Abstract
Engaging cancer patients and community members as partners in research helps ensure that the scientific evidence generated is useful to patients and, more importantly, trusted. This bidirectional engagement between patients/community members and research investigators fosters a collaborative and ethical foundation for scientific discovery. While community engaged research (CER) has been in existence for decades, more recent attention by national organizations to embed cancer patients and community members into the research process has accelerated the importance of these efforts. Here, we describe the importance of patient and community engagement (PCE) in cancer research. We outline key principles in undertaking PCE in cancer research, a provide a framework for PCE throughout the cancer research continuum, review metrics for evaluating the effectiveness of PCE in cancer research, and share opportunities for PCE in cancer research going forward.
Summary
Currently, patient and community members have more frequent interaction with clinical and population scientists than basic scientists, leading to gaps in their engagement across the cancer research continuum. Engaging patients and community members in all types of cancer research can bring personal experiences and societal factors to the forefront, informing scientists about these concerns, leading to research that is more responsive to patient and community needs.
Introduction
Patient and community engagement (PCE) in research is recognized as important but is differentially implemented across the research continuum. Laboratory-based scientists may be more comfortable communicating their research to peer audiences in technical publications than to members of the lay community. This disconnect between scientists and the lay community contributes to a widespread lack of scientific literacy and even active distrust of biomedical research within the general community, as vividly illustrated by the current phenomena of COVID-19 hoax theories and vaccine hesitancy in the United States. Furthermore, levels of trust in science remain low in some communities, often stemming from historical events, health system policies, and discriminatory practices. For these reasons and many others, engaging patients and community members in the research process can bring personal experiences and societal factors to the forefront, informing scientists about these concerns, leading to research that is more responsive to patient and community needs.
Cancer Health Disparities
While the scientific community has made great strides against cancer, progress has not benefited everyone equally and some populations bear a disproportionate burden of disease. The first report of differences in cancer outcomes by race or ethnicity was published almost fifty years ago.1,2 Black Americans have had the highest overall cancer death rate of any racial or ethnic group in the United States for more than four decades. Socioeconomic status also drives cancer disparities; it is estimated that eliminating socioeconomic disparities could prevent 34 percent of cancer deaths among all U.S. adults between the ages of 25 to 74.3 Americans living in rural communities face obstacles to cancer care: rural patients are diagnosed later and less likely to receive standard-of-care treatment, follow-up or supportive services than patients in metropolitan areas.4 Older adults tend to have lower levels of health literacy and greater difficulty navigating the health care system.5 Highlighting these and other disparities, as well as their implications on cancer outcomes, will not only raise awareness among investigators but could potentially lead to new research and methods to address them.
Trust in Science
Science can be a powerful and positive force in society, as it shapes innovations not only in health care but in daily life. Yet science in society can only be effective if built on public trust. Advancements in personalized medicine, genetics, and genomics all rely on patients and community members sharing deeply personal information and their biological samples with clinicians and scientists. Prior to the COVID-19 pandemic, Americans’ trust in science had remained relatively stable over time, with a 2018 report finding that about 40 percent of Americans felt a great deal of confidence in the leaders of scientific institutions, a number that has changed little since the surveys began in 1973.6 However, levels of trust in science have always been diminished in minority communities, stemming from events including the Tuskegee syphilis study and reinforced by health system inadequacies and discriminatory events that continue to this day.7 More recently, the COVID-19 pandemic and the rapid development of vaccines against the virus have brought concerns about trust in science to new light. New data show that certain communities are less accepting of the vaccines, often citing mistrust in the science as a primary factor.8 PCE in research has the potential to restore trust in science by making scientific processes more transparent and leveling the balance of power in the relationship between lay members and investigators.
Call to Action
In 2017, the National Cancer Institute (NCI), the American Cancer Society (ACS), the American Association for Cancer Research (AACR), and the American Society of Clinical Oncology (ASCO) released a position statement about the future of cancer health disparities research.9 The report noted that despite breakthrough successes in cancer detection and treatment, the impact of these successes has been hampered by poor dissemination to and uptake in vulnerable and underserved communities. These limitations have the potential to widen, not reduce, cancer health disparities. The report highlighted the promise that PCE in cancer research has in reversing this trend, and that cancer centers and investigators should invest in understanding and utilizing principles and practices of PCE in their research. This recommendation is further articulated by the NCI, which added community outreach and engagement (COE) as a major evaluation criterion in 2016 to its requirements for cancer center designation. As part of this requirement, NCI-designated Cancer Centers are required to understand and be responsive to the needs of their catchment area; more recently, NCI added a focus on diversity and equity to the criterion. PCE in all aspects of cancer research can facilitate a cancer center’s bidirectional relationship with its catchment area and initiatives in diversity and equity.
Community-Engaged Research
In the past decade, the use of patient-reported outcomes (PROs) has become common in cancer care; this trend highlights the role of the patient experience as a key measure of health-care quality.10 Incorporating patient and community experience in the research process dates back even farther, with roots in community advocacy and organizing.11 Community-engaged research (CER) utilizes a partnership approach that includes community members throughout the research process.12 Involving stakeholders in research allows for a deeper understanding of community needs and creates a pathway to disseminate findings back to the communities they are intended to serve. Community-engaged approaches have demonstrated a range of benefits, including improved health outcomes, community capacity building, and empowerment of community members.13 While CER often fits most likely with clinical or population science research, there is increasing interest to engage patients and community members in basic and laboratory-based research.
Challenges to Engaging Patients and Community Members in Cancer Research
Despite the promise of CER in cancer, there remain many challenges to its success. One study found that only 5% of cancer survivors reported participating in research although 26% expressed interest in participating.14 Population science researchers and clinicians have historically engaged patients and community members in research, often involving them in aspects of recruitment, questionnaire design, study intervention delivery, and/or participant retention. Basic scientists are less likely to involve patients and community members in their research, often having difficulty finding common ground, overcoming logistical challenges in a laboratory-based setting, and feeling less comfortable working with community members. Indeed, a recent systematic review of patient and public involvement in cancer research found that it was most common during recruitment and study materials development, and was less likely to be encountered in basic science research.15 Training and educating investigators across the research continuum, particularly basic science, in the value and principles of CER will potentially eradicate challenges in the future and lead to greater PCE in all types of cancer research.
Guiding Tenets for PCE in Cancer Research
The Patient Centered Outcomes Research Institute (PCORI) funds comparative effectiveness research that actively engages patients and other stakeholders in setting research priorities; reviewing research applications; designing, conducting, and disseminating research; and evaluating research outcomes. PCORI research has set a standard for PEC in federally funded research and is guided by four engagement principles: reciprocal relationships; partnerships; co-learning; and transparency, honesty and trust.16 Reciprocal relationships, or when the roles, decision and authority of all stakeholders are clear and well-defined, could be ensured by denoting patients and community members as key personnel on a grant proposal. Co-learning, or when both patients and community members, as well as the investigators, acknowledge that they both bring expertise to the research, can be enhanced through frequent opportunities to share experiences and recommendations. Partnerships should be representative of the local population, accommodating to all, and sustainable. This includes fair compensation for all involved, thoughtful requests of time, and dedication to cultural competency. Transparency, honest and trust is demonstrated when major decisions are inclusive and information is freely shared among all members of the research team. All cancer research, whether basic, clinical, translational or population science, that includes PCE should be designed with these guiding principles in mind.
Opportunities for PCE across the Cancer Research Continuum
Engaging patients and community members in cancer research may be easier for population or translational scientists than basic or clinical scientists, but there are ways to increase community and investigator interactions across all points in the cancer research continuum. (Figure 1). In basic science research, community members can attend laboratory meetings or interview laboratory members about their research to understand the science and convey its importance to community members. They can also serve on basic science study sections and advisory boards to contribute patient priorities and perspectives to investigators. Conversely, basic scientists can attend patient advocacy meetings, to better understand the implications and impact of their research. In clinical science, stakeholders can decide on which PROs to include in the protocol, or how often they should be measured to reduce the burden of study participation. Clinician scientists can give presentations to patient groups about the latest updates in therapies and outcomes while highlighting the role of their research. Population scientists can collaboratively work with patients and community members in the recruitment and retention of participants, as often there are shared living experiences that may help or hinder recruitment and retention. Across the entire cancer research spectrum, there are numerous opportunities to include patients and community members on advisory boards and grant review committees, an aspect that is becoming increasingly common but not fully integrated into the cancer research process.
Figure 1.
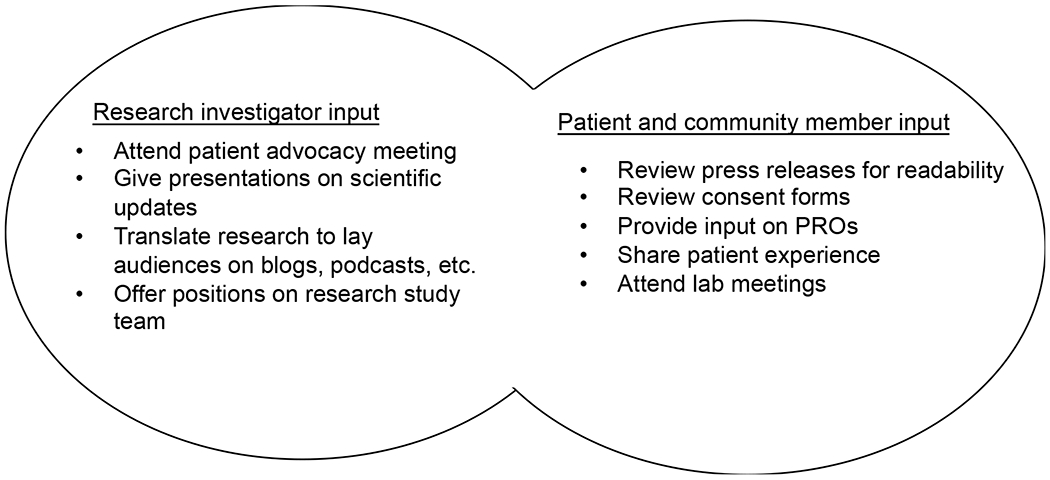
Examples of PCE across the Cancer Research Continuum
Evaluating PCE in the Cancer Research Continuum
While integrating patient and community members into the research process is important, evaluating the impact of such a collaboration may be even more important. In the early stages of this work, most evaluation was short-term, process-oriented and measured by the number of stakeholders and researchers involved in the program, joint meetings attended, and grants or consent forms reviewed by patient advocates. More recently, efforts have been made to shift the focus to more mid-range evaluation outcomes such as perceptions of the stakeholder feeling contributory, the investigator in feeling that adding a community member brought value to the research, that there were shared values and reciprocity in the collaboration, and intentions to continue the collaboration in the future. As interest in the field grows, there will be increased attention to measuring long-term evaluation outcomes that could transfer to communities and society, like changes in perceptions about the transparency of scientific discovery or trust in researchers.
The Future of CER and PCE
At the heart of cancer research are patients, families, and community members. It only makes sense that we, as investigators, would work to integrate them into our research so that their voice is guiding our efforts. As a scientific community, we have made tremendous advances in PCE in research but there is room to improve. To date, PCE in cancer research is more readily accessible in clinical and population research. Here, we outline possible avenues for researchers to locate opportunities to collaborate with patients and community members in ways that will benefit their research and patients in a bi-directional manner. Challenges in the logistics of working with patients and community members will persist, as well as the time it takes to meaningfully engage outsiders in our work. We must overcome these hurdles and recognize the immense value that patients and community members add to the cancer research process and experience. Ultimately, we all have a shared goal – to reduce the burden of cancer for everyone.
Acknowledgments
Funding provided by the Sidney Kimmel Cancer Center NCI Core Grant (5P30CA056036-17)
Authors’ Disclosures
A.E. Aplin reports receiving a commercial research grant from Pfizer Inc. (2013-2017) and has ownership interest in patent number 9880150.
REFERENCES
- 1.Fontaine SA, Henschke UK, Leffall LD Jr, Mason CH, Reinhold AW, Schneider R, et al. Comparison of the cancer deaths in the black and white U.S.A. population from 1949 to 1967. Med Ann Dist Columbia. 1972;41(5):293–298. [PubMed] [Google Scholar]
- 2.Burbank F, Fraumeni JF Jr. U.S. cancer mortality: nonwhite predominance. J Natl Cancer Inst. 1972;49(3):649–659. [PubMed] [Google Scholar]
- 3.Siegel RL, Jemal A, Wender RC, Gansler T, Ma J, Brawley OW. An assessment of progress in cancer control. CA Cancer J Clin 2018;68:329–39. [DOI] [PubMed] [Google Scholar]
- 4.Levit LA, Byatt L, Lyss AP, Paskett ED, Levit K, Kirkwood K, et al. Closing the rural cancer care gap: three institutional approaches. J Oncol Pract. 2020;16(7):422–430. [DOI] [PubMed] [Google Scholar]
- 5.Kadambi S, Loh KP, Dunne R, Magnuson A, Maggiore R, Zittel J, et al. Older adults with cancer and their caregivers - current landscape and future directions for clinical care. Nat Rev Clin Oncol. 2020;17(12):742–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause N, Brossard D, Scheufele DA, Xenos MA, Franke K. Trends—Americans’ Trust in Science and Scientists, Public Opinion Quarterly, Volume 83, Issue 4, Winter 2019, Pages 817–836 [Google Scholar]
- 7.Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21(3):879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Momplaisir F, Haynes N, Nkwihoreze H, Nelson M, Werner RM, Jemmott J. Understanding Drivers of COVID-19 Vaccine Hesitancy Among Blacks [published online ahead of print, 2021 Feb 9]. Clin Infect Dis. 2021. February 9;ciab102. doi: 10.1093/cid/ciab102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polite BN, Adams-Campbell LL, Brawley OW, Bickell N, Carethers JM, Flowers CR, et al. Charting the future of cancer health disparities research: A position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. CA Cancer J Clin. 2017;67(5):353–361. [DOI] [PubMed] [Google Scholar]
- 10.LeBlanc TW, Abernethy AP. Patient-reported outcomes in cancer care - hearing the patient voice at greater volume. Nat Rev Clin Oncol. 2017;14(12):763–772. [DOI] [PubMed] [Google Scholar]
- 11.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. [DOI] [PubMed] [Google Scholar]
- 12.Michener L, Cook J, Ahmed SM, Yonas MA, Coyne-Beasley T, Aguilar-Gaxiola S. Aligning the goals of community-engaged research: Why and how academic health centers can successfully engage with communities to improve health. Acad Med. 2012;87(3):285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyril S, Smith BJ, Possamai-Inesedy A, Renzaho AM. Exploring the role of community engagement in improving the health of disadvantaged populations: A systematic review. Global Health Action 2015; 8(1): 29842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubas MM, Lu Y, Gehr AW, Ghabach B, Tanna B, Narra K, et al. Adult cancer survivors’ engagement and interest in patient-centered research. Cancer Epidemiol Biomarkers Prev. 2020;29(2):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pii KH, Schou LH, Piil K, Jarden M. Current trends in patient and public involvement in cancer research: A systematic review. Health Expect. 2019;22(1):3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PCORI Engagement Rubric. Patient Centered Outcomes Research Institute; website. https://www.pcori.org/sites/default/files/Engagement-Rubric.pdf. Published February 4, 2014, updated June 6, 2016. Accessed on June 3, 2021. [Google Scholar]


