information provision, motivational enhancement, inhaler skills training and behavioural skills training | Routine care offered by the HCP that included medication education written information medication, medication education provided by pharmacist, demonstration of inhaler technique given by nurses (on request by doctor). After the intervention group completed the programme, the control group was offered the intervention (wait list group) |
To 2020 4 weeks' intervention (results collected at 6 weeks) |
||
| Pharmacist‐led intervention: protocolised 2‐session intervention (1:1 sessions) including verbal and written structured education about COPD, medication, inhaler technique and demonstration, importance on maintenance therapy adherence, and current barriers preventing adherence, adverse effects, self‐management (lifestyle), smoking cessation | Control group: non‐protocolised usual pharmacist care |
Tommelein 2014 13 weeks |
||
| Comprehensive clinical pharmacist‐led care programme: structured individualised education, telephone counselling on effective use of inhalers, information about COPD, medication management, discussion of medical test results, patient preference, barriers to medication adherence. Telephone calls by the clinical pharmacist aimed to ascertain treatment effects, address participants' misconceptions about adverse effects, reminders for next clinical appointment | Control group: general counselling without individualised education and telephone follow‐up |
Wei 2014 52 weeks |
||
| Health coaching intervention: with 100 hours of training for COPD‐specific content, health coaches addressed barriers to medication adherence and inhaler technique (teach‐back) once every 3 weeks. Health coaches accompanied participants to their visits with primary care clinicians, pulmonary clinicians or both, in community or at home, and conducted telephone calls between face‐to‐face visits | Usual care: any resources provided by clinic as part of standard care but were not limited to visits with the GP, pulmonary specialist, COPD education classes, PR, smoking cessation resources |
Thom 2018 39 weeks |
||
COPD: chronic obstructive pulmonary disease; GP: general practitioner; HCP: healthcare professional; MI: motivational interview; NR: not reported; PR: pulmonary rehabilitation; SEPAR: Spanish Society of Pulmonology and Thoracic Surgery.
We categorised studies according to how many intervention components there were. Any interventions with one component were grouped as single component interventions. Any interventions with two or more components were categorised as multi‐component interventions. Seven studies investigated single component interventions (De Tullio 1987; Hagedorn 2013; Mochizuki 2013; Criner 2018; Naderloo 2018; Park 2019; NCT03379233), and nine studies investigated multi‐component interventions (Leiva‐Fernandez 2014; Khdour 2009; Jarab 2012; Margolis 2013; Tommelein 2014; Wei 2014; Grandos‐Santiago 2020; To 2020; Thom 2018).
Single component interventions
Seven studies compared a single component intervention to usual care, conventional treatment or a control group (De Tullio 1987; Hagedorn 2013; Mochizuki 2013; Criner 2018; Naderloo 2018; Park 2019; NCT03379233).
Criner 2018 investigated the use of a 'BreatheMe' Bluetooth inhaler monitoring device with reminders, and linked to a mobile application, compared to current care, medication usage monitoring device without any reminders at 26 weeks.
De Tullio 1987 included a pharmacist‐led educational intervention consisting of verbal instruction on importance of medication adherence (and theophylline adherence to maintain blood levels for achieving therapeutic effects) lasting for three to five minutes. The control group did not receive a pharmacist‐led session but were asked by the pharmacist if they had any questions about their medication. The study duration was 26 weeks.
Hagedorn 2013 compared a single Diskus inhaler (containing salmeterol xinafoate 50 µg and fluticasone propionate 500 µg inhalation powder at dosage of one inhalation twice daily plus existing therapy) to salmeterol xinafoate 50 µg and fluticasone propionate 500 µg inhalation powder at dosage of one inhalation twice daily via two separate Diskus inhalers plus existing therapy. The study duration was 52 weeks.
Mochizuki 2013 compared a tulobuterol patch 2 mg once daily to inhaled salmeterol 50 µg twice daily for 12 weeks. Participants received individualised instructions on inhaler technique and how to apply the patch by trained health professionals at the start of the trial. Inhalation technique was reinforced after four weeks of use of salmeterol inhaler.
Naderloo 2018 consisted of a motivational interview intervention based on five one‐to‐one sessions over two consecutive days. Sessions focused on participants' feelings and helping towards motivation for change; resolving uncertainties; creating a desire to change; and identifying, clarifying and acknowledging participants' values. Two further sessions covered information on medication, lifestyle and respiratory chest physiotherapy that the control group received. The study duration was 8.6 weeks.
NCT03379233 included a fixed combination containing indacaterol maleate 110 μg and glycopyrronium bromide 50 μg capsule added to a Concept 2 inhaler with an additional application used for monitoring for 24 weeks. This was compared to the same inhaler without the additional monitoring application.
Park 2019 was a dose‐escalation study comparing roflumilast 250 µg once daily for four weeks followed by a dose of 500 µg for 12 weeks. This was compared to the conventional dose of 500 µg for 12 weeks. The study compared adherence rates to roflumilast as dose‐escalation to a conventional dose strategy.
Multi‐component interventions
Nine studies compared a multi‐component intervention to usual care, or a control group with no further information (Khdour 2009; Jarab 2012; Margolis 2013; Leiva‐Fernandez 2014; Tommelein 2014; Wei 2014; Grandos‐Santiago 2020; To 2020; Thom 2018).
Grandos‐Santiago 2020 consisted of an individualised shared decision‐making and patient engagement intervention that included pharmacological management, symptom control and healthy lifestyle promotion in addition to standard treatment. The control group received standard treatment that included medical and pharmacological care, review of systemic steroids, antibiotics, inhaled bronchodilators and oxygen therapy. The duration was from discharge from hospital to 13 weeks' follow‐up.
Five studies consisted of a pharmacist‐led intervention (Khdour 2009; Jarab 2012; Margolis 2013; Tommelein 2014; Wei 2014). Jarab 2012 included patient education, management of symptoms and medication management (delivered by a clinical pharmacist). An information booklet was also provided, and the pharmacist used motivational interviewing to help participants adhere to their prescribed medication. Similarly, Khdour 2009 also included these components, with additional education and demonstration of pursed lip breathing and expectoration techniques followed by asking participants to perform the techniques. The pharmacist also provided motivational interviewing to help to increase self‐efficacy, and advice on smoking cessation. Individual action plans were developed for each participant and the pharmacist reinforced COPD education via clinic visits and telephone calls. Margolis 2013 consisted of a tele‐pharmacy intervention focusing on improving inhaler technique. The pharmacist asked participants about their knowledge of medication, inhaler technique and any barriers to adherence. Participants were given education as reminder techniques through motivational interviewing. Pharmacists made follow‐up telephone calls twice after the intervention phase ended. Tommelein 2014 and Wei 2014 provided all components as described in the other pharmacist‐led interventions but did not include motivational interviewing. The durations of studies ranged from 13 to 52 weeks.
Leiva‐Fernandez 2014 was a 52‐week study that consisted of group and individual sessions to help improve adherence through motivational interviewing. They provided information on COPD, daily treatments, and training and skills on inhaler technique.
To 2020 included an information, motivation, behavioural‐based intervention consisting of three sessions: an individual face‐to‐face session, and two telephone follow‐up sessions at two weeks apart over four weeks of the intervention. The sessions focused on providing information, motivational enhancement, inhaler technique training and behaviour skills training. The control group received routine care; however, they were offered the intervention after the intervention group completed the programme.
Thom 2018 was a 39‐week study focusing on a Health Coaching intervention that included training for health coaches to enable them to address barriers to medication adherence, inhaler technique with teach‐back methods (closing the loop) once every three weeks. Participants received telephone calls between face‐to‐face visits. The control group received standard care.
Excluded studies
We excluded 44 trials from the review. Details are presented in the Characteristics of excluded studies table.
Risk of bias in included studies
An overview of the risk of bias assessments across individual studies is presented in Figure 2. Support for judgements are presented in the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 16 included studies, 14 were at low risk of bias for random sequence generation, with two studies unclear for this domain due to limited information about how the randomisation process was performed (Hagedorn 2013; Naderloo 2018). Only four studies reported allocation concealment methods (Tommelein 2014; To 2020; Thom 2018; NCT03379233), and 12 studies were unclear for this domain as there was no further information.
Blinding
Fifteen studies were not blinded, that is, participants were aware of the treatment they received, and personnel were aware of the treatment they were providing (performance bias). One study was unclear for blinding of participants or personnel (Jarab 2012). Blinding of outcome assessors (detection bias) varied across studies, with five studies at low risk (Park 2019; Grandos‐Santiago 2020; To 2020; Thom 2018; NCT03379233), and eight studies at high risk (Khdour 2009; Hagedorn 2013; Margolis 2013; Mochizuki 2013; Leiva‐Fernandez 2014; Tommelein 2014; Criner 2018; Naderloo 2018). We judged three studies unclear for this domain due to insufficient information (De Tullio 1987; Jarab 2012; Wei 2014).
Incomplete outcome data
Six studies were at low risk of bias with low rates of attrition (Khdour 2009; Margolis 2013; Tommelein 2014; Wei 2014; Naderloo 2018; Grandos‐Santiago 2020), whereas five were at high risk of bias due to higher rates of attrition in the intervention group (Hagedorn 2013; Mochizuki 2013; Leiva‐Fernandez 2014; Park 2019; Thom 2018). Possible reasons for the attrition rates observed could have included differences in COPD severity (i.e. very severe COPD subgroups) as seen in Hagedorn 2013, or differences in progression of illness demonstrated by further decline in FEV1 and FVC from baseline to end of study visit (Leiva‐Fernandez 2014). Mochizuki 2013 reported discontinuation due to acute exacerbations of COPD, loss to follow‐up and participant desire. Participants were also excluded from the analysis for the six‐minute walk distance because of complaints of severe knee joint pain with or without back pain (Mochizuki 2013). Park 2019 reported high discontinuation rates that could not be explained by dose‐escalation of roflumilast alone. Thom 2018 reported high attrition in the intervention group but did not give reasons. Baseline characteristics were similar across both treatment groups (Thom 2018). Five studies were at unclear risk of attrition (De Tullio 1987; Jarab 2012; Criner 2018; To 2020; NCT03379233).
Selective reporting
Only three studies were at low risk of bias of selective reporting as we identified a prospective trial registration and all outcomes were reported as planned (Tommelein 2014; Naderloo 2018; Grandos‐Santiago 2020). Nine studies were unclear due to lack of information about their protocols and it was unclear if outcomes were reported as planned. Four studies were at high risk of bias due to lack of reporting of outcomes in the publication or protocol (Hagedorn 2013; Criner 2018; Park 2019; Thom 2018).
Other potential sources of bias
There were no other potential sources of bias except for one study in which participants received financial incentives at baseline and at the end of the study as acknowledgement for participation (Thom 2018).
Effects of interventions
Summary of findings 1. Single component intervention compared to control for chronic obstructive pulmonary disease (COPD).
| Single component intervention compared to control for chronic obstructive pulmonary disease (COPD) | |||||||
| Patient or population: COPD Setting: multi‐centre, outpatient pulmonary clinic, medical centre Intervention: single component intervention (change to pharmacotherapy, adherence aids) Comparison: control | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with control | Risk with single component intervention | ||||||
|
Adherence to pharmacotherapy: number of people completing treatment |
Change to pharmacotherapy 16 weeks' follow‐up |
429 people per 1000 | 334 people per 1000 (142 to 599) | OR 0.67 (0.22 to 1.99) | 55 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
|
Quality of life: SGRQ total or CCQ |
Change to pharmacotherapy SGRQ total score: 52 weeks' follow‐up Scale 0–100 lower scores represent better outcome |
The mean change in the SGRQ total score was –2.6 | MD 0.80 higher (3.12 lower to 4.72 higher) | — | 212 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | MID was 4 points change (Jones 2005; Welling 2015) |
|
Adherence aids CCQ total: 26 weeks' follow‐up Scale 0–7, lower scores represent better outcome |
The mean change in the CCQ total score was –0.2 | MD 0.4 higher (0.07 higher to 0.73 higher) | — | 137 (1 RCT) |
⊕⊝⊝⊝ Very lowe,f | MID was 0.4 (Kocks 2006) | |
|
Hospital service utilisation: number of people admitted to hospital (all cause) |
Change to pharmacotherapy 52 weeks' follow‐up |
171 people per 1000 | 233 people per 1000 (134 to 375) | OR 1.47 (0.75 to 2.90) | 212 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | — |
|
Exacerbations: number of people experiencing exacerbations |
Change to pharmacotherapy Mean 43.7 weeks' follow‐up** |
338 people per 1000 | 308 people per 1000 (207 to 434) | OR 0.87 (0.51 to 1.50) | 267 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c | — |
|
Adverse events: number of people experiencing adverse events (all cause or COPD‐related), serious adverse events, mortality |
Change to pharmacotherapy or adherence aids Adverse events (all‐cause) Mean 37.6 weeks' follow‐upg |
433 people per 1000 | 410 people per 1000 (311 to 517) | OR 0.92 (0.60 to 1.41) | 404 (3 RCTs) | ⊕⊝⊝⊝ Very lowc,h,i | — |
|
Change to pharmacotherapy Adverse events (COPD‐related) 52 weeks' follow‐up |
314 people per 1000 | 306 people per 1000 (198 to 441) | OR 0.96 (0.54 to 1.72) | 213 (1 RCT) | ⊕⊝⊝⊝ Very lowc,i | — | |
|
Change to pharmacotherapy or adherence aids Serious adverse events Mean 41.6 weeks follow‐upg |
120 people per 1000 | 176 people per 1000 (105 to 281) | OR 1.57 (0.86 to 2.87) | 350 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c | — | |
|
Change to pharmacotherapy Mortality 52 weeks' follow‐up |
19 people per 1000 | 9 people per 1000 (1 to 96) | OR 0.49 (0.04 to 5.44) | 212 (1 RCT) | ⊕⊝⊝⊝ Very lowc,i | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;CCQ: Clinical COPD Questionnaire; COPD: chronic obstructive pulmonary disease; MD: mean difference; MID: minimal important difference; OR: odds ratio; RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for limitations in risk of bias performance, detection and selective reporting bias. bDowngraded one level for imprecision due to confidence interval crossing the line of no effect. cDowngraded two levels for limitations due to performance, detection, attrition and selective reporting bias. dDowngraded two levels for imprecision due to very wide confidence intervals. eDowngraded two levels for limitations in risk of bias due to performance and detection, selective reporting, and unclear risk for allocation concealment and attrition. fDowngraded one level for imprecision due to optimal information size less than 200 participants. gWeighted mean duration (weeks). hDowngraded one level for indirectness due to one trial that compared roflumilast at different dosages, whereas the other two trials compared different inhaler types. iDowngraded two levels for imprecision due to very wide confidence intervals.
Summary of findings 2. Multi‐component intervention compared to control for chronic obstructive pulmonary disease (COPD).
| Multi‐component intervention compared to control for chronic obstructive pulmonary disease (COPD) | |||||||
| Patient or population: COPD Setting: pulmonary clinic, outpatient COPD clinic, health centre, community pharmacies, primary care clinics, telephone Intervention: multi‐component intervention Comparison: control | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with control | Risk with multi‐component intervention | ||||||
|
Adherence to pharmacotherapy: number of people achieving adherence to medication |
Mean 40.5 weeks' follow‐upa | 502 people per 1000 | 688 per 1000 (593 to 799) | RR 1.37 (1.18 to 1.59) | 446 (4 RCTs) | ⊕⊕⊝⊝ Lowb | Data converted to RR to allow incorporation of a study reporting RR and 95% CI. |
|
Quality of life: SGRQ total, SF CRDQ total, CAT total |
SGRQ total: Mean 35.9 weeks follow‐upa Scale 0–100, lower scores represent better outcome |
The mean SGRQ total score range was 52.2 to 65.3 | MD 2.96 lower (6.36 lower to 0.44 higher) | — | 374 (3 RCTs) | ⊕⊕⊝⊝ Lowc,d | — |
|
CAT total: 17.5 weeks' follow‐upa Scale 0–40, lower scores represent better outcome |
The mean CAT total score was 20.2 | MD 0.22 lower (1.26 lower to 0.81 higher) | — | 879 (3 RCTs) |
⊕⊝⊝⊝ Very lowd,e | MCID was 2 points. Control group data taken from Thom 2018. | |
|
SF CRDQ total: 39 weeks' follow‐up Scale 0–7, higher scores represent better outcomes |
The mean SF CRDQ total score was 4.43 | MD 0.15 higher (0.24 lower to 0.54 higher) | — | 158 (1 RCT) | ⊕⊕⊝⊝ Lowd,e | MCID was 0.5 points (Wijkstra 1994), < 200 participants |
|
|
Hospital service utilisation: number of people admitted to hospital (all‐cause or COPD‐related) |
All‐cause: Mean 19.4 weeks weeks' follow‐up |
129 people per 1000 | 52 per 1000 (31 to 85) | OR 0.37 (0.22 to 0.63) | 877 (2 RCTs) | ⊕⊕⊝⊝ Lowb | — |
|
COPD‐related: Mean 36 weeks' follow‐upa |
438 people per 1000 | 104 per 1000 (52 to 214) | OR 0.15 (0.07 to 0.34) | 220 (2 RCTs) | ⊕⊕⊕⊝ Moderatec | — | |
|
Exacerbations: number of people experiencing moderate or severe exacerbations |
Moderate exacerbations: 13 weeks' follow‐up |
344 people per 1000 | 338 per 1000 (272 to 408) | OR 0.97 (0.71 to 1.31) | 734 (1 RCT) | ⊕⊝⊝⊝ Very lowd,e | — |
|
Severe exacerbations: 13 weeks' follow‐up |
91 people per 1000 | 51 per 1000 (29 to 88) | OR 0.54 (0.30 to 0.97) | 734 (1 RCT) | ⊕⊕⊝⊝ Lowe | — | |
|
Adverse events: number of people experiencing adverse events (all cause or COPD‐related), serious adverse events, mortality |
Adverse events: 39 weeks' follow‐up |
467 per 1000 | 480 per 1000 (345 to 619) | OR 1.05 (0.60 to 1.85) | 192 (1 RCT) |
⊕⊕⊝⊝ Lowd,f | — |
|
Serious adverse events: 39 weeks' follow‐up |
272 per 1000 | 230 per 1000 (135 to 365) | OR 0.80 (0.42 to 1.54) | 192 (1 RCT) |
⊕⊕⊝⊝ Lowd,f | — | |
|
Mortality: Mean 41 weeks' follow‐upa |
40 people per 1000 | 30 per 1000 (12 to 77) | OR 0.79 (0.28 to 2.26) | 462 (3 RCTs) | ⊕⊝⊝⊝ Very lowd,g | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAT: COPD Assessment Test; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CRDQ: Chronic Respiratory Disease Questionnaire; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio; SF: Short Form; SGRQ: St George's Respiratory Questionnaire. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aWeighted mean duration. bDowngraded two levels for limitations in risk of bias due to allocation, performance and detection bias. cDowngraded one level for limitations in risk of bias due to performance bias and other unclear domains. dDowngraded one level for imprecision due to upper confidence interval crossed the line of no effect. eDowngraded two level s for limitations in risk of bias due to performance bias, attrition and selective reporting bias. fDowngraded one level for limitations in risk of bias due to performance bias. gDowngraded two levels for limitations in risk of bias due to allocation, performance, detection and attrition.
We included 16 studies in this review but only 14 studies contributed to the meta‐analyses. Two trials did not contribute to the main analyses (Grandos‐Santiago 2020; NCT03379233).
Of the 14 analysed trials, six reported single component interventions (De Tullio 1987; Hagedorn 2013; Mochizuki 2013; Criner 2018; Naderloo 2018; Park 2019), and eight reported multi‐component interventions (Khdour 2009; Jarab 2012; Margolis 2013; Leiva‐Fernandez 2014; Tommelein 2014; Wei 2014; To 2020; Thom 2018). We described the studies and their interventions with overall direction of effect of interventions in Table 4 and Table 5.
3. Direction of effects of single or multi‐component interventions on outcomes.
| Study ID/outcomes | Adherence (n) | Quality of life | Hospital admissions (n) | Exacerbations (n) | Self‐efficacy (validated scale) | AEs (n) | Participant acceptability | Correct inhaler technique (n) | Mortality |
| Single component interventions | |||||||||
| Criner 2018 | — | ↑ | — | — | — | → | — | — | — |
| De Tullio 1987 | — | — | — | — | — | — | — | — | — |
| Hagedorn 2013 | — | → | → | → | — | → | — | — | → |
| Naderloo 2018 | ↑ | — | — | — | — | — | — | — | — |
| Park 2019 | → | — | — | → | — | → | — | — | — |
| Multi‐component interventions | |||||||||
| Jarab 2012 | ↑ | → | ↓ | — | — | — | — | — | — |
| Khdour 2009 | ↑ | → | ↓ | — | — | — | — | — | → |
| Leiva‐Fernandez 2014 | ↑ | — | — | — | — | — | — | — | — |
| Margolis 2013 | — | — | — | — | — | — | — | → | → |
| To 2020 | → | — | — | — | — | — | — | — | — |
| Tommelein 2014 | — | — | ↓ | →a ↓b |
— | — | — | ↑ | — |
| Wei 2014 | — | → | ↓ | — | — | — | — | — | — |
| Thom 2018 | — | → | — | — | → | — | — | ↑ | → |
| ↑ Effect favouring intervention; positive effect ↓ Reduction of cases, favours intervention → No difference between intervention or control — Not reported aPeople experiencing moderate exacerbations bPeople experiencing severe exacerbations | |||||||||
AE: adverse event; N: number of people.
1. Single component interventions
Primary outcome: adherence to pharmacotherapy
Based on one study, a change in dose (roflumilast 250/500 mg versus roflumilast 500 mg) may make little to no difference to the number of people completing treatment at 12 weeks (OR 0.67, 95% CI 0.22 to 1.99; studies = 1, participants = 55; Analysis 1.1; Table 1) (Park 2019). The evidence is uncertain.
1.1. Analysis.

Comparison 1: Single component intervention versus control, Outcome 1: Adherence: number of people completing treatment (12 weeks' duration)
Two studies measured adherence using prescription refills (De Tullio 1987; Criner 2018). We did not pool the data from these studies due to variation of interventions. One study had a duration of 21.6 weeks (De Tullio 1987), and the other study had a duration of 26 weeks (Criner 2018). Criner 2018 compared a Bluetooth inhaler monitoring device plus a mobile application for support. De Tullio 1987 compared a pharmacist counselling session with no counselling session. There may be little to no difference on adherence with an adherence aid (Bluetooth monitoring device), but an educational intervention (pharmacist counselling session) did improve adherence (mean prescription refill) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Single component intervention versus control, Outcome 2: Adherence: compliance by prescription refill (21.6–26 weeks' duration)
One study used motivational interviewing as an intervention to improve adherence (Naderloo 2018). Adherence was measured using the Adherence among Patients with Chronic Disease questionnaire (APCD). On a scale of 0 to 100, higher scores represented better treatment adherence. Motivational interviewing improved mean adherence scores on the APCD scale at eight weeks compared to control at eight weeks (Analysis 1.3).
1.3. Analysis.

Comparison 1: Single component intervention versus control, Outcome 3: Adherence: Adherence among Patients with Chronic Disease scale (8 weeks)
We analysed Mochizuki 2013 separately as it was a cross‐over study without a washout period. Authors stated that the lack of a washout period was unlikely to affect outcome measures because the carryover effects of treatments would only last for a few days. We were uncertain about these effects. Adherence was measured at the end of the second phase using a different calculation (counting the number of unused doses of medication as a mean percentage of doses used as prescribed). At 12 weeks, adherence improved in people in the transdermal patch tulobuterol condition compared to the inhaled salmeterol condition (Analysis 1.4).
1.4. Analysis.

Comparison 1: Single component intervention versus control, Outcome 4: Adherence % (change to pharmacotherapy) (12 weeks)
Other measures of adherence
Criner 2018 reported adherence by measuring the mean number of adherent sets of budesonide/formoterol puffs per day for each group over 26 weeks. Adherent set of puffs was defined as two sets of budesonide/formoterol puffs per day, and the set must have been taken within one hour of each other. A mean of 2 equated to 100% adherence. The mean set of adherent puffs in the intervention group was 1.6 (standard deviation (SD) 0.39) among 67 participants, and 1.3 (SD 0.51) among 70 participants in the control group (P < 0.001).
Primary outcome: quality of life
One study reported the SGRQ total score (Hagedorn 2013). The SGRQ scale is from 0 to 100 with lower scores indicating better quality of life. Very low‐certainty evidence showed little to no difference in quality of life with a single component intervention (single Diskus inhaler plus individual existing therapy) compared to standard therapy (two separate Diskus inhalers plus individual existing therapy). There was no clear difference between a single component intervention and control at 52 weeks (MD 0.80, 95% CI –3.12 to 4.72; studies = 1, participants = 212; Analysis 1.5; Table 1).
1.5. Analysis.

Comparison 1: Single component intervention versus control, Outcome 5: Quality of life: St George's Respiratory Questionnaire or Clinical COPD Questionnaire total scores (26–52 weeks)
One study reported the effect of a Bluetooth inhaler monitoring device plus mobile application on quality of life measured by the CCQ (a scale from 0 to 7 with lower scores indicating better quality of life) (Criner 2018). At 26 weeks there may be slight improvement in quality of life, however, the evidence was uncertain (Analysis 1.5; Table 1).
Primary outcome: hospital service utilisation
One study reported the number of participants admitted to hospital (Hagedorn 2013). Very uncertain evidence suggests that there is little to no difference in effect of single inhaler compared to two separate inhalers at 52 weeks (OR 1.47, 95% CI 0.75 to 2.90; studies = 1, participants = 212; Analysis 1.6; Table 1).
1.6. Analysis.

Comparison 1: Single component intervention versus control, Outcome 6: Hospital service utilisation: number of people admitted to hospital (all cause) (52 weeks)
Studies not included in the main analysis
One study showed that there may be little to no difference in effect of a single inhaler plus individual existing therapy on mean length of stay (days) in hospital at 52 weeks (Table 6) (Hagedorn 2013).
4. Single or multi‐component interventions: data not included in main analyses.
| Outcome | Effect estimate and 95% CI | Number of studies and study ID | Participants (n) |
| Single component interventions | |||
| Adherence: APCD scale | MD 22.00 (95% CI 8.40 to 36.00) | 1 study (Naderloo 2018) | 54 |
| HA: length of stay (mean days) | MD 3.80 (95% CI –1.77 to 9.37) | 1 study (Hagedorn 2013) | 212 |
| Multi‐component interventions | |||
| Adherence: mean MRA score | MD 8.20 (95% CI 4.34 to 12.06) | 1 study (Tommelein 2014) | 692 |
| Adherence: mean TAI score (change from baseline to hospital discharge) | MD 1.02 (95% CI –1.21 to 3.25) | 1 study (Grandos‐Santiago 2020) | 42 |
| HA: non‐COPD hospitalisations HA: COPD‐related hospitalisations |
MD –0.05 (95% CI –0.25 to 0.15) MD –0.25 (95% CI –0.55 to 0.05 |
1 study (Thom 2018) | 192 |
| HA: number of people admitted to ED | MD 0.87 (95% CI 0.48 to 1.57) | 2 studies (Jarab 2012; Tommelein 2014) | 825 |
| HA: non‐COPD ED visits HA: COPD‐related ED visits |
MD 0.15 (95% CI –0.45 to 0.75) MD –0.09 (95% CI –0.61 to 0.43) |
1 study (Thom 2018) | 192 |
| HA: rate of ED visits per patient per year | RR 0.59 (95% CI 0.27 to 1.29) | 1 study (Tommelein 2014 | 692 |
| HA: length of stay per participant | MD –5.54 (95% CI –11.61 to 0.53) | 1 study (Wei 2014) | 87 |
| HA: rate of hospital days per year | RR, IV 0.27 (95% CI 0.21 to 0.35) | 1 study (Tommelein 2014) | 692 |
| Exacerbations: mean exacerbations per year | MD –0.27 (95% CI –6.01 to 5.47) | 1 study (Thom 2018) | 192 |
| Exacerbations: rate of severe exacerbations per participant per year | RR 0.45 (95% CI 0.25 to 0.81) | 1 study (Tommelein 2014) | 692 |
APCD: Adherence among Patients with Chronic Disease; CAT: COPD Assessment Test; CI: confidence interval; COPD: chronic obstructive pulmonary disease; ED: emergency department; HA: hospital admissions; IV: inverse variance; MD: mean difference; MRA: Medication Refill Adherence scale; n: number of participants; OR: odds ratio; QoL: quality of life; RR: rate ratio; TAI: Test of Adherence to Inhalers.
Secondary outcome: exacerbations
Very uncertain evidence from two studies reported the number of people who experienced exacerbations (Hagedorn 2013; Park 2019). There may be little to no difference in the number of people experiencing exacerbations with a change in dose of roflumilast or single inhaler compared to conventional roflumilast dose or two separate inhalers at mean 43.7 weeks (OR 0.87, 95% CI 0.51 to 1.50; studies = 2, participants = 267; I2 = 0%; Analysis 1.7; Table 1), and the result was not influenced by duration of the studies.
1.7. Analysis.

Comparison 1: Single component intervention versus control, Outcome 7: Exacerbations: number of people experiencing exacerbations (16–52 weeks)
Secondary outcome: self‐efficacy
There were no studies that reported self‐efficacy.
Secondary outcome: adverse events: number of people experiencing adverse events (all‐cause)
Three studies reported all‐cause non‐serious AEs (Hagedorn 2013; Criner 2018; Park 2019). There may be little to no effect of single interventions (adherence aids or changes to pharmacotherapy) compared to control treatment (current care, active control or a conventional dose) at mean 37.6 weeks (OR 0.92, 95% CI 0.60 to 1.41; studies = 3, participants = 4045; I2 = 0%; Analysis 1.8; Table 1). The evidence is very low certainty and the interventions were clinically heterogeneous.
1.8. Analysis.

Comparison 1: Single component intervention versus control, Outcome 8: Adverse events: number of people experiencing adverse events (16–52 weeks)
Secondary outcome: adverse events: number of people adverse events (COPD‐related)
There was very uncertain evidence from one study for the number of people experiencing COPD‐related AEs as there may be little to no difference between single or two separate inhalers at 52 weeks (OR 0.96, 95% CI 0.54 to 1.72; studies = 1, participants = 213; Analysis 1.9; Table 1) (Hagedorn 2013).
1.9. Analysis.

Comparison 1: Single component intervention versus control, Outcome 9: Adverse events: number of people experiencing adverse events (COPD‐related) (52 weeks)
Secondary outcome: adverse events: number of people experiencing a serious adverse event
Based on two studies, very low certainty evidence from two clinically heterogeneous trials suggests there may be little to no difference between a single component intervention (adherence aids or changes to pharmacotherapy) compared with control treatment on the number of people experiencing a serious adverse event (SAE) at mean 41.6 weeks (OR 1.57, 95% CI 0.86 to 2.87; studies = 2, participants = 350; I2 = 0%; Analysis 1.10; Table 1).
1.10. Analysis.

Comparison 1: Single component intervention versus control, Outcome 10: Adverse events: number of people experiencing a serious adverse event (26–52 weeks)
Secondary outcome: mortality
One study reported mortality (Hagedorn 2013). At 52 weeks evidence suggests there is no difference in deaths between a single component intervention (single Diskus inhaler plus individual existing therapy) compared to standard therapy (two separate Diskus inhalers plus individual existing therapy) (Peto OR 0.490, 95% CI 0.04 to 5.44; studies = 1, participants = 212; Analysis 1.11). We presented the analysis as Peto OR because the rate of mortality was lower than 1% in the multi‐component group.
1.11. Analysis.

Comparison 1: Single component intervention versus control, Outcome 11: Mortality (52 weeks)
Secondary outcome: patient acceptability of intervention
There were no studies identified that reported patient acceptability of intervention.
Secondary outcome: inhaler technique
There were no studies identified that reported inhaler technique.
2. Multi‐component interventions
Primary outcome: adherence to pharmacotherapy
Four studies contributed to the analysis (Khdour 2009; Jarab 2012; Leiva‐Fernandez 2014; To 2020). We used RR to analyse the data to allow incorporation of a study reporting RR and 95% CI, rather than the number of participants who were adherent. There may be an increase in the number of people adhering to medication in the multi‐component group compared with a control intervention, regardless of duration of treatment (RR 1.37, 95% CI 1.18 to 1.59; studies = 4, participants = 446; I2 = 0%; Analysis 2.1; Table 2). The evidence was low certainty.
Studies not included in the main analysis
Tommelein 2014 measured mean adherence to maintenance therapy using the Medication Refill Adherence scale (MRA). We did not analyse the data as we preferred using the number of people adhering to medication rather than mean adherence. The study authors calculated MRA by dividing the total days' supply by the number of days the participant was in the study. The number of study participation days represented the number of days between the inclusion date and the date of the second follow‐up visit after 13 weeks (90 days). At 13 weeks, there was an improvement in the MRA in the pharmacist care group compared to control treatment (Table 6).
Grandos‐Santiago 2020 reported the mean Test of the Adherence to Inhalers (TAI) score as change from baseline to hospital discharge. At hospital discharge there was little to no difference in the mean TAI score between multi‐component and control treatment (Table 6).
Primary outcome: quality of life
Three studies reported SGRQ total scores (Khdour 2009; Jarab 2012; Wei 2014). The SGRQ scale is from 0 to 100 with lower scores indicating better quality of life. Low‐certainty evidence suggested there may be little to no difference between multi‐component and control interventions in improving quality of life on the SGRQ total score at 26 to 52 weeks (MD –2.96, 95% CI –6.36 to 0.44; studies = 3, participants = 374; I2 = 0%; Analysis 2.2; Table 2).
2.2. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 2: Quality of life: St George's Respiratory Questionnaire total scores (26–52 weeks)
One multi‐component intervention study reported Chronic Respiratory Disease Questionnaire (CRDQ) score and three reported CAT scores. The CRDQ is measured on a seven‐point scale with higher scores indicating better quality of life. The CAT is measured on a scale from 0 to 40 with lower scores indicating better quality of life. Multi‐component interventions may result in little to no effect on CRDQ score at 39 weeks (MD 0.15, 95% CI –0.24 to 0.54; studies = 1, participants = 158; Analysis 2.3; Table 2) or CAT at 39 weeks (MD –0.22, 95% CI –1.26 to 0.81; studies = 3, participants = 879; I2 = 0%; Analysis 2.4; Table 2). Evidence was of low to very low certainty.
2.3. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 3: Quality of life: Short Form Chronic Respiratory Disease Questionnaire total score (39 weeks)
2.4. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 4: Quality of life: COPD Assessment Test (4–39 weeks)
Primary outcome: hospital service utilisation
Two studies reported hospital service utilisation (Khdour 2009; Tommelein 2014). Uncertain evidence suggests multi‐component interventions likely reduce the number of people experiencing all‐cause hospital admissions (OR 0.37, 95% CI 0.22 to 0.63; studies = 2, participants = 877; I2 = 0%; Analysis 2.5; Table 2). Multi‐component interventions also likely result in a reduction of the number of people experiencing COPD‐related hospital admissions (OR 0.15, 95% CI 0.07 to 0.34; studies = 2, participants = 220; I2 = 0%; Analysis 2.6; Table 2; moderate‐certainty evidence).
2.5. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 5: Hospital service utilisation: number of people admitted to hospital (all‐cause) (13–52 weeks)
2.6. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 6: Hospital service utilisation: number of people admitted to hospital (COPD‐related) (26–52 weeks)
Studies not included in the main analysis
We identified numerous different measures for hospital service utilisation that we did not include in the main analyses, but we reported the data in Table 6. There was no difference in mean hospital admissions (all‐cause or COPD‐related) (Thom 2018) or mean length of stay per participant (Wei 2014). There was no difference in the number of people admitted to an emergency department (Jarab 2012; Tommelein 2014), and there was no difference in mean all‐cause and COPD‐related emergency department visits (Thom 2018). The rate of emergency department visits per participant per year in the multi‐component intervention arm was no different from control treatment (Tommelein 2014). Tommelein 2014 reported the rate of hospital days per year. There was a lower rate of hospital days in the multi‐component intervention arm (Table 6).
Secondary outcome: exacerbations
Based on one study (Tommelein 2014), very uncertain evidence suggests there may be little to no impact of a multi‐component intervention on the number of people experiencing moderate exacerbations compared with control at 13 weeks' duration (OR 0.97, 95% CI 0.71 to 1.31; studies = 1, participants = 734; Analysis 2.7; Table 2). However, uncertain evidence suggests that a multi‐component intervention may result in a reduction in the number of people experiencing severe exacerbations compared to control at 13 weeks (OR 0.54, 95% CI 0.30 to 0.97; studies = 1, participants = 734; Analysis 2.7; Table 2).
2.7. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 7: Exacerbations: number of people experiencing exacerbations (moderate or severe) (13 weeks)
Studies not included in the main analysis
There may be little to no difference in mean exacerbations per year (Thom 2018); however, multi‐component interventions may be of benefit in reducing the rate of severe exacerbations per person per year (Tommelein 2014; Table 6).
Secondary outcome: self‐efficacy
One study (Thom 2018) measured self‐efficacy using the validated Stanford Self‐Efficacy for Managing Chronic Disease Scale (Lorig 2001). At 39 weeks, uncertain evidence suggests there may be little to no difference between multi‐component intervention or control on improving self‐efficacy (MD 0.30, 95% CI –0.33 to 0.93; Analysis 2.8).
2.8. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 8: Self‐efficacy: Stanford Self‐Efficacy for Managing Chronic Disease Scale (39 weeks)
Secondary outcome: adverse events: number of people experiencing adverse events (all‐cause)
One study reported the number of people experiencing all‐cause AEs (Thom 2018). There may be little or no difference between multi‐component interventions or control at 39 weeks and evidence is uncertain (OR 1.05, 95% CI 0.60 to 1.85; Analysis 2.9; Table 2).
2.9. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 9: Adverse events: number of people experiencing adverse events (39 weeks)
Secondary outcome: adverse events: number of people experiencing a serious adverse event
One study reported the number of people experiencing an SAE (Thom 2018). There may be little or no difference between a multi‐component intervention or control at 39 weeks and evidence is uncertain (OR 0.80, 95% CI 0.42 to 1.54; Analysis 2.10; Table 2).
2.10. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 10: Serious adverse events: number of people experiencing a serious adverse event (52 weeks)
Secondary outcome: mortality
Three studies reported mortality (Khdour 2009; Margolis 2013; Thom 2018). Very uncertain evidence shows there may be little to no difference between a multi‐component intervention or control treatment on deaths at mean 41 weeks (OR 0.79, 95% CI 0.28 to 2.26; studies = 3, participants = 462; I2 = 0%; Analysis 2.11; Table 2).
2.11. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 11: Mortality (26–52 weeks)
Secondary outcome: patient acceptability of intervention
There were no studies identified that reported patient acceptability of intervention.
Secondary outcome: inhaler technique
Three studies reported inhaler technique (Margolis 2013; Tommelein 2014; Thom 2018). Multi‐component interventions may improve inhaler technique but the evidence is very uncertain (OR 3.00, 95% CI 1.35 to 6.64; studies = 3, participants = 927; I2 = 74%; Analysis 2.12).
2.12. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 12: Inhaler technique: number of people achieving correct inhaler technique (13–39 weeks)
We investigated reasons for the high heterogeneity in the analysis further. Removal of the unpublished Margolis 2013 study in a sensitivity analysis reduced overall heterogeneity to 0%, tightened the CIs and increased the effect estimate from 3.00 to 4.45 (Analysis 2.13).
2.13. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 13: Inhaler technique: number of people achieving correct inhaler technique; sensitivity analysis (13–39 weeks)
By grouping studies that were published or unpublished (Analysis 2.14), we found that there was an improvement in inhaler technique in two studies that were published (Tommelein 2014; Thom 2018), but one unpublished study (Margolis 2013) showed little to no difference in inhaler technique between a multi‐component intervention and control.
2.14. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 14: Inhaler technique: published versus unpublished; SGA, number of people achieving correct inhaler technique (13–39 weeks)
Discussion
Summary of main results
This systematic review evaluated RCTs that assessed effectiveness of interventions aimed to improve adherence to COPD pharmacotherapy and other outcomes including quality of life and hospitalisations.
We included 16 studies in this review but only 14 studies contributed to the meta‐analyses.
Single component interventions
Of the six studies identified, reporting of adherence as well as types of interventions to promote adherence were varied. Three studies reporting adherence showed little to no difference between the intervention and control treatment (De Tullio 1987; Criner 2018; Park 2019). Park 2019 showed that there was little to no difference in between dose escalation or standard dosage of roflumilast regimens on the number of people completing their treatment. A Bluetooth inhaler monitoring device linked to a mobile application providing reminders (beeps and flashes) was no better than a monitoring device without these reminders in promoting compliance (Criner 2018). A counselling session provided by a pharmacist did improve compliance to medication as measured by prescription refills, as did a transdermal tulobuterol patch compared to the inhaled salmeterol; both results are based on single studies (De Tullio 1987; Mochizuki 2013).
Evidence for quality of life measures was very uncertain for the SGRQ total score and CCQ. There was little to no benefit of a single Diskus inhaler compared to two separate inhalers (standard therapy) (Hagedorn 2013). A Bluetooth inhaler monitoring device may have some benefit in improving quality of life when measured by the CCQ, however, we were uncertain about this result as it was based on one small study (Criner 2018).
We found little to no benefit of single versus two separate inhalers on the number of people with hospital admissions due to high uncertainty of the evidence. Similarly, there was no benefit of change to regimen of pharmacotherapy (whether that be single versus separate inhalers (Hagedorn 2013) or roflumilast dose escalation (Park 2019) on the number of people experiencing exacerbations.
There was little to no benefit in change of pharmacotherapy or adherence aids on AEs (all‐cause and COPD related), SAEs and mortality.
Multi‐component interventions
Eight studies investigated multi‐component interventions and were included in our main analyses. From a pooled result of four studies, adherence improved with multi‐component interventions compared to a control treatment (Analysis 2.2).
Multi‐component interventions were no better than control treatments in improving quality of life as measured by SGRQ total, CRDQ and CAT (Analysis 2.2; Analysis 2.3; Analysis 2.4). There was a reduced number of people with hospital admissions (all cause) as a result of a multi‐component intervention at 13 and 52 weeks. The number of people admitted to hospital due to COPD was also reduced at 26 and 52 weeks (Analysis 2.5; Analysis 2.6).
Multi‐component interventions were not of benefit in reducing the number of people experiencing moderate exacerbations, however, there may be a small benefit for people experiencing severe exacerbations at 13 weeks based on one study (Analysis 2.7).
There were no differences in AEs, SAE, and mortality between multi‐component interventions and control treatment at short‐ or long‐term durations (three studies), although events were rare for mortality. A multi‐component intervention had little or no impact on self‐efficacy as reported by one study (Thom 2018).
There was no evidence for patient acceptability of the intervention.
Three studies reported inhaler technique. There was a benefit with a multi‐component intervention on the number of people achieving the correct inhaler technique (Analysis 2.12). Margolis 2013 was the only unpublished study in the analysis that showed little to no difference in the outcome, and in the sensitivity analysis it was clear that this study was the outlier (Analysis 2.14).
Overall completeness and applicability of evidence
We identified seven studies that investigated single component interventions, and nine studies for multi‐component interventions but there were significant gaps in the evidence (Table 5). We were not clear about effects of single component interventions on adherence, quality of life or hospital admissions due to limited evidence and variation of interventions. In addition, the tools used to measure adherence were different, making it difficult to pool data. We could not determine which of the single component interventions would be of benefit and were safe to use. Equally, we could not determine any harms. An educational session given by a pharmacist on adherence (measured by prescription refill) was found to be of benefit but based on 60 participants with mild‐to‐severe COPD, we could not be certain that this effect would be observed in the wider COPD population. As the data were not segregated by COPD severity, we could not determine which COPD severity population would benefit from the intervention. Similarly, a change in regimen (transdermal patch versus inhaled salmeterol) was effective in improving adherence, but this was based on one study including 44 people with moderate‐to‐severe COPD. Improvements in adherence measured by prescription refills were not seen with a Bluetooth inhaler monitor in one study, but did show a small improvement in quality of life (Criner 2018; Analysis 1.2; Analysis 1.5). The study did show improvement in adherence when the set number of inhaler puffs were measured but we did not analyse the data further and were uncertain about the intervention as the results were based on one study. We could not determine adverse effects that may result from these interventions (non‐serious, serious or fatal) due to the limited number of studies reporting them.
There was marginally more evidence for multi‐component interventions, for example, more people with COPD adhered to pharmacotherapy (pooled result of four studies with 446 participants). However, there was uncertainty in the evidence because of risk of bias across the studies. As COPD severity among three of the studies included participants with moderate‐to‐severe COPD and one with mild‐to‐moderate severity, multi‐component interventions could possibly help people with mild‐to‐severe COPD. Education‐based, or health professional‐led interventions could benefit, but we acknowledge that more trials are needed to determine whether effects are robust and can be applied to most COPD severity groups. We could not determine which individual component of the intervention may have been the driver of effects observed.
Two studies that were pooled in the analysis found positive effects on the number of people hospitalised for any cause over 13 to 52 weeks. The multi‐component interventions were pharmacist‐led but there were limitations due to risk of bias mainly due to blinding of participants, personnel and those assessing outcome measures in both studies, which led to the evidence being rated as low certainty. One study reported that the population had moderate‐to‐severe COPD whereas the second study did not report severity status, therefore, we are unclear which COPD subgroup would benefit from the intervention and more segregated data are needed. Regardless of the issues of blinding (which may be unlikely to be resolved due to the nature of these complex interventions), there is likely to be a potential for health professional‐led individualised input to help people with COPD to self‐manage their medication. Further research is required to confirm these findings. The evidence for COPD‐related hospitalisations was more certain, showing that a multi‐component intervention can help to reduce the number of people admitted to hospital despite limitations due to lack of participant or personnel blinding to the interventions (structured education or pharmacist‐led care). Populations were of either mild‐to‐severe or moderate‐to‐severe COPD, which suggests that the interventions could help across most COPD severity groups. We could not find any difference of effect on AEs (non‐serious, serious or fatal) based on three studies.
Most studies were conducted in high‐income settings, which may limit the applicability of the findings to lower‐ and middle‐income counties. As the majority of the global burden of disease from COPD, including 90% of deaths, occurs in lower‐ and middle‐income countries, this is an important limitation of the evidence (WHO 2017).
We could not determine the influence of personal behaviours towards interventions as we did not plan to investigate this in the review, but it is likely that behaviours do play a role in uptake of the intervention whether that is a simple change to pharmacotherapy, or provision of a multi‐component intervention.
Quality of the evidence
Studies that contributed to evidence for key outcomes including adherence, quality of life, hospital service utilisation and AEs were of high risk of bias due to lack of blinding (performance bias). This judgement was included in the GRADE assessment of key outcomes, which ranged from low to very low certainty for single component interventions, and moderate to very low certainty for multi‐component interventions. Reasons for downgrading mainly included risk of bias, imprecision (including wide or very wide CIs, and small participant numbers), and high heterogeneity in some analyses. The only evidence that was of moderate certainty was the number of people with a COPD‐related hospital admission as there were no issues with imprecision, inconsistency or indirectness (Table 2). We prespecified that we would use the random effects model for our analyses and that we would perform subgroup analyses if we found high levels of heterogeneity. We were unable to perform subgroup analyses due to limited numbers of studies. Publication bias could not be investigated because of the small number of studies in analyses.
High discontinuation rates due to AEs were not dissimilar to other studies that included Asian participants. In comparison, dose‐escalation studies that did not include Asian participants were more effective in improving adherence rates. One study suggested that dose‐escalation may not be effective in Asian populations (Park 2019).
Potential biases in the review process
This review is based on a published protocol (Janjua 2019). Any deviations from the published protocol were noted in Differences between protocol and review, and we provided reasons for the changes made.
We planned to contact authors of trials with mixed populations to request disaggregated data for the participants with COPD, but we did not do this due to time restrictions. Therefore, we only included studies in which the population had a diagnosis of COPD only. Most of the interventions in the mixed population studies that we identified were pharmacist‐led interventions focusing on inhaler technique or counselling. Our analysis of data showed that pharmacist‐led interventions helped to reduce the number of people admitted to hospital (all‐cause or COPD‐related) (Analysis 2.5; Analysis 2.6) and those experiencing exacerbations (Analysis 2.7). While the direction of effect on these outcomes may not change, addition of disaggregated COPD data from the mixed population studies may make the CIs tighter around the effect estimates. It is unclear what additional effects these interventions may have on other outcomes.
We categorised interventions into those that were single component or multi‐component (two or more components) based on Jordan 2015 classifications for ease of management of studies. We did not include all outcome measures in the main analyses because they were unlikely to provide meaningful conclusions as they were based on single studies (e.g. hospitalisations) but we did provide the data in an additional table. We gave preference to the number of people experiencing an outcome rather than the mean number per outcome, due to likely skew in these outcomes, especially hospitalisations. Studies measured adherence in different ways, which made it difficult to compare effects of interventions across studies, and the lack of a gold standard to measure adherence prompted to report the number of people adhering to pharmacotherapy.
We did not include data from studies in the main review that only included information on the intervention group, or if authors did not provide outcome data on request.
Agreements and disagreements with other studies or reviews
Nearly half of medications may be missed because of non‐adherence or poor adherence in high‐income countries and even lower rates in lower‐ and middle‐income countries (Sabaté 2003). Poor adherence may result in loss of benefit of pharmacotherapy to the individual, cost implications for healthcare services and those funding healthcare systems. The WHO report outlines guidance for improving adherence to treatment for people with long‐term chronic conditions. This includes constant review of methods to measure adherence, support for individuals, identifying social and economic factors, the health care system itself, severity of the condition and any comorbidities that an individual may have (Sabaté 2003).
Clinical practice guidelines recommend a multi‐component approach for managing COPD, which could include approaches for adherence using pharmacological and non‐pharmacological techniques (Qaseem 2011).
Our review of the evidence found that multi‐component interventions (individualised or tailored) had some benefit in more people adhering to medication mid to long term (Analysis 2.1) Two of the studies were pharmacist‐led interventions and two studies were multi‐factorial behavioural interventions in people with moderate‐to‐severe COPD, and included face‐to‐face education on COPD, medication management, management of symptoms, written information, motivational interview technique to increase self‐efficacy, development of an individualised action plan and demonstration of the pursed‐lip technique as part of the intervention. Pharmacist‐led multi‐component interventions were also found to be effective in helping people with COPD to adherence as reported by Bryant 2013.
2.1. Analysis.

Comparison 2: Multi‐component intervention versus control, Outcome 1: Adherence: risk ratio and 95% CI (6‐52 weeks)
We found that multi‐component interventions, specifically pharmacist‐led interventions that included education programmes and disease management protocols, helped to reduce all‐cause and COPD‐related hospital admissions (Analysis 2.5; Analysis 2.6). Our findings are in line with another systematic review that showed reduced hospital admissions as a result of pharmacist‐led interventions (Zhong 2014).
We found no differences of effect of interventions on quality of life as measured by SGRQ, CRDQ or CAT. This somewhat reflects findings from other studies in which a clear association between medication adherence and effects on quality of life was not established (Agh 2015).
We found improvements in inhaler technique with multi‐component intervention that had included education on inhaler technique as part of the intervention. However, the evidence was limited to three studies of short to medium duration of follow‐up, and considerable variation that led to uncertainty in the results. Similar results have been reported in another systematic review (Klijn 2017).
Authors' conclusions
Implications for practice.
Single component interventions such as provision of education or motivational interviewing by a health professional can help to improve adherence to pharmacotherapy. Similarly, single component interventions may also help to improve quality of life, but evidence is uncertain to very uncertain. There is no difference in people experiencing exacerbations, adverse events, serious adverse events or deaths. However, these findings are based on very uncertain, limited evidence.
Multi‐component interventions that include structured education, motivational or behavioural components provided by a health professional may help to improve medication adherence in people who have mild‐to‐severe chronic obstructive pulmonary disease (COPD) for medium‐ to long‐term duration, but evidence is uncertain. Low‐ to moderate‐certainty evidence shows that pharmacist‐led approaches may be beneficial in reducing number of people admitted to hospital (for any reason or COPD‐related). A reduced number of people experienced severe exacerbations, but evidence is uncertain and based on one study. There is some uncertainty about adverse events, serious adverse events or deaths, because the evidence is based on a limited number of studies.
The evidence presented in this review should be interpreted with caution as larger studies are needed to demonstrate effects of single and multi‐component interventions, in different COPD populations and optimal intervention durations. Discussions between health professionals and patients may be important to consider which of these approaches may be beneficial to individuals.
Implications for research.
Future trials should include:
larger well‐characterised trial populations, with data subgrouped by COPD severity and longer follow‐up durations;
single and multi‐component interventions with a primary aim to investigate adherence but should also consider the knock‐on effects on outcomes such as quality of life and hospital service utilisation as a consequence of adherence;
outcomes measuring behaviour change where behavioural interventions are being investigated;
information on adverse events and self‐efficacy.
Future trial methods should include:
standardised approaches for measuring adherence and quality of life applicable to the COPD population as well as validated scales to measure patient acceptability;
robust methods in randomised trials which should be reported with transparency;
an active comparator in trials, as blinding was an issue across most studies;
a component network meta‐analysis may help to provide more information about which component of multi‐component interventions are drivers for effects on outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 13 September 2021 | Amended | Corrected typo in abstract. |
History
Protocol first published: Issue 8, 2019 Review first published: Issue 9, 2021
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
The authors and Airways Editorial Team are grateful to Job van Boven (the Netherlands) for his peer review and a consumer reviewer who wishes to remain anonymous.
The author team would like to thank the following for their assistance with translating trial papers: Miguel Maldonado Fernandez, Matthias Perleth, Anja Lieder, Julia Bidonde and Rodrigo Torres.
This project was funded by the National Institute for Health Research (NIHR) Systematic Reviews Programme (project number 16/114/21). This project was also supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Health Research Systematic Reviews Programme, NIHR, National Health Service or the Department of Health and Social Care.
Appendices
Appendix 1. Database search strategies
| Database/search platform/date of last search | Search strategy | Results (number of hits) |
| Airways Register (via Cochrane Register of Studies) Date of most recent search: 1 May 2020 | 1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All AND INREGISTER 2 MeSH DESCRIPTOR Bronchitis, Chronic AND INREGISTER 3 ((obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)):ti,ab AND INREGISTER 4 COPD:MISC1 AND INREGISTER 5 (COPD OR COAD OR COBD OR AECOPD):TI,AB AND INREGISTER 6 #1 OR #2 OR #3 OR #4 OR #5 AND INREGISTER 7 MeSH DESCRIPTOR Patient Compliance Explode All AND INREGISTER 8 MeSH DESCRIPTOR Patient Acceptance of Health Care Explode All AND INREGISTER 9 MeSH DESCRIPTOR Patient Dropouts AND INREGISTER 10 (adhere* or nonadhere* or non‐adhere*):ti,ab,kw AND INREGISTER 11 (complian* or noncomplian* or non‐complian*):ti,ab,kw AND INREGISTER 12 refusal or refuse*:ti,ab,kw AND INREGISTER 13 concord*:ti,ab,kw AND INREGISTER 14 conform*:ti,ab,kw AND INREGISTER 15 accept*:ti,ab,kw AND INREGISTER 16 comply*:ti,ab,kw AND INREGISTER 17 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 AND INREGISTER 18 #6 AND #17 AND INREGISTER | June 2019 (938) May 2020 (229) |
| CENTRAL (via Cochrane Register of Studies) Date of most recent search: 1 May 2020 | 1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All AND CENTRAL:TARGET 2 MeSH DESCRIPTOR Bronchitis, Chronic AND CENTRAL:TARGET 3 ((obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)):ti,ab AND CENTRAL:TARGET 4 COPD:MISC1 AND CENTRAL:TARGET 5 (COPD OR COAD OR COBD OR AECOPD):TI,AB AND CENTRAL:TARGET 6 #1 OR #2 OR #3 OR #4 OR #5 AND CENTRAL:TARGET 7 MeSH DESCRIPTOR Patient Compliance Explode All AND CENTRAL:TARGET 8 MeSH DESCRIPTOR Patient Acceptance of Health Care Explode All AND CENTRAL:TARGET 9 MeSH DESCRIPTOR Patient Dropouts AND CENTRAL:TARGET 10 (adhere* or nonadhere* or non‐adhere*):ti,ab,kw AND CENTRAL:TARGET 11 (complian* or noncomplian* or non‐complian*):ti,ab,kw AND CENTRAL:TARGET 12 refusal or refuse*:ti,ab,kw AND CENTRAL:TARGET 13 concord*:ti,ab,kw AND CENTRAL:TARGET 14 conform*:ti,ab,kw AND CENTRAL:TARGET 15 accept*:ti,ab,kw AND CENTRAL:TARGET 16 comply*:ti,ab,kw AND CENTRAL:TARGET 17 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 AND CENTRAL:TARGET 18 #6 AND #17 AND CENTRAL:TARGET | June 2019 (1715) May 2020 (564) |
| MEDLINE (Ovid) ALL Date of most recent search: 1 May 2020 | 1 exp Pulmonary Disease, Chronic Obstructive/ 2 Bronchitis, Chronic/ 3 (obstruct* adj3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)).ti,ab. 4 (COPD or AECB or AECOPD).ti,ab. 5 or/1‐4 6 exp Patient Compliance/ 7 "Patient Acceptance of Health Care"/ 8 Patient Dropouts/ 9 (adhere* or nonadhere* or non‐adhere*).ti,ab. 10 (complian* or noncomplian* or non‐complian*).ti,ab. 11 (refusal or refuse*).ti,ab. 12 concord*.ti,ab. 13 conform*.ti,ab. 14 accept*.ti,ab. 15 comply.ti,ab. 16 or/6‐15 17 (treatment* or therapy or drug* or medicat* or pharmacolog*).tw. 18 16 and 17 19 5 and 18 20 (controlled clinical trial or randomized controlled trial).pt. 21 (randomized or randomised).ab,ti. 22 placebo.ab,ti. 23 dt.fs. 24 randomly.ab,ti. 25 trial.ab,ti. 26 groups.ab,ti. 27 or/20‐26 28 Animals/ 29 Humans/ 30 28 not (28 and 29) 31 27 not 30 32 19 and 31 | June 2019 (1678) May 2020 (116) |
| Embase (Ovid) Date of most recent search: 1 May 2020 | 1 chronic obstructive lung disease/ 2 chronic bronchitis/ 3 (obstruct* adj3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)).ti,ab. 4 (COPD or AECB or AECOPD).ti,ab. 5 or/1‐4 6 exp patient compliance/ 7 patient attitude/ 8 patient dropout/ 9 treatment refusal/ 10 (adhere* or nonadhere* or non‐adhere*).ti,ab. 11 (complian* or noncomplian* or non‐complian*).ti,ab. 12 (refusal or refuse*).ti,ab. 13 concord*.ti,ab. 14 conform*.ti,ab. 15 accept*.ti,ab. 16 comply.ti,ab. 17 or/6‐16 18 (treatment* or therapy or drug* or medicat* or pharmacolog*).tw. 19 17 and 18 20 5 and 19 21 Randomized Controlled Trial/ 22 randomization/ 23 controlled clinical trial/ 24 Double Blind Procedure/ 25 Single Blind Procedure/ 26 Crossover Procedure/ 27 (clinica$ adj3 trial$).tw. 28 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 29 exp Placebo/ 30 placebo$.ti,ab. 31 random$.ti,ab. 32 ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 33 (crossover$ or cross‐over$).ti,ab. 34 or/21‐33 35 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 36 human/ or normal human/ or human cell/ 37 35 and 36 38 35 not 37 39 34 not 38 40 20 and 39 | June 2019 (2091) May 2020 (189) |
| ClinicalTrials.gov Date of most recent search: 1 May 2020 | Study type: Interventional
Condition: COPD Intervention: adherence OR compliance |
June 2019 (53) May 2020 (6) |
| WHO trials portal Date of most recent search: 1 May 2020 | Condition: COPD Intervention: adherence OR compliance |
June 2019 (15) May 2020 (not searched) |
Data and analyses
Comparison 1. Single component intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Adherence: number of people completing treatment (12 weeks' duration) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.1 Change to pharmacotherapy | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Adherence: compliance by prescription refill (21.6–26 weeks' duration) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.1 Adherence aids | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.2 Education | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Adherence: Adherence among Patients with Chronic Disease scale (8 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Adherence % (change to pharmacotherapy) (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Quality of life: St George's Respiratory Questionnaire or Clinical COPD Questionnaire total scores (26–52 weeks) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.1 Change to pharmacotherapy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.2 Adherence aids | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Hospital service utilisation: number of people admitted to hospital (all cause) (52 weeks) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.1 Change to pharmacotherapy | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Exacerbations: number of people experiencing exacerbations (16–52 weeks) | 2 | 267 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.51, 1.50] |
| 1.7.1 Change to pharmacotherapy | 2 | 267 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.51, 1.50] |
| 1.8 Adverse events: number of people experiencing adverse events (16–52 weeks) | 3 | 404 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.60, 1.41] |
| 1.8.1 Adherence aids | 1 | 137 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.38, 1.83] |
| 1.8.2 Change to pharmacotherapy | 2 | 267 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.50, 2.17] |
| 1.9 Adverse events: number of people experiencing adverse events (COPD‐related) (52 weeks) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9.1 Change to pharmacotherapy | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.10 Adverse events: number of people experiencing a serious adverse event (26–52 weeks) | 2 | 350 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.86, 2.87] |
| 1.10.1 Change to pharmacotherapy | 1 | 213 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [0.74, 3.04] |
| 1.10.2 Adherence aids | 1 | 137 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.55, 5.69] |
| 1.11 Mortality (52 weeks) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.11.1 Change to pharmacotherapy | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Multi‐component intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Adherence: risk ratio and 95% CI (6‐52 weeks) | 4 | Risk Ratio (IV, Random, 95% CI) | 1.37 [1.18, 1.59] | |
| 2.2 Quality of life: St George's Respiratory Questionnaire total scores (26–52 weeks) | 3 | 374 | Mean Difference (IV, Random, 95% CI) | ‐2.96 [‐6.36, 0.44] |
| 2.3 Quality of life: Short Form Chronic Respiratory Disease Questionnaire total score (39 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.1 At 6 to < 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Quality of life: COPD Assessment Test (4–39 weeks) | 3 | 879 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐1.26, 0.81] |
| 2.5 Hospital service utilisation: number of people admitted to hospital (all‐cause) (13–52 weeks) | 2 | 877 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.22, 0.63] |
| 2.6 Hospital service utilisation: number of people admitted to hospital (COPD‐related) (26–52 weeks) | 2 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.07, 0.34] |
| 2.7 Exacerbations: number of people experiencing exacerbations (moderate or severe) (13 weeks) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.7.1 At 3 to < 6 months, moderate exacerbations | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.7.2 At 3 to < 6 months, severe exacerbations | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.8 Self‐efficacy: Stanford Self‐Efficacy for Managing Chronic Disease Scale (39 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.8.1 At 6 to < 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.9 Adverse events: number of people experiencing adverse events (39 weeks) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.10 Serious adverse events: number of people experiencing a serious adverse event (52 weeks) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.11 Mortality (26–52 weeks) | 3 | 462 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.28, 2.26] |
| 2.12 Inhaler technique: number of people achieving correct inhaler technique (13–39 weeks) | 3 | 927 | Odds Ratio (M‐H, Random, 95% CI) | 3.00 [1.35, 6.64] |
| 2.13 Inhaler technique: number of people achieving correct inhaler technique; sensitivity analysis (13–39 weeks) | 2 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 4.45 [3.28, 6.05] |
| 2.14 Inhaler technique: published versus unpublished; SGA, number of people achieving correct inhaler technique (13–39 weeks) | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.14.1 Published | 2 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 4.45 [3.28, 6.05] |
| 2.14.2 Unpublished | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.60, 2.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Criner 2018.
| Study characteristics | ||
| Methods |
Design: RCT (unpublished data) Duration: 26 weeks Location: USA Setting: 8 research sites (multi‐centre) |
|
| Participants |
Population: 138 participants with COPD randomly assigned to BreatheMate device and application (n = 68) or BreatheMate device without application (n = 70) Baseline characteristics: COPD severity: moderate to severe Age (mean): BreatheMate device and application 66.7 (SD 8.72) years; BreatheMate device 66.6 (SD 8.21) years % male: BreatheMate device and application 59.7%; BreatheMate device 52.9% Inclusion criteria: outpatient adults aged ≥ 40 years, COPD diagnosis by postbronchodilator FEV1/FVC < 0.70 in the last 3 years, moderate‐to‐severe COPD, taking ICS/LABA combination therapy for ≥ 3 months prior to screening, current or previous smoker, willing to discontinue all medications containing both LABA and ICS and to begin Symbicort 160/4.5 μg, 2 inhalations twice daily, life expectancy > 12 months, compliance with protocol Exclusion criteria: asthma, drug or alcohol abuse, AECOPDs 28 days before visit 1, a COPD exacerbation during run‐in period, hospital admissions (ischaemic heart disease or heart failure) within 3 months of study enrolment, significant disease/disorder, lung or upper airway cancer and other malignancy not in remission for ≥ 5 years, except for people who have had basal cell carcinoma, or in situ carcinoma of the cervix, systemic corticosteroids as a maintenance treatment, planned hospitalisation or surgical procedure, pregnancy, breastfeeding or planned pregnancy, fertile women not using acceptable contraceptive measures, any clinically relevant abnormal findings that puts the patient at risk, hypersensitivity to Symbicort, unable or unwilling to use mobile communication devices with cellular connectivity, thoracic surgery within 6 months of visit 1, lung transplant or on lung transplant waiting list. |
|
| Interventions |
Treatment arms
Allowed co‐medications: not reported |
|
| Outcomes |
Primary outcome: mean number of adherent sets of budesonide/formoterol puffs per day over the 26‐week study period Secondary outcomes: mean CCQ scores at baseline, EOT, and mean change in score over the 26‐week study period, mean total and domain weekly CCQ scores over each 2‐month study interval for the intervention group |
|
| Notes |
Funding: AstraZeneca Identifiers: DOI: 10.1183/13993003.congress‐2018.PA1988 (ERS International Congress abstract); NCT02864342 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation schedule consisting of 250 blocks with block size of four was created and implemented by a third party vendor." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Unblinded trial. |
| Incomplete outcome data (attrition bias) all outcomes | Unclear risk | Attrition rates were similar in both groups (21% with BreatheMate device and application vs 28% with BreatheMate device without application ), however, the study was terminated early due to "smart phone syncing issues," and it was unclear how this affected outcomes as the "resulting impact on statistical power due to reduced sample size was likely to impose limits on the interpretability of this study." |
| Selective reporting (reporting bias) | High risk | The trial was registered on the website, and outcomes were reported as planned. However, early termination of the study may have impacted on the interpretation of findings. Also, there was an agreement between the principal investigators and the sponsor that restricted the principal investigator's rights to discuss or publish trial results after the trial was completed. |
| Other bias | Low risk | None identified. |
De Tullio 1987.
| Study characteristics | ||
| Methods |
Design: RCT Duration: 26 weeks Location: USA Setting: 1 outpatient pulmonary clinic at VA medical centre |
|
| Participants |
Population: 60 participants with COPD randomly assigned to counselling (n = 30) or control (n = 30) Baseline characteristics COPD severity: mild to severe Age (mean): counselling 62.1 (SD 9.3) years; control 63.2 (SD 6.7) years % male: 100% Pulmonary disease severity (1 = mild, 5 = severe) (mean): counselling 2.1 (SD 1.3); control 2.0 (SD 1.0) Inclusion criteria: adult men with COPD; theophylline established 1 month prior to entering; dosing stabilise for at least 1 week; able to take medications unassisted Exclusion criteria: none stated but only uncomplicated CHF in study |
|
| Interventions |
Treatment arms
Allowed co‐medications: number of other medications (mean): counselling 6.0 (SD 2.9), control 4.9 (SD 2.3) |
|
| Outcomes |
Primary outcomes: adherence (ratio of actual to predicted serum levels; number of refills in 155 days) Secondary outcomes: not reported |
|
| Notes |
Funding: not reported Identifiers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to intervention or control group, based on social security numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Not reported although due to the nature of the intervention blinding would be an issue. |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) all outcomes | Unclear risk | The number of participants not completing prescription refills was similar: 30% in the intervention arm and 23% in the control arm. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not found, unclear if outcomes were reported as planned. |
| Other bias | Low risk | None identified. |
Grandos‐Santiago 2020.
| Study characteristics | ||
| Methods |
Design: RCT Duration (mean): hospitalised duration: SDM‐PE 8.53 (SD 2.29) days; standard 9.17 (3.93) days; 13‐week follow‐up Location: Spain Setting: Pulmonology Service of Virgen de las Nieves and San Cecilio hospitals |
|
| Participants |
Population: 42 hospitalised people with AECOPD randomised into individualised SDM‐PE or standard care Baseline characteristics COPD severity: moderate to severe Age (mean): SDM‐PE 69.33 (SD 9.89) years; standard care 74.20 (SD 9.25) years Pulmonary disease: FEV1 %, SDM‐PE 33.24 (SD 12.88); standard care 37.15 (SD 16.33) Overall health status: EQ‐5D, SDM‐PE 44.55 (SD 20.67); standard care 46.50 (SD 21.30) Inclusion criteria: people hospitalised due to AECOPD Exclusion criteria: inability to provide informed consent, the presence of psychiatric or cognitive disorders, progressive neurological disorders, organ failure, cancer or inability to co‐operate and people who had experienced another exacerbation of COPD in the previous month |
|
| Interventions |
Treatment arms
Allowed co‐medications: systemic steroids, antibiotics, inhaled bronchodilators, oxygen therapy, diuretics, anticoagulants and cardiovascular agents |
|
| Outcomes |
Primary outcome: perceived health status (EQ‐5D) Secondary outcomes: knowledge of the disease, pharmacological management, general functionality, and healthy lifestyle |
|
| Notes | Funding: Fundación Progreso y Salud (FPS), Boehringer Ingelheim España, S.A. (project code: PI‐0370‐2014), and Oximesa, Praxair. Identifiers: doi.org/10.1016/j.pec.2019.12.004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation sequence was prepared by a statistician using computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered, sealed envelopes were used to conceal allocation of participants, the researcher opened the envelopes to determine participant allocation to either the intervention arm or control group. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Trial was single blind, only the outcome assessor was blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Outcome assessor was blind at all time points of outcome assessments. |
| Incomplete outcome data (attrition bias) all outcomes | Low risk | No participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Trial was registered on website and outcomes were reported as planned. Duration of baseline to discharge was not reported for outcomes (contacted authors). |
| Other bias | Low risk | None identified. |
Hagedorn 2013.
| Study characteristics | ||
| Methods |
Design: RCT (unblinded) Duration: 52 weeks Location: Germany Setting: multi‐centre |
|
| Participants |
Population: 212 adults with COPD Baseline characteristics COPD severity: severe to very severe (GOLD stages III and IV) Age (mean): SFC 65.5 (SD 8.3) years; sal/FP 64.2 (SD 8.9) years % male: SFC 69.2%; sal/FP 72.4% Duration of COPD (mean): SFC 12.4 (SD 7.3) years; sal/FP 13.3 (SD 8.3) years Pack‐years: SFC 39.2 (SD 16.6); sal/FP 40.4 (SD 24.1) Exacerbations in last 12 months: SFC 2.2 (0.5); sal/FP 2.3 (0.6) COPD hospitalisations in last 12 months: SFC 84; sal/FP 87 Inclusion criteria: aged ≥ 40 years, COPD diagnosis (ATS, ERS) GOLD stage III or IV, postbronchodilator < 50% predicted, FEV1/FVC ratio < 70%, ≥ 2 moderate or severe exacerbations leading to medical consultation, stable COPD 4 weeks prior to study start, ≥ 10 pack‐years Exclusion criteria: other respiratory conditions, significant inflammatory condition other than COPD, chronic or prophylactic antibiotic use, moderate or severe COPD exacerbation, lower tract infection 4 weeks before randomisation Maintenance systemic steroids were not allowed at start of study |
|
| Interventions |
Treatment arms
Allowed co‐medications: individual existing therapy |
|
| Outcomes | Primary outcome: compliance to inhalers | |
| Notes |
Funding: GlaxoSmithKline Identifiers: NCT00527826 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, but no further information. |
| Allocation concealment (selection bias) | Unclear risk | No further information. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Unblinded trial. |
| Incomplete outcome data (attrition bias) all outcomes | High risk | Higher rate of attrition in the treatment group (24%) vs control (19.4%). |
| Selective reporting (reporting bias) | High risk | Trial was registered on website; however, some outcomes were not reported in the publication, but were reported on the trial website. Mortality was not reported on the trial website but was reported in the publication. |
| Other bias | Low risk | None identified. |
Jarab 2012.
| Study characteristics | ||
| Methods |
Design: RCT Duration: 26 weeks Location: Jordan Setting: 1 outpatient COPD Clinic at the Royal Medical Services Hospital |
|
| Participants |
Population: 133 participants with COPD randomly assigned to structured patient education (n = 66) or a control group (n = 67) Baseline characteristics COPD severity: moderate to severe Age (median): structured patient education 61 (IQR 14) years; control 64 (IQR 15) years % male: structured patient education 39.4%; control 41.8% FEV1 (mean) each group 1.1 (SD 0.5) L Inclusion criteria: people attending the outpatient COPD clinic at the Royal Medical Services, confirmed diagnosis of COPD by the hospital consultant for ≥ 1 year, aged > 35 years, FEV1 30–80% of the predicted normal value and hospital consultant agreement that the person was suitable for entering the trial Exclusion criteria: moderate‐to‐severe learning difficulties, mobility problems, confusion, disorientation or terminal illness, congestive heart failure or if patients attended a pulmonary rehabilitation programme or had consulted a pulmonary nurse or clinical pharmacist in the last 6 months |
|
| Interventions |
Treatment arms
Allowed co‐medications: median 8 (IQR 5) in each group |
|
| Outcomes |
Primary outcome: HRQoL improvement (SGRQ) Secondary outcomes: healthcare utilisation, COPD knowledge, medication adherence (self‐report) |
|
| Notes |
Funding: Alzaytoonah University of Jordan Identifiers: DOI: 10.1007/s11096‐011‐9585‐z |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to intervention and control groups via minimisation technique using MINIM software." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) all outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) all outcomes | Unclear risk | Attrition rate similar in both groups; however, there was no explanation of the people who withdrew, and the analysis was not ITT. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not found, unclear if outcomes were reported as planned. |
| Other bias | Low risk | None identified. |
Khdour 2009.
| Study characteristics | ||
| Methods |
Design: RCT Duration: 52 weeks Location: Northern Ireland Setting: outpatient COPD clinic, Mater Hospital, Belfast |
|
| Participants |
Population: 173 people with COPD (86 intervention; 87 control) recruited from the outpatient clinic Baseline characteristics COPD severity: moderate to severe Age (mean): intervention 65.63 (SD 10.1) years; control 67.3 (SD 9.2) years % females: intervention 55.8%; control 56.3% FEV1: intervention 0.95 (SD 0.48); control 1.1 (SD 0.50) Inclusion criteria: confirmed diagnosis of COPD for ≥ 1 year, FEV1 30–80% predicted normal value, aged > 45 years Exclusion criteria: congestive heart failure, moderate‐to‐severe learning difficulties, attended a pulmonary rehabilitation programme in the last 6 months, severe mobility problems or terminal illness |
|
| Interventions |
Treatment arms
Allowed co‐medications: SABA, LABA, LAAC, ICS, oral steroids |
|
| Outcomes |
Primary outcomes: hospital admissions, HRQoL (SGRQ) Secondary outcomes: COPD Knowledge and Morisky Adherence Questionnaires |
|
| Notes |
Funding: not reported Identifiers: DOI:10.1111/j.1365‐2125.2009.03493.x |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out using the minimisation method by Gore 1981, and a software program was used in the minimisation process. |
| Allocation concealment (selection bias) | Unclear risk | No further information. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Unblinded trial. |
| Incomplete outcome data (attrition bias) all outcomes | Low risk | Similar rates of attrition at 1‐year follow‐up: 17.4% in the intervention group, 17.2% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not found, unclear if outcomes were reported as planned. |
| Other bias | Low risk | None identified. |
Leiva‐Fernandez 2014.
| Study characteristics | ||
| Methods |
Design: RCT Duration: 52 weeks Location: Spain Setting: multi‐centre |
|
| Participants |
Population: 146 people with COPD block randomised into intervention (n = 72) or control (n = 74) groups Baseline characteristics Age (mean): intervention 69.57 years; control 68.59 years % males: intervention 91.7%; control 91.9% COPD severity: mild: intervention 40.6%; control 34.8%; moderate: intervention 43.5%; control 43.5%; severe: intervention. 15.9%; control 21.7% Inclusion criteria: confirmed COPD diagnosis registered in the patient's clinical record, clinical assistance at primary care centres in the Malaga area, prescription of scheduled inhalation therapy and written informed consent Exclusion criteria: respiratory conditions that are not included in the COPD definition (bronchiectasis, asthma or cystic fibrosis) or cognitive impairment |
|
| Interventions |
Treatment arms
Allowed co‐medications: anticholinergic, β2‐adrenergic, ICS, xanthine |
|
| Outcomes |
Primary outcome: treatment adherence (evaluated using dose or tablet recount) Secondary outcomes: functional status (spirometry), quality of life (Spanish version of SGRQ, and the specifically developed SeguiEPOC Questionnaire) |
|
| Notes |
Funding: Carlos III Institute of Health (Instituto de Salud Carlos III) (Spain) Health Research Fund Ministry of Science and Innovation. Identifiers: ISRCTN18841601 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation achieved by applying the block randomisation method using a spreadsheet to create a sequence of random numbers in blocks of 4. |
| Allocation concealment (selection bias) | Unclear risk | Researcher allocating participants had an "open‐list" of randomisation. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Not reported, but unlikely to be blinded due to nature of intervention. |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Not reported, but unlikely to be blinded due to nature of intervention. |
| Incomplete outcome data (attrition bias) all outcomes | High risk | Higher attrition in the intervention group (34%) compared to control group (22%), there were differences in spirometry, % FEV1, FVC and severity of COPD. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were reported as planned in the published protocol, but not adequately reported in the publication. |
| Other bias | Low risk | None identified. |
Margolis 2013.
| Study characteristics | ||
| Methods |
Design: prospective, randomised, single‐blinded intervention study (unpublished) Duration: 26 weeks Location: USA Setting: counselling by telephone |
|
| Participants |
Population: 97 adult veterans with COPD, randomised into a counselling intervention (n = 49) or control group (n = 48) Baseline characteristics COPD severity: unclear Age (mean): intervention 71.6 years; control 70.9 years % males: 97.9% of sample Oxygen use (%): intervention 26.5%; control 26.7% Inclusion criteria: aged ≥ 60 years were identified by pharmacy EMR for tiotropium or LABA (or both) with or without an ICS, low adherence by refilling ≥ 1 daily inhaler < 80% of the time over the previous 6 months Exclusion criteria: age < 60 years, cognitive disorder, severe hearing impairment, < 4 months from initial prescription date, activated healthcare power of attorney |
|
| Interventions |
Treatment arms
Allowed co‐medications: tiotropium or LABA (or both) with or without ICS as part of inclusion. No other medications reported |
|
| Outcomes |
Primary outcomes: incorrect inhaler directions, LABA directions, ICS directions, MDI directions, holding breath, exhaling, LABA breath, MDI inhalation, MDI: wait between puffs, ICS breath, ICS: rinse and spit Secondary outcomes: not reported |
|
| Notes |
Funding: ASHP Research and Education Foundation Fostering Young Investigators Federal Services Junior Investigator Research Grant Identifiers: DOI 10.2146/ajhp120241 (1 letter, 2 posters) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Not possible to blind participants or personnel due to nature of intervention. |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Not reported, but unlikely to be blinded due to nature of intervention. |
| Incomplete outcome data (attrition bias) all outcomes | Low risk | No dropouts or deaths in the intervention group, 2 deaths and 1 dropout in the control group, but overall low (6%). |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered on trial registry website; however, results were reported in a conference poster and a published letter only with no further publication. |
| Other bias | Low risk | None identified. |
Mochizuki 2013.
| Study characteristics | ||
| Methods |
Design: randomised cross‐over study Duration: 12 weeks Location: Japan Setting: single centre |
|
| Participants |
Population: 44 adults with moderate‐to‐severe COPD Baseline characteristics COPD severity: moderate to severe Age (mean): 75.9 (SE 0.9) years Male 72% Smoking history (mean): Hokunalin tape 59.1 (SE 7.0) pack‐years; salmeterol 51.0 (SE 5.8) pack‐years FEV1: Hokunalin tape 1.14% (SE 0.09%); salmeterol 1.34% (SE 0.11%) Inclusion criteria: aged ≥ 65 years, moderate‐to‐severe COPD, spirometry postbronchodilator FEV1:FVC ratio < 0.70, FEV1 30–80% Exclusion criteria: aged < 65 years, no airway limitation, dementia, previous inhaled medication (β2 agonist, anticholinergics, corticosteroids, patched β2 agonists, skin disorders, severe heart disease, unstable hypertension, other conditions in which patch or treatment with β2 agonists used |
|
| Interventions |
Treatment arms
Allowed co‐medications: previous inhaled medications not allowed |
|
| Outcomes |
Primary outcomes: adherence (duration for which a participant took a given medication as prescribed), quality of life (SGRQ) Secondary outcomes: 6MWT, adverse events |
|
| Notes |
Funding: none Identifiers: UMIN000001503 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a random sequence generator. |
| Allocation concealment (selection bias) | Unclear risk | No further information. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded study design. |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Unblinded study design. |
| Incomplete outcome data (attrition bias) all outcomes | High risk | Attrition was higher in the control group compared to the intervention (22% vs 9%). |
| Selective reporting (reporting bias) | Unclear risk | Unable to find a protocol for the trial, so unclear if outcomes were reported as planned. Authors reported that outcome measures were conducted at the end of the second phase as it was (quote) "unlikely that they would be affected by the absence of a washout period." |
| Other bias | Low risk | None identified. |
Naderloo 2018.
| Study characteristics | ||
| Methods |
Design: RCT, 2‐group repeated measures design Duration: 2 months after final session Location: Iran Setting: Masih‐Daneshvari Hospital |
|
| Participants |
Population: 60 hospitalised patients with COPD block randomised into an intervention group (n = 27) and a control group (n = 27) (6 dropped out) Baseline characteristics COPD severity: mild to very severe Age (mean): intervention 53.07 (SD 10.06) years; control 55.04 (SD 7.8) years % males: intervention 51.9%; control 63% Inclusion criteria: positive diagnosis of COPD by a pulmonologist, aged < 65 years, no comorbid serious health condition, ability to speak and understand Persian, no history of mental disorder or Alzheimer’s disease, and basic literacy skills Exclusion criteria: not reported |
|
| Interventions |
Treatment arms
Allowed co‐medications: not reported |
|
| Outcomes |
Primary outcomes: only demographic questionnaire and the APCD questionnaire reported Secondary outcomes: not reported |
|
| Notes |
Funding: not reported Identifiers: IRCT201604128650N7 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation carried out using block randomisation method; however, there was no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Unblinded trial. |
| Incomplete outcome data (attrition bias) all outcomes | Low risk | There were similar drop‐outs in each group (10%). |
| Selective reporting (reporting bias) | Low risk | Trial was registered on website and outcomes were reported as planned. |
| Other bias | Low risk | None identified. |
NCT03379233.
| Study characteristics | ||
| Methods |
Design: RCT Duration: 24 weeks Location: Germany and the Netherlands Setting: unclear |
|
| Participants |
Population: 7 participants with COPD randomly assigned to Concept2 inhaler and application (1 participant) or Concept2 inhaler without application (6 participants) Baseline characteristics COPD severity: NR Number of people aged 18–65 years: telehealth 1; usual care 2 Number of people aged ≥ 65 years: telehealth 0; usual care 4 % male: 57% Inclusion criteria: consent to participate in the trial, > 10% and < 70% adherence during screening process, COPD diagnosis, using Ultbro Breezehaler for ≥ 12 weeks before first visit, been in screening process for > 35 days Exclusion criteria: hypersensitivity or reactions to study drugs or to other ingredients in the study drugs, other co‐existing conditions, COPD exacerbation 6 weeks before first visit or randomisation, using other experimental drugs at the time of study, not able or being willing to use digital system |
|
| Interventions |
Treatment arms
|
|
| Outcomes |
Primary outcomes: change in participant's on‐time adherence, change in participant's total adherence Secondary outcomes: change from participant's 6 week on‐time adherence to participant's on‐time adherence over the last 4 weeks of the intervention: percentage days on which inhaler was taken on time |
|
| Notes | Funding: Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation method was described in the protocol: a randomisation list was generated by interactive response technology and a validated automated system was used to assign participants. |
| Allocation concealment (selection bias) | Low risk | The randomisation numbers were concealed from participants and staff, as described in the protocol. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Participants and personnel were blinded to the randomisation list, but were allowed to be unblinded at the individual level. Only the drug supply randomisation office was blinded to safety events, but participants were unblinded on an individual basis. |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | All staff were blinded to adherence data (inhaler use). |
| Incomplete outcome data (attrition bias) all outcomes | Unclear risk | The trial was terminated early, only 7 participants were in the trial. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported on the ClinicalTrials.gov website but no data were available as the trial was terminated early. |
| Other bias | Low risk | None identified. |
Park 2019.
| Study characteristics | ||
| Methods |
Design: RCT Duration: 12 weeks Location: South Korea Setting: 1 Asian medical centre |
|
| Participants |
Population: 55 participants with COPD randomly assigned to dose‐escalation strategy (n = 27) or conventional dose strategy (n = 28) Baseline characteristics COPD severity: severe to very severe Age (mean): dose‐escalation strategy 70.5 (SD 5.9) years; conventional dose strategy 69.8 (SD 7.6) years % male: dose‐escalation strategy 96.3%; conventional dose strategy 96.4% FEV1 (mean): 1 (SD 0.3) L each group Inclusion criteria: aged ≥ 40 years, clinical diagnosis of severe or very severe COPD (postbronchodilator FEV1/FVC ratio, 70% and postbronchodilator FEV1, 50% of the predicted value) ≥ 4 weeks prior to the study; smoking history ≥ 10 pack‐years, ≥ 1 episode of exacerbation (visits to outpatient clinic or emergency department (or both) or hospitalisation due to purulent sputum, increase in sputum amount, or worsened dyspnoea) in the previous year, chronic bronchitis (cough and sputum production for ≥ 3 months within 2 years), ability to provide written informed consent Exclusion criteria: COPD exacerbation or respiratory infection 4 weeks prior to baseline visit, known α1‐antitrypsin deficiency, long‐term oxygen therapy, moderate‐to‐severe liver impairment (Child‐Pugh B or C), severe immunological diseases (e.g. HIV infection, multiple sclerosis, lupus erythematosus), severe acute infectious diseases, cancers, immunosuppressive medicinal treatment (i.e. methotrexate, azathioprine, infliximab, etanercept or oral corticosteroids to be taken long‐term) except for short‐term systemic corticosteroids, latent infections (e.g. tuberculosis), viral hepatitis, herpes viral infection, herpes zoster, congestive heart failure, history of depression associated with suicidal ideation or behaviour, clinically meaningful bronchiectasis, pregnancy or breastfeeding, or women of childbearing potential unwilling to use effective contraception, hypersensitivity to roflumilast or rescue medication and their ingredients, previous roflumilast therapy within past 3 months, rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose‐galactose malabsorption |
|
| Interventions |
Treatment arms
Allowed co‐medications: medications for COPD, including LAMA, LABA, or combinations of LAMA and LABA, LABA and ICS, LAMA, LABA and ICS were continued. Systemic corticosteroids were not allowed except for the treatment of acute exacerbations |
|
| Outcomes |
Primary outcomes: proportion of participants who prematurely discontinued roflumilast due to adverse events during the entire study period Secondary outcomes: proportion of people prematurely discontinuing roflumilast due to adverse events during the first 8 weeks, time to discontinuation due to adverse events during the study period, and changes in pre‐ and postbronchodilator FEV1 from visit 1 (baseline) to visits 4 (week 4) and 6 (week 12) |
|
| Notes |
Funding: supported by grants (2018‐7043 and 20180352) from the Asan Institute for Life Sciences, Asian Medical Center, Seoul, Korea Identifiers: NCT02018432 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised into two groups (1:1), using a randomisation table generated by SAS software." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | The outcome assessor was blinded (as reported on registry website). |
| Incomplete outcome data (attrition bias) all outcomes | High risk | The attrition rate was higher in the dose‐escalation group compared to conventional treatment (59.3% with dose‐escalation vs 46.6% with conventional treatment). Due to the high attrition rate (adverse events), authors stated that the study was terminated early. Instead of recruiting 84 participants, the end number of participants randomised was 55. |
| Selective reporting (reporting bias) | High risk | Trial was registered on website; however, not all outcomes were reported in the publication, such as quality of life and other outcomes such as laboratory tests. The authors aimed to measure adherence, which was reported as discontinuation rates of roflumilast. |
| Other bias | Low risk | None identified. |
Thom 2018.
| Study characteristics | ||
| Methods |
Design: single blind RCT Duration: 39 weeks Location: USA Setting: 7 urban, county‐operated primary care clinics, including 2 academic residency teaching practices, that primarily serve a low‐income, publicly insured patient population |
|
| Participants |
Population: 192 adults with COPD randomised to receive 39 weeks of health coaching (n = 100) or usual care (n = 92) Baseline characteristics COPD severity: moderate to severe Age (mean): intervention 60.7 (SD 8.0) years; control 61.9 (SD 7.2) years % males: intervention 67%; control 64.1% FEV1 % predicted (mean): intervention 55% (SD 19%); control 60% (SD 20%) High COPD symptom score: CAT ≥ 10: intervention 90.9%; control 94.6% Inclusion criteria: moderate‐to‐severe COPD, confirmed by a postbronchodilator FEV1:FVC ratio < 0.70 or by review by a pulmonologist Exclusion criteria: unable to participate in the study due to mental or physical impairment, severe or terminal illness that precludes focus on COPD, no telephone (from protocol) |
|
| Interventions |
Treatment arms
Allowed co‐medications: SABA, SAAC, LABA, LAMA, ICS |
|
| Outcomes |
Primary outcomes: COPD‐related quality of life on the CRDQ‐ SF scale: total and dyspnoe domain Secondary outcomes: rate of COPD exacerbations per year, excercise capacity (6MWT), Self‐efficacy to Manage Chronic Disease Scale Additional outcomes: SF PACIC, CAT, FEV1 (% predicted), percentage of participants reporting current cigarette use, COPD‐related function (bed days due to respiratory problems), percentage of people demonstrating adequate inhaler use (as a measure of adherence); self‐reported adherence to inhaled controller medications in the past 7 days and observed technique, percentage of people with correct answer to knowledge questions 1 to 4, rates of outpatient visits, ED visits and hospitalisations (non‐COPD and COPD‐related) |
|
| Notes |
Funding: Patient‐Centered Outcomes Research Institute (PCORI AD‐1306‐03900) and supported by the NIH/NCRR Colorado CTSI (grant number UL1 RR025780) Identifiers: NCT02234284 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random binary sequence created by the project manager and stratified by site. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered envelopes were used to mask allocation. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Single blind; participants were aware of assignment and reported adherence. |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "Study investigators and data safety monitoring board were blinded to assignment until analyses were finalised." |
| Incomplete outcome data (attrition bias) all outcomes | High risk | Higher attrition in the intervention group (29%) vs usual care (14%); ITT was implemented; however, adherence outcome was based on number of 98 instead of 138. It is unclear if imputation is based on such low sample sizes/information. |
| Selective reporting (reporting bias) | High risk | The trial was registered on website; however, there were some inconsistencies of reporting of outcomes between the protocol and the publication. For example, number of days (of the last 7) participants reported taking medications as prescribed (measured by % adherence), which was not mentioned in the protocol but was reported in the publication. Morisky Adherence Scale was planned in the protocol but was deleted from the findings because of licence issues. |
| Other bias | Unclear risk | Participants received up to USD 30 at baseline and USD 60 at 9 months for acknowledgement of participation. |
To 2020.
| Study characteristics | ||
| Methods |
Design: single‐blind RCT Duration: 4‐week programme; data collected at 6 weeks Location: Hong Kong Setting: outpatient clinic of a government‐funded regional hospital |
|
| Participants |
Population: 30 outpatients with COPD randomly assigned to 15 IMB model group and 15 usual care Baseline characteristics COPD severity: moderate to very severe Age: IMB 75.6 (SD 5.0) years; usual care 75.9 (SD 4.7) years % males: IMB 14% (93.3%); usual care 15% (100.0%) FEV1 % predicted: IMB 43.7 (SD 10.6); usual care 43.4 (SD 13.3) Inclusion criteria: confirmed diagnosis of COPD by spirometry, with bronchodilator FEV1/FVC < 0.7 (70% predicted) and presence of airflow limitation, aged ≥ 18 years, currently prescribed regular self‐administered inhaled medication and able to communicate in Cantonese Exclusion criteria: clinical record of cognitive impairment, psychiatric problems, too frail to participate and already attending a disease management programme for COPD |
|
| Interventions |
Treatment arms
Allowed co‐medications: LABA, LAAC, ICS, combined LABA and ICS (1 inhaler) |
|
| Outcomes |
Primary outcomes: adherence to inhalation therapy and inhalation techniques Secondary outcomes: symptom severity and quality of life |
|
| Notes |
Funding: not reported Identifiers: 10.1111/ijn.12799 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence of codes was used for randomisation process, prepared by (quote) "an independent statistician to determine group allocation for each participant." |
| Allocation concealment (selection bias) | Low risk | Allocation of sequence was concealed in opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | This was a single‐blind RCT, it was not possible to blind participants or personnel due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "Another research nurse who did not have any information about the assignment collected the outcome data." |
| Incomplete outcome data (attrition bias) all outcomes | Unclear risk | 30 participants completed the study, although the attrition rate was 14.3%. This percentage referred to those participants who completed consent forms and agreed to participate, but then withdrew before baseline data collection. It was unclear why these participants were not included in the ITT analysis if they had been randomised in the study. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not found, unclear if outcomes were reported as planned. |
| Other bias | Low risk | None identified. |
Tommelein 2014.
| Study characteristics | ||
| Methods |
Design: single‐blind, parallel‐group RCT Duration: 13 weeks Location: Belgium Setting: 170 community pharmacies |
|
| Participants |
Population: 734 participants with COPD randomised into an intervention group (n = 371), receiving protocol‐defined pharmacist care or a control group (n = 363), receiving usual pharmacist care Baseline characteristics COPD severity: unclear Age (mean): intervention 68.4 (SD 9.6) years; control 68.9 (SD 9.7) years % males: intervention 64%; control 69% CAT score (mean): intervention 16.7 (SD 7.8); control 16.4 (SD 7.6) Inclusion criteria: prescription for daily COPD maintenance medication; aged ≥ 50 years, smoking history ≥ 10 pack‐years, regular visitor to the pharmacy, and providing written informed consent Exclusion criteria: current asthma, inability to read and write |
|
| Interventions |
Treatment arms
Allowed co‐medications: theophylline, oral corticosteroids, ICS, LAAC, LABAs, SAAC, SABA, SAAC+SABA, ICS+LABA, triple therapy (LAAC+LABA+ICS) |
|
| Outcomes |
Primary outcomes: inhalation technique and medication adherence Secondary outcomes: exacerbation rate, dyspnoea, COPD‐specific and generic health status and smoking behaviour |
|
| Notes |
Funding: Ghent University, Liège University and GlaxoSmithKline Identifiers: DOI:10.1111/bcp.12242 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by pharmacists through a central web‐based randomisation system, created by an independent investigator. |
| Allocation concealment (selection bias) | Low risk | Quote: "to conceal assignments, pharmacists performed allocation through a central web‐based randomisation system, created by an independent investigator." |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Unblinded trial. |
| Incomplete outcome data (attrition bias) all outcomes | Low risk | Attrition rates were similar in both groups (6% protocol‐defined pharmacist care vs 4% usual care ), ITT was used for analyses |
| Selective reporting (reporting bias) | Low risk | Trial was registered on website. All outcomes were reported as planned. |
| Other bias | Low risk | None identified. |
Wei 2014.
| Study characteristics | ||
| Methods |
Design: RCT Duration: 52 weeks Location: China Setting: The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China |
|
| Participants |
Population: 117 people with COPD randomly assigned to pharmaceutical care (n = 58) or usual care (n = 59) Baseline characteristics COPD severity: mild to severe Age (mean): pharmaceutical care 65.2 (SD 8.1) years; usual care 63.9 (SD 6.2) years % male: pharmaceutical care 65.5%; usual care 65% FEV1 (mean): pharmaceutical care 1.07 (SD 0.41) L; usual care 1.15 (SD 0.47) L Number of medications (mean): pharmaceutical care 6.2 (SD 4.3); usual care 6.6 (SD 5.0) Inclusion criteria: stable COPD (respiratory symptoms and medication unchanged for ≥ 4 weeks before enrolment); postbronchodilator FEV1:FVC ratio < 0.70 and FEV1 25–79% predicted value); ≥ 2 consecutive visits to our hospital for the treatment of COPD; no participation in a respiratory rehabilitation in the past year; no previous diagnosis of asthma, dementia, uncontrolled psychiatric disease, and severe heart, liver and kidney disease Exclusion criteria: adherence patients (taken > 80% of the daily dose prescribed), refusal to participate the study |
|
| Interventions |
Treatment arms
Allowed co‐medications: anticholinergic agents, LABA, ICS, xanthines, carbocisteine |
|
| Outcomes |
Primary outcome: medication adherence which was measured by tablet counts + direct interview Secondary outcomes: severe exacerbation rate and HRQoL (SGRQ) |
|
| Notes |
Funding: not reported Identifiers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation codes were computer‐generated." |
| Allocation concealment (selection bias) | Unclear risk | Allocation was concealed using sealed envelopes labelled with consecutive numbers. Envelopes were opened and patients were allocated to treatment group. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Participants were aware of which group they were allocated to; however, pharmacists were blinded to the randomisation codes. |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | It was not clear if the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) all outcomes | Low risk | Similar withdrawal numbers in each arm, 9 in the intervention group and 8 in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Could not find a trial protocol, unclear of outcomes were reported as planned, time points on the CONSORT diagram were not clear. |
| Other bias | Low risk | None identified. |
6MWT: six‐minute walk test; AECOPD: acute exacerbation of COPD; APCD: Adherence among Patients with Chronic Disease; ATS: American Thoracic Society; CAT: COPD Assessment Test; CCQ: Clinical COPD Questionnaire; CHF: chronic heart failure; COPD: chronic obstructive pulmonary disease; EMR: electronic medical record; EOT: end of treatment; ERS: European Respiratory Society; EQ‐5D: EuroQol 5 dimension; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HRQoL: health‐related quality of life; ICS: inhaled corticosteroid; IMB: information‐motivation‐behavioural‐based model; IQR: interquartile range; ITT: intention‐to‐treat; LAAC: long‐acting anticholinergic; LABA: long‐acting beta2‐adrenoceptor agonist; LAMA: long‐acting muscarinic receptor antagonist; MDI: metered dose inhaler; MI: motivational interviewing; n: number of people; PACIC: Patient Assessment of Quality of Care; pMDI: pressurised metered dose inhaler; RCT: randomised controlled trial; SAAC: short‐acting anticholinergic; SABA: short‐acting beta2‐adrenoceptor agonist; sal/FP: salmeterol/fluticasone propionate; SD: standard deviation; SDM‐PE: shared decision‐making and patient engagement programme; SE: standard error; SF: short form; SFC: salmeterol–fluticasone combination; SGRQ: St George's Respiratory Questionnaire; VA: Veterans Affairs.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexopoulos 2016 | Wrong study design (aimed at depressive symptoms). |
| Anonymous 2007 | Wrong study design. |
| Berbecaru‐Iovan 2015 | Wrong intervention (not aimed at adherence). |
| Bock 2012 | Wrong intervention (not aimed at adherence). |
| Boland 2016 | Wrong intervention (not aimed at adherence). |
| Botvinikova 2003 | Wrong population (asthma). |
| Buist 1997 | Wrong intervention (smoking cessation). |
| Bunnag 2007 | Wrong intervention (patient perception). |
| Cabedo Garcia 2010 | Wrong intervention (not aimed at adherence). |
| Chrystyn 2014 | Wrong intervention (patient preference and satisfaction). |
| Collier 2017 | Wrong study design (adherence reported as a post hoc outcome). |
| Dahl 2003 | Wrong intervention (aimed at patient perception). |
| EUCTR2013‐001788‐21‐SK | Wrong study design. |
| Evans 2019 | Wrong intervention (aimed at re‐admissions to hospital). |
| Fischer 2000 | Wrong study design (not an RCT). |
| Gallefoss 1999 | Wrong population (< 50% of the study population was COPD). |
| Garcia‐Aymerich 2007 | Wrong study design (not aimed at adherence). |
| Hernandez 2001 | Wrong study design (not an RCT). |
| Holzer 1988 | Wrong study design (not an RCT). |
| Kirby 2016 | Wrong intervention (not aimed at adherence). |
| Kripalani 2007 | Wrong study design (systematic review; no individual studies included COPD). |
| Mutterlein 1990 | Wrong study design (no relevance to current management). |
| NCT00005717 | Wrong study design (not an RCT). |
| NCT00462540 | Wrong study design (not aimed at adherence). |
| NCT04052906 | Wrong study design (not aimed at inhaler technique or adherence). |
| Nishimura 2011 | Wrong study design (not an RCT). |
| Pongchaidecha 2005 | Wrong study design (not an RCT). |
| Press 2016 | Wrong study design (not aimed at inhaler improvement). |
| Riar 2016 | Wrong study design (not an RCT). |
| Schulte 2008 | Wrong study design (not an RCT). |
| Smith‐McLallen 2015 | Wrong study design (cost‐effectiveness, mixed population). |
| Sung 1998 | Unavailable. |
| Tack 2011 | Wrong study design (not an RCT). |
| Tashkin 1991 | Wrong intervention (main intervention was a smoking cessation programme). |
| Trivedi 2012 | Wrong study design (not an RCT). |
| Van Ganse 2016 | Wrong study design (not an RCT). |
| Van Grunsven 2000 | Wrong population. |
| Vanhaecht 2010 | Wrong intervention (not aimed at adherence). |
| Van Wijk 2005 | Wrong study design (systematic review; no individual studies include COPD‐only population). |
| Vestbo 2009 | Wrong intervention (not aimed at adherence). |
| Viejo 2001 | Wrong study design (not an RCT). |
| Weinstein 2011 | Wrong study design (not an RCT). |
| Yildirim 2015 | Wrong study design (not an RCT). |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12618000410257.
| Methods |
Study design: cluster RCT Duration: 5 months Location: Spain |
| Participants |
Inclusion criteria: aged ≥ 18 years, able to complete questionnaires EQ‐5D, Morisky 4 item scale, ACQ, CAT, blood pressure medication classified according to Anatomical Therapeutic Chemical classification system (C02, C03, C07, C09), asthma medication (group R03), or COPD medication (R03) Exclusion criteria: people collecting somebody else's medication, pregnancy, those unable to visit the pharmacy on a regular basis, previous participation in an education programme or in a study to improve adherence to medication, communication limits or any other impairment preventing participation in the study |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcomes: adherence to medication (self‐report and proportion of days covered) Secondary outcomes: asthma control (ACQ), CCQ, blood pressure levels, EQ‐5D, cost‐effective analysis |
| Notes |
Funding: CINFA laboratory, Spain Identifier: www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000410257 |
Bookser 2018.
| Methods |
Study design: RCT Duration: 26 weeks Location: USA |
| Participants |
Population: people with asthma or COPD or both Baseline characteristics: NR Inclusion criteria: consenting to being included in the study Exclusion criteria: NR |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome: spirometry test results Secondary outcomes: inhaler technique, CAT, ACQ, Morisky scale, number of exacerbations and hospitalisations |
| Notes |
Funding: NR Identifier: NR Ongoing study, only the conference abstract was available |
Davis 2016.
| Methods |
Study design: cluster RCT (protocol) Duration: 6 months Location: Canada |
| Participants |
Population: NA Baseline characteristics: NA Inclusion criteria: aged ≥ 40 years, physician diagnosed COPD, able to answer questions in English Exclusion criteria: severe COPD, dementia diagnosis, terminal illness, asthma diagnosis, enrolled in another clinical trial, do not provide consent |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcomes: change in adherence to medication (Medication Possession Ratio), MMAS‐8 Secondary outcomes: number of participants with a clinically significant change in adherence, number of participants who had 'good adherence', mean MPR between groups, SGRQ, medication inhalation technique, healthcare utilisation, antibiotic and orally administered corticosteroid use for COPD exacerbations |
| Notes | Identifier: ISNRCTN78138190 |
Elliot 2016.
| Methods |
Study design: RCT Duration: 10 weeks Location: UK |
| Participants |
Population: asthma/COPD (n = 117), hypertension (n = 249), T2DM (n = 95) Baseline characteristics: mean age 59 years; male 48%, mean number of other medication 3.5 Inclusion criteria: community‐dwelling, aged ≥ 14 years, able to consent to new medicine service, study and able to provide written consent Exclusion criteria: NR |
| Interventions |
Treatment arms
|
| Outcomes | Primary outcomes: adherence, adherence score (MMAS‐8; self‐report), medication stopped or changed, health status (EQ‐5D‐3L), BMQ, healthcare resource use |
| Notes |
Funding: Department of health policy research programme Other identifier: NCT01635361 |
EUCTR2016‐001435‐13‐FR.
| Methods |
Study design: cross‐over randomised trial Duration: 7 days Location: France |
| Participants |
Population: COPD (GOLD) Baseline characteristics: NR Inclusion criteria: adults aged ≥ 40 years, written informed consent, COPD diagnosis (GOLD 2012), health insurance or social security, women of childbearing age Exclusion criteria: previous use of Breezhaler, Diskus or Respimat or similar device; QT ≥ 450 ms; hypersensitivity to any of the study medications (tiotropium, indacatérol, salmeterol, fluticasone, glycopyrronium); exacerbations in the last 6 months; long‐term psychiatric conditions; inability to use inhaler devices; protected adult; excluded from other study; pregnant or breastfeeding |
| Interventions |
Treatment arms
All groups received all 3 inhalers in cross‐over format. All participants received 2 video recordings:
|
| Outcomes |
Primary outcome: assessment of the presence of at least a major error in the use of the inhalation system, from standardised checklist Secondary outcomes: number of non‐critical errors from standardised checklist, number of non‐device‐dependent errors from standardised checklist, measure of time for necessary drug administration, FEV1, adverse events |
| Notes |
Funding: University Hospital Bordeaux Identifier: NCT02813200 or CHUBX 2014/22 Principal investigator: Dr Pierre‐Olivier Girodet (pierre‐olivier.girodet@chu‐bordeaux.fr) |
Godycki‐Cwirko 2014.
| Methods |
Study design: cluster RCT (protocol) Duration: 9 months Location: Poland |
| Participants |
Population: NA Baseline characteristics: NA Inclusion criteria: people treated in the same practice at baseline (including those with COPD) Exclusion criteria: terminal illness, cognitive impairment |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcomes: GP adherence to recommendations Secondary outcomes: patient health outcomes (e.g. change in smoking status, number of COPD medications prescribed, dyspnoea perception, number of exacerbations in the past and during the study) |
| Notes | Other identifier: NCT01893476 |
Gregoriano 2015.
| Methods |
Study design: RCT Duration: 26 weeks Location: Switzerland |
| Participants |
Population: asthma (GINA), COPD (GOLD) or asthma‐COPD overlap Baseline characteristics: mean age 69 years, males 61% Inclusion criteria: aged ≥ 18 years, established asthma or COPD diagnosis (GINA or GOLD criteria), prescribed daily inhaled maintenance medication, ≥ 1 exacerbation in the last 12 months Exclusion criteria: malignancies, severe diseases, unable to speak German sufficiently, pregnancy or breastfeeding |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome: time to next asthma or COPD exacerbation Secondary outcomes: frequency of exacerbations, number of severe exacerbations leading to hospitalisation, timing and taking adherence, health‐related quality of life |
| Notes |
Funding: Gottfried and Julia Bangerter‐Rhyner Foundation, Swiss Academy of Medical Sciences, the Freiwillige Akademische Gesellschaft (FAG), Swiss Lung League, unrestricted grants from Boehringer Ingelheim GmbH, AstraZeneca AG, and Mundipharma AG, Switzerland Other identifier: NCT02386722 |
Hesselink 2004.
| Methods |
Study design: RCT Duration: 104 weeks Location: Netherlands |
| Participants |
Population: asthma or COPD Baseline characteristics: NR Inclusion criteria: aged 16–75 years, clinical diagnosis of asthma or COPD or mixed (asthma with persisting airway obstruction), treated by GP, no other respiratory or terminal illness, current asthma or COPD medication use, experienced symptoms in the last 12 months Exclusion criteria: NR |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcomes: degree of dyspnoea (mMRC questionnaire), quality of life in respiratory illness questionnaire Secondary outcomes: Inhalation technique, self‐efficacy, coping questionnaire |
| Notes |
Funding: NR Other identifier: NR |
ISRCTN10844309 2019.
| Methods |
Study design: cluster RCT Duration: 69 weeks Location: community pharmacies in 3 locations of Portugal (Faro, Lisboa and Setubal) |
| Participants |
Population: adults with asthma or COPD Baseline characteristics: NR Inclusion criteria: aged ≥ 18 years; self‐reported asthma or COPD; using Breezhaler, Ellipta, Spiromax, Turbohaler, pMDI or Respimat Exclusion criteria: NR |
| Interventions |
Treatment arms
|
| Outcomes | Proportion of people with asthma or COPD achieving 100% in assessment of inhaler technique, inhaler technique score, quality of life (CAT), ACT (for asthma) and mMRC (for COPD), adverse drug events; disease‐related exacerbations, healthcare utilisation and cost |
| Notes |
Funding: Associação Nacional das Farmácias (ANF, Portuguese National Association of Pharmacies) Identifier: ISRCTN10844309 |
ISRCTN62025354.
| Methods |
Study design: RCT Duration: 13 weeks Location: GP practices in Manchester, UK |
| Participants |
Population: adults with depression, COPD, T2DM, arthritis or coronary heart disease Baseline characteristics: NR Inclusion criteria: people attending primary care aged ≥ 50 years, ≥ 2 of 5 common conditions, fluent in written English Exclusion criteria: unable to consent, or considered unsuitable by the primary care team |
| Interventions |
Treatment arms
|
| Outcomes | Recruitment, retention, characteristics of responders vs non‐responders, engagement with intervention (intervention group only), number of negative comments made about the experience of the intervention (intervention group only) and participation (both groups), number of questionnaires completed correctly |
| Notes |
Funding: NIHR Greater Manchester Primary Care Patient Safety Translational Research Centre (GM PSTRC) Identifiers: MINIMA; ISRCTN62025354 |
Kristeller 2017.
| Methods |
Study design: RCT Duration: 26 weeks Location: hospital, USA |
| Participants |
Population: 69 people with COPD or heart failure Baseline characteristics: mean age: treatment group 64 years; control group 69 years; male %: treatment group 46%; control group 44%; education level (graduated from high school): treatment group 74%; control group 79%; hospitalisation in the last year %: treatment group 54%; control group 62% Inclusion criteria: aged ≥ 18 years, hospital admission with primary or secondary diagnosis of COPD or heart failure, agreed to take part in monthly counselling sessions (treatment group only) Exclusion criteria: cognitive impairment, dementia, non‐English, discharge to long‐term care or permanent skilled nursing facility, surgery, hospice, at end of life |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome: medication adherence Secondary outcomes: medication‐related issues, patient satisfaction, hospital readmissions or ED visits |
| Notes |
Funding: Community Pharmacy Foundation Identifier: NCT02047448 |
Maricoto 2019.
| Methods |
Study design: RCT Duration: 52 weeks Location: NR |
| Participants |
Population: adults with COPD or asthma Baseline characteristics: NR Inclusion criteria: aged ≥ 65 years, diagnosis of COPD or asthma, taking any inhaler device (pMDI with or without spacer, DPI or Soft Mist), regular primary care users, to diagnose asthma or COPD at study baseline by GINA and GOLD guidelines Exclusion criteria: severe or acute conditions (unstable cardiovascular status, unstable angina, recent myocardial infarction or pulmonary embolism, unknown haemoptysis, pneumothorax, thoracic, abdominal or eye surgery, acute nausea/vomiting, severe respiratory distress, dementia), intermittent asthma or COPD with mild severity (GOLD I) |
| Interventions |
Treatment arms
|
| Outcomes | Adverse events, quality of life (CAT, mMRC), CARAT, ACT, AQLQ, SGRQ, CCQ, FEV1, FVC, PEF, MEF 25–75, FEV1/FVC, adherence rate (BMQ), inhaler technique performance |
| Notes | Identifier: NCT03449316 |
Navarre 2007.
| Methods |
Study design: RCT Duration: NR (short‐term intervention) Location: USA |
| Participants |
Population: 34 adults with respiratory disease Baseline characteristics: mean age: intervention 57 years; control 51.8 years; male %: intervention 33.3%; control 25%; education (college, %): intervention 61%; control 66%; mean length of respiratory disease: intervention 9.7 (SD 9.4) years ; control 24.5 (SD 16.2) years ; computer use: intervention 69%; control 71.4% Inclusion criteria: age ≥ 18 years, using at ≥ 1 respiratory medication daily for ≥ 6 months, receiving care from a university affiliated respiratory clinic or inhaled medication from a community pharmacy located in the same city in the last 3 months Exclusion criteria: requiring spacer devices to aid inhaled drug delivery |
| Interventions |
Treatment arms
|
| Outcomes | Primary outcomes: observed inhaler technique score, inhaler technique knowledge test, patient evaluation of computer‐based tutorial |
| Notes |
Funding: University of Michigan Office of Information Technology Fund for Innovations that Enhance the Quality of Student Learning via Computer‐Based Learning Grant Identifier: none |
NCT04195191.
| Methods |
Study design: RCT Duration: 10 weeks Location: Spain |
| Participants |
Population: adults with COPD, arterial hypertension, diabetes mellitus Baseline characteristics: NR Inclusion criteria: diagnosis of COPD, arterial hypertension or diabetes mellitus, using anticoagulants Exclusion criteria: not fluent in Spanish, physical or mental disorder hindering participation in the study |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome: adherence to treatment at 10 and 26 weeks Secondary outcome: satisfaction with the service |
| Notes |
Funding: Andalusian School of Public Health Identifier: NCT04195191, 2018713122010 |
Nimmo 1993.
| Methods |
Study design: randomised cross‐over trial Duration: 3 days Location: Canada (tertiary care hospital) |
| Participants |
Population: 20 adults with asthma or COPD Baseline characteristics: hospitalised, mean age 58 years, 60% male, ≥ 1 previous MDI instruction episodes, education level (most, vocational: 9 of total study participants) Inclusion criteria: clinically stable asthma or COPD, using albuterol MDI for ≥ 1 month before study start, able to read English, informed consent obtained Exclusion criteria: NR |
| Interventions |
Treatment arms Pharmacist‐led inhaler technique instruction and assessment
|
| Outcomes | Primary outcomes: inhaler technique (mean performance score %, incorrect inhaler technique demonstration assessment %, steps performed incorrectly %), % participants rating each inhaler step as easy |
| Notes |
Funding: Astra Pharma (part‐funded) Identifier: none |
NTR5187.
| Methods |
Study design: RCT Duration: 6–8 weeks Location: NR |
| Participants |
Population: 100 adults with asthma or COPD Baseline characteristics: mean age 63 years, 49% male Inclusion criteria: NR Exclusion criteria: NR |
| Interventions |
Treatment arms
|
| Outcomes | Primary outcomes: inhalation technique (inhaler‐specific checklists), ACQ or CCQ |
| Notes |
Funding: NR Identifier: none |
O'Dwyer 2016.
| Methods |
Study design: RCT Duration: 26 weeks Location: Ireland |
| Participants |
Population: 152 adults with asthma, COPD, asthma‐COPD overlap or unknown Baseline characteristics: mean age: biofeedback group 54 years; demonstration group 53 years; control group 55 years; male %: biofeedback group 42%; demonstration group 57%; control group 50%; mean exacerbations in the last 6 months: biofeedback group 09 (SD 1.2); demonstration group 1.0 (SD 1.5); control group 0.9 (SD 1.4); asthma diagnosis > 50% across groups; COPD diagnosis 30%; asthma‐COPD overlap 4% Inclusion criteria: aged ≥ 18 years, consenting and understanding study protocol, willing and able to take inhaled medication, valid prescription of or already using a Seratide Diskus inhaler, pharmacy attendance history demonstrating collection of 3 prescriptions for any medication in the last 6 months Exclusion criteria: discontinuation of fluticasone/salmeterol (authorised by physician) in the last 6 months |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome: rate of adherence Secondary outcomes: inhaled medication use (rescue), antibiotic with or without steroid medication (rescue), quality of life, adherence, technique adherence |
| Notes |
Funding: Boots retail (Ireland) Limited, Beaumont Hospital, Royal College of Surgeons (Ireland) Identifier: NCT02203266, RC004 |
Qin 2016.
| Methods |
Study design: RCT Duration: 26 weeks Location: China |
| Participants |
Population: 100 adults with asthma or COPD Baseline characteristics: NR Inclusion criteria: aged 18–75 years, diagnosis of asthma or COPD, using inhaled devices, able to read, write and consenting to be contacted Exclusion criteria: NR |
| Interventions |
Treatment arms
|
| Outcomes | Primary outcomes: correct inhalation technique, drug compliance, exacerbations, adverse events |
| Notes |
Funding: NR Identifier: none |
RBR‐5bw2wt.
| Methods |
Study design: RCT Duration: 52 weeks Location: Brazil |
| Participants |
Population: 75 people with COPD Baseline characteristics: NR Inclusion criteria: inpatients with AECOPD Exclusion criteria: cognitive impairment, psychiatric illness, metastatic disease, severe heart disease |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome: rate of hospitalisation due to AECOPD Secondary outcomes: quality of life, knowledge and techniques for inhaled medication, drug‐related issue rates |
| Notes | Funding: Hospital de Clínicas de Porto Alegre – UFRGS, Universidade Federal do Rio Grande do Sul |
SAM30001 GSK.
| Methods |
Study design: cross‐over RCT Duration: 12 weeks Location: 45 centres across the Netherlands |
| Participants |
Population: 77 people with asthma or COPD Baseline characteristics: mean age: NR; male %: intervention group 49%; control group 49%; ethnicity (white %): intervention group 100%; control group 100% Inclusion criteria: age ≥ 12 years with asthma or COPD as diagnosed according to European Respiratory Society criteria Exclusion criteria: NR |
| Interventions |
Treatment arms
|
| Outcomes | Primary outcome: percentage compliance with use of medication |
| Notes |
Funding: GSK Identifier: SAM30001 GSK |
Serra‐Batlles 2002.
| Methods |
Study design: cross‐over RCT Duration: 8 weeks Location: Spain |
| Participants |
Population: children with asthma and adults with COPD Baseline characteristics: NR Inclusion criteria: children (asthma) or adults (COPD) with history of condition for > 6 months' duration, only taking inhaled medication by another delivery system Exclusion criteria: myopathy, neuropathy leading to muscle weakness, tracheostomy, laryngectomy, mental health issues |
| Interventions |
Treatment arms
|
| Outcomes | Primary outcomes: inhaler preference, percentage correct inhalation technique, percentage achieving correct technique after education |
| Notes | Funding: NR |
Suhaj 2016.
| Methods |
Study design: RCT Duration: 156 weeks Location: India |
| Participants |
Population: 260 adults with COPD Baseline characteristics: mean age: intervention: 60.6 (SD 7.9) years; control: 61.1 (SD 8.4) years; male %: intervention 96%; control 94%; FEV1%: intervention 44.4 (SD 14.5); control 41.9 (SD 14.7); pack‐years: intervention 23.2 (SD 11.4); control 21.7 (SD 12.6); COPD severity %: mild: intervention 13.8; control 12.7; moderate: intervention 20.1; control 21.9; severe: intervention 47.6; control 45.4; very severe: intervention 18.5; control 20.0; number of medications: intervention 6.3 (SD 1.7); control 7.2 (SD 2.1); % comorbidities: intervention 69; control 74 Inclusion criteria: COPD diagnosis (GOLD), informed consent Exclusion criteria: NR |
| Interventions |
Treatment arms
|
| Outcomes | Primary outcomes: health‐related quality of life, medication adherence |
| Notes |
Funding: none Identifiers: NR |
Xin 2016.
| Methods |
Design: RCT Duration: 52 weeks Location: China Setting: Tongde Hospital of Zhejiang Province, located in the Hangzhou City of the Zhejiang Province |
| Participants |
Population: 244 participants with COPD randomly assigned to PMC (n = 122) or usual care (n = 122) Baseline characteristics: age (mean): PMC 64.2 (SD 14.2) years; usual care 64.6 (SD 14.5) years % male: PMC 38.6%; usual care 37.1% FEV % predicted (mean): PMC 55.4 (SD 15.7); usual care 54.7 (SD 14.9) Inclusion criteria: age 35 years, diagnosis of COPD, regular visit to the pharmacist, no previous diagnosis of uncontrolled psychiatric disease and no previous diagnosis of severe liver or kidney disease Exclusion criteria: pregnant women or analphabetic people |
| Interventions |
Treatment arms
Allowed co‐medications: SABA, LABA/ICS, LABA, LAMA, oral steroids, anticholinergic agents, xanthines |
| Outcomes |
Primary outcomes: patient's adherence to maintenance therapy (Medication Refill Adherence score) and SGRQ scores Secondary outcomes: acute exacerbation rate, hospitalisation rate due to acute exacerbation, and smoking behaviour |
| Notes |
Funding: supported by Zhejiang Provincial Natural Science Foundation of China (LY14H280003) and Zhejiang Pharmaceutical Association (2012ZYY11) Identifiers: NR |
ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; ADR: adverse drug reaction; AECOPD: acute exacerbation of COPD; AQLQ: Asthma Quality of Life Questionnaire; BMQ: Beliefs about Medications Questionnaire; CAT: COPD Assessment Test; CCQ: Clinical COPD Questionnaire; COPD: chronic obstructive pulmonary disease; ED: emergency department; EQ‐5D: EuroQoL‐5 dimension; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GINA: Global Initiative for Asthma; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GP: general practice; ICS: inhaled corticosteroid; LABA: long‐acting beta2‐adrenoceptor agonist; LAMA: long‐acting muscarinic receptor antagonist; MDI: metered dose inhaler; MEF 25–75: maximal expiratory flow 25–75%; MMAS‐8: Morisky Medication Adherence Scale; MPR: medication possession ratio; mMRC: modified Medical Research Council dyspnoea scale; NA: not applicable; NR: not reported; PEF: peak expiratory flow; PMC: pharmacist‐managed clinic; PR: pulmonary rehabilitation; QT: measurement made on electrocardiogram to assess electrical properties of the heart. Calculated as time from start of the Q wave to end of the T wave (measure of time taken from when cardiac ventricles start to contract to when they finish relaxing; RCT: randomised controlled trial; SABA: short‐acting beta2‐adrenoceptor agonist; SD: standard deviation; SGRQ: St George's Respiratory Questionnaire; T2DM: type 2 diabetes mellitus.
Characteristics of ongoing studies [ordered by study ID]
ISRCTN10567920.
| Study name | A pragmatic, cluster randomized trial evaluating the impact of an enhanced adherence package (dual bronchodilator+add‐on+app) on time to treatment failure and other clinical outcomes in exacerbating COPD patients with poor adherence to mono or dual therapy over one year (MAGNIFY) |
| Methods | Randomly assigned participants from selected GP practices to either use the technology when taking their daily inhaler dose or usual patient care for 12 months |
| Participants | People with COPD |
| Interventions | Device to monitor inhalation attached to Ultibro inhaler, reminders sent via smartphone application |
| Outcomes | Exacerbations, medication use |
| Starting date | 13 June 2019 |
| Contact information | rupert.jones@plymouth.ac.uk |
| Notes |
ISRCTN77785397.
| Study name | Using the internet to help individuals stay healthy and prevent further reductions in health from existing chronic diseases |
| Methods | Participants receive a web‐based self‐management programme including educational information, drug and medical visit information in addition to usual care. The control group receive usual care only over 12 months |
| Participants | People with COPD (men and women, age ≥ 40 years, already using or started "Living with COPD" programme, stable COPD, access to computer and Internet, current or previous smoker (≥ 10 pack‐years), FEV1 25–70% predicted value after bronchodilator use, FVC < 70%, 2 exacerbations in the last year |
| Interventions | Access to COPD web‐based self‐management intervention in addition to usual care |
| Outcomes | Improvement in participants' adherence to action plan on exacerbation event, action plan adherence (defined as participant taking antibiotic or prednisolone within 3 days of acute exacerbation), medication adherence, self‐efficacy, health‐related quality of life, adverse events |
| Starting date | 2011 |
| Contact information | NR |
| Notes |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; GP: general practitioner; NR: not reported.
Differences between protocol and review
In the review, we did not include studies that consisted of mixed populations (including COPD) but only included studies in which the populations had COPD alone as the main condition. We added exacerbations as an important outcome and reported results in the summary of findings tables.
We clarified the methods to explain that we planned to extract data at all reported time points and for studies that reported outcomes at multiple time points we planned to analyse data in the following groupings by time from baseline: less than three months; three months or greater but less than six months; six months or greater but less than 12 months and 12 months or greater.
In a change to our protocol, we analysed one outcome as a risk ratio (RR) rather than odds ratio to allow incorporation of a study reporting RR and 95% confidence intervals, rather than numbers of participants with an event.
Contributions of authors
SJ, KP, RC and RF performed sifting of search results.
SJ, KP, MB and RC performed data extractions and risk of bias assessments.
SJ completed write‐up of the draft.
AC, KP, MB, RC and RF provided comments before final submission of the review.
Both RC and KP provided clinical input in the discussion of the results.
Contributions of editorial team
Chris Cates (Co‐ordinating Editor): checked the planned methods.
Han Ni: edited the review; advised on content, assisted with sign‐off.
Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on content; edited the review, signed off review for publication.
Sarah Hodgkinson (Circulation and Breathing Network Associate Editor) assisted with sign‐off.
Emma Jackson (Assistant Managing Editor): conducted peer review, edited the references and other sections of the review.
Elizabeth Stovold (Information Specialist): designed the search strategy.
Lucy Goldsmith (Statistician): checked the data entry prior to the full write up of the review.
Sources of support
Internal sources
-
All authors, Other
The authors declare that no such support was received for this systematic review
-
St George's, University of London, UK
Rebecca Fortescue and Sadia Janjua were supported by their employer, St George's, University of London
External sources
-
National Institute for Health Research, UK
Cochrane Programme Grant 16/114/21: NHS priorities in the management of chronic respiratory disease
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health and Social Care.
Declarations of interest
SJ: is employed full‐time as a systematic reviewer by a National Institute for Health Research (NIHR) Programme Grant to complete work on this review.
KP: is a senior clinical lecturer in paediatric medicine at Great Ormond Street Hospital, London, UK; has acted as a consultant on an advisory board for Respiri for development of a symptom monitoring device for asthma; and has given a lecture paid by GlaxoSmithKline. KP has also attended a Novartis severe asthma consultation to develop educational material.
RC: retired in 2018 as a general practitioner and has given lectures to primary care staff funded by GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, and Chiesi in the last 36 months.
AC: has COPD and is part of the patient advisory group for the current NIHR Programme Grant. He has given advice on the development of the protocol, and will provide further advice in the reviewing process.
RF: is a UK qualified general practitioner and Co‐ordinating Editor of Cochrane Airways. She is funded by grants from the NIHR.
MB: none known
Edited (no change to conclusions)
References
References to studies included in this review
Criner 2018 {published and unpublished data}
- Criner GJ, Cole T, Hahn K, Kastango K, Eudicone J, Gilbert I. A randomized clinical study to assess the impact of budesonide/formoterol (BUD/FM) pMDI medication reminders on adherence in COPD patients. European Respiratory Journal 2018;52:PA1988. [Google Scholar]
- NCT02864342. Adherence study in COPD patients [A randomized clinical study to assess the impact of Symbicort® pMDI medication reminders on adherence in COPD patients]. clinicaltrials.gov/ct2/show/NCT02864342 (first received 12 August 2018).
De Tullio 1987 {published data only}
- De Tullio PL, Kirking DM, Arslanian C, Olson DE. Compliance measure development and assessment of theophylline therapy in ambulatory patients. Journal of Clinical Pharmacy and Therapeutics 1987;12(1):19-26. [PubMed] [Google Scholar]
Grandos‐Santiago 2020 {published data only}
- Grandos-Santiago M, Valenza MC, Lopez-Lopez L, Prados-Roman E, Rodriguez-Torres J, Cabrera-Martos I. Shared decision-making and patient engagement program during acute exacerbation of COPD hospitalization: a randomized control trial. Patient Education and Counselling 2020;103:702-8. [DOI] [PubMed] [Google Scholar]
- Sanchez IR, Grandos-Santiago M, López-López L, Del Mar Lucena-Aguilera M, Romero-Fernández R, Valenza MC. Efficacy of a comprehensive education program including inhaler training and COPD management during hospitalization of AECOPD, a randomized controlled clinical. European Respiratory Journal 2018;52:PA3836. [Google Scholar]
Hagedorn 2013 {published data only}
- Hagedorn C, Kassner F, Banik N, Ntampakas P, Fielder K. Influence of salmeterol/fluticasone via single versus separate inhalers on exacerbations in severe/very severe COPD. Respiratory Medicine 2013;107(4):542-9. [DOI] [PubMed] [Google Scholar]
Jarab 2012 {published data only}
- Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. International Journal of Clinical Pharmacy 2012;34(1):53-62. [DOI] [PubMed] [Google Scholar]
Khdour 2009 {published data only}
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. British Journal of Clinical Pharmacology 2009;68(4):588-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leiva‐Fernandez 2014 {published and unpublished data}
- Barnestein-Fonseca P, Leiva-Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D, Leiva-Fernandez F. Efficacy and safety of a multifactor intervention to improve therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): protocol for the ICEPOC study. Trials 2011;12(40):1-9. [DOI] [PMC free article] [PubMed]
- ISRCTN18841601. Educational intervention to improve the treatment adherence in patients with chronic obstructive pulmonary disease (COPD) [Efficacy and safety of a multifactorial intervention to improve treatment adherence in patients with chronic obstructive pulmonary disease (COPD)]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN18841601 (first received 28 April 2010).
- Leiva-Fernandez F, Barnestein-Fonseca P, Leiva- Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D. Effectiveness of a multifactorial intervention to improve adherence in patients with chronic obstructive pulmonary disease (COPD). ICEPOC study. Value in Health 2011;14:A587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva-Fernandez F, Leiva-Fernandez J, Prados-Torres D, Garcia-Ruiz AJ, Barnestein-Fonseca P. Factors related to adherence after a multifactorial intervention to improve it in patients with chronic obstructive pulmonary disease (COPD). ICEPOC study. Value in Health 2013;16:A373-4. [Google Scholar]
- Leiva-Fernandez J, Leiva-Fernandez F, Garcia-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of a multifactorial intervention on therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled trial. BMC Pulmonary Medicine 2014;14(70):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Margolis 2013 {published and unpublished data}
- Margolis A, Young H, Lis J, Schuna A, Sorkness CA. A telepharmacy intervention to improve inhaler adherence in veterans with chronic obstructive pulmonary disease. American Journal of Health-System Pharmacy 2013;70(21):1875-6. [DOI] [PubMed] [Google Scholar]
- NCT01270594. A pilot study to evaluate a telepharmacy intervention to improve inhaler adherence in veterans with COPD. clinicaltrials.gov/show/nct01270594 (first received 5 January 2011).
Mochizuki 2013 {published data only}
- Mochizuki H, Nanjo Y, Takehashi H. Better adherence to a transdermal tulobuterol patch than inhaled salmeterol in elderly chronic obstructive pulmonary disease patients. Geriatrics and Gerontology International 2013;13(2):398-404. [DOI] [PubMed] [Google Scholar]
- UMIN000001503. Comparison of adherence and efficacy between inhaled salmeterol and transdermal tulobuterol patch in elderly COPD patients. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000001503 (first received 13 November 2008).
Naderloo 2018 {published data only}
- IRCT201604128650N7. The effect of motivational interviewing (MI) on adherence to therapy in COPD patients. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=IRCT201604128650N7 (first received 27 July 2016).
- Naderloo H, Vafadar Z, Eslaminejad A, Ebadi A. Effects of motivational interviewing on treatment adherence among patients with chronic obstructive pulmonary disease: a randomized controlled clinical trial. Tanaffus 2018;17(4):241-9. [PMC free article] [PubMed] [Google Scholar]
NCT03379233 {published data only}
- EUCTR2017-001593-42-BE. A 24-week randomized, single blinded study in subjects with chronic obstructive pulmonary disease (COPD) to evaluate the effect of reminders and motivational/adaptive messages on treatment adherence tracked by the Concept 2 inhaler. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2017-001593-42-BE (first received 5 March 2018).
- NCT03379233. A randomized study to evaluate the effect of reminder notifications and motivational/adaptive messaging on treatment adherence (ADVICE) [A 24-week randomized, controlled, multicenter, open-label study to evaluate the effect of reminder notifications and motivational/adaptive messaging on treatment adherence of COPD subjects receiving Ultibro® Breezhaler® treatment using the Concept2 inhaler for dose administration and tracking]. clinicaltrials.gov/show/nct03379233 (first received 20 December 2017).
Park 2019 {published data only}
- Park TS, Kang J, Lee JS, Oh Y-M, Lee S-D, Lee SW. Adherence to roflumilast under dose-escalation strategy in Korean patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2019;14:871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thom 2018 {published data only}
- NCT02234284. Aides in respiration health coaching for COPD (AIR). https://clinicaltrials.gov/ct2/show/results/NCT02234284 2014.
- Thom DH, Willard-Grace R, Tsao S, Hessler D, Huang B, DeVore D et al. Randomised controlled trial of health coaching for vulnerable patients with chronic obstructive pulmonary disease. Annals of the American Thoracic Society 2018;15(10):1159-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard-Grace R, Chirinos C, Wolf J, DeVore D, Huang B, Hessler D. Lay health coaching to increase appropriate inhaler use in COPD: a randomized controlled trial. Annals of Family Medicine 2020;18(1):5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
To 2020 {published data only}
- To KW, Lee IF, Choi KC, Cheung YT, Yu DS. An information-motivation-behavioural-based model and adherence to inhalation therapy and other health outcomes in patients with chronic obstructive pulmonary disease: a pilot randomized controlled trial. International Journal of Nursing Practice 2020;26(2):e12799. [DOI] [PubMed] [Google Scholar]
- To KW. The effects of an education-based adherence intervention on adherence of inhalation therapy among patients with chronic obstructive pulmonary disease. Unpublished doctoral thesis 2017:37-113.
Tommelein 2014 {published data only}
- Tommelein E, Mehuys E, Van Hees T, Adriaens E, Van Bortel L, Christiaens T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. British Journal of Clinical Pharmacology 2014;77(5):756-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boven JF, Tommelein E, Boussery K, Mehuys E, Vegter S, Brusselle GGO, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respiratory Research 2014;15(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2014 {published data only}
- Wei L, Yang X, Li J, Liu L, Luo H, Zheng Z, et al. Effect of pharmaceutical care on medication adherence and hospital admission in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled study. Journal of Thoracic Disease 2014;6(6):656-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexopoulos 2016 {published data only}
- Alexopoulos GS, Sirey JA, Banerjee S, Dimitris KN, Pollari C, Novitch RS, et al. Two behavioural interventions for patients with major depression and severe COPD. American Journal of Geriatric Psychiatry 2016;24(11):964-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2007 {published data only}
- Anonymous. Innovative inhalation system can improve compliance. Long-term therapy becomes simpler. MMW Fortschritte der Medizin 2007;149(47):55. [PubMed] [Google Scholar]
Berbecaru‐Iovan 2015 {published data only}
- Berbecaru-Iovan S, Berbecaru-Iovan A, Stanciulescu EC, Ceausu I, Rau G, Pisoschi CG. Efficacy of indacaterol, a modern ultralong-acting bronchodilator, in elderly patients with chronic obstructive pulmonary disease. Farmacia 2015;63(2):306-12. [Google Scholar]
Bock 2012 {published data only}
- Bock D, Kroneberg P, Mullinger B, Roller J, Meyer M, Schierhorn K. Phase II multi-centre trial with inhaled bimosiamose in ambulatory patients with moderate to severe COPD: evaluation of dose compliance. American Journal of Respiratory and Critical Care Medicine 2012;185:A2952. [Google Scholar]
Boland 2016 {published data only}
- Boland M, Van Boven J, Kruis A, Chavannes N, Van Der Molen T, Goossens L, et al. COPD medication adherence and quality of life. European Respiratory Journal 2016;48:PA3961. [DOI] [PubMed] [Google Scholar]
Botvinikova 2003 {published data only}
- Botvinikova LA, Garagulya AA, Botvinikova LA, Garagulya AA. The improvement in quality of life in patients with COPD: the influence of long term regular monitoring and good compliance. European Respiratory Journal 2003;22(Suppl 45):1609. [Google Scholar]
Buist 1997 {published data only}
- Buist AS. The US Lung Health Study. Respirology 1997;2(4):303-7. [DOI] [PubMed] [Google Scholar]
Bunnag 2007 {published data only}
- Bunnag C, Fuangtong R, Pothirat C, Punyaratabandhu P. A comparative study of patients' preferences and sensory perceptions of three forms of inhalers among Thai asthma and COPD patients. Asian Pacific Journal of Allergy and Immunology 2007;25(2):99-109. [PubMed] [Google Scholar]
Cabedo Garcia 2010 {published data only}
- Cabedo Garcia VR, Garces Asemany CR, Cortes Berti A, Oteo Elso JT, Ballester Salvador FJ. Effectiveness of the correct use of inhalation devices in patients with COPD: randomized clinical trial. Medicina Clinica 2010;135(13):586-91. [DOI] [PubMed] [Google Scholar]
Chrystyn 2014 {published data only}
- Chrystyn H, Pascual S, Feimer J, De Soyza A, Sauleda Roig J, Haughney J. Preference, satisfaction and critical errors with Genuair® and Breezhaler® in patients with COPD. European Respiratory Journal 2014;44:P928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collier 2017 {published data only}
- Collier S, Browning D, New JP, Gibson JM, Stephens L, Diar Bakerly N, et al. Describing adherence data in a clinical effectiveness trial: the Salford lung study in COPD (SLS COPD). Thorax 2017;72:A229. [Google Scholar]
Dahl 2003 {published data only}
- Dahl R, Backer V, Ollgaard B, Gerken F, Kesten S. Assessment of patient performance of the HandiHaler compared with the metered dose inhaler four weeks after instruction. Respiratory Medicine 2003;97(10):1126-33. [DOI] [PubMed] [Google Scholar]
EUCTR2013‐001788‐21‐SK {published data only}
- EUCTR2013-001788-21-SK. Study to evaluate how to optimise the use of Roflumilast in subjects who have a lung disease called chronic obstructive pulmonary disease (COPD) [A multicenter, randomized, double-blind phase 3 study to evaluate tolerability and pharmacokinetics of 500µg roflumilast once daily with an up-titration regimen in COPD, including an open-label down-titration period evaluating tolerability and pharmacokinetics of 250µg roflumilast once daily in subjects not tolerating 500µg roflumilast once-daily. Evaluation of tolerability and pharmacokinetics of roflumilast (250µg and 500µg)]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2013-001788-21-GB (first received 30 December 2013).
Evans 2019 {published data only}
- Evans CN, Volpp KG, Polsky D, Small DS, Kennedy EH, Karpink K. Prediction using a randomized evaluation of data collection integrated through connected technologies (PREDICT): design and rationale of a randomized trial of patients discharged from the hospital to home. Contemporary Clinical Trials 2019;83:53-6. [DOI] [PubMed] [Google Scholar]
Fischer 2000 {published data only}
- Fischer LR, Scott LM, Boonstra DM, DeFor TA, Cooper S, Elkema MA, et al. Pharmaceutical care for patients with chronic conditions. Journal of the American Pharmaceutical Association 2000;40(2):174-80. [DOI] [PubMed] [Google Scholar]
Gallefoss 1999 {published data only}
- Gallefoss F, Bakke P. Effect of patient education on compliance in asthmatics and chronic obstructive lung disease. American Journal of Respiratory and Critical Care Medicine 1998;157:A275. [DOI] [PubMed] [Google Scholar]
- Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? American Journal of Respiratory and Critical Care Medicine 1999;160(6):2000-5. [DOI] [PubMed] [Google Scholar]
Garcia‐Aymerich 2007 {published data only}
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101(7):1462-9. [DOI] [PubMed] [Google Scholar]
Hernandez 2001 {published data only}
- Hernandez C, Casas A, Celorrio N, Collvinent B, Farrero E, Fornas C. Impact of a home care program on adherence to treatment in patients with COPD. European Respiratory Journal 2001;18:207s. [Google Scholar]
Holzer 1988 {published data only}
- Holzer R. Compliance improving measures in the treatment of obstructive lung diseases. Praxis und Klinik der Pneumologie 1988;42(7):535-6. [PubMed] [Google Scholar]
Kirby 2016 {published data only}
- Kirby S, Zhu C-Q, Kerwin EM, Stanford RH, Georges G. A randomized controlled trial comparing two dry powder inhalers: more patients with COPD prefer ellipta compared to diskus based on device-specific attributes. American Journal of Respiratory and Critical Care Medicine 2014;189:A3037. [Google Scholar]
Kripalani 2007 {published data only}
- Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Archives of Internal Medicine 2007;167(6):540-50. [DOI] [PubMed] [Google Scholar]
Mutterlein 1990 {published data only}
- Mutterlein R, Schmidt G, Fleischer W, Freund E. A new inhalation system for bronchodilatation. Study of the acceptance of the Ingelheim M inhaler in chronic obstructive respiratory tract diseases. Fortschritte der Medizin 1990;108(11):225-8. [PubMed] [Google Scholar]
NCT00005717 {published data only}
- NCT00005717. Medication adherence in COPD – a self-regulation study. clinicaltrials.gov/show/nct00005717 (first received 26 May 2000).
NCT00462540 {published data only}
- NCT00462540. A crossover study in the treatment of patients with COPD. clinicaltrials.gov/show/NCT00462540 (first received 19 April 2007).
- Sutherland ER, Brazinksy S, Feldman G, McGinty J, Tomlinson L, Denis-Mize K. Nebulised formoterol effect on bronchodilation and satisfaction in COPD patients compared to QID ipratropium/albuterol MDI. Current Medical Research and Opinion 2009;25(3):653-61. [DOI] [PubMed] [Google Scholar]
NCT04052906 {published data only}
- NCT04052906. The effect on self-care and self-efficacy of inhaler training in COPD. clinicaltrials.gov/show/nct04052906 (first received 12 August 2019).
Nishimura 2011 {published data only}
- Nishimura N, Asakura Y, Jinta T, Suda R, Yamao S, Tomishima Y, et al. Therapeutic effect of switching tiotropium Handihaler To Respimat Soft MistTM; inhaler in the patients with COPD: the difference of adverse events and adherence to manipulations between inhaler devices. American Journal of Respiratory and Critical Care Medicine 2011;183:A1604. [Google Scholar]
Pongchaidecha 2005 {published data only}
- Pongchaidecha N, Trakarnkitwichit U, Pituknitinunth K. Effects of inpatient drug counseling on compliance of asthmatic and COPD patients. Thai Journal of Hospital Pharmacy 2005;15(1):28-37. [Google Scholar]
Press 2016 {published data only}
- NCT01456494. Teaching use of respiratory inhalers (TURI). clinicaltrials.gov/ct2/show/NCT01456494 (first received 20 October 2011).
- Press VG, Arora VM, Shah LM, Lewis SL, Charbeneau J, Naureckas ET, et al. Teaching the use of respiratory inhalers to hospitalised patients with asthma or COPD: a randomized trial. Journal of General Internal Medicine 2012;27(10):1317-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press VG, Arora VM, Trela KC, Adhikari R, Zadravecz FJ, Liao C, et al. Effectiveness of interventions to teach metered-dose and diskus inhaler techniques: a randomized trial. Annals of the American Thoracic Society 2016;13(6):816-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riar 2016 {published data only}
- Riar R, Carrasco L, Olibrice M, Ayinla R. Patient education with inhaler technique to prevent readmissions and emergency room (ER) visits in asthma and COPD: a quality improvement project at an inner-city hospital. Chest 2016;150(4):632A. [Google Scholar]
Schulte 2008 {published data only}
- Schulte M, Osseiran K, Betz R, Wencker M, Brand P, Meyer T, et al. Handling of and preferences for available dry powder inhaler systems by patients with asthma and COPD. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2008;21(4):321-8. [DOI] [PubMed] [Google Scholar]
Smith‐McLallen 2015 {published data only}
- Smith-McLallen A, Fensterheim L, Cruz S, Fu X, Solak E, Marks A, et al. A randomized clinical trial of medication therapy management in a commercial population. Journal of Managed Care and Specialty Pharmacy 2015;21(10):S77-8. [Google Scholar]
Sung 1998 {published data only}
- Sung SJ, Gray DR. Effectiveness and economics of a formulary switch from two separate inhalation aerosols to 2-in-1 Combivent inhaler in a veteran COPD population. American Journal of Health-System Pharmacy 1998;33:P347E. [Google Scholar]
Tack 2011 {published data only}
- Tack G, Tjia-Leong E, Davies L, Warburton CJ. Factors affecting inhaler choice and adherence in urban Liverpool. Thorax 2011;66:A161. [Google Scholar]
Tashkin 1991 {published data only}
- Nides MA, Tashkin DF, Simmons MS, Wise RA, Li VC, Rand CS. Improving inhaler adherence in a clinical trial through the use of the Nebuliser Chronolog. Chest 1993;104(2):501-7. [DOI] [PubMed] [Google Scholar]
- O'Hara P, Grill J, Rigdon MA, Connett JE, Lauger GA, Johnston JJ. Design and results of the initial intervention program for the Lung Health Study. The Lung Health Study Research Group. Preventative medicine 1993;22(3):304-15. [DOI] [PubMed] [Google Scholar]
- Rand CS, Nides M, Cowles MK, Wise RA, Connett J. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. American Journal of Respiratory and Critical Care Medicine 1995;152(2):580-8. [DOI] [PubMed] [Google Scholar]
- Rand CS, Wise RA, Nides M, Simmons MS, Bleecker ER, Kusek JW, et al. Metered-dose inhaler adherence in a clinical trial. American Review of Respiratory Disease 1992;146(6):1559-64. [DOI] [PubMed] [Google Scholar]
- Simmons MS, Nides MA, Rand CS, Wise RA, Tashkin DP. Trends in compliance with bronchodilator inhaler use between follow-up visits in a clinical trial. Chest 1996;109(4):963-8. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Rand C, Nides M, Simmons M, Wise R, Coulson AH, et al. A nebulizer chronolog to monitor compliance with inhaler use. American Journal of Medicine 1991;91(4):33S-6S. [DOI] [PubMed] [Google Scholar]
Trivedi 2012 {published data only}
- Trivedi RB, Bryson CL, Udris E, Au DH. The influence of informal caregivers on adherence in COPD patients. Annals of Behavioral Medicine 2012;44(1):66-72. [DOI] [PubMed] [Google Scholar]
Van Ganse 2016 {published data only}
- Van Ganse E, Price D. Respiratory medication adherence: toward a common language and a shared vision. Journal of Allergy and Clinical Immunology in Practice 2016;4(5):799-801. [DOI] [PubMed] [Google Scholar]
Van Grunsven 2000 {published data only}
- Van Grunsven PM, Van Schayck CP, Van Deuveren M, Van Herwaarden CLA, Akkermans RP, Van Weel C. Compliance during long-term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease: results of the detection, intervention, and monitoring programme of COPD and asthma (DIMCA) study. Journal of Asthma 2000;37(3):225-34. [DOI] [PubMed] [Google Scholar]
Vanhaecht 2010 {published data only}
- Vanhaecht K, Lodewijckx C, Sermeus W, Decramer M, Deneckere S, Leigheb F, et al. Impact of a care pathway for COPD on adherence to guidelines and hospital readmission: a cluster randomized trial. International Journal of COPD 2016;11(1):2897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaecht K, Sermeus W, Peers J, Lodewijckx C, Deneckere S, Leigheb F, et al. The impact of care pathways for exacerbation of chronic obstructive pulmonary disease: rationale and design of a cluster randomized controlled trial. Trials 2010;11(111):1-7. [DOI] [PMC free article] [PubMed]
Van Wijk 2005 {published data only}
- Van Wijk BL, Klungel OH, Heerdink ER, Boer A. Effectiveness of interventions by community pharmacists to improve patient adherence to chronic medication: a systematic review. Annals of Pharmacotherapy 2005;39(2):319-28. [DOI] [PubMed] [Google Scholar]
Vestbo 2009 {published data only}
- Vestbo J, Anderson J, Calverley P, Celli B, Ferguson G, Jenkins C. Adherence to medication, mortality and severe exacerbations in the TORCH study. European Respiratory Society 18th Annual Congress; 2008 Oct 3-7; Berlin 2008:383.
- Vestbo J, Anderson JA, Calverley PM, Celli B, Ferguson GT, Jenkins C, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 2009;64:939-43. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Anderson JA, Willits LR, Celli B, Ferguson GT, Jenkins CR, et al. The TOwards a Revolution in COPD Health (TORCH) study, the effects of compliance on mortality over 3 years. American Thoracic Society International Conference; 2008 May 16-21; Toronto.
- Vestbo J, TORCH study group. The TORCH (towards a revolution in COPD health) survival study protocol. European Respiratory Journal 2004;24(2):206-10. [DOI] [PubMed] [Google Scholar]
Viejo 2001 {published data only}
- Viejo JI, Alcazar B, Garcia I, Guallar J, Hernandez S, Martin P, et al. A realistic study assessing inhaled treatment compliance in COPD patients. European Respiratory Journal 2001;18:25s. [Google Scholar]
Weinstein 2011 {published data only}
- Weinstein C, Staudinger H, Scott I, Amar NJ, LaForce C. Dose counter performance of mometasone furoate/formoterol inhalers in subjects with asthma or COPD. Respiratory Medicine 2011;105(7):979-88. [DOI] [PubMed] [Google Scholar]
Yildirim 2015 {published data only}
- Yildirim N, Demir T, Gemicioglu B, Kiyan E, Oguzulgen K, Polatli M, et al. Acute exacerbation in COPD and asthma. Tuberkuloz ve Toraks 2015;63(2):111-31. [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12618000410257 {published data only}
- ACTRN12618000410257. Medication adherence management in a community pharmacy setting (AdherenciaMED): a cluster randomised controlled trial [The effect of a medication adherence management service in a community pharmacy setting on patient's adherence to medications: a cluster randomized controlled trial]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ACTRN12618000410257 (first received 21 March 2018).
Bookser 2018 {published data only}
- Bookser M, Drennen C, Leonard E, McConaha J. Pharmacist-led medication intervention for patients with asthma and COPD within a primary care setting. Journal of the American Pharmacists Association 2018;58(3):e24. [Google Scholar]
Davis 2016 {published data only}
- Davis E, Marra C, Gamble J-M, Farrell J, Lockyer J, FitzGerald JM, et al. Effectiveness of a pharmacist-driven intervention in COPD (EPIC): study protocol for a randomized controlled trial. Trials 2016;17(1):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliot 2016 {published data only}
- Boyd M, Waring J, Barber N, Mehta R, Chuter A, Avery AJ et al. Protocol for the new medicine service study: a randomised controlled trial and economic evaluation with qualitative appraising comparing the effectiveness and cost-effectiveness of the new medicine service in community pharmacies in England. Trials 2013;14:411. [DOI] [PMC free article] [PubMed]
- Elliott RA, Boyd MJ, Salema NE, Davies J, Barber N, Mehta RL. Supporting adherence for people starting a new medication for a long-term condition through community pharmacies: a pragmatic randomised controlled trial of the new medicine service. British Medical Journal Quality and Safety 2016;25(10):747-58. [DOI] [PubMed] [Google Scholar]
- Elliott RA, Boyd MJ, Tanajewski L, Barber N, Gkountouras G, Avery AJ et al. New medicine service: supporting adherence in people starting a new medication for a long-term condition: 26-week follow-up of a pragmatic randomised controlled trial. British Medical Journal 2020;29(4):286-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RA, Tanajewski L, Gkountouras G, Avery AJ, Barber N, Mehta R et al. Cost-effectiveness of support for people starting a new medication for a long-term condition through community pharmacies: an economic evaluation of the new medicine service (NMS) compared with normal practice. Pharmacoeconomics 2017;35(12):1237-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01635361. Understanding and appraising the new medicine service in England (NMS). https://clinicaltrials.gov/ct2/show/NCT01635361 2012.
EUCTR2016‐001435‐13‐FR {published data only}
- EUCTR2016-001435-13-FR. Description of the ability to learn how to handle inhaler devices in COPD [Description of the ability to learn how to handle inhaler devices in COPD – INTUITIVE]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2016-001435-13-FR (first received 17 June 2016).
Godycki‐Cwirko 2014 {published data only}
- Godycki-Cwirko M, Zakowska I, Kosiek K, Wensing M, Krawczyk J, Kowalczyk A. Evaluation of a tailored implementation strategy to improve the management of patients with chronic obstructive pulmonary disease in primary care: a study protocol of a cluster randomized trial. Trials 2014;15(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregoriano 2015 {published data only}
- Gregoriano C, Dieterle T, Breitenstein AL, Durr S, Baum A, Giezendanner S, et al. Does a tailored intervention to promote adherence in patients with chronic obstructive pulmonary disease affect exacerbations? A randomised controlled trial. Respiratory Research 2019;23(6):e204. [DOI: 10.2196/resprot.7522] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriano C, Dieterle T, Durr S, Arnet I, Hersberger KE, Leuppi JD. Impact of an electronic monitoring intervention to improve adherence to inhaled medication in patients with asthma and chronic obstructive pulmonary disease. JMIR Research Protocols 2017;3(20):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriano C, Dieterle T, Flamm A-L, Durr S, Maier S, Arnet I. Objective adherence to inhaled medication and health-related outcomes in asthma and chronic obstructive pulmonary disease after an electronic monitoring intervention. Respiration 2018;95(6):486. [Google Scholar]
- Gregoriano C, Henny-Reinalter S, Handschin A, Flamm A-L, Dieterle T, Arnet I, et al. Patients' adherence to chronic treatment of pulmonary diseases. European Respiratory Journal 2015;46:PA1061. [Google Scholar]
- Gregoriano C, Henny-Reinalter S, Han d schin A, Lisa Flamm A, Dieterle T, Arnet I, et al. How do patients adhere to chronic treatment of pulmonary diseases? Baseline data from an adherence study. Praxis 2015;104:127-8. [Google Scholar]
- Gregoriano C, Henny-Reinalter S, Maier S, Flamm A-L, Dieterle T, Arnet I, et al. How do patients adhere to chronic treatment of pulmonary diseases? Preliminary data from a randomized controlled trial. Kardiovaskulare Medizin 2016;0:91S. [Google Scholar]
- NCT02386722. Intervention to improve inhalative adherence [Impact of a pharmaceutical care intervention to improve adherence of inhaled medication in asthma and COPD patients]. clinicaltrials.gov/ct2/show/NCT02386722 (first received 12 March 2017).
Hesselink 2004 {published data only}
- Hesselink AE, Penninx BW, Van der Windt DA, Van Duin BJ, Vries P, Twisk JW, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Education and Counselling 2004;55(1):121-8. [DOI] [PubMed] [Google Scholar]
ISRCTN10844309 2019 {published data only}
- ISRCTN10844309. Study of inhaler use in asthma and COPD [Cluster randomized controlled trial of the effectiveness and the cost-effectiveness of a pharmacist-led educational inhaler technique intervention on asthma and chronic obstructive pulmonary disease (COPD) patients (INspira)]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN10844309 (first received 3 May 2019).
ISRCTN62025354 {published data only}
- ISRCTN62025354. Minimal interventions to improve medication adherence in people with multiple long-term conditions (MINIMA) study. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN62025354 (first received 24 January 2017).
Kristeller 2017 {published data only}
- Kristeller J, Snyder F, Kong F, Musheno M. Collaboration between hospital and community pharmacists to improve medication management from hospital to home. Innovations in Pharmacy 2017;8(2):1-9. [Google Scholar]
- NCT02047448. Improving medication adherence through a transitional care pharmacy practice model. www.clinicaltrials.gov/ct2/show/NCT02047448 (first received 28 January 2014).
Maricoto 2019 {published data only}
- Maricoto T, Correla-de-Sousa, Taborda-Barata L. Inhaler technique education in elderly patients with asthma or COPD: impact on disease exacerbations-a protocol for a single-blinded randomised controlled trial. BMJ Open 2019;9:e022685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Navarre 2007 {published data only}
- Navarre M, Patel H, Johnson CE, Durance A, McMorris M, Bria W, et al. Influence of an interactive computer-based inhaler technique tutorial on patient knowledge and inhaler technique. Annals of Pharmacotherapy 2007;41:216-21. [DOI] [PubMed] [Google Scholar]
NCT04195191 {published data only}
- NCT04195191. Intervention to improve the adherence in community pharmacies [Effectiveness of an intervention to improve the adherence to treatment of new medicines from the community pharmacist]. clinicaltrials.gov/ct2/show/NCT04195191 (first received 11 December 2019).
Nimmo 1993 {published data only}
- Nimmo CJ, Chen DN, Martinusen SM, Ustad TL, Ostrow DN. Assessment of patient acceptance and inhalation technique of a pressurized aerosol inhaler and two breath-actuated devices. Annals of Pharmacotherapy 1993;27(7):922-7. [DOI] [PubMed] [Google Scholar]
NTR5187 {published data only}
- Kemerink A, Steenhuis LH, Klemmeier TJ, Vroegop JS, Schokker S. Inhalation technique education in asthma or COPD: the value of a visual instruction card. European Respiratory Journal 2016;48:PA5006. [DOI: 10.1183/13993003congress-2016.PA5006] [DOI] [Google Scholar]
O'Dwyer 2016 {published data only}
- NCT02203266. Teaching inhaler use with the INCA device in a community pharmacy setting [A randomised, parallel-group, multi-centre trial using a novel INCA tracker device to measure and monitor compliance and technique of Seretide Diskus inhaler in a community pharmacy setting]. www.clinicaltrials.gov/ct2/show/NCT02203266 (first received 29 July 2014).
- O'Dwyer S, Greene G, MacHale E, Cushen B, Sulaiman I, Boland F, et al. Personalised biofeedback on inhaler adherence and technique by community pharmacists: a cluster randomised clinical trial. Journal of Allergy and Clinical Immunology Practice 2020;8:635-44. [DOI] [PubMed] [Google Scholar]
Qin 2016 {published data only}
- Qin Q, Chen R, Lei W, Bian Y-C, Gu B-C, Bao J-A. Evaluation and analysis of inhaler drug device adherence in asthma and COPD patients. Chinese Pharmaceutical Journal 2016;51(5):413-6. [Google Scholar]
RBR‐5bw2wt {published data only}
- RBR-5bw2wt. The role of the pharmacist in the team care of hospitalised patients with chronic bronchitis or emphysema [Pharmaceutical care programme for inpatients with chronic obstructive pulmonary disease in a tertiary teaching hospital in southern Brazil – PHARBE: PHARmaceutical Care Programme for Inpatients with COPD in a Tertiary Teaching Hospital in Southern Brazil]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=RBR-5bw2wt (first received 1 May 2012).
SAM30001 GSK {published data only}
- SAM30001 GSK. Compliance in asthma and COPD. www.gsk-studyregister.com/en/trial-details/?id=SAM30001#documents-section (first received 1 May 1999).
Serra‐Batlles 2002 {published data only}
- Serra-Batlles J, Plaza V, Badiola C, Morejon E, Inhalation Devices Study Group, Morejon E, et al. Patient perception and acceptability of multidose dry powder inhalers: a randomized crossover comparison of Diskus/Accuhaler with Turbuhaler. Journal of Aerosol Medicine 2002;15(1):59-64. [DOI] [PubMed] [Google Scholar]
Suhaj 2016 {published data only}
- Abdulsalim S, Unnikrishnan K, Manu MK, Alrasheedy AA, Godman B, Morisky DE. Structured pharmacist-led intervention programme to improve medication adherence in COPD patients: a randomised controlled trial. Research in Social and Administrative Pharmacy 2018;14:909-14. [DOI] [PubMed] [Google Scholar]
- Suhaj A, Manu MK, Unnikrishnan MK, Vijayanarayana K, Mallikarjuna CR. Effectiveness of clinical pharmacist intervention on health-related quality of life in chronic obstructive pulmonary disorder patients – a randomized controlled study. Journal of Clinical Pharmacy and Therapeutics 2016;41(1):78-83. [DOI] [PubMed] [Google Scholar]
- Suhaj A, Unnikrishnan M, Mohan MK, Rao CM, Vijayanarayana K. The effectiveness of clinical pharmacist intervention on health related quality of life in chronic obstructive pulmonary disorder patients – a randomized controlled study. Respirology 2015;20:58. [DOI] [PubMed] [Google Scholar]
Xin 2016 {published data only}
- Xin C, Xia Z, Jiang C, Lin M, Li G. The impact of pharmacist-managed clinic on medication adherence and health-related quality of life in patients with COPD: a randomized controlled study. Patient Preference and Adherence 2016;10:1197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN10567920 {published data only}
- ISRCTN10567920. Maximising adherence and gaining new information for your COPD [A pragmatic, cluster randomized trial evaluating the impact of an enhanced adherence package (dual bronchodilator + add-on + app) on time to treatment failure and other clinical outcomes in exacerbating COPD patients with poor adherence to mono or dual therapy over one year]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN10567920 (first received 25 July 2019).
ISRCTN77785397 {published data only}
- ISRCTN77785397. Using the internet to help individuals stay healthy and prevent further reductions in health from existing chronic diseases [Maximizing the effects of self-management interventions on chronic disease outcomes: the development of a chronic obstructive pulmonary disease (COPD) web-based patient portal]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN10567920 (first received 8 November 2011).
Additional references
Agh 2015
- Ágh T, Dömötör P, Bártfai Z, Inotai A, Fujsz E, Mészáros Á. Relationship between medication adherence and health related quality of life in subjects with COPD: a systematic review. Respiratory Care 2015;60(2):297-303. [DOI] [PubMed] [Google Scholar]
Blackstock 2016
- Blackstock FC, ZuWallack R, Nici L, Lareau SC. Why don't our patients with chronic obstructive pulmonary disease listen to us? Annals of the American Thoracic Society 2016;13(3):317-23. [DOI] [PubMed] [Google Scholar]
Blakey 2018
- Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. European Respiratory Journal 2018;52:1801147. [DOI] [PMC free article] [PubMed] [Google Scholar]
BMJ 2012
- Healthcare professionals as bad as patients at good respiratory inhaler technique: editorial. www.sciencedaily.com/releases/2012/10/121003195134.htm (accessed prior to 19 May 2021).
Brandstetter 2017
- Brandstetter S, Finger T, Fischer W, Brandl M, Bohmer M, Pfeifer M, et al. Differences in medication adherence are associated with beliefs about medicines in asthma and COPD. Clinical and Translational Allergy 2017;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryant 2013
- Bryant J, McDonald VM, Boyes A, Sanson-Fisher R, Paul C, Melville J. Improving medication adherence in chronic obstructive pulmonary disease: a systematic review. Respiratory Research 2013;14:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2017
- Chen W, FitzGerald JM, Sin DD, Sadatsafavi M, Canadian Respiratory Research Network. Excess economic burden of co-morbidities in COPD: a 15 year population-based study. European Respiratory Journal 2017;50(1):1700393. [DOI: 10.1183/13993003.00393-2017] [DOI] [PubMed] [Google Scholar]
Cochrane Airways 2019
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 12 March 2019).
COPD Foundation 2018
- COPD Foundation. What is COPD? www.copdfoundation.org/What-is-COPD/Understanding-COPD/What-is-COPD.aspx (accessed 5 February 2019).
De Geest 2018
- De Geest S, Zullig LL, Dunbar-Jacob J, Helmy R, Hughes DA, Wilson IB, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Annals of Internal Medicine 2018;169(1):30-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duncan 2015
- Duncan D. Medication adherence in chronic obstructive pulmonary disease. Practice Nursing 2015;26(10):493-8. [Google Scholar]
Ejiofor 2013
- Ejiofor S, Turner AM. Pharmacotherapies for COPD. Clinical Medical Insights: Circulatory, Respiratory and Pulmonary Medicine 2013;7:17-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furmedge 2018
- Furmedge DS, Stevenson JM, Schiff R, Davies JG. Evidence and tips on the use of medication compliance aids. BMJ 2018;362:k2801. [DOI] [PubMed] [Google Scholar]
GBD 2015 Chronic Respiratory Disease Collaborators
- Global Burden of Disease 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease study 2015. Lancet Respiratory Medicine 2017;5:691-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2015 Incidence and Prevalence Collaborators
- Global Burden of Disease 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2021
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2021 global strategy for the diagnosis, management, and prevention of COPD. goldcopd.org/ (accessed 5 May 2021).
Gore 1981
- Gore SM. Assessing clinical trials – restricted randomisation. BMJ 1981;282:2114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 5 March 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Herath 2018
- Herath SC, Normansell R, Maisey S, Poole PX. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD009764. [DOI: 10.1002/14651858.CD009764.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler K, Cumpston M, Li T, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Second edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Humenberger 2018
- Humenberger M, Horner A, Labek A, Kaiser B, Frechinger R, Brock C, et al. Adherence to inhaled therapy and its impact on chronic obstructive pulmonary disease (COPD). BioMed Central Pulmonary Medicine 2018;18:163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones W. St. George’s Respiratory Questionnaire: MCID. Journal of Chronic Obstructive Pulmonary Disease 2005;2:75-9. [Google Scholar]
Jordan 2015
- Jordan RE, Majothi S, Heneghan NR, Blissett DB, Riley RD, Sitch AJ, et al. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technology Assessment 2015;19(36):1-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klijn 2017
- Klijn SL, Hiligsmann M, Evers SM, Román-Rodríguez M, Van der Molen T, Van Boven JFM. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. Primary Care Respiratory Medicine 2017;27:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocks 2006
- Kocks JW, Tuinenga MG, Uil SM, Van den Berg JW, Ståhl E, Van der Molen T. Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respiratory Research 2006;7:62-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorig 2001
- Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program for patients with chronic disease. Effective Clinical Practice 2001;4:256-62. [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. Global Evidence Summit; 2017 Sept 13-16; Cape Town, South Africa.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHLBI 1982
- National Heart, Lung and Blood Institute Working Group on Patient Compliance. Management of patient compliance in the treatment of hypertension. Hypertension 1982;4:415-53. [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management [CG101]. www.nice.org.uk/guidance/cg101 (accessed prior to 5 March 2019). [PubMed]
Nieuwlaat 2014
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD000011. [DOI: 10.1002/14651858.CD000011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Storr 2018
- Noel-Storr AH, Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 Jun 18-20; Oxford, UK. 2018.
Normansell 2017
- Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD012226. [DOI: 10.1002/14651858.CD012226.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poole 2019
- Poole P, Sathananthan K, Fortescue R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD001287. [DOI: 10.1002/14651858.CD001287.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qaseem 2011
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155:179-91. [DOI] [PubMed] [Google Scholar]
Quaderi 2018
- Quaderi SA, Hurst JR. The unmet global burden of COPD. Global Health, Epidemiology and Genomics 2018;3:e4. [DOI: 10.1017/gheg.2018.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Restrepo 2008
- Restrepo RD, Alvarez MT, Wittnebel LD, Sorenson H, Wettstein R, Vines DL, et al. Medication adherence issues in patients treated for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2008;3(3):371-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rootmensen 2010
- Rootmensen GN, Van Keimpema ARJ, Jansen HM, Haan RJ. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2010;23(5):323-8. [DOI] [PubMed] [Google Scholar]
Sabaté 2003
- Sabaté E. Adherence to long-term therapies: evidence for action. https://apps.who.int/iris/bitstream/handle/10665/42682/9241545992.pdf?sequence=1&isAllowed=y [accessed September 2021] 2003.
Sulaiman 2017
- Sulaiman I, Cushen B, Greene G, Seheult J, Seow D, Rawat F, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2017;195(10):1333-43. [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI] [PubMed] [Google Scholar]
Van Boven 2014
- Van Boven JF, Chavannes NH, Van der Molen T, Rutten-van Molken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respiratory Medicine 2014;108(1):103-13. [DOI] [PubMed] [Google Scholar]
Vestbo 2013
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2013;187(4):347-65. [DOI] [PubMed] [Google Scholar]
Vogelmeier 2017
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Borbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2017;195(5):557-82. [DOI] [PubMed] [Google Scholar]
Vrijens 2016
- Vrijens B, Dima AL, Van Ganse E, Van Boven JF, Eakin MN, Foster JM, et al. What we mean when we talk about adherence in respiratory medicine. Journal of Allergy and Clinical Immunology: In Practice 2016;4(5):802-12. [DOI] [PubMed] [Google Scholar]
Walters 2014
- Walters JA, Tan DJ, White CJ, Gibson PG, Wood‐Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD001288. [DOI: 10.1002/14651858.CD001288.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welling 2015
- Welling JB, Hartman JE, Ten Hacken NH, Klooster K, Slebos D-J. The minimal important difference for the St George’s Respiratory Questionnaire in patients with severe COPD. European Respiratory Journal 2015;46:1598-604. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Adherence to long-term therapies: evidence for action. apps.who.int/medicinedocs/pdf/s4883e/s4883e.pdf (accessed prior to 5 March 2019).
WHO 2017
- World Health Organization. Chronic obstructive pulmonary disease (COPD). www.who.int/en/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed prior to 29 March 2019).
Wijkstra 1994
- Wijkstra PJ, TenVergert EM, Van Altena R, Otten V, Postma DS, Kraan J, et al. Reliability and validity of the chronic respiratory questionnaire (CRQ). Thorax 1994;49:465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiśniewski 2014
- Wiśniewski D, Porzezińska M, Gruchała-Niedoszytko M, Niedoszytko M, Słomiński JM, Jassem E. Factors influencing adherence to treatment in COPD patients and its relationship with disease exacerbations. Pneumonologia i Alergologia Polska 2014;82:96-104. [DOI] [PubMed] [Google Scholar]
Zhong 2014
- Zhong H, Ni X-J, Cui M, Liu X-Y. Evaluation of pharmacist care for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. International Journal of Clinical Pharmacy 2014;36(6):1230-40. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Janjua 2019
- Janjua S, Pike KC, Carr R, Coles A, Fortescue R. Interventions to improve adherence to pharmacological therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD013381. [DOI: 10.1002/14651858.CD013381] [DOI] [PMC free article] [PubMed] [Google Scholar]


