Abstract
Introduction
Survivors of COVID-19 infection may develop post-covid pulmonary fibrosis (PCF) and suffer from long term multi-system complications. The magnitude and risk factors associated with these are unknown.
Objectives
We investigated the prevalence and risk factors associated with PCF and other complications in patients discharged after COVID-19 infection.
Methods
Patients had phone assessment 6 weeks post hospital discharge after COVID-19 infection using a set protocol. Those with significant respiratory symptoms were investigated with a CTPA, Pulmonary Function Tests and echocardiogram. Prevalence of myalgia, fatigue, psychological symptoms and PCF was obtained. Risk factors associated with these were investigated.
Results
A large number of patients had persistent fatigue (45.1%), breathlessness (36.5%), myalgia (20.5%) and psychological symptoms (19.5%). PCF was seen in 9.5% of the patients and was associated with persistent breathlessness at 6 weeks and inpatient ventilation [adjusted OR 5.02(1.76–14.27) and 4.45(1.27–15.58)] respectively. It was more common in men and in patients with peak CRP >171.5 mg/L, peak WBC count ≥12 × 10 9/L, severe inpatient COVID-19 CXR changes and CT changes. Ventilation was also a risk factor for persisting fatigue and myalgia, the latter was also more common in those with severe cytokine storm and severe COVID-19 inpatient CXR changes.
Conclusions
All the patients discharged after COVID-19 should be assessed using a set protocol by a multidisciplinary team. Patients who had severe COVID-19 infection particularly those who were intubated and who have persistent breathlessness are at risk of developing PCF. They should have a CT Chest and have respiratory follow-up.
Keywords: COVID-19, Post COVID Fibrosis, Long term complications of COVID-19
List of abbreviations
- ARDS
Acute Respiratory Distress Syndrome
- BMI
Body Mass Index
- BSTI
British Society of Thoracic Imaging
- COPD
Chronic Obstructive Pulmonary Disease
- CRP
C-Reactive Protein
- CT
Computed Tomography
- CTPA
CT Pulmonary Angiogram
- CXR
Chest X-ray
- DLCOc
Diffusion Capacity for Carbon Monoxide Corrected for Hemoglobin
- FEV1
Forced Expiratory Volume in one second
- FVC
Forced Vital Capacity
- HRCT
High Resolution CT
- ICU
Intensive Care Unit
- IPF
Idiopathic Pulmonary Fibrosis
- LV
Left ventricular
- CAT
COPD Assessment Tool
- MERS
Middle East Respiratory Syndrome
- MRC
Medical Research Council
- MRI
Magnetic Resonance Imaging
- PE
Pulmonary Embolus
- PCF
Post-COVID Fibrosis
- PCVCT
Post COVID-19 CT Chest Codes
- PFT
Pulmonary Function Test
- RT-PCR
Real Time – Polymerase Chain Reaction
- SARS-CoV1
Severe Acute Respiratory Syndrome – Coronavirus 1
- TLC
Total Lung Capacity
- WBC
White Blood Cell
1. Introduction
The COVID-19 pandemic has been the biggest challenge facing the world in modern times. Globally more than 69 million cases and 1.5 million deaths have been recorded as of December 10th, 2020 [1]. The initial focus has rightly been on the provision of health care facilities and developing treatments for hospitalised COVID-19 patients. There have been encouraging results with Dexamethasone [2] and Remdesivir [3,4] in reducing mortality and morbidity respectively. As survival improves, the next challenge we face is identifying and managing longer term complications of COVID-19 infection [5,6].
There are indications that a significant number of COVID-19 survivors develop longer term respiratory [[7], [8], [9]] cardiovascular [10] and psychological [11] sequalae. Many patients also suffer from fatigue, myalgia and memory impairment [[12], [13], [14]]. There is a need to identify the magnitude of these complications so that health care resources can be suitably allocated [5]. There is a particular concern that a proportion of the patients could develop post-COVID fibrosis (PCF) in their lungs similar to the survivors of MERS and SARS-CoV1 [8,15,16]. Patients developing pulmonary fibrosis often suffer from progressive breathlessness and need long term follow up [17]. There is a need to identify risk factors for developing PCF so that those at risk can be promptly investigated and managed or entered into future therapeutic clinical trials.
In May 2020, the British Society of Thoracic Imaging (BSTI) published codes (0–3) for assessing and reporting post-COVID changes on CT scans [18]. This has helped in providing standardisation while reporting the CT scans and identifying post-COVID fibrosis on thoracic CT scans.
Eight hundred and ninety-eight patients were admitted to our institution with COVID-19 between March and July 2020. We developed a pathway for the follow-up of patients discharged after COVID-19 infection which is presented in this observational study. We have also described the persisting symptoms and the magnitude of systemic effects, particularly PCF, in our cohort. We have further identified risk factors associated with the development of PCF.
2. Methods
All patients admitted to our hospital with COVID-19 (defined as admission to an acute inpatient bed, with either a positive SARS-COV2 naso-pharyngeal swab on RT-PCR, or a clinico-radiological diagnosis of COVID-19), were identified. We developed a pathway for the outpatient follow up of all the patients who were successfully discharged. Patients deemed unable to manage a phone consultation (those with significant memory loss, nursing home residents and those patients who were discharged with a palliative intent) were excluded. The rest of the patients were offered a phone consultation with a respiratory physician 6 weeks after discharge. The patients who answered the phone consultation were included. The patients who could not be contacted over the phone were further excluded from this analysis. We wrote to the general practitioners of all the excluded patients asking them to refer the patients back to us if there was a concern in the community.
2.1. First consultation by telephone
We used a standardised proforma to assess the patients which is described in the supplement. We collected laboratory investigation data during the inpatient admission with COVID-19 particularly, the peak levels of CRP, ferritin, D-dimer, WBC and the lowest lymphocyte count which were used as biomarkers of cytokine storm. Chest X rays during their inpatient stay were reviewed and the worst CXR was divided into low and high risk as described by Toussie et al. [19] Those patients who were clinically judged to have persistent significant respiratory symptoms were investigated with a CTPA with high resolution reconstruction, PFT and echocardiogram and offered a follow-up outpatient appointment with a respiratory consultant.
Patients without respiratory symptoms at the time of the phone clinic were asked to have a follow up CXR 10 weeks after discharge if they had COVID-19 changes on their inpatient CXR. If this CXR showed persistent changes, they were investigated and reviewed using the same pathway as the symptomatic patients. The rest of the patients were discharged from respiratory follow up and referred to appropriate other specialities if required.
2.2. Second outpatient assessment
CTPA, PFT and echocardiogram findings were reviewed. CT chest was reported by two thoracic radiologists and the post-COVID pulmonary changes were coded as PCVCT0-3 as per the BSTI coding [18]. Only those patients whose CT scans showed established fibrosis ± ground glass abnormalities (PCVCT3) were classified as having post-COVID fibrosis. The extent of the interstitial abnormality on both this (follow up) and the worst inpatient CT chest was scored between 0 and 25, a score ≥18 was classified as high risk CT, as described previously [20]. This scoring was performed by an experienced respiratory consultant with an interest in interstitial lung diseases.
We estimated the persistent disease burden by calculating the number of patients with anxiety, depression, fatigue, myalgia and PCF. We assessed the association of PCF, fatigue and myalgia with a number of variables including demographics (gender, race, age and BMI), markers of cytokine storm, high risk inpatient CXR, history of ICU admission and invasive ventilation use during the inpatient stay. The following additional factors were also assessed for association with the development of PCF – premorbid respiratory disease, co-morbidities (diabetes, hypertension, ischemic heart disease, chronic liver disease and history of long term dialysis), smoking status, inpatient CT score, PFT parameters, MRC, ΔMRC (Difference in post and pre-COVID MRC dyspnoea scores), CAT score and persistent symptoms. The association between the inpatient and outpatient CT chest score was also assessed.
2.3. Statistical analysis
Data analysis was performed using STATA version 16.1 [21]. The categorical data is expressed as frequency (%) and compared using the Chi-squared test. Univariate logistic regression analysis was undertaken to examine associations with significant outcomes. Results are expressed as odds ratio (OR) and 95% confidence intervals (CI). Continuous data is presented as medians (interquartile range) and compared using the Mann-Whitney U test. Optimal cut points for discriminating outcomes with significant differences were determined using the Liu method [22], then univariate logistic regression was used to assess associations. Those variables with significant associations and where we had data from ≥150 observations were entered into step wise logistic regression analysis. Association between the outpatient and inpatient CT score was assessed using Pearson's correlation coefficient. P values of <0.05 are considered significant.
The data presented here was collected as part of a new service evaluation, and judged by the St George's NHS Clinical Research committee to be exempt from NHS Research Ethics Committee review, and as per Health Research Authority's COVID guidelines ‘anonymised information can then be used in health and care research’ [23].
3. Results
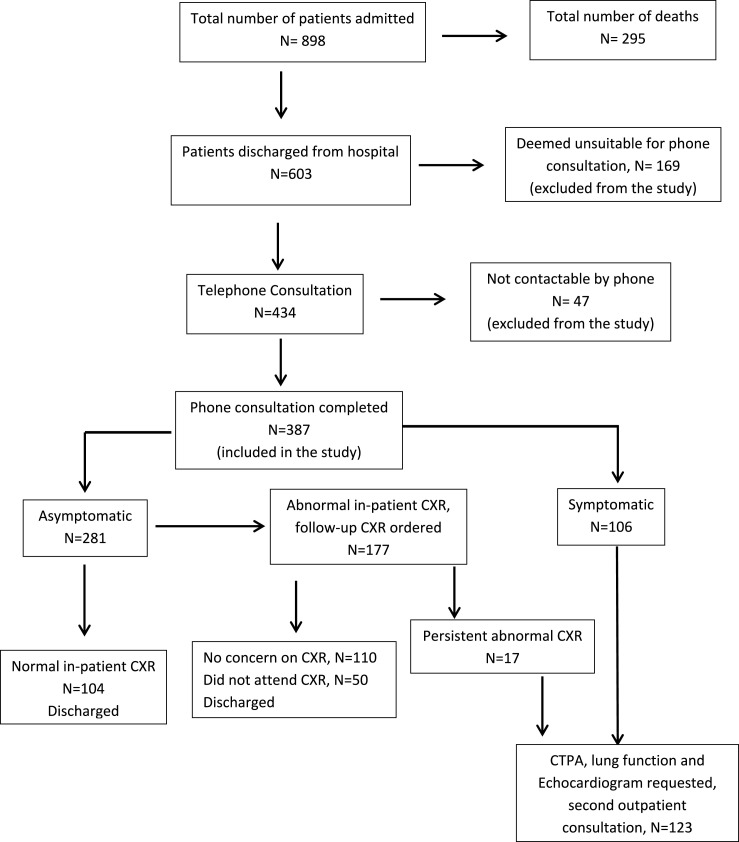
Outcomes of hospital discharged patients (Fig. 1 ).
Fig. 1.
Flow chart Showing outcomes of patients admitted to our institution with COVID-19.
A total of 603 COVID-19 patients were successfully discharged from our institution. A total of 387 (64.2%) of these patients completed the phone consultation and were included in this analysis. One hundred and twenty-three patients (31.8% of those phoned) were judged to need further investigations and a second outpatient respiratory review.
3.1. Results at the phone (first) clinic
The median age of the patients was 63 (50–75) years, 114 (31.2%) of the patients were smokers. Two hundred and nineteen (56.6%) were male. Eighty-four (21.7%) patients had an ICU admission, 60 (15.6%) patients required invasive ventilation.
The symptom burden of patients is shown in Table 1 . The commonest symptom, fatigue, was present in 165 (45.1%) patients. One hundred and thirty-five (36.5%) of the patients had persistent breathlessness with a median CAT score of 6 (2–12), 112 (32.2%) of the patients had a clinically significant CAT score of ≥10. Median post COVID-19 MRC score was 3 (2–4). We could only reliably assess ΔMRC score in 140 of the patients, the median ΔMRC score was 1 (0–2). Thirty-nine (12.5%) patients had anxiety, 49 (15.7%) had depression, 27 (8.6%) patients had both so a total of 61 (19.5%) had ongoing psychological symptoms.
Table 1.
Symptom burden.
| Symptom | Frequency (%) |
|---|---|
| Breathlessness | 135 (36.5%)a |
| Cough | 83 (22.7%)a |
| Chest Pain | 39 (10.77%)b |
| Fatigue | 165 (45.1%)b |
| Myalgia | 75 (20.5%)b |
Data available for.
N = 370.
N = 366.
3.2. Results from the second consultation
CT scan findings: A CTPA was requested for 123 patients, of which 6 patients did not attend. Of those who had a CTPA, 33 (28.2%) had normal lung parenchyma (PCVCT0) on high resolution reconstruction images with no ground glass abnormality, tractional bronchiectasis or honeycombing, 45 (38.5%) had improving ground glass abnormalities as compared to inpatient CT and no tractional bronchiectasis or honeycombing (PCVCT1). Three patients (2.6%) had persisting significant residual ground glass abnormalities and no tractional bronchiectasis or honeycombing. (PCVCT2 changes). Thirty-six (30.8%) patients had both ground glass abnormalities and established fibrosis in the form of tractional bronchiectasis (PCVCT3) which represents 9.3% of the patients included in this analysis, none of these patients developed honeycombing. Median CT score on the inpatient and outpatient CT was 15 (11–20) and 8 (0–13) respectively. The extent of persisting interstitial abnormalities (outpatient CT score) was moderately associated with the inpatient CT score r = 0.58, p < 0.001. CT scores in each of the BSTI categories (PCVCT1-3) are shown in Table 2 .
Table 2.
Extent of Interstitial Abnormality on outpatient CT scans.
| BSTI CT Stage | CT Score | no. of observations |
|---|---|---|
| PCVCT1 | 7 (5–9.5) | 48 |
| PCVCT2 | 14 (12–15) | 3 |
| PCVCT3 | 14 (12.5–17) | 36 |
3.3. Data presented as median (interquartile range)
Pulmonary embolus (PE) was seen in 6 patients (7.7% of those who had a CT scan and 1.6% of the study population), of these 4 were known to have PE during their inpatient stay and 2 new cases were identified.
Lung function: We only observed only a mild reduction in lung volumes in the PCF group with a median TLC (% predicted) of 73.4% (71.3–81.7) and moderate reduction in gas transfer DLCOc (%predicted) of 59% (52.7–67.8). DLCOc% and TLC% was lower in these patients as compared to those without PCF, there was no difference in FVC or FVC% in the two groups (Table 3 ).
Table 3.
Lung function data in fibrotic and non fibrotic patients.
| No Fibrosis | Post-Covid Fibrosis | P value (no. of observations) | |
|---|---|---|---|
| FEV1 (L) | 2.61 (2.07–3.23) | 2.59 (2.08–3.13) | ns (109) |
| FEV1 (%predicted) | 90 (74–97) | 89.9 (80–103) | ns (109) |
| FVC (L) | 3.28 (2.76–3.92) | 3.11 (2.38–3.7) | ns (109) |
| FVC (%predicted) | 89.2 (76.8–98) | 85.4 (74.7–96.7) | ns (109) |
| DLCOc (mmol/min/kPa) | 5.94 (4.62–7.52) | 5.10 (4.05–6.22) | 0.07 (103) |
| DLCOc (%predicted) | 70.4 (58.7–79.6) | 59 (52.7–67.8) | 0.03* (103) |
| TLC (%predicted) | 87 (76–97.5) | 73.4 (71.3–81.7) | 0.027* (62) |
Data presented as Median (interquartile range) compared using Mann-Whitney U test *significant.
Echocardiogram findings: Five patients (1.3%) had LV dysfunction and 9 (2.3%) were found to have pulmonary hypertension.
3.4. Factors associated with PCF (Table 4b, Table 4c, Table 4a)
Table 4b.
Persistent symptoms in fibrotic and non fibrotic patients.
| No Fibrosis | Post Covid Fibrosis | P value (no. of observations) | OR (95% CI) unadjusted | OR (95% CI) adjusted | |
|---|---|---|---|---|---|
| Breathlessness | 106 (31.6%) | 29 (82.9%) | <0.001* (370) | 10.44 (4.21–25.91) | 5.25 (1.86–14.81)b |
| Cough | 64 (19.1%) | 19 (54.3%) | <0.001*(370) | 5.03 (2.45–10.32) | |
| Chest pain | 28 (8.5%) | 11 (31.4%) | <0.001*(366) | 4.96 (2.2–11.17) | |
| Fatigue | 136 (40.7%) | 29 (82.9%) | <0.001*(366) | 7.04 (2.84–17.41) | |
| Myalgia | 54 (16.3%) | 21 (61.8%) | <0.001*(366) | 8.32 (3.93–17.62) | 1.68 (0.65–4.36)b |
| MRC pre-Covida | 1 (1–2) | 1 (1–1) | 0.008* (320) | ||
| MRC post-Covida | 2 (2–4) | 3 (2–4) | 0.099 (141) | ||
| Δ MRCa | 1 (0–1) | 2 (1–3) | 0.001* (140) | ||
| CAT scorea | 6 (2–10) | 14 (7–18) | 0.001* (348) | ||
| CAT ≥10 | 89 (28.4%) | 24 (68.6%) | 0.001* (348) | 5.49 (2.58–11.68) |
Data presented as Frequency (%) compared using Chi square test except.
Median (interquartile range) compared using Mann-Whitney U test *significant.
Adjusted for male sex, persistent breathlessness, myalgia, invasive ventilation, Peak WBC and high risk CXR during COVID-19 admission.
Table 4c.
In-patient ICU admission and investigations in Fibrotic and Non Fibrotic Subjects.
| No Fibrosis | Post Covid Fibrosis | P value (no. of observations) | OR (95% CI) unadjusted | OR (95% CI) adjusted | |
|---|---|---|---|---|---|
| Admitted to ICU | 61 (17.4%) | 23 (63.9%) | <0.001* (387) | 8.38 (4.02–17.46) | |
| Intubated | 38 (10.7%) | 22 (61.1%) | <0.001* (387) | 12.9 (6.09–27.31) | 3.48 (1.16–10.49) b |
| Duration intubated a (days) | 14 (7–34) | 27.5 (19–49) | 0.004* (387) | ||
| Peak CRPa (mg/L) | 130.5 (70–218) | 214 (122–379) | 0.001* (375) | ||
| Peak WBC a (109/L) | 9.4 (7.1–13.4) | 17.1 (11.9–21.3) | <0.001* (375) | 6.16 (2.8–13.5) c | 2.57 (0.85–7.79) b |
| Peak Ferritin a (μg/L) | 906 (526–1620) | 1250 (932–2735.5) | 0.036 *(145) | ||
| Peak D-Dimera (ng/ml) | 619 (310–2633) | 3026 (1530–5467) | 0.001 *(145) | ||
| Minimum Lymphocytesa (109/L) | 0.7 (0.5–1.0) | 0.5 (0.4–0.8) | 0.006* (375) | ||
| High risk Inpatient CXR | 136 (46.7%) | 29 (85.3%) | <0.001* (325) | 6.61 (2.49–17.6) | 3.31 (0.82–13.33) b |
| In patient CT scorea | 14 (11–19) | 21 (17–23) | 0.001 * (89) | ||
| High risk inpatient CT (score ≥18) | 21 (28%) | 9 (64.3%) | 0.008 * (89) | 4.63 (1.39–15.4) |
Data presented as Frequency (%) compared using Chi square test except.
Median (interquartile range) compared using Mann-Whitney U test * significant.
Adjusted for male sex, persistent breathlessness, myalgia, invasive ventilation, Peak WBC and high risk CXR during COVID-19 admission.
Unadjusted OR for Peak WBC ≥12 × 106/L.
Table 4a.
Demographic and comorbidity data in non fibrotic and fibrotic group.
| No Fibrosis | Post-COVID Fibrosis | P value (no. of observations) | OR (95% CI) unadjusted | OR (95% CI) adjusted | |
|---|---|---|---|---|---|
| Agea | 63 (50–76) | 61.5 (54.5–66.5) | ns (387) | ||
| Sex (male) | 191 (54.4%) | 28 (77.8%) | p = 0.007* (387) | 2.93 (1.3–6.6) | 1.61 (0.58–4.47)b |
| Chronic lung disease | 67 (19.3%) | 7 (19.4%) | ns (383) | ||
| COPD | 13 (3.7%) | 1 (2.8%) | ns (383) | ||
| Asthma | 28 (8%) | 4 (11.1%) | ns (383) | ||
| Diabetes mellitus | 95 (27.1%) | 6 (16.7%) | ns (386) | ||
| Hypertension | 131 (37.5%) | 13 (36%) | ns (385) | ||
| Ischemic heart disease | 40 (11.4%) | 1 (2.8%) | ns (386) | ||
| Chronic liver disease | 4 (1.2%) | 0 (0%) | ns (385) | ||
| Chronic Dialysis | 15 (4.3%) | 0 (0%) | ns (387) | ||
| Smokers | 226 (68.3%) | 25(73.5%) | ns (383) | ||
| BMIa | 27.4 (23.7–31.7) | 26.4 (23.4–30.3) | ns (337) | ||
| Ethnic - White | 114 (38.8%) | 10 (34.5%) | ns (323) | ||
| Ethic - Black | 62 (21.1%) | 6 (20.7%) | ns (323) | ||
| Ethnic -Asian | 54 (18.4%) | 7 (24.1%) | ns (323) | ||
| Ethnic - other | 64 (21.8%) | 6 (20.7%) | ns (323) |
Data presented as Frequency (%) compared using Chi square test except.
Median (interquartile range) compared using Mann-Whitney U test * significant.
Adjusted for male sex, persistent breathlessness, myalgia, invasive ventilation, Peak WBC count and high risk CXR during COVID-19 admission.
The strongest association was seen with invasive ventilation during the inpatient admission, OR 12.9 (6.09–27.31). Patients who developed PCF were also on the ventilator for a longer period of time. ICU admission itself was associated with a risk of developing fibrosis, but this was no longer significant when adjusted for invasive ventilation.
Men were 2.93 (1.3–6.6) times more likely to develop PCF. All the on-going symptoms were associated with a risk of developing fibrosis, with the strongest association seen with persistent breathlessness, OR 10.44 (4.21–25.91). Patients with fibrosis had a higher ΔMRC and CAT score. Only the presence of breathlessness and myalgia [adjusted OR 3.6 (1.18–10.98) and 3.11 (1.27–7.61) respectively] were associated with the risk of PCF when adjusted for all the symptoms and CAT score of ≥10.
Patients with PCF had higher values of peak WBC count, CRP, ferritin, D-dimer and lower minimum lymphocyte count. Those with a peak WBC count of ≥12 × 109/L and CRP >171.5 mg/l were 6.16 (2.8–13.5) and 2.51 (1.24–5.1) times more likely to develop PCF. Only peak WBC ≥12 × 109/L was associated with a significant risk [adjusted OR 5.61 (2.44–12.92)] of PCF when corrected for other markers of cytokine storm. Patients with inpatient CT score ≥18 and high risk inpatient CXR changes were also more likely to develop PCF.
On multiple step logistic analysis inpatient invasive ventilation and persistent breathlessness were the only two independent risk factors [adjusted OR 3.48 (1.16–10.49) and 5.25 (1.86–14.81) respectively] associated with post-COVID fibrosis when adjusted for these, male gender, persistent myalgia, peak WBC count and high risk CXR during inpatient COVID-19 admission.
Age, BMI and both respiratory and non-respiratory co morbidities were not associated with the development of PCF.
3.5. Risk factors associated with fatigue
Persistent fatigue was associated with ICU admission and strongly associated with invasive ventilation, OR 3.1 (1.72–5.6) (Table 5 ). Although patients with fatigue had a lower median BMI (26.5) compared to those without fatigue (28.9), this is not clinically significant as both lie in the overweight range. There was no significant association seen with any of the other studied variables although there was a trend towards a higher CRP in the fatigued patients. ICU admission was no longer significant when adjusted for invasive ventilation.
Table 5.
Risk Factors associated with Fatigue.
| No Fatigue | Fatigue present | P value (no. of observations) | OR (95% CI) unadjusted | |
|---|---|---|---|---|
| Age | 64 (50–76) | 61 (49–72) | 0.12 (369) | |
| Sex (male) | 119 (57.2%) | 89 (42.8%) | 0.4 (369) | |
| Ethnic - White | 63 (53.4%) | 55 (46.6%) | ns (311) | |
| Ethic - Black | 40 (60.6%) | 26 (39.4%) | ns (311) | |
| Ethnic -Asian | 33 (54.1%) | 28 (45.9%) | ns (311) | |
| Ethnic - other | 37 (56.1%) | 29 (43.9%) | ns (311) | |
| BMIa | 28.9 (23.9–32.7) | 26.5 (23.5–30) | 0.035* (319) | |
| Admitted to ICU | 34 (41%) | 49 (59%) | 0.003* (369) | 2.1(1.28–3.45) |
| Intubated | 19 (32.2%) | 40 (67.8%) | <0.001* (369) | 3.1 (1.72–5.6) |
| Number of days intubated a | 17 (7–26) | 22 (11–45) | 0.097 (369) | |
| Minimum lymphocytesa(109/L) | 0.7 (0.5–1.0) | 0.7 (0.5–1.0) | 0.64 (369) | |
| Peak WBC counta(109/L) | 9.8 (7.2–13.7) | 10.1 (7.1–15.6) | 0.37 (369) | |
| Peak CRPa (mg/L) | 133 (73–212) | 147 (81–276) | 0.081 (369) | |
| Peak ferritina (μg/L) | 961.5 (559–1625) | 999 (562.5–2053.5) | 0.68 (145) | |
| Peak D-dimera (ng/ml) | 657.5 (328–2473) | 1122 (326–3821) | 0.138 (145) | |
| High risk inpatient CXR | 78 (47.9%) | 83 (55.7%) | 0.167 (312) |
Data presented as Frequency (%) compared using Chi square test except.
Median (interquartile range) compared using Mann-Whitney U test * significant.
3.6. Risk factors associated with myalgia
Peak CRP, D-dimer and WBC count were significantly higher in patients with persistent myalgia (Table 6 ). Of these peak WBC count >11 × 109/L, was the only risk factor associated with myalgia when adjusted for all the other markers of cytokine storm, adjusted OR 2.4 (1.37–4.21). High risk inpatient CXR was also associated with myalgia. Invasive ventilation [OR 6.0 (3.3–10.94)] was also associated with myalgia but ICU admission was no longer significant when corrected for invasive ventilation. The number of days on the ventilator was also higher in those with myalgia. Invasive ventilation and high risk CXR were the two factors associated with myalgia [adjusted OR of 2.49 (1.16–5.35) and 2.64 (1.33–5.24) in multiple regression model comprising these and peak WBC count.
Table 6.
Risk Factors associated with Myalgia.
| No myalgia | Myalgia | P value (no. of observations) | OR (95% CI) unadjusted | OR (95% CI) adjusted | |
|---|---|---|---|---|---|
| Agea | 64 (50–76) | 60 (50–64) | 0.09 (366) | ||
| Sex male | 158 (76.7%) | 48 (23.3%) | 0.13 (366) | ||
| Ethnic -White | 94 (80.3%) | 23 (19.7%) | ns (308) | ||
| Ethic - Black | 52 (80%) | 13 (20%) | ns (308) | ||
| Ethnic -Asian | 46 (76.7%) | 14 (23.3%) | ns (308) | ||
| Ethnic - other | 56 (84.6%) | 10 (15.2%) | ns (308) | ||
| BMIa | 27.5 (23.7–31.2) | 26.5 (23.6–32.4) | 0.53 (317) | ||
| Admitted to ICU | 49 (16.9%) | 34 (45%) | <0.001* (366) | 4.1 (2.36–7.1) | |
| Intubated | 29 (10%) | 30 (40%) | <0.001* (336) | 6.0 (3.3–10.94) | 2.49 (1.16–5.35) [3] |
| Duration intubated (days)a | 12 (7–26) | 35 (15–48) | 0.008* | ||
| Minimum Lymphocytesa(109/L) | 0.7 (0.5–1.0) | 0.7 (0.5–0.9) | 0.47 (366) | ||
| Peak WBCa(109/L) | 9.4 (7–13.4) | 12.2 (8.5–19.8) | 0.001* (366) | 2.79 (1.6–4.7)b | 1.79 (0.92–3.49) [3] |
| Peak CRPa (mg/L) | 131 (73–214) | 181 (83–302) | 0.008* (366) | ||
| Peak ferritina (μg/L) | 961.5 (491–1564) | 1053.5 (602–2432) | 0.26 (145) | ||
| Peak D-Dimera (ng/ml) | 671 (312–2997) | 1987.5 (553–3979) | 0.025* (145) | ||
| High Risk Inpatient CXR | 107 (44.6%) | 53 (76.8%) | <0.001* (309) | 4.12 (2.23–7.61) | 2.64 (1.33–5.24) [3] |
Data presented as Frequency (%) compared using Chi square test except.
Median (interquartile range) compared using Mann-Whitney U test * significant.
Unadjusted OR for peak WBC >11 × 109/L, 3Adjusted for invasive ventilation, Peak WBC count and high risk CXR during COVID-19 admission.
4. Discussion
There is no validated pathway available for investigating patients for long term complications after discharge from hospital with COVID-19 infection. British Thoracic Society's guidance suggests using a CXR 12 weeks post discharge, with a further clinical assessment recommended only in those with persistently abnormal x-rays or those patients who needed critical care admission [24]. However, a recent study has shown that a follow up x-ray on its own is insufficient for assessing recovery in post-COVID patients as many patients with a normal CXR have a significant symptom burden [25]. In this study we have described a simple holistic outpatient follow up pathway, which uses an initial phone clinic to assess symptoms and targeting investigations in symptomatic patients with onward referral to appropriate specialities for timely management.
4.1. Post COVID-19 symptom burden
A large number (36.5%) of patients suffer from persistent breathlessness, many patients also have fatigue, myalgia, and psychological symptoms 6 weeks after discharge from COVID-19 infection. This is similar to the results seen in other studies [12,13,26,27]. The degree of breathlessness is moderate to severe with a median MRC dyspnea score of 3 (2–4), which was an average of 1(0–2) point higher than the pre-COVID score. We also found that a majority of the patients (56.3%) had difficulty in estimating their MRC dyspnea score because they had not started to exercise or go outdoors since discharge from hospital. CAT score has been validated in assessing symptom burden in patients with COPD and IPF [[28], [29], [30]], patients in this study were able to estimate this score easily.
We found that CAT score ≥10 was useful in predicting risk of developing post-COVD fibrosis, making it a useful simple tool in assessing respiratory symptoms in this setting. As post-COVID-19 patients have a diverse symptom burden, they should be assessed in a multi-disciplinary clinic comprising of respiratory physicians, physiotherapists, occupational therapists and psychologists.
4.2. Estimation of Post-COVID fibrosis
9.3% of our study population had post-COVID fibrosis. One study has described prevalence of post-COVID pulmonary abnormalities on CT in 63% of the patients, no patient in that study had pulmonary fibrosis [27]. Other studies from previous pandemics have shown that at 12 months there is 20–45% prevalence of pulmonary fibrosis on CT scans after H7N9 [31] and SARS [32] respectively. The higher rate reported in these studies is likely because these studies did not use specific criteria to define interstitial changes and fibrosis. Our study is the first study to specifically describe prevalence of post-COVID-19 fibrotic lung disease as we selected patients with definite fibrotic change on CT (PCVCT3 changes as per BSTI coding [18]). The extent of interstitial abnormalities on the outpatient follow up CT Chest (CT score) was related to the COVID-19 inpatient CT score (r = 0.58). It is difficult to be certain whether these changes will persist or resolve with time and longer term follow up over a year will be useful. This is one of the reasons why we did not classify patients with isolated ground glass changes (PCVCT1 and 2) as having PCF.
Patients with PCF had a moderate reduction in DLCOc, only mild reduction in TLC and had a normal FVC. Other studies have also shown that changes in DLCO are most sensitive in predicting post-COVID pulmonary disease [27,33]. Therefore, spirometry on its own is insufficient for screening for PCF. Gas transfers are essential if lung physiology is used as a screening tool, this is challenging because these require trained physiologists and access to lung function is restricted due to cross-infection risk.
Similar to a previous study [27] we only found 2 (0.5%) new cases of PE in our study and these patients had high risk factors for thrombo-embolism. One of these had deep vein thrombosis few weeks before the CTPA and the other had suspected gall bladder cancer. This is in contrast to a higher incidence of PE in acute COVID-19 infection [34]. The low incidence is likely to be due to prompt diagnosis, management and widespread use of thrombo-prophylaxis during acute COVID. Therefore, non-contrast HRCT would suffice as the imaging modality in this cohort and CTPA can be reserved for patients with a high risk of PE.
Only 1.2% patients were found to have LV dysfunction in our study which is similar to another recent study [27]. Previous studies have estimated a much higher (20%) incidence of cardiac damage during acute COVID-19 infection [35,36]. The low incidence of cardiac dysfunction in our study could be because we used echocardiogram rather than superior modalities such as cardiac MRI [37].
4.3. Risk factors for developing PCF
This study shows that patients developing PCF are symptomatic with breathlessness, myalgia, cough, chest pain and fatigue. Men are more likely to develop PCF, men are also more likely to have more severe acute COVID-19, so perhaps this is simply a reflection of severity of COVID in men [[38], [39], [40]].
ICU admission, especially invasive ventilation is the strongest risk associated with development of PCF. This is similar to the findings in the SARS epidemic, [41,42]. Patients developing PCF have higher markers of cytokine storm and peak WBC count during their inpatient course. This is similar to other studies which have shown that these are risk factors for mortality with COVID [39,43,44]. High risk inpatient CXR and higher score on inpatient CT Chest were also associated with the development of post COVID fibrosis. Previous studies have also shown that severity of changes on both inpatient CXR and CT Chest are good predictors of mortality and severity of acute COVID [20,45,46]. It is possible that patients who suffer a severe cytokine storm during acute COVID-19 infection develop more severe CXR and CT changes and are more likely to be intubated and later develop PCF.
We have identified that receipt of invasive ventilation and persistent breathlessness are the two independent risk factors associated with developing PCF when corrected for all the other risk factors. Therefore, these group of patients should have a CT Chest and respiratory follow up.
4.4. Risks for myalgia and fatigue
Patients with persisting myalgia had higher levels of markers of cytokine storm, high risk inpatient CXR and there was a trend towards a higher CRP in those with persistent fatigue. Patients admitted to ICU, especially those who were ventilated are at very high risk of developing myalgia and fatigue. Cytokine storm is the mechanism behind severe illness, ICU admission and ventilation during acute COVID [39,47] and this along with muscle wasting and deconditioning in ICU are likely to be the mechanisms behind persistent myalgia and fatigue.
4.5. Limitations of the study
We did not include any questionnaires to screen for memory impairment as we were apprehensive that these would be difficult to administer over the phone. Similarly, we do not have data on Vitamin D levels in our study. Vitamin D deficiency may be a risk factor associated with long term complications of COVID-19 particularly fatigue. We would suggest that post-COVID-19 follow up protocols include screening for memory impairment [25,48] and assess Vitamin D levels especially in those with persistent fatigue and myalgia.
We did not use 6-min walk test [49] or 1-min sit-stand test [50] to screen patients for further cardio-respiratory investigations as these require a face to face encounter. There may be a role of these tests in screening patients for PCF and need further research.
We performed CTPA with high resolution reconstruction in this study. CTPA was chosen as the imaging modality so that we did not miss pulmonary emboli. We acknowledge that the contrast used in CTPA can sometimes give a false impression of ground glass change on the images, but it does not over estimate tractional bronchiectasis, the presence of which is necessary for the CT scans to be classified as PCVCT3 (the criteria used in this study to define PCF). Therefore, the radio-contrast and imaging modality of CTPA instead of high resolution CT Chest would not affect the accuracy of assessment of PCF. We are confident that we screened the patients meticulously and only those patients who had no respiratory symptoms and no COVID-19 changes on their inpatient or follow up CXR did not have CT imaging. It is unlikely that these patients would have PCF.
5. Conclusions
Patients discharged from hospital after COVID-19 infection should be screened with standardised questionnaires (such as used in this paper) for respiratory symptoms, fatigue, myalgia and psychological disorders. Questions directed at screening for memory impairment should also be incorporated.
As these patients have a large and diverse symptom burden, they should be managed by a MDT comprising of respiratory physicians, physiotherapists, occupational therapists and psychologists with access to other specialities as needed.
All patients who received invasive ventilation and those who have persistent breathlessness (especially men), those who had high risk inpatient CXR, high inpatient CT scores and high markers of cytokine storm (CRP >171.5 mg/L and WBC ≥12 × 109/L) are at risk of developing post-COVID fibrosis and should have a HRCT Chest and respiratory review.
Spirometry on its own is not helpful in screening patients for post-COVID fibrosis, patients should instead have gas transfer and lung volumes performed if feasible.
Author contributes
All the co-authors took part in data collection, carrying out study procedures, the conception and design of the study, acquisition of data, drafting the article or revising it critically for important intellectual content and final approval of the version submitted. In addition, Dr Raminder Aul, Dr Jessica Gates, Dr Adrian Draper and Dr Yee Ean Ong took part in data analysis, interpretation and writing the manuscript.
Declaration of competing interest
No Conflicts of interest for all authors.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
No prior publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106602.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dexamethasone in hospitalized patients with covid-19 — preliminary report. N. Engl. J. Med. July 2020 doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 3.Grein J., Ohmagari N., Shin D., et al. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/nejmoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandar V., Mahalaxmi I., Subramaniam M., et al. Follow-up studies in COVID-19 recovered patients - is it mandatory? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee L., Iyer S., Jose R.J., Manuel A. COVID-19 follow-up planning: what will we be missing? ERJ Open Res. 2020;6(2) doi: 10.1183/23120541.00198-2020. 198-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser E. Long term respiratory complications of covid-19. BMJ. 2020;370 doi: 10.1136/bmj.m3001. [DOI] [PubMed] [Google Scholar]
- 8.George P.M., Barratt S.L., Condliffe R., et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 9.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;55(6) doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K.Y., Li T., Gong F.H., Zhang J.S., Li X.K. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front. Psychiatr. 2020;11(July):1–6. doi: 10.3389/fpsyt.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal S., Barnett J., Brill S.E., et al. “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA, J. Am. Med. Assoc. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho-Schneider C., Laurent E., Lemaignen A., et al. Follow-up of adults with non-critical COVID-19 two months after symptoms' onset. Clin. Microbiol. Infect. October 2020 doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salehi S., Reddy S., Gholamrezanezhad A. Long-term pulmonary consequences of coronavirus disease 2019 (COVID-19): what we know and what to expect. J. Thorac. Imag. 2020;35(4):W87–W89. doi: 10.1097/RTI.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed H., Patel K., Greenwood D.C., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J. Rehabil. Med. 2020;52(5) doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 17.Directors AB of, Committie E.E. American thoracic society idiopathic pulmonary Fibrosis : diagnosis and treatment. Am. J. Respir. Crit. Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 18.BSTI. BSTI reportPost-COVID-19 CT Report Codes. BSTI.
- 19.Toussie D., Voutsinas N., Finkelstein M., et al. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology. 2020;297(1):E197–E206. doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franconi M., Iafrate F., Masci G.M., et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur. Radiol. 2020;30:6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.StataCorp. Stata Stat. Softw.: Release. 2019;16 [Google Scholar]
- 22.Liu X. Classification accuracy and cut pointselection. Stat. Med. 2012;31(23):2676–2686. doi: 10.1002/sim.4509. [DOI] [PubMed] [Google Scholar]
- 23.HRA . NHS Health Research Authority; 2020. Guidance for Using Patient Data.https://www.hra.nhs.uk/covid-19-research/guidance-using-patient-data/ Published. Accessed. [Google Scholar]
- 24.British Thoracic Society British thoracic society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia. Br. Thorac. Soc. 2020;(May) https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/resp-follow-up-guidance-post-covid-pneumonia/ [Google Scholar]
- 25.D'Cruz R.F., Waller M.D., Perrin F., et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2020 doi: 10.1183/23120541.00655-2020. 655-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D., Zhang W., Pan F., et al. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir. Res. 2020;21(1) doi: 10.1186/s12931-020-01385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonnweber T., Sahanic S., Pizzini A., et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur. Respir. J. 2020 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata K., Tomii K., Otsuka K., et al. Evaluation of the chronic obstructive pulmonary disease assessment test for measurement of health-related quality of life in patients with interstitial lung disease. Respirology. 2012;17(3):506–512. doi: 10.1111/j.1440-1843.2012.02131.x. [DOI] [PubMed] [Google Scholar]
- 29.Burhan Shaker S., Kjeldgaard H., Konradsen H. Clinical application of the COPD assessment test (CAT) in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2017;50(suppl 61) doi: 10.1183/1393003.congress-2017.PA4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kon S.S.C., Canavan J.L., Jones S.E., et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir. Med. 2014;2(3):195–203. doi: 10.1016/S2213-2600(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 31.Chen J., Wu J., Hao S., et al. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci. Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-17497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L., Liu Y., Xiao Y., et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127(6):2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.10.013. S2531-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meiler S., Hamer O.W., Schaible J., et al. Computed tomography characterization and outcome evaluation of COVID-19 pneumonia complicated by venous thromboembolism. PloS One. 2020;15(11 November):1–16. doi: 10.1371/journal.pone.0242475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C., Yeming W., Xingwang L., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(January):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA, J. Am. Med. Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stawicki S., Jeanmonod R., Miller A., et al. The 2019–2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: a joint american. college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group consensus paper. J. Global Infect. Dis. 2020;12(2):47–93. doi: 10.4103/jgid.jgid_86_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murillo-Zamora E., Hernandez-Suarez C.M. Survival in adult inpatients with COVID-19. Publ. Health. 2020;190:1–3. doi: 10.1016/j.puhe.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peckham H., De Gruijter N., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020:1–10. doi: 10.21203/rs.3.rs-23651/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hui D.S., Joynt G.M., Wong K.T., et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonio G.E., Wong K.T., Hui D.S.C., et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228(3):810–815. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]
- 43.Izcovich A., Ragusa M.A., Tortosa F., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PloS One. 2020;15(11 November):1–30. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int. J. Infect. Dis. 2020;95(April):304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mushtaq J., Pennella R., Lavalle S., et al. Initial chest radiographs and artificial intelligence (AI) predict clinical outcomes in COVID-19 patients: analysis of 697 Italian patients. Eur. Radiol. 2020 doi: 10.1007/s00330-020-07269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balbi M., Caroli A., Corsi A., et al. Chest X-ray for predicting mortality and the need for ventilatory support in COVID-19 patients presenting to the emergency department. Eur. Radiol. 2020 doi: 10.1007/s00330-020-07270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrigues E., Janvier P., Kherabi Y., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Directors AB of. American thoracic society ATS Statement : guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/rccm.166/1/111. [DOI] [PubMed] [Google Scholar]
- 50.Crook S., Büsching G., Schultz K., et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur. Respir. J. 2017;49(3):1–11. doi: 10.1183/13993003.01871-2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.