Abstract
The systemic illness associated with SARS-CoV-2 infection results in hospitalization rate of 380.3 hospitalizations per 100,000 population, overwhelming health care systems. Vitamin D regulates expression of approximately 11,000 genes spanning many physiologic functions that include regulation of both innate and adaptive immune function. We investigate potential benefit of calcitriol therapy given to patients hospitalized with COVID-19.
This was an open label, randomized clinical trial of calcitriol or no treatment given to hospitalized adult patients with COVID-19. Subjects were randomly assigned treatment with calcitriol 0.5 μg daily for 14 days or hospital discharge; or no treatment (1:1) at time of enrollment.
We enrolled 50 consecutive patients, 25 per trial arm. The change in peripheral arterial oxygen saturation to the inspired fraction of oxygen (SaO2/FIO2 ratio) was calculated on admission and discharge between the groups. The control group had an average increase of +13.2 (±127.7) on discharge and the calcitriol group had an increase of +91.04 (±119.08) (p = .0305), suggesting an improvement in oxygenation among subjects who received calcitriol. Additionally, 12 patients in the control group required oxygen supplementation on admission and 21 of them were discharged on room air. 14 subjects needed oxygen supplementation in the calcitriol group on admission while all 25 were discharged on room air.
Other clinical markers showed the average length of stay was 9.24 (±9.4) in the control group compared to 5.5 (±3.9) days in the calcitriol group (p = .14). The need for ICU transfer was 8 in the control group and 5 in the calcitriol group. There were 3 deaths and 4 readmissions in the control group and 0 deaths and 2 readmissions in the calcitriol group.
This pilot study illustrates improvement in oxygenation among hospitalized patients with COVID-19 treated with calcitriol and suggests the need for a larger randomized trial.
Keywords: Calcitriol, Vitamin D, COVID-19, Endocrine, Therapy
1. Introduction
The systemic illness that manifests SARS-CoV-2 infection has resulted in a cumulative hospitalization rate of 380.3 hospitalizations per 100,000 population according to the Centers for Disease Control (CDC). A triggered cytokine storm commonly results in acute respiratory distress syndrome (ARDS), multiorgan system failure, and is potentially fatal in approximately 23% of cases, including over 50% of patients who require mechanical ventilation [1]. Emerging therapies including remdesivir and corticosteroids demonstrate modest benefits [2].
Vitamin D regulates expression of approximately 11,000 genes spanning many physiologic functions that include regulation of both innate and adaptive immune function, pulmonary cytokine release, and induction of autophagy as an immune defense [3]. Synthesis of 1,25-dihydroxyvitamin D (1,25(OH)2D) is integral to the normal development of antigen-presenting cells [4]. Moreover, through induction of macrophage expression of cathelicidin and β-Defensin2, 1,25(OH)2D stimulates chemotaxis of neutrophils, monocytes, macrophages, and T cells, promotes the clearance of respiratory pathogens through apoptosis and autophagy of infected epithelial cells. Vitamin D-induced autophagy modulates the immune response in influenza A infection, and diminishes clinical severity of rotavirus infection [3].
Hospitalized and severely ill patients exhibit high rates of vitamin D deficiency, potentially impacting these immune pathways [[5], [6], [7]]. Lower serum concentration of 25-hydroxyvitamin D is associated with adverse outcomes including increased susceptibility to SARS-CoV-2 infection, and increased severity of symptoms in the course of COVID-19 [8,9].
Activation of vitamin D-dependent pathways may provide clinical benefit to patients with SARS-CoV-2 infection. As the biochemically active metabolite, 1,25(OH)2D or calcitriol, is useful over other forms of vitamin D as therapy in patients with hypocalcemia or with secondary hyperparathyroidism due to renal insufficiency [3]. We investigate potential activation of vitamin D-dependent pathways in a trial of calcitriol therapy given to patients who are hospitalized with COVID-19.
2. Materials and methods
The study protocol was approved by the Mount Sinai Program for the protection of Human subjects/Institutional Review Board (IRB) for enrollment at all sites involved. Verbal consent was obtained from all patients about the outcomes that would be measured, and potential benefits and side effects with subsequent acceptance recorded in the electronic medical record.
2.1. Study design and participants
This was an open label, randomized clinical trial investigating the use of calcitriol in hospitalized patients with COVID-19. We planned to enroll 50 consecutive hospitalized adult patients with COVID-19 admitted to Mount Sinai Beth Israel, Mount Sinai Morningside, and Mount Sinai West Hospitals. Patients were excluded if they are admitted directly to the intensive care unit (ICU), if they had any of the following: hypercalcemia and/or hyperphosphatemia on admission blood tests, untreated disorders of calcium metabolism including hyperparathyroidism, hypoparathyroidism, chronic renal insufficiency with glomerular filtration rate < 30 ml/min, or if they are prescribed calcitriol for any reason outside of the study. Patients were also excluded if they declined to participate prior to enrollment. Any subject who withdrew from the trial or who left the hospital against medical advice would be included in intention to treat analysis.
2.2. Procedures
Eligible patients were allocated at a 1:1 calcitriol, no calcitriol ratio through electronic randomization on the day of admission. Enrolled subjects were assigned treatment with calcitriol 0.5 μg daily for 14 days or hospital discharge, whichever came first; or no treatment. The remainder of the patient's care was determined by the primary team and may include treatment with remdesivir (200 mg for one day followed by 100 mg for 4 days), dexamethasone (6 mg daily for 10 days), or convalescent plasma, as well as supplemental O2. Medication was supplied by the inpatient pharmacy.
2.3. Outcomes
Outcomes of the effectiveness of calcitriol in the treatment of COVID-19 included oxygen requirements, length of hospital stay, need for ICU admission, mortality, and readmission.
2.4. Laboratory analysis and respiratory function test
Clinical samples for SARS-CoV-2 diagnostic testing were obtained for all hospital admissions at each medical center by nasopharyngeal exudate sampling. Procedures for RNA extraction and real-time RT-PCR were undertaken at on-site testing using Roche Cobas 6800 System or Agena Mass ARRAY System.
Hematology analyses included a complete blood count (DYN Sapphire, Siemens Healthineers, Erlangen, Germany) and coagulation studies included D-Dimer (Sta R max, 5070194, 5070195). Biochemical tests included renal function (Abbott Architect Analyzer), liver function (Abbott Architect Analyzer), lactate dehydrogenase (Abbott Architect Analyzer Siemens Healthineers, Erlangen, Germany), ferritin, and, ESR (Excite 40 Random access ESR Analyzer 8060), C-reactive protein (Abbott Architect Analyzer, Siemens Healthineers, Erlangen, Germany), IL-6 (Siemens Healthineers, Erlangen, Germany). Respiratory function was assessed by peripheral oxygen saturation.
To assess respiratory status and oxygen requirement, the ratio of peripheral arterial oxygen saturation to the inspired fraction of oxygen (SpO2/FIO2) was calculated. The peripheral arterial oxygenation was completed with SpO2 pulse oximeter. The validity of this measure as a surrogate for the pressure arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) ratio has been demonstrated in numerous studies [10].
2.5. Statistical analysis
Descriptive statistics were used for demographic, laboratory, and clinical prognostic factors related to COVID-19 for each treatment arm. This included age, race, sex, body mass index, comorbidities, COVID-19 treatments utilized, and baseline IL-6 and procalcitonin levels.
The comparison between groups of quantitative variables were performed by using t-test for qualitative variables, χ2 tests and Fisher exact tests (with frequencies <5) were used as well as a Mann-Whitney test for length of stay. The comparison for the improvement in SpO2/FIO2 ratio was performed using t-test.
Univariate and multivariate logistic regressions were used to estimate odds ratio and 95% CIs for the length of stay, rate of readmission, probability of admission to ICU, and mortality rate. Significant p-value was considered when p < .05. All the analysis has been done using IBM SPSS Statistics software (SPSS).
3. Results
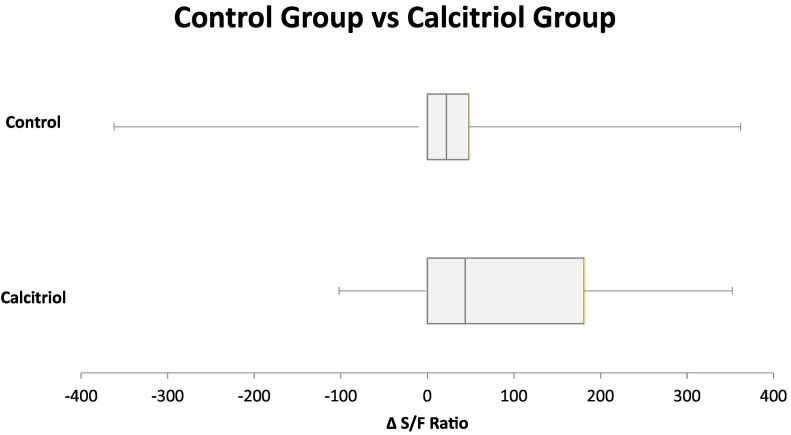
We enrolled 50 consecutive patients, 25 per trial arm between September 2020 and December 2020. Baseline characteristics of each group are given in Table 1 . No subject withdrew from the study. Primary outcome and other clinical outcomes are given in Fig. 1 and Table 2 . The average length of stay was 9.24 (±9.4) in the control group compared to 5.5 (±3.9) days in the calcitriol group (p = .14). The need for transfer to ICU was 8 in the control group and 5 in the calcitriol group (p = .33). There were 3 deaths and 4 readmissions in the control group and 0 deaths and 2 readmissions in the calcitriol group. 12 patients in the control group required oxygen supplementation on admission and 21 of them were discharged on room air. 14 subjects needed oxygen supplementation in the calcitriol group on admission while all 25 were discharged on room air.
Table 1.
Baseline characteristics.
| Control group | Calcitriol group | p-Value | |
|---|---|---|---|
| Demographics | 25 | 25 | |
| Age (y) | 64 ± 16 | 69 ± 18 | 0.16 |
| Above age 65 | 19 | 14 | 0.14 |
| Male gender | 13 | 12 | 0.77 |
| Race | 0.90 | ||
| Caucasian | 5 | 7 | |
| Non-White Latino | 15 | 14 | |
| Black | 3 | 2 | |
| Asian | 2 | 2 | |
| Poor prognostic factors | |||
| Body mass index (kg/m2) | 27.2 ± 5 | 27.3 ± 6 | 0.48 |
| BMI > 30 kg/m2 | 6 | 4 | 0.48 |
| Hypertension | 17 | 13 | 0.24 |
| Type 2 diabetes | 11 | 9 | 0.56 |
| Cancer history | 1 | 1 | 1.00 |
| Asthma/COPD | 3 | 5 | 0.44 |
| Admission levels | |||
| Procalcitonin | 0.21 ± 0.27 | 0.11 ± 0.1 | 0.14 |
| IL-6 | 65 ± 114 | 38 ± 27 | 0.29 |
| Lymphocytes (absolute number) | 1.25 ± 0.68 | 1.14 ± 0.55 | 0.51 |
| C-reactive protein | 160.7 ± 381 | 78.9 ± 76.8 | 0.33 |
| Ferritin | 570 ± 667.2 | 902.6 ± 981.8 | 0.18 |
| d-Dimer | 1.36 ± 0.91 | 1.65 ± 2.08 | 0.55 |
| Glomerular filtration rate | 75 ± 41 | 69 ± 23 | 0.28 |
| Oxygen requirements on admission | 0.74 | ||
| Room air | 13 | 11 | |
| Nasal canula | 8 | 12 | |
| High flow nasal canula | 1 | 0 | |
| Non-rebreather | 3 | 1 | |
| Bipap | 0 | 1 | |
| Treatments during admission | |||
| Remdesivir | 11 | 15 | 0.25 |
| Dexamethasone | 12 | 13 | 0.78 |
| Convalescent plasma | 2 | 4 | 0.38 |
| Medical center | 0.67 | ||
| MS Beth Israel | 13 | 15 | |
| MS Morningside | 10 | 7 | |
| MS West | 2 | 3 |
Fig. 1.
SaO2/FIO2.
Table 2.
Clinical outcomes.
| Control | Calcitriol | p value | |
|---|---|---|---|
| Length of hospital stay (days) | 9.24 ± 9.4 | 5.5 ± 3.9 | 0.14 |
| ICU admission | 8 | 5 | 0.33 |
| Endotracheal intubation | 2 | 0 | 0.48 |
| Mortality | 3 | 0 | 0.23 |
| Readmission within 30 days | 4 | 2 | 0.67 |
When comparing change in oxygen saturation using SaO2/FIO2 ratio on admission and discharge between the groups, the control group had an average increase of +13.2 (±127.7) on discharge and the calcitriol group had an increase of +91.04 (±119.08) (p = .0305) (Fig. 1).
No adverse effects were observed (Table 3 ), including no hypercalcemia or hyperphosphatemia.
Table 3.
Adverse effects.
| Control | Calcitriol | p value | |
|---|---|---|---|
| Hypercalcemia | 0 | 0 | – |
| Hyperphosphatemia | 0 | 0 | – |
| Renal calculus | 0 | 0 | – |
| Reduction in glomerular filtration rate by >10% | 4 | 0 | 0.1 |
4. Discussion
Our study shows a significant reduction in oxygen requirements in patients hospitalized with COVID-19 who received calcitriol. Since ICU patients were not included, routine arterial blood gas assessment was not available. The PaO2/FIO2 ratio is a highly regarded parameter of respiratory function and serves as a predictor of ICU mortality in patients with sepsis and ARDS [11]. Moreover, SaO2/FIO2 is commonly used as an end point in clinical trials for patients with COVID-19, with success and is considered an accurate predictor of PaO2/FIO2 ratio [10]. ARDS severity when measured by PaO2/FiO2 is classified as mild if 200–300, moderate if 100–200, and severe is less than 100. SaO2/FIO2 ratios of 181 and 235 corresponded approximately to PaO2/FIO2 ratios of 300 and 200, respectively [12]. While infected with COVID-19, an average increase in SaO2/FIO2 of 91.04 ± 119.08, as noted with calcitriol therapy, demonstrates a significant clinical improvement, and may render a typical patient to a more mild case of ARDS. The SaO2/FIO2 ratio is a reliable noninvasive surrogate of the PaO2/FIO2 ratio with the advantage of replacing invasive arterial blood gases [12].
Effects of calcitriol therapy on other clinical parameters, including hospital length of stay, need for ICU admission, endotracheal intubation, hospital readmission, and mortality did not show significant benefit. This may be secondary to the small size of the trial. In each case, numerical results may favor calcitriol therapy. A larger trial is warranted to investigate further.
On the basis of improved oxygenation, our results are somewhat consistent with those reported by Castillo, et al., who conducted a similar open-label pilot trial examining the effects of calcifediol in hospitalized patients with COVID-19 [13]. A significant reduction in ICU admission is noted with calcifediol therapy, again suggesting potential respiratory benefit of therapy with vitamin D derivatives.
In contrast, results of a recent trial demonstrate no clinical benefit from a single dose of 200,000 IU cholecalciferol in patients with moderate to severe COVID-19 [14]. While increased serum levels of 25-hydroxyvitamin D were observed, levels of 1,25(OH)2D were not reported. The efficiency of renal and other systemic 1α-hydroxylase activity to generate 1,25-dihydroxyvitamin D during acute illness is unknown [15]. Moreover, reduced circulation of vitamin D binding protein (VDBP) during acute illness may diminish clinical activity of cholecalciferol [16]. Calcitriol, but not cholecalciferol, can restore calcium homeostasis in patients with inherited VDBP deficiency [17]. These potential mechanisms suggest administration of calcitriol may be more effective than cholecalciferol in severe acute illness such as COVID-19.
Several preclinical and clinical studies suggest therapy with vitamin D is beneficial in ARDS specifically. Animal models demonstrate vitamin D sufficient state mitigates ARDS that is induced by lipopolysaccharide, compared to vitamin D deficiency [18]. Moreover, animal models of ARDS with knockout mutations of the vitamin D receptor (VDR) show significantly worse lung histology and nearly twice the mortality rate as wild type mice [19]. Results of animal model of ARDS in VDR-knockout mice that were also given losartan suggest that vitamin D functions, in part, by up-regulating the pulmonary renin/angiotensin system to mitigate lung damage [19]. This same system is disrupted by COVID-19 infection [20].
Human trials with cholecalciferol therapy for ARDS have mixed results. In a randomized trial, patients that underwent esophagectomy demonstrated a transient reduction in markers of ARDS with 300,000 IU treatment of cholecalciferol compared to placebo [21]. The authors argue that declining levels of VDBP that were observed in this trial may explain the short-lived benefit of cholecalciferol that was observed.
In another randomized trial of 475 mechanically ventilated ICU patients given 540,000 units cholecalciferol vs placebo, a 44% reduction in hospital mortality was observed in the cholecalciferol treated patients among the subgroup of patients with 25-hydroxyvitamin D levels less than 12 ng/ml [22]. In addition, the cholecalciferol treated patients demonstrated a further reduction in mortality that persisted for 6 months of follow up [22]. A larger, multicenter trial is currently underway [23].
Limitations of our trial include lack of a placebo and lack of blinding. It is unlikely this would affect the SaO2/FIO2 endpoint. Additionally, 25-hydroxyvitamin D levels were not measured in our study. Enrollment of patients without regard to vitamin D status strengthens the generalizability of the results.
Sample size was another limitation. The patients in the control group had a higher percentage of patients above 65 years of age and certain co morbidities such as hypertension and diabetes mellitus were higher in that group. The statistical non significance in these baseline characteristics could be due to the small sample size.
The low cost and high availability make calcitriol an attractive candidate for therapy in hospitalized patients with moderate to severe COVID-19. Further study of this approach is warranted.
Data availability statement
The data that support the findings of this study will be openly available in a public archive.
CRediT authorship contribution statement
Yasmine M. Elamir: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Hajira Amir: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Steven Lim: Formal analysis, Investigation. Yesha Patel Rana: Formal analysis, Investigation. Carolina Gonzalez Lopez: Formal analysis, Investigation. Natalia Viera Feliciano: Formal analysis, Investigation. Ali Omar: Formal analysis, Investigation. William Paul Grist: Formal analysis, Investigation. Michael A. Via: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision.
Declaration of competing interest
None of the authors have anything to disclose regarding the below.
-
1.
Service on Board of Directors
-
2.
Consulting (other than Advisory Boards or Board of Directors)
-
3.
Position in a company, employment or executive position in pharmaceutical, medical device, or diagnostic companies; including industry scientists in the bone and mineral field
-
4.
Serving as an expert witness or consultant in litigation for commercial entities
-
5.
Honoraria or royalties for books or publications or for lectures (speaker fees) or participating in a speakers bureau
-
6.
Research grants, direct salary support or other financial support from commercial entities
-
7.
Stock holdings and/or stock options in pharmaceutical, medical device, or diagnostic companies
-
8.
Partnerships, warrants, royalties for inventions (licensing revenues) or other ownership interest
-
9.
No institutional investigation regarding conflict of interest, responsible conduct in research, animal welfare, human subjects, or laboratory safety compliance exists
-
10.
Or any other situation or transaction or formal role where we have interest
Acknowledgements
There are no acknowledgements.
References
- 1.Guan W.J., Zhong N.S. Clinical characteristics of Covid-19 in China. Reply. N. Engl. J. Med. 2020;382(19):1861–1862. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 2.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hewison M., Freeman L., Hughes S.V., Evans K.N., Bland R., Eliopoulos A.G., et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003;170(11):5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 5.Nierman D.M., Mechanick J.I. Bone hyperresorption is prevalent in chronically critically ill patients. Chest. 1998;114(4):1122–1128. doi: 10.1378/chest.114.4.1122. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berghe G., Van Roosbroeck D., Vanhove P., Wouters P.J., De Pourcq L., Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J. Clin. Endocrinol. Metab. 2003;88(10):4623–4632. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]
- 7.Lee P., Eisman J.A., Center J.R. Vitamin D deficiency in critically ill patients. N. Engl. J. Med. 2009;360(18):1912–1914. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 8.Laird E., Rhodes J., Kenny R.A. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir. Med. J. 2020;113(5):81. [PubMed] [Google Scholar]
- 9.Rhodes J., Dunstan F., Laird E., Subramanian S., Kenny R.A. COVID-19 mortality increases with northerly latitude after adjustment for age suggesting a link with ultraviolet and vitamin D. BMJ Nutr. Prev. Health. 2020;3(1):118–120. doi: 10.1136/bmjnph-2020-000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catoire P., Tellier E., de la Riviere C., Beauvieux M.C., Valdenaire G., Galinski M., et al. Assessment of the SpO2/FiO2 ratio as a tool for hypoxemia screening in the emergency department. Am. J. Emerg. Med. 2021;44:116–120. doi: 10.1016/j.ajem.2021.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santana A.R., de Sousa J.L., Amorim F.F., Menezes B.M., Araújo F.V.B., Soares F.B., et al. SaO2/FiO2 ratio as risk stratification for patients with sepsis. Crit. Care. 2013;17(4):P51. [Google Scholar]
- 12.Bilan N., Dastranji A., Ghalehgolab Behbahani A. Comparison of the spo2/fio2 ratio and the pao2/fio2 ratio in patients with acute lung injury or acute respiratory distress syndrome. J. Cardiovasc. Thorac. Res. 2015;7(1):28–31. doi: 10.15171/jcvtr.2014.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcala Diaz J.F., Lopez Miranda J., Bouillon R., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zehnder D., Bland R., Williams M.C., McNinch R.W., Howie A.J., Stewart P.M., et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 16.Waldron J.L., Ashby H.L., Cornes M.P., Bechervaise J., Razavi C., Thomas O.L., et al. Vitamin D: a negative acute phase reactant. J. Clin. Pathol. 2013;66(7):620–622. doi: 10.1136/jclinpath-2012-201301. [DOI] [PubMed] [Google Scholar]
- 17.Henderson C.M., Fink S.L., Bassyouni H., Argiropoulos B., Brown L., Laha T.J., et al. Vitamin D-binding protein deficiency and homozygous deletion of the GC gene. N. Engl. J. Med. 2019;380(12):1150–1157. doi: 10.1056/NEJMoa1807841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dancer R.C., Parekh D., Lax S., D’Souza V., Zheng S., Bassford C.R., et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70(7):617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong J., Zhu X., Shi Y., Liu T., Chen Y., Bhan I., et al. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol. Endocrinol. 2013;27(12):2116–2125. doi: 10.1210/me.2013-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parekh D., Dancer R.C.A., Scott A., D’Souza V.K., Howells P.A., Mahida R.Y., et al. Vitamin D to prevent lung injury following esophagectomy—a randomized, placebo-controlled trial. Crit. Care Med. 2018;46(12):e1128–e1135. doi: 10.1097/CCM.0000000000003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amrein K., Schnedl C., Holl A., Riedl R., Christopher K.B., Pachler C., et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312(15):1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 23.Amrein K., Parekh D., Westphal S., Preiser J.C., Berghold A., Riedl R., et al. Effect of high-dose vitamin D3 on 28-day mortality in adult critically ill patients with severe vitamin D deficiency: a study protocol of a multicentre, placebo-controlled double-blind phase III RCT (the VITDALIZE study) BMJ Open. 2019;9(11):e031083. doi: 10.1136/bmjopen-2019-031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study will be openly available in a public archive.