Abstract
Objective
A better understanding of the immune response against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is critical to predict its dynamics within the general population and its impact on the vaccination strategy. This study assessed the persistence of neutralizing antibody (Nab) activity and SARS-CoV-2 serology in serum samples of mild and asymptomatic patients 9 months post symptom onset (PSO) in a primary care context among immunocompetent adults.
Methods
A longitudinal cohort of crew members (CMs) exposed to coronavirus disease 2019 (COVID-19) during an outbreak of SARS-CoV-2 on the French aircraft carrier ‘Charles de Gaulle’ in April 2020 was created. CMs infected with COVID-19 and with positive serology at the end of quarantine were tested 9 months PSO. Samples were collected 18 and 280 days PSO. For each patient, both serology and serum viral neutralizing activity were performed.
Results
In total, 86 CMs were analysed. Samples were collected 18 and 280 days PSO. The seroconversion rates were 100% and 93% (82/86) at 18 and 280 days PSO, respectively, and 72.7% of patients exhibited persistent Nab activity at 9 months, regardless of disease severity.
Conclusion
Nab activity persists for up to 9 months following asymptomatic/mild COVID-19 among young adults, regardless of serological results.
Keywords: SARS-CoV-2 infection, Neutralizing antibodies, Outbreak, Serology
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of coronavirus disease 19 (COVID-19), emerged in late 2019 and rapidly spread worldwide, causing a global pandemic (Lipsitch et al., 2020). Despite many insights on the virus, data regarding the long-term immune response are quite scarce (Huang et al., 2020), although this issue is of high clinical relevance.
SARS-CoV-2 infection elicits a humoral response with specific antibodies against the viral surface spike (S) protein. These antibodies can be detected 2 weeks after disease onset, and persist for >6 months post symptom onset (PSO) (Gaebler et al., 2021). Neutralizing antibodies (Nabs) play a crucial role in the immune response, and their steadiness over time has been the subject of discission. Correlations have been reported between Nab levels and both anti-SARS-CoV-2 IgG titres and clinical status.
Antibody dynamics over time have been less well described among mild and asymptomatic patients, for whom interpretation of the humoral response is hazardous as time since seroconversion may be impossible to define. It is also less clear whether or not serological results are predictive for Nab remaining potential with a strong correlation in mild and asymptomatic patients.
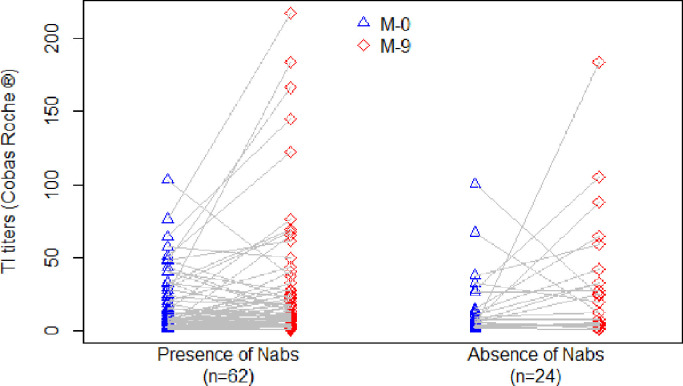
As such, this study investigated the persistence of Nab activity in serum samples in mild and asymptomatic patients 9 months PSO among a cohort of French crew members (CMs) following a large outbreak onboard the nuclear aircraft carrier ‘Charles de Gaulle’ (Figure 1 ).
Figure 1.
Severe acute respiratory syndrome coronavirus-2 serology titre kinetics according to the persistence of neutralizing antibodies (Nabs) 9 months post symptom onset. TI, total immunoglobulin.
Methods
Study design and participants
An outbreak of COVID-19 occurred in April 2020 on the French nuclear aircraft carrier ‘Charles de Gaulle’. Among the CMs, 64% (1121/1769) tested positive for SARS-CoV-2 by reverse transcriptase polymerase chain reaction. Genomic analysis has identified SARS-CoV-2 viruses from clade 20C. SARS-CoV-2 serological testing performed in 1706 CMs revealed that 60% were antibody-positive at the end of quarantine. Among the 682 CMs who agreed to participate in the longitudinal follow-up cohort study COV-PA (IDRCB 2020-A02397-32), 86 CMs selected were retested in January 2021 (9 months PSO). The severity of acute infection was assessed by the World Health Organization's Ordinal Clinical Progression/Improvement Scale. All data were collected in the context of care from completely anonymized files, in accordance with French and European laws, including General Data Protection Regulation. All patients were informed and provided written consent to use their data. COV-PA was approved by the regional research ethics committee on 7 December 2020.
SARS-COV-2 serology
The Elecsys anti-SARS-CoV-2 assay (Roche, Basel, Switzerland) is an electrochemiluminescence immunoassay for the detection of total immunoglobulins (TIs) (IgM, IgG and IgA) to SARS-CoV-2 on the Cobas automatized system (Roche). This sandwich immunoassay is designed to preferentially detect mature, high-affinity antibodies to the highly immunogenic SARS-CoV-2 nucleocapsid protein. The test is non-reactive when the sample index/cut-off index (COI) is <1.0 and positive when it is ≥1.0. Specificity and sensitivity have been described as excellent and show good correlation with viral neutralizing activity (To et al., 2020).
Virus production
Vero cells (ATCC CCL-81) were grown in DMEM medium (Gibco Cat. No. 31966021, ThermoFisher Scientific, Waltham, MA, USA). At confluence, the Vero cells were harvested and subsequently seeded at 1.5 × 105/mL in 96-well plates in order to reach confluence after 72 h at 37°C in 5% CO2. Serial dilutions of stock viruses were made in the infection medium [DMEM supplemented with 50 U/mL penicillin, 50 mg/mL streptomycin (Gibco Cat. No. 15070063, ThermoFisher Scientific) and TPCK-trypsin (Sigma Cat. No. T1426) at a final concentration of 1 µg/mL]. For the neutralization test, SARS-CoV-2 (BetaCoV/France/IDF0372/2020 strain) from clade 19A was isolated by the National Reference Centre (CNR) for Respiratory Viruses (Institut Pasteur), as described previously (Lescure et al., 2020). The BetaCoV/France/IDF0372/2020 strain was supplied by CNR for Respiratory Viruses hosted by Institut Pasteur, headed by Pr. Sylvie van der Werf. The human sample from which the BetaCoV/France/IDF0372/2020 strain was isolated was provided by Dr. X. Lescure and Pr. Y. Yazdanpanah from Bichat Hospital, Paris. Moreover, the BetaCoV/France/IDF0372/2020 strain was supplied through the European Virus Archive goes Global (Evag) platform, a project that has received funding from the European Union's Horizon 2020 research and innovation programme under Grant No. 653316.
Virus titration
Briefly, 50 µL of 10-fold serial dilutions of SARS-CoV-2 were inoculated into eight replicate wells. The 96-well microplates were incubated at 37°C in a 5% CO2 atmosphere, and the cytopathic effect was checked 5 days after inoculation. The virus titre was calculated according to the method of Reed and Muench (1938).
Neutralizing antibody
Nab tests were performed in flat-bottomed microtitre plates (96 wells), 3 days after seeding the Vero cells. Two-fold serial dilutions of inactivated sera, starting at a 1/40 dilution, were mixed with an equal volume of SARS-CoV-2 (100 TCID50/50 µL), and incubated for 1 h at 37°C. One hundred microlitres of the serum–virus mixture and 100 µL of infection medium were inoculated in each well (four wells per dilution), and the plates were incubated for 5 days at 37°C in 5% CO2. The cytopathic effect was checked 5 days after inoculation. Neutralization titres were expressed as the inverse of the final dilution of serum that neutralized 50% of the inoculated wells. As no cytopathic effects due to serum cytotoxicity were observed at a dilution of 1/60, a neutralization titre of 60 was considered as positive.
Statistical methods
Continuous variables are expressed as mean and interquartile range (IQR), and discrete variables are expressed as number and percentage. Two-sided Wilcoxon signed-rank test was used to compare serum TI and Nab titres for CMs between M0–M9 and TI vs Nab titres 9 months PSO, respectively. All analyses were computed using R Version 4.3.0. A 5% significance level was used for all analyses.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the 86 participants analysed and their COVID-19 status are reported in Table 1 . All participants presented with COVID-19 infection during the outbreak, of whom 17.4% were asymptomatic. Three CMs (3.4%) were hospitalized for oxygen administration. All CMs had detectable positive SARS-CoV-2 serology at the end of quarantine. The first and second samples were collected 18 and 280 days after PSO, respectively.
Table 1.
Characteristics of the crew members with severe acute respiratory virus infection.
| Characteristic | Results (n=86) |
|---|---|
| Male, n (%) | 75 (87.2%) |
| Age (years), median (IQR) | 31 (25–38) |
| COVID infection Symptomatic PCR positive PCR negative Asymptomatic |
71 (82.5%) 67 (77.9%) 4 (4.6%) 15 (17.4%) |
| Reported symptom, n (%) Myalgia Headache Anosmia Asthenia Cough Fever Aguesia Rhinitis Dyspnoea Diarrhoea |
42 (48.8%) 40 (46.5%) 36 (41.8%) 35 (40.7%) 31 (36.0%) 26 (30.2%) 26 (30.2%) 21 (24.3%) 18 (20.0%) 6 (6.9%) |
| Duration of symptom median (IQR) | 7 (3–12) |
| Hospitalization Medicine with O2 |
3 (3.5%) |
COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; IQR, interquartile range.
Kinetics of total anti-SARS-CoV-2 immunoglobulin
The average rate for COI was 18.0 (IQR 3.1–25.8) in the first sample. At 9 months PSO, the seropositive rate was 93.0 % (80/86) and the mean COI level was 31.0 (IQR 4.8–32.7).
For the three CMs who required hospitalization, a 100% seropositive rate was observed at 9 months PSO with an average COI of 124.2, vs 27.8 for the non-hospitalized CMs. No significant difference was observed in the positivity kinetics of the serology depending on the severity of COVID-19 infection.
Six CMs had COI <1.0 at 9 months PSO. Of these, five CMs presented with symptomatic COVID-19 and one patient presented with asymptomatic COVID-19. At 9 months PSO, these seronegative patients had lower COI levels than others (mean 7.0 vs 18.8; P<0.01).
Neutralizing antibody
The median Nab titre 9 months PSO was 1:96 (IQR 1:57–1:113). With a 1:60 threshold, 72.1% (62/84) were considered positive. Table 2 details the characteristics of CMs depending on the presence of Nabs. No significant difference was found in terms of type of infection, symptoms or anti-IgG-SARS-CoV-2 rate. Among the six patients who no longer showed anti-IgG-SARS-CoV-2, five had a neutralizing antibody titre >1:60.
Table 2.
Characteristics of crew members depending on the presence of neutralizing antibodies (Nabs).
| Characteristic | Presence of Nabs (n=62) | Absence of Nabs (n=24) | P-value |
|---|---|---|---|
| Male, n (%) | 54 (87.0%) | 21 (87.5) | 1 |
| Age (years), median (IQR) | 31 (19.0–37.7) | 33 (22.0–36.3) | 0.25 |
| COVID infection Symptomatic Asymptomatic |
52 (83.8%) 10 (16.1%) |
19 (79.2) 5 (10.8) |
0.54 |
| Reported symptom, n (%) Myalgia Headache Asthenia Anosmia Cough Fever Aguesia Rhinitis Dyspnoea Diarrhoea |
29 (46.7%) 33 (53.2%) 26 (41.9%) 30 (48.3%) 25 (40.3%) 20 (32.2%) 20 (32.2%) 14 (22.5%) 13 (20.9%) 5 (8.0%) |
13 (54.1%) 7 (29.2%) 9 (37.5%) 7 (29.2%) 7 (29.2%) 6 (25.0%) 6 (25.2%) 7 (29.2%) 5 (20.8%) 1 (4.1%) |
0.63 0.05 0.80 0.14 0.21 0.60 0.60 0.58 1 1 |
| Duration of symptom mean | 9.04 | 7.31 | 0.17 |
| Hospitalization Medicine |
2 (4.5%) |
1 (4.2%) |
1 |
| First TI titres (Cobas Roche) mean (IQR) | 18.1 (3.1–30.2) | 17.9 (3.6–17.4) | 0.84 |
| Second TI titres mean Positive (>1.0) Negative (<1.0) |
30.2 57 (92.0%) 5 (8.0%) |
33.5 23 (95.8%) 1 (4.2%) |
0.52 |
| Hospitalization Medicine with O2 |
3 (3.5%) |
COVID-19, coronavirus disease 2019; TI, total immunoglobulin; IQR, interquartile range.
Discussion
Studies focusing on the detectability of Nabs over time have mainly reported data from the first year of a COVID-19 outbreak, thus mainly following exposure to the initial strain of SARS-CoV-2. Nabs last for up to 12 months PSO, with discrepancies between studies with regards to waning (Betton et al., 2021; Choe et al., 2021; Dispinseri et al., 2021; Moriyama et al., 2021; Terpos et al., 2021; Yao et al., 2021). These studies involved 16–188 patients, mainly with mild or moderate COVID-19, with a median age of 41–63 years. De Giorgi et al. (2021) reported detectable Nabs in 63% of 162 patients at 11 months PSO. An open question remains about the extended efficacy of Nabs and their cross-reactivity against emerging viral variants, and re-infection rates among convalescent patients carrying Nabs are notably unknown.
Passive transfer of Nabs is protective against severe SARS-CoV-2 infection or re-infection in multiple animal models (Kim et al., 2021; McMahan et al., 2021; Rogers et al., 2020). A fatal outcome was correlated with Nab deficiency in a cohort of 162 patients (Dispinseri et al., 2021). Moriyama et al. (2021) reported limited efficacy of Nabs toward B.1.351 (501&Y.V2) and P.1 (501Y.V3) variants using 188 sera obtained from symptomatic patients between June 2020 and February 2021. On the other hand, the administration of convalescent plasma to patients with COVID-19 improves both morbidity and mortality, particularly when used in the early stages of the disease, even for critical cases (Hegerova et al., 2020; Kim et al., 2021). More recently, treatment with Nab bamlanivimab in association with etesevimab has been shown to reduce viral load, hospitalization and death (Dougan et al., 2021; Gottlieb et al., 2021). mRNA-based vaccines have been shown to elicit a high Nab rate (Chu et al., 2021; Pratesi et al., 2021). A randomized controlled trial reported the efficacy of the mRNA-1273 SARS-CoV-2 vaccine to prevent COVID-19, but with a median follow-up duration of 2 months (Baden et al., 2021). Whereas SARS-CoV-2 B.1.1.7 (alpha) and B.1.526 (iota) variants remain susceptible to vaccine- or infection-elicited neutralizing activity, the more recent variants [B.1.617.1 (kappa) and B.1.617.2 (delta)] are less susceptible to Nabs (Liu et al., 2020; Edara et al., 2021). Thus, it remains unknown how the persistence of the neutralization response following vaccination or SARS-CoV-2 infection confers protection against new variants.
This study included individuals infected during the same epidemic event and followed them prospectively, allowing reliable chronological analysis. While the stability of binding antibodies is known to be linked to the severity of disease, this cluster still exhibited a high and persistent seroconversion level. An increased titre range was observed at 9 months compared with 1 month PSO, but the timing at first sample collection may have been too early to capture the initial IgG peak as a whole. Although not likely to be correlated with protective efficacy, the low seroreversion rate (7%, 6/86) of CMs at 9 months PSO strongly suggests the persistence of a significant humoral response against SARS-COV-2 within a young population. The mean COI was higher for three hospitalized CMs, but the serological response level was irrespective of both symptom intensity and duration among other CMs.
Usually assessed during shorter follow-up periods, Nabs persisted at a detectable level 9 months PSO for the majority of patients in the present study (Chen et al., 2020; Iyer et al., 2020; Reynolds et al., 2020; Wajnberg et al., 2020; Crawford et al., 2021; Gaebler et al., 2021; Legros et al., 2021). Likewise, persistent serum neutralizing activity was observed for asymptomatic patients whose contamination period was clearly determined (Brochot et al., 2020; Reynolds et al., 2020; Jonsdottir et al., 2021).
Former studies including patients with severe SARS-CoV-2 infection identified a significant difference in Nabs according to disease severity (Chen et al., 2020; Iyer et al., 2020; Wajnberg et al., 2020). However, this correlation might be unstable over time (Crawford et al., 2021). Like other publications enrolling mild and/or asymptomatic patients, no difference in Nab titre was observed between the groups in the present study (Reynolds et al. 2020; Jonsdottir et al., 2021).
Both IgG stability and neutralizing activity rely on targeted antigens (Wajnberg et al., 2020). This correlation is inconstantly observed, and appears to be mainly influenced by time of Nab assessment PSO and patient profile (Iyer et al., 2020; Wang et al., 2020). Five patients showed persistent Nab activity and negative serology. Nevertheless, as this study used nucleocapsid-based serology, its correlation with Nab titres targeting the S protein was judged to be irrelevant and was not assessed.
Due to homogeneous patient profiles, the present study may not reflect characteristics of the general population, but provides crucial information on a group not usually targeted as high priority by vaccination programmes, highlighting the persistence of Nabs 9 months PSO, as well as an upward trend in nucleocapsid-based serology titres. No cases of re-infection had been observed at the time of publication, reducing doubt about protective immunity among non-severe cases.
Nabs persist for up to 9 months PSO following asymptomatic/mild COVID-19 among young adults, regardless of serological results.
Author contributions
Study design: OB, DD, AM, AB, CM, FJ and J-NT.
Data collection: OB, JC, NM, EV-B and OF.
Data analysis: OB, DD, AM, AF, OF, FJ and J-NT.
Writing: OB, DD, AM, AB, CM, FJ and J-NT.
Declaration of Competing Interest
None declared.
Acknowledgments
Acknowledgements
The authors wish to thank all the crew members who participated in the study and the entire staff of HIA Saint Anne Clinical Unit Research who actively contributed to the data collection.
Funding
The COV-PA cohort is supported by the Military National Social Security ‘Caisse Nationale Militaire Securité Sociale’.
Ethical approval
The COV-PA study was approved by the regional research ethics committee on 7 December 2020 (IDRCB 2020-A02397-32).
References
- Baden LR, et al. Efficacy and safety of the MRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betton M, et al. Sera neutralizing activities against SARS-CoV-2 and multiple variants six months after hospitalization for COVID-19. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochot E, et al. Anti-spike, anti-nucleocapsid and neutralizing antibodies in SARS-CoV-2 inpatients and asymptomatic individuals. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.584251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. SARS-CoV-2 Neutralizing antibody levels are correlated with severity of COVID-19 pneumonia. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe PG, et al. Persistence of neutralizing antibody response up to one year after asymptomatic or symptomatic SARS-CoV-2 infection. J Infect Dis. 2021 doi: 10.1093/infdis/jiab339. [DOI] [PubMed] [Google Scholar]
- Chu L, et al. A preliminary report of a randomized controlled Phase 2 trial of the safety and immunogenicity of MRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39:2791–2799. doi: 10.1016/j.vaccine.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford KHD, et al. Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection. J Infect Dis. 2021;223:197–205. doi: 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi V, et al. Naturally acquired SARS-CoV-2 immunity persists for up to 11 months following infection. J Infect Dis. 2021 doi: 10.1093/infdis/jiab295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dispinseri S, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Comm. 2021;12:2670. doi: 10.1038/s41467-021-22958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edara VV, et al. Neutralizing antibodies against SARS-CoV-2 variants after infection and vaccination. JAMA. 2021;325:1896–1898. doi: 10.1001/jama.2021.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RL, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerova L, et al. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood. 2020;136:759–762. doi: 10.1182/blood.2020006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Comm. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AS, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5:eabe0367. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir HR, et al. Titers of neutralizing antibodies against SARS-CoV-2 are independent of symptoms of non-severe covid-19 in young adults. Viruses. 2021;13 doi: 10.3390/v13020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-I, et al. Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerg Microb Infect. 2021;10:152–160. doi: 10.1080/22221751.2021.1872352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros V, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F-X, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of COVID-19 – studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- Liu L, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- McMahan K, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama S, et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity. 2021;54:1841–1852. doi: 10.1016/j.immuni.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratesi F, et al. BNT162b2 MRNA SARS-CoV-2 vaccine elicits high avidity and neutralizing antibodies in healthcare workers. Vaccines. 2021;9:672. doi: 10.3390/vaccines9060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- Reynolds CJ, et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol. 2020;5:eabf3698. doi: 10.1126/sciimmunol.abf3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TF, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E, et al. SARS-CoV-2 antibody kinetics eight months from COVID-19 onset: persistence of spike antibodies but loss of neutralizing antibodies in 24% of convalescent plasma donors. Eur J Intern Med. 2021;89:87–96. doi: 10.1016/j.ejim.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK-W, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajnberg A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Neutralizing antibody responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin Infect Dis. 2020;71:2688–2694. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, et al. Persistence of antibody and cellular immune responses in COVID-19 patients over nine months after infection. J Infect Dis. 2021 doi: 10.1093/infdis/jiab255. [DOI] [PMC free article] [PubMed] [Google Scholar]