Abstract
AML patients often undergo allogeneic hematopoietic cell transplantation (alloHCT) in first complete remission (CR). We examined effect of depth of clinical response, including incomplete count recovery (CRi) and/or measurable residual disease (MRD), in patients from the Center for International Blood and Marrow Transplantation Research (CIBMTR) registry. We identified 2492 adult patients (1799 CR and 693 CRi) who underwent alloHCT between January 1, 2007 and December 31, 2015. The primary outcome was overall survival (OS). Multivariable analysis was performed to adjust for patient-, disease-, and transplant-related factors. Baseline characteristics were similar. Patients in CRi compared to those in CR had an increased likelihood of death (HR 1.27; 95% confidence interval 1.13–1.43). Compared to CR, CRi was significantly associated with increased non-relapse mortality (NRM), shorter disease-free survival (DFS), and a trend toward increased relapse. Detectable MRD was associated with shorter OS, shorter DFS, higher NRM, and increased relapse compared to absence of MRD. The deleterious effects of CRi and MRD were independent. In this large CIBMTR cohort, survival outcomes differ among AML patients based on depth of CR and presence of MRD at the time of alloHCT. Further studies should focus on optimizing post-alloHCT outcomes for patients with responses less than CR.
Introduction
Adult acute myeloid leukemia (AML) patients with intermediate or high-risk features often undergo allogeneic hematopoietic cell transplantation (alloHCT) during first complete remission (CR1). Due to competing risks of relapse and non-relapse mortality, most patients with favorable genomic risk stratification do not benefit from alloHCT in CR1.(1, 2) What remains less clear is which other patients in morphologic CR derive benefit from the procedure. The 2017 European LeukemiaNet AML guidelines categorize morphologic CR (<5% marrow blasts) according to whether it is accompanied by blood count recovery (CR rather than CRi) or presence of measurable residual disease (MRD).(3)
CRi has been associated with an increased risk of relapse in AML patients receiving chemotherapy in several retrospective analyses,(4, 5) although one study observed pre-HCT blood counts did not affect post-HCT outcomes.(6) The presence of MRD, commonly assessed by multiparameter flow cytometry (MFC) and molecular methods, is generally accepted as leading to increased relapse risk and decreased likelihood of survival regardless of receipt of HCT.(4, 7–10) This information has led some physicians to recommend against HCT given MRD or responses less than CR. Nonetheless, alternatives to HCT are also unsatisfactory in such patients. The graft-versus-leukemia effect of HCT seems beneficial in patients in CR, with or without MRD.(11, 12)
In this analysis, we used data collected through the Center for International Blood and Marrow Transplantation Research (CIBMTR) registry to examine the relative roles of pre-HCT blood counts and presence or absence of MRD in determining post-HCT outcomes.
Methods
Data source
Study data were obtained from the CIBMTR registry, a voluntary network of over 450 blood and marrow transplant centers in the US and around the world. Participating centers contributed transplant-related information to the central data management and statistical centers at the Medical College of Wisconsin in Milwaukee and “Be the Match” in Minneapolis, Minnesota. As mandated for observational research conducted by CIBMTR, this study adhered to federal regulations for the protection of human research subjects. Protected health information was collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
Patients
Eligible cases were identified from 7 346 adults with AML who underwent first alloHCT from any donor source between January 1, 2007 and December 31, 2015. We excluded patients who at time of transplant were classified as primary induction failure (n=736), CR2 (n=1438), CR3 or beyond (n=124), relapse (n=758), or missing disease status (n=10), leaving a population of 4 280 patients in CR1. We also excluded patients with syngeneic twin donors (n=16), without appropriate data on comprehensive research forms (CRF; n=435), without consent for data analysis (n=45), or from embargoed centers (n=78). We further excluded patients with AML transformed from MDS (n=749) and those with missing or contradictory data (n=465), leaving a population of 2492.
We used standard CRFs for baseline characteristics including blood counts prior to alloHCT. Blood counts and MRD status were defined at the pre-alloHCT patient evaluation. We defined CR as absolute neutrophil count ≥ 1000/μl and platelet count ≥ 100 000/μl along with no peripheral blasts and <5% blasts on morphologic assessment of the bone marrow; patients also needed normal maturation of all cellular components in the bone marrow. CRi was defined as <5% blast percentage in the marrow as in CR, but peripheral blood neutrophils, platelets, or both remained below the above stated levels. MRD was assessed based on answers to qualitative CRF questions that ask if the patient is in molecular, cytogenetic, and/or MFC remission (see Supplemental Material for full operational definition).
Statistical analysis
The primary outcome was overall survival (OS), defined as time from HCT to death due to any cause, and secondary outcomes were non-relapse mortality (NRM), defined as time to death without evidence of relapse, relapse, defined as the reappearance of at least 5% blasts on morphological / cytogenetic / flow / molecular evaluation in bone marrow, blood, or an extramedullary site as per the reporting center, and disease-free survival (DFS), defined as time to relapse or death due to any cause. The Kaplan-Meier method was used to estimate survival and cumulative incidence function was used to estimate relapse and NRM. Multivariable analysis (MVA) was performed using the Cox proportional hazards model to adjust for patient-, disease-, and transplant-related factors. The covariates considered in the Cox models included age at transplant, Karnofsky performance score, transplant comorbidity index (HCT-CI), MRD at time of transplant, white blood count at diagnosis, cytogenetic risk group,(13) time to achieve first CR, de novo vs. therapy-related AML, number of cycles of induction and consolidation prior to transplant, conditioning intensity (using standard CIBMTR operational definitions), type of donor, and year of transplant. All clinically relevant patient-, disease-, and transplant-related variables were considered in the Cox model, and those that were significant to corresponding outcomes were kept in the final model. Adjusted probabilities of DFS and OS and adjusted cumulative incidence curves of NRM and relapse were generated from final regression models stratified on CR vs. CRi and weighted averages of covariate values using pooled sample proportion as weight function. Interactions between main effect (CR vs. CRi) and all covariates were tested at a significance level of p=0.01.
Results
Characteristics of study population
The study population included 2492 patients (CR, n=1799; CRi, n=693). Patients with CRi were more likely than those with CR to have a Karnofsky score <90 (39% vs. 33%) and an HCT-CI score of 3 or higher (47% vs. 40%), but other demographic variables were similarly distributed (Table 1; Supplementary Table 1). The time to achieve remission, type of pre-transplant therapy, and number of cycles of pre-HCT chemotherapy were similar. Most patients received 7+3 chemotherapy for induction (87.3%). However, positive MRD at the time of HCT was more common in the CRi group (18% vs. 12%, p<0.001), as was older age (p = 0.02). CR patients were more likely to undergo myeloablative conditioning (62% vs. 53% in the CRi group, p<0.001).
Table 1:
Baseline characteristics, n (%) unless otherwise specified.
| Characteristic | CR | CRi | P Value | Total |
|---|---|---|---|---|
|
| ||||
| No. of patients | 1799 | 693 | 2492 | |
| Age at HCT - median (min-max) | 52.1 (18–81.1) | 54.3 (18.1–77.7) | 52.8 (18–81.1) | |
| Male sex | 896 (49.8) | 364 (52.5) | 0.22a | 1260 (50.6) |
| KPS ≥ 90 | 1212 (67.4) | 422 (60.9) | 0.007a | 1634 (65.6) |
| HCT-CI ≥ 3 | 724 (40.2) | 323 (46.6) | 0.004a | 1047 (42) |
| WBC at diagnosis ≥ 10×109/L | 772 (42.9) | 285 (41.1) | 0.42a | 1057 (42.4) |
| Therapy-linked AML | 175 (9.7) | 77 (11.1) | 0.30a | 252 (10.1) |
| 7+3 for induction | 1589 (88.3) | 587 (84.7) | 0.01a | 2176 (87.3) |
| Total cycles of pre-HCT chemotherapy | ||||
| Median | 3 | 2 | 0.94b | 2 |
| 25th-75th pctl | 2–3 | 2–4 | 2–3 | |
| Time to achieve CR1 (weeks) | ||||
| Median | 6 | 6 | 0.02b | 6 |
| 25th-75th pctl | 4–9 | 4–10 | 4–9 | |
| Time from CR1 to HCT (months) | ||||
| Median | 3 | 3 | 0.19b | 3 |
| 25th-75th pctl | 2–4 | 2–5 | 2–4 | |
| Cytogenetic score | 0.62a | |||
| Favorable | 62 (3.4) | 22 (3.2) | 84 (3.4) | |
| Intermediate | 1135 (63.1) | 421 (60.8) | 1556 (62.4) | |
| Poor | 538 (29.9) | 226 (32.6) | 764 (30.7) | |
| Missing | 64 (3.6) | 24 (3.5) | 88 (3.5) | |
| Positive MRD at time of HCT | 214 (11.9) | 126 (18.2) | < 0.001a | 340 (13.6) |
| Conditioning intensity | < 0.001a | |||
| MAC w/ TBI | 416 (23.1) | 130 (18.8) | 546 (21.9) | |
| MAC w/o TBI | 707 (39.3) | 240 (34.6) | 947 (38) | |
| RIC/NMA | 643 (35.7) | 303 (43.7) | 946 (38) | |
| Missing | 33 (1.8) | 20 (2.9) | 53 (2.1) | |
| Type of donor | < 0.001a | |||
| HLA-identical sibling | 558 (31) | 156 (22.5) | 714 (28.7) | |
| Other related | 116 (6.4) | 58 (8.4) | 174 (7) | |
| Well-matched URD | 691 (38.4) | 290 (41.8) | 981 (39.4) | |
| Other URD | 151 (8.4) | 65 (9.4) | 216 (8.7) | |
| UCB | 283 (15.7) | 124 (17.9) | 407 (16.3) | |
| Type of post-HCT planned therapy | 0.04a | |||
| No therapy | 1551 (86.2) | 580 (83.7) | 2131 (85.5) | |
| HMA (±other) | 138 (7.7) | 75 (10.8) | 213 (8.5) | |
| Other therapy | 110 (6.1) | 38 (5.5) | 148 (5.9) | |
| Follow-up (months) - median (min-max) | 60.72 (4.44–125.76) | 50.1 (11.94–122.53) | 60.26 (4.44–125.76) | |
Hypothesis testing:
Pearson chi-square test
Kruskal-Wallis test
Abbreviations: complete remission (CR); complete remission with incomplete count recovery (CRi); hematopoietic cell transplantation (HCT); Karnofsky performance score (KPS); hematopoietic cell transplantation-comorbidity index (HCT-CI); white blood cell (WBC); acute myeloid leukemia (AML); first complete remission (CR1); measurable residual disease (MRD); human leukocyte antigen (HLA); unrelated donor (URD); umbilical cord blood (UCB); hypomethylating agent (HMA); myeloablative conditioning (MAC); total body irradiation (TBI); reduced-intensity conditioning (RIC); nonmyeloablative (NMA)
Effect of incomplete count recovery on outcomes
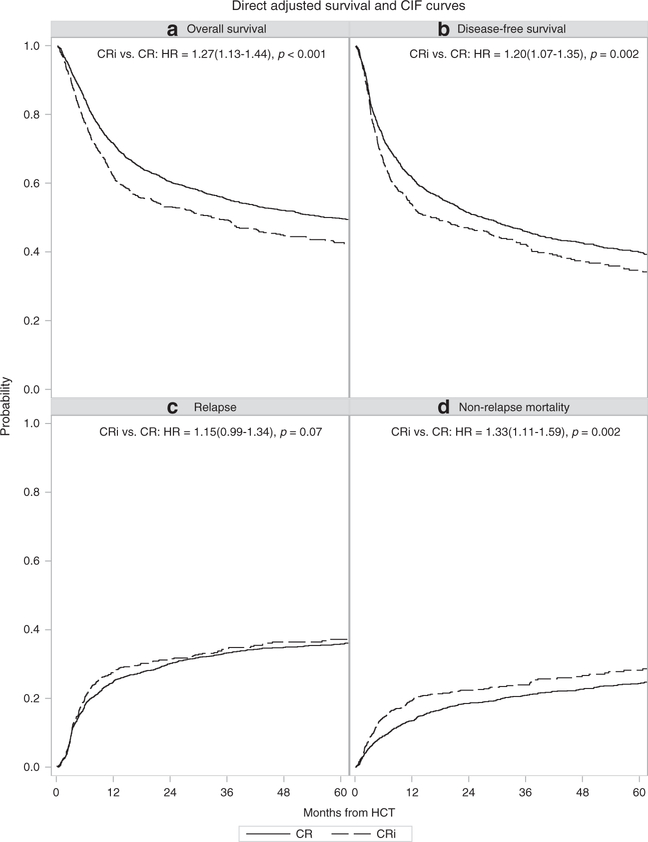
Multivariate analysis found CRi was associated with a statistically significant increased risk of death with HR 1.27 (95%CI 1.13–1.44) even after accounting for other associated covariates such as older age, non-favorable cytogenetics, lower Karnofsky score, higher HCT-CI, MRD, and higher WBC count at diagnosis (Table 2; Figure 1). Conditioning intensity was not independently associated with survival, suggesting increasing conditioning intensity may not be sufficient to abrogate the deleterious effects of CRi. Donor type did not differentially affect survival in the CR or CRi groups.
Table 2:
Multivariable analysis results. Other significant co-variates were considered in the final Cox model but estimates are not presented here.
| Outcomes | HR (95% CI) | p-value |
|---|---|---|
| Overall Survival | ||
| CRi vs. CR | 1.27 (1.13–1.44) | < 0.001 |
| MRD + vs. MRD − | 1.52 (1.31–1.77) | < 0.001 |
| Disease-Free Survival | ||
| CRi vs. CR | 1.20 (1.07–1.35) | 0.002 |
| MRD + vs. MRD − | 1.64 (1.42–1.89) | < 0.001 |
| Relapse | ||
| CRi vs. CR | 1.15 (0.99–1.34) | 0.07 |
| MRD + vs. MRD− | 1.78 (1.48–2.12) | < 0.001 |
| Non-Relapse Mortality | ||
| CRi vs. CR | 1.33 (1.11–1.59) | 0.002 |
| MRD + vs. MRD − | 1.34 (1.06–1.69) | 0.01 |
Abbreviations: hazard ratio (HR); confidence interval (CI); complete remission with incomplete count recovery (CRi); complete remission (CR); measurable residual disease (MRD)
Figure 1.
Adjusted survival curves for patients in CR vs. CRi prior to alloHCT.
A longer time to achieve CR1 was associated with shorter survival. In the 622 patients who did not achieve CR1 within 8 weeks, the HR for death was 1.32 (95%CI 1.11–1.57) compared to those who achieved CR1 in ≤4 weeks, suggesting that slower recovery after chemotherapy may identify patients with less responsive disease as has been shown previously.(14) Presence of MRD at time of HCT was also independently associated with a higher HR for death of 1.52 (95%CI 1.31–1.77) compared to absence of MRD (independent of CR vs. CRi status). The adjusted OS probabilities at five years post-HCT, after accounting for factors from the MVA model, are 50% (95%CI 47–52) for patients with CR and 43% (95%CI 39–47) for patients with CRi. The deleterious effect of CRi was also seen with DFS, relapse, and NRM (Table 2; Figure 1; Supplementary Tables 2a, 2b, 3, and 4). Though data regarding peri-transplant infections are limited, it is notable that 12% of CRi patients had an infection requiring continuation of antimicrobial treatment after transplant day 0, compared to 6.7% of CR patients (p<0.001). Although patients who received reduced-intensity or nonmyeloablative conditioning (RIC/NMA) had less NRM in the MVA [HR of 0.73 (95%CI 0.56–0.94)], they also had a statistically significantly higher risk of relapse than those who underwent MAC with TBI [HR 1.69, 95%CI (1.39–2.05)].
Effect of MRD on outcomes, and interaction with CRi
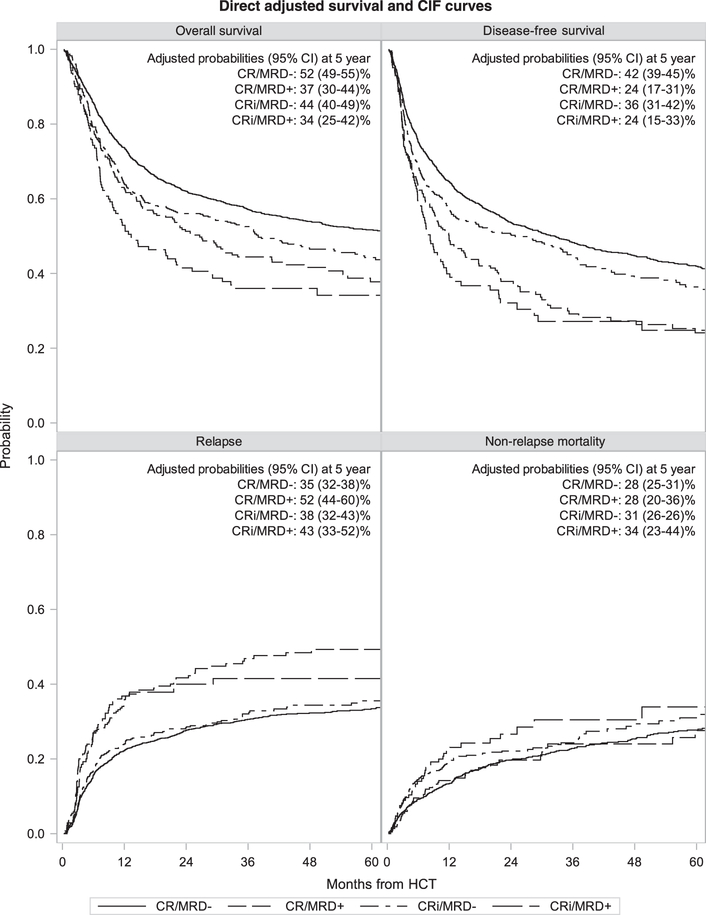
MRD status was available in 2267 (91%) patients who were classified as: CR/MRD- (n=1450), CR/MRD+ (n=214), CRi/MRD- (n=477), and CRi/MRD+ (n=126). As expected, presence of MRD was associated with shorter OS, shorter DFS, higher NRM, and increased relapse compared to absence of MRD (Table 3; Figure 2). Notably, the effect of MRD was similar in those in CR and those in CRi and the unfavorable effect of CRi was the same regardless of MRD status. Pairwise interactions between the main effects (CR vs. CRi) and MRD status were not significant at a level of p <0.01, demonstrating independently significant negative effects of CRi and presence of MRD. Older age was associated with a greater likelihood of positive MRD (p-value <0.001 using Pearson chi-square test).
Table 3.
Pairwise comparisons of remission and MRD status. Other significant co-variates were considered in the final Cox model but estimates are not presented here.
| Contrast | HR (95% CI) | p-value | |
|---|---|---|---|
| OS | CR/MRD+ vs. CR/MRD− | 1.51 (1.25–1.83) | < 0.001 |
| CRi/MRD+ vs. CRi/MRD− | 1.51 (1.18–1.94) | 0.001 | |
| DFS | CR/MRD+ vs. CR/MRD− | 1.66 (1.39–1.98) | < 0.001 |
| CRi/MRD+ vs. CRi/MRD− | 1.62 (1.27–2.05) | < 0.001 | |
| Relapse | CR/MRD+ vs. CR/MRD− | 1.86 (1.50–2.32) | < 0.001 |
| CRi/MRD+ vs. CRi/MRD− | 1.62 (1.19–2.23) | 0.003 | |
| NRM | CR/MRD+ vs. CR/MRD− | 1.25 (0.92–1.69) | 0.15 |
| CRi/MRD+ vs. CRi/MRD− | 1.48 (1.03–2.13) | 0.03 |
Abbreviations: hazard ratio (HR); confidence interval (CI); complete remission with incomplete count recovery (CRi); complete remission (CR); measurable residual disease (MRD); overall survival (OS); disease-free survival (DFS); non-relapse mortality (NRM)
Figure 2.
Adjusted survival curves for patients in CR vs. CRi, MRD+ vs. MRD- prior to alloHCT
Discussion
Analysis of this large CIBMTR cohort with 2492 patients demonstrates that survival outcomes differ significantly among AML patients in morphologic CR at the time of alloHCT. In our analysis, patients with CRi or MRD prior to HCT had worse outcomes than those with CR or without MRD, respectively; the negative effects on survival of incomplete count recovery and presence of MRD were independent. Most clinical trial reporting combines the endpoints of CR and CRi, but our analysis suggests that morphologic CR with fewer than 5% marrow blasts is an inadequate assessment of disease status and that both count recovery and MRD status also need to be considered. DFS was significantly lower in the CRi patients, who also showed a trend toward increased risk of relapse. Data from pediatric AML patients indicate that MRD detected by flow cytometry is more important than morphologic assessments in determining outcomes,(15) and we may reach a point when sensitive methodology such as flow cytometry or molecular analyses take precedence over morphologic evaluation in adult AML patients as well.(16)
In our cohort, CRi was not only associated with lower DFS; patients with CRi also had an increased rate of NRM, meaning that the negative effects of responses less than CR were not solely a sign of persistent disease that was more likely to relapse. The reasons for poorer survival outcomes in CRi patients are not fully elucidated by our dataset. Rate of infection may be higher in patients with incomplete count recovery prior to HCT, since many of those patients would have had a prolonged duration of neutropenia prior to HCT; in fact, CRi patients were significantly more likely to have an infection requiring antimicrobial therapy past transplant day 0 than CR patients. However, infection may not fully explain the increased NRM rate in CRi patients. One study of 459 patients who underwent non-myeloablative HCT indicated that depth of neutrophil nadir in the first 21 days after transplant was associated with higher NRM because of higher rates of GVHD.(17) In our study, due to incomplete information, we could not adequately evaluate the effect of any post-HCT interventions such as maintenance chemotherapy, which are increasingly being employed in high-risk patients.
Our retrospective study used a definition of incomplete count recovery prior to alloHCT as that was directly available in our dataset. Importantly, we found that CRi on the pre-HCT assessment had significant survival implications and thus may be generalizable to the broad population of AML HCT recipients. The completeness of available MRD data was variable though missing data was generally <10%. Additionally, CIBMTR CRFs collect only qualitative data and rely on the transplant centers’ testing methodology without centralized confirmation, so it is possible that MRD status would be interpreted and reported in different ways at different centers. Definitions and standards in MRD terminology remain a moving target in the AML field, though most experts would agree that flow cytometric and molecular assessments (at least of NPM1 and the core-binding factor fusion proteins) are well-validated.(18, 19) We used a binary operational definition of MRD with any detectable disease identified as “positive” (further described in the Supplemental Information); ideally, we would have had more granular information about methodology and cutoffs for positivity at the level of reporting centers.(19) High rates of false positive and false negative results with MRD testing should be taken into account when making decisions about referral to alloHCT.(20) Even given these limitations, the assessment of CR and MRD status utilized in our study (those performed immediately pre-transplant) may be useful in decision-making as well as guidance about treatment options and prognosis at the time of alloHCT.
Overall, AML patients with CRi or those with presence of MRD at the time of the pre-transplant evaluation have inferior outcomes after alloHCT compared to those in CR or those without MRD. Prognostic counseling should be offered to patients so that they are aware of the increased risks of both NRM and relapse following HCT though with limited other treatment options, alloHCT often remains the best available choice. In the future, inclusion of CRi and MRD status could strengthen prognostic models that evaluate the effect of disease-specific characteristics including cytogenetic risk and CR status on post-HCT outcomes, such as the disease risk index (DRI) or the HCT-composite risk (HCT-CR).(21, 22)
Strategies to eliminate MRD prior to alloHCT seem appealing, but are unproven since it is unknown whether additional therapy to eliminate MRD can lead to improvement in post-HCT outcomes. Additionally, no drugs in AML have shown the promise of blinatumomab, which is approved for MRD-level disease in acute lymphoblastic leukemia. Further, efficacy of MRD eradication pre-alloHCT is unknown; that is, presence of MRD following chemotherapy may denote more resistant AML, an unfavorable bone marrow microenvironment, or other unknown factor, any of which may predispose to worse post-transplant outcomes even if MRD is temporarily eradicated.
Our data suggest that MAC with TBI is associated with a decreased risk of relapse compared to NMA conditioning for patients with MRD prior to alloHCT, which is consistent with findings from previous retrospective analyses.(23–25) However, conditioning intensity did not seem to affect outcomes for patients with incomplete count recovery prior to alloHCT. Additionally, CR and CRi prior to HCT appear to define prognostic groups with significantly different outcomes, and these patients should be analyzed separately in future analyses. Further prospective studies should focus on limiting NRM and reducing relapse to optimize post-alloHCT outcomes for AML patients with CRi or MRD.
Supplementary Material
ACKNOWLEDGEMENTS
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Gilead; GlaxoSmithKline; Incyte Corporation; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karyopharm Therapeutics; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Medac GmbH; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncopeptides, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Tscan; Vertex; Vor Biopharma; Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Data Sharing:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Conflicts of Interest:
Dr. Rybka: Merck, Seattle Genetics, Spark Therapeutics; Dr. Luger: Biosight, Celgene, Hoffman La Roche, Kura, Onconova, Acceleron, Agios, Daichii-Sankyo, Bristol Myers Squibb, Jazz; Dr. Kansagra: Takeda, Jansen, Pfizer, Karyopharm, Celgene/BMS, Sanofi; Dr. Nishihori: Novartis, Karyopharm; Dr. Bhatt: Agios, Incyte, Takeda, Partner Therapeutics, Omeros, Abbvie, Jazz, Tolero Pharmaceuticals, National Marrow Donor Program, Oncoceutics, Partnership for health analytic research, LLC, Pfizer, CSL Behring;Dr. Strair: Janssen; Dr. Ganguly: Seattle Genetics, Kadmon; Dr. Rizzieri: Amgen, Kite, AROG, Pharmacyclics, Seattle Genetics, Pfizer, Novartis, Sanofi-Aventis, Incyte, Gilead, Jazz, Abbvie, Celltron/Teva, Mustang, Bayer, Stemline, Celegene; Dr. Grunwald: Incyte, Amgen, Alexion, ARIAD, Abbvie, Astellas, BMS/Celegene, Merck, Pfizer, Premier, Trovagene, Daiichi Sankyo, Cardinal Health, Novartis, Janssen, Genentech/Roche, Forma Therapeutics; Dr. Kharfan-Dabaja: Daiichi Sankyo; Dr. Olsson: AstraZeneca; Dr. Liu: BMS, Karyopharm, Agios
References
- 1.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum FR. Indications for allogeneic hematopoietic cell transplantation for acute myeloid leukemia in the genomic era. Am Soc Clin Oncol Educ Book. 2014:e327–33. [DOI] [PubMed] [Google Scholar]
- 3.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Xie H, Wood BL, Walter RB, Pagel JM, Becker PS, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol. 2015;33(11):1258–64. [DOI] [PubMed] [Google Scholar]
- 5.Walter RB, Kantarjian HM, Huang X, Pierce SA, Sun Z, Gundacker HM, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol. 2010;28(10):1766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vu K, Manjappa S, DiPersio JF, Gao F, Westervelt P, Vij R, et al. Hematologic Recovery after Pretransplant Chemotherapy Does Not Influence Survival after Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia Patients. Biol Blood Marrow Transplant. 2015;21(8):1425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? J Clin Oncol. 2016;34(4):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Othus M, Wood BL, Stirewalt DL, Estey EH, Petersdorf SH, Appelbaum FR, et al. Effect of measurable (‘minimal’) residual disease (MRD) information on prediction of relapse and survival in adult acute myeloid leukemia. Leukemia. 2016;30(10):2080–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Versluis J, Cornelissen JJ. Risks and benefits in a personalized application of allogeneic transplantation in patients with AML in first CR. Semin Hematol. 2019;56(2):164–70. [DOI] [PubMed] [Google Scholar]
- 12.Versluis J, Kalin B, Zeijlemaker W, Passweg J, Graux C, Manz MG, et al. Graft-Versus-Leukemia Effect of Allogeneic Stem-Cell Transplantation and Minimal Residual Disease in Patients With Acute Myeloid Leukemia in First Complete Remission. JCO Precision Oncology. 2017(1):1–13. [DOI] [PubMed] [Google Scholar]
- 13.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–83. [PubMed] [Google Scholar]
- 14.Estey EH, Shen Y, Thall PF. Effect of time to complete remission on subsequent survival and disease-free survival time in AML, RAEB-t, and RAEB. Blood. 2000;95(1):72–7. [PubMed] [Google Scholar]
- 15.Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY, et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. 2012;30(29):3625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Wood BL, Walter RB, Becker PS, Percival ME, Bar M, et al. Is there a need for morphologic exam to detect relapse in AML if multi-parameter flow cytometry is employed? Leukemia. 2017;31(11):2536–7. [DOI] [PubMed] [Google Scholar]
- 17.Storb R, Gyurkocza B, Storer BE, Maloney DG, Sorror ML, Mielcarek M, et al. Allogeneic hematopoietic cell transplantation following minimal intensity conditioning: predicting acute graft-versus-host disease and graft-versus-tumor effects. Biol Blood Marrow Transplant. 2013;19(5):792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N Engl J Med. 2018;378(13):1189–99. [DOI] [PubMed] [Google Scholar]
- 19.Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Othus M, Gale RP, Hourigan CS, Walter RB. Statistics and measurable residual disease (MRD) testing: uses and abuses in hematopoietic cell transplantation. Bone Marrow Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kongtim P, Parmar S, Milton DR, Perez JMR, Rondon G, Chen J, et al. Impact of a novel prognostic model, hematopoietic cell transplant-composite risk (HCT-CR), on allogeneic transplant outcomes in patients with acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2019;54(6):839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ustun C, Courville EL, DeFor T, Dolan M, Randall N, Yohe S, et al. Myeloablative, but not Reduced-Intensity, Conditioning Overcomes the Negative Effect of Flow-Cytometric Evidence of Leukemia in Acute Myeloid Leukemia. Biol Blood Marrow Transplant. 2016;22(4):669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilleece MH, Labopin M, Yakoub-Agha I, Volin L, Socie G, Ljungman P, et al. Measurable residual disease, conditioning regimen intensity, and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: A registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood and Marrow Transplantation. Am J Hematol. 2018;93(9):1142–52. [DOI] [PubMed] [Google Scholar]
- 25.Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia With Genomic Evidence of Residual Disease. J Clin Oncol. 2020;38(12):1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.