Abstract
The effectiveness of cisplatin, a mainstay in the treatment of many solid organ cancers, is hindered by dose-limiting nephrotoxicity. Cisplatin causes AKI in 30% of patients. Patients who do not develop AKI by clinical standards during treatment are still at risk for long-term decline in kidney function and the development of CKD. The connection between AKI and CKD has become increasingly studied, with renal fibrosis a hallmark of CKD development. To prevent both the short- and long-term effects of cisplatin, researchers must use models that reflect both types of pathology. Although a lot is known about cisplatin-induced AKI, very little is known about the mechanisms by which repeated low levels of cisplatin lead to fibrosis development. In this review, strategies used in various rodent models to prevent kidney injury, its progression to fibrosis, or both, are examined to gain mechanistic insights and identify potential therapeutic targets for cisplatin-induced kidney pathologies. Reviewing the results from these models highlights the diverse and highly complex role of cell death, cell senescence, endoplasmic reticulum stress, autophagy, and immune cell activation in acute and chronic kidney injuries. The use of several models of kidney injury is needed for development of agents that will prevent all aspects of cisplatin-induced kidney injury.
Keywords: cisplatin, acute kidney injury, chronic kidney disease, fibrosis
Cisplatin is an inorganic platinum-based drug that was FDA approved as an anticancer agent in 1978.1 It is a standard therapy used to treat many solid organ cancers.2 The major mechanism of action of cisplatin involves binding DNA and forming adducts, leading to apoptosis or cell cycle arrest. Cisplatin effectiveness is reduced by its dose-limiting nephrotoxicity. Approximately 30% of patients administered cisplatin develop AKI.2 There are no approved agents to treat AKI, and thus its development requires suspending cisplatin treatment.
After AKI, the kidney initiates wound-healing processes to recover. Although the repair processes after AKI can restore function, in some patients, the normal repair processes go awry, causing progressive renal damage and leading to development of CKD.3 The primary features of such maladaptive repair include cell cycle arrest, profibrotic cytokine production, myofibroblast accumulation, chronic immune cell activation, and chronic vascular impairment.3
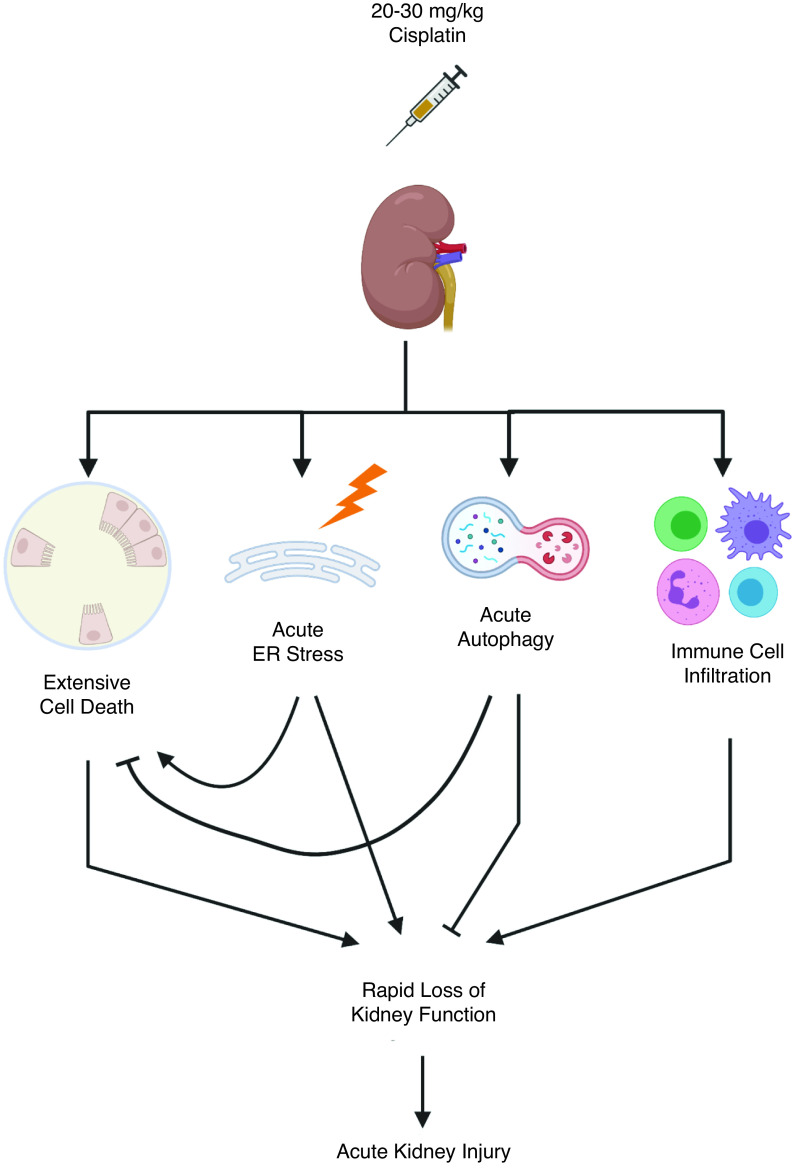
Cisplatin-induced AKI is commonly modeled in rodents via a single high dose (20–30 mg/kg) of cisplatin. After treatment, renal function sharply declines, and the animals must be euthanized within 3–4 days. This model mirrors the development of AKI in the clinic; however, it does not allow for long-term studies of the effects of cisplatin-induced kidney injury and represents only patients with severe AKI.1,4
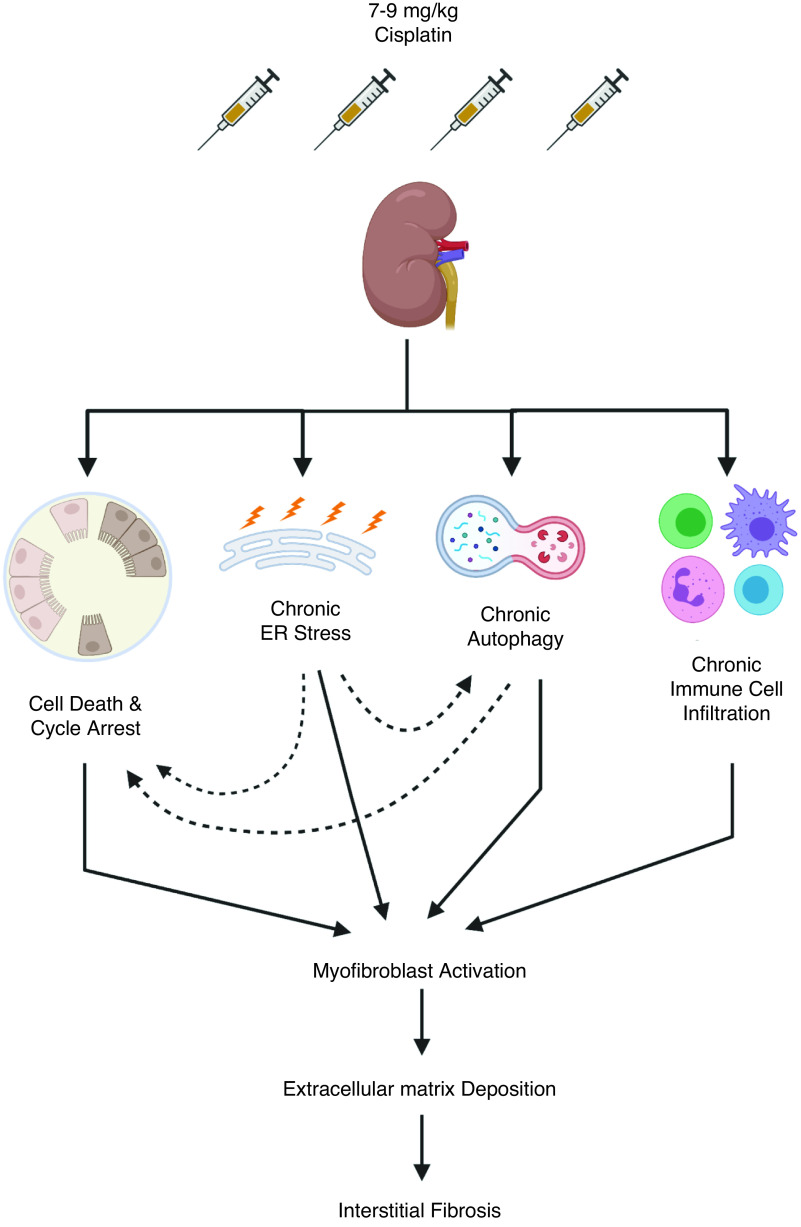
Approximately 70% of patients treated with cisplatin do not develop AKI by clinical criteria, but may still be at risk for long-term renal impairment. Skinner et al. followed pediatric patients treated with cisplatin who did not develop clinical AKI; however, 10 years after treatment, 11% had reduced GFR and 15% displayed symptoms of nephrotoxicity.5 To model this, our laboratory6–9 and others10–15 developed an animal model to study cisplatin-induced kidney injury that involves repeated weekly low doses of cisplatin (7–9 mg/kg) for 4 weeks. A repeated low-dose cisplatin (RLDC) regimen allows mice to survive <6 months after treatment without showing clinical signs of AKI. Mice develop renal fibrosis during the dosing window and have progressive loss of kidney function after cisplatin treatment.8
Together, these models facilitate study of renal pathologies on either end of the spectrum of cisplatin-induced kidney injury. Models are needed that allow for AKI development and recovery, using intermediate doses of cisplatin (10–15 mg/kg), but optimization is required so that animal survival is not an obstacle.10,15 The RLDC and high-dose models of cisplatin-induced kidney injury are well characterized and depict how different biologic processes are triggered after acute or chronic cisplatin treatment. Common therapeutic targets of both cisplatin-induced AKI and fibrosis are needed, and potential drug candidates need testing in both models.
In this review, we compare how targeting cell death, senescence, endoplasmic reticulum (ER) stress, autophagy, and immune cell activation has different effects on renal outcomes in the high-dose cisplatin model and models of renal fibrosis (Supplemental Table 1). Because the RLDC model is fairly new, we examine the results from studies of fibrosis following the ischemia-reperfusion (IR) injury and unilateral ureteral obstruction (UUO) models. It is important to note these models of fibrosis may differ from the RLDC model of fibrosis. A discussion of their key similarities and differences may be found elsewhere.16
Cell Death, Cell Cycle Arrest, and Senescence
In the high-dose cisplatin model, AKI development depends on cell death.2,17,18 Cisplatin induces necrosis and intrinsic, extrinsic, and ER stress–associated pathways of apoptosis.17,18 Intrinsic apoptosis is a key driver of AKI in the high-dose cisplatin model (Figure 1). Global knockout of the proapoptotic Bcl-2–associated X protein conferred resistance to cisplatin-induced AKI in mice by decreasing the number of apoptotic cells.19 Additionally, inhibition and genetic depletion of tumor protein 53 (p53)–attenuated cisplatin-induced AKI in mice, demonstrating that DNA damage is a major contributor to intrinsic apoptosis in acute cisplatin nephrotoxicity.20
Figure 1.
Mechanisms of cisplatin-induced AKI after a single high dose (20–30 mg/kg) of cisplatin. Pointed arrow heads denote promotion of process, solid block lines indicate inhibition of process. Supporting references: 19, 20, 31, 37, 38, 45, 46, and 58-60.
The RLDC model does not induce robust apoptotic or necrotic cell death,7 but whether other forms of cell death are involved has not been examined. A variety of cell death pathways (for example, apoptosis, necrosis, necroptosis, ferroptosis, NETosis, and mitophagy21–24) have been documented in other models of renal fibrosis and CKD, but the extent to which they drive fibrotic development is unclear. Proximal tubule–specific knockout of proapoptotic Bcl-2 proteins, Bcl-2–associated X protein and Bcl-2 homologous antagonist/killer, protected from apoptosis and renal fibrosis in the UUO model.25 Proximal tubule–specific p53 deletion reduced apoptosis and interstitial fibrosis after IR injury.26 However, in the UUO model, global deletion of p53 attenuated apoptosis but did not confer protection from fibrosis 20 days after injury.27 Attenuation of fibrosis with p53 inhibition has been observed at earlier points in time after UUO, but protection was attributed to reduction of cell cycle arrest at the G2/M phase rather than apoptosis.28 These results suggest apoptosis plays complex and cell type–specific roles in renal fibrosis. As with apoptosis, in-depth studies of nonapoptotic forms of cell death in fibrosis development are greatly needed.
A single high dose of cisplatin induces widespread cell death, whereas RLDC causes very little cell death, with surviving cells undergoing sublethal changes, such as cell cycle arrest (Figure 2). This may be due to repeated insults to tubule epithelial cells. Administration of diphtheria toxin to transgenic mice expressing proximal tubule–specific simian diphtheria toxin receptors directly injures proximal tubules.29 A single dose of diphtheria toxin allowed for replacement of lost tubule cells and recovery of kidney function. Repeated injury to tubules via multiple doses of diphtheria toxin induced either senescence or a prolonged dedifferentiated state of the proximal tubules, inducing maladaptive repair, fibrosis, and glomerulosclerosis.29 This study suggests fibrosis is driven by proximal tubule cells that survive injury rather than the loss of proximal tubule cells itself.
Figure 2.
Mechanisms of cisplatin-induced fibrosis after repeated low dose (7–9 mg/kg) cisplatin treatment. Solid line arrows indicate relationships directly examined in the literature. Dotted line arrows represent relationships studied in other models of renal fibrosis that we propose may also be occurring in the RLDC model. Supporting references: 25-30, 39-42, 47-49, and 61-67.
Cell cycle arrest at the G2/M phase contributes to fibrotic outcomes in renal models of injury. It is associated with high levels of JNK signaling and secretion of profibrotic cytokines, including TGFβ. Pharmacologic inhibition of JNK reduces fibrosis after IR injury.30 Data suggest that RLDC causes cell cycle arrest and senescence mediated by JNK signaling.7 Cisplatin is known to induce JNK phosphorylation and activation.17 In the high-dose model of cisplatin-induced kidney injury, JNK activation promotes apoptosis and inflammation, whereas inhibition of JNK reduces renal damage.31 JNK activation also promotes G2/M cell cycle arrest when activated by TGFβ. This alternative activation identifies JNK signaling as a balancing point between apoptosis and cell cycle arrest, with environmental stimuli determining the outcome.32 In RLDC, p-JNK and TGFβ are increased, accompanying elevated expression of cyclin-dependent kinase inhibitor 2a, a known marker of cellular senescence.7,8 Therefore, RLDC-induced fibrosis may be driven by tubule G2/M cell cycle arrest, senescence, or both, as repair of renal damage is attempted. This needs more in-depth investigation.
ER Stress and Autophagy
ER stress can lead to outcomes ranging from apoptosis to adaptation. Responses to ER stress depend on the extent of cellular damage and the duration of the insult. With acute insults, cells need only to withstand stress for a short period of time. Recovery depends on how quickly the cell can mount the unfolded protein response and clear accumulated unfolded proteins. With chronic stress, the cell must undergo a functional change that is more permanent, with a prolonged unfolded protein response allowing cells to adapt and escape cell death.33 The level and duration of ER stress determines if cells die or adapt and survive. One method of ER stress adaptation is autophagy34 (discussed below).
In models of AKI, ER stress is induced, and the development of fibrosis and its pathologic effects are attributed to apoptotic and autophagic induction.35 The role of ER stress is studied in vivo using many strategies, including pharmacologic intervention and C/EBP homologous protein (CHOP)–deficient mice. Although CHOP classically is studied as an inducer of proapoptotic genes, it also regulates prosurvival genes.36
In the high-dose cisplatin model, the α2 adrenergic agonist dexmedetomidine attenuated ER stress via decreased CHOP expression,37 preventing apoptosis and AKI in rats. Similarly, the GPR120 activator TUG891 inhibited ER stress induction, blocked apoptosis, and protected from cisplatin-induced AKI.38 Although these pharmacologic agents might act through alternative mechanisms, both studies attributed the protective effects to ameliorating ER stress, decreasing CHOP expression, and reducing apoptosis.
In models of renal fibrosis, the role of ER stress may be more complex. In the IR model of injury and fibrosis, both CHOP knockout mice and pharmacologic inhibition of ER stress were protective, accompanied by reductions in apoptosis, inflammation, and tubule autophagy.39,40 Compared with wild-type mice, CHOP knockout mice have reduced expression of autophagy-associated proteins and mitigated fibrosis after UUO.41,42 Noh et al. suggest that excessive autophagy can contribute to kidney injury via induction of apoptosis.41 These studies in models of renal fibrosis demonstrate how the ER stress response can lead to both autophagy and apoptosis in kidney disease, processes that may function in opposition or coordination, depending on the length and strength of stimuli. In the high-dose model of cisplatin, high levels of ER stress activation accompany apoptosis (Figure 1). In contrast, the RLDC model may result in sustained, low levels of ER stress, leading to long-term upregulation of autophagy that may be maladaptive (Figure 2).
Autophagy is a form of cellular “self-eating” that can be both protective and detrimental.43,44 Studies have shown that cisplatin-induced AKI is associated with induction of autophagy, and data suggest it is protective.18 Autophagy can help reduce levels of cisplatin-induced apoptosis by ameliorating the effects of cellular damage from oxidative stress and mitochondrial dysfunction.43 In the high-dose cisplatin model, autophagy inhibition exacerbated kidney injury by increasing DNA damage, p53 activation, protein aggregation, and apoptosis.45,46
In models of fibrosis, the role of autophagy is still unclear. It has been argued that long-term upregulation of autophagy might contribute to renal damage.44 In a model of IR injury, mice with specific deletion of autophagy related 5 in proximal tubule cells of the S3 segment had reduced interstitial fibrosis and cellular senescence 30 days after reperfusion. Interestingly, at 2 hours postreperfusion, autophagy-deficient mice had increased levels of cell death. This led to the hypothesis that fibrosis is being driven by proximal tubule cells that survive initial injury via increased autophagy and become senescent later.47
Mice with distal tubule–specific conditional knockout of autophagy-related 7 had increased levels of TGFβ and renal fibrosis after UUO.48 In contrast to the findings of this study, mice with proximal tubule–specific conditional knockout of autophagy-related 7 had decreased levels of renal fibrosis after UUO.49 Pharmacologic inhibitors of autophagy (chloroquine and 3-methyladenine) also prevented UUO-induced fibrosis.49 Interestingly, TGFβ expression was not altered when autophagy was inhibited.49 These studies indicate the autophagy in renal fibrosis may play roles that depend on context and cell type.43 The majority of evidence suggests elevated autophagy can decrease cell death, providing protection from AKI (Figure 1),45,46 but under chronic stress, the cells that escape death become sublethally injured and undergo senescence, contributing to fibrosis development (Figure 2).47,50,51
Immune Response
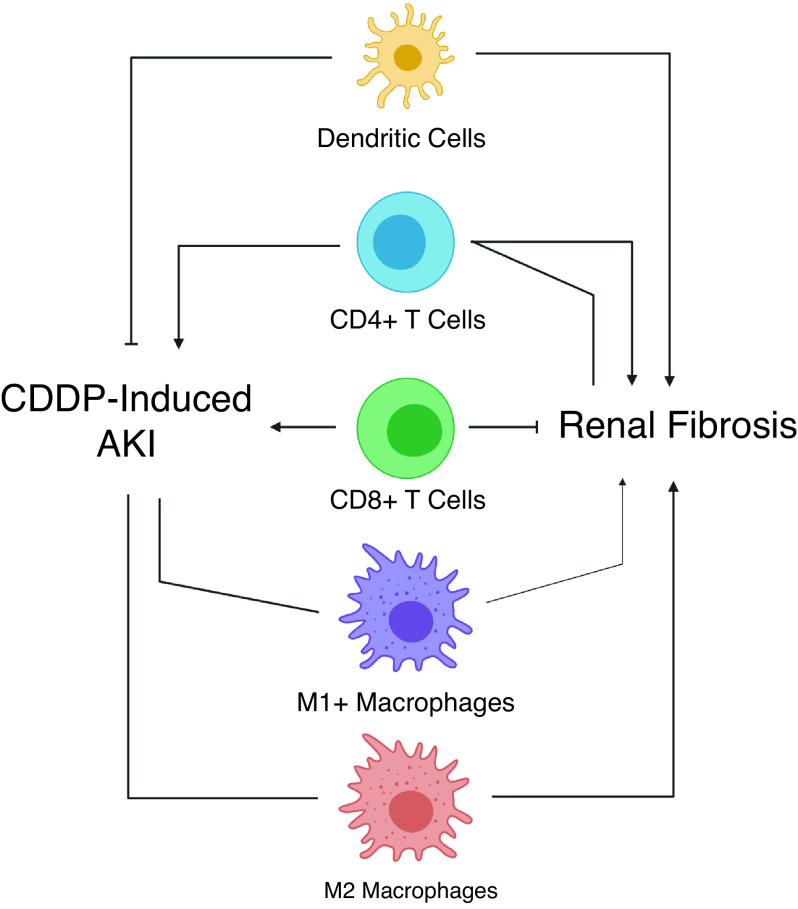
Renal immune cell infiltration and activation may differ in high-dose cisplatin and RLDC models (Figure 3). Inflammation is a major mediator of AKI in the high-dose cisplatin model. Inflammation and renal fibrosis are also known to be closely linked.52 Cisplatin treatment induces upregulation of many different proinflammatory cytokines and chemokines, including TNFα.9,17,18
Figure 3.
Immune cell involvement in cisplatin-induced AKI and renal fibrosis, ointed arrow heads denote promotion of process, solid block lines indicate inhibition of process, lines with no arrow ending denote no effect on process. Supporting references: 59-67. CDDP, cisplatin.
Inhibition of TNFα signaling attenuated cisplatin-induced kidney injury in the high-dose model and decreased production of other inflammatory mediators, indicating it plays a central role in activation of the cytokine response.53,54 In contrast, TNFα-deficient mice had worse fibrosis compared with wild-type mice 4 weeks after UUO.55 However, neutralization of TNFα reduced renal fibrosis and improved function up to 1 week after UUO.56 These conflicting results could be due to the different points in time observed, indicating TNFα may be pathogenic in early processes but important for longer-term recovery processes. These studies demonstrate the diverse functions the immune response can have at different stages of renal injury and progression to fibrosis, highlighting the importance of more in-depth studies of immune responses in chronic injury models.
We have observed increased expression of TNFα and other inflammatory cytokines in the mouse kidney after RLDC treatment.8,9 This cytokine response attracts immune cells to the kidney. Neutrophils, macrophages, T cells, and dendritic cells are the major responders to high-dose cisplatin-induced injury.2,18 Our laboratory has shown RLDC leads to a significant increase in immune cells in the kidney after four doses8; analysis of myeloid cells revealed a significant increase in M2 macrophages.9 Although further study is needed to evaluate the role of these inflammatory responders in RLDC, studies in other models of both AKI and renal fibrosis can provide some insight into which targets should be examined.
T cell–deficient (nu/nu) mice were protected from injury after a single high dose of cisplatin.57 Furthermore, CD4 T cell–deficient mice showed greater protection compared with CD8 T cell–deficient mice.57 In contrast, depletion of CD11c dendritic cells using the diphtheria toxin receptor model–sensitized mice to cisplatin-induced AKI, indicating dendritic cells may play a nephroprotective role.58 Lastly, although macrophages were shown to infiltrate the kidney after cisplatin treatment, depletion of macrophages with liposome-encapsulated clodronate had no effect on development of AKI.59
The role of immune cells in renal fibrosis may be more complex. RAG-1 mice deficient in T and B cells had less fibrosis development after UUO compared with wild-type mice. Furthermore, reconstitution of CD4 T cells reversed this protection, whereas CD8 T cell reconstitution did not.60 Additionally, CD8 T cell–knockout mice were reported to have increased renal fibrosis after UUO, whereas pharmacologic depletion of CD4 T cells decreased renal fibrosis.61 These results suggest a pathogenic role for CD4 T cells in renal fibrosis. However, a study by Ravichandran et al. found that depletion of CD4 T cells did not protect mice from RLDC-induced fibrosis, and it hindered the efficacy of cisplatin, accelerating subcutaneous tumor growth.62 This study indicates CD4 T cells would not be a viable target in cisplatin-induced kidney injury. It also demonstrates that immune cells may play different roles in the different models.
Studies of IR injury showed that global depletion of macrophages via liposome-encapsulated clodronate attenuated development of renal fibrosis. Adoptive transfer of M2 macrophages after global depletion reversed this beneficial effect, whereas M1 transfer did not, suggesting a role for M2 macrophages in the development of fibrosis post-IR injury.63 Global depletion of macrophages with liposome-encapsulated clodronate after development of IR-induced AKI prevented recovery from injury, indicating the timing and coordination of M1 and M2 responses play a role in injury and recovery.64 Depletion of macrophages and dendritic cells with liposome-encapsulated clodronate before UUO-attenuated renal fibrosis.65,66 Altogether, these studies indicate the diverse and complex role of immune cells in kidney disease. The role of the immune system in renal fibrosis is made more complex by the transitions that occur from proinflammatory to prorepair phenotypes. Using only the high-dose model of cisplatin-induced kidney injury does not allow for the study of immune cell clearance and tissue repair. Further studies are needed to elucidate the role of specific immune cell subtypes in the different phases of RLDC-induced fibrosis.
Nephroprotective agents can have differential success in the high-dose cisplatin model and the RLDC model. This is likely due to the inherent biologic differences in these two models with respect to the induction of cell death/senescence, autophagy, ER stress induction and response, and immune-cell involvement. The high-dose cisplatin-induced kidney injury model displays high levels of cell death and ER stress, whereas the RLDC model of injury causes lower levels of ER stress and cell death, leading to more sublethal injury and cellular senescence. The RLDC model is also complicated by chronic immune-cell activity in the kidney during recovery. Evaluating the success of different therapeutic strategies in these models may help shed light on the different nephrotoxic mechanisms underlying high-dose cisplatin and RLDC treatment.
Modeling cisplatin-induced kidney injury in rodents is challenging. One challenge is overcoming the differences in pharmacokinetics between rodents and humans. Mice are known to have a higher peak plasma concentration and shorter half-life of platinum compared with humans.67 With the differences in cisplatin handling, the best way to model human nephrotoxicity is to match pathologies induced by different dosing regimens. Because cisplatin is administered with different dosing regimens, depending on the cancer type, it follows that many models of nephrotoxicity should be used in rodent studies.
Another challenge in modeling cisplatin-induced kidney injury is comparing manifestations of kidney injury in mice and humans. Although kidney histopathology can be readily evaluated in rodents, this is not the case in humans, because patients who develop AKI after cisplatin treatment rarely undergo kidney biopsy. Blood urea nitrogen and serum creatinine are relied on as markers of kidney function for both rodents and humans. Electrolyte disturbances such as hypokalemia and hypomagnesemia are also closely monitored in human patients,68 but are largely ignored in rodents, although it is possible to perform these analyses.69 Finally, patients who receive cisplatin have cancer, and the effect of cancer on cisplatin-induced kidney pathologies has not been well studied in rodents. In sum, rodent studies largely ignore some key aspects of human cisplatin-induced AKI, which may be essential for understanding this disease.
Despite these challenges, notable similarities in rodent and human AKI development after cisplatin treatment have been observed. Sex differences are noted in both mice and humans, with females more sensitive than males to cisplatin-induced AKI.70,71 Studies have also been undertaken to identify genetic predictors of cisplatin nephrotoxicity in humans,72,73 but direct comparisons to rodent models have not been investigated in depth. Although controversy exists over methods of analysis, some studies have identified a polymorphism (rs316019) in the organic cation transporter 2 gene SLC22A2 as a predictor of cisplatin nephrotoxicity.74,75 Similarly, organic cation transporter 2 deletion or inhibition in rodents confers resistance to cisplatin-induced AKI.74–76 This suggests similar cisplatin handling in rodents and humans.
It is important to consider both acute and chronic renal injury when developing nephroprotective agents. Patients exhibit a wide range of responses to cisplatin in the clinic. Some develop AKI after a single low dose, whereas others may receive multiple doses without displaying signs of clinical AKI. Understanding the biologic processes occurring in both the high-dose cisplatin and RLDC models will elucidate the pathologic processes occurring in all categories of patients, leading to better treatment of AKI and prevention of CKD.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK124112 (to L.J. Siskind) and F31DK126400 (to S.M. Sears).
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020101455/-/DCSupplemental.
Supplemental Table 1. Strategies to prevent cisplatin-induced AKI and fibrosis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Kelland L: The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7: 573–584, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferenbach DA, Bonventre JV: Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharp CN, Siskind LJ: Developing better mouse models to study cisplatin-induced kidney injury. Am J Physiol Renal Physiol 313: F835–F841, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner R, Parry A, Price L, Cole M, Craft AW, Pearson AD: Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: Relevance of age and dose as risk factors. Eur J Cancer 45: 3213–3219, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Sharp CN, Doll M, Dupre TV, Beverly LJ, Siskind LJ: Moderate aging does not exacerbate cisplatin-induced kidney injury or fibrosis despite altered inflammatory cytokine expression and immune cell infiltration. Am J Physiol Renal Physiol 316: F162–F172, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp CN, Doll MA, Dupre TV, Shah PP, Subathra M, Siow D, et al.: Repeated administration of low-dose cisplatin in mice induces fibrosis. Am J Physiol Renal Physiol 310: F560–F568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp CN, Doll MA, Megyesi J, Oropilla GB, Beverly LJ, Siskind LJ: Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am J Physiol Renal Physiol 315: F161–F172, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sears SM, Sharp CN, Krueger A, Oropilla GB, Saforo D, Doll MA, et al.: C57BL/6 mice require a higher dose of cisplatin to induce renal fibrosis and CCL2 correlates with cisplatin-induced kidney injury. Am J Physiol Renal Physiol 319: F674–F685, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katagiri D, Hamasaki Y, Doi K, Negishi K, Sugaya T, Nangaku M, et al.: Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int 89: 374–385, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Landau SI, Guo X, Velazquez H, Torres R, Olson E, Garcia-Milian R, et al.: Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int 95: 797–814, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi M, McMillan KL, Wu J, Gillings N, Flores B, Moe OW, et al.: Cisplatin nephrotoxicity as a model of chronic kidney disease. Lab Invest 98: 1105–1121, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black LM, Lever JM, Traylor AM, Chen B, Yang Z, Esman SK, et al.: Divergent effects of AKI to CKD models on inflammation and fibrosis. Am J Physiol Renal Physiol 315: F1107–F1118, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Cai J, Li F, Liu Z, Shu S, Wang Y, et al.: Chronic effects of repeated low-dose cisplatin treatment in mouse kidneys and renal tubular cells. Am J Physiol Renal Physiol 317: F1582–F1592, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Torres R, Velazquez H, Chang JJ, Levene MJ, Moeckel G, Desir GV, et al.: Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J Am Soc Nephrol 27: 1102–1112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z: Rodent models of AKI-CKD transition. Am J Physiol Renal Physiol 315: F1098–F1106, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Ozkok A, Edelstein CL: Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res Int 2014: 967826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Q, Dong G, Franklin J, Dong Z: The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72: 53–62, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z: Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293: F1282–F1291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatia D, Choi ME: The emerging role of mitophagy in kidney diseases. J Life Sci (Westlake Village) 1: 13–22, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronze-da-Rocha E, Santos-Silva A: Neutrophil elastase inhibitors and chronic kidney disease. Int J Biol Sci 14: 1343–1360, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Z, Zhang H, Yang SK, Wu X, He D, Cao K, et al.: Emerging role of ferroptosis in acute kidney injury. Oxid Med Cell Longev 2019: 8010614, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priante G, Gianesello L, Ceol M, Del Prete D, Anglani F: Cell death in the kidney. Int J Mol Sci 20: 3598, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mei S, Li L, Wei Q, Hao J, Su Y, Mei C, et al.: Double knockout of Bax and Bak from kidney proximal tubules reduces unilateral urethral obstruction associated apoptosis and renal interstitial fibrosis. Sci Rep 7: 44892, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying Y, Kim J, Westphal SN, Long KE, Padanilam BJ: Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J Am Soc Nephrol 25: 2707–2716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YJ, Mendoza L, Rha SJ, Sheikh-Hamad D, Baranowska-Daca E, Nguyen V, et al.: Role of p53-dependent activation of caspases in chronic obstructive uropathy: Evidence from p53 null mutant mice. J Am Soc Nephrol 12: 983–992, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Zhang P, Bai M, He L, Zhang L, Liu T, et al.: p53 upregulated by HIF-1α promotes hypoxia-induced G2/M arrest and renal fibrosis in vitro and in vivo. J Mol Cell Biol 11: 371–382, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, et al.: Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francescato HDC, Costa RS, Júnior FB, Coimbra TM: Effect of JNK inhibition on cisplatin-induced renal damage. Nephrol Dial Transplant 22: 2138–2148, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Grynberg K, Ma FY, Nikolic-Paterson DJ: The JNK signaling pathway in renal fibrosis. Front Physiol 8: 829, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutkowski DT, Kaufman RJ: That which does not kill me makes me stronger: Adapting to chronic ER stress. Trends Biochem Sci 32: 469–476, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Rashid HO, Yadav RK, Kim HR, Chae HJ: ER stress: Autophagy induction, inhibition and selection. Autophagy 11: 1956–1977, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cybulsky AV: Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13: 681–696, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Hu H, Tian M, Ding C, Yu S: The C/EBP Homologous Protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol 9: 3083, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chai Y, Zhu K, Li C, Wang X, Shen J, Yong F, et al.: Dexmedetomidine alleviates cisplatin-induced acute kidney injury by attenuating endoplasmic reticulum stress-induced apoptosis via the α2AR/PI3K/AKT pathway. Mol Med Rep 21: 1597–1605, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z, Guo F, Xia Z, Liang Y, Lei S, Tan Z, et al.: Activation of GPR120 by TUG891 ameliorated cisplatin-induced acute kidney injury via repressing ER stress and apoptosis. Biomed Pharmacother 126: 110056, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Shu S, Zhu J, Liu Z, Tang C, Cai J, Dong Z: Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 37: 269–280, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noh MR, Kim JI, Han SJ, Lee T-J, Park KM: C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim Biophys Acta 1852: 1895–1901, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Noh MR, Woo C-H, Park M-J, In Kim J, Park KM: Ablation of C/EBP homologous protein attenuates renal fibrosis after ureteral obstruction by reducing autophagy and microtubule disruption. Biochim Biophys Acta Mol Basis Dis 1864: 1634–1641, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Liu SH, Wu CT, Huang KH, Wang CC, Guan SS, Chen LP, et al.: C/EBP homologous protein (CHOP) deficiency ameliorates renal fibrosis in unilateral ureteral obstructive kidney disease. Oncotarget 7: 21900–21912, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin TA, Wu VC, Wang CY: Autophagy in chronic kidney diseases. Cells 8: 61, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Choi ME: Autophagy in kidney health and disease. Antioxid Redox Signal 20: 519–537, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi A, Kimura T, Takabatake Y, Namba T, Kaimori J, Kitamura H, et al.: Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol 180: 517–525, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z: Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baisantry A, Bhayana S, Rong S, Ermeling E, Wrede C, Hegermann J, et al.: Autophagy induces prosenescent changes in proximal tubular S3 segments. J Am Soc Nephrol 27: 1609–1616, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nam SA, Kim W-Y, Kim JW, Park SH, Kim HL, Lee M-S, et al.: Autophagy attenuates tubulointerstital fibrosis through regulating transforming growth factor-β and NLRP3 inflammasome signaling pathway. Cell Death Dis 10: 78, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z: Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12: 976–998, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baisantry A, Bhayana S, Wrede C, Hegermann J, Haller H, Melk A, et al.: The impact of autophagy on the development of senescence in primary tubular epithelial cells. Cell Cycle 15: 2973–2979, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang C, Livingston MJ, Liu Z, Dong Z: Autophagy in kidney homeostasis and disease. Nat Rev Nephrol 16: 489–508, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black LM, Lever JM, Agarwal A: Renal inflammation and fibrosis: A double-edged sword. J Histochem Cytochem 67: 663–681, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramesh G, Reeves WB: TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B, Ramesh G, Norbury CC, Reeves WB: Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-α produced by renal parenchymal cells. Kidney Int 72: 37–44, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Morimoto Y, Gai Z, Tanishima H, Kawakatsu M, Itoh S, Hatamura I, et al.: TNF-alpha deficiency accelerates renal tubular interstitial fibrosis in the late stage of ureteral obstruction. Exp Mol Pathol 85: 207–213, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Meldrum KK, Misseri R, Metcalfe P, Dinarello CA, Hile KL, Meldrum DR: TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol 292: R1456–R1464, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, et al.: A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol 17: 765–774, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Tadagavadi RK, Reeves WB: Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol 21: 53–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, et al.: Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther 324: 111–117, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Tapmeier TT, Fearn A, Brown K, Chowdhury P, Sacks SH, Sheerin NS, et al.: Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int 78: 351–362, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Dong Y, Yang M, Zhang J, Peng X, Cheng J, Cui T, et al.: Depletion of CD8+ T cells exacerbates CD4+ T cell-induced monocyte-to-fibroblast transition in renal fibrosis. J Immunol 196: 1874–1881, 2016 [DOI] [PubMed] [Google Scholar]
- 62.Ravichandran K, Wang Q, Ozkok A, Jani A, Li H, He Z, et al.: CD4 T cell knockout does not protect against kidney injury and worsens cancer. J Mol Med (Berl) 94: 443–455, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Kim MG, Kim SC, Ko YS, Lee HY, Jo SK, Cho W: The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One 10: e0143961, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al.: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitamoto K, Machida Y, Uchida J, Izumi Y, Shiota M, Nakao T, et al.: Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci 111: 285–292, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Wang K, Liang X, Li Y, Zhang Y, Zhang C, et al.: Complement C3 produced by macrophages promotes renal fibrosis via IL-17A secretion. Front Immunol 9: 2385, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Hennik MB, van der Vijgh WJ, Klein I, Elferink F, Vermorken JB, Winograd B, et al.: Comparative pharmacokinetics of cisplatin and three analogues in mice and humans. Cancer Res 47: 6297–6301, 1987 [PubMed] [Google Scholar]
- 68.Oronsky B, Caroen S, Oronsky A, Dobalian VE, Oronsky N, Lybeck M, et al.: Electrolyte disorders with platinum-based chemotherapy: Mechanisms, manifestations and management. Cancer Chemother Pharmacol 80: 895–907, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Angelen AA, Glaudemans B, van der Kemp AWCM, Hoenderop JGJ, Bindels RJM: Cisplatin-induced injury of the renal distal convoluted tubule is associated with hypomagnesaemia in mice. Nephrol Dial Transplant 28: 879–889, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Chen W-Y, Hsiao C-H, Chen Y-C, Ho C-H, Wang J-J, Hsing C-H, et al.: Cisplatin nephrotoxicity might have a sex difference: An analysis based on women’s sex hormone changes. J Cancer 8: 3939–3944, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL: Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci 20: 3011, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zazuli Z, Vijverberg S, Slob E, Liu G, Carleton B, Veltman J, et al.: Genetic variations and cisplatin nephrotoxicity: A systematic review. Front Pharmacol 9: 1111, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia SL, Lauritsen J, Zhang Z, Bandak M, Dalgaard MD, Nielsen RL, et al.: Prediction of nephrotoxicity associated with cisplatin-based chemotherapy in testicular cancer patients. JNCI Cancer Spectr 4: a032, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A: Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 86: 396–402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katsuda H, Yamashita M, Katsura H, Yu J, Waki Y, Nagata N, et al.: Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol Pharm Bull 33: 1867–1871, 2010 [DOI] [PubMed] [Google Scholar]
- 76.Sprowl JA, Lancaster CS, Pabla N, Hermann E, Kosloske AM, Gibson AA, et al.: Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res 20: 4026–4035, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]