The extracellular matrix (ECM) is a network of collagen fibers and interconnecting proteins that surrounds the cells, providing structural support and the capacity to adhere to and communicate with neighboring cells.1 The ECM is a dynamic structure undergoing balanced remodeling. In aging and disease, this balance is disrupted in response to injury, leading to abnormal remodeling. Aberrant ECM dynamics has been linked to inflammation.1 Decreased ECM leads to increased matrix porosity, which facilitates the infiltration of immune cells into the interstitial space.1 In addition to providing a scaffold, ECM sequesters growth factors for controlled outside-in signaling, which dictates cell function, fate, and phenotype. Age and disease can perturb this “identity reminder,” leading to pathologic changes. Breakdown products of ECM are increased in CKD and often act as damage-associated molecular patterns stimulating immune cells.2 Furthermore, collagen fragments can bind integrin receptors and regulate cell migration, proliferation, and apoptosis. Abnormal ECM remodeling also induces senescence, increases cytokine secretion, and promotes a proinflammatory microenvironment.
Targeting early ECM remodeling could help ameliorate inflammation and the subsequent progression to irreversible kidney damage.
Proteomics-based strategies applied to clinical specimens enable identification of kidney ECM changes in the setting of disease3 that are not typically captured using transcriptomics. Protease-mediated cleavage of ECM proteins affects their connectivity and induces matrix remodeling. Protease prediction studies utilizing proteomics data suggest that this concept applies to the kidney.3 Resolving the components of the ECM and its breakdown products is thus warranted to improve understanding of matrix remodeling role in kidney disease.
Altered kidney ECM remodeling may result in lesions in the glomerular basement membrane (GBM) and the tubular basement membrane. GBM is the interface between glomerular endothelial cells and podocytes that participates in the filtration barrier. Tubular basement membrane supports the tubular epithelial cells. There are common but also distinct features in the ECM composition and remodeling between glomeruli and tubulointerstitium.4 Defining ECM changes in each compartment will improve understanding of kidney aging and disease.
In this issue of JASN, Randles et al.5 applied an unbiased proteomics approach to the study of kidney ECM changes in mice of different ages and disease phenotypes. This work has expanded the temporal proteomic resolution of ECM remodeling in the healthy kidney and in disease models. The authors analyzed the kidney proteome of three murine models with collagen IV defects: Col4a1 disease (Col4a1+/SVC mice), autosomal recessive Alport syndrome (Col4a3−/− mice), and X-linked Alport syndrome (Col4a5−/− mice). The strengths of the study include the identification of compartment-specific molecular alterations and ECM components consistently altered by age and/or disease. The glomerular ECM-enriched fractions led to the identification of 707 (Col4a1+/svc), 334 (Col4a3−/−), and 600 (Col4a5−/−) proteins. The first major observation of the study is that kidney aging and disease are linked to both deposition and downregulation of ECM components. Glomerular defects in Col4-deficient mice were associated with significantly increased collagen VI chains (Col6a1, Col6a2, and Col6a3), nephronectin, and fibrinogen and significantly decreased collagen IV chains (Col4a3, Col4a4, and Col4a5) and laminin subunits (Lamb1, Lamb2, and Lamc1). Although these alterations were more pronounced in older mice, they were already evident in young mice with early injury. The second major observation is that molecular changes in the kidney ECM predated overt ultrastructural changes, suggesting that they could play a major role in the development of ECM-related diseases.
The recognition that ECM remodeling early in disease is characterized by decreased matrix components is a novel concept gaining traction in kidney research. Our group identified decreased protein levels of COL4A4, LAMB2, LAMC1, and other basement membrane components in the glomeruli of patients with kidney transplants and early antibody-mediated rejection.3 In line with the observations of Randles et al.,5 this ECM remodeling was detected before histologic signs of GBM injury or fibrosis.3 Early detection of ECM changes might provide additional information about the risk of progression. Given that increased urinary levels of COL6A and COL4A peptides are linked to more severe CKD,6 these assays may represent a noninvasive method to monitor intrarenal ECM remodeling.
In the study by Randles et al.,5 the external validation through data mining reinforces the translational potential of the work. However, the authors did not convincingly validate their protein-level findings in human kidney samples. This shortcoming is related to the limited access to human kidney specimens and limited availability of kidney proteomic datasets. Although still scarce, proteomic studies of human clinical biopsies are shedding new light on ECM protein alterations in kidney disease.3,7,8 Novel methods of analyzing ultrasmall compartments of paraffin-embedded biopsies3 and cataloging of all proteins9 in these clinical specimens may yield new insights into the link between ECM remodeling and disease.
The observations of Randles et al.5 expand the picture of ECM dynamics in the mouse kidney, but whether these molecular changes are relevant to the human kidney remains unanswered. Unfortunately, there is still a paucity of standardized methods to accurately recapitulate the human kidney ECM. Similarly, the main molecular events that trigger abnormal ECM remodeling are currently unknown. Recent advances in bioengineering are increasing our capacity to study ECM-producing kidney cells ex vivo. Moreover, methods using multichannel time-lapse confocal imaging can be adapted to the study of leukocyte infiltration and basement membrane turnover.10 These approaches will enable the study of the interplay between distinct cell types and the dynamics of ECM processing, such as the release of collagen fragments upon injury. To identify and target these events, it is crucial to determine the contribution of proteases and each cell type to the remodeling of the kidney ECM. Strategies on the basis of differential isotope labeling, mass spectrometry, and activity-based profiling of kidney cells in coculture may help address this gap (Figure 1).
Figure 1.
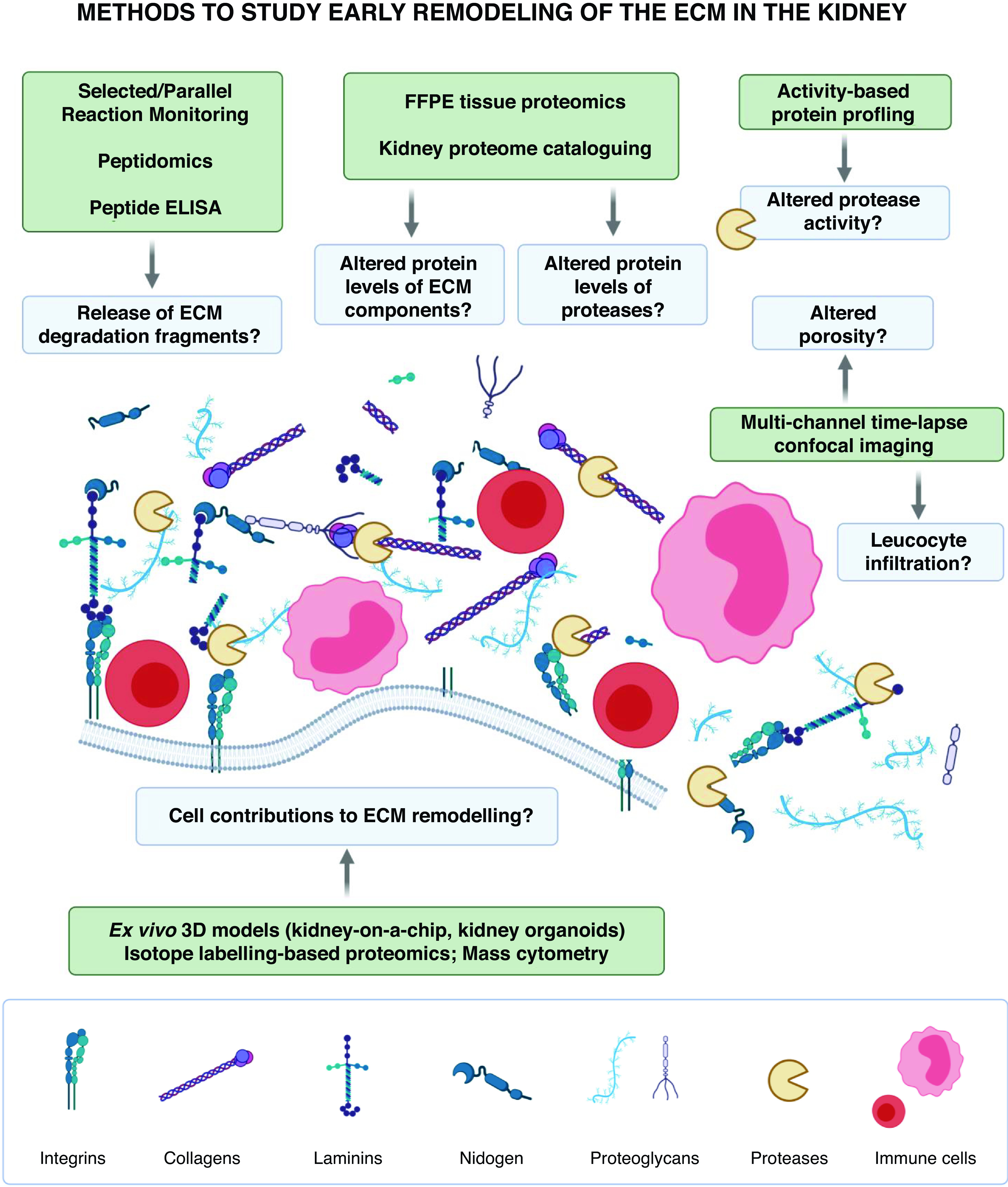
Proposed methods to study early remodeling of the ECM in the kidney. Biological processes likely involved in early ECM remodeling in kidney aging and disease are illustrated and shown in the blue boxes. The proposed methods to study these processes are summarized in the green boxes. 3D, three-dimensional; FFPE, formalin fixed, paraffin embedded. The figure was created using BioRender.com.
In conclusion, the findings from Randles et al.5 support emerging paradigms in nephrology, which conceptualize the ECM as a dynamic entity, and describe early ECM changes in kidney aging and disease. Integration of compartment-specific proteomics with protease activity and substrate profiling, as well as larger numbers of carefully characterized clinical samples, may lead to new insights into kidney ECM remodeling.
Disclosures
All authors have nothing to disclose.
Funding
S. Clotet-Freixas is supported by Kidney Research Scientist Core Education and National Training (KRESCENT) Fellowship Award 2019KPPDF637713. A. Konvalinka is supported by Canada Foundation for Innovation grant 37205; and Kidney Foundation of Canada grants KRES160004 and KRES160005. A. Konvalinka has also received funding from Toronto General and Western Hospital Research Foundation grants TGTWF 1617-464 and TGTWF MKFTR 1718-1268.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or JASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Sorokin L: The impact of the extracellular matrix on inflammation. Nat Rev Immunol 10: 712–723, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Anders HJ, Schaefer L: Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clotet-Freixas S, McEvoy CM, Batruch I, Pastrello C, Kotlyar M, Van JAD, et al. : Extracellular matrix injury of kidney allografts in antibody-mediated rejection: A proteomics study. J Am Soc Nephrol 31: 2705–2724, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miner JH: Renal basement membrane components. Kidney Int 56: 2016–2024, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Randles M, Lausecker F, Kong Q, Suleiman H, Reid G, Kolatsi-Joannou M, et al. : Identification of an altered matrix signature in kidney aging and disease [published online ahead of print May 28, 2021]. J Am Soc Nephrol 32: 2483–2493, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen DGK, Boesby L, Nielsen SH, Tepel M, Birot S, Karsdal MA, et al. : Collagen turnover profiles in chronic kidney disease. Sci Rep 9: 16062, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakatani S, Wei M, Ishimura E, Kakehashi A, Mori K, Nishizawa Y, et al. : Proteome analysis of laser microdissected glomeruli from formalin-fixed paraffin-embedded kidneys of autopsies of diabetic patients: Nephronectin is associated with the development of diabetic glomerulosclerosis. Nephrol Dial Transplant 27: 1889–1897, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Paunas TIF, Finne K, Leh S, Marti HP, Mollnes TE, Berven F, et al. : Glomerular abundance of complement proteins characterized by proteomic analysis of laser-captured microdissected glomeruli associates with progressive disease in IgA nephropathy. Clin Proteomics 14: 30, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo T, Kouvonen P, Koh CC, Gillet LC, Wolski WE, Röst HL, et al. : Rapid mass spectrometric conversion of tissue biopsy samples into permanent quantitative digital proteome maps. Nat Med 21: 407–413, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley LC, Wang Z, Hagedorn EJ, Wang L, Shen W, Lei S, et al. : Live-cell confocal microscopy and quantitative 4D image analysis of anchor-cell invasion through the basement membrane in Caenorhabditis elegans. Nat Protoc 12: 2081–2096, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]


