Visual Abstract
Keywords: vaccination, COVID-19, SARS-CoV-2, dialysis, humoral response, hemodialysis
Abstract
Background and objectives
Patients receiving hemodialysis are at high risk for both severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe coronavirus disease 2019. A lifesaving vaccine is available, but sensitivity to vaccines is generally lower in patients on dialysis. Little is yet known about antibody responses after coronavirus disease 2019 (COVID-19) vaccination in this vulnerable group.
Design, setting, participants, and measurements
In this prospective single-center study, we included 22 patients on dialysis and 46 healthy controls from Heidelberg University Hospital between December 2020 and February 2021. We measured anti-S1 IgG with a threshold index for detection greater than one, neutralizing antibodies with a threshold for viral neutralization of ≥30%, and antibodies against different SARS-CoV2 fragments 17–22 days after the first dose and 18–22 days after the second dose of the mRNA vaccine BNT162b2.
Results
After the first vaccine dose, four of 22 (18%) patients on dialysis compared with 43 of 46 (93%) healthy controls developed positive anti-S1 IgG, with a median anti-S1 IgG index of 0.2 (interquartile range, 0.1–0.7) compared with nine (interquartile range, 4–16), respectively. SARS-CoV2 neutralizing antibodies exceeded the threshold for neutralization in four of 22 (18%) patients on dialysis compared with 43 of 46 (93%) healthy controls, with a median percent inhibition of 11 (interquartile range, 3–24) compared with 65 (interquartile range, 49–75), respectively. After the second dose, 14 of 17 (82%) patients on dialysis developed neutralizing antibodies exceeding the threshold for viral neutralization and antibodies against the receptor binding S1 domain of the spike protein, compared with 46 of 46 (100%) healthy controls, respectively. The median percent inhibition was 51 (interquartile range, 32–86) compared with 98 (interquartile range, 97–98) in healthy controls.
Conclusions
Patients receiving long-term hemodialysis show a reduced antibody response to the first and second doses of the mRNA vaccine BNT162b2. The majority (82%) develop neutralizing antibodies after the second dose but at lower levels than healthy controls.
Introduction
Patients receiving long-term hemodialysis treatment for kidney failure are at high risk of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection (1). Because self-isolation is not feasible in this cohort, an extremely high incidence of SARS-CoV-2 infection is reported from countries around the world, ranging from 5% to 20%, with even higher seroprevalence rates of up to 36% (2–5). Patients on dialysis are not only particularly susceptible to SARS-CoV-2 infection but are also at high risk for more severe disease progression compared with hospitalized patients without kidney failure. The generally impaired immune response together with a high prevalence of comorbidities results in mortality rates of up to 32% (6). In the absence of disease-specific drugs, it is hoped that sustained immunity following SARS-CoV-2 vaccination will reduce this substantially high mortality rate.
As kidney function declines, there seems to be lower vaccine responsiveness (7,8). Various strategies have been explored to improve responses to hepatitis B and influenza A vaccination, such as higher doses of vaccine, use of adjuvants, or additional immunizations (9–12). However, there are few data available on the efficacy of coronavirus disease 2019 (COVID-19) vaccination, also because large vaccine trials excluded patients on dialysis (13). Moreover, data on humoral and cellular response after COVID-19 disease are also limited in patients on dialysis. Although one dialysis center in New York reported detectable SARS-CoV-2 IgG antibodies against the nucleocapsid protein in all of their patients 2 months after SARS-CoV-2 infection, a French group noted a lack of seroconversion, particularly in immunosuppressed patients (14,15).
SARS-CoV-2 has four major structural proteins called spike, envelope, membrane, and nucleocapsid protein. The spike protein consists of the S1 subunit, which mediates cell surface binding via the receptor binding domain, and the S2 subunit, which induces viral-host cell membrane fusion (16). Many antibodies result from SARS-CoV-2 infection. However, antibodies to the highly immunogenic receptor binding domain could account for up to 90% of neutralizing SARS-CoV-2–specific antibodies, whereas there is little evidence of neutralizing antibodies to viral structural proteins, such as the nucleocapsid protein (16).
Given the lack of data in these high-risk patients, together with a persistent threat of infection, there is an urgent need to determine vaccination success in patients on dialysis. There is no “one-size-fits-all” approach to vaccination, and cohort-adapted immunization protocols might be required (17,18). Here, we provide a thorough characterization of the early humoral response following vaccination with BNT162b2 mRNA vaccine in patients on dialysis.
Methods and Materials
Study Design and Cohorts
In this prospective, single-center study, we included 22 patients on long-term hemodialysis and 46 healthy controls without CKD who had received two doses of the mRNA vaccine BNT162b2 (BioNTech) 19–22 days apart between December 2020 and February 2021 at the Division of Nephrology of the University Hospital of Heidelberg. Key inclusion and exclusion criteria are given in Supplemental Table 1. In addition, we performed a subgroup analysis with age-matched patients on dialysis (n=20) and healthy controls (n=15) by frequency matching. Antibodies to the nucleocapsid protein were measured before enrollment and after the first and the second vaccinations, and individuals with positivity were excluded because of suspected recent SARS-CoV-2 infection. In addition, SARS-CoV-2 antigen rapid tests were performed in patients on dialysis before every single dialysis session. These measures were taken to exclude individuals who developed infection during follow-up to detect an unbiased seroresponse due to vaccination rather than infection. The anti-S1 IgG index and SARS-CoV-2–specific neutralizing antibodies were measured after a median of 18 (17–21) and 19 (18–22) days after the first dose and after a median of 20 (18–21) and 20 (18–22) days after the second dose in patients on dialysis and in healthy controls, respectively. In addition, a multiplex assay was performed to detect different SARS-CoV-2 target antibodies at the same time point after the second dose. The study was approved by the ethics committee of the University of Heidelberg and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Anti-Severe Acute Respiratory Syndrome Coronavirus Type 2 Immunoglobulin G Enzyme-Linked Immunosorbent Assay
For determination of the IgG response against the S1 protein, the SARS-CoV-2 Total Assay (Siemens, Eschborn, Germany) was performed according to the manufacturer’s instructions, and for determination of the IgG response against the nucleocapsid protein, the ECLIA assay (Roche, Mannheim, Germany) was performed according to the manufacturer’s instructions. The value of the anti-S1 antibody test is expressed as a dimensionless index. Semiquantitative index of less than one was classified as negative, and a value of greater than or equal to one was classified as positive. According to the manufacturer, the cutoff value was set three SDs above the mean of the negatives. This cutoff for detection gives a specificity of 100% with a sensitivity of 89%.
Detection of Severe Acute Respiratory Syndrome Coronavirus Type 2 Neutralizing Antibodies
The binding inhibition potency of serum samples was investigated by a plate-based SARS-CoV-2 surrogate virus neutralizing assay (Medac, Wedel, Germany) (19,20). Using purified receptor binding domain protein from the viral spike protein and the host cell receptor angiotensin-converting enzyme 2 (ACE2), this test mimics the virus-host interaction by direct protein-protein interaction. In brief, antibodies in serum samples were first conjugated with soluble SARS-CoV-2 receptor binding domain-horseradish peroxidase and incubated with ACE2-coated wells. Unbound receptor binding domain-horseradish peroxidase was washed out twice, and the reactions were developed using 3,3′,5,5′-tetramtheylnezdine as substrate. OD at 450 nm was measured in each well, and the percent inhibition was calculated as follows:
A cutoff of ≥30% inhibition of receptor binding domain: ACE2 binding was applied according to the manufacturer’s instructions (19). Tan et al. (19) validated the SARS-CoV-2 surrogate virus neutralizing assay (Medac) in their previous publication and chose a final cutoff for viral neutralization of 30%, resulting in a specificity of 100% with a sensitivity of 98%.
Bead-Based Multiplex Assay for Severe Acute Respiratory Syndrome Coronavirus Type 2 Antibody Detection
For the detection of IgG antibodies against SARS-CoV-2 target antigens, a multiplex bead-based assay for the Luminex platform (LabScreen COVID Plus) was performed (One Lambda Inc., West Hill, CA) (21). The assay includes, other than the SARS-CoV-2 nucleocapsid protein, four distinct fragments of the SARS-CoV-2 spike protein, namely the full spike protein, the S1 protein, the receptor binding domain of the spike protein, and the S2 protein. Additionally, the test incorporates S1 fragments from six other coronaviruses, namely HCoV-229E, HCoV-HKU1, HCoV-NL63, HCoV-OC43, MERS-CoV, and SARS-CoV-1. Antibody detection on antigen-coated microparticles was performed according to the manufacturer’s instructions, and the mean fluorescence intensity (MFI) was analyzed on a Luminex 200 device (Luminex Corporation, Noord-Brabant, The Netherlands). The MFI cutoff values for each of the 11 proteins are given in Supplemental Table 2.
Statistical Analyses
Data are expressed as median and interquartile range (IQR) or number (N) and percent. Continuous and categorial variables were analyzed using the nonparametric t test with the Well correction or the Mann–Whitney U test, respectively. The correlation of SARS-CoV-2–specific antibody indices and neutralizing antibodies with age was examined using the Spearman correlation analysis. Statistical significance was assumed at a P value =0.05. The statistical analysis was performed using GraphPad Prism version 9.0.0 (GraphPad Software, San Diego, CA).
Results
Baseline Characteristics
From December 29, 2020 to February 2, 2021, we prospectively enrolled 22 patients on dialysis and 46 healthy controls before a first vaccination with the mRNA vaccine BNT162b2. Baseline characteristics are given in Table 1. Antibodies against the nucleocapsid protein remained negative in all individuals during follow-up. Patients on dialysis were older than healthy controls (median age 74 versus 48 years). To exclude a decisive effect of age on antibody results, we performed a subgroup analysis with age-matched patients on dialysis (n=20) and healthy controls (n=15) (Table 1). Assays were performed after the first and second doses in all healthy control subjects and in 22 and 17 subjects, respectively, in the dialysis cohort. In the dialysis cohort, two patients aged 87 and 89 died due to acute myocardial infarction 12 and 19 days after the first vaccination, respectively. Two patients were hospitalized due to an intestinal perforation and pneumonia 5 and 17 days after the first vaccination. Both patients missed their second vaccination. One patient changed the dialysis center. There are no missing results for any of the assays.
Table 1.
Characteristics of participants in a prospective cohort study of early humoral responses to coronavirus disease 2019 vaccination with BNT162b2 among patients on hemodialysis
| Characteristic | Healthy Controls, n=46 | Patients on Dialysis, n=22 | Healthy Controls: Age Matched, n=15 | Patients on Dialysis: Age Matched, n=20 |
|---|---|---|---|---|
| Age, yr, median (range) | 48a (28–90) | 74a (51–92) | 67 (54–90) | 72 (51–82) |
| Sex, n (%) | ||||
| Men | 19 (41) | 12 (55) | 11 (73) | 10 (50) |
| Women | 27 (59) | 10 (45) | 4 (27) | 10 (50) |
| Times from dialysis initiation, yr, median (range) | N/A | 5 (2–14) | N/A | 8 (5–14) |
| Cause of kidney failure, n (%) | ||||
| Diabetes or vascular nephropathy | N/A | 14 (64) | N/A | 14 (70) |
| Glomerular disease | N/A | 4 (18) | N/A | 3 (15) |
| Other | N/A | 4 (18) | N/A | 3 (15) |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | N/A | 14 (64) | N/A | 3 (15) |
| Hypertension | N/A | 21 (95) | N/A | 20 (100) |
| Obesity, BMI>25 kg/m2 | N/A | 16 (73) | N/A | 15 (75) |
| Coronary heart disease | N/A | 8 (36) | N/A | 7 (35) |
| Peripheral artery disease | N/A | 6 (27) | N/A | 4 (20) |
| Chronic heart failure | N/A | 3 (14) | N/A | 3 (15) |
| COPD | N/A | 1 (5) | N/A | 1 (5) |
| Cirrhosis | N/A | 1 (5) | N/A | 0 (0) |
N/A, not available; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
aP<0.001.
Spike Antigen–Specific Severe Acute Respiratory Syndrome Coronavirus Type 2 Immunoglobulin G Levels Are Lower in Patients on Dialysis after the First and Second Vaccinations than in Healthy Controls
Patients on dialysis had significantly lower anti-S1 IgG levels as compared with total as well as age-matched healthy controls (Figure 1, A and B, Table 2). Only four of 20 (20%) age-matched patients on dialysis had detectable anti-S1 IgG antibodies after the first vaccination with index levels above the cutoff for detection with a median index of 0.2 (IQR, 0.1–0.7). In contrast, age-matched healthy controls had a median anti-S1 IgG index of six (IQR, 1–16), with 12 of 15 (80%) patients exceeding the cutoff for detection (Figure 1, A and B, Table 2).
Figure 1.
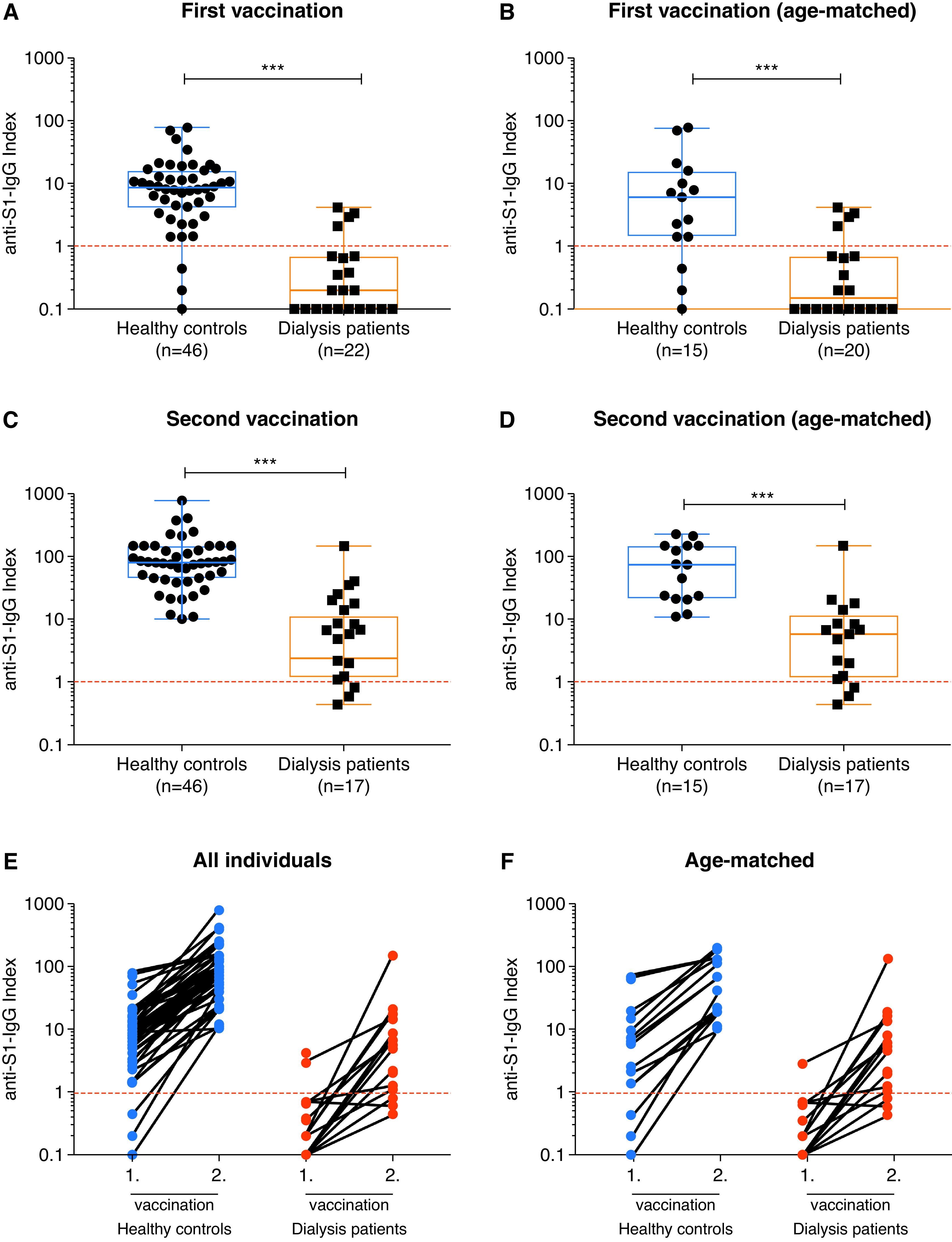
Spike antigen–specific severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG levels of patients on hemodialysis and healthy controls after vaccination with BNT162b2. SARS-CoV-2 IgG antibodies represented logarithmically as an anti–S1-IgG index in all and in age-matched healthy controls and patients on dialysis after the first (A and B) and after the second (C and D) coronavirus disease 2019 (COVID-19) vaccination. Individual SARS-CoV-2 IgG antibody courses in all (E) and in age-matched (F) individuals after the first and after the second COVID-19 vaccination. The dashed red lines represent the cutoff for detection according to the manufacturer’s instructions. A semiquantitative index of less than one was classified as negative, and a value of one or higher was classified as positive. ***P<0.001.
Table 2.
Severe acute respiratory syndrome coronavirus 2 IgG antibody response and severe acute respiratory syndrome coronavirus 2 neutralizing capacity of patients on hemodialysis and healthy controls after vaccination with BNT162b2
| Humoral Responses | All Participants | Age-Matched Participants | ||||
|---|---|---|---|---|---|---|
| Healthy Controls | Patients on Dialysis | P Value | Healthy Controls | Patients on Dialysis | P Value | |
| SARS-CoV-2 IgG antibody response | ||||||
| Participants meeting threshold for positive response, N (%) | ||||||
| After first vaccine | 43 (93) | 4 (18) | <0.001 | 12 (80) | 4 (20) | <0.001 |
| After second vaccine | 46 (100) | 14 (82) | <0.001 | 15 (100) | 14 (82) | <0.001 |
| Anti-S1 IgG index, median (IQR) | ||||||
| After first vaccine | 9 (4–16) | 0.2 (0.1–0.7) | <0.001 | 6 (1–16) | 0.2 (0.1–0.7) | <0.001 |
| After second vaccine | 81 (45–150) | 6 (1–11) | <0.001 | 74 (21–150) | 6 (1–11) | <0.001 |
| SARS-CoV-2 neutralizing capacity | ||||||
| Participants meeting threshold for viral neutralization, N (%) | ||||||
| After first vaccine | 43 (93) | 4 (18) | <0.001 | 12 (80) | 4 (20) | <0.001 |
| After second vaccine | 46 (100) | 14 (82) | <0.001 | 15 (100) | 14 (82) | <0.001 |
| Inhibition (%), median (IQR) | ||||||
| After first vaccine | 65 (49–75) | 11 (3–24) | <0.001 | 62 (37–75) | 16 (7–24) | <0.001 |
| After second vaccine | 98 (97–98) | 51 (32–86) | <0.001 | 98 (92–98) | 51 (32–86) | <0.001 |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IQR, interquartile range.
After the second vaccination, we measured anti-S1 IgG levels in 46 healthy controls and 17 patients on dialysis. The anti-S1 IgG index after the second vaccination remained significantly lower in patients on dialysis as compared with total and age-matched healthy controls (Figure 1, C and D, Table 2). Patients on dialysis had a median index of six (IQR, 1–11) compared with a median index of 74 (IQR, 21–150) in age-matched healthy controls. All healthy individuals had measurable anti-S1 IgG antibodies with no participant testing below the detection cutoff, whereas three of 17 (18%) age-matched patients on dialysis had no detectable anti-S1 IgG antibodies after the second vaccination (Figure 1, C and D). The individual anti-S1 IgG course after the first and second vaccinations in every participant is shown in Figure 1, E and F.
In addition, we determined the IgG reactivity against four different fragments of the SARS-CoV-2 S protein, SARS-CoV-2 nucleocapsid protein, and S1 proteins from six other coronaviruses in patients on dialysis (n=17) and age-matched healthy controls (n=15) 18–22 days after the second vaccination (Figure 2). All 15 individuals of the healthy control group showed MFI values above the cutoff for the full spike protein, the S1 spike protein, and the spike receptor binding domain protein, with median MFIs of 24,640 (IQR, 23,866–25,576), 16,850 (IQR, 14,153–19,071), and 21,188 (IQR, 19,358–22,955), respectively (Figure 2, A–C). Patients on dialysis had significantly lower MFI values for the same three proteins, with median MFIs of 20,513 (IQR, 14,256–23,926), 7808 (IQR, 4398–10,171), and 11,902 (IQR, 6814–15,319), respectively (for all P<0.001) (Figure 2, A–C). The MFI values against the S2 spike protein were lower compared with all other S-protein fragments in both groups. Six of 15 healthy controls (40%) and 14 of 17 patients on dialysis (82%) showed values under the MFI cutoff, with median MFIs of 6369 (IQR, 2613–12,224) and 1305 (IQR, 429–2349), respectively (P=0.01) (Figure 2D). The exclusion of patients with recent SARS-CoV-2 infection was confirmed by MFI values clearly under the given cutoff for the nucleocapsid protein in all individuals (Figure 2E). We also detected a reactivity against the S1 spike protein of SARS-CoV-1, which was again higher in healthy controls (median MFI, 959; IQR, 493–1278) compared with patients on dialysis (median MFI, 160; IQR, 59–522; P<0.001) (Supplemental Figure 1). No differences were found between the groups in IgG reactivities against other coronaviruses (Supplemental Figure 1).
Figure 2.

IgG antibodies against different SARS-CoV-2 target antigens of patients on hemodialysis and healthy controls after vaccination with BNT162b2. Detection of antibodies against the full spike protein (A), the S1 spike protein (B), the receptor binding domain (RBD) of the spike protein (C), the S2 spike protein (D), and the nucleocapsid protein (E) of SARS-CoV-2 in patients on dialysis and age-matched healthy controls after a median of 20 days after the second vaccine dose. The x axes represent the sample number, and the y axes represent the mean fluorescence intensity (MFI) value of the reactivity. The dashed red lines represent the cutoffs. ***P<0.001.
Lower Severe Acute Respiratory Syndrome Coronavirus Type 2 Neutralizing Capacity in Patients on Dialysis
We determined the SARS-CoV-2 neutralizing capacity of vaccine-induced antibodies by an assay that measures the antibody-mediated inhibition of SARS-CoV-2 receptor binding domain:ACE2 interaction. The OD values are given in Supplemental Table 3. After the first vaccination, sera of patients on dialysis showed a significantly lower inhibition than sera of age-matched healthy controls with a median percent inhibition of 16 (IQR, 7–24) compared with 62 (IQR, 37–75), respectively. Only four of 20 (20%) patients on dialysis compared with 12 of 15 (80%) age-matched healthy controls had an inhibition exceeding the cutoff for viral neutralization of 30% (Figure 3, A and B, Table 2). After the second vaccination, most patients on dialysis had a markedly higher inhibition compared with the first vaccination, with 14 of 17 (82%) patients exceeding the cutoff for viral neutralization, but it was still significantly lower than the 46 of 46 (100%) rate in the healthy control group (Figure 3, C and D, Table 2). Notably, two of these three patients on dialysis with low inhibition (patients 4 and 6) had no detectable antibodies against the spike receptor binding domain protein.
Figure 3.
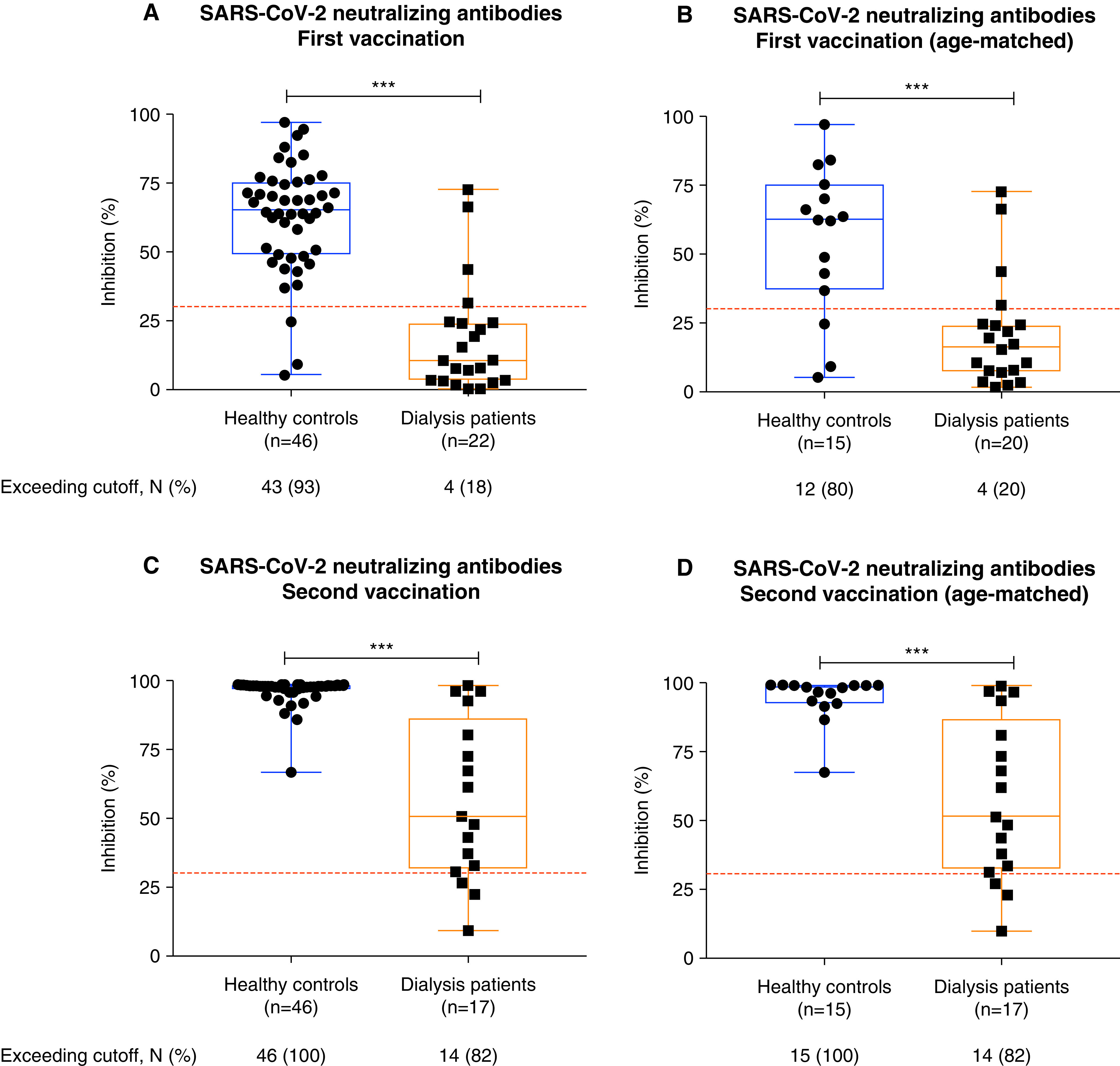
SARS-CoV-2 neutralizing capacity of patients on hemodialysis and healthy controls after vaccination with BNT162b2. SARS-CoV-2 neutralizing capacity of vaccine-induced antibodies was determined by a virus neutralization test. Antibody-mediated inhibition of the SARS-CoV-2 receptor binding domain:angiotensin-converting enzyme 2 interaction is expressed as a percentage. Inhibition capacity of vaccine-induced antibodies in all and in age-matched healthy controls and patients on dialysis after the first (A and B) and after the second (C and D) COVID-19 vaccination. The dashed red lines represent the cutoffs for viral neutralization in this assay according to the manufacturer’s instructions. A cutoff of <30% binding inhibition indicates absence or a level of SARS-CoV-2 neutralizing antibodies below the limit of detection of this test. The number (N) of individuals exceeding the cutoff is expressed as a percentage. ***P<0.001.
Age Dependency of Spike Antigen–Specific Severe Acute Respiratory Syndrome Coronavirus Type 2 Immunoglobulin G Levels and the Neutralizing Capacity of Antibodies
We investigated the age-dependent humoral immune response in patients on dialysis and healthy controls after the first and second vaccine doses. After the first dose, anti-S1 IgG levels did not significantly correlate with the age of individuals in both groups (Figure 4). However, the level of neutralizing antibodies was significantly lower at older age in the healthy control group (Figure 4A). After the second vaccination, the development of both anti-S1 IgG and neutralizing antibody levels correlated inversely with increasing age in healthy controls (Figure 4A). Although only a slightly impaired increase of anti-S1 IgG and neutralizing antibody levels was observed between 20 and 60 years of age, >80-years-old healthy individuals showed a markedly impaired increase (Figure 4A). In patients on dialysis, we did not detect age-related differences in anti-S1 IgG or neutralizing antibody levels; however, patients on dialysis <50 years of age are missing in our study (Figure 4B).
Figure 4.
Age dependency of spike antigen–specific SARS-CoV-2 IgG levels and the neutralizing capacity of antibodies after vaccination with BNT162b2. Age-dependent courses of SARS-CoV-2 IgG antibodies and neutralizing antibodies after the first and after the second COVID-19 vaccinations in healthy controls (A) and patients on dialysis (B).
Discussion
A recent review by Glenn et al. (13) showed that inclusion of patients with CKD in completed and ongoing COVID-19 vaccine trials remains low, with most studies specifically excluding individuals with CKD. Because vaccine efficacy is known to decline with decreasing kidney function, there is an urgent need to determine the immunogenicity of COVID-19 vaccines in patients on dialysis and provide much needed information to assess the level of protection in these high-risk patients (13). This is one of the first prospective studies to examine the humoral response after COVID-19 vaccination in patients on hemodialysis.
We demonstrated that patients on dialysis developed significantly fewer SARS-CoV-2 anti-S1 IgG antibodies compared with age-matched healthy controls 3 weeks after the first vaccination. Only four of 20 age-matched patients on dialysis showed an antibody index above the cutoff for detection, with a specificity of 100% and a sensitivity of 89%. Most importantly, the SARS-CoV-2 neutralizing capacity of induced antibodies was also significantly lower, with only 20% of patients on dialysis having neutralizing antibodies exceeding the predefined threshold for viral neutralization after the first vaccination. Notably, after the second vaccination, most patients on dialysis developed SARS-CoV-2 anti-S1 IgG and neutralizing antibodies (for both, 14 of 17; 82%), which is, however, still significantly lower compared with age-matched healthy controls (15 of 15; 100%).
There are no further data on COVID-19 vaccine response in patients on dialysis, but our results are consistent with studies that have examined the immune response to influenza A vaccination in patients on dialysis. Three weeks after a single dose of influenza vaccine, the humoral response rate to hemagglutinin-1 antigen was significantly lower in patients on dialysis compared with healthy controls (12). The level of anti–hemagglutinin-1 antibody protection was 40% in vaccinated patients on dialysis compared with 65% in healthy controls (12). Another study also showed a below-average influenza A vaccine response in patients on dialysis compared with the age-matched general population (22). Humoral hepatitis B vaccine response also appears to be worse in long-term patients on dialysis compared with a healthy control group. Ghadiani et al. (23) showed a gradual decline in seroconversion rates with lower kidney function after hepatitis B vaccination, with rates of 44%, 90%, and 96% in patients on dialysis, patients with CKD stages 3–4, and healthy medical personnel, respectively. No direct conclusions should be drawn from comparisons of responses to other vaccines due to disease-specific diagnostic assays, different immunization protocols, and differences in immunogenicity of pathogens and vaccines. However, detectable SARS-CoV-2–specific neutralizing antibodies in all patients on dialysis with 82% exceeding the threshold for viral neutralization after the second vaccination are encouraging findings. Shaikh et al. (14) showed that after a median of 35 days from the first COVID-19 diagnosis, all patients on dialysis (19 of 19) had detectable SARS-CoV-2–specific IgG antibodies, whereas neutralizing antibodies were not measured. In a recent study, 89% (74 of 83) of patients on dialysis developed nucleocapsid IgG antibodies after a median of 67 days after COVID-19 disease (24). Antibodies to the spike protein and neutralizing antibodies were not measured. Studies with SARS-CoV and MERS-CoV have shown that various fragments of the spike protein serve as targets for neutralizing antibodies, but receptor binding domain–specific antibodies have the greatest potency to neutralize infection (25). We showed that in agreement with the detected neutralizing antibodies, 82% of patients on dialysis had detectable receptor binding domain–specific antibodies after the second vaccination.
In healthy controls, we demonstrated an impairment of SARS-CoV-2 anti-S1 IgG antibody response after the second vaccination and of neutralizing antibodies after the first as well as second vaccination with increasing age. The age effect may explain, at least in part, the lower humoral response in patients on dialysis. We and others have shown that kidney failure and long-term dialysis are associated with premature aging of the immune system (26). Progressive immunosenescence, in turn, is associated with both lower humoral response and lower memory T cell activity, potentially leading to lower vaccine protection (27). Interestingly, the first real-world data from Scotland and Israel showed comparable protection in all age groups after the first dose of ChAdOx1 or BNT162b2 mRNA vaccine, independent of antibody levels, respectively (E. Vasileiou, C.R. Simpson, C. Robertson, et al., unpublished article) (28). These observations suggest that antibody levels alone are not able to fully reflect the complex immunologic response and level of protection after vaccination.
Although our data conclusively demonstrate a lower humoral response in patients on dialysis, they should be interpreted with caution. The extent to which humoral response contributes to vaccine protection is known neither in COVID-19 nor in hepatitis B or influenza A (8,10,23). In addition, there are no universally validated and accepted antibody cutoffs that correlate with protection against severe COVID-19 courses. Patients on dialysis who did not exceed the respective threshold but had detectable antibodies may be at least partially protected. Remarkably, although 18% of patients on dialysis had neutralizing antibody levels below the threshold for viral neutralization after the second vaccination, neutralizing antibodies were detected in all patients on dialysis. It is also possible that in patients on dialysis with undetectable humoral immunity, the cellular immune response may protect against infection or at least reduce the likelihood of severe COVID-19 disease. Other limitations of our study are the relatively small number of patients on dialysis and the exclusion of patients with previous SARS-CoV-2 infection. Our preliminary data need to be confirmed by further testing, including the cellular immunity and the response against other available vaccines.
This is one of the first studies to examine in detail the humoral response of patients on dialysis after vaccination with the two-dose mRNA vaccine BNT162b2. After the first vaccination, the seroconversion rate including neutralizing antibodies was low in patients on dialysis. Health care professionals should be aware that this high-risk cohort may not be adequately protected after the first COVID-19 vaccine dose, and hygienic measures remain essential. It may be advisable to avoid strategies that achieve more rapid population vaccination coverage by withholding the second vaccination in patients on dialysis. Further data on vaccine response, including cellular response and efficacy studies, are urgently needed to adapt immunization protocols. However, encouraging levels of neutralizing antibody can be measured in patients on dialysis who have received both BNT162b2 mRNA doses.
Disclosures
L. Benning reports employment with University Clinic Heidelberg. M. Buylaert reports employment with University Hospital Heidelberg. D. Göth reports employment with University Hospital Heidelberg. J. Grenz reports employment with Heidelberg University Hospital. A. Hidmark reports employment with NZ Heidelberg. K. Klein reports employment with Nierenzentrum. M. Kreysing reports employment with Uniklinik Heidelberg. C. Morath reports employment with Nierenzentrum Heidelberg and TolerogenixX GmbH; having ownership interest in TolerogenixX GmbH; receiving research funding from BMBF, BMWi, and TolerogenixX GmbH; and having patents and inventions with TolerogenixX GmbH. C. Nusshag reports employment with University Hospital Heidelberg and receiving research funding from the Physician Scientist Program of Heidelberg Faculty of Medicine. G. Ponath reports employment with the University of Heidelberg, Department of Nephrology, Heidelberg, Germany. P. Reichel reports employment with NZ Heidelberg. M. Schaier reports employment with the University of Heidelberg and TolerogenixX GmbH and having ownership interest in TolerogenixX GmbH. P. Schnitzler reports employment with Virology Heidelberg. C. Speer reports employment with Universitätsklinikum Heidelberg. C. Süsal reports employment with the University of Heidelberg; receiving research funding from Chiesi; receiving honoraria from Hansa; an International Patent Application for Mitomycin C Induced Cells (MIC) therapy for specific immunosuppression in transplantation (PCT/EP2019/062857); serving on the boards of Eurotransplant, the German Society for Immungenetics, and the German Transplantation Society; and serving as an associate editor of Transplantation Journal. M. Töllner reports employment with the University of Heidelberg. M. Zeier reports employment with Universitätsklinikum Heidelberg. The remaining author has nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We thank Prof. Jochen Reiser (Department of Internal Medicine, Rush University Medical Center, Chicago, IL, US), for critical review of the manuscript. We also thank Ms. Iris Arnold and Ms. Sabine Bönisch of the Department of Nephrology (both at Heidelberg University Hospital, Heidelberg, Germany) for their technical support.
Data Sharing Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “mRNA COVID-19 Vaccine for People with Kidney Failure: Hope but Prudence Warranted,” on pages 996–998.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03700321/-/DCSupplemental.
Supplemental Figure 1. Detection of antibodies against the S1 spike protein of the four community coronaviruses HCoV-229E (A), HCoV- HKU1 (B), HCoV-NL63 (C), HCoV-OC43 (D), MERS-CoV (E), and SARS-CoV-1 (F).
Supplemental Table 1. Exclusion and inclusion criteria.
Supplemental Table 2. Cutoff values of the SARS-CoV-2–specific bead-based multiplex assay.
Supplemental Table 3. OD values of the SARS-CoV-2 virus neutralizing assay.
References
- 1.Weiss S, Bhat P, Fernandez M del P, Bhat JG, Coritsidis GN: COVID-19 infection in ESKD: Findings from a prospective disease surveillance program at dialysis facilities in New York City and Long Island. J Am Soc Nephrol 31: 2517–2521, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu CM, Weiner DE, Aweh G, Miskulin DC, Manley HJ, Stewart C, Ladik V, Hosford J, Lacson EC, Johnson DS, Lacson E Jr.: COVID-19 among US dialysis patients: Risk factors and outcomes from a national dialysis provider. Am J Kidney Dis 77: 748–756.e1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, Frimat L, Galland R, Hourmant M, Laurain E, Lobbedez T, Mercadal L, Moranne O; French REIN registry: Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 98: 1519–1529, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbett RW, Blakey S, Nitsch D, Loucaidou M, McLean A, Duncan N, Ashby DR; West London Renal and Transplant Centre: Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol 31: 1815–1823, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatri M, Islam S, Dutka P, Carson J, Drakakis J, Imbriano L, Jawaid I, Mehta T, Miyawaki N, Wu E, Yang S, Ali N, Divers J, Grant C, Masani N: COVID-19 antibodies and outcomes among outpatient maintenance hemodialysis patients. Kidney360 2: 263–269, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng JH, Hirsch JS, Wanchoo R, Sachdeva M, Sakhiya V, Hong S, Jhaveri KD, Fishbane S; Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium: Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int 98: 1530–1539, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krueger KM, Ison MG, Ghossein C: Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am J Kidney Dis 75: 417–425, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Sit D, Esen B, Atay AE, Kayabaşı H: Is hemodialysis a reason for unresponsiveness to hepatitis B vaccine? Hepatitis B virus and dialysis therapy. World J Hepatol 7: 761–768, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drachman R, Isacsohn M, Rudensky B, Drukker A: Vaccination against hepatitis B in children and adolescent patients on dialysis. Nephrol Dial Transplant 4: 372–374, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Dikow R, Eckerle I, Ksoll-Rudek D, Hampel H, Schwenger V, Zeier M, Schnitzler P, Sommerer C: Immunogenicity and efficacy in hemodialysis patients of an AS03(A)-adjuvanted vaccine for 2009 pandemic influenza A (H1N1): A nonrandomized trial. Am J Kidney Dis 57: 716–723, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Schulte K, Schierke H, Tamayo M, Hager L, Engehausen R, Raspe M, Hübner R-H, Schlieper G, Borzikowsky C, Urbschat A, Auerswald S, Kunzendorf U, Feldkamp T: Strategies for improving influenza vaccination rates in patients with chronic renal disease. Dtsch Arztebl Int 116: 413–419, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastalerz-Migas A, Steciwko A, Brydak LB: Immune response to influenza vaccine in hemodialysis patients with chronic renal failure. Adv Exp Med Biol 756: 285–290, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Glenn DA, Hegde A, Kotzen E, Walter EB, Kshirsagar AV, Falk R, Mottl A: Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease [published online ahead of print February 9, 2021]. Kidney Int Rep 10.1016/j.ekir.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaikh A, Zeldis E, Campbell KN, Chan L: Prolonged SARS-CoV-2 viral RNA shedding and IgG antibody response to SARS-CoV-2 in patients on hemodialysis. Clin J Am Soc Nephrol 16: 290–292, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candon S, Guerrot D, Drouot L, Lemoine M, Lebourg L, Hanoy M, Boyer O, Bertrand D: T cell and antibody responses to SARS-CoV-2: Experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant 21: 854–863, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccoli L, Park Y-J, Tortorici MA, Czudnochowski N, Walls AC, Beltramello M, Silacci-Fregni C, Pinto D, Rosen LE, Bowen JE, Acton OJ, Jaconi S, Guarino B, Minola A, Zatta F, Sprugasci N, Bassi J, Peter A, De Marco A, Nix JC, Mele F, Jovic S, Rodriguez BF, Gupta SV, Jin F, Piumatti G, Lo Presti G, Pellanda AF, Biggiogero M, Tarkowski M, Pizzuto MS, Cameroni E, Havenar-Daughton C, Smithey M, Hong D, Lepori V, Albanese E, Ceschi A, Bernasconi E, Elzi L, Ferrari P, Garzoni C, Riva A, Snell G, Sallusto F, Fink K, Virgin HW, Lanzavecchia A, Corti D, Veesler D: Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 183: 1024–1042.e21, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Combe C, Kirsch AH, Alfano G, Luyckx VA, Shroff R, Kanbay M, van der Sande F, Basile C; EUDIAL Working Group of the ERA-EDTA: At least 156 reasons to prioritize COVID-19 vaccination in patients receiving in-centre haemodialysis. Nephrol Dial Transplant 36: 571–574, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis A, Baigent C, Ikizler TA, Cockwell P, Jha V: The urgent need to vaccinate dialysis patients against SARS-CoV-2: A call to action. Kidney Int 99: 791–793, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan CW, Chia WN, Qin X, Liu P, Chen MI-C, Tiu C, Hu Z, Chen VC-W, Young BE, Sia WR, Tan Y-J, Foo R, Yi Y, Lye DC, Anderson DE, Wang L-F: A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 38: 1073–1078, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Wacharapluesadee S, Tan CW, Maneeorn P, Duengkae P, Zhu F, Joyjinda Y, Kaewpom T, Chia WN, Ampoot W, Lim BL, Worachotsueptrakun K, Chen VC-W, Sirichan N, Ruchisrisarod C, Rodpan A, Noradechanon K, Phaichana T, Jantarat N, Thongnumchaima B, Tu C, Crameri G, Stokes MM, Hemachudha T, Wang L-F: Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat Commun 12: 972, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bray RA, Lee J-H, Brescia P, Kumar D, Nong T, Shih R, Woodle ES, Maltzman JS, Gebel HM: Development and validation of a multiplex, bead-based assay to detect antibodies directed against SARS-CoV-2 proteins. Transplantation 105: 79–89, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Chang Y-T, Wang J-R, Lin M-T, Wu C-J, Tsai M-S, Wen-Chi CL, Shih T-E, Kuo T-H, Song E-J, Sung J-M: Changes of immunogenic profiles between a single dose and one booster influenza vaccination in hemodialysis patients - An 18-week, open-label trial. Sci Rep 6: 20725, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghadiani MH, Besharati S, Mousavinasab N, Jalalzadeh M: Response rates to HB vaccine in CKD stages 3-4 and hemodialysis patients. J Res Med Sci 17: 527–533, 2012 [PMC free article] [PubMed] [Google Scholar]
- 24.Sakhi H, Dahmane D, Attias P, Kofman T, Bouvier M, Lapidus N, Fourati S, El Karoui K; Mondor NephroCov Study Group: Kinetics of anti–SARS-CoV-2 IgG antibodies in hemodialysis patients six months after infection [published online ahead of print February 26, 2021]. J Am Soc Nephrol 10.1681/ASN.2020111618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S, Hillyer C, Du L: Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol 41: 355–359, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaier M, Leick A, Uhlmann L, Kälble F, Eckstein V, Ho A, Meuer S, Mahnke K, Sommerer C, Zeier M, Steinborn A: The role of age-related T-cell differentiation in patients with renal replacement therapy. Immunol Cell Biol 95: 895–905, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Pawelec G: Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun Ageing 9: 15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD: BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384: 1412–1423, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




