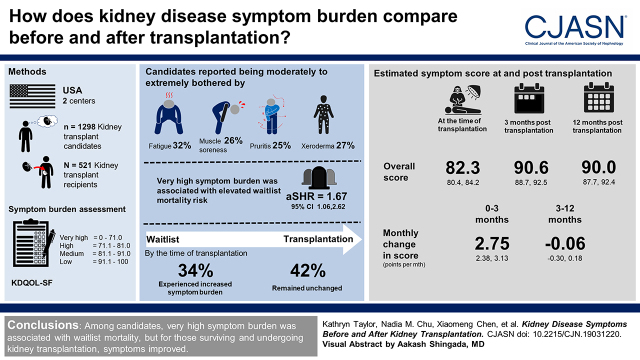
Visual Abstract
Keywords: kidney transplantation
Abstract
Background and objectives
Patients with kidney failure report a high symptom burden, which likely increases while on dialysis due to physical and mental stressors and decreases after kidney transplantation due to restoration of kidney function.
Design, setting, participants, & measurements
We leveraged a two-center prospective study of 1298 kidney transplant candidates and 521 recipients (May 2014 to March 2020). Symptom scores (0–100) at evaluation and admission for transplantation were calculated using the Kidney Disease Quality of Life Short-Form Survey, where lower scores represent greater burden, and burden was categorized as very high: 0.0–71.0; high: 71.1–81.0; medium: 81.1–91.0; and low: 91.1–100.0. We estimated adjusted waitlist mortality risk (competing risks regression), change in symptoms between evaluation and transplantation (n=190), and post-transplantation symptom score trajectories (mixed effects models).
Results
At evaluation, candidates reported being moderately to extremely bothered by fatigue (32%), xeroderma (27%), muscle soreness (26%), and pruritus (25%); 16% reported high and 21% reported very high symptom burden. Candidates with very high symptom burden were at greater waitlist mortality risk (adjusted subdistribution hazard ratio, 1.67; 95% confidence interval, 1.06 to 2.62). By transplantation, 34% experienced an increased symptom burden, whereas 42% remained unchanged. The estimated overall symptom score was 82.3 points at transplantation and 90.6 points at 3 months (10% improvement); the score increased 2.75 points per month (95% confidence interval, 2.38 to 3.13) from 0 to 3 months, and plateaued (−0.06 points per month; 95% confidence interval, −0.30 to 0.18) from 3 to 12 months post-transplantation. There were early (first 3 months) improvements in nine of 11 symptoms; pruritus (23% improvement) and fatigue (21% improvement) had the greatest improvements.
Conclusions
Among candidates, very high symptom burden was associated with waitlist mortality, but for those surviving and undergoing kidney transplantation, symptoms improved.
Introduction
Patients with kidney failure frequently report a wide range of common symptoms (1–4). Approximately 63%–72% of patients undergoing dialysis report being bothered by pruritus (5), 77%–89% report being bothered by fatigue (5), 33%–78% report being bothered by cramping (6,7), 30% report being bothered by muscle soreness (6), 27% report being bothered by numbness (6), 35% report being bothered by dizziness (6), and 44% report being bothered by loss of appetite (5). Qualitative studies suggest that patients with a high symptom burden experience reduced mobility, sleep interference, declines in social relationships, and diminished quality of life (8–11). Understanding the burden of symptoms is a crucial part of patient-centered care that adds important information above and beyond other clinical and laboratory measures (12). This is why symptoms are core outcomes for patients undergoing hemodialysis according to the Standardized Outcomes in Nephrology initiative as part of KidneyX (13,14). Although symptoms have been studied among patients undergoing hemodialysis and peritoneal dialysis (1,15,16), the symptom burden among the select and often healthier group of patients with kidney failure who are evaluated for and undergo kidney transplantation is unclear.
Among patients undergoing dialysis, symptom burden is associated with lower quality of life, depression, early termination of dialysis/underdialysis, and greater risk of both hospitalization and mortality (5,7,15–17). While undergoing dialysis and waiting for transplantation, these symptoms likely persist or worsen, putting candidates at risk for these adverse outcomes. However, cross-sectional studies suggest that restoration of kidney function after kidney transplantation improves symptoms (18–22) and overall health-related quality of life (21). Key questions surrounding post-transplantation symptoms remain: which symptoms improve, how soon after transplantation they will improve, who is likely to experience the greatest improvements, and will certain populations experience greater improvement after transplantation. Understanding how symptoms change and for whom they improve after transplantation can help inform shared decision making surrounding future care post-transplantation (23).
In a cohort of kidney transplant candidates enrolled at evaluation and a cohort of recipients enrolled at admission, we tested whether symptom burden is associated with waitlist mortality and whether symptoms change pre- and post-transplantation.
Materials and Methods
Study Design
We leveraged a two-center prospective study composed of a cohort of 1298 patients with kidney failure being evaluated and subsequently listed for kidney transplant and a cohort of 521 recipients with reported symptoms of kidney disease, as described below. Participants were enrolled from the Johns Hopkins Hospital (candidates enrolled May 2014 to March 2020, n=1298; recipients enrolled May 2014 to February 2020, n=441) and the University of Michigan University Hospital (recipients enrolled March 2015 to May 2017, n=80) (Supplemental Figure 1). All English-speaking candidates and recipients aged 18 years and older were eligible. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Candidates were enrolled at time of evaluation, and recipients were enrolled at admission for transplantation; participants enrolled in both cohorts were also identified, and records were linked. Candidates reported symptoms status at evaluation and were followed for waitlist mortality and transplantation. Recipients reported symptoms status at admission and at routine clinical follow-up approximately 1, 3, and 6 months and yearly post-transplantation.
Both cohorts used the same study protocol for data collection and measured the same factors. Participant characteristics were self-reported, measured, or abstracted from medical records (age, sex, education, body mass index [BMI], dialysis type, and months on dialysis). Race and ethnicity were self-reported. Additional participant characteristics consistent with data from the Scientific Registry of Transplant Recipients were also assessed (cause of kidney failure). We abstracted comorbidity information for the Charlson Comorbidity Index adapted for kidney failure (24) from patient electronic medical records and supplemented with participant self-reported comorbidities data. Additionally, measures of functional and cognitive status included the frailty phenotype (25) (three or more of the five frailty components), global cognitive impairment using the Modified Mini-Mental State examination (26) (score <80), lower extremity impairment (27) (Short Physical Performance Battery score less than or equal to ten), and dependence in activities of daily living (28) and instrumental activities of daily living (29). Finally, trained research assistants administered the Kidney Disease Quality of Life Short-Form Survey (KDQOL-SF) (30,31).
The Institutional Review Boards of Johns Hopkins and the University of Michigan approved the study. All participants provided written informed consent.
Symptoms of Kidney Disease
KDQOL-SF (30,31) is a commonly used instrument for the self-report of physical and mental health of patients with kidney failure that contains a kidney disease targeted scale assessing symptoms and problems of kidney disease and has been validated among US patients on dialysis (32). Participants reported how much they were bothered by each symptom/problem during the past 4 weeks with five response options ranging from “not at all bothered” to “extremely bothered.” Individual symptom scores were calculated using the symptoms subscale: muscle soreness, chest pain, cramps, pruritus, xeroderma, dyspnea, dizziness, anorexia, fatigue, numbness, upset stomach, and site-related problems for patients on dialysis. The individual symptom scores were transformed to a scale of 0–100 range, where a higher score represents a lower symptom burden. The overall symptom score was calculated by averaging across the 12 individual scores, so a higher overall score represents a lower overall symptom burden (30). Symptom burden was categorized as very high: 0.0–71.0; high: 71.1–81.0; medium: 81.1–91.0; and low: 91.1–100.0 on the basis of the reference quartiles among US patients on dialysis (33). The quartile scores in candidates and recipients were consistent with those in the reference population (Supplemental Table 1). A modified overall symptom score was calculated to assess overall symptoms change after transplantation by averaging 11 individual scores, excluding dialysis site–related symptoms.
Descriptive Statistics
Differences in characteristics by symptom burden were tested using ANOVA tests for normally distributed continuous variables, Kruskal–Wallis tests for non-normally distributed continuous variables, and Fisher exact tests for categorical variables. Prevalence ratios (PRs) of very high symptom burden by risk factors (age, sex, race, dialysis type, time on dialysis, and BMI) and functional and cognitive status were estimated using a single modified Poisson regression model.
Risk of Waitlist Mortality by Symptom Burden among Candidates
Among candidates, the time origin was date of listing, and participants were followed until either death or administrative censoring (August 2020). Proportional hazard assumptions were confirmed by visually inspecting log-log plots. Fine and Gray competing risks models (adjusted for age, sex, race, dialysis type, time on dialysis, BMI, and cause of kidney failure) were used to estimate the adjusted subdistribution hazard ratios (aSHRs) of waitlist mortality by symptom burden, accounting for transplantation as a competing risk.
Change in Symptom Burden between Evaluation and Transplantation
For candidates who received kidney transplants (n=190), we calculated the difference in overall symptom scores between evaluation and transplantation. Given that the differences in score were normally distributed, we assessed the mean difference in scores with SD. Additionally, we used a pairwise Pearson correlation to compare the symptom scores at evaluation and at transplantation.
Post-Transplantation Trajectories of Symptom Scores among Recipients
Among recipients (n=521), we described the post-transplantation trajectories of the 11 individual symptom scores (excluding dialysis site–related symptoms) and the overall symptom score using mixed effects models (adjusted for age, sex, race, diabetes, and time on dialysis) with random slope and random intercept for person and time; we used maximum likelihood estimation and selected an unstructured correlation structure for the random effects to generate the best possible model fit. A knot was fit at 3 months post-transplantation to account for the curve-linear trajectories observed in exploratory data analyses using lowess plots. From these models, we obtained the estimated scores for the “reference population” (65-year-old non-Black man without diabetes and with preemptive transplant). We also assessed whether the post-transplantation overall symptom score trajectories differed by age, sex, Black race, diabetes, and frailty using a Wald test for interaction between time and each factor in separate models.
Statistical Analyses
All analyses were performed using Stata version 15 (StataCorp, College Station, TX). All outcomes were nonmissing, and model covariates were mostly nonmissing with few exceptions (<1% missing); therefore, we used complete patient analysis for all adjusted models.
Sensitivity Analyses
As sensitivity analyses, we (1) excluded patients who were preemptive candidates to test whether the number of months on dialysis affects inferences related to risk of waitlist mortality, (2) adjusted for additional factors that may confound the relationship between symptom burden and risk of waitlist mortality, and (3) fitted a random effects Tobit model (34) for post-transplantation trajectory of symptom score to address ceiling effects in symptoms.
Results
Kidney Transplant Candidates
Characteristics.
Among the 1298 candidates, the mean age was 55 years (SD=13), 40% were women, and 41% were Black patients. The median months on dialysis by the time of evaluation were 3 months (interquartile range [IQR], 0–20). In this cohort, 46% of participants were on hemodialysis, 13% were on peritoneal dialysis, and 42% were preemptive candidates who were not on dialysis.
Kidney Disease Symptoms at Evaluation.
At evaluation, candidates had a median symptom score of 85.4 (IQR, 75.0–93.2), with 32% reporting low, 31% reporting medium, 16% reporting high, and 21% reporting very high symptom burden (Table 1). Candidates reported being moderately to extremely bothered by fatigue (32%), xeroderma (27%), muscles soreness (26%), and pruritus (25%) at evaluation (Figure 1). Only 8% reported no symptoms (overall score =100).
Table 1.
Characteristics of kidney transplant candidates by kidney disease symptom burden at evaluation (n=1298)
| Characteristics | Kidney Disease Symptom Burden | |||
|---|---|---|---|---|
| Low, n=420 | Medium, n=403 | High, n=202 | Very High, n=273 | |
| Age at evaluation, yr, mean (SD) | 56 (13) | 55 (13) | 55 (13) | 52 (13) |
| Women, n (%) | 146 (35%) | 140 (35%) | 98 (49%) | 131 (48%) |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | 187 (45%) | 197 (49%) | 99 (49%) | 120 (44%) |
| Non-Hispanic Black | 189 (45%) | 150 (37%) | 84 (42%) | 114 (42%) |
| Hispanic | 17 (4%) | 16 (4%) | 7 (4%) | 16 (6%) |
| Other | 27 (6%) | 40 (10%) | 12 (6%) | 23 (8%) |
| High school or above, n (%) | 395 (94%) | 389 (97%) | 186 (92%) | 259 (95%) |
| BMI, kg/m2, mean (SD) | 28.1 (5.5) | 27.9 (5.8) | 28.6 (6.3) | 28.9 (6.1) |
| Frail, n (%) | 32 (8%) | 51 (13%) | 33 (18%) | 57 (22%) |
| Cognitive impairment, n (%) | 30 (8%) | 20 (5%) | 10 (5%) | 11 (4%) |
| Lower extremity impairment, n (%) | 202 (50%) | 210 (54%) | 118 (62%) | 163 (62%) |
| Functional impairment, n (%) | ||||
| Activities of daily living | 7 (2%) | 13 (3%) | 19 (10%) | 25 (9%) |
| Instrumental activities of daily living | 34 (8%) | 68 (17%) | 53 (27%) | 78 (29%) |
| Comorbidities, n (%) | ||||
| Myocardial infarction | 32 (8%) | 29 (7%) | 22 (11%) | 24 (9%) |
| Peripheral vascular | 18 (4%) | 27 (7%) | 12 (6%) | 13 (5%) |
| Cerebral vascular | 14 (3%) | 24 (6%) | 13 (7%) | 16 (6%) |
| Dementia | 2 (0.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Chronic lung disease | 4 (1%) | 8 (2%) | 9 (5%) | 8 (3%) |
| Rheumatologic disease | 21 (5%) | 24 (6%) | 11 (6%) | 30 (11%) |
| Peptic ulcer | 11 (3%) | 10 (3%) | 8 (4%) | 11 (4%) |
| Diabetes | 152 (36%) | 155 (39%) | 94 (47%) | 107 (39%) |
| Diabetes with complication | 60 (14%) | 81 (20%) | 55 (28%) | 74 (27%) |
| Moderate/severe liver | 9 (2%) | 13 (3%) | 10 (5%) | 12 (4%) |
| Metastatic cancer | 4 (1%) | 2 (0.5%) | 2 (1%) | 1 (0.4%) |
| Leukemia | 1 (0.2%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Lymphoma | 2 (0.5%) | 5 (1%) | 1 (0.5%) | 2 (0.7%) |
| HIV | 14 (3%) | 9 (2%) | 6 (3%) | 11 (4%) |
| Congestive heart failure | 53 (14%) | 60 (16%) | 32 (18%) | 48 (20%) |
| Charlson Comorbidity Index, n (%) | ||||
| 0 | 191 (46%) | 169 (42%) | 63 (31%) | 100 (37%) |
| 1 | 27 (6%) | 24 (6%) | 16 (8%) | 21 (8%) |
| 2 | 95 (23%) | 75 (19%) | 44 (22%) | 40 (15%) |
| 3 | 52 (12%) | 59 (15%) | 35 (17%) | 53 (19%) |
| 4+ | 55 (13%) | 76 (19%) | 44 (22%) | 59 (22%) |
| Dialysis type, n (%) | ||||
| Preemptive transplant | 178 (43%) | 175 (44%) | 84 (42%) | 99 (36%) |
| Hemodialysis | 181 (44%) | 181 (45%) | 86 (43%) | 141 (52%) |
| Peritoneal dialysis | 57 (14%) | 46 (11%) | 28 (14%) | 33 (12%) |
| Months on dialysis, median (IQR) | 3 (0–21) | 3 (0–19) | 2 (0–19) | 5 (0–25) |
| Cause of kidney failure, n (%) | ||||
| Glomerular disease | 83 (20%) | 87 (22%) | 33 (16%) | 66 (24%) |
| Diabetes | 70 (17%) | 60 (15%) | 46 (23%) | 52 (19%) |
| Cystic disease | 37 (9%) | 35 (9%) | 18 (9%) | 25 (9%) |
| Hypertension | 149 (36%) | 128 (32%) | 61 (30%) | 77 (28%) |
| Others | 81 (19%) | 92 (23%) | 44 (22%) | 53 (19%) |
Candidates were enrolled at evaluation for transplant. The overall symptom score ranged from zero to 100 points and was on the basis of self-reported burden of individual symptom/problem of kidney disease with five response options ranging from “not at all bothered” to “extremely bothered.” A higher symptom score represents a lower burden. Characteristics were presented by category of symptom burden at evaluation for transplant: very high: 0.0–71.0; high: 71.1–81.0; medium: 81.1–91.0; and low: 91.1–100.0. BMI, body mass index; IQR, interquartile range.
Figure 1.
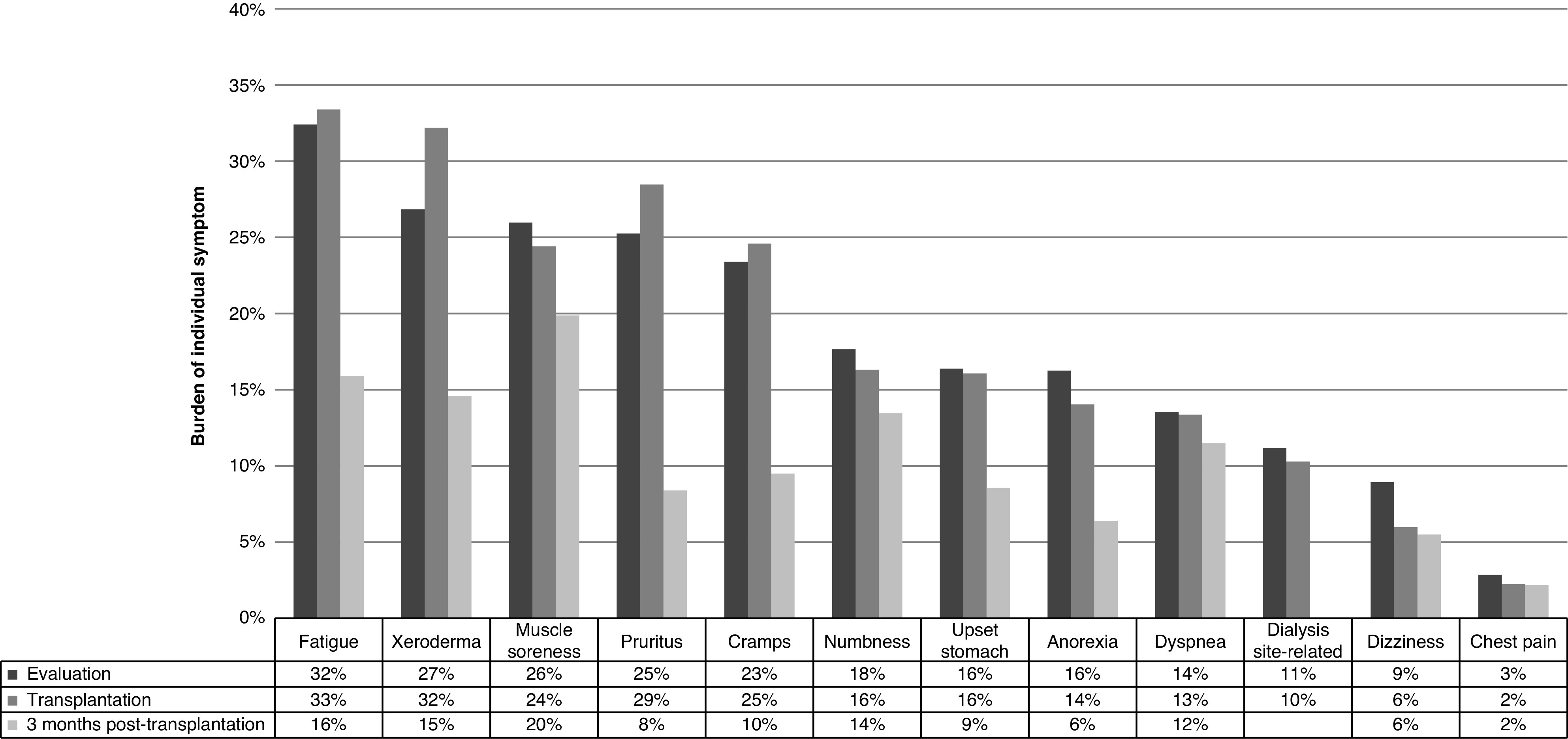
Comparison of individual symptom burdens at evaluation (n=1298), admission for kidney transplantation (n=521), and 3 months post-transplantation (n=452). Burden at evaluation was examined among candidates who were enrolled at evaluation for kidney transplant, and burden at admission for transplantation and 3 months post-transplantation was examined among recipients who were enrolled at admission for kidney transplantation and followed up approximately 1, 3, and 6 months and yearly post-transplantation. Participants reported burdens of individual symptoms with five response options ranging from “not at all bothered” to “extremely bothered.” The prevalence of each individual symptom was defined as participants reported being moderately to extremely bothered by the symptom. Dialysis site–related symptom was not assessed after transplantation.
Participants with high and very high symptom burden were more likely to be younger, be women, and have a high comorbidity burden (Table 1). Additionally, after adjustment, patients who were frail (PR, 1.76; 95% confidence interval [95% CI], 1.37 to 2.26), had impairment in lower extremity function (PR, 1.29; 95% CI, 1.03 to 1.61), had dependence in activities of daily living (PR, 1.89; 95% CI, 1.36 to 2.61), or had dependence in instrumental activities of daily living (PR, 1.76; 95% CI, 1.41 to 2.19) were more likely to report very high symptom burden (Supplemental Table 2).
Risk of Waitlist Mortality by Symptom Burden.
During a median follow-up period of 1.9 years (IQR, 0.8–3.5), 12% of the cohort (n=152) died on the waitlist. After adjustment, ten-point reductions (worsening) in individual symptom scores for muscle soreness (aSHR, 1.07; 95% CI, 1.02 to 1.13), chest pain (aSHR, 1.18; 95% CI, 1.07 to 1.29), cramps (aSHR, 1.05; 95% CI, 1.00 to 1.10), dyspnea (aSHR, 1.07; 95% CI, 1.01 to 1.14), anorexia (aSHR, 1.08; 95% CI, 1.02 to 1.14), and fatigue (aSHR, 1.06; 95% CI, 1.01 to 1.12) were independently associated with a greater risk of waitlist mortality (Table 2).
Table 2.
Risk of waitlist mortality by kidney disease symptom burden among kidney transplant candidates
| Exposure | Crude Subdistribution Hazard Ratios (95% Confidence Interval), n=1298 | Adjusted Subdistribution Hazard Ratios (95% Confidence Interval), n=1282 |
|---|---|---|
| Individual symptom scores (range 0–100, per 10-points worse) | ||
| Muscle soreness | 1.06 (1.01 to 1.11) | 1.07 (1.02 to 1.13) |
| Chest pain | 1.14 (1.05 to 1.24) | 1.18 (1.07 to 1.29) |
| Cramps | 1.04 (1.00 to 1.10) | 1.05 (1.00 to 1.10) |
| Pruritus | 1.00 (0.96 to 1.06) | 1.00 (0.95 to 1.06) |
| Xeroderma | 0.99 (0.94 to 1.04) | 0.99 (0.95 to 1.05) |
| Dyspnea | 1.05 (0.99 to 1.11) | 1.07 (1.01 to 1.14) |
| Dizziness | 0.99 (0.91 to 1.08) | 1.01 (0.93 to 1.11) |
| Anorexia | 1.04 (0.98 to 1.09) | 1.08 (1.02 to 1.14) |
| Fatigue | 1.02 (0.97 to 1.08) | 1.06 (1.01 to 1.12) |
| Numbness | 1.06 (1.01 to 1.12) | 1.05 (1.00 to 1.11) |
| Upset stomach | 1.02 (0.96 to 1.08) | 1.05 (0.99 to 1.12) |
| Site related (patients on dialysis only) | 0.99 (0.91 to 1.08) | 1.00 (0.91 to 1.10) |
| Overall symptom score (range 0–100) | ||
| Continuous score per 10-points worse | 1.10 (0.99 to 1.21) | 1.16 (1.05 to 1.29) |
| Symptom burden | ||
| Low | Reference | Reference |
| Medium | 1.24 (0.81 to 1.88) | 1.32 (0.87 to 2.00) |
| High | 1.36 (0.84 to 2.21) | 1.48 (0.91 to 2.40) |
| Very high | 1.35 (0.87 to 2.11) | 1.67 (1.06 to 2.62) |
Candidates were enrolled at evaluation for transplant. The individual and overall symptom scores ranged from zero to 100 points and were on the basis of self-reported burden of individual symptom/problem of kidney disease with five response options ranging from “not at all bothered” to “extremely bothered.” The individual symptom scores were transformed to a scale of 0–100 possible range, where a higher score represents lower burden. The overall symptom score was calculated by averaging across the 12 individual scores, so a higher symptom score represents a lower burden. Symptom burden was defined as very high: 0.0–71.0; high: 71.1–81.0; medium: 81.1–91.0; and low: 91.1–100.0. Crude subdistribution hazard ratios and adjusted subdistribution hazard ratios with 95% confidence intervals are presented from competing risks models (competing risk of transplantation). Associations that are statistically significant at P=0.05 are in bold. Adjusted models were adjusted for age at evaluation, sex, Black race, dialysis type, time on dialysis, body mass index, and cause of kidney failure.
Additionally, patients with very high symptom burden were at greater risk of waitlist mortality (aSHR, 1.67; 95% CI, 1.06 to 2.61) compared with their low-burden counterparts (Table 2). Furthermore, a ten-point worsening in the overall symptom score was associated with a 1.16-fold (95% CI, 1.05 to 1.29) higher risk of waitlist mortality.
Change in Symptom Burden between Evaluation and Transplantation.
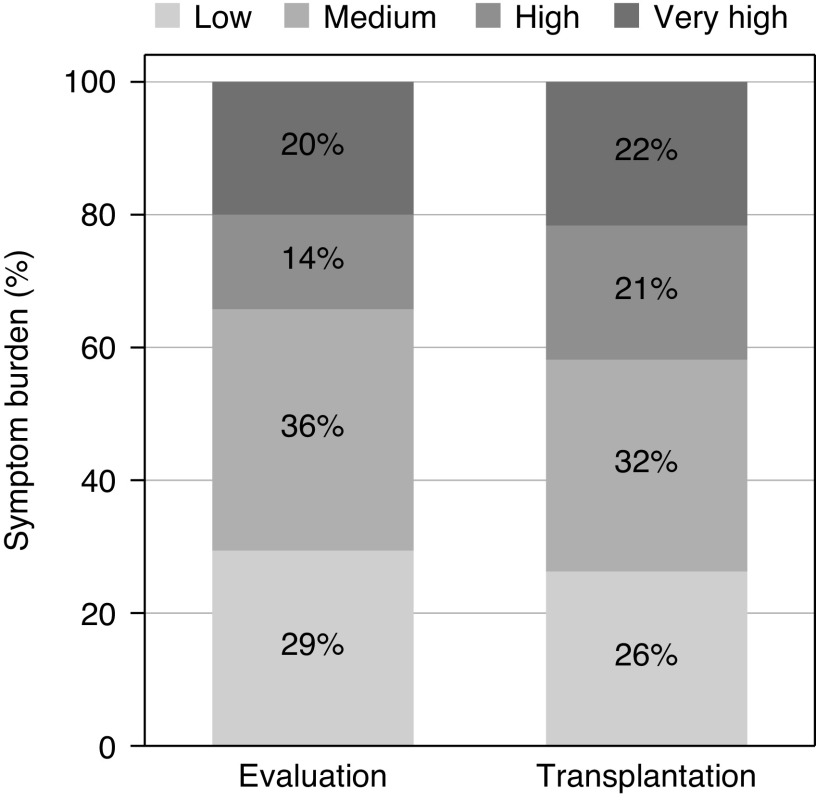
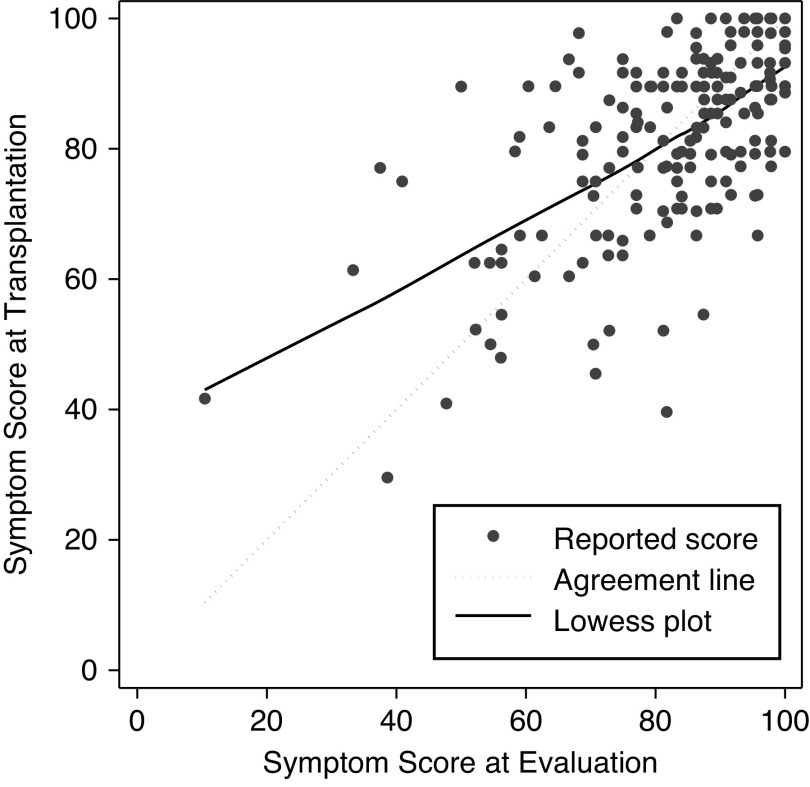
Among 190 candidates who ultimately underwent transplantation and had symptoms recorded at admission for transplantation (median time between evaluation and transplantation =1.1 years; IQR, 0.6–1.8), the proportions of high (21%) and very high symptom burden (22%) at transplantation were higher than the proportions at evaluation (14% high and 20% very high burden) (Figure 2). Of these patients, 25% experienced a decline in symptom burden, 34% experienced an increase in symptom burden, and 42% remained unchanged by the time of transplantation (Supplemental Table 3). The unadjusted mean overall symptom score decreased from 82.2 points at evaluation to 81.5 points at transplantation (difference =−0.7 points; SD=12.9). The symptom scores at evaluation and at transplantation were strongly correlated (r=0.60; P<0.001) (Figure 3).
Figure 2.
Change in symptom burden at evaluation and admission for kidney transplantation among the combined kidney transplant candidate and recipient cohorts (n=190). Transplant candidates were enrolled at evaluation; recipients were enrolled at admission for transplantation. The overall symptoms score ranged from zero to 100 points and was on the basis of self-reported burden of individual symptom/problem of kidney disease with five response options ranging from “not at all bothered” to “extremely bothered.” Symptom burden was defined as very high: 0.0–71.0; high: 71.1–81.0; medium: 81.1–91.0, and low: 91.1–100.0.
Figure 3.
Symptom scores at evaluation and at kidney transplantation among the combined candidate and recipient cohorts (n=190). Candidates were enrolled at evaluation for transplant; recipients were enrolled at admission for transplantation.
Kidney Transplant Recipients
Characteristics.
Recipients were followed up for a median of 11 months (IQR, 4–14) and four (IQR, 3–5) visits. Among the 521 recipients, the mean age was 52 years (SD=13), 39% were women, and 38% were Black patients. The median time on dialysis was 28 months (IQR, 5–67). In this cohort, 63% of participants underwent hemodialysis, 18% underwent peritoneal dialysis, and 19% were preemptive recipients.
Similar to candidates, recipients reported being moderately to extremely bothered by fatigue (33%), xeroderma (32%), and pruritus (29%) at admission for transplantation; however, they reported lower burden by each of the 11 individual symptoms at 3 months post-transplantation (Figure 1). At transplantation, 26% of recipients reported low, 33% reported medium, 21% reported high, and 20% reported very high symptom burden (Table 3).
Table 3.
Characteristics of kidney transplant recipients by kidney disease symptom burden at admission for transplantation (n=521)
| Characteristics | Kidney Disease Symptom Burden | |||
|---|---|---|---|---|
| Low, n=137 | Medium, n=174 | High, n=107 | Very High, n=103 | |
| Age at evaluation, mean (SD) | 52 (14) | 53 (13) | 54 (13) | 51 (13) |
| Women, n (%) | 55 (40%) | 65 (37%) | 44 (41%) | 37 (36%) |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | 64 (47%) | 90 (52%) | 59 (55%) | 58 (56%) |
| Non-Hispanic Black | 56 (41%) | 66 (38%) | 39 (36%) | 36 (35%) |
| Hispanic | 9 (7%) | 7 (4%) | 3 (3%) | 3 (3%) |
| Other | 8 (6%) | 11 (6%) | 6 (6%) | 6 (6%) |
| High school or above, n (%) | 130 (95%) | 170 (98%) | 103 (96%) | 100 (97%) |
| BMI kg/m2, mean (SD) | 27.6 (5.5) | 27.8 (5.4) | 27.6 (5.4) | 27.5 (6.3) |
| Frail, n (%) | 10 (7%) | 20 (12%) | 11 (10%) | 20 (19%) |
| Cognitive impairment, n (%) | 8 (6%) | 6 (4%) | 7 (7%) | 7 (7%) |
| Lower extremity impairment, n (%) | 61 (53%) | 90 (59%) | 49 (51%) | 58 (62%) |
| Functional impairment, n (%) | ||||
| Activities of daily living | 2 (2%) | 3 (2%) | 7 (7%) | 5 (5%) |
| Instrumental activities of daily living | 19 (14%) | 25 (15%) | 18 (18%) | 32 (32%) |
| Comorbidities, n (%) | ||||
| Myocardial infarction | 5 (4%) | 7 (4%) | 8 (8%) | 8 (8%) |
| Peripheral vascular | 4 (3%) | 6 (3%) | 5 (5%) | 5 (5%) |
| Cerebral vascular | 4 (3%) | 6 (4%) | 5 (5%) | 5 (5%) |
| Dementia | 1 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Chronic lung disease | 1 (0.7%) | 2 (1%) | 3 (3%) | 2 (2%) |
| Rheumatologic disease | 5 (4%) | 21 (12%) | 9 (9%) | 12 (12%) |
| Peptic ulcer | 7 (5%) | 4 (2%) | 2 (2%) | 3 (3%) |
| Diabetes | 40 (29%) | 49 (28%) | 34 (32%) | 28 (28%) |
| Diabetes with complication | 13 (10%) | 25 (15%) | 15 (14%) | 15 (15%) |
| Moderate/severe liver | 4 (3%) | 5 (3%) | 5 (5%) | 6 (6%) |
| Metastatic cancer | 1 (0.7%) | 1 (0.6%) | 0 (0%) | 2 (2%) |
| Leukemia | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Lymphoma | 0 (0%) | 2 (1%) | 0 (0%) | 0 (0%) |
| HIV | 3 (2%) | 7 (4%) | 3 (3%) | 1 (1%) |
| Congestive heart failure | 3 (3%) | 13 (10%) | 7 (8%) | 4 (5%) |
| Charlson Comorbidity Index, n (%) | ||||
| 0 | 78 (57%) | 85 (49%) | 53 (50%) | 54 (52%) |
| 1 | 9 (7%) | 18 (10%) | 7 (7%) | 10 (10%) |
| 2 | 26 (19%) | 32 (18%) | 22 (21%) | 11 (11%) |
| 3 | 16 (12%) | 22 (13%) | 13 (12%) | 14 (14%) |
| 4+ | 8 (6%) | 17 (10%) | 12 (11%) | 14 (14%) |
| Dialysis type, n (%) | ||||
| Preemptive transplant | 27 (21%) | 35 (20%) | 19 (18%) | 17 (17%) |
| Hemodialysis | 81 (62%) | 108 (62%) | 65 (63%) | 64 (64%) |
| Peritoneal dialysis | 23 (18%) | 30 (17%) | 19 (18%) | 19 (19%) |
| Months on dialysis, median (IQR) | 28 (4–72) | 23 (4–68) | 29 (6–66) | 30 (7–56) |
| Cause of kidney failure, n (%) | ||||
| Glomerular disease | 36 (26%) | 36 (21%) | 30 (28%) | 22 (21%) |
| Diabetes | 18 (13%) | 26 (15%) | 16 (15%) | 16 (16%) |
| Cystic disease | 16 (12%) | 25 (15%) | 14 (13%) | 10 (10%) |
| Hypertension | 43 (31%) | 58 (34%) | 28 (26%) | 33 (32%) |
| Others | 24 (18%) | 28 (16%) | 19 (18%) | 22 (21%) |
The overall symptom score ranged from zero to 100 points and was on the basis of self-reported burden of individual symptom/problem of kidney disease with five response options ranging from “not at all bothered” to “extremely bothered.” A higher symptom score represents a lower burden. Characteristics were presented by category of symptom burden at admission for transplantation: very high: 0.0–71.0; high: 71.1–81.0; medium: 81.1–91.0; and low: 91.1–100.0. BMI, body mass index; IQR, interquartile range.
Post-Transplantation Trajectories of Symptom Scores.
Of 11 individual symptom scores, nine (muscle soreness, cramps, pruritus, xeroderma, dizziness, anorexia, fatigue, numbness, and upset stomach) significantly increased (improved) in the first 3 months post-transplantation; peak improvements were observed at 3 months (Figure 4). The greatest improvements were observed in pruritus (23% improvement), fatigue (21%), xeroderma (20%), and cramps (17%). For example, after adjustment, the estimated pruritus score was 75.6 at transplantation and increased to 93.2 by 3 months post-transplantation, with a 5.86–points per month (95% CI, 5.00 to 6.72) increase for the reference population. For 3–12 months post-transplantation, only dyspnea score increased significantly (slope =0.49 points per month; 95% CI, 0.02 to 0.96) (Table 4).
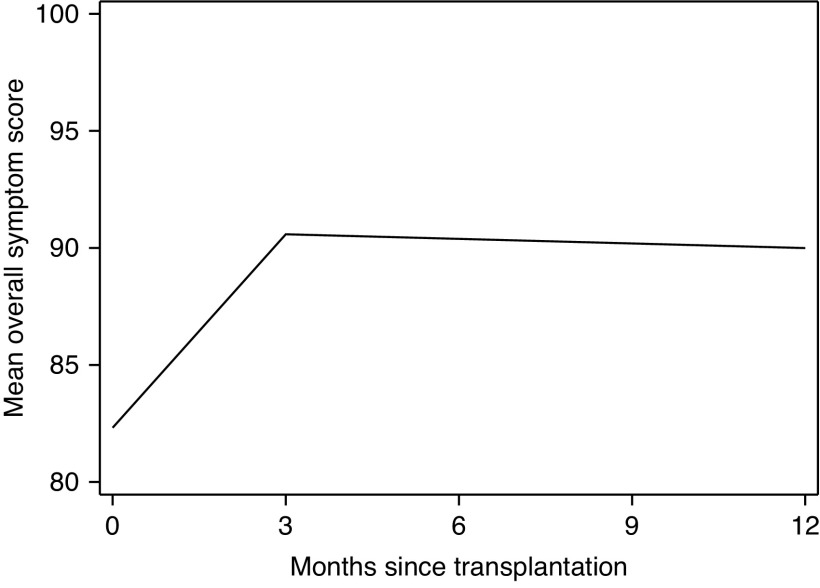
Figure 4.
Estimated postkidney transplantation change in overall symptom score by months since transplantation among recipients (n=521). Recipients were enrolled at admission for kidney transplantation. The overall symptom score ranged from zero to 100 points and was on the basis of self-reported burden of individual symptom/problem of kidney disease with five response options ranging from “not at all bothered” to “extremely bothered.” A higher symptom score represents a lower burden. Symptom burden was defined as very high: 0.0–71.0; high: 71.1–81.0; medium: 81.1–91.0; and low: 91.1–100.0. A knot was fit at 3 months post-transplantation on the basis of exploratory data analysis using lowess plots. The score was calculated from adjusted mixed effects models and was estimated for the “reference population” in which age is set to 65, sex is set to men, race is set to non-Black, diabetes status is set to nondiabetic, and time on dialysis is set to zero.
Table 4.
Estimated symptom burden at admission for kidney transplantation and post-transplantation trajectories among recipients (n=521)
| Analysis | Estimated Score, Points (95% Confidence Interval) | Estimated Monthly Change in Score, Points per Month (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|---|
| At Transplantation | 3 months Post-Transplantation | 12 months Post-Transplantation | 0–3 Months Post-Transplantation | Pinteraction Value | 3–12 Months Post-Transplantation | Pinteraction Value | |
| Individual symptom scores (range 0–100) | |||||||
| Muscle soreness | 76.6 (72.9 to 80.2) | 82.4 (78.5 to 86.2) | 80.5 (75.1 to 85.9) | 1.92 (0.93 to 2.92) | −0.21 (–0.81 to 0.40) | ||
| Chest pain | 97.4 (95.9 to 99.0) | 98.2 (96.5 to 99.8) | 98.0 (95.7 to 100.0) | 0.25 (–0.21 to 0.70) | −0.02 (–0.29 to 0.26) | ||
| Cramps | 79.6 (76.5 to 82.7) | 92.9 (89.7 to 96.0) | 91.2 (86.9 to 95.5) | 4.43 (3.56 to 5.31) | −0.19 (–0.71 to 0.33) | ||
| Pruritus | 75.6 (72.6 to 78.7) | 93.2 (90.2 to 96.3) | 91.2 (87.0 to 95.3) | 5.86 (5.00 to 6.72) | −0.23 (–0.74 to 0.28) | ||
| Xeroderma | 72.6 (68.9 to 76.2) | 87.0 (83.3 to 90.6) | 86.3 (81.6 to 91.1) | 4.80 (3.86 to 5.73) | −0.07 (–0.62 to 0.48) | ||
| Dyspnea | 84.6 (81.4 to 87.7) | 85.8 (82.7 to 89.0) | 90.2 (85.9 to 94.6) | 0.42 (–0.32 to 1.15) | 0.49 (0.02 to 0.96) | ||
| Dizziness | 90.2 (87.9 to 92.4) | 92.2 (89.9 to 94.5) | 93.1 (89.9 to 96.4) | 0.67 (0.07 to 1.26) | 0.11 (–0.27 to 0.48) | ||
| Anorexia | 87.5 (84.7 to 90.4) | 96.2 (93.3 to 99.0) | 94.6 (90.7 to 98.6) | 2.88 (2.07 to 3.70) | −0.17 (–0.65 to 0.31) | ||
| Fatigue | 69.2 (65.5 to 72.9) | 83.8 (80.0 to 87.6) | 81.6 (76.6 to 86.7) | 4.86 (3.92 to 5.80) | −0.24 (–0.80 to 0.31) | ||
| Numbness | 85.2 (81.6 to 88.8) | 89.0 (85.3 to 92.6) | 88.6 (84.1 to 93.1) | 1.26 (0.50 to 2.01) | −0.04 (–0.49 to 0.41) | ||
| Upset stomach | 86.1 (83.1 to 89.0) | 93.9 (90.9 to 96.9) | 93.4 (89.3 to 97.6) | 2.60 (1.80 to 3.41) | −0.05 (–0.54 to 0.44) | ||
| Overall symptom score (range 0–100) | |||||||
| Overall score | 82.3 (80.4 to 84.2) | 90.6 (88.7 to 92.5) | 90.0 (87.7 to 92.4) | 2.75 (2.38 to 3.13) | −0.06 (–0.30 to 0.18) | ||
| Age | |||||||
| 18–49 yr a [a] | 81.9 (79.8 to 84.1) | 91.7 (89.6 to 93.8) | 90.9 (87.8 to 94.0) | 3.26 (2.67 to 3.84) | −0.09 (–0.47 to 0.28) | ||
| 50–64 yr [b] | 83.4 (81.2 to 85.6) | 91.1 (88.9 to 93.3) | 91.1 (87.9 to 94.2) | 2.57 (1.96 to 3.18) | −0.01 (–0.39 to 0.37) | ||
| Difference [b−a] | 1.5 (–1.0 to 3.9) | −0.6 (–3.1 to 1.9) | 0.2 (–3.9 to 4.2) | −0.68 (–1.53 to 0.16) | 0.11 | 0.09 (–0.45 to 0.62) | 0.75 |
| ≥65 yr [c] | 83.5 (80.7 to 86.3) | 89.8 (87.0 to 92.6) | 89.0 (84.9 to 93.1) | 2.09 (1.26 to 2.92) | −0.09 (–0.60 to 0.42) | ||
| Difference [c−a] | 1.6 (–1.5 to 4.6) | −1.9 (–5.0 to 1.2) | −1.9 (–6.8 to 3.0) | –1.17 (–2.18 to –0.15) | 0.02 | 0.00 (–0.63 to 0.64) | 0.99 |
| Sex | |||||||
| Men a | 81.7 (79.8 to 83.7) | 91.1 (89.1 to 93.1) | 89.8 (87.1 to 92.4) | 3.12 (2.64 to 3.60) | −0.15 (–0.46 to 0.16) | ||
| Women | 81.9 (79.6 to 84.2) | 88.3 (86.0 to 90.7) | 89.1 (85.9 to 92.3) | 2.14 (1.52 to 2.75) | 0.08 (–0.29 to 0.45) | ||
| Difference | 0.2 (–2.1 to 2.5) | –2.7 (–5.1 to –0.4) | −0.7 (–4.3 to 3.0) | –0.98 (–1.76 to –0.21) | 0.01 | 0.23 (–0.25 to 0.71) | 0.35 |
| Race | |||||||
| Non-Black a | 81.8 (79.8 to 83.7) | 90.9 (89.0 to 92.9) | 90.0 (87.4 to 92.6) | 3.06 (2.59 to 3.53) | −0.10 (–0.40 to 0.19) | ||
| Black | 82.1 (79.6 to 84.6) | 88.8 (86.3 to 91.2) | 88.8 (85.2 to 92.3) | 2.22 (1.60 to 2.84) | 0.00 (–0.40 to 0.40) | ||
| Difference | 0.3 (–2.0 to 2.6) | −2.19 (–4.5 to 0.1) | −1.3 (–5.1 to 2.6) | –0.84 (–1.62 to –0.06) | 0.03 | 0.10 (–0.40 to 0.60) | 0.69 |
| Diabetes status | |||||||
| Nondiabetic a | 81.7 (79.7 to 83.6) | 90.7 (88.8 to 92.6) | 90.8 (88.3 to 93.3) | 3.01 (2.57 to 3.46) | 0.01 (–0.27 to 0.28) | ||
| Diabetic | 82.6 (80.3 to 84.9) | 88.9 (86.6 to 91.3) | 86.4 (82.7 to 90.0) | 2.10 (1.39 to 2.80) | −0.28 (–0.74 to 0.17) | ||
| Difference | 1.0 (–1.5 to 3.5) | −1.8 (–4.3 to 0.8) | –4.4 (–8.5 to –0.3) | –0.92 (–1.75 to –0.09) | 0.03 | −0.29 (–0.82 to 0.23) | 0.27 |
| Frailty | |||||||
| Nonfrail a | 82.9 (81.0 to 84.8) | 90.9 (89.0 to 92.7) | 90.8 (89.0 to 92.5) | 2.66 (2.29 to 3.03) | −0.01 (–0.13 to 0.11) | ||
| Frail | 77.9 (74.5 to 81.4) | 86.7 (83.1 to 90.2) | 88.1 (84.8 to 91.3) | 2.90 (1.88 to 3.93) | 0.16 (–0.18 to 0.49) | ||
| Difference | –4.9 (–8.3 to –1.6) | –4.2 (–7.6 to –0.9) | −2.7 (–5.7 to 0.3) | 0.24 (–0.84 to 1.33) | 0.66 | 0.17 (–0.18 to 0.52) | 0.35 |
Recipients were enrolled at admission for transplantation. The overall symptom score ranged from zero to 100 points and was on the basis of self-reported burden of individual symptom/problem of kidney disease with five response options ranging from “not at all bothered” to “extremely bothered.” The individual symptom scores were transformed to a scale of 0–100 possible range, where a higher score represents lower burden. The overall symptom score was calculated by averaging across the 12 individual scores, so a higher symptom score represents a lower burden. Symptom burden was defined as very high: 0.0–71.0; high: 71.1–81.0; medium: 81.1–91.0; and low: 91.1–100.0. Estimated scores and monthly change (slope per unit time of 1 month) with 95% confidence intervals are presented from separate adjusted mixed effects models. The scores were estimated for the “reference population” in which age is set to 65 years, sex is set to men, race is set to non-Black, diabetes status is set to nondiabetic, and time on dialysis is set to zero, and they are estimated for the last day for the time interval. Monthly changes and differences that are statistically significant at P=0.05 are in bold. All models were adjusted for age, sex, Black race, diabetes status, and time on dialysis.
Reference group.
The estimated overall symptom score was 82.3 points at transplantation, 90.6 points at 3 months (10% improvement) post-transplantation, and 90.0 points at 12 months (9% improvement) post-transplantation (Table 4) for the reference population. This score increased 2.75 points per month (95% CI, 2.38 to 3.13) during 0–3 months post-transplantation; it plateaued (−0.06 points per month; 95% CI, −0.30 to 0.18) from 3 to 12 months post-transplantation (Table 4). Although trajectories between frail and nonfrail recipients did not significantly differ between 0–3 (P=0.66) or 3–12 months (P=0.35), frail recipients had significantly lower (worse) scores at transplantation and 3 months post-transplantation compared with nonfrail recipients. Specifically, frail recipients had estimated symptom scores of 4.9 points (95% CI, 1.6 to 8.3) lower at transplantation and 4.2 points (95% CI, 0.9 to 7.6) lower at 3 months compared with nonfrail counterparts. Additionally, younger, male, non-Black, nondiabetic recipients showed a greater rate of improvement in symptom score (all Pinteraction values =0.04) (Table 4).
Sensitivity Analyses
In spite of a few changes in significance, the direction and magnitude of our inferences remained robust after (1) excluding patients who were preemptive candidates (Supplemental Table 4), (2) additionally adjusting waitlist mortality models for additional potential confounders (Supplemental Table 5), and (3) accounting for ceiling effects of symptom score (Supplemental Table 6).
Discussion
In this prospective cohort study of 1298 patients being evaluated for kidney transplant, 16% of participants reported high and 21% reported very high symptom burden. After adjustment, patients with very high symptom burden were at greater waitlist mortality risk (aSHR, 1.67; 95% CI, 1.06 to 2.62). By transplantation (n=190), 25% experienced decline in symptom burden, and 34% experienced an increase in symptom burden. In the first 3 months post-transplantation (n=521), nine of the 11 symptoms improved; the greatest improvements were in pruritus (23% improvement) and fatigue (21% improvement) after adjustment. Overall, symptom score improved 10% in the first 3 months post-transplantation. Our study suggests that kidney disease symptoms remain unchanged or worsen until transplantation, but then, they improve post-transplantation; this reduction in symptom burden is greatest in younger, male, non-Black, and nondiabetic recipients.
To our knowledge, this is the first longitudinal study of symptoms in kidney transplant candidates and recipients rather than patients on dialysis (1–7,15). Interestingly, the symptom burden was higher in younger patients, possibly due to changing expectations about how they perceive symptoms as they age. A systematic review found that approximately 55% of patients on dialysis report being moderately bothered by pruritus, 71% report being moderately bothered by fatigue, and 49% report being moderately bothered by loss of appetite (anorexia) (1). In contrast, we found a much lower burden of symptoms. One potential reason for this difference may be that patients undergoing evaluation are typically healthier than general dialysis populations and includes preemptive transplant candidates. Therefore, these results are only generalizable to transplant candidates and may not be generalizable to all patients with kidney failure.
In a previous study, symptoms were associated with a higher mortality risk among patients undergoing dialysis in Norway (16); we extended these findings to the candidate population and found that those with a high symptom burden were at a 1.67-fold higher waitlist mortality risk. Some of these symptoms may result from uremia, which is associated with higher mortality via multiple pathways, including lipid abnormalities, insulin resistance, and subsequent cardiovascular disease (35–38). Given that dialysis centers are required to administer Kidney Disease Quality of Life surveys annually (39), adding the symptoms assessment to the transplant evaluation would help inform the decision making surrounding post-transplantation care and allow for discussion of the potential benefit of transplantation in reducing symptoms. Additional information about how symptoms will change over time after transplantation can correctly inform expectations about life post-transplantation and, thus, allow patients and clinicians to make more informed decisions and necessary preparations for their future care after transplantation.
We built upon previous cross-sectional studies (18–22) and identified longitudinal changes in symptoms pre- and post-transplantation. We found that 75% of candidates on the waitlist had worsened or remained unchanged in symptom scores between evaluation and transplantation. However, there were profound short-term improvements in nine of the 11 symptom scores and in the overall score after transplantation; there was a 10% improvement in overall symptom burden in just 3 months post-transplantation, similar to the short-term health-related quality of life, cognition, and frailty improvements after transplantation (20,21,40). It is unclear whether the plateau in symptoms after 3 months is related to a lack of precision in the data during follow-up or is due to other transplant-related factors, such as choice of immunosuppression (18–22,41). These findings highlight the importance of restoration of kidney function on changes in symptoms, provide estimates of percentage reduction for each individual symptom, and clarify the time frame for these reductions in symptoms.
Strengths of this study include the large number of patients enrolled in this two-center prospective cohort study and the measurement of KDQOL-SF to assess symptoms at evaluation and admission. Symptoms were self-reported using a standard assessment for quality of life in this population; this patient-centered measure is available to clinicians and open to interpretation in routine clinical practice. Furthermore, the multiple measures of symptoms in the same patients allowed for the assessment of pre- and post-transplantation changes in symptoms. The main limitation of this study is that we were limited to two transplant centers, so generalizability of inferences may be limited. However, our cohorts were similar to the national transplant population. Additionally, the study did not collect hours of dialysis per week, Kt/V, urea reduction ratio, or other measures of dialysis adequacy. Finally, future studies should estimate the minimally important differences in symptom scores for the KDQOL-SF.
In conclusion, although symptom burden was most likely to be unchanged or worsen pretransplantation and be associated with waitlist mortality, there was substantial and clinically significant early improvement in specific individual symptoms and the overall symptom scores in the first 3 months post-transplantation. In fact, nine of the 11 symptoms improved, and in particular, there was a 23% decline in pruritus burden. Discussion of expected reductions in symptoms should be a part of the shared decision making for future care post-transplantation. Our findings on the post-transplantation change in symptoms can help inform this important discussion, clarify the timeline for improvement, identify populations that are most likely to benefit, and promote patient-centered care.
Disclosures
P. Butz reports ownership interest in Clene, Progenity, Spectrum Pharmaceuticals, Vaxart, Veru, and Viking Therapeutics. D.C. Crews reports consultancy agreements with Yale New Haven Health Services Corporation Center for Outcomes Research and Evaluation; receiving research funding from Somatus, Inc.; serving on the editorial boards of CJASN, JASN, and Journal of Renal Nutrition; serving as an associate editor of Kidney360; serving as a cochair of Bayer HealthCare Pharmaceuticals Inc. Patient and Physician Advisory Board Steering Committee for Disparities in Chronic Kidney Disease Project; serving as a scientific advisor or member of the advisory group of the Health Equity Collaborative, Partner Research for Equitable System Transformation after COVID-19, Optum Labs; and other interests/relationships with the Council of Subspecialist Societies of the American College of Physicians, the board of directors of the National Kidney Foundation of Maryland/Delaware, and the Nephrology Board of the American Board of Internal Medicine. A. Mathur reports serving as an editorial board member of Thyroid. S. Norman reports receiving research funding from Allo-Vir, Astellas, and Natera; serving as a scientific advisor or member of the American Kidney Fund Board of Trustees, the Minority Organ and Tissue Transplant Education Program Detroit Foundation Board, and the National Kidney Foundation of Michigan Scientific Advisory Board and Board of Directors; and other interests/relationships with the Mitzvah Circle Foundation. D.L. Segev reports consultancy agreements with, receiving honoraria from, and speakers bureau for CSL Behring, Genzyme/Sanofi, and Novartis. T. Shafi reports consultancy agreements with Siemens; receiving research funding from Baxter (clinical trial); receiving honoraria from Cara Therapeutics, the National Institutes of Health, and Siemens; and serving as a scientific advisor or member of American Journal of Kidney Diseases and CJASN. K. Taylor reports serving as the Corporate Vice President of Quality for Fresenius Kidney Care from September 2016 through December 2017. K. Taylor is currently a PhD student/candidate at the Johns Hopkins University School of Nursing and does not currently have any financial interests, relationship, or commitment with Fresenius Kidney Care. All remaining authors have nothing to disclose.
Funding
Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Diseases and National Institute on Aging grants K01AG064040 (Principal Investigator [PI]: N.M. Chu), R01AG055781 (PI: M.A. McAdams-DeMarco), R01DK114074 (PI: M.A. McAdams-DeMarco), K23AG053429 (PI: A. Mathur), and K24AI144954 (PI: D.L. Segev). D.C. Crews was supported by National Heart, Lung, and Blood Institute grant K24HL148181. K.I. Greenberg was supported by National Heart, Lung, and Blood Institute grant R01HL132372 and National Institute on Aging grant UH3AG056933. T. Shafi was supported by National Heart, Lung, and Blood Institute grant R01HL132372 and National Institute of Nursing Research grant R01NR017399. K. Taylor was supported by National Center for Advancing Translational Sciences grant TL1TR003100.
Supplementary Material
Acknowledgments
The funders had no role in the study design, data collection, analysis, reporting, or decision to submit for publication.
Footnotes
K.T. and N.M.C. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice, “Kidney Disease Burden and Kidney Transplantation: A True Story,” on pages 989–990.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.19031220/-/DCSupplemental.
Supplemental Figure 1. Derivation of the kidney transplant candidate and recipient cohorts.
Supplemental Table 1. Distributions of symptoms of kidney disease scores from the KDQOL-SF among kidney transplant candidates (at evaluation) and recipients (at admission) as compared with the reference population.
Supplemental Table 2. Prevalence of very high symptom burden by risk factors and functional and cognitive status among kidney transplant candidates (n=1298).
Supplemental Table 3. Changes in kidney disease symptom burden between evaluation and admission for kidney transplantation using the combined candidate and recipient cohorts (n=190).
Supplemental Table 4. Risk of waitlist mortality by individual and overall symptom scores among kidney transplant candidates undergoing pretransplantation dialysis.
Supplemental Table 5. Risk of waitlist mortality and symptoms of kidney disease among kidney transplant candidates (n=1282).
Supplemental Table 6. Estimated overall symptom score at admission for kidney transplantation and post-transplantation trajectories among recipients using the Tobit model (n=521).
References
- 1.Murtagh FE, Addington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Caplin B, Kumar S, Davenport A: Patients’ perspective of haemodialysis-associated symptoms. Nephrol Dial Transplant 26: 2656–2663, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT: Physical symptoms and quality of life in patients on chronic dialysis: Results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD). Nephrol Dial Transplant 14: 1163–1170, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Levy AR, Xing S, Brunelli SM, Cooper K, Finkelstein FO, Germain MJ, Kimel M, Platt RW, Belozeroff V: Symptoms of secondary hyperparathyroidism in patients receiving maintenance hemodialysis: A prospective cohort study. Am J Kidney Dis 75: 373–383, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Rhee EP, Guallar E, Hwang S, Kim N, Tonelli M, Moe SM, Himmelfarb J, Thadhani RI, Powe NR, Shafi T: Prevalence and persistence of uremic symptoms in incident dialysis patients. Kidney360 1: 86–92, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Kader K, Unruh ML, Weisbord SD: Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 4: 1057–1064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moledina DG, Perry Wilson F: Pharmacologic treatment of common symptoms in dialysis patients: A narrative review. Semin Dial 28: 377–383, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Flythe JE, Dorough A, Narendra JH, Forfang D, Hartwell L, Abdel-Rahman E: Perspectives on symptom experiences and symptom reporting among individuals on hemodialysis. Nephrol Dial Transplant 33: 1842–1852, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox KJ, Parshall MB, Hernandez SHA, Parvez SZ, Unruh ML: Symptoms among patients receiving in-center hemodialysis: A qualitative study. Hemodial Int 21: 524–533, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Pilsum Rasmussen SE, Warsame F, Eno AK, Ying H, Covarrubias K, Haugen CE, Chu NM, Crews DC, Harhay MN, Schoenborn NL, Segev DL, McAdams-DeMarco MA: Perceptions, barriers, and experiences with successful aging before and after kidney transplantation: A focus group study. Transplantation 104: 603–612, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flythe JE, Hilliard T, Lumby E, Castillo G, Orazi J, Abdel-Rahman EM, Pai AB, Rivara MB, St Peter WL, Weisbord SD, Wilkie CM, Mehrotra R; Kidney Health Initiative Prioritizing Symptoms of ESRD Patients for Developing Therapeutic Interventions Stakeholder Meeting Participants: Fostering innovation in symptom management among hemodialysis patients: Paths forward for insomnia, muscle cramps, and fatigue. Clin J Am Soc Nephrol 14: 150–160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perl J, Dember LM, Bargman JM, Browne T, Charytan DM, Flythe JE, Hickson LJ, Hung AM, Jadoul M, Lee TC, Meyer KB, Moradi H, Shafi T, Teitelbaum I, Wong LP, Chan CT; American Society of Nephrology Dialysis Advisory Group: The use of a multidimensional measure of dialysis adequacy-moving beyond small solute kinetics. Clin J Am Soc Nephrol 12: 839–847, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flythe JE, Hilliard TS, Ikeler K, Keller S, Gipson DS, Grandinetti AC, Nordyke RJ, Perrone RD, Roy-Chaudhury P, Unruh M, West M, Bocell F, Hurst FP: Toward patient-centered innovation: A conceptual framework for patient-reported outcome measures for transformative kidney replacement devices. Clin J Am Soc Nephrol 15: 1522–1530, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standardised Outcomes in Nephrology: SONG-HD. 2015. Available at: https://songinitiative.org/projects/song-hd/. Accessed May 3, 2020
- 15.Amro A, Waldum B, Dammen T, Miaskowski C, Os I: Symptom clusters in patients on dialysis and their association with quality-of-life outcomes. J Ren Care 40: 23–33, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Amro A, Waldum B, von der Lippe N, Brekke FB, Dammen T, Miaskowski C, Os I: Symptom clusters predict mortality among dialysis patients in Norway: A prospective observational cohort study. J Pain Symptom Manage 49: 27–35, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Bossola M, Di Stasio E, Antocicco M, Panico L, Pepe G, Tazza L: Fatigue is associated with increased risk of mortality in patients on chronic hemodialysis. Nephron 130: 113–118, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Ozcan H, Yucel A, Avşar UZ, Cankaya E, Yucel N, Gözübüyük H, Eren F, Keles M, Aydınlı B: Kidney transplantation is superior to hemodialysis and peritoneal dialysis in terms of cognitive function, anxiety, and depression symptoms in chronic kidney disease. Transplant Proc 47: 1348–1351, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Molnar MZ, Novak M, Ambrus C, Szeifert L, Kovacs A, Pap J, Remport A, Mucsi I: Restless legs syndrome in patients after renal transplantation. Am J Kidney Dis 45: 388–396, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Chu NM, Gross AL, Shaffer AA, Haugen CE, Norman SP, Xue QL, Sharrett AR, Carlson MC, Bandeen-Roche K, Segev DL, McAdams-DeMarco MA: Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol 30: 336–345, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams DeMarco M, Olorundare I, Ying H, Warsame F, Haugen C, Hall R, Garonzik Wang JM, Desai NM, Walston J, Norman SP, Segev D: Frailty and post-kidney transplant health-related quality of life. Transplantation 102: 291–299, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs AZ, Molnar MZ, Szeifert L, Ambrus C, Molnar-Varga M, Szentkiralyi A, Mucsi I, Novak M: Sleep disorders, depressive symptoms and health-related quality of life--A cross-sectional comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis. Nephrol Dial Transplant 26: 1058–1065, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Gordon EJ, Butt Z, Jensen SE, Lok-Ming Lehr A, Franklin J, Becker Y, Sherman L, Chon WJ, Beauvais N, Hanneman J, Penrod D, Ison MG, Abecassis MM: Opportunities for shared decision making in kidney transplantation. Am J Transplant 13: 1149–1158, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group: Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Teng EL, Chui HC: The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48: 314–318, 1987 [PubMed] [Google Scholar]
- 27.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB: A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49: M85–M94, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW: Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 185: 914–919, 1963 [DOI] [PubMed] [Google Scholar]
- 29.Lawton MP, Brody EM: Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: 179–186, 1969 [PubMed] [Google Scholar]
- 30.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB: Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 3: 329–338, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Hays RD, Kallich JD, Mapes DL, Coons SJ, Amin N, Carter WB, Kamberg C: Kidney Disease Quality of Life Short Form (KDQOL-SF), Version 1.3: A Manual for Use and Scoring, Santa Monica, CA, Rand, 1997 [Google Scholar]
- 32.Korevaar JC, Merkus MP, Jansen MA, Dekker FW, Boeschoten EW, Krediet RT; NECOSAD-study group: Validation of the KDQOL-SF: A dialysis-targeted health measure. Qual Life Res 11: 437–447, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Peipert JD, Bentler PM, Klicko K, Hays RD: Psychometric properties of the Kidney Disease Quality of Life 36-Item Short-Form Survey (KDQOL-36) in the United States. Am J Kidney Dis 71: 461–468, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Tobin J: Estimation of relationships for limited dependent variables. Econometrica 26: 24–36, 1958 [Google Scholar]
- 35.Massy ZA: The role of lipids and uremic toxins in cardiovascular disease in CKD. Clin Exp Nephrol 18: 255–256, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Melamed ML, Plantinga L, Shafi T, Parekh R, Meyer TW, Hostetter TH, Coresh J, Powe NR: Retained organic solutes, patient characteristics and all-cause and cardiovascular mortality in hemodialysis: Results from the retained organic solutes and clinical outcomes (ROSCO) investigators. BMC Nephrol 14: 134, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Himmelfarb J, Ikizler TA: Hemodialysis. N Engl J Med 363: 1833–1845, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Meyer TW, Hostetter TH: Uremia. N Engl J Med 357: 1316–1325, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Centers for Medicare & Medicaid Services (CMS), Department of Health and Human Services: Conditions of coverage for end-stage renal disease facilities: Interpretive guidance. Baltimore, MD. April 15, 2008
- 40.McAdams-DeMarco MA, Isaacs K, Darko L, Salter ML, Gupta N, King EA, Walston J, Segev DL: Changes in frailty after kidney transplantation. J Am Geriatr Soc 63: 2152–2157, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolonko A, Jurys M, Sirek S, Dwulit T, Pojda-Wilczek D, Więcek A: The effect of maintenance treatment with twice-daily or prolonged once-daily tacrolimus formulation on visual evoked potentials in stable kidney transplant recipients. J Clin Med 9: 1827, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.