Abstract
Background:
Clinical trials involving individuals with mild cognitive impairment (MCI) have reported mixed results for the effects of cholinesterase inhibitors on cognitive outcomes. Our previous work demonstrated that a visuospatial problem-solving task was sensitive to non-memory impairments in individuals with MCI.
Objective:
To determine whether the same task is also sensitive to the effects of cholinesterase inhibitors in individuals with amnestic MCI (aMCI).
Method:
We gave 22 individuals with aMCI (clinical dementia rating of 0.5) and Mini-Mental State Examination (MMSE) scores of at least 24 the following measures at baseline and at follow-up 1 year later: Hopkins Verbal Learning Test, Boston Naming Test, Rey Complex Figures Test copying task, anagrams task, and visuospatial problem-solving task. The MMSE was also given at the 1-year follow-up. Twelve of the individuals were drug naïve, having never taken cholinesterase inhibitors before, and donepezil was initiated and titrated to 10 mg daily after baseline in an open-label manner. Ten of the individuals had already been taking donepezil, and there was no change in treatment. We compared the two groups for amount of performance change over 1 year.
Results:
Individuals for whom donepezil was initiated performed significantly better on the visuospatial problem-solving task after 1 year compared with individuals who had already been taking donepezil. No difference was observed for any of the other variables.
Conclusion:
The visuospatial problem-solving task appeared to be more sensitive than memory measures to the effects of cholinesterase inhibitors in individuals with aMCI, perhaps due to the high attentional demand of the task.
Keywords: mild cognitive impairment, problem-solving, donepezil, visuospatial, memory, language, executive function
Mild cognitive impairment (MCI) is characterized by impaired memory and preserved activities of daily living (Petersen et al, 1999, 2001). Individuals with this diagnosis are at significantly increased risk for developing dementia (Bennett et al, 2002; Morris et al, 2001; Storandt et al, 2002). In most individuals, amnestic MCI (aMCI) represents a milder end point on the spectrum of Alzheimer disease (AD) before the development of dementia (Morris et al, 2001), where memory impairment is the most prominent cognitive feature. With increasing advances in treatment options for individuals with AD, much attention has been directed at intervention at earlier stages of the disease.
Some studies have suggested that treatment with cholinesterase inhibitors may have a beneficial effect on individuals with MCI (Petersen et al, 2005), but not all studies have revealed such an effect (Feldman et al, 2007). In fact, the most recent practice guidelines from the American Academy of Neurology recommended that clinicians choose to not offer cholinesterase inhibitors to individuals with MCI, or, if they do offer them, to discuss with the individuals that cholinesterase inhibitors are an off-label prescription that is not currently backed by empirical evidence (Petersen et al, 2018).
In order to better define the effects of cholinesterase inhibitors on individuals with aMCI, it is important to first identify cognitive markers that are particularly sensitive to the effects of these drugs. Some studies that focused primarily on the effects of cholinesterase inhibitors on cognition and global assessment outcomes in individuals with MCI have revealed benefits on global assessment, psychomotor speed, and attention, but not on specific memory tasks (Salloway et al, 2004). However, most studies investigating the effects of cholinesterase inhibitors have found improvement on memory measures (Salloway et al, 2008). It is important to determine if there are non-memory measures that might be sensitive to the effects of cholinesterase inhibitors in individuals with aMCI.
Research examining the role of acetylcholine in animal behavior revealed a primary effect on attention (Sarter et al, 2005). Tasks that demand an individual’s attention may therefore be the most sensitive for detecting the effects of cholinesterase inhibitors on individuals with MCI. In our previous research (Beversdorf et al, 2007), we demonstrated that a visuospatial problem-solving (VPS) task that is sensitive to both frontal and posterior cortical damage of the brain (Miller and Tippett, 1996) and requires spatial, cognitive flexibility/executive function, and sustained attention (Beversdorf et al, 2007), was significantly more sensitive at detecting non-memory impairments in individuals with MCI than measures that are directed more toward language or more limited aspects of visuospatial function such as figure copying and recall.
Compensatory mechanisms, such as executive function, can often help preserve an individual’s functional ability when specific cognitive domains, such as memory, are mildly impaired. However, individuals with AD may have deficits in so many domains that they may lose their ability to compensate. Some researchers have suggested that measures that assess cognitive flexibility may be particularly sensitive for detecting early cognitive impairments (Albert, 1996). Executive function effects, which are not always evaluated in the assessment of MCI outside of specialty care centers, may also play an important role in compromising function in the daily life of individuals with MCI (Guarino et al, 2020).
Cognitive flexibility is considered an integrated function of the frontal lobes (Duncan et al, 1995; Eslinger and Grattan, 1993; Karnath and Wallesch, 1992; Vilkki, 1992), and frontal lobe-related findings are common in most forms of dementia, including AD (Albert, 1996; Beversdorf and Heilman, 1998). Albert (1996) proposed that these frontal lobe findings may be more apparent when the posterior cortical regions are impaired in addition to the anterior cortical regions due to disordered interactions between the frontal and parietal lobes, thus further diminishing an individual’s ability to functionally compensate for any one particular impairment.
In order to assess multiple cognitive domains within a task, we used a problem-solving task that requires participants to rearrange a set of matchsticks to form a new shape. This visuospatial task involves a multi-step command and a high degree of sustained attention, which includes cognitive flexibility. It was previously shown to be sensitive to non-memory impairments in individuals with MCI (Beversdorf et al, 2007), and it is also used within the Self-administered Gerocognitive Examination (Scharre et al, 2010). The task has identified impairments in individuals with frontal lobe lesions due to impaired strategy-shifting (cognitive flexibility) as well as in individuals with parietal lobe lesions due to difficulty manipulating visual material (Miller and Tippett, 1996). Thus, it would seem optimal for examining sustained attention and the interaction of frontal and posterior cortical regions in individuals with aMCI. We hypothesized that the VPS task would be sensitive to the effects of cholinesterase inhibitors on individuals with aMCI.
METHOD
Participants
Consecutive individuals from a convenience sample of individuals who had been referred to one of the authors (D.Q.B.) at the Ohio State University Memory Disorders Clinic for a chief complaint of memory loss were monitored over 1 year. Inclusion criteria were a family member report of no impairment of activities of daily living; a clinical dementia rating (Berg, 1988; Morris, 1993) of 0.5; a Mini-Mental State Examination (MMSE; Folstein et al, 1975) score of ≥24; and confirmation of a significant memory impairment that met the Peterson criteria for MCI (Petersen et al, 1999, 2001).
The 22 participants were divided into two groups based on whether or not they were taking a cholinesterase inhibitor. Donepezil is one of three cholinesterase inhibitors that is approved for use in AD, along with rivastigmine and galantamine. Because all of the individuals in our clinic who had already been taking cholinesterase inhibitors were on donepezil, this drug was the focus of study monitoring the convenience sample. Twelve of the individuals were drug naïve at the time of the initial evaluation, and 10 had already been taking donepezil 10 mg for at least 6 months. Table 1 shows the participant demographics for both groups.
TABLE 1.
Participant Demographics
| Characteristic | Rx Naïve MCI | Already Rx MCI | P |
|---|---|---|---|
| n | 12 | 10 | |
| Gender | 7 male, 5 female | 6 male, 4 female | 0.67 |
| Age (years) | 68.2 (8.0) | 66.4 (9.8) | 0.65 |
| Education level (years) | 14.5 (3.1) | 14.8 (2.7) | 0.81 |
Values are presented as M ± SD unless otherwise noted. Gender was compared using the Fisher exact test, and age and education level were compared using the Welch test.
MCI = mild cognitive impairment. Rx = treatment.
The study protocol was approved by the institutional review board of The Ohio State University and was performed according to the ethical guidelines of the Declaration of Helsinki and its later amendments. All individuals provided informed written consent before enrolling in the study.
Assessments
At the first visit, and again at the 1-year follow-up, we gave all of the individuals the MMSE to assess their global function across several domains, including memory. Additionally, the Hopkins Verbal Learning Test (Brandt, 1991) was administered to assess their verbal memory. We also gave them the following non-memory measures: the Boston Naming Test, Second Edition (Kaplan et al, 1983) to assess language, specifically naming; the Rey Complex Figure Test copy task (CFT; Corwin and Bylsma, 1993; Osterrieth, 1944; Rey, 1941) to assess visuospatial ability; a series of anagrams to assess verbal and divergent cognitive flexibility (Beversdorf et al, 1999) (Rearrange these letters to form an English word: OGRF, RDWO, FALC, LANI, MHBTU, HTRSI, DSLEI, TMLAE); and the VPS task, which was adapted from the “matchstick” problems task (Guilford, 1967; Miller and Tippett, 1996) (Figure 1), to assess visuospatial divergent cognitive flexibility.
FIGURE 1.
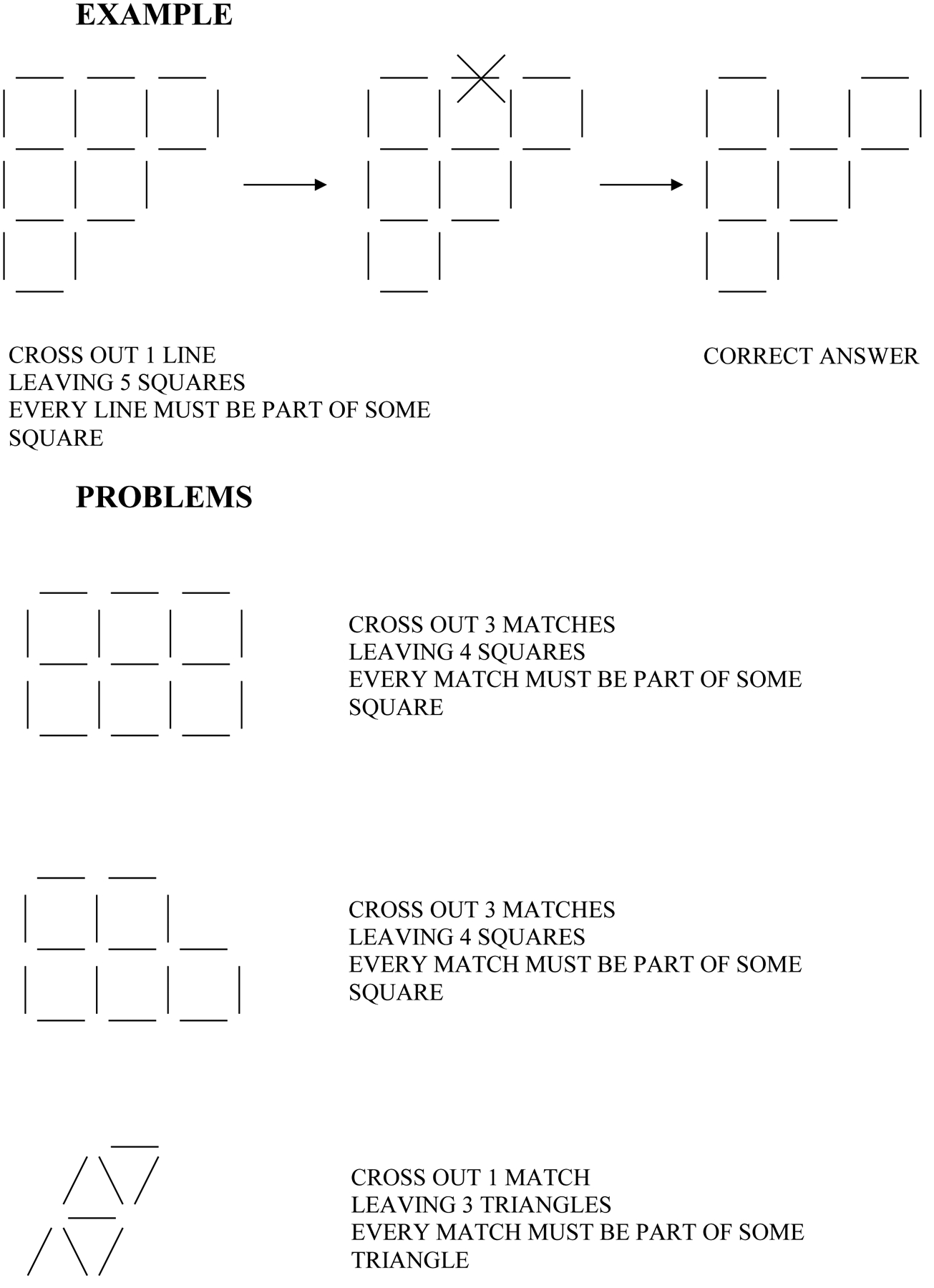
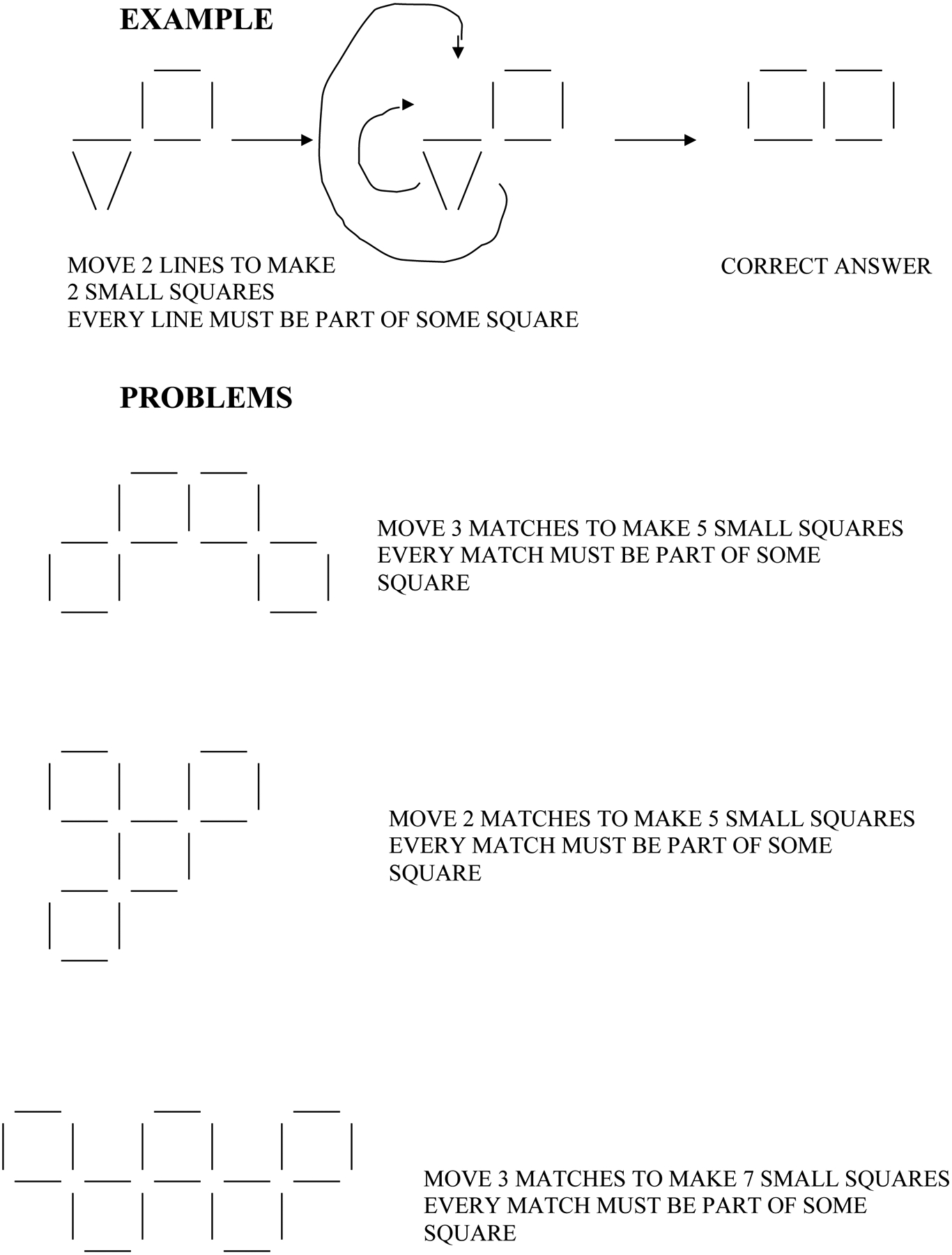
Visuospatial problem-solving task example and sample problems.
We gave the individuals sample problems for the anagrams and VPS tasks before testing and allowed them to see examples of solutions for these samples until they understood what they needed to do. As with our previous work with cognitive flexibility problem-solving tasks (Beversdorf et al, 1999, 2007), solution latencies were recorded as the measure of performance for both the series of anagrams tasks (eight anagrams) and the series of VPS tasks (six VPS tasks), and a maximum time of 2 minutes was allowed for each anagram and 4 minutes for each VPS problem. For purposes of analysis of solution latencies, failed anagrams were recorded as 2 minutes, and failed VPS problems were recorded as 4 minutes.
All of the measures were administered within 1-hour testing sessions, with breaks offered as needed, in the order presented in the description of the assessments. For those individuals who were accompanied by their caregiver, functional ability was also assessed with the Instrumental Activities of Daily Living scale (IADL; Lawton and Brody, 1969).
For the 10 individuals who had already been taking donepezil, the drug was continued. For the 12 individuals who were drug naïve, donepezil was initiated and titrated to 10 mg daily in an open-label manner.
Statistical Analysis
We compared the baseline variables using the robust t test (Welch test) or Fisher exact test for proportions and the Wilcoxon test for the amount of percentage change. The effect of the baseline CFT on its 1-year change was examined using an analysis of covariance. The significance level was set at 0.05. Single predictor logistic regression models were examined to classify the two groups. Using the resulting significant predictors, a two-predictor model was developed. The χ2 test was used to evaluate the adequacy of these logistic models.
RESULTS
The results of the two groups’ assessments at baseline, as well as the percentage change from baseline to the 1-year follow-up, are shown in Table 2. Performance between the two groups did not differ significantly at baseline except that the CFT score was significantly lower for the drug naïve group compared with the already treated group. In order to determine whether there was a significant difference in percentage change in performance on the CFT score between the two groups, we used an analysis of covariance, with baseline CFT score as a covariate. Neither baseline score nor group was a significant predictor of the relative change in the CFT score. The magnitude of the percentage change in performance of the newly treated drug naïve group compared with the percentage change in performance of the already treated group was significant only for the VPS task. This finding supported our a priori hypothesis that the VPS task is sensitive to the effects of cholinesterase inhibitors on individuals with aMCI.
TABLE 2.
Comparison of Baseline and Percentage Change Over 1 Year Between the Drug Naïve and the Already Treated Groups on Each Measure
| Measure | Rx Naïve MCI Baseline | Already Rx MCI Baseline | P | Rx Naïve MCI % change | Already Rx MCI % change | P |
|---|---|---|---|---|---|---|
| MMSE | 25.6 (1.6) | 26.3 (2.0) | 0.37 | 6.6% (14.2) | 8.4% (5) | 0.44 |
| HVLT (total items) | 14.4 (4.7) | 15.1 (6.2) | 0.78 | 3.7% (34.5) | −9.3% (39.6) | 0.72 |
| Rey CFT | 15.0 (5.2) | 21.2 (6.7) | 0.029* | −20.6% (58) | 17.2% (24.8) | 0.13 |
| BNT | 51.0 (10.4) | 49.7 (5.8) | 0.72 | −2.6% (11.3) | 8.8% (17.5) | 0.17 |
| VPS | ||||||
| (seconds) | 865.7 (409.1) | 729.8 (320.3) | 0.39 | 9.2% (41.1) | −34.3% (50.7) | 0.0377* |
| (# failed) † | 2.7 (2.2) | 1.6 (1.5) | 0.21 | 0.45 (1.9) | 1.4 (1.8) | 0.25 |
| Anagrams | ||||||
| (seconds) | 503.3 (246.1) | 449.9 (162.9) | 0.55 | −4.5% (27.9) | −20.3% (20.9) | 0.28 |
| (# failed) † | 3.3 (2.2) | 2.6 (1.5) | 0.43 | 0.64 (1.1) | 1.8 (1.9) | 0.11 |
| IADL | 11.2 (3.8) | 14.7 (5.2) | 0.11 | −73.4% (72) | −20.1% (33.9) | 0.09 |
Values are M ± SD unless otherwise noted. Baseline variables were compared using the Welch test or Fisher exact test for proportions, and percentage changes were compared using the Wilcoxon test.
Significant at P < 0.05.
The number failed is the difference (year 1 minus baseline) and not the percentage change, and P is the Welch test and not the Wilcoxon test.
BNT = Boston Naming Test. CFT = Complex Figure Test. HVLT = Hopkins Verbal Learning Test. IADL = Instrumental Activities of Daily Living. MCI = mild cognitive impairment. MMSE = Mini-Mental State Examination. Rx = prescription. VPS = visuospatial problem-solving task.
In order to determine, in an exploratory manner, how well each measure discriminated between the newly treated and already treated groups, we examined the percentage change in their responses as predictors using logistic regression and computer area under the receiver operating characteristic curve. Only the IADL scale (n = 18, χ2(1) = 4.2, P = 0.039, area under the curve = 0.74) and the VPS task (n = 22, χ2(1) = 4.8, P = 0.028, area under the curve = 0.77) turned out to be significant. With a 2-predictor logistic model based on the IADL scale and the VPS task, a sensitivity of 80% and a specificity of 87.5% were achieved with area under the curve equal to 0.875. Of the 18 available individuals with aMCI who had data for all of the measures (four had no data available for the IADL scale), only three were misclassified, with one false positive and two false negatives. However, due to the small sample size, this finding should be interpreted with significant caution. The correlation between percentage changes in the IADL score and the VPS task score with donepezil was not significant for the newly treated group (Pearson r = 0.2610, Spearman r = 0.0).
By the following year, three of the 12 newly treated individuals with MCI had progressed to meet the criteria for dementia (clinical dementia rating of at least 1), and three of the 10 already treated individuals with MCI had developed dementia. All of the patients who developed dementia had probable AD.
DISCUSSION
Our previous results regarding individuals with MCI demonstrated that their most severe impairments were on the Hopkins Verbal Learning Test (verbal memory measure) and the MMSE (which includes a significant memory component—both orientation and verbal memory), as expected (Beversdorf et al, 2007). However, in our current study, neither of these test results were differentially affected by initiation of a cholinesterase inhibitor in drug naïve individuals with aMCI. Also, no change was observed from baseline to the 1-year follow-up in the individuals who had stayed on the drug.
Among the non-memory measures for which the individuals with MCI in our previous work received low scores (Beversdorf et al, 2007), only the VPS task demonstrated a significant benefit with drug treatment. In our present study, an exploratory logistic regression model using change in the IADL scale and the VPS task performance as predictors resulted in only three individuals each being misclassified into the newly treated or already treated group. However, this exploratory analysis must be interpreted with significant caution because the sample size was very small.
Due to the memory impairment that individuals with MCI exhibit, most studies examining treatment effects on cognition have focused on memory outcomes (Salloway et al, 2004). The results described here suggest that the assessment of other cognitive domains may also be important for detecting cognitive effects in individuals with aMCI, at least for those individuals who are taking a cholinesterase inhibitor.
Given the well-established effects of the cholinergic system on attention (Sarter et al, 2005), one possible explanation for our findings for the VPS task is that the length of sustained attention required to complete the VPS task, with a duration of up to 4 minutes per task, might have yielded a significant effect due to the cholinergic-dependent attentional demands. This finding may also have relevance for the anagrams task, which has a duration of up to 2 minutes per task. However, no specific attentional task was included in this study for comparison.
The VPS task was designed to assess the cumulative burden of frontal and posterior damage, including cognitive flexibility (ability to shift strategy), within one task (Miller and Tippett, 1996). Based on our results, it is possible that the VPS task may provide a more sensitive assessment of the cumulative effect of non-memory cognitive impairments than can be revealed by measures that assess the specific domains in a more targeted manner. Another possible reason for the sensitivity of the VPS task to cholinesterase inhibitor treatment effects is that a task that simultaneously recruits anterior and posterior processes, such as the VPS task (Beversdorf et al, 2007), allows a wider distribution of brain regions upon which cholinesterase inhibitors can act and affect performance. Future research will have to further explore problem-solving tasks, executive function tasks, and attentional tasks in order to determine the optimal tasks or combination of tasks for detecting cognitive effects in individuals with aMCI.
Study Limitations
We cannot exclude the possibility that some other aspect of the timed nature of the VPS, aside from its demands on sustained attention, may have been a major factor of our findings. For example, because each individual task could take as long as 4 minutes, fatigue may have been a factor. The limited nature of the neuropsychological battery that we used also served as a limitation in this pilot study. Additionally, the lack of memory findings are likely related to the small sample size in this exploratory pilot, particularly given the positive results from previous studies (Salloway et al, 2004).
The small sample size may also have been a factor for the lack of a correlation between the IADL scale and VPS task changes with drug treatment; therefore, larger studies to monitor the relationship between problem-solving and activities of daily living will be critical for the implications of this finding. The small sample size may also be an important reason why the other task that assesses the cumulative effect of the non-memory cognitive impairments, the anagrams task, did not reveal a significant finding. Despite the lack of a difference in solution latency on anagrams between the two groups, the drug naïve group, who had donepezil added to their treatment, did have a borderline trend toward a smaller increase in unsolved anagrams over time. Future work will need to determine whether the VPS task is, in fact, more sensitive than the anagrams task for problem-solving. Both the anagrams task and the VPS task should engage a significant component of attention, which would be expected to be significantly targeted due to acetylcholine’s critical role in attentional control (Sarter and Lustig, 2019).
Finally, due to the preponderance of individuals with aMCI in our clinic desiring drug treatment, after discussion of the mixed data and the risks and benefits, we did not have an adequate convenience sample of drug-free individuals. Inclusion of such a group would be needed to further examine the sensitivity of the VPS task to cholinesterase inhibitor treatment effects in future studies.
CONCLUSION
Based on the results of this pilot study, the VPS task may be a sensitive measure of an MCI individual’s response to cholinesterase inhibitors. Future research is needed to find the optimal tasks for monitoring treatment response in individuals with aMCI. Specifically, future work is needed to examine whether the VPS task or other tasks that are designed in a similar manner (ie, tasks that assess the cumulative burden of frontal and posterior damage) or with a greater emphasis on attention are not only more sensitive to cholinesterase inhibitors, but perhaps are also the most sensitive predictors of progression to dementia in individuals with aMCI.
It will be of interest to examine how the VPS task differs in sensitivity between individuals with aMCI and those with nonamnestic MCI. One might presume that a task that targets non-memory domains might be more sensitive to the effects of cholinesterase inhibitors in the nonamnestic (compared with amnestic) MCI population. However, the presence or absence of Alzheimer pathology might be a critical covariate in such a hypothesis. Future work, though, will need to be approached with caution because tolerability issues with cholinesterase inhibitors may be greater in individuals with aMCI than in individuals with AD (Doody et al, 2010), and because more recently identified polymporhisms in butyrylcholinesterase have been associated with cognitive decline with treatment with donepezil in individuals with aMCI (Sokolow et al, 2017). As a result, a recent meta-analysis suggested slight efficacy for cholinesterase inhibitors in individuals with aMCI, but the benefits were limited by the drug’s side effects (Matsunaga et al, 2019). Future aMCI studies should also consider the inclusion of biomarkers of AD for greater targeted specificity.
Acknowledgments
Supported in part by a seed grant from The Ohio State University Research Foundation; a grant (M01-RR-00034) from the National Institutes of Health to The Ohio State University in support of H.N.N.; grants from Glaxo Smith Kline, Pfizer, Eisai, Novartis, Myriad, Takeda, and Sanofi-Aventis to D.W.S.; and grants from Pfizer and Eisai and from the National Institutes of Health (NS045222 and DA15734) to D.Q.B.
Glossary
- AD
Alzheimer disease
- aMCI
amnestic mild cognitive impairment
- CFT
Complex Figure Test
- IADL
Instrumental Activities of Daily Living
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- VPS
visuospatial problem-solving
Footnotes
D.W.S. was on the speaker’s bureau for Ortho-McNeil, Pfizer, Eisai, Forest, and Novartis at the time of the study, and D.Q.B. was on the speaker’s bureau for Pfizer and Eisai at the time of the study. The remaining authors declare no conflicts of interest.
REFERENCES
- Albert MS. 1996. Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci. 93:13547–13551. doi: 10.1073/pnas.93.24.13547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, et al. 2002. Natural history of mild cognitive impairment in older persons. Neurology. 59:198–205. doi: 10.1212/wnl.59.2.198 [DOI] [PubMed] [Google Scholar]
- Berg L 1988. Clinical dementia rating (CDR). Psychopharmacol Bull. 24:637–639. [PubMed] [Google Scholar]
- Beversdorf DQ, Ferguson JLW, Hillier A, et al. 2007. Problem solving ability in patients with mild cognitive impairment. Cogn Behav Neurol. 20:44–47. doi: 10.1097/WNN.0b013e31802e5101 [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Heilman KM. 1998. Facilitory paratonia and frontal lobe functioning. Neurology. 51:968–971. doi: 10.1212/wnl.51.4.968 [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Hughes JK, Steinberg BA, et al. 1999. Noradrenergic modulation of cognitive flexibility in problem solving. Neuroreport. 10:2763–2767. doi: 10.1097/00001756-199909090-00012 [DOI] [PubMed] [Google Scholar]
- Brandt J 1991. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 5:125–142. doi: 10.1080/13854049108403297 [DOI] [Google Scholar]
- Corwin J, Bylsma FW. 1993. Translations of excerpts from André Rey’s Psychological Examination of Traumatic Encephalopathy and P.A. Osterrieth’s The Complex Figure Copy Test. Clin Neuropsychol. 7:3–21. [Google Scholar]
- Doody RS, Ferris S, Salloway S, et al. 2010. Safety and tolerability of donepezil in mild cognitive impairment: an open-label extension study. Am J Alzheimers Dis Other Demen. 25:155–159. doi: 10.1177/1533317509352334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Burgess P, Emslie H. 1995. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 33:261–268. doi: 10.1016/0028-3932(94)00124-8 [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM. 1993. Frontal lobe and frontal-striatal substrates for different forms of human cognitive flexibility. Neuropsychologia. 31:17–28. doi: 10.1016/0028-3932(93)90077-d [DOI] [PubMed] [Google Scholar]
- Feldman HH, Ferris S, Winblad B, et al. 2007. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 6:501–512. doi: 10.1016/S1474-4422(07)70109-6 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Guarino A, Forte G, Giovannoli J, et al. 2020. Executive functions in the elderly with mild cognitive impairment: a systematic review on motor and cognitive inhibition, conflict control, and cognitive flexibility. Aging Ment Health. 24:1028–1045. doi: 10.1080/13607863.2019.1584785 [DOI] [PubMed] [Google Scholar]
- Guilford JP. 1967. The Nature of Human Intelligence. New York, New York: McGraw-Hill. [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. 1983. The Boston Naming Test (2nd ed.). Philadelphia, Pennsylvania: Lea & Febiger. [Google Scholar]
- Karnath HO, Wallesch CW. 1992. Inflexibility of mental planning: a characteristic disorder with prefrontal lobe lesions? Neuropsychologia. 30:1011–1016. doi: 10.1016/0028-3932(92)90052-N [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. 1969. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 9:179–186. [PubMed] [Google Scholar]
- Matsunaga S, Fujishiro H, Takechi H. 2019. Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: a systematic review and meta-analysis. J Alzheimers Dis. 71:513–523. doi: 10.3233/JAD-190546 [DOI] [PubMed] [Google Scholar]
- Miller LA, Tippett LJ. 1996. Effects of focal brain lesions on visual problem-solving. Neuropsychologia. 34:387–398. doi: 10.1016/0028-3932(95)00116-6 [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, et al. 2001. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 58:397–405. doi: 10.1001/archneur.58.3.397 [DOI] [PubMed] [Google Scholar]
- Morris JC. 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 43:2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. 1944. Le test de copie d’une figure complexe. Archives de Psychologie. 30:206–356. [Google Scholar]
- Petersen RC, Doody R, Kurz A, et al. 2001. Current concepts in mild cognitive impairment. Arch Neurol. 58:1985–1992. doi: 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Lopez O, Armstrong MJ, et al. 2018. Practice guideline update summary: mild cognitive impairment. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 90:126–135. doi: 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, et al. 1999. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 56:303–308. doi: 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, et al. 2005. Vitamin E and donepezil for the treatment of mild cognitive impairment. New Engl J Med. 352:2379–2388. doi: 10.1056/NEJMoa050151 [DOI] [PubMed] [Google Scholar]
- Rey A 1941. L’examen psychologique dans les cas d’encéphalopathie traumatique. Archives de Psychologie. 28:286–340. [Google Scholar]
- Salloway S, Correia S, Richardson S. 2008. Key lessons learned from short-term treatment trials of cholinesterase inhibitors for amnestic MCI. Int Psychogeriatr. 20:40–46. doi: 10.1017/S1041610207005650 [DOI] [PubMed] [Google Scholar]
- Salloway S, Ferris S, Kluger A, et al. 2004. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 63:651–657. doi: 10.1212/01.wnl.0000134664.80320.92 [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, et al. 2005. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 48:98–111. doi: 10.1016/j.brainresrev.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C. 2019. Cholinergic double duty: cue detection and attentional control. Curr Opin Psychol. 29:102–107. doi: 10.1016/j.copsyc.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharre DW, Chang SI, Murden RA, et al. 2010. Self-administered gerocognitive examination (SAGE): a brief cognitive assessment instrument for mild cognitive impairment (MCI) and early dementia. Alzheimer Dis Assoc Disord. 24:64–71. doi: 10.1097/WAD.0b013e3181b03277 [DOI] [PubMed] [Google Scholar]
- Sokolow S, Li X, Chen L, et al. 2017. Deleterious effect of butyrylcholinesterase K-variant in donepezil treatment of mild cognitive impairment. J Alzheimers Dis. 56:229–237. doi: 10.3233/JAD-160562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, et al. 2002. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 59:1034–1041. doi: 10.1212/wnl.59.7.1034 [DOI] [PubMed] [Google Scholar]
- Vilkki J 1992. Cognitive flexibility and mental programming after closed head injuries and anterior or posterior cerebral excisions. Neuropsychologia. 30:807–814. doi: 10.1016/0028-3932(92)90084-Y [DOI] [PubMed] [Google Scholar]


