Abstract
CKD is common in patients with heart failure, associated with high mortality and morbidity, which is even higher in people undergoing long-term dialysis. Despite increasing use of evidence-based drug and device therapy in patients with heart failure in the general population, patients with CKD have not benefitted. This review discusses prevalence and evidence of kidney replacement, device, and drug therapies for heart failure in CKD. Evidence for treatment with β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor neprilysin inhibitors, and sodium-glucose cotransporter inhibitors in mild-to-moderate CKD has emerged from general population studies in patients with heart failure with reduced ejection fraction (HFrEF). β-Blockers have been shown to improve outcomes in patients with HFrEF in all stages of CKD, including patients on dialysis. However, studies of HFrEF selected patients with creatinine <2.5 mg/dl for ACE inhibitors, <3.0 mg/dl for angiotensin-receptor blockers, and <2.5 mg/dl for mineralocorticoid receptor antagonists, excluding patients with severe CKD. Angiotensin receptor neprilysin inhibitor therapy was successfully used in randomized trials in patients with eGFR as low as 20 ml/min per 1.73 m2. Hence, the benefits of renin-angiotensin-aldosterone axis inhibitor therapy in patients with mild-to-moderate CKD have been demonstrated, yet such therapy is not used in all suitable patients because of fear of hyperkalemia and worsening kidney function. Sodium-glucose cotransporter inhibitor therapy improved mortality and hospitalization in patients with HFrEF and CKD stages 3 and 4 (eGFR>20 ml/min per 1.73 m2). High-dose and combination diuretic therapy, often necessary, may be complicated with worsening kidney function and electrolyte imbalances, but has been used successfully in patients with CKD stages 3 and 4. Intravenous iron improved symptoms in patients with heart failure and CKD stage 3; and high-dose iron reduced heart failure hospitalizations by 44% in patients on dialysis. Cardiac resynchronization therapy reduced death and hospitalizations in patients with heart failure and CKD stage 3. Peritoneal dialysis in patients with symptomatic fluid overload improved symptoms and prevented hospital admissions. Evidence suggests that combined cardiology-nephrology clinics may help improve management of patients with HFrEF and CKD. A multidisciplinary approach may be necessary for implementation of evidence-based therapy.
Keywords: chronic kidney disease, heart failure, dialysis
Epidemiology of CKD in Heart Failure
Patients with heart failure frequently suffer from coexisting CKD (1). A large meta-analysis suggests that approximately half (49%) of patients with heart failure suffer from CKD (excluding registry studies) (2). The incidence of heart failure in patients with CKD was 18/1000 person-years in a large population-based study in the United States (3). Prevalence of heart failure is higher with decreasing kidney function, and approximately 44% of patients on dialysis suffer from heart failure, and half of these have reduced ejection fraction (1). The prognosis of patients with heart failure and CKD is poor, and worsens with deteriorating kidney function, with a higher mortality (odds ratio, 2.34; 95% confidence interval [95% CI], 2.20 to 2.50; P<0.001) (4). The coexisting CKD is usually caused by diabetes, hypertension, or ischemic kidney disease (see case in Box 1). Consider a 54-year-old man with diabetes, hypertension, and heart failure owing to severe coronary artery disease who presents with fluid overload and near ESKD. In patients like this, prognosis is poor and management is difficult. Patients with CKD were older in most studies, which may be because of age-related decline in GFR; however, presence of CKD was associated with higher mortality when adjusted for age (5). In addition, a significant number of patients with heart failure also suffer from AKI resulting from a variety of conditions, such as sepsis, kidney hypoperfusion, and drug toxicity, with significant adverse outcomes (6). However, the worsening of eGFR because of initiation of renin-angiotensin-aldosterone system inhibitors (RAASis) does not bear the same long-term consequences as persistent AKI owing to sepsis or hypovolemia (7,8).
Box 1.
Case discussion
| Presentation |
| A 54-year-old man was referred to a joint cardiology-nephrology clinic presenting with progressive edema, increasing breathlessness (New York Heart Association class 3), decreased urine output, and stage 5 CKD. On examination, he had leg edema, his weight since the last hospital visit had increased by 9 kg, his BP was 158/70 mm Hg, his pulse rate was 74 beats/min, his jugular venous pressure was elevated, and bibasilar chest crepitations were audible. He had no ascites. Four years ago, he was diagnosed with biopsy proven, stage 3 diabetic nephropathy; multivessel, inoperable coronary artery disease; and heart failure with reduced ejection fraction. He suffered from hypertension and hypercholesterolemia. |
| Investigation |
| His echocardiogram showed reduced ejection fraction of 20%, and an electrocardiogram showed sinus rhythm with QRS duration of 100 ms. His blood tests showed sodium 130 mmol/L, potassium 5.7 mmol/L, creatinine 4.2 mg/dl (372 µmol/L), eGFR 15 ml/min per 1.73 m2, and N-Terminal pro-B-type natriuretic peptide 2742 ng/L. |
| Management |
| He was previously treated with aspirin, clopidogrel, bisoprolol, ramipril, atorvastatin, metformin, and insulin; and hospital admissions were prevented using variable doses furosemide, with intermittent metolazone and careful monitoring of weight and electrolytes. |
| In the joint clinic, his furosemide dose was increased, he was started on daily metolazone, his β-blocker dose increased, intravenous iron was administered, and metformin was stopped. He was informed about long-term KRT and visited the peritoneal dialysis unit. All of this was only possible because he was seen in a joint CKD-heart failure clinic with access to specialist nurses. |
The Interdependence of the Heart and Kidney
The heart and the kidney are interlinked in physiologic states to maintain salt-water homeostasis and normal BP. In health, the crosstalk between the two organs helps the body to respond to changes in kidney perfusion resulting from volume depletion or overload, to maintain appropriate blood flow to vital organs and thereby avoid ischemia or hyperperfusion injury. In disease, the kidney and heart can adversely affect each other’s function (9). On the one hand, inability to excrete salt and water and abnormal renin secretion by the diseased kidney increases cardiac preload, afterload, and heart failure; on the other hand, poor kidney perfusion owing to low cardiac output, and renal venous congestion owing to right heart failure, causes kidney failure. When both organs are diseased, they adversely affect each other’s function, which poses significant challenges in the management of patients with compromised heart and kidney function. Common pathologic mechanisms may affect both organs and cause simultaneous dysfunction of the kidney and heart (10).
The neurohumoral interactions between the two organs are complex in disease states. The natriuretic peptides, which induce diuresis with cardiac volume overload, are upregulated with kidney disease so as to help with associated fluid retention; however, they can be elevated because of poor elimination of the peptide molecules by the kidneys themselves. During treatment of heart failure with diuretics, although congestive symptoms and brain natriuretic peptide (BNP) concentrations improve, kidney function may worsen because of a reduction in kidney plasma flow (11). Stopping of diuretics may improve kidney function but worsen cardiac volumes and levels of BNP.
In heart failure with reduced ejection fraction (HFrEF), the renin, angiotensin, and aldosterone levels increase with hypotension, in response to low kidney perfusion, to improve GFR by efferent arteriolar vasoconstriction and increase systemic BP. However, the excess fluid retention caused by increased levels of renin, angiotensin, and aldosterone may increase the volume overload of the heart and cause further deterioration of heart failure.
Treatment with angiotensin-converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs) lowers intraglomerular pressure caused by efferent arteriolar vasodilation, presenting as apparent worsening of kidney function, which causes anxiety in treating physicians. However, with ACEis, eGFR decline up to 35% has been associated with improved heart failure hospitalization rates (12).
Progression of CKD in patients with heart failure is perhaps faster than in patients without heart failure in the absence of AKI; for example, in the recently completed EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR)-reduced trial, eGFR declined by 4.2 ml/min per 1.73 m2 (95% CI, 3.1 to 5.3) in the placebo arm, over a median follow-up of 16 months, with most patients on ACEis and a baseline eGFR of 62±21 ml/min per 1.73 m2 (13).
AKI in Patients with Heart Failure and CKD
The incidence of AKI is high in patients with heart failure. Incidence of AKI among acute heart failure admissions at St George’s Hospital was 17% in 1094 patients admitted with acute decompensated heart failure, and inpatient mortality was 21% with stage 1, 36% with stage 2, and 48% with stage 3 AKI (P<0.001) (6). In a meta-analysis of 28 trials with 49,890 patients, the incidence of AKI or worsening of kidney function was 23%, and the mortality was higher (odds ratio, 1.81; 95% CI, 1.55 to 2.12; P<0.001) over 488±569 days (2). In a unique situation in cardiology, when the kidney insult is known and preventive measures are used (i.e., with coronary angiogram), the incidence was 7.1% in 985,737 US patients undergoing percutaneous coronary interventions, and was higher with the presence of CKD and heart failure (14). The other causes of AKI in patients with heart failure are infection, sepsis, volume depletion, drug toxicity, and obstruction of the urinary tract in older men with enlarged prostate.
Certain classes of drugs decrease kidney function at therapy initiation, which is distinguished from true AKI by the term “permissive AKI,” or “hemodynamic AKI.” ACEis and ARBs work by dilating the efferent arteriole, thereby lowering intraglomerular pressure and preventing long-term harmful effects of hyperfiltration in individual nephrons (e.g., glomerulosclerosis) in patients with CKD. Sodium-glucose cotransporter 2 inhibitors (SGLT2i), a recently introduced class of oral hypoglycemics, cause a drop in eGFR at therapy initiation. With canagliflozin, a SGLT2i in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation Trial (CREDENCE) study (baseline eGFR of 56 ml/min per 1.73 m2), the initial drop of eGFR was followed by a slowing of progression of CKD and improvement in heart failure outcomes. The drop in eGFR at 3 weeks in the canagliflozin arm was 3.17 ml/min per 1.73 m2 (95% CI, 3.87 to 2.47) more than in the placebo arm, yet the decline in eGFR was less over longer follow-up (15). The drop in eGFR in the first 4 weeks was similar with empagliflozin in the EMPEROR-reduced trial, where the baseline eGFR was 62±22 ml/min per 1.73 m2 (13). The initial worsening of GFR is likely a result of increased delivery of salt and water to the distal tubule, which, in turn, decreases glomerular filtration pressure through the crosstalk between the distal tubule and efferent arteriole of the same nephron by a mechanism known as “tubuloglomerular feedback.”
Heart Failure Therapy in Patients with CKD
Management of chronic heart failure in the general population has changed over the past 3 decades. Novel agents such as ivabradine, angiotensin receptor neprilysin inhibitors, mineralocorticoid receptor inhibitors, and cardiac resynchronization therapy have improved survival. Many trials have included patients with mild-to-moderate CKD, and evidence has emerged for newer agents in the treatment of patients with heart failure and CKD, which is discussed below, and summarized in Figure 1 and Table 1 (1).
Figure 1.
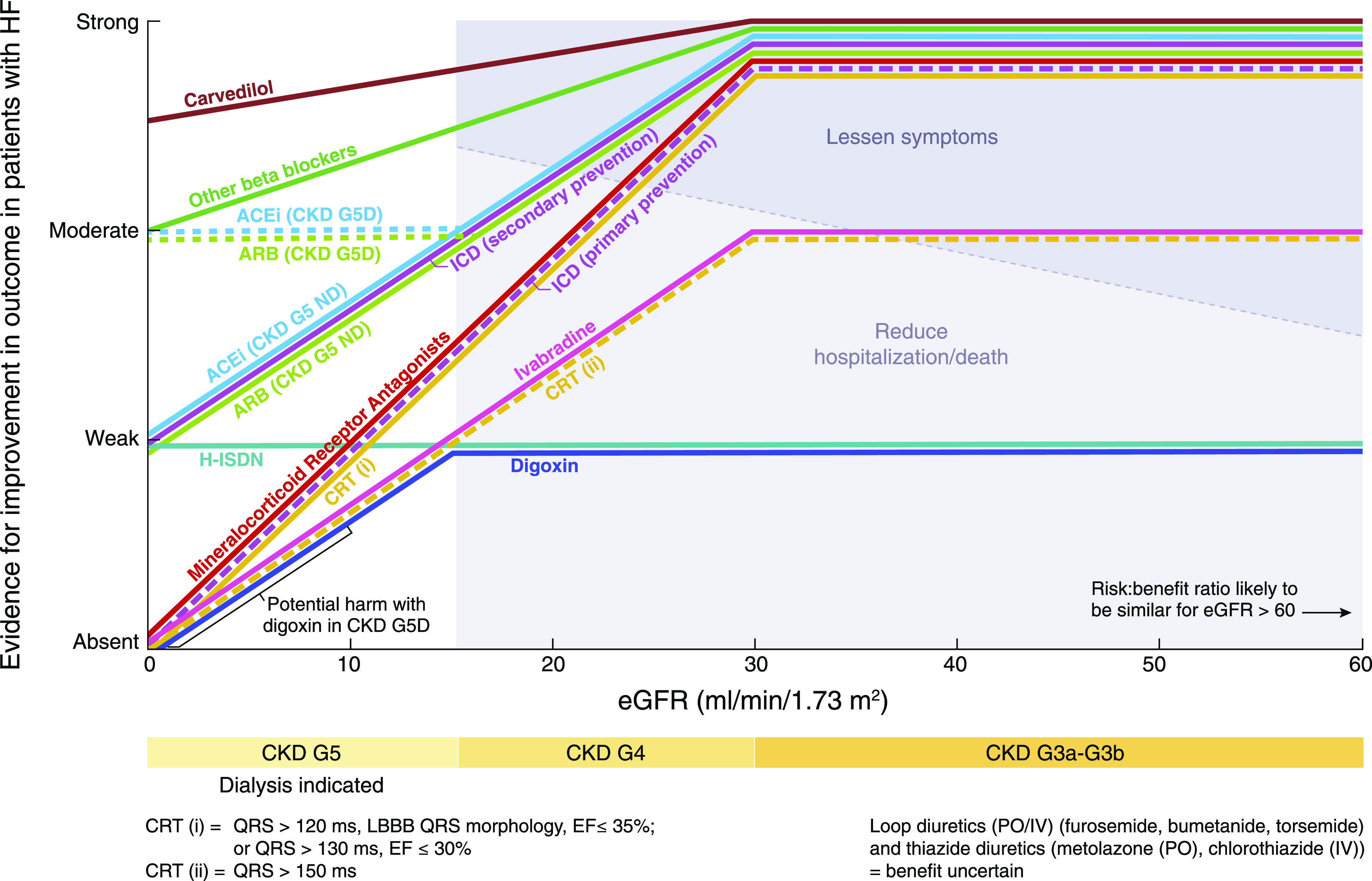
The figure shows level of evidencefor heart failure management in patients with CKD with different levels of kidney function. Adapted from the Kidney Disease Improving Global Outcomes Consensus Conference report (1). The increasing levels of evidence for improved outcomes (mortality and hospitalizations) are shown for each therapy on the y axis, with increasing levels of GFR on the x axis. Evidence is strong for BBs, ACEis, ARBs, and MRAs and moderate for CRTs and ivabradine for eGFR>30 ml/min per 1.73 m2. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; CKD G3a–3b, chronic kidney disease grade 3a and 3b; CKD G4, chronic kidney disease grade 4; CKD G5D, chronic kidney disease GFR category 5 patient on dialysis; CKD G5 ND, chronic kidney disease GFR category 5 patient not on dialysis; CRT, cardiac resynchronization therapy; EF, ejection fraction; H-ISDN, hydralazine-isosorbide dinitrate; ICD, implantable cardioverter defibrillator; IV, intravenous; LBBB, left bundle branch block; MRA, mineralocorticoid receptor antagonist; PO, oral; QRS, QRS wave length in electrocardiogram.
Table 1.
Heart failure studies in general population with clinical characteristics including creatinine- or eGFR-based inclusion criterion
| Trial, yr | Age and Diabetes | < Creatinine (mean) or > GFR |
|---|---|---|
| Angiotensin-converting enzyme inhibitors | ||
| SAVE 1992 (53) | 59 yr, 29% | <2.5 mg/dl |
| SOLVD 1991 (54) | 61 yr, 26% | <2.5 mg/dl (1.2 mg/dl) |
| SOLVD prevent 1992 (55) | 59 yr, 15% | <2.5 mg/dl (1.2 mg/dl) |
| Angiotensin receptor blockers | ||
| CHARM 2003 (56) | 66 yr, 28% | <3 mg/dl |
| β-Blockers | ||
| CIBIS II 1999 (30) | 61 yr, 12% | <3.4 mg/dl |
| COPERNICUS 2001 (31) | 63 yr, 26% | <2.8 mg/dl (1.4 mg/dl) |
| MERIT HF 1999 (29) | 63 yr, 25% | — |
| SENIORS (32) | 76 yr, 27% | <2.8 mg/dl (1.15 mg/dl) |
| Mineralocorticoid receptor antagonists | ||
| RALES 1999 (57) | 65 yr, NA | <2.5 mg/dl |
| EMPHASIS-HF 2011 (58) | 69 yr, 34% | >30 ml/min (1.1 mg/dl) |
| EPHESUS 2003 (59) | 64 yr, 32% | <2.5 mg/dl (1.1 mg/dl) |
| Angiotensin receptor neprilysin inhibitors | ||
| PARADIGM HF 2014 (26). | 64 yr, 35% | >30 ml/min (1.1 mg/dl) |
| Ivabradine | ||
| SHIFT 2010 (60) | 61 yr, 30% | (74 ml/min per 1.73 m2) |
| Cardiac resynchronization therapy | ||
| RAFT 2010 (45) | 66 yr, 30% | 51% patients <60 ml/min per 1.73 m2 a |
| SGLT2 inhibitor | ||
| DAPA-HF 2019 (36) | 66 yr, 41% | >30 ml/min per 1.73 m2 |
| EMPEROR-reduced 2020 | 67 yr, 50% | >20 ml/min per 1.73 m2 |
The table includes pivotal studies that have helped us to draw our conclusions on therapy of patients with HFrEF and CKD (particularly stage 3). It highlights that patients included in these studies had relatively high creatinine and low eGFR, owing to the fact that CKD is common in patients with heart failure. Hence, significant numbers of patients with CKD were included. Studies included a significant proportion of patients with diabetes. SAVE, Survival and Ventricular Enlargement; SOLVD, Studies of Left Ventricular Dysfunction; CHARM, Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity; CIBIS II, Cardiac Insufficiency Bisoprolol Study II; COPERNICUS, Carvedilol Prospective Randomized Cumulative Survival; MERIT HF, Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure; SENIORS, Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure; RALES, Randomized Aldactone Evaluation Study; NA, not available; EMPHASIS-HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; EPHESUS, Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; PARADIGM HF, Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure; SHIFT, Systolic Heart Failure Treatment with the IF inhibitor ivabradine trial; RAFT, Resynchronization–Defibrillation for Ambulatory Heart Failure Trial; DAPA-HF, Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure; EMPEROR reduced, EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction.
51% of the included patients had eGFR<60 ml/min per 1.73 m2.
Diuretic Therapy
As seen in the case in Box 1, water retention and lung congestion in patients with heart failure and CKD causes breathlessness, poor exercise tolerance, and multiple hospital admissions, resulting in poor quality of life. Diuretic therapy is challenging in these patients because of the need for higher doses, frequently causing transient worsening kidney function and electrolyte imbalances such as hyponatremia and hypokalemia (16). These challenges result in the patient needing to visit a variety of specialty doctors, each changing diuretic agents and diuretic doses, often to minimize adverse electrolyte and creatinine changes, and resulting in poor symptom control for the volume-overloaded patient. Renal venous congestion and consequent kidney dysfunction owing to elevated right heart pressure is an often mentioned, poorly understood, and difficult to manage condition, requiring careful escalation of diuretic doses with close monitoring of weight, electrolytes, and creatinine (17). With appropriate use of diuretic therapy, working on different segments of the nephron, symptoms can be controlled and survival may be better. Commonly used thiazide diuretics may not be effective, and loop diuretics are often used with metolazone, as necessary. Intravenous diuretics are used for admitted patients with acute decompensated heart failure. However, there is no significant difference between continuous infusion or bolus administration of intravenous diuretics (16). Spironolactone in patients with acute heart failure can be natriuretic and relieve congestion without significant adverse effect on serum potassium levels (18). A study by the National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network has demonstrated that in 360 patients with acute heart failure and CKD stages 3 and 4, rapid diuresis of 8425 ml (interquartile range 6341–10,528) over 72 hours, with 560 mg (interquartile range 300–815) of furosemide, was safe and was not associated with elevation of markers of tubular injury despite some worsening of creatinine (19). Aggressive diuresis may be useful, provided that the patient is adequately decongested, as evidenced by improvement of physical symptoms and decreased BNP and hemoconcentration, despite the rise in creatinine (20).
Renin-Angiotensin-Aldosterone System Inhibitor Therapy in Patients with Heart Failure with Reduced Ejection Fraction and CKD
Randomized controlled trials have shown improvement of survival in patients with heart failure with use of ACEis (or ARBs) and mineralocorticoid receptor antagonists. Most of these studies included patients with mild-to-moderate (i.e., stages 1–3) CKD (21). Patients with severe CKD have been excluded from most ACEi studies (Figure 1, Tables 1 and 2).
Table 2.
Pharmacotherapy of heart failure in patients with CKD
| Agents | CKD Stages 1– 3 | CKD Stages 4 and 5 |
|---|---|---|
| ACEis | Should be used in all patients with HFrEF, with monitoring of creatinine and potassium | May be used in HFrEF, with monitoring of creatinine and potassium. Dose modification may be necessary |
| β-Blockers | Should be used in all patients with HFrEF | May be used in HFrEF |
| Mineralocorticoid receptor antagonists | Should be used in HFrEF, with careful monitoring of potassium | May be used in HFrEF, with caution and monitoring of potassium |
| ARBs | Should be used in all patients with HFrEF with caution | May be used in HFrEF, with monitoring of creatinine and potassium |
| Ivabradine | May be used in patients with HFrEF with sinus rhythm and who are stable on β-blockers | Unknown effects |
| Angiotensin receptor and neprilysin inhibitor | May be used in patients with HFrEF instead of ACEis/ARBs | Unknown effects |
| Sodium-glucose cotransporter 2 inhibitor | Can be used patients with HFrEF used in or without diabetes | Unknown effects |
| Hydralazine and isosorbide dinitrate | Should be considered in patients with HFrEF who are intolerant to ACEis/ARBs | May be considered in patients with HFrEF who are intolerant to ACEis/ARBs |
ACEi, angiotensin-converting enzyme inhibitor; HFrEF, heart failure with reduced ejection fraction; ARB, angiotensin receptor blocker.
Mineralocorticoid receptor antagonist therapy has been associated with improved survival of patients with symptomatic heart failure; in a randomized controlled trial of spironolactone, 50% of patients had a GFR of <60 ml/min per 1.73 m2, hence the benefits of the trial extended in CKD patients. Similarly, eplerenone was beneficial in patients with postmyocardial infarction heart failure and CKD. However, hyperkalemia was not infrequent and was associated with discontinuation of the therapy.
The use of ACEis and mineralocorticoid receptor antagonists, particularly in patients with CKD, is often associated with hyperkalemia and rising creatinine. Rising creatinine of up to 40% has been observed in ACEi trials, without significant effects on long-term outcomes (12,22). A rise of up to 30% can be viewed as resulting from hemodynamic changes owing to RAASis (23). In fact, such a change may be beneficial and is named permissive AKI as opposed to true AKI due to other reasons in patients with HFrEF, which requires careful history taking and physical examination (7). RAASis should be stopped when kidney blood flow autoregulation is necessary in the patient, e.g., with diarrhea causing volume depletion.
Patients are referred back and forth, between nephrologists and cardiologists, with RAASi-induced changes in creatinine and potassium, resulting in multiple hospital attendances and, often, discontinuation of the RAASi. Analysis of 194,456 patient records showed increasing frequency of hyperkalemia of up to 30% in patients with CKD stages 4 and 5 if measured more than four times a year, and discontinuation of ACEis/ARBs in 24% of patients (24). Nephrologists traditionally have managed hyperkalemia with judicious use of diuretics and correction of acidosis, but oral potassium binders such as patiromer or sodium zirconium cyclosilicate may be useful (25). Randomized controlled studies of new potassium binders for maximization of RAASi therapy in patients with advanced CKD and heart failure are necessary. A close collaboration between nephrologists and cardiologists is necessary for successful initiation and continuation of RAASi in patients with CKD and heart failure.
Angiotensin Receptor and Neprilysin Inhibitor Therapy in Patients with Heart Failure with Reduced Ejection Fraction and CKD
A large randomized controlled trial of dual angiotensin receptor and neprilysin inhibitor therapy excluded patients with eGFR<30 ml/min per 1.73 m2 (see Figure 1, Tables 1 and 2), but included patients with only mild CKD (26). A randomized controlled trial of angiotensin receptor neprilysin inhibitors, including patients with eGFR as low as 20 ml/min per 1.73 m2, demonstrated safety and efficacy similar to irbesartan (27). More recently, angiotensin receptor and neprilysin inhibitor therapy has been shown to slow the progression of CKD in patients with heart failure with preserved ejection fraction (HFpEF) more effectively than valsartan (28). The recommended starting dosage for patients with eGFR<60 ml/min per 1.73 m2 is 24 mg sacubitril and 26 mg valsartan, administered twice per day, at least 36 hours after stopping ACEis or ARBs; the dose is then increased, with careful monitoring of creatinine, potassium, and BP.
Ivabradine and β-Blocker Therapy in Patients with Heart Failure with Reduced Ejection Fraction and CKD
β-Blockers are beneficial in patients with HFrEF and CKD, as evidenced in studies of CKD and the general population (Figures 1 and 2, Tables 1 and 2) (29–33). Carvedilol has been shown to be beneficial in patients with heart failure with CKD stage 5 who are receiving dialysis (34).
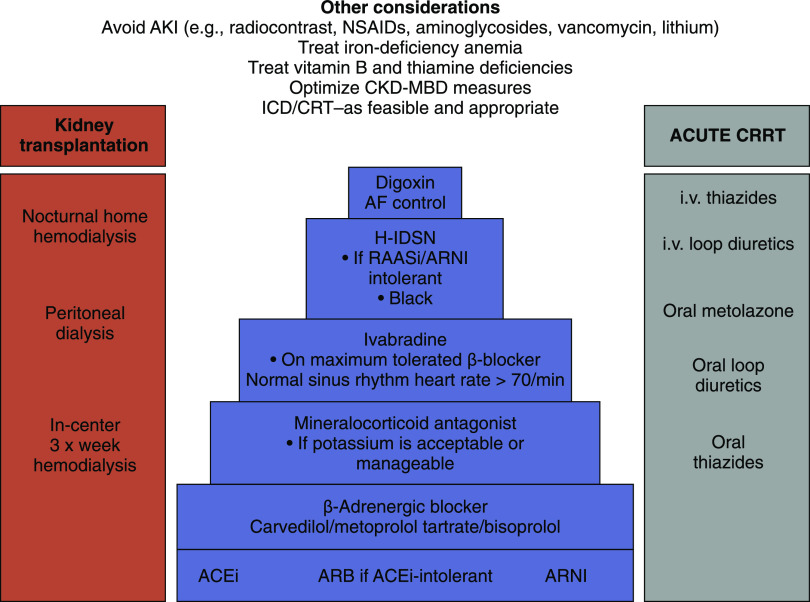
Figure 2.
The figure shows management strategy for patients with heart failure and CKD, including KRT. Adapted from the Kidney Disease Improving Global Outcomes Consensus Conference report (1). Stepwise, evidence-based drug therapy (increasing levels of evidence from the top to bottom of the pyramid) is shown in the middle. The bars on either side of the pyramid show relevant supportive treatment. AF, atrial fibrillation; ARNI, angiotensin receptor neprilysin inhibitor; CKD-MBD, CKD with mineral and bone disorder; CRRT, continuous KRT; i.v., intravenous; NSAID, nonsteroidal anti-inflammatory drug; RAASi, renin-angiotensin-aldosterone system inhibitor.
The Ivabradine and outcomes in chronic heart failure (SHIFT) study demonstrated improved hospitalization and deaths owing to heart failure with ivabradine treatment in patients with HFrEF with a heart rate >70 beats per minute despite β-blocker therapy, which included patients with creatinine <2.5 mg/dl. The study included 1589 patients with CKD stage 3, and benefits in patients with CKD were similar to patients without CKD (35). Ivabradine is metabolized by the CYP3A4 enzyme in the liver and gut, and elimination by kidneys is minimal. The dosage is 2.5–7.5 mg twice per day and does not require dose adjustment with creatinine clearance of >15 ml/min.
Sodium-Glucose Cotransporter 2 Inhibitor Therapy in Patients with Heart Failure with Reduced Ejection Fraction and CKD
SGLT2i therapy in patients with heart failure has been recently shown to reduce mortality and hospitalizations. The recently completed dapagliflozin study included 1926 patients with CKD stage 3 and was able to achieve a risk reduction for composite end points of 28% versus 24% in patients with eGFR>60 ml/min per 1.73 m2 (see Box 1, Table 1). Adverse kidney events with dapagliflozin was not higher compared with placebo (36). More recently, empagliflozin was shown to reduce heart failure hospitalization and eGFR decline, including in patients with eGFR as low as 20 ml/min per 1.73 m2 (48% had eGFR<60 ml/min per 1.73 m2) (13). The Dapagliflozin in Patients with Chronic Kidney Disease (DAPA-CKD) trial has reported lower heart failure admissions in patients with CKD (eGFR >25 but <75 ml/min per 1.73 m2) and proteinuria.
Management of Iron Deficiency and Anemia in Patients with Heart Failure with Reduced Ejection Fraction and CKD
Nephrologists have been using intravenous iron for the past 3 decades to treat anemia in patients with CKD, both before and during dialysis. In the United Kingdom, a recent study in patients receiving dialysis has shown benefits of high-dose intravenous iron in reducing mortality and morbidity, together with heart failure hospitalizations by 44% (37). A previous trial of benefits of intravenous iron in patients with HFrEF included patients with early stages of CKD (38). A collaboration between cardiologists and nephrologists may assist the management of iron deficiency in patients with HFrEF and CKD.
KRT
CKD is a progressive disease with declining kidney function over time, causing frequent episodes of fluid overload and hyperkalemia, further complicated by episodes of AKI. With eGFR<20 ml/min per 1.73 m2, decisions need to be made, in discussion with the patient, about indications, appropriateness, and modality of KRT. Careful consideration of patient expectations, comorbidities, frailty, and quality of life are necessary factors to consider when starting KRT. Patients with heart failure on dialysis have very poor prognosis, with a 5-year survival rate of 12.5% (39). There may be symptomatic relief and fewer hospitalizations with peritoneal dialysis in carefully selected patients (40). In a study of 118 patients with heart failure and CKD, peritoneal dialysis was associated with improvement in quality of life and New York Heart Association class (41). A meta-analysis of 23 studies demonstrated benefits of peritoneal dialysis in patients with heart failure and CKD, with improved hospitalization rates and heart function (42). In patients with refractory heart failure, overnight ultrafiltration with icodextrin solution improved quality of life, heart function, and New York Heart Association class (43). Hemodialysis may be tricky in patients with low BP, but more frequent dialysis and longer nocturnal dialysis may be useful for fluid removal. However, creation of arteriovenous fistula or graft for hemodialysis may cause dilation of left atrium and right ventricle, and associated heart failure (44). For patients with acute heart failure and low BP, slow ultrafiltration with continuous KRT may be useful.
Device Therapy
Rate of CKD progression to dialysis, associated blood stream infection, patient’s age, and general health determine the benefits of other treatments, such as use of cardiac resynchronization therapy and implantation of defibrillators. Cardiac synchronization therapy was beneficial in patients with CKD stage 3 in a Canadian study, of which more than half of patients had eGFR<60 ml/min per 1.73 m2 (45). In a meta-analysis of five retrospective studies, defibrillator therapy was associated with improvement in mortality of patients at high risk of sudden cardiac death (46). Defibrillator therapy in dialysis was associated with high infection rates, and a recent randomized trial in patients on hemodialysis has failed to show any significant benefit (47,48).
Heart Failure with Preserved Ejection Fraction in Patients with CKD
The diagnosis of HFpEF in patients with CKD may be challenging. The BNP levels may be elevated because of the CKD and may not be diagnostic. The fluid overload may be a result of CKD itself. These patients have similar risk of poor outcomes and suffer frequent hospital admissions because of fluid overload, similar to patients with heart failure with reduced ejection function (49). Careful use of diuretics is necessary to control the symptoms of volume overload. Nonsteroidal anti-inflammatory drugs should be avoided because they can cause AKI and fluid retention in patients with CKD, and mimic HFpEF. There is no compelling evidence for effective therapy in HFpEF to improve outcomes such as cardiovascular mortality or heart failure hospitalizations. However, large trials are underway, such as FINEARTS-HF (ClinicalTrials.gov identifier NCT04435626) [Study to Evaluate the Efficacy (Effect on Disease) and Safety of Finerenone on Morbidity (Events Indicating Disease Worsening) & Mortality (Death Rate) in Participants With Heart Failure and Left Ventricular Ejection Fraction (Proportion of Blood Expelled Per Heart Stroke) Greater or Equal to 40%], a randomized controlled trial of finerenone (a nonsteroidal mineralocorticoid receptor antagonist) in patients with CKD and HFpEF.
Multidisciplinary Care
Patients with CKD and heart failure need multidisciplinary care to minimize the number of health care visits. A close working relationship between nephrologists and cardiologists is the key to controlling symptoms and prolonging life where possible; treating with diuretics, ACEis, and mineralocorticoid receptor antagonists, while avoiding electrolyte abnormalities and AKI; and advising appropriately for dialysis and device therapy (Figure 2) (1). In a recent study in St George’s Hospital, multidisciplinary care was associated with improvement in iron stores and RAASi use in 124 patients with CKD and heart failure (50). A multidisciplinary cardiology-nephrology service with doctors and specialist nurses in the United Kingdom has demonstrated better utilization of evidence-based therapy and health care resources (51). A similar multidisciplinary meeting for prekidney transplant patients, with cardiologists, nephrologists, transplant surgeons, and specialist nurses, showed that high-cardiac-risk patients can be safely transplanted (52).
The Way Forward
To achieve appropriate therapy and the best possible outcome, combined cardiology-nephrology clinics are necessary, as demonstrated by the success of the kidney and heart failure clinic at St George’s Hospital, which manages kidney and heart care, together with intravenous iron and advice on dialysis therapy (50). Patients appreciate the multiple benefits from a single visit and provide excellent feedback. The multidisciplinary “one-stop shop” clinic is very patient centered and helps with decision making regarding RAASi, device therapy, and intravenous iron administration, but requires proper resources, including the presence of a nephrologist, cardiologist, anemia nurse, and appropriate space. However, once economic and outcome benefits of a combined clinic are established, such clinics need to be replicated in other institutions. With improved knowledge among cardiologists and nephrologists, multidisciplinary approach, evidenced-based therapy can be implemented in patients with early-to-moderate CKD. However, we recognize that more studies are necessary to strengthen the evidence base in patients with advanced CKD.
Disclosures
D. Banerjee reports receiving lecture fees from Pfizer and ViforPharma; grants and lecture fees from AstraZeneca; and grants from BHF, KRUK, and Wellcome Trust, outside the submitted work. C.A. Herzog reports receiving honoraria from UpToDate; receiving consultation fees from Abbvie, Amgen, Corvidia, Diamedica, Fibrogen, Janssen, NxStage, Pfizer, Relypsa, Sanifit, and University of Oxford; receiving grants from Amgen, National Heart, Lung, and Blood Institute/National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Relapsa, and University of British Columbia; being a stockholder of Boston Scientific, Bristol-Myers Squibb, General Electric, Johnson & Johnson, and Merck; and being an employee of Hennepin Healthcare, outside the submitted work. The remaining author has nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, Kasiske BL, Deswal A, deFilippi CR, Cleland JGF, Anker SD, Herzog CA, Cheung M, Wheeler DC, Winkelmayer WC, McCullough PA; Conference Participants: Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 95: 1304–1317, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL: Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur Heart J 35: 455–469, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J: Reduced kidney function as a risk factor for incident heart failure: The Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Nephrol 18: 1307–1315, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Damman K, Testani JM: The kidney in heart failure: An update. Eur Heart J 36: 1437–1444, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amsalem Y, Garty M, Schwartz R, Sandach A, Behar S, Caspi A, Gottlieb S, Ezra D, Lewis BS, Leor J: Prevalence and significance of unrecognized renal insufficiency in patients with heart failure. Eur Heart J 29: 1029–1036, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Jenkins R, Mandarano L, Gugathas S, Kaski JC, Anderson L, Banerjee D: Impaired renal function affects clinical outcomes and management of patients with heart failure. ESC Heart Fail 4: 576–584, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Testani JM, Kimmel SE, Dries DL, Coca SG: Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 4: 685–691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnamoorthy A, Greiner MA, Sharma PP, DeVore AD, Johnson KW, Fonarow GC, Curtis LH, Hernandez AF: Transient and persistent worsening renal function during hospitalization for acute heart failure. Am Heart J 168: 891–900, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R: Cardiorenal syndrome. J Am Coll Cardiol 52: 1527–1539, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Zannad F, Rossignol P: Cardiorenal syndrome revisited. Circulation 138: 929–944, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, Testani JM: Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: Insights from the DOSE trial. J Card Fail 22: 753–760, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCallum W, Tighiouart H, Ku E, Salem D, Sarnak MJ: Acute declines in estimated glomerular filtration rate on enalapril and mortality and cardiovascular outcomes in patients with heart failure with reduced ejection fraction. Kidney Int 96: 1185–1194, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators: Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383: 1413–1424, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, Spertus JA: Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv 7: 1–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM; NHLBI Heart Failure Clinical Research Network: Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364: 797–805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testani JM, Damman K: Venous congestion and renal function in heart failure ... it’s complicated. Eur J Heart Fail 15: 599–601, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, Tang WHW, Droogné W, Mullens W: Spironolactone to increase natriuresis in congestive heart failure with cardiorenal syndrome. Acta Cardiol 74: 100–107, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, Felker GM, Hernandez AF, O’Connor CM, Sabbisetti VS, Bonventre JV, Wilson FP, Coca SG, Testani JM: Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation 137: 2016–2028, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada T, Ueyama H, Chopra N, Yamaji T, Azushima K, Kobayashi R, Kinguchi S, Urate S, Suzuki T, Abe E, Saigusa Y, Wakui H, Partridge P, Burger A, Bravo CA, Rodriguez MA, Ivey-Miranda J, Tamura K, Testani J, Coca S: Systematic review of the association between worsening renal function and mortality in patients with acute decompensated heart failure. Kidney Int Rep 5: 1486–1494, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowling CB, Sanders PW, Allman RM, Rogers WJ, Patel K, Aban IB, Rich MW, Pitt B, White M, Bakris GC, Fonarow GC, Ahmed A: Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: Insights from the SOLVD Treatment trial. Int J Cardiol 167: 151–156, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCallum W, Tighiouart H, Ku E, Salem D, Sarnak MJ: Trends in kidney function outcomes following RAAS inhibition in patients with heart failure with reduced ejection fraction. Am J Kidney Dis 75: 21–29, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark AL, Kalra PR, Petrie MC, Mark PB, Tomlinson LA, Tomson CR: Change in renal function associated with drug treatment in heart failure: National guidance. Heart 105: 904–910, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang AR, Sang Y, Leddy J, Yahya T, Kirchner HL, Inker LA, Matsushita K, Ballew SH, Coresh J, Grams ME: Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension 67: 1181–1188, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitt B, Bushinsky DA, Kitzman DW, Ruschitzka F, Metra M, Filippatos G, Rossignol P, Du Mond C, Garza D, Berman L, Lainscak M; Patiromer-204 Investigators: Evaluation of an individualized dose titration regimen of patiromer to prevent hyperkalaemia in patients with heart failure and chronic kidney disease. ESC Heart Fail 5: 257–266, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees: Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993–1004, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, Kalra PA, McMurray JJV, Taal M, Wheeler DC, Landray MJ, Baigent C: Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation 138: 1505–1514, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Mc Causland FR, Lefkowitz MP, Claggett B, Anavekar NS, Senni M, Gori M, Jhund PS, McGrath MM, Packer M, Shi V, Van Veldhuisen DJ, Zannad F, Comin-Colet J, Pfeffer MA, McMurray JJV, Solomon SD: Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation 142: 1236–1245, 2020 [DOI] [PubMed] [Google Scholar]
- 29.MERIT-HF Study Group: Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353: 2001–2007, 1999 [PubMed] [Google Scholar]
- 30.CIBIS-II Investigators and Committees : The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 353: 9–13, 1999. 10023943 [PubMed]
- 31.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival Study Group: Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 344: 1651–1658, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Böhm M, Anker SD, Thompson SG, Poole-Wilson PA; SENIORS Investigators: Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26: 215–225, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Lunney M, Ruospo M, Natale P, Quinn RR, Ronksley PE, Konstantinidis I, Palmer SC, Tonelli M, Strippoli G, Ravani P: Pharmacological interventions for heart failure in people with chronic kidney disease. Cochrane Database Syst Rev 2: CD012466, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cice G, Ferrara L, D’Andrea A, D’Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabrò R: Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol 41: 1438–1444, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Voors AA, van Veldhuisen DJ, Robertson M, Ford I, Borer JS, Böhm M, Komajda M, Swedberg K, Tavazzi L; SHIFT Investigators: The effect of heart rate reduction with ivabradine on renal function in patients with chronic heart failure: An analysis from SHIFT. Eur J Heart Fail 16: 426–434, 2014 [DOI] [PubMed] [Google Scholar]
- 36.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators: Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995–2008, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, McMurray JJV, Murray H, Tomson CRV, Wheeler DC, Winearls CG, Ford I; PIVOTAL Investigators and Committees: Intravenous iron in patients undergoing maintenance hemodialysis [published correction appears in N Engl J Med 380: 502, 2019 10.1056/NEJMx180044]. N Engl J Med 380: 447–458, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P; FAIR-HF Trial Investigators: Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 361: 2436–2448, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Banerjee D, Ma JZ, Collins AJ, Herzog CA: Long-term survival of incident hemodialysis patients who are hospitalized for congestive heart failure, pulmonary edema, or fluid overload. Clin J Am Soc Nephrol 2: 1186–1190, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Núñez J, González M, Miñana G, Garcia-Ramón R, Sanchis J, Bodí V, Núñez E, Puchades MJ, Palau P, Merlos P, Llàcer A, Miguel A: Continuous ambulatory peritoneal dialysis as a therapeutic alternative in patients with advanced congestive heart failure. Eur J Heart Fail 14: 540–548, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Koch M, Haastert B, Kohnle M, Rump LC, Kelm M, Trapp R, Aker S: Peritoneal dialysis relieves clinical symptoms and is well tolerated in patients with refractory heart failure and chronic kidney disease. Eur J Heart Fail 14: 530–539, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Lu R, Muciño-Bermejo MJ, Ribeiro LC, Tonini E, Estremadoyro C, Samoni S, Sharma A, Zaragoza Galván JJ, Crepaldi C, Brendolan A, Ni Z, Rosner MH, Ronco C: Peritoneal dialysis in patients with refractory congestive heart failure: A systematic review. Cardiorenal Med 5: 145–156, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wojtaszek E, Grzejszczak A, Niemczyk S, Malyszko J, Matuszkiewicz-Rowińska J: Peritoneal ultrafiltration in the long-term treatment of chronic heart failure refractory to pharmacological therapy. Front Physiol 10: 310, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy YNV, Obokata M, Dean PG, Melenovsky V, Nath KA, Borlaug BA: Long-term cardiovascular changes following creation of arteriovenous fistula in patients with end stage renal disease. Eur Heart J 38: 1913–1923, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL; Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators: Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 363: 2385–2395, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Makki N, Swaminathan PD, Hanmer J, Olshansky B: Do implantable cardioverter defibrillators improve survival in patients with chronic kidney disease at high risk of sudden cardiac death? A meta-analysis of observational studies. Europace 16: 55–62, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Charytan DM, Patrick AR, Liu J, Setoguchi S, Herzog CA, Brookhart MA, Winkelmayer WC: Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis 58: 409–417, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Jukema JW, Timal RJ, Rotmans JI, Hensen LCR, Buiten MS, de Bie MK, Putter H, Zwinderman AH, van Erven L, Krol-van Straaten MJ, Hommes N, Gabreëls B, van Dorp W, van Dam B, Herzog CA, Schalij MJ, Rabelink TJ; ICD2 Trial Investigators: Prophylactic use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation 139: 2628–2638, 2019 [DOI] [PubMed] [Google Scholar]
- 49.Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH: Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail 19: 1606–1614, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Nguyen M, Rumjaun S, Lowe-Jones R, Ster IC, Rosano G, Anderson L, Banerjee D: Management and outcomes of heart failure patients with CKD: Experience from an inter-disciplinary clinic. ESC Heart Fail 7: 3225–3230, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sankaranarayanan R, Douglas H, Wong C: Cardio-nephrology MDT meetings play an important role in the management of cardiorenal syndrome. Br J Cardiol 27: 80–82, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Junarta J, Fernandez M, Chung I, Salha A, Klaud Francheska BD, Lowe-Jones R, Sharma R, Firoozi S, Banerjee D: Role of a cardio-renal multi-disciplinary team meeting in managing cardiovascular risk in patients on kidney transplant waitlists. Clin Transplant 34: e14061, 2020 [DOI] [PubMed] [Google Scholar]
- 53.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr., Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM: Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 327: 669–677, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN; SOLVD Investigators: Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293–302, 1991 [DOI] [PubMed] [Google Scholar]
- 55.Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN; SOLVD Investigators: Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions [published correction appears in N Engl J Med 327: 1768, 1992]. N Engl J Med 327: 685–691, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S; CHARM Investigators and Committees: Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-Overall programme [published correction appears in Lancet: 1744, 2009]. Lancet 362: 759–766, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J; Randomized Aldactone Evaluation Study Investigators: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group: Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11–21, 2011. 21073363 [Google Scholar]
- 59.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators: Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction [published correction appears in N Engl J Med 348: 2271, 2003]. N Engl J Med 348: 1309–1321, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L; SHIFT Investigators: Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study [published correction appears in Lancet 376: 1988, 2010]. Lancet 376: 875–885, 2010 [DOI] [PubMed] [Google Scholar]