ABSTRACT
Bispecific antibodies have recently attracted intense interest. CrossMab technology was described in 2011 as novel approach enabling correct antibody light-chain association with their respective heavy chain in bispecific antibodies, together with methods enabling correct heavy-chain association using existing pairs of antibodies. Since the original description, CrossMab technology has evolved in the past decade into one of the most mature, versatile, and broadly applied technologies in the field, and nearly 20 bispecific antibodies based on CrossMab technology developed by Roche and others have entered clinical trials. The most advanced of these are the Ang-2/VEGF bispecific antibody faricimab, currently undergoing regulatory review, and the CD20/CD3 T cell bispecific antibody glofitamab, currently in pivotal Phase 3 trials. In this review, we introduce the principles of CrossMab technology, including its application for the generation of bi-/multispecific antibodies with different geometries and mechanisms of action, and provide an overview of CrossMab-based therapeutics in clinical trials.
KEYWORDS: Bispecific, CrossMab, Immunotherapy, Oncology, Ophthalmology, TCB, T cell engager
Introduction into bispecific antibodies
Antibodies, or so-called immunoglobulins, are Y-shaped proteins of ca. 150 kDa generated by B and plasma cells of the immune system as response to infection. They consist of two identical heavy and two identical light chains forming: 1) two variable antigen-binding sites within the antigen-binding fragments (Fabs) that serve for the specific recognition of (foreign) antigens; and 2) a constant Fc domain that serves for the recruitment of the human immune system.
Recombinant antibodies have been used therapeutically for over 30 years, and today more than 120 therapeutic antibodies are approved or under regulatory review by health authorities for use in humans (source: https://www.antibodysociety.org/resources/approved-antibodies/). Since the advent of recombinant antibody technologies, there has been substantial interest in the generation of engineered and bispecific antibodies that are characterized by having two independent specificities in the Fabs, resulting in novel mechanisms of action that typically cannot be achieved with conventional monospecific antibodies. More than 100 bispecific antibodies are currently being tested in clinical trials.1–6
While uncountable approaches for the generation of bispecific antibodies have been described, 1–6 some of the most broadly applied technologies for the generation of bispecific antibodies include ART-Ig,7–10 BEAT,11 BiTE,12,13 common light chains,9,10,14–16 DAF,17 DART,18 DuoBody,19 DutaFab,20 DVD-Ig,21 Fab arm exchange,22 Fcab,23–25 FORCE,26 half antibody assembly,27 Hetero-Ig,28,29 IgG-scFv,30,31,131κλ-bodies,32 Multiclonics,14 orthogonal Fab interface,33 Tandab,34 XmAb,35 VELOCI-Bi,15 and WuxiBODY.36
As of August 2021, three bispecific antibodies have been approved, the tandem single-chain variable fragment (Fv)-based CD19/CD3 Bispecific T-cell Engager (BiTE) blinatumomab developed by Amgen for the treatment of acute lymphocytic leukemia (ALL),37 the heterodimeric ART-Ig-based coagulation factor IX/X bispecific IgG antibody emicizumab developed by Chugai and Roche for the treatment of hemophilia A,7,8,10 and the heterodimeric DuoBody-based EGFR/c-Met bispecific IgG antibody amivantamab developed by Janssen for treatment of non-small cell lung cancer harboring EGFR exon 20 insertion mutations.38–40
CrossMab technology for the generation of bispecific antibodies
We have developed an alternative technology, known as CrossMab technology, which together with methods enabling correct heavy-chain association such as the so-called knobs-into-holes technology (KiH),16 that enables the correct association of the different antibody light chains with their respective counterparts. This is achieved in different antibody formats and geometries by the exchange or crossover of antibody domains.41–43 Here, we give a brief overview of the basic principles of CrossMab technology and its application for the generation of various CrossMabs with different molecular formats and mechanisms of action.42,44,45 In fact, back in 2011, this approach was the first technology described allowing the conversion of two pre-existing antibodies into heterodimeric bispecific antibodies of the bivalent IgG format without the need to rely on so-called common light-chain antibodies that have identical light chains in each Fab.42
Since in bispecific antibodies the two heavy chains as well as the two light chains are different and can randomly associate, expression of these four chains leads to the formation of ten different antibody variants.46 Correct heavy-chain association resulting in a heterodimeric Fc can be enforced using KiH technology by introducing a bulky tryptophan (Trp) residue in one Fc fragment and forming a corresponding cavity on the other Fc fragment that can accommodate the Trp residue.16,47,48 More recently, multiple alternative approaches to enable correct heavy-chain association have been described, such as relying on charge interactions.7–11,14,29,49
Although KiH technology was developed in the late 1990s,16 enabling correct light-chain association remained a major problem, and the only approach to achieve this at the time relied on the use of common light chains for both specificities.9,10,14–16 However, the use of a common light chain requires the de novo identification of the corresponding antibody pairs, which can be challenging and/or time-consuming depending on the desired target, and restricts the availability and diversity of antibodies that can be used; thus, methods allowing the generation of bispecific antibodies from pre-existing antibody pairs were highly desired.
Figure 1 shows the basic principle of the domain crossover applied in CrossMab technology to enable correct light-chain association in bispecific antibodies.41 By incorporating the original heavy chain VH-CH1 domains in the Fab of the second specificity of the bispecific antibody as the novel “light chain” and the original light chain VL-CL domains for the novel “heavy chain” by fusing them to the hinge region of the Fc fragment, correct light-chain association can be enforced in the CrossMabFab format. This format has recently also been described as Fabs-in-Tandem Ig (FIT-Ig).50,51
Figure 1.

Principles of CrossMab technology: The four major CrossMab formats as applied to 1 + 1 heterodimeric bispecific antibodies are depicted as well as potential side products. On the bottom, the structure of mono- and duomabs is indicated. Heavy-chain domains are depicted in dark colors and respective light-chain domains are depicted with corresponding bright colors. Created with BioRender.com
Alternatively, only the VH-VL or only the CH1-CL domains can be exchanged in the CrossMabVH-VL and CrossMabCH1−CL formats (Figure 1). In the case of the CH1-CL crossover, no theoretical side product due to domain crossover is expected and crystal structure analysis confirmed the structural integrity of the crossed Fab domain in the CrossMabCH11−CL format.52 In the case of the Fab crossover in the CrossMabFab, two heavy and light chain-based monovalent side products can be observed. However, the correct preferential formation of the CrossMabFab can be fostered by relative over-expression of the respective light chains so that the respective undesired monovalent and binding inactive side products do not form in significant amounts. Similarly, in the case of VH-VL crossover, a Bence-Jones-like side product based on VL-VL together with the CH1-CL interaction can be observed. In order to avoid formation of this side product, natural charge pairs in the Fab were identified and the respective orthogonal charge interactions were introduced into the non-exchanged antibody CH1-CL domains.53 As a consequence, the undesired Bence-Jones-like side product does not form due to repulsive charge interactions, whereas the desired light-chain pairs correctly in the non-crossed Fab due to attractive charge interactions so that the corresponding CrossMabVH-VL± constructs can subsequently be obtained in high yields and purity without major side products.
Notably, these design principles can be applied not only to heterodimeric antibodies where one arm is directed to the first antigen, the other arm to the second antigen (1 + 1 format), but the CrossMab technology also allows generation of so-called MonoMabs, monovalent antibodies with one Fc portion, and DuoMabs, bivalent antibodies with two Fc portions (Figure 1).54 Furthermore, it can be applied to enable the correct light-chain association in hetero-/homodimeric bi-/multispecific antibody appended or tandem-Fab formats with, for example, 2 + 1, 2 + 2, 3 + 1, 4 + 1 or 4 + 2 valencies and in antibody fusion proteins (Figure 2).44,45 In line with this, Wu and colleagues from Lilly applied Fab crossover to generate orthogonal Fab-based trispecific antibody formats termed “OrthoTsAbs”.55,56 Interestingly, domain crossover has also been described as a means to prevent mispairing of T-cell receptor (TCR) domains in adoptive T-cell therapy.57
Figure 2.
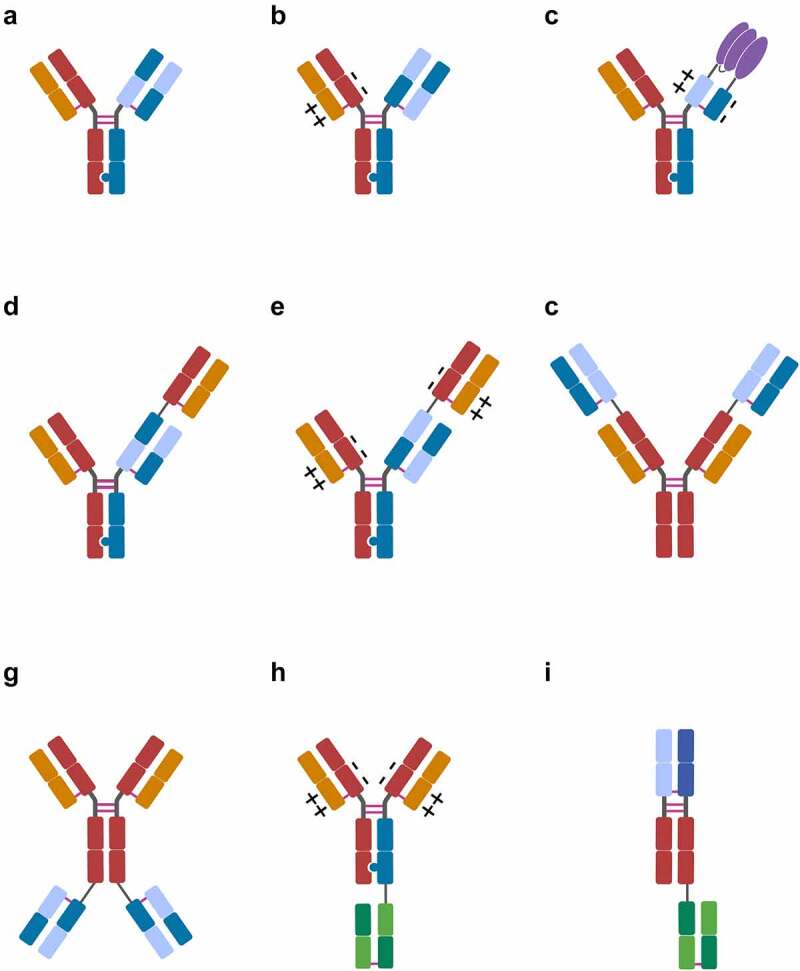
Major CrossMab formats: A) 1 + 1 CrossMab:CH1−CL vanucizumab, faricimab, 10E8.4/iMab 1 + 1; B) 1 + 1 CrossMabVH-VL±: PD1-TIM3, PD1-LAG3; C) CrossMabCH1−CL+/–based FAP-4-1BBL, CD19-4-1BBL fusion proteins; D) 2 + 1 CrossMab:CH1−CL cibisatamab; E) 2 + 1 CrossMabVH-VL±: glofitamab, CC-93269, TYRP1-TCB, WT1-TCB, RG6123; F) 2 + 2 CrossMabCH1−CL-based FIT-Ig EMB-01, EMB-02, EMB-06; G) 2 + 2 CrossMab:CH1−CL FAP-DR5; H) 2 + 1 CrossMab VH-VL±: BS-GANT, FAP-CD40; I) 1 + 1 CrossMabCH1−CL-based Nkp46-based NK cell engager (NKCE). Heavy-chain domains are depicted in dark colors and respective light-chain domains are depicted with corresponding bright colors. Fusion protein depicted in purple. Note: Differences in variable regions and/or isotype and Fc engineering are not depicted. Created with BioRender.com
Because the CrossMab approach showed advantages in terms of production, stability, developability, and versatility over analogous formats based on either single-chain Fv58–60 or single-chain Fab61–67 building blocks, it was ultimately chosen as the antibody engineering approach of choice for the generation of various clinical development candidates. Obviously, in order to develop CrossMabs for therapeutic use, the establishment of various methods covering CMC (Chemistry, Manufacturing, and Controls) aspects, including upstream and downstream processing (USP, DSP) and the establishment of the respective bioassay and (bio-) analytical methods was and is essential.68–80,81 When considering the formation of (undesired) side products, it has to be taken into account that, independent of CrossMab technology, other unrelated side products can occur, such as half- or ¾-antibodies missing two or one light chains, respectively, or hole-hole/knob-knob heavy-chain homodimers. In order to avoid the formation of these side products, achieving equal expression levels for the four heavy and light chains during transient expression and/or stable cell line generation by selecting suitable clones is advantageous. Based on the general advancement in the field of therapeutic antibody manufacturing, as well as considering these specific learnings, bispecific antibodies of different formats based on CrossMab technology can generally be manufactured in a consistent and reproducible fashion with volumetric yields in the several g/L range and in quality comparable to conventional therapeutic antibodies using established USP and DSP platforms.
Consequently, since the original description of this concept, the technology has evolved in the past decade into one of the most mature, versatile, and broadly applied technologies in the field for the generation of various bispecific antibody formats. As of mid-2021, at least 19 bispecific antibodies and fusion proteins based on CrossMab technology developed by Roche and others have entered clinical trials, of which 16 continue to be evaluated in active clinical trials (Table 1 and Figure 2). In the following sections, an overview of therapeutic bispecific antibodies and fusion proteins based on CrossMab technology is provided, with a focus on those in clinical trials.
Table 1.
CrossMabs in clinical trials (status July 2021), FP: Fusion protein, FIT-Ig: Fabs-in-tandem Ig, EIH: Entry into human date
| Name | Target A/B | Format | Indication | Stage | Company | EIH | Clinical trial | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Vanucizumab (RG7221) | Ang-2/VEGF-A | 1 + 1 CrossMabCH1−CL | Oncology | Terminated Ph 2 | Roche | 2012 | NCT02141295, NCT01688206, NCT02665416 | 81 |
| 2 | Faricimab (RG7716) | Ang-2/VEGF-A | 1 + 1 CrossMabCH1−CL | DME, wAMD | Ph 3 | Roche | 2013 | NCT03823287, NCT03823300, NCT03622580, NCT03622593 | 82,83 |
| 3 | Cibisatamab (RG7802) | CEA/CD3ε | 2 + 1 CrossMabCH11−CL | Oncology | Ph 1b | Roche | 2014 | NCT03866239, NCT04826003 | 84 |
| 4 | FAP-DR5 (RG7386) | FAP/DR5 | 2 + 2 CrossMabCH11−CL | Oncology | Terminated Ph 1 | Roche | 2015 | NCT02558140 | 85 |
| 5 | Glofitamab, RG6026) | CD20/CD3ε | 2 + 1 CrossMabVH-VL± | NHL | Ph 2/3 | Roche | 2017 | NCT04703686, NCT04914741 NCT04077723, NCT04408638 | 86 |
| 6 | PD1-TIM3 (RG7769) | PD-1/TIM-3 | 1 + 1 CrossMabVH-VL± | Oncology | Ph 1/2 | Roche | 2018 | NCT03708328, NCT04785820 | 87 |
| 7 | RG6123 | CEACAM5/CD3ε | 2 + 1 CrossMabVH-VL± | Oncology | Terminated Ph 1 | Roche | 2018 | NCT03539484 | - |
| 8 | BCMA TCE (CC-93269) | BCMA/CD3ε | 2 + 1 CrossMabVH-VL± | MultipleMyeloma | Ph 1 | BMS | 2018 | NCT03486067 | 88 |
| 9 | FAP-4-1BBL (RG7827) | FAP/4-1BB | 1 + 3 CrossMabCH1−CL± 4-1BBL FP | Oncology | Ph 1b | Roche | 2018 | NCT03869190, NCT04826003 | 89 |
| 10 | 10E8.4/iMab, TMB-370 | HIV-1 Env/CD4 | 1 + 1 CrossMabCH1−CL | HIV-1 | Ph 1 | TaiMed | 2019 | NCT03875209 | 90 |
| 11 | EMB-01 | EGFR/c-Met | 2 + 2 CrossMabFab /FIT-Ig | Oncology | Ph 1 | EpimAb | 2019 | NCT03797391 | 50,151 |
| 12 | BS-GANT (RG6102) | Abeta/TfR | 2 + 1 CrossMabVH-VL± | Alzheimer’s | Ph 2 | Roche | 2019 | NCT04639050 | |
| 13 | CD19-4-1BBL (RG6076) | CD19/4-1BB | 1 + 3 CrossMabCH1−CL± 4-1BBL FP | NHL | Ph 1b | Roche | 2019 | NCT04077723 | 89 |
| 14 | PD1-LAG3 (RG6139) | PD-1/LAG-3 | 1 + 1 CrossMabVH-VL± | Oncology | Ph 1/2 | Roche | 2019 | NCT04140500, NCT04785820 | 91 |
| 15 | TYRP1-TCB (RG6232) | TYRP1/CD3ε | 2 + 1 CrossMabVH-VL± | Melanoma | Ph 1 | Roche | 2020 | NCT04551352 | 92 |
| 16 | WT1-TCB (RG6007) | WT1/CD3ε | 2 + 1 CrossMabVH-VL± | AML | Ph 1 | Roche | 2020 | NCT04580121 | 93 |
| 17 | EMB-02 | PD-1/LAG-3 | 2 + 2 CrossMabFab /FIT-Ig | Oncology | Ph 1 | EpimAb | 2020 | NCT04618393 | - |
| 18 | EMB-06 | BCMA/CD3ε | 2 + 2 CrossMabFab /FIT-Ig | Multiple Myeloma | Ph 1 | EpimAb | 2021 | NCT04735575 | - |
| 19 | FAP-CD40 | FAP/CD40 | 2 + 1 CrossMabVH-VL± | Oncology | Ph 1 | Roche | 2021 | NCT04857138 | 94 |
Applications in targeted cancer therapy: Angiogenesis, receptor tyrosine kinases, and death receptor signaling
For many years, anti-angiogenesis approaches blocking the vascular endothelial growth factor-A (VEGF-A) have been a major area of targeted cancer therapy.95–96 One of the first IgG-based antibodies and the first bispecific CrossMab to enter clinical trials, in 2012, was the heterodimeric 1 + 1 VEGF/Ang-2 CrossMabCH1−CL vanucizumab (RG7221) (Figure 2a) targeting the pro-angiogenic ligands VEGF-A and angiopoietin-2 (Ang-2), which are involved in (tumor) angiogenesis.95,96,97 Vanucizumab, as well as a mouse-specific surrogate bispecific, mediated potent anti-tumoral and anti-angiogenic efficacy in various preclinical models as monotherapy and in combination with chemotherapy,81,98–101 as well as combined with PD-1 checkpoint inhibition102–104 and CD40 agonism.105,106 Vanucizumab was generally well tolerated as a monotherapy in a Phase 1 clinical trial and demonstrated promising anti-tumor efficacy, IgG-like pharmacokinetics and low immunogenicity,107 as well as the anticipated pharmacodynamic mechanism of action.108 Based on the negative outcome of the randomized McCAVE Phase 2 study, where it was compared to bevacizumab in combination with FOLFOX-6 chemotherapy in patients with untreated metastatic colorectal carcinoma, clinical development was discontinued.109 Similarly, in spite of promising preclinical data, Phase 1b studies of vanucizumab in combination with the PD-L1 antibody atezolizumab (NCT01688206) and the CD40 antibody selicrelumab (NCT02665416) were ultimately discontinued. Recently, preclinical data demonstrated that dual inhibition of VEGF and Ang-2 by the vanucizumab mouse-specific surrogate bispecific in murine sepsis models improved the outcomes, making it a potential therapeutic against vascular barrier breakdown.110 Similarly, Zhou and colleagues reported on an alternative anti-angiogenic approach for cancer therapy using a heterodimeric 1 + 1 VEGF/DLL4 CrossMabCH1−CL called HB-32 that mediated potent anti-angiogenic activity in vitro, as well as in vivo anti-tumor activity in breast cancer xenograft models.111
In addition to anti-angiogenesis, targeting receptor tyrosine kinases (RTKs) like EGFR, HER2 or c-Met has been a major area for cancer therapy during the past decades.112 Accordingly, various preclinical-stage bispecific CrossMabs targeting RTKs have been developed during the past years, but none of these have advanced to clinical trials so far. Zhang and colleagues created a biparatopic HER2/HER2 1 + 1 CrossMabFab based on trastuzumab and an avidity-improved variant L56TY derived from pertuzumab called Tras-Permut CrossMab. Tras-Permut CrossMab mediated improved activity against trastuzumab-resistant breast cancer and enhanced calreticulin exposure, which may contribute to the induction of tumor-specific T-cell responses.113 Lu and colleagues, in turn, generated a bispecific HER2/EGFR 1 + 1 CrossMabCH1−CL based on the trastuzumab and cetuximab.72 Interestingly, Hu and colleagues went a step further and generated so-called four-in-one antibodies that exhibited four different specificities against EGFR, HER2, HER3, and VEGF by generating a 1 + 1 CrossMAbCH1−CL using dual-acting Fabs (DAF) as building blocks in the FL518 bispecific or by combining CrossMab and DVD-Ig technology in the tetraspecific, tetravalent antibody CRTB6 to enable correct light-chain association in the DVD format.114 Not surprisingly, these tetraspecific antibodies showed superior efficacy as compared to the respective bispecific antibodies.114 Finally, different bispecific EGFR/Notch CrossMabs were described to block EGFR signaling together with Notch signaling. The first of these antibodies, termed CT16, combined the EGFR antibody cetuximab and the Notch 2/3 antibody tarextumab using the prototypical heterodimeric 1 + 1 CrossMabCH11−CL format, which served as a radiosensitizer and prevented acquisition of resistance to EGFR inhibitors and radiation in cell line models of non–small cell lung cancer and patient-derived xenograft tumors.115 In a second publication from the same group, three heterodimeric bispecific 1 + 1 CrossMabCH1−CL antibodies (PTG12, RTB3, MTJ16) were generated from panitumumab/tarextumab, RG7116/tarextumab, and MEHD7945A/tarextumab and were shown to increase the response to PI3K inhibition with GDC-0941 by inhibiting stem cell–like subpopulation, reducing tumor-initiating cell frequency, and downregulating mesenchymal gene expression.116
Another major field in targeted cancer therapy has been and continues to be apoptosis induction through death receptor (DR) signaling.117,118 As conventional DR5 antibodies have not been successful in clinical trials, approaches for tumor-targeted DR5 agonism have been pursued. Expression of the fibroblast activation protein (FAP) on tumor fibroblasts is found in the majority of solid tumors, making FAP an attractive antigen for tumor targeting.119,120 Based on this rationale, FAP-targeted bispecific antibodies and fusion proteins have been created using CrossMab technology that rely on FAP binding with one moiety to induce, with their second moiety, hyper-clustering of tumor necrosis factor (TNF) receptor superfamily members121 like DR5 for apoptosis induction,85 4–1BB/CD137 for T cell activation,89 or CD40 for activation of antigen-presenting cells,94,122 as described below. The first of these conditional FAP-targeted TNFR agonistic antibodies entering Phase 1 clinical trials was the symmetric tetravalent C-terminally fused FAP/DR5 targeted 2 + 2 CrossMabCH1−CL RG7386 (Figure 2g). Preclinical data demonstrated that RG7386 effectively triggered FAP-dependent, avidity-driven DR5 hyper-clustering and subsequent tumor cell apoptosis,85 but ultimately, clinical development of RG7383 was not further continued after the completed Phase 1 study (NCT02558140) due to portfolio reprioritization.
Finally, Tung and colleagues described novel HER2 or CD19 tumor-targeted heterodimeric 1 + 1 CrossMabCH1−CL antibodies that recognize with their second specificity PEGylated proteins, liposomes, and nanoparticles. Using these bispecific antibodies, cytotoxic cargo such as PEGylated liposomal doxorubicin can be delivered to tumor cells.123
Applications in cancer immunotherapy: Dual checkpoint inhibitors, T and innate cell engaging bispecifics and tumor-targeted co-stimulation
With the advent of cancer immunotherapy and checkpoint inhibitor antibodies during the past decade, the development of bispecific antibodies for immunotherapy has attracted substantial attention in industry and academia, whereas the interest in anti-angiogenic and pro-apoptotic therapies has declined. In this context, bispecific monovalent dual checkpoint inhibitory PD-1 antibodies co-targeting the checkpoint inhibitory receptors TIM-3 or LAG-3 have been designed based on a bispecific 1 + 1 CrossMabVH-VL± format (Figure 2b), allowing avidity-mediated selectivity gain and thus enhanced selectivity for PD-1+ and PD-1+ TIM-3+/LAG-3+ double-positive T cells. Both of these bispecific dual checkpoint inhibitory antibodies, PD1-TIM3 (RG7769) 87 and PD1-LAG3 (RG6139),91 are currently in Phase 1 and 2 clinical trials (NCT03708328, NCT04140500, NCT04785820).124,125 Preclinically, a heterodimeric 1 + 1 PD-1/RANKL CrossMabCH1−CL was shown to demonstrate potent tumor growth inhibition as a monotherapy and combined with CTLA-4 antibodies, particularly in models showing checkpoint inhibitor resistance to PD-1 antibodies.126
Many of bispecific antibodies currently being developed are bispecific T-cell engagers.22,127–131 One of the first IgG-based, and Roche’s first, T-cell bispecific antibody (TCB) to enter clinical trials was the heterodimeric and trivalent CEA/CD3ε 2 + 1 TCB cibisatamab (RG7802). It is a heterodimeric CEA/CD3ε bispecific antibody in the 1 + 1 CrossMabCH1−CL format to which a single additional Fab targeting CEA is fused to the N-terminus of the knob-containing heavy chain (Figure 2d).84,132 FcγR and C1q binding are abolished by introduction of P329G LALA mutations.133 This so-called 2 + 1 TCB format provides advantages over conventional heterodimeric 1 + 1 TCB formats through the highly flexible head-to-tail fusion in the tandem Fab arm and by being bivalent for the tumor antigen, allowing a better differentiation between tumor and normal cells due to avidity-mediated affinity tuning.132 Cibisatamab demonstrated tumor targeting and in vitro and in vivo anti-tumor efficacy dependent on CEA over-expression due to the bivalent binding mode in models of colorectal and gastric cancer,84,134–137 which was further enhanced when combined with PD-L1 inhibition.138 Based on these data and using a MABEL approach due to the lack of cross-reactive toxicology species,139,140 clinical studies were initiated in relapsed/refractory CEA-positive colorectal cancer patients. Cibisatamab is currently in Phase 1b clinical trials in combination with the PD-L1 antibody atezolizumab (NCT03866239) and with FAP-4-1-BBL (NCT04826003) (see below). Pre-treatment with obinutuzumab is being clinically explored to mitigate the potential development and impact of anti-drug antibodies that could be observed in patients treated with cibisatamab.
The most advanced 2 + 1 T cell bispecific antibody is glofitamab (RG6026), which, in contrast to cibisatamab, is based on a 2 + 1 CrossMabVH-VL format with charge interactions using variable regions derived from obinutuzumab (Figure 2e). Glofitamab showed potent tumor cell killing and antitumor efficacy in preclinical in vitro, ex vivo and in vivo lymphoma models, as well as superiority over the respective conventional heterodimeric 1 + 1 TCB formats as a consequence of its head-to-tail orientation and bivalent binding to CD20, allowing pre-treatment with obinutuzumab, in this case as a strategy to reduce the incidence of cytokine-release syndrome by glofitamab.86,141,142 Based on the clinical efficacy and safety in the Phase 1 clinical trial in relapsed/refractory non-Hodgkin lymphoma (NHL) patients and particularly the high rate of durable complete responses,143,144 glofitamab is currently being evaluated in multiple clinical trials in lymphoma patients, including trials in patients relapsed after CAR-T cell therapy (NCT04703686) and in Phase 3 clinical trials in relapsed/refractory diffuse large B cell lymphoma patients (NCT04077723, NCT04408638).145 No anti-drug antibodies recognizing glofitamab were detected in the Phase 1 clinical study.143
Additional analogous 2 + 1 T cell bispecific antibodies using this technology have entered early clinical Phase 1 trials, including the BCMA-TCE CC-93269 for the treatment of multiple myeloma (NCT03486067)88 and the TYRP1-TCB (RG6232) for the treatment of TYRP1-expressing melanoma (NCT04551352).92 Recently, the WT1-peptide-MHC-specific TCR-like WT1-TCB (RG6007) for the treatment of acute myeloid leukemia (AML) became the first TCR-like bispecific antibody to enter a clinical trial (NCT04580121).93 While Immunocore pioneered the field of targeting peptide-MHC complexes with recombinant TCR-based bispecific T-cell engagers, the so-called ImmTACs,146 WT1-TCB is based on a TCR-like antibody fragment recognizing the RMF WT1 peptide-HLA-A*02 complex. WT1-TCB can mediate specific killing of AML cell lines and primary AML cells, and it has anti-tumor activity in humanized mice bearing SKM-1 tumors.93
Additional preclinical stage 2 + 1 TCBs based on this format have been described, including ones that target HER2147,148 or the p95 HER2 fragment.149 The p95 HER2 fragment is only found on a portion of ~ 30–40% of HER2+++ tumor cells, and as such can be considered a highly tumor-specific neoantigen. Thus, 2 + 1 TCBs targeting specifically p95 HER2 are of particular interest as they do not mediate T cell killing of normal cells that express HER2, such as cardiomyocytes or breast epithelial cells, as opposed to conventional HER2-TCBs.149 An alternative approach to overcome the on-target off-tumor killing of normal cells is tumor-specific activation of TCBs by protease expressed in the tumor. For this purpose, a protease-activated mesothelin-TCB using CrossMab technology has been described that is blocked by an anti-CD3 anti-idiotypic mask that is cleaved in the tumor-microenvironment.150 Alternatively, to counteract and manage any undesired T cell activation, it was shown that the Src/lck inhibitor dasatinib is able to reversibly switch off cytokine release and T-cell cytotoxicity following stimulation with different 2 + 1 TCBs targeting CEA, CD19 and WT1.151 Finally, related to the TCB approach, 2 + 1 bispecific antibodies designed based on CrossMab technology have been developed specifically for the recruitment of synthetic agonistic receptor transduced T-cells (SAR-T) in adoptive T-cell therapy together with Kobold and colleagues.152–154
In order to further boost the potency of T-cell bispecific antibodies, the tumor (stroma)-targeted FAP-4-1BBL (RG7827), CD19-4-1BBL (RG6076) and CEA-4-1BBL fusion proteins have been developed for solid tumors and NHL. These molecules are used to provide the co-stimulatory TNF receptor superfamily-mediated signal 2 to T cells in combination with the T-cell bispecific antibodies cibisatamab or glofitamab, which provide the signal 1.89,155,156 These 4–1BBL fusion proteins contain a split trimeric 4–1BB ligand fused to the CH1 and CL domains, and constant chain mispairing is abolished by CH1-CL domain crossover in conjunction with the respective charge pairs (Figure 2c).89 FAP-4-1BBL and CD19-4-1BBL have been designed to trigger 4–1BB/CD137 hyper-clustering specifically in the tumor microenvironment, but not in circulation or in the liver, with the goal to overcome typical 4–1BB antibody-mediated toxicities.89 Tumor-targeted 4–1BBL fusion proteins were shown to mediate improved T-cell activation, superior tumor control in combination with TCBs and checkpoint inhibitors, and strong T-cell infiltration in preclinical models.89,155,157 Clinical Phase 1b studies combining cibisatamab with FAP-4-1-BBL (NCT04826003) and glofitamab with CD19-4-1BBL (NCT04077723) are currently ongoing.
A similar rationale was applied to trigger the TNF receptor superfamily member CD40 on antigen-presenting cells (APCs) through a trivalent C-terminally fused FAP/CD40 2 + 1 bispecific antibody in a 2 + 1 CrossMabVH-VL± format with charges (Figure 2h). This design was chosen to make FAP-CD40 (RG6189), a FAP-targeted CD40 agonistic bispecific antibody, with the goal of abrogating systemic toxicity and enabling administration of doses sufficiently high to result in highly tumor- and lymph node-specific activation of APCs with subsequent induction of antitumor immunity.94,122 Phase 1 clinical trials have been initiated to validate this approach in the clinic (NCT04857138).
Notably, the domain crossover/CrossMab technology has also been used by researchers outside of Roche for the development of bispecific antibodies for cancer immunotherapy. This includes the so-called Fabs-in-tandem Ig (FIT-Ig) approach developed by Gong and colleagues from EpimAb, which relies on Fab crossover to enable correct light-chain association for the generation of symmetric tetravalent N-terminally fused bispecific antibodies in the 2 + 2 CrossMabFab format (Figure 2f).50,51 Three different bispecific FIT-Igs have reached clinical Phase 1 trials to date co-targeting: 1) EGFR/c-Met for receptor tyrosine kinase inhibition (EMB-01) (NCT03797391), 2) PD-1/LAG-3 for dual checkpoint inhibition (EMB-02) (NCT04618393), and 3) BCMA/CD3ε for T cell engagement in multiple myeloma (EMB-06) (NCT04735575).
In order to recruit innate immune cells for cancer cell killing, Gauthier and colleagues from Innate Pharma recently described an advanced preclinical approach to generate multifunctional natural killer cell engagers (NKCE) targeting a tumor antigen and the NK cell ligand NKp46 in a FcγRIII-binding competent monovalent C-terminally fused 1 + 1 antibody format using CH1-CL crossover to ensure correct light-chain association (Figure 2i).158 Trifunctional NKCEs targeting CD19, CD20, or EGFR as tumor antigens triggered tumor killing by human primary NK cells in vitro and induced NK cell infiltration and anti-tumor efficacy, as well as protective tumor immunity in vivo.158
Zhao and colleagues demonstrated that a bispecific heterodimeric CD20/HLA-DR 1 + 1 CrossMabCH1−CL termed CD20–243 CrossMab for the treatment of NHL patients co-expressing CD20 and HLA-DR mediated strong complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity and anti-proliferative activity.159 Similarly, Rajendran and colleagues generated a bispecific heterodimeric CD30/CD137 1 + 1 CrossMabCH1−CL to target specifically these two co-expressed antigens on Hodgkin and Reed-Sternberg cells without inducing CD137 signaling.160
In an alternative approach to activate innate immunity, Du and colleagues devised a bispecific heterodimeric GPC3/CD47 1 + 1 CrossMabCH1−CL to bind to GPC3 and CD47 on hepatocellular cancer cells, and at the same time inhibit the CD47 interaction with SIRP1α responsible for the “do-not-eat-me signal” to recruit myeloid cells for phagocytosis.161 The GPC3/CD47 CrossMab induced enhanced Fc-mediated effector functions by both macrophages and neutrophils toward dual antigen-expressing hepatocellular carcinoma (HCC) cells in vitro, and strong in vivo efficacy against xenograft HCC tumors in a fashion superior to the respective monotherapies and combination thereof.161
In order to further boost antigen presentation and foster the generation of a secondary anti-tumor immune response, again Zhao and colleagues created a novel CD20/Flt3 ligand antibody fusion protein, termed CD20-Flex BiFP using CrossMab technology.162 CD20-Flex BiFP not only eliminated lymphoma temporarily but also potentiated tumor-specific T-cell immunity by expanding and fostering infiltration of antigen-presenting dendritic cells into the tumor tissue.162
Most recently, Panina and colleagues described a novel bispecific heterodimeric HER2/IFNα-1 + 1 CrossMabCH1−CL with the ultimate goal to deliver IFNα into HER2 expressing tumors.163
Applications in therapy of viral infections and autoimmune diseases
The application of CrossMab technology has become quite popular for the generation of bispecific and multispecific antibodies targeting various viruses. During the past years, multiple highly potent bispecific antibodies targeting HIV-1 have been generated using CrossMab technology for the prevention and treatment of HIV-1.164,165 Examples of these approaches are: 1) four different 1 + 1 CrossMabCH1−CL-based bispecific antibodies, of which the one based on VRC07 and PG9-16 displayed the most favorable neutralization profile and IgG-like pharmacokinetic properties in monkeys;166 2) 1 + 1 CrossMabCH1−CL-based bispecific antibodies that, however, did not allow intra-spike binding;167 3) unique bispecific antibodies based on the broadly neutralizing antibodies (bNAbs) 3BNC117 and 10–1074 with a modified hinge region of human IgG3 isotype for increased Fab flexibility and improved neutralization potency based on a 1 + 1 CrossMabCH1−CL format;168 4) a 1 + 1 CrossMabCH1−CL-based bispecific antibody targeting two non-competing epitopes on the HIV-1 co-receptor CCR5 based on RoAb13 and PRO 140 to increase avidity;169 5) the 1 + 1 CrossMabCH1−CL-based bispecific antibody iMab‑CAP256 comprising the highly potent CAP256.VRC26.25 bNAb and the host-directed CD4 antibody, ibalizumab (iMab);170 and 6) the 1 + 1 CrossMabCH1−CL-based bispecific antibody BICM-1A for simultaneous recognition of two critical V2-and V3-glycan epitopes of the single HIV-1 envelope glycoprotein.171 Of all these approaches, the heterodimeric bispecific 1 + 1 CrossMabCH1−CL antibody 10E8.4/iMab showed exquisite potency and breadth against various HIV-1 strains, including activity in HIV-1 in vivo treatment and prevention models,90,172 and compared very favorably to conventional antibodies and other bispecific bNAbs.173 Based on these data, 10E8.4/iMab is currently being evaluated in a Phase 1 clinical trial (NCT03875209).174
Wang and colleagues generated a symmetric and tetravalent FIT-Ig-based bispecific antibody against Zika virus that showed high in vitro and in vivo potency, and prevented viral escape, supporting its potential use for the therapy of Zika virus prevention or infections.175
Interestingly, and most recently, De Gasparo and colleagues described the first bispecific antibody targeting SARS-CoV-2 based on a 1 + 1 CrossMabCH1−CL format targeting two non-overlapping sites on the receptor binding domain of SARS-CoV-2 and blocking binding to angiotensin-converting enzyme 2 (ACE2).176 The respective bispecific antibody CoV-X2 was designed using C121 and C135, two antibodies derived from donors who had recovered from COVID-19. Most notably, CoV-X2 neutralized wild-type SARS-CoV-2 and variants of concern and escape, protected mice from disease and suppressed viral escape.176 Along these lines, Jette and colleagues described a subset of donor-derived neutralizing bispecific CrossMabs with broad cross-reactivity to sarbecoviruses.177
Bispecific CrossMab-based antibodies have also been generated with the goal of treating autoimmune diseases.178,179 Fischer and colleagues showed that combined inhibition of TNFα and IL-17 was more effective in inhibiting the development of inflammation and bone and cartilage destruction in arthritic mice compared to the respective monotherapies. For this purpose, bispecific TNFα/IL-17 1 + 1 and 2 + 2 CrossMabCH1−CL antibodies were prepared that showed superior efficacy in blocking cytokine and chemokine responses in vitro.180 Similarly, Xu and colleagues showed that a tetravalent bispecific TNFα/IL-17 1 + 1 CrossMabVH-VL together with electrostatic steering for heavy-chain heterodimerization significantly decreased the expression level of neutrophil and Th17 chemokines, and the secretion of IL-6/IL-8 on fibroblast-like synoviocytes. Moreover, combined inhibition of both cytokines by the bispecific antibody was superior to inhibition of either cytokine alone.181 Based on these data, dual-targeting bispecific antibodies neutralizing pro-inflammatory cytokines may provide novel treatment options for autoimmune diseases. However, as they are not necessarily differentiated from the combination of the respective monotherapies, the benefit of using a bispecific antibody over a combination therapy needs to be assessed on a case-by-case basis.
Applications in ophthalmology and therapy of central nervous system diseases
The heterodimeric 1 + 1 VEGF/Ang-2 CrossMabCH1−CL vanucizumab (RG7221) was the first anti-angiogenic bispecific antibody to enter clinical trials with the goal of suppressing tumor angiogenesis via simultaneous blockade of the pro-angiogenic ligands VEGF-A and Ang-2. VEGF and Ang-2 have also been shown to play an important role in ocular angiogenesis in diseases like wet age-related macular degeneration (wAMD) and diabetic macular edema (DME).96,182–184 However, until now only the VEGF blocking antibody fragments ranibizumab and brolucizumab and the VEGFR1/2-ECD-Fc fusion protein aflibercept are approved for use in ophthalmology.185
Faricimab (RG7716) is a heterodimeric 1 + 1 VEGF/Ang-2 CrossMabCH1−CL specifically optimized for intraocular use and high concentration formulation in ophthalmology indications by use of optimized anti-VEGF and anti-Ang-2 Fabs, as compared to vanucizumab, and by the introduction of P329G LALA and Triple A mutations in the KiH-containing IgG1 Fc portion to abolish FcγR-mediated effector functions and FcRn recycling for low systemic exposure.82,83,186–189 While faricimab neutralizes two soluble ligands, particularly in the field of ophthalmology the use of such a bispecific antibody provides advantages in terms of intraocular administration via a single injection due to the simultaneous inhibition of two different angiogenic pathways with a single agent. Importantly, as compared to VEGF inhibition alone, faricimab mediated improved anti-angiogenic activity in various preclinical models to limit pathological angiogenesis in the eye.82,83,190,191 Based on these data, faricimab was the first bispecific antibody worldwide entering Phase 1 clinical trials in ophthalmology, where it was well tolerated and exhibited a favorable safety profile with evidence of improvements in best-corrected visual acuity (BCVA) and anatomic parameters supporting further clinical investigation.192 Subsequently, faricimab was compared head-to-head to ranibizumab in the BOULEVARD Phase 2 randomized clinical trial in patients with DME, where it met the primary end point and demonstrated statistically superior visual acuity gains versus ranibizumab, suggesting a benefit of simultaneous inhibition of angiopoietin-2 and VEGF-A.193 In the AVENUE Phase 2 randomized clinical trial in patients with AMD, it did not meet the primary end point of superiority over ranibizumab in BCVA at week 36, but visual and anatomical gains observed with faricimab supported pursuing Phase 3 trials for an alternative to monthly anti-VEGF therapy.194 This was taken into account together with the data from the STAIRWAY Phase 2 randomized clinical trial in AMD where faricimab dosed every 16 weeks or 12 weeks resulted in maintenance of initial vision and anatomic improvements comparable with monthly ranibizumab.195,196 Recently, positive outcomes were reported from four independent pivotal Phase 3 trials in wAMD and DME patients where faricimab was compared to aflibercept and met the primary endpoints (NCT03823287, NCT03823300, NCT03622580, NCT03622593). Based on these data, marketing applications for faricimab have been filed with health authorities for approval in DME and wAMD, with FDA granting it a priority review.197
The treatment of central nervous system (CNS) diseases with monoclonal antibodies is hampered by the low penetration of antibodies through the blood-brain barrier, and the field still is in its infancy.198 To overcome this limitation, Niewoehner and colleagues have generated transferrin receptor-targeted bispecific antibodies that allowed delivery of these antibodies through the blood-brain barrier and showed improved brain exposure and prevented plaque formation.66,67 Using this approach, BS-GANT (RG6102) was generated based on the amyloid-beta antibody gantenerumab199 as a trivalent C-terminally fused amyloid-beta/TfR 2 + 1 bispecific antibody in a 2 + 1 CrossMabVH-VL± format with charges (Figure 2h). BS-GANT (RG6102) recently entered Phase 2 clinical trials in patients with prodromal or mild-to-moderate Alzheimer’s disease (NCT04639050).
Conclusions
During the past 20 years, numerous technologies have been developed to generate bispecific antibodies, and these molecules represent a rapidly growing class of biopharmaceuticals in clinical trials and on the market. CrossMab technology was first described in 2011 as a novel approach enabling correct antibody light-chain association with their respective heavy chain in bi-/multispecific antibodies, together with methods enabling correct heavy-chain association.
As briefly mentioned in the introduction, alternative technologies to achieve correct heavy-light-chain pairing are currently being applied for the generation of prototypical (heterodimeric) IgG-like bispecific antibodies. These include in vitro assembly approaches, where the two bispecific antibodies are produced separately and subsequently assembled in vitro like DuoBody,19 Fab arm exchange,22 FORCE26 or half antibody assembly,27 as well as approaches allowing the production of bispecific antibodies in one cell line, for example via the use of common light chains or orthogonal Fab interfaces. Recently, several groups have also reported that the specific pairing preferences of selected heavy and light chain pairs can be used to drive the assembly of correct bispecific antibodies.200,201 In the field of common light chains, much progress has been made in the selection of suitable common light-chain antibodies from common light-chain-bearing animals or use of in vitro display technologies.9,10,14–16,202–205 Based on this progress, several bispecific common light-chain-based IgG antibodies are currently in clinical trials, including odronextamab, REGN4018, REGN5678, REGN7075, MCLA-145, MCLA-158, and others.7,8,10,15,206–213 Alternatively, the correct light-chain-heavy-chain association can be enforced using orthogonal Fab interfaces by introduction of (several) mutations in the Fab interface.28,29,33,55,56,214,215
CrossMab technology continues to represent a simple, straightforward and clinically validated antibody engineering solution to achieve correct light-chain association with minimal engineering using existing pairs of antibodies. In fact, since its original description, it has evolved into one of the most mature, versatile, and broadly applied technologies in industry and academia, in conjunction with the KiH technology. Until now ~20 bispecific antibodies and fusion proteins based on CrossMab technology developed by Roche and others have entered clinical trials. Based on the available clinical data, CrossMabs show favorable IgG-like properties in terms of pharmacokinetics and immunogenicity similar to conventional therapeutic monoclonal antibodies. The most advanced of these bispecific antibodies are: 1) the 1 + 1 heterodimeric Ang-2/VEGF bispecific antibody faricimab for the treatment of DME and wAMD, which is currently undergoing regulatory review, and 2) the 2 + 1 heterodimeric CD20/CD3 T-cell bispecific antibody glofitamab for the treatment of relapsed/refractory DLBCL or follicular NHL, which is currently in pivotal Phase 3 clinical trials.
Based on the progress in making bi- and multispecific antibodies, we anticipate that this class of therapeutics with novel mechanisms of actions as compared to conventional therapeutic antibodies will have a major impact on the treatment of various diseases, including oncology, infectious diseases, autoimmunity, CNS, and metabolic diseases. Taken together, CrossMab technology has proven to be very useful for the fast and straightforward generation of bispecific antibody formats to tackle novel biological challenges and help to develop novel therapeutic concepts for patients in need.
Acknowledgments
The authors want to thank all contributors and project team members from Roche Innovation Centers Zurich, Munich and Basel, Roche pRED as well as all other collaborators within and outside of Roche contributing to the development of CrossMab technology and individual drug candidates for their support and effort. The authors apologize to those authors whose work could not be cited due to space restrictions.
Disclosure statement
MS declares employment with Roche, WS declares patents/royalties with Roche and CK declares employment, stock ownership, and patents/royalties with Roche. CROSSMAB® is a registered trademark by Genentech/Roche.
Abbreviations
| ACE2 | Angiotensin-Converting Enzyme 2 |
| ALL | Acute Lymphocytic Leukemia |
| AML | acute myeloid leukemia |
| Ang-2 | Angiopoietin-2 |
| BCVA | Best-Corrected Visual Acuity |
| BiTE | Bispecific T cell Engager |
| bNAbs | broadly Neutralizing Antibodies |
| CEA | Carcinoembryonic antigen |
| CMC | Chemistry, Manufacturing, and Controls |
| CNS | Central Nervous System |
| DAF | Dual Acting Fab |
| DME | Diabetic Macular Edema |
| DSP | Downstream Processing |
| DVD-Ig | Dual Variable Domain-Ig |
| FDA | Federal Drug Administration |
| FAP | Fibroblast Activation Protein |
| FIT-Ig | Fabs-In-Tandem-Ig |
| Ig | Immunoglobulin |
| KiH | Knobs-into-Holes |
| NKCE | NK Cell Engager |
| SAR | Synthetic Agonistic Receptor |
| TCB | T-Cell Bispecific Antibody |
| TCR | T-Cell Receptor |
| TfR | Transferrin Receptor |
| TNFR | TNF Receptor |
| USP | Upstream Processing |
| VEGF-A | Vascular Endothelial Growth Factor-A |
| wAMD | wet Age-Related Macular Degeneration |
References
- 1.Brinkmann U, Kontermann RE.. The making of bispecific antibodies. MAbs. 2017;9(2):182–17. PubMed PMID: 28071970; PubMed Central PMCID: PMCPMC5297537. doi: 10.1080/19420862.2016.1268307. Feb/Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labrijn AF, Janmaat ML, Reichert JM, et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019Aug;18(8):585–608. 10.1038/s41573-019-0028-1: PubMed PMID: 31175342 [DOI] [PubMed] [Google Scholar]
- 3.Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat Rev Drug Discov. 2018Mar;17(3):197–223. PubMed PMID: 29192287. doi: 10.1038/nrd.2017.227. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan C. Bispecific antibodies poised to deliver wave of cancer therapies. Nat Biotechnol. 2021Mar;39(3):251–54. PubMed PMID: 33692520. doi: 10.1038/s41587-021-00850-6. [DOI] [PubMed] [Google Scholar]
- 5.Suurs FV, Lub-de Hooge MN, De Vries EGE, et al. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Ther. 2019Sep;201:103–19. doi: 10.1016/j.pharmthera.2019.04.006. PubMed PMID: 31028837. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Yi J, Zhou P. Development of bispecific antibodies in China: overview and prospects. Antib Ther. 2020Apr;3(2):126–45. doi: 10.1093/abt/tbaa011. PubMed PMID: 33928227; PubMed Central PMCID: PMCPMC7990247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012Oct;18(10):1570–74. 10.1038/nm.2942: PubMed PMID: 23023498 [DOI] [PubMed] [Google Scholar]
- 8.Kitazawa T, Esaki K, Tachibana T, et al. Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost. 2017Jun28;117(7):1348–57. PubMed PMID: 28451690; PubMed Central PMCID: PMCPMC6292136. doi: 10.1160/TH17-01-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igawa T. Next generation antibody therapeutics using bispecific antibody technology. Yakugaku Zasshi. 2017;137(7):831–36. doi: 10.1248/yakushi.16-00252-3. PubMed PMID: 28674296 [DOI] [PubMed] [Google Scholar]
- 10.Sampei Z, Igawa T, Soeda T, et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS One. 2013;8(2):e57479. PubMed PMID: 23468998; PubMed Central PMCID: PMCPMC3585358. doi: 10.1371/journal.pone.0057479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skegro D, Stutz C, Ollier R, et al. Immunoglobulin domain interface exchange as a platform technology for the generation of Fc heterodimers and bispecific antibodies. J Biol Chem. 2017Jun9;292(23):9745–59. PubMed PMID: 28450393; PubMed Central PMCID: PMCPMC5465497. doi: 10.1074/jbc.M117.782433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Einsele H, Borghaei H, Orlowski RZ, et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer. 2020Jul15;126(14):3192–201. PubMed PMID: 32401342. doi: 10.1002/cncr.32909 [DOI] [PubMed] [Google Scholar]
- 13.Wolf E, Hofmeister R, Kufer P, et al. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today. 2005Sep15;10(18):1237–44. PubMed PMID: 16213416. doi: 10.1016/S1359-6446(05)03554-3 [DOI] [PubMed] [Google Scholar]
- 14.De Nardis C, Hendriks LJA, Poirier E, et al. A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G1. J Biol Chem. 2017Sep1;292(35):14706–17. PubMed PMID: 28655766; PubMed Central PMCID: PMCPMC5582861. doi: 10.1074/jbc.M117.793497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith EJ, Olson K, Haber LJ, et al. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep. 2015Dec11;5(1):17943. PubMed PMID: 26659273; PubMed Central PMCID: PMCPMC4675964. doi: 10.1038/srep17943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merchant AM, Zhu Z, Yuan JQ, et al. An efficient route to human bispecific IgG. Nat Biotechnol. 1998Jul;16(7):677–81. 10.1038/nbt0798-677: PubMed PMID: 9661204 [DOI] [PubMed] [Google Scholar]
- 17.Bostrom J, Yu SF, Kan D, et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science. 2009Mar20;323(5921):1610–14. PubMed PMID: 19299620. doi: 10.1126/science.1165480 [DOI] [PubMed] [Google Scholar]
- 18.Johnson S, Burke S, Huang L, et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol. 2010Jun11;399(3):436–49. PubMed PMID: 20382161. doi: 10.1016/j.jmb.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 19.Labrijn AF, Meesters JI, De Goeij BE, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013Mar26;110(13):5145–50. PubMed PMID: 23479652; PubMed Central PMCID: PMCPMC3612680. doi: 10.1073/pnas.1220145110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckmann R, Jensen K, Fenn S, et al. DutaFabs are engineered therapeutic Fab fragments that can bind two targets simultaneously. Nat Commun. 2021Jan29;12(1):708. PubMed PMID: 33514724; PubMed Central PMCID: PMCPMC7846786. doi: 10.1038/s41467-021-20949-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Ying H, Grinnell C, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007Nov;25(11):1290–97. 10.1038/nbt1345: PubMed PMID: 17934452 [DOI] [PubMed] [Google Scholar]
- 22.Strop P, Ho WH, Boustany LM, et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol. 2012Jul13;420(3):204–19. PubMed PMID: 22543237. doi: 10.1016/j.jmb.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 23.Leung KM, Batey S, Rowlands R, et al. A HER2-specific modified fc fragment (fcab) induces antitumor effects through degradation of HER2 and Apoptosis. Mol Ther. 2015Nov;23(11):1722–33. 10.1038/mt.2015.127: PubMed PMID: 26234505; PubMed Central PMCID: PMCPMC4817942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woisetschlager M, Antes B, Borrowdale R, et al. In vivo and in vitro activity of an immunoglobulin Fc fragment (Fcab) with engineered Her-2/neu binding sites. Biotechnol J. 2014Jun;9(6):844–51. 10.1002/biot.201300387: PubMed PMID: 24806546 [DOI] [PubMed] [Google Scholar]
- 25.Wozniak-Knopp G, Stadlmayr G, Perthold JW, et al. Designing Fcabs: well-expressed and stable high affinity antigen-binding Fc fragments. Protein Eng Des Sel. 2017Sep1;30(9):657–71. PubMed PMID: 28981753. doi: 10.1093/protein/gzx042 [DOI] [PubMed] [Google Scholar]
- 26.Dengl S, Mayer K, Bormann F, et al. Format chain exchange (FORCE) for high-throughput generation of bispecific antibodies in combinatorial binder-format matrices. Nat Commun. 2020Oct2;11(1):4974. PubMed PMID: 33009381; PubMed Central PMCID: PMCPMC7532213. doi: 10.1038/s41467-020-18477-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiess C, Merchant M, Huang A, et al. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat Biotechnol. 2013Aug;31(8):753–58. 10.1038/nbt.2621: PubMed PMID: 23831709 [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Leng EC, Gunasekaran K, et al. A novel antibody engineering strategy for making monovalent bispecific heterodimeric IgG antibodies by electrostatic steering mechanism. J Biol Chem. 2015Mar20;290(12):7535–62. PubMed PMID: 25583986; PubMed Central PMCID: PMCPMC4367261. doi: 10.1074/jbc.M114.620260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunasekaran K, Pentony M, Shen M, et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem. 2010Jun18;285(25):19637–46. PubMed PMID: 20400508; PubMed Central PMCID: PMCPMC2885242. doi: 10.1074/jbc.M110.117382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997Feb;15(2):159–63. PubMed PMID: 9035142. doi: 10.1038/nbt0297-159. [DOI] [PubMed] [Google Scholar]
- 31.Dong J, Sereno A, Aivazian D, et al. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs. 2011May-Jun;3(3):273–88. 10.4161/mabs.3.3.15188: PubMed PMID: 21393993; PubMed Central PMCID: PMCPMC3149708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer N, Elson G, Magistrelli G, et al. Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG. Nat Commun. 2015Feb12;6:6113. 10.1038/ncomms7113: PubMed PMID: 25672245; PubMed Central PMCID: PMCPMC4339886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis SM, Wu X, Pustilnik A, et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol. 2014Feb;32(2):191–98. 10.1038/nbt.2797: PubMed PMID: 24463572 [DOI] [PubMed] [Google Scholar]
- 34.Cochlovius B, Kipriyanov SM, Stassar MJ, et al. Cure of Burkitt’s lymphoma in severe combined immunodeficiency mice by T cells, tetravalent CD3 x CD19 tandem diabody, and CD28 costimulation. Cancer Res. 2000Aug15;60(16):4336–41. PubMed PMID: 10969772. [PubMed] [Google Scholar]
- 35.Moore GL, Bernett MJ, Rashid R, et al. A robust heterodimeric Fc platform engineered for efficient development of bispecific antibodies of multiple formats. Methods. 2019Feb1;154:38–50. 10.1016/j.ymeth.2018.10.006: PubMed PMID: 30366098 [DOI] [PubMed] [Google Scholar]
- 36.Guo G, Han J, Wang Y, et al. A potential downstream platform approach for WuXiBody-based IgG-like bispecific antibodies. Protein Expr Purif. 2020Sep;173:105647. doi: 10.1016/j.pep.2020.105647. PubMed PMID: 32334139. [DOI] [PubMed] [Google Scholar]
- 37.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008Aug15;321(5891):974–77. PubMed PMID: 18703743. doi: 10.1126/science.1158545 [DOI] [PubMed] [Google Scholar]
- 38.Neijssen J, Cardoso RMF, Chevalier KM, et al. Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J Biol Chem. 2021Apr;8:100641. doi: 10.1016/j.jbc.2021.100641. PubMed PMID: 33839159; PubMed Central PMCID: PMCPMC8113745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijayaraghavan S, Lipfert L, Chevalier K, et al. Amivantamab (JNJ-61186372), an Fc Enhanced EGFR/cMet Bispecific Antibody, Induces Receptor Downmodulation and Antitumor Activity by Monocyte/Macrophage Trogocytosis. Mol Cancer Ther. 2020Oct;19(10):2044–56. 10.1158/1535-7163.MCT-20-0071: PubMed PMID: 32747419 [DOI] [PubMed] [Google Scholar]
- 40.Yun J, Lee SH, Kim SY, et al. Antitumor Activity of Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR Exon 20 Insertion-Driven NSCLC. Cancer Discov. 2020Aug;10(8):1194–209. 10.1158/2159-8290.CD-20-0116: PubMed PMID: 32414908 [DOI] [PubMed] [Google Scholar]
- 41.Schaefer W, Regula JT, Bahner M, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011Jul5;108(27):11187–92. PubMed PMID: 21690412; PubMed Central PMCID: PMCPMC3131342. doi: 10.1073/pnas.1019002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein C, Sustmann C, Thomas M, et al. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs. 2012Nov-Dec;4(6):653–63. 10.4161/mabs.21379: PubMed PMID: 22925968; PubMed Central PMCID: PMCPMC3502232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grote M, Haas AK, Klein C, et al. Bispecific antibody derivatives based on full-length IgG formats. Methods Mol Biol. 2012;901:247–63. PubMed PMID: 22723106. doi: 10.1007/978-1-61779-931-0_16. [DOI] [PubMed] [Google Scholar]
- 44.Klein C, Schaefer W, Regula JT. The use of CrossMAb technology for the generation of bi- and multispecific antibodies. MAbs. 2016Aug-Sep;8(6):1010–20. PubMed PMID: 27285945; PubMed Central PMCID: PMCPMC4968094. doi: 10.1080/19420862.2016.1197457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein C, Schaefer W, Regula JT, et al. Engineering therapeutic bispecific antibodies using CrossMab technology. Methods. 2019Feb1;154:21–31. 10.1016/j.ymeth.2018.11.008: PubMed PMID: 30453028 [DOI] [PubMed] [Google Scholar]
- 46.Schaefer W, Volger HR, Lorenz S, et al. Heavy and light chain pairing of bivalent quadroma and knobs-into-holes antibodies analyzed by UHR-ESI-QTOF mass spectrometry. MAbs. 2016;8(1):49–55. PubMed PMID: 26496506; PubMed Central PMCID: PMCPMC4966523. doi: 10.1080/19420862.2015.1111498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996Jul;9(7):617–21. doi: 10.1093/protein/9.7.617. PubMed PMID: 8844834. [DOI] [PubMed] [Google Scholar]
- 48.Kuglstatter A, Stihle M, Neumann C, et al. Structural differences between glycosylated, disulfide-linked heterodimeric Knob-into-Hole Fc fragment and its homodimeric Knob-Knob and Hole-Hole side products. Protein Eng Des Sel. 2017Sep1;30(9):649–56. PubMed PMID: 28985438. doi: 10.1093/protein/gzx041 [DOI] [PubMed] [Google Scholar]
- 49.Stutz C, Blein S. A single mutation increases heavy-chain heterodimer assembly of bispecific antibodies by inducing structural disorder in one homodimer species. J Biol Chem. 2020Jul10;295(28):9392–408. PubMed PMID: 32404368; PubMed Central PMCID: PMCPMC7363136. doi: 10.1074/jbc.RA119.012335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong S, Ren F, Wu D, et al. Fabs-in-tandem immunoglobulin is a novel and versatile bispecific design for engaging multiple therapeutic targets. MAbs. 2017Oct9;7:1118–28. doi: 10.1080/19420862.2017.1345401. PubMed PMID: 28692328; PubMed Central PMCID: PMCPMC5627593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong S, Wu C. Generation of Fabs-in-tandem immunoglobulin molecules for dual-specific targeting. Methods. 2019Feb1;154:87–92. doi: 10.1016/j.ymeth.2018.07.014. PubMed PMID: 30081078. [DOI] [PubMed] [Google Scholar]
- 52.Fenn S, Schiller CB, Griese JJ, et al. Crystal structure of an anti-Ang2 CrossFab demonstrates complete structural and functional integrity of the variable domain. PLoS One. 2013;8(4):e61953. PubMed PMID: 23613981; PubMed Central PMCID: PMCPMC3629102. doi: 10.1371/journal.pone.0061953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regula JT, Imhof-Jung S, Molhoj M, et al. Variable heavy-variable light domain and Fab-arm CrossMabs with charged residue exchanges to enforce correct light chain assembly. Protein Eng Des Sel. 2018Jul1;31(7–8):289–99. PubMed PMID: 30169707; PubMed Central PMCID: PMCPMC6277175. doi: 10.1093/protein/gzy021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sustmann C, Dickopf S, Regula JT, et al. DuoMab: a novel CrossMab-based IgG-derived antibody format for enhanced antibody-dependent cell-mediated cytotoxicity. MAbs. 2019Nov-Dec;11(8):1402–14. 10.1080/19420862.2019.1661736: PubMed PMID: 31526159; PubMed Central PMCID: PMCPMC6816436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, Yuan R, Bacica M, et al. Generation of orthogonal Fab-based trispecific antibody formats. Protein Eng Des Sel. 2018Jul1;31(7–8):249–56. PubMed PMID: 29718394. doi: 10.1093/protein/gzy007 [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Demarest SJ. Building blocks for bispecific and trispecific antibodies. Methods. 2019Feb1;154:3–9. doi: 10.1016/j.ymeth.2018.08.010. PubMed PMID: 30172007. [DOI] [PubMed] [Google Scholar]
- 57.Bethune MT, Gee MH, Bunse M, et al. Domain-swapped T cell receptors improve the safety of TCR gene therapy. Elife. 2016Nov;8:5. doi: 10.7554/eLife.19095. PubMed PMID: 27823582; PubMed Central PMCID: PMCPMC5101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Croasdale R, Wartha K, Schanzer JM, et al. Development of tetravalent IgG1 dual targeting IGF-1R-EGFR antibodies with potent tumor inhibition. Arch Biochem Biophys. 2012Oct15;526(2):206–18. PubMed PMID: 22464987. doi: 10.1016/j.abb.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 59.Scheuer W, Thomas M, Hanke P, et al. Anti-tumoral, anti-angiogenic and anti-metastatic efficacy of a tetravalent bispecific antibody (TAvi6) targeting VEGF-A and angiopoietin-2. MAbs. 2016;8(3):562–73. PubMed PMID: 26864324; PubMed Central PMCID: PMCPMC4966847. doi: 10.1080/19420862.2016.1147640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schanzer J, Jekle A, Nezu J, et al. Development of tetravalent, bispecific CCR5 antibodies with antiviral activity against CCR5 monoclonal antibody-resistant HIV-1 strains. Antimicrob Agents Chemother. 2011May;55(5):2369–78. 10.1128/AAC.00215-10: PubMed PMID: 21300827; PubMed Central PMCID: PMCPMC3088204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castoldi R, Ecker V, Wiehle L, et al. A novel bispecific EGFR/Met antibody blocks tumor-promoting phenotypic effects induced by resistance to EGFR inhibition and has potent antitumor activity. Oncogene. 2013Dec12;32(50):5593–601. PubMed PMID: 23812422; PubMed Central PMCID: PMCPMC3898114. doi: 10.1038/onc.2013.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castoldi R, Jucknischke U, Pradel LP, et al. Molecular characterization of novel trispecific ErbB-cMet-IGF1R antibodies and their antigen-binding properties. Protein Eng Des Sel. 2012Oct;25(10):551–59. 10.1093/protein/gzs048: PubMed PMID: 22936109; PubMed Central PMCID: PMCPMC3449402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castoldi R, Schanzer J, Panke C, et al. TetraMabs: simultaneous targeting of four oncogenic receptor tyrosine kinases for tumor growth inhibition in heterogeneous tumor cell populations. Protein Eng Des Sel. 2016Oct;29(10):467–75. 10.1093/protein/gzw037: PubMed PMID: 27578890; PubMed Central PMCID: PMCPMC5036864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schanzer JM, Wartha K, Croasdale R, et al. A novel glycoengineered bispecific antibody format for targeted inhibition of epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor type I (IGF-1R) demonstrating unique molecular properties. J Biol Chem. 2014Jul4;289(27):18693–706. PubMed PMID: 24841203; PubMed Central PMCID: PMCPMC4081915. doi: 10.1074/jbc.M113.528109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schanzer JM, Wartha K, Moessner E, et al. XGFR*, a novel affinity-matured bispecific antibody targeting IGF-1R and EGFR with combined signaling inhibition and enhanced immune activation for the treatment of pancreatic cancer. MAbs. 2016May-Jun;8(4):811–27. 10.1080/19420862.2016.1160989: PubMed PMID: 26984378; PubMed Central PMCID: PMCPMC4966845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niewoehner J, Bohrmann B, Collin L, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014Jan8;81(1):49–60. PubMed PMID: 24411731. doi: 10.1016/j.neuron.2013.10.061 [DOI] [PubMed] [Google Scholar]
- 67.Weber F, Bohrmann B, Niewoehner J, et al. Brain shuttle antibody for alzheimer’s disease with attenuated peripheral effector function due to an inverted binding mode. Cell Rep. 2018Jan2;22(1):149–62. PubMed PMID: 29298417. doi: 10.1016/j.celrep.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 68.Dengl S, Wehmer M, Hesse F, et al. Aggregation and chemical modification of monoclonal antibodies under upstream processing conditions. Pharm Res. 2013May;30(5):1380–99. 10.1007/s11095-013-0977-8: PubMed PMID: 23322133 [DOI] [PubMed] [Google Scholar]
- 69.Haberger M, Leiss M, Heidenreich AK, et al. Rapid characterization of biotherapeutic proteins by size-exclusion chromatography coupled to native mass spectrometry. MAbs. 2016;8(2):331–39. PubMed PMID: 26655595; PubMed Central PMCID: PMCPMC4966600. doi: 10.1080/19420862.2015.1122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee YF, Kluters S, Hillmann M, et al. Modeling of bispecific antibody elution in mixed-mode cation-exchange chromatography. J Sep Sci. 2017Sep;40(18):3632–45. 10.1002/jssc.201700313: PubMed PMID: 28714211 [DOI] [PubMed] [Google Scholar]
- 71.Meschendoerfer W, Gassner C, Lipsmeier F, et al. SPR-based assays enable the full functional analysis of bispecific molecules. J Pharm Biomed Anal. 2017Jan5;132:141–47. 10.1016/j.jpba.2016.09.028: PubMed PMID: 27721070 [DOI] [PubMed] [Google Scholar]
- 72.Lu Y, Zhou Q, Han Q, et al. Inactivation of deubiquitinase CYLD enhances therapeutic antibody production in Chinese hamster ovary cells. Appl Microbiol Biotechnol. 2018Jul;102(14):6081–93. 10.1007/s00253-018-9070-x: PubMed PMID: 29766242 [DOI] [PubMed] [Google Scholar]
- 73.Gstottner C, Nicolardi S, Haberger M, et al. Intact and subunit-specific analysis of bispecific antibodies by sheathless CE-MS. Anal Chim Acta. 2020Oct16;1134:18–27. 10.1016/j.aca.2020.07.069: PubMed PMID: 33059862 [DOI] [PubMed] [Google Scholar]
- 74.Gstottner C, Reusch D, Haberger M, et al. Monitoring glycation levels of a bispecific monoclonal antibody at subunit level by ultrahigh-resolution MALDI FT-ICR mass spectrometry. MAbs. 2020Jan-Dec;12(1):1682403. 10.1080/19420862.2019.1682403: PubMed PMID: 31630606; PubMed Central PMCID: PMCPMC6927770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graf T, Heinrich K, Grunert I, et al. Recent advances in LC-MS based characterization of protein-based bio-therapeutics - mastering analytical challenges posed by the increasing format complexity. J Pharm Biomed Anal. 2020Jul15;186:113251. 10.1016/j.jpba.2020.113251: PubMed PMID: 32251978 [DOI] [PubMed] [Google Scholar]
- 76.Filep C, Szigeti M, Farsang R, et al. Multilevel capillary gel electrophoresis characterization of new antibody modalities. Anal Chim Acta. 2021Jun29;1166:338492. 10.1016/j.aca.2021.338492: PubMed PMID: 34023000 [DOI] [PubMed] [Google Scholar]
- 77.Chen SW, Zhang W. Current trends and challenges in the downstream purification of bispecific antibodies. Antib Ther. 2021Apr;4(2):73–88. doi: 10.1093/abt/tbab007. PubMed PMID: 34056544; PubMed Central PMCID: PMCPMC8155696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Register AC, Tarighat SS, Lee HY. Bioassay development for bispecific antibodies-challenges and opportunities. Int J Mol Sci. 2021May19;22(10). PubMed PMID: 34069573; PubMed Central PMCID: PMCPMC8160952. doi: 10.3390/ijms22105350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haberger M, Heidenreich AK, Hook M, et al. Multiattribute monitoring of antibody charge variants by cation-exchange chromatography coupled to native mass spectrometry. J Am Soc Mass Spectrom. 2021Mar9 [PubMed PMID: 33687195]. doi: 10.1021/jasms.0c00446. [DOI] [PubMed] [Google Scholar]
- 80.Wan Y, Wang Y, Zhang T, et al. Application of pH-salt dual gradient elution in purifying a WuXiBody-based bispecific antibody by MMC ImpRes mixed-mode chromatography. Protein Expr Purif. 2021May;181:105822. doi: 10.1016/j.pep.2021.105822. PubMed PMID: 33429037. [DOI] [PubMed] [Google Scholar]
- 81.Kienast Y, Klein C, Scheuer W, et al. Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer Res. 2013Dec15;19(24):6730–40. PubMed PMID: 24097868. doi: 10.1158/1078-0432.CCR-13-0081 [DOI] [PubMed] [Google Scholar]
- 82.Regula JT, Lundh von Leithner P, Foxton R, et al. Targeting key angiogenic pathways with a bispecific Cross MAb optimized for neovascular eye diseases. EMBO Mol Med. 2016Nov8;8(11):1265–88. PubMed PMID: 27742718; PubMed Central PMCID: PMCPMC5090659. doi: 10.15252/emmm.201505889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Regula JT, Lundh von Leithner P, Foxton R, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2019May;11(5): 10.15252/emmm.201910666. PubMed PMID: 31040127; PubMed Central PMCID: PMCPMC6505574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bacac M, Fauti T, Sam J, et al. A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin Cancer Res. 2016Jul1;22(13):3286–97. PubMed PMID: 26861458. doi: 10.1158/1078-0432.CCR-15-1696 [DOI] [PubMed] [Google Scholar]
- 85.Brunker P, Wartha K, Friess T, et al. RG7386, a novel tetravalent FAP-DR5 antibody, effectively triggers fap-dependent, avidity-driven dr5 hyperclustering and tumor cell apoptosis. Mol Cancer Ther. 2016May;15(5):946–57. 10.1158/1535-7163.MCT-15-0647: PubMed PMID: 27037412 [DOI] [PubMed] [Google Scholar]
- 86.Bacac M, Colombetti S, Herter S, et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018Oct1;24(19):4785–97. PubMed PMID: 29716920. doi: 10.1158/1078-0432.CCR-18-0455 [DOI] [PubMed] [Google Scholar]
- 87.Deak LLC, Seeber S, Perro M, et al. Abstract 2270: RG7769 (PD1-TIM3), a novel heterodimeric avidity-driven T cell specific PD-1/TIM-3 bispecific antibody lacking Fc-mediated effector functions for dual checkpoint inhibition to reactivate dysfunctional T cells. Cancer Research. 2020;80(16 Supplement):2270–2270. doi: 10.1158/1538-7445.am2020-2270. [DOI] [Google Scholar]
- 88.Seckinger A, Delgado JA, Moser S, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017Mar13;31(3):396–410. PubMed PMID: 28262554. doi: 10.1016/j.ccell.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 89.Claus C, Ferrara C, Xu W, et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med. 2019Jun12;11(496):eaav5989. PubMed PMID: 31189721; PubMed Central PMCID: PMCPMC7181714. doi: 10.1126/scitranslmed.aav5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang Y, Yu J, Lanzi A, et al. Engineered Bispecific Antibodies with Exquisite HIV-1-Neutralizing Activity. Cell. 2016Jun16;165(7):1621–31. PubMed PMID: 27315479; PubMed Central PMCID: PMCPMC4972332. doi: 10.1016/j.cell.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deak LC, Weber P, Seeber S, et al. editors. A novel bispecific checkpoint inhibitor antibody to preferentially block PD-1 and LAG-3 on dysfunctional TILs whilst sparing Treg activation. In: JOURNAL FOR IMMUNOTHERAPY OF CANCER. ENGLAND: BMC CAMPUS, 4 CRINAN ST, LONDON N1 9XW; 2019. [Google Scholar]
- 92.Nicolini VG, Waldhauer I, Freimoser-Grundschober A, et al. Abstract LB-389: combination of TYRP1-TCB, a novel T cell bispecific antibody for the treatment of melanoma, with immunomodulatory agents. Cancer Research. 2020;80(16Supplement):LB-389-LB-389. doi: 10.1158/1538-7445.am2020-lb-389. [DOI] [Google Scholar]
- 93.Augsberger C, Hanel G, Xu W, et al. Targeting intracellular WT1 in AML with a novel RMF-peptide-MHC specific T-cell bispecific antibody. Blood. 2021Jul19 [PubMed PMID: 34280257]. doi: 10.1182/blood.2020010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sum E, Rapp M, Frobel P, et al. Fibroblast activation protein alpha-targeted CD40 agonism abrogates systemic toxicity and enables administration of high doses to induce effective antitumor immunity. Clin Cancer Res. 2021Mar26;27(14):4036–53. PubMed PMID: 33771854. doi: 10.1158/1078-0432.CCR-20-4001 [DOI] [PubMed] [Google Scholar]
- 95.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010Jul;10(7):505–14. PubMed PMID: 20574450. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 96.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016Jun;15(6):385–403. PubMed PMID: 26775688. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 97.Parmar D, Apte M. Angiopoietin inhibitors: a review on targeting tumor angiogenesis. Eur J Pharmacol. 2021May15;899:174021. doi: 10.1016/j.ejphar.2021.174021. PubMed PMID: 33741382. [DOI] [PubMed] [Google Scholar]
- 98.Kloepper J, Riedemann L, Amoozgar Z, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A. 2016Apr19;113(16):4476–81. PubMed PMID: 27044098; PubMed Central PMCID: PMCPMC4843473. doi: 10.1073/pnas.1525360113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baker LCJ, Boult JKR, Thomas M, et al. Acute tumour response to a bispecific Ang-2-VEGF-A antibody: insights from multiparametric MRI and gene expression profiling. Br J Cancer. 2016Sep6;115(6):691–702. PubMed PMID: 27529514; PubMed Central PMCID: PMCPMC5023775. doi: 10.1038/bjc.2016.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solecki G, Osswald M, Weber D, et al. Differential effects of ang-2/VEGF-A inhibiting antibodies in combination with radio- or chemotherapy in glioma. Cancers (Basel). 2019Mar6;11(3): 10.3390/cancers11030314. PubMed PMID: 30845704; PubMed Central PMCID: PMCPMC6468722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mueller T, Freystein J, Lucas H, et al. Efficacy of a bispecific antibody co-targeting VEGFA and Ang-2 in combination with chemotherapy in a chemoresistant colorectal carcinoma xenograft model. Molecules. 2019Aug7;24(16): 10.3390/molecules24162865. PubMed PMID: 31394786; PubMed Central PMCID: PMCPMC6719918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmittnaegel M, Rigamonti N, Kadioglu E, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017Apr12;9(385): 10.1126/scitranslmed.aak9670. PubMed PMID: 28404865. [DOI] [PubMed] [Google Scholar]
- 103.Killock D. Immunotherapy: combine and conquer - antiangiogenic immunotherapy. Nat Rev Clin Oncol. 2017Jun;14(6):327. PubMed PMID: 28466876. doi: 10.1038/nrclinonc.2017.65. [DOI] [PubMed] [Google Scholar]
- 104.Schmittnaegel M, De Palma M. Reprogramming tumor blood vessels for enhancing immunotherapy. Trends Cancer. 2017Dec;3(12):809–12. doi: 10.1016/j.trecan.2017.10.002. PubMed PMID: 29198436. [DOI] [PubMed] [Google Scholar]
- 105.Kashyap AS, Schmittnaegel M, Rigamonti N, et al. Optimized antiangiogenic reprogramming of the tumor microenvironment potentiates CD40 immunotherapy. Proc Natl Acad Sci U S A. 2020Jan7;117(1):541–51. PubMed PMID: 31889004; PubMed Central PMCID: PMCPMC6955310. doi: 10.1073/pnas.1902145116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ragusa S, Prat-Luri B, Gonzalez-Loyola A, et al. Antiangiogenic immunotherapy suppresses desmoplastic and chemoresistant intestinal tumors in mice. J Clin Invest. 2020Mar2;130(3):1199–216. PubMed PMID: 32015230; PubMed Central PMCID: PMCPMC7269598. doi: 10.1172/JCI129558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hidalgo M, Martinez-Garcia M, Le Tourneau C, et al. First-in-human phase i study of single-agent vanucizumab, a first-in-class bispecific anti-angiopoietin-2/Anti-VEGF-A antibody, in adult patients with advanced solid tumors. Clin Cancer Res. 2018Apr1;24(7):1536–45. PubMed PMID: 29217526. doi: 10.1158/1078-0432.CCR-17-1588 [DOI] [PubMed] [Google Scholar]
- 108.Heil F, Babitzki G, Julien-Laferriere A, et al. Vanucizumab mode of action: serial biomarkers in plasma, tumor, and skin-wound-healing biopsies. Transl Oncol. 2021Feb;14(2):100984. 10.1016/j.tranon.2020.100984: PubMed PMID: 33338877; PubMed Central PMCID: PMCPMC7749407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bendell JC, Sauri T, Gracian AC, et al. The McCAVE Trial: vanucizumab plus mFOLFOX-6 Versus Bevacizumab plus mFOLFOX-6 in patients with previously untreated metastatic colorectal carcinoma (mCRC). Oncologist. 2020Mar;25(3):e451–e459. 10.1634/theoncologist.2019-0291: PubMed PMID: 32162804; PubMed Central PMCID: PMCPMC7066709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hauschildt J, Schrimpf C, Thamm K, et al. Dual Pharmacological Inhibition of Angiopoietin-2 and VEGF-A in murine experimental sepsis. J Vasc Res. 2020;57(1):34–45. PubMed PMID: 31726451. doi: 10.1159/000503787. [DOI] [PubMed] [Google Scholar]
- 111.Zhou R, Wang S, Wen H, et al. The bispecific antibody HB-32, blockade of both VEGF and DLL4 shows potent anti-angiogenic activity in vitro and anti-tumor activity in breast cancer xenograft models. Exp Cell Res. 2019Jul15;380(2):141–48. PubMed PMID: 31034805. doi: 10.1016/j.yexcr.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 112.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004May;4(5):361–70. doi: 10.1038/nrc1360. PubMed PMID: 15122207. [DOI] [PubMed] [Google Scholar]
- 113.Zhang F, Zhang J, Liu M, et al. Combating HER2-overexpressing breast cancer through induction of calreticulin exposure by Tras-Permut CrossMab. Oncoimmunology. 2015Mar;4(3):e994391. 10.4161/2162402X.2014.994391: PubMed PMID: 25949918; PubMed Central PMCID: PMCPMC4404837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu S, Fu W, Xu W, et al. Four-in-one antibodies have superior cancer inhibitory activity against EGFR, HER2, HER3, and VEGF through disruption of HER/MET crosstalk. Cancer Res. 2015Jan1;75(1):159–70. PubMed PMID: 25371409. doi: 10.1158/0008-5472.CAN-14-1670 [DOI] [PubMed] [Google Scholar]
- 115.Hu S, Fu W, Li T, et al. Antagonism of EGFR and Notch limits resistance to EGFR inhibitors and radiation by decreasing tumor-initiating cell frequency. Sci Transl Med. 2017Mar8;9(380):eaag0339. PubMed PMID: 28275151. doi: 10.1126/scitranslmed.aag0339 [DOI] [PubMed] [Google Scholar]
- 116.Fu W, Lei C, Yu Y, et al. EGFR/notch antagonists enhance the response to inhibitors of the PI3K-Akt pathway by decreasing tumor-initiating cell frequency. Clin Cancer Res. 2019May1;25(9):2835–47. PubMed PMID: 30670492. doi: 10.1158/1078-0432.CCR-18-2732 [DOI] [PubMed] [Google Scholar]
- 117.Lim B, Greer Y, Lipkowitz S, et al. Novel Apoptosis-Inducing Agents for the Treatment of Cancer, a New Arsenal in the Toolbox. Cancers (Basel). 2019Jul31;11(8):1087. PubMed PMID: 31370269; PubMed Central PMCID: PMCPMC6721450. doi: 10.3390/cancers11081087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020Jul;17(7):395–417. PubMed PMID: 32203277; PubMed Central PMCID: PMCPMC8211386. doi: 10.1038/s41571-020-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davidson S, Coles M, Thomas T, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021Apr28 [PubMed PMID: 33911232]. doi: 10.1038/s41577-021-00540-z. [DOI] [PubMed] [Google Scholar]
- 120.Kratochwil C, Flechsig P, Lindner T, et al. (68)Ga-FAPIPET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019Jun;60(6):801–05. 10.2967/jnumed.119.227967: PubMed PMID: 30954939; PubMed Central PMCID: PMCPMC6581228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001Feb23;104(4):487–501. PubMed PMID: 11239407. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 122.Labiano S, Roh V, Godfroid C, et al. CD40 agonist targeted to fibroblast activation protein alpha synergizes with radiotherapy in murine hpv-positive head and neck tumors. Clin Cancer Res. 2021Apr26;27(14):4054–65. PubMed PMID: 33903200. doi: 10.1158/1078-0432.CCR-20-4717 [DOI] [PubMed] [Google Scholar]
- 123.Tung HY, Su YC, Chen BM, et al. Selective delivery of PEGylated compounds to tumor cells by anti-PEG hybrid antibodies. Mol Cancer Ther. 2015Jun;14(6):1317–26. 10.1158/1535-7163.MCT-15-0151: PubMed PMID: 25852063 [DOI] [PubMed] [Google Scholar]
- 124.Herrera-Camacho I, Anaya-Ruiz M, Perez-Santos M, et al. Cancer immunotherapy using anti-TIM3/PD-1 bispecific antibody: a patent evaluation of EP3356411A1. Expert Opin Ther Pat. 2019Aug;29(8):587–93. 10.1080/13543776.2019.1637422: PubMed PMID: 31241380 [DOI] [PubMed] [Google Scholar]
- 125.Cebada J, Flores A, Bandala C, et al. Bispecific anti-PD-1/LAG-3 antibodies for treatment of advanced or metastatic solid tumors: a patent evaluation of US2018326054. Expert Opin Ther Pat. 2020Jul;30(7):487–94. 10.1080/13543776.2020.1767071: PubMed PMID: 32397849 [DOI] [PubMed] [Google Scholar]
- 126.Dougall WC, Roman Aguilera A, Smyth MJ. Dual targeting of RANKL and PD-1 with a bispecific antibody improves anti-tumor immunity. Clin Transl Immunology. 2019;8(10):e01081. doi: 10.1002/cti2.1081. PubMed PMID: 31572609; PubMed Central PMCID: PMCPMC6763724 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.De Miguel M, Umana P, Gomes de Morais AL, et al. T-cell-engaging Therapy for Solid Tumors. Clin Cancer Res. 2021Mar15;27(6):1595–603. PubMed PMID: 33082210. doi: 10.1158/1078-0432.CCR-20-2448 [DOI] [PubMed] [Google Scholar]
- 128.Singh A, Dees S, Grewal IS. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer. 2021Mar;124(6):1037–48. PubMed PMID: 33469153; PubMed Central PMCID: PMCPMC7960983. doi: 10.1038/s41416-020-01225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Crawford A, Chiu D. Targeting solid tumors using CD3 bispecific antibodies. Mol Cancer Ther. 2021May27;20(8):1350–58. PubMed PMID: 34045228. doi: 10.1158/1535-7163.MCT-21-0073. [DOI] [PubMed] [Google Scholar]
- 130.Kaiser J. Forced into battle. Science. 2020May29;368(6494):930–33. PubMed PMID: 32467373. doi: 10.1126/science.368.6494.930. [DOI] [PubMed] [Google Scholar]
- 131.Wu Z, Cheung NV. T cell engaging bispecific antibody (T-BsAb): from technology to therapeutics. Pharmacol Ther. 2018Feb;182:161–75 . PubMed PMID: 28834699; PubMed Central PMCID: PMCPMC5785550. doi: 10.1016/j.pharmthera.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bacac M, Klein C, CEA TCB: UP. A novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. Oncoimmunology. 2016Aug5;5(8):e1203498. PubMed PMID: 27622073; PubMed Central PMCID: PMCPMC5007959. doi: 10.1080/2162402X.2016.1203498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schlothauer T, Herter S, Koller CF, et al. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng Des Sel. 2016Oct;29(10):457–66. 10.1093/protein/gzw040: PubMed PMID: 27578889 [DOI] [PubMed] [Google Scholar]
- 134.Lehmann S, Perera R, Grimm HP, et al. In Vivo Fluorescence Imaging of the Activity of CEA TCB, a novel T-cell bispecific antibody, reveals highly specific tumor targeting and fast induction of T-cell-mediated tumor killing. Clin Cancer Res. 2016Sep1;22(17):4417–27. PubMed PMID: 27117182. doi: 10.1158/1078-0432.CCR-15-2622 [DOI] [PubMed] [Google Scholar]
- 135.Van De Vyver AJ, Weinzierl T, Eigenmann MJ, et al. Predicting tumor killing and T-cell activation by T-cell bispecific antibodies as a function of target expression: combining in vitro experiments with systems modeling. Mol Cancer Ther. 2021Feb;20(2):357–66. 10.1158/1535-7163.MCT-20-0269: PubMed PMID: 33298591 [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez-Exposito R, Semiannikova M, Griffiths B, et al. CEA expression heterogeneity and plasticity confer resistance to the CEA-targeting bispecific immunotherapy antibody cibisatamab (CEA-TCB) in patient-derived colorectal cancer organoids. J Immunother Cancer. 2019Apr15;7(1):101. PubMed PMID: 30982469; PubMed Central PMCID: PMCPMC6463631. doi: 10.1186/s40425-019-0575-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Teijeira A, Etxeberria I, Ponz-Sarvise M, et al. Immunotherapy of cancer visualized by live microscopy: seeing is believing. Clin Cancer Res. 2016Sep1;22(17):4277–79. PubMed PMID: 27330056. doi: 10.1158/1078-0432.CCR-16-1072 [DOI] [PubMed] [Google Scholar]
- 138.Sam J, Colombetti S, Fauti T, et al. Combination of T-cell bispecific antibodies With PD-L1 checkpoint inhibition elicits superior anti-tumor activity. Front Oncol. 2020;10:575737. PubMed PMID: 33330050; PubMed Central PMCID: PMCPMC7735156. doi: 10.3389/fonc.2020.575737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dudal S, Hinton H, Giusti AM, et al. Application of a MABEL approach for a T-cell-bispecific monoclonal antibody: CEA TCB. J Immunother. 2016Sep;39(7):279–89. 10.1097/CJI.0000000000000132: PubMed PMID: 27404941 [DOI] [PubMed] [Google Scholar]
- 140.Kamperschroer C, Shenton J, Lebrec H, et al. Summary of a workshop on preclinical and translational safety assessment of CD3 bispecifics. J Immunotoxicol. 2020Dec;17(1):67–85. 10.1080/1547691X.2020.1729902: PubMed PMID: 32100588 [DOI] [PubMed] [Google Scholar]
- 141.Cremasco F, Menietti E, Speziale D, et al. Cross-linking of T cell to B cell lymphoma by the T cell bispecific antibody CD20-TCB induces IFNgamma/CXCL10-dependent peripheral T cell recruitment in humanized murine model. PLoS One. 2021;16(1):e0241091. PubMed PMID: 33406104; PubMed Central PMCID: PMCPMC7787458 and SC declare ownership of Roche stocks. MP, PU, MB, CK, SC, JS are inventors on patent applications related to the CD20-TCB. This affiliation and patent participation do not alter our adherence to PLOS ONE policies on sharing data and materials. doi: 10.1371/journal.pone.0241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prakash A, Diefenbach CS. Immunity War: a Novel Therapy for Lymphoma Using T-cell Bispecific Antibodies. Clin Cancer Res. 2018Oct1;24(19):4631–32. PubMed PMID: 29884742. doi: 10.1158/1078-0432.CCR-18-1363. [DOI] [PubMed] [Google Scholar]
- 143.Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a novel, bivalent CD20-Targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol. 2021Jun20;39(18):1959–70. PubMed PMID: 33739857; PubMed Central PMCID: PMCPMC8210975. doi: 10.1200/JCO.20.03175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Killock D. Engaging results with glofitamab. Nat Rev Clin Oncol. 2021May;18(5):257. PubMed PMID: 33828233. doi: 10.1038/s41571-021-00510-3. [DOI] [PubMed] [Google Scholar]
- 145.Minson A, Glofitamab DM. CD20-TCB bispecific antibody. Leuk Lymphoma. 2021Jul15. 1–11. PubMed PMID: 34263696. doi: 10.1080/10428194.2021.1953016. [DOI] [PubMed] [Google Scholar]
- 146.Liddy N, Bossi G, Adams KJ, et al. Monoclonal TCR-redirected tumor cell killing. Nat Med. 2012Jun;18(6):980–87. 10.1038/nm.2764: PubMed PMID: 22561687 [DOI] [PubMed] [Google Scholar]
- 147.Arenas EJ, Martinez-Sabadell A, Rius Ruiz I, et al. Acquired cancer cell resistance to T cell bispecific antibodies and CAR T targeting HER2 through JAK2 down-modulation. Nat Commun. 2021Feb23;12(1):1237. PubMed PMID: 33623012; PubMed Central PMCID: PMCPMC7902842. doi: 10.1038/s41467-021-21445-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Loo Yau H, Bell E, Ettayebi I, et al. DNA hypomethylating agents increase activation and cytolytic activity of CD8(+) T cells. Mol Cell. 2021Apr1;81(7):1469–1483e8. PubMed PMID: 33609448. doi: 10.1016/j.molcel.2021.01.038 [DOI] [PubMed] [Google Scholar]
- 149.Rius Ruiz I, Vicario R, Morancho B, et al. p95HER2-T cell bispecific antibody for breast cancer treatment. Sci Transl Med. 2018Oct3;10(461):eaat1445. PubMed PMID: 30282693; PubMed Central PMCID: PMCPMC6498439. doi: 10.1126/scitranslmed.aat1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Geiger M, Stubenrauch KG, Sam J, et al. Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody. Nat Commun. 2020Jun24;11(1):3196. PubMed PMID: 32581215; PubMed Central PMCID: PMCPMC7314773. doi: 10.1038/s41467-020-16838-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leclercq G, Haegel H, Schneider A, et al. Src/lck inhibitor dasatinib reversibly switches off cytokine release and T cell cytotoxicity following stimulation with T cell bispecific antibodies. J Immunother Cancer. 2021Jul9;9(7):e002582. PubMed PMID: 34326166. doi: 10.1136/jitc-2021-002582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Karches CH, Benmebarek MR, Schmidbauer ML, et al. Bispecific antibodies enable synthetic agonistic receptor-transduced t cells for tumor immunotherapy. Clin Cancer Res. 2019Oct1;25(19):5890–900. PubMed PMID: 31285373. doi: 10.1158/1078-0432.CCR-18-3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Darowski D, Kobold S, Jost C, et al. Combining the best of two worlds: highly flexible chimeric antigen receptor adaptor molecules (CAR-adaptors) for the recruitment of chimeric antigen receptor T cells. MAbs. 2019;11(4):621–31. PubMed PMID: 30892136; PubMed Central PMCID: PMCPMC6601549. doi: 10.1080/19420862.2019.1596511. May/Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kobold S, Steffen J, Chaloupka M, et al. Selective bispecific T cell recruiting antibody and antitumor activity of adoptive T cell transfer. J Natl Cancer Inst 2015Jan;107(1):364. 10.1093/jnci/dju364: PubMed PMID: 25424197 [DOI] [PubMed] [Google Scholar]
- 155.Griessinger CM, Olafsen T, Mascioni A, et al. The PET-tracer (89) Zr-Df-IAB22M2CEnables monitoring of intratumoral CD8 T-cell infiltrates in tumor-bearing humanized mice after t-cell bispecific antibody treatment. Cancer Res. 2020Jul1;80(13):2903–13. PubMed PMID: 32409308. doi: 10.1158/0008-5472.CAN-19-3269 [DOI] [PubMed] [Google Scholar]
- 156.Villanueva MT. Building on bispecifics. Nat Rev Drug Discov. 2019Jul;18(8):582. PubMed PMID: 31367057. doi: 10.1038/d41573-019-00117-5. [DOI] [PubMed] [Google Scholar]
- 157.Trub M, Uhlenbrock F, Claus C, et al. Fibroblast activation protein-targeted-4-1BB ligand agonist amplifies effector functions of intratumoral T cells in human cancer. J Immunother Cancer. 2020Jul;8(2):e000238. 10.1136/jitc-2019-000238: PubMed PMID: 32616554; PubMed Central PMCID: PMCPMC7333869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gauthier L, Morel A, Anceriz N, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019Jun13;177(7):1701–1713e16. PubMed PMID: 31155232. doi: 10.1016/j.cell.2019.04.041 [DOI] [PubMed] [Google Scholar]
- 159.Zhao L, Xie F, Tong X, et al. Combating non-Hodgkin lymphoma by targeting both CD20 and HLA-DR through CD20-243 CrossMab. MAbs. 2014May-Jun;6(3):740–48. 10.4161/mabs.28613: PubMed PMID: 24670986; PubMed Central PMCID: PMCPMC4011918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rajendran S, Li Y, Ngoh E, et al. Development of a bispecific antibody targeting CD30 and CD137 on hodgkin and reed-sternberg cells. Front Oncol. 2019;9:945. PubMed PMID: 31616638; PubMed Central PMCID: PMCPMC6768943. doi: 10.3389/fonc.2019.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Du K, Li Y, Liu J, et al. A bispecific antibody targeting GPC3 and CD47 induced enhanced antitumor efficacy against dual antigen-expressing HCC. Mol Ther. 2021Apr7;29(4):1572–84. PubMed PMID: 33429083; PubMed Central PMCID: PMCPMC8058486. doi: 10.1016/j.ymthe.2021.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao L, Tong Q, Qian W, et al. Eradication of non-Hodgkin lymphoma through the induction of tumor-specific T-cell immunity by CD20-Flex BiFP. Blood. 2013Dec19;122(26):4230–36. PubMed PMID: 24178967. doi: 10.1182/blood-2013-04-496554 [DOI] [PubMed] [Google Scholar]
- 163.Panina AA, Rybchenko VS, Solopova ON, et al. Recombinant bispecific antibodies to the human erbb2 receptor and interferon-beta. Acta Naturae. 2020Apr-Jun;12(2):95–104. 10.32607/actanaturae.10903: PubMed PMID: 32742732; PubMed Central PMCID: PMCPMC7385087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Montefiori DC. Bispecific Antibodies Against HIV. Cell. 2016Jun16;165(7):1563–64. PubMed PMID: 27315470. doi: 10.1016/j.cell.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 165.Kingwell K. Infectious diseases: two-pronged attack on HIV. Nat Rev Immunol. 2016Jul27;16(8):465. PubMed PMID: 27461148. doi: 10.1038/nri.2016.87. [DOI] [PubMed] [Google Scholar]
- 166.Asokan M, Rudicell RS, Louder M, et al. Bispecific Antibodies Targeting Different Epitopes on the HIV-1 Envelope Exhibit Broad and Potent Neutralization. J Virol. 2015Dec;89(24):12501–12. 10.1128/JVI.02097-15: PubMed PMID: 26446600; PubMed Central PMCID: PMCPMC4665248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Galimidi RP, Klein JS, Politzer MS, et al. Intra-spike crosslinking overcomes antibody evasion by HIV-1. Cell. 2015Jan29;160(3):433–46. PubMed PMID: 25635457; PubMed Central PMCID: PMCPMC4401576. doi: 10.1016/j.cell.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bournazos S, Gazumyan A, Seaman MS, et al. Bispecific Anti-HIV-1 antibodies with enhanced breadth and potency. Cell. 2016Jun16;165(7):1609–20. PubMed PMID: 27315478; PubMed Central PMCID: PMCPMC4970321. doi: 10.1016/j.cell.2016.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Khan SN, Sok D, Tran K, et al. Targeting the HIV-1 spike and coreceptor with bi- and trispecific antibodies for single-component broad inhibition of entry. J Virol. 2018Sep15;92(18): 10.1128/JVI.00384-18. PubMed PMID: 29976677; PubMed Central PMCID: PMCPMC6146690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moshoette T, Ali SA, Papathanasopoulos MA, et al. Engineering and characterising a novel, highly potent bispecific antibody iMab-CAP256 that targets HIV-1. Retrovirology. 2019Nov8;16(1):31. PubMed PMID: 31703699; PubMed Central PMCID: PMCPMC6842167. doi: 10.1186/s12977-019-0493-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Davis-Gardner ME, Alfant B, Weber JA, et al. A Bispecific Antibody That Simultaneously Recognizes the V2- and V3-Glycan Epitopes of the HIV-1 envelope glycoprotein is broader and more potent than its parental antibodies. mBio. 2020Jan14;11(1): 10.1128/mBio.03080-19. PubMed PMID: 31937648; PubMed Central PMCID: PMCPMC6960291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guttman M, Padte NN, Huang Y, et al. The influence of proline isomerization on potency and stability of anti-HIV antibody 10E8. Sci Rep. 2020Aug31;10(1):14313. PubMed PMID: 32868832; PubMed Central PMCID: PMCPMC7458915. doi: 10.1038/s41598-020-71184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wagh K, Seaman MS, Zingg M, et al. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathog. 2018Mar;14(3):e1006860. 10.1371/journal.ppat.1006860: PubMed PMID: 29505593; PubMed Central PMCID: PMCPMC5854441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Padte NN, Yu J, Huang Y, et al. Engineering multi-specific antibodies against HIV-1. Retrovirology. 2018Aug29;15(1):60. PubMed PMID: 30157871; PubMed Central PMCID: PMCPMC6114543. doi: 10.1186/s12977-018-0439-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang J, Bardelli M, Espinosa DA, et al. A human bi-specific antibody against zika virus with high therapeutic potential. Cell. 2017Sep21;171(1):229–241e15. PubMed PMID: 28938115; PubMed Central PMCID: PMCPMC5673489. doi: 10.1016/j.cell.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.De Gasparo R, Pedotti M, Simonelli L, et al. Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice. Nature. 2021May;593(7859):424–28. 10.1038/s41586-021-03461-y: PubMed PMID: 33767445 [DOI] [PubMed] [Google Scholar]
- 177.Jette CA, Cohen AA, Gnanapragasam PNP, et al. Broad cross-reactivity across sarbecoviruses exhibited by a subset of COVID-19 donor-derived neutralizing antibodies. bioRxiv. 2021Apr26 [PubMed PMID: 33948592; PubMed Central PMCID: PMCPMC8095199]. doi: 10.1101/2021.04.23.441195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Taylor PC, Williams RO. Combination cytokine blockade: the way forward in therapy for rheumatoid arthritis? Arthritis Rheumatol. 2015Jan;67(1):14–16. PubMed PMID: 25302944. doi: 10.1002/art.38893. [DOI] [PubMed] [Google Scholar]
- 179.Buckland J. Rheumatoid arthritis: anti-TNF and anti-IL-17 antibodies–better together! Nat Rev Rheumatol. 2014Dec;10(12):699. PubMed PMID: 25348041. doi: 10.1038/nrrheum.2014.183. [DOI] [PubMed] [Google Scholar]
- 180.Fischer JA, Hueber AJ, Wilson S, et al. Combined inhibition of tumor necrosis factor alpha and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 2015Jan;67(1):51–62. 10.1002/art.38896: PubMed PMID: 25303306 [DOI] [PubMed] [Google Scholar]
- 181.Xu T, Ying T, Wang L, et al. A native-like bispecific antibody suppresses the inflammatory cytokine response by simultaneously neutralizing tumor necrosis factor-alpha and interleukin-17A. Oncotarget. 2017Oct10;8(47):81860–72. PubMed PMID: 29137228; PubMed Central PMCID: PMCPMC5669854. doi: 10.18632/oncotarget.19899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Heier JS, Singh RP, Wykoff CC, et al. The angiopoietin/tie pathway in retinal vascular diseases: a Review. Retina. 2021Jan1;41(1):1–19. PubMed PMID: 33136975. doi: 10.1097/IAE.0000000000003003 [DOI] [PubMed] [Google Scholar]
- 183.Hussain RM, Neiweem AE, Kansara V, et al. Tie-2/Angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert Opin Investig Drugs. 2019Oct;28(10):861–69. 10.1080/13543784.2019.1667333: PubMed PMID: 31513439 [DOI] [PubMed] [Google Scholar]
- 184.Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021May6;7(1):31. PubMed PMID: 33958600. doi: 10.1038/s41572-021-00265-2 [DOI] [PubMed] [Google Scholar]
- 185.Hussain RM, Shaukat BA, Ciulla LM, et al. Vascular endothelial growth factor antagonists: promising players in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther. 2021;15:2653–65. PubMed PMID: 34188445; PubMed Central PMCID: PMCPMC8232378. doi: 10.2147/DDDT.S295223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sharma A, Kumar N, Kuppermann BD, et al. Faricimab: expanding horizon beyond VEGF. Eye (Lond). 2020May;34(5):802–04. 10.1038/s41433-019-0670-1: PubMed PMID: 31695160; PubMed Central PMCID: PMCPMC7182558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jakubiak P, Alvarez-Sanchez R, Fueth M, et al. Ocular pharmacokinetics of intravitreally injected protein therapeutics: comparison among standard-of-care formats. Mol Pharm. 2021Jun7;18(6):2208–17. PubMed PMID: 34014104. doi: 10.1021/acs.molpharmaceut.0c01218 [DOI] [PubMed] [Google Scholar]
- 188.Joussen AM, Ricci F, Paris LP, et al. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye (Lond). 2021May;35(5):1305–16. 10.1038/s41433-020-01377-x: PubMed PMID: 33564135; PubMed Central PMCID: PMCPMC8182896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nicolo M, Ferro Desideri L, Vagge A, et al. Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021Mar;30(3):193–200. 10.1080/13543784.2021.1879791: PubMed PMID: 33471572 [DOI] [PubMed] [Google Scholar]
- 190.Foxton RH, Uhles S, Gruner S, et al. Efficacy of simultaneous VEGF-A/ANG-2 neutralization in suppressing spontaneous choroidal neovascularization. EMBO Mol Med. 2019May;11(5): 10.15252/emmm.201810204. PubMed PMID: 31040126; PubMed Central PMCID: PMCPMC6505683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wolf A, Langmann T. Anti-VEGF-A/ANG2 combotherapy limits pathological angiogenesis in the eye: a replication study. EMBO Mol Med. 2019May;11(5): 10.15252/emmm.201910362. PubMed PMID: 31040129; PubMed Central PMCID: PMCPMC6505573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chakravarthy U, Bailey C, Brown D, et al. Phase I trial of anti-vascular endothelial growth factor/anti-angiopoietin 2 bispecific antibody rg7716 for neovascular age-related macular degeneration. Ophthalmol Retina. 2017Nov - Dec;1(6):474–85. 10.1016/j.oret.2017.03.003: PubMed PMID: 31047438 [DOI] [PubMed] [Google Scholar]
- 193.Sahni J, Patel SS, Dugel PU, et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-a with faricimab in diabetic macular edema: BOULEVARD phase 2 Randomized Trial. Ophthalmology. 2019Aug;126(8):1155–70. 10.1016/j.ophtha.2019.03.023: PubMed PMID: 30905643 [DOI] [PubMed] [Google Scholar]
- 194.Sahni J, Dugel PU, Patel SS, et al. Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration: the avenue phase 2 randomized clinical trial. JAMA Ophthalmol. 2020Sep1;138(9):955–63. PubMed PMID: 32729888; PubMed Central PMCID: PMCPMC7393587. doi: 10.1001/jamaophthalmol.2020.2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Khanani AM, Patel SS, Ferrone PJ, et al. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the stairway phase 2 randomized clinical trial. JAMA Ophthalmol. 2020Sep1;138(9):964–72. PubMed PMID: 32729897; PubMed Central PMCID: PMCPMC7489851. doi: 10.1001/jamaophthalmol.2020.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.De La Huerta I, Kim SJ, Sternberg P Jr. Faricimab combination therapy for sustained efficacy in neovascular age-related macular degeneration. JAMA Ophthalmol. 2020Sep1;138(9):972–73. PubMed PMID: 32729885. doi: 10.1001/jamaophthalmol.2020.2723. [DOI] [PubMed] [Google Scholar]
- 197.Sharma A, Kumar N, Parachuri N, et al. Faricimab: two in the bush is proving better than one in the hand? Ocul Immunol Inflamm. 2021Jul;9:1–3. doi: 10.1080/09273948.2021.1931350. PubMed PMID: 34242102. [DOI] [PubMed] [Google Scholar]
- 198.Terstappen GC, Meyer AH, Bell RD, et al. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discov. 2021May;20(5):362–83. 10.1038/s41573-021-00139-y: . PubMed PMID: 33649582 [DOI] [PubMed] [Google Scholar]
- 199.Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti-Abeta antibody demonstrates sustained cerebral amyloid-beta binding and elicits cell-mediated removal of human amyloid-beta. J Alzheimers Dis. 2012;28(1):49–69. PubMed PMID: 21955818. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 200.Joshi KK, Phung W, Han G, et al. Elucidating heavy/light chain pairing preferences to facilitate the assembly of bispecific IgG in single cells. MAbs. 2019Oct;11(7):1254–65. 10.1080/19420862.2019.1640549: PubMed PMID: 31286843; PubMed Central PMCID: PMCPMC6748609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gong D, Riley TP, Bzymek KP, et al. Rational selection of building blocks for the assembly of bispecific antibodies. MAbs. 2021Jan-Dec;13(1):1870058. 10.1080/19420862.2020.1870058: PubMed PMID: 33397191; PubMed Central PMCID: PMCPMC7808324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tustian AD, Endicott C, Adams B, et al. Development of purification processes for fully human bispecific antibodies based upon modification of protein A binding avidity. MAbs. 2016May-Jun;8(4):828–38. 10.1080/19420862.2016.1160192: PubMed PMID: 26963837; PubMed Central PMCID: PMCPMC4966828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bogen JP, Carrara SC, Fiebig D, et al. Expeditious generation of biparatopic common light chain antibodies via chicken immunization and yeast display screening. Front Immunol. 2020;11:606878. PubMed PMID: 33424853; PubMed Central PMCID: PMCPMC7786285. doi: 10.3389/fimmu.2020.606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bogen JP, Carrara SC, Fiebig D, et al. Design of a Trispecific Checkpoint Inhibitor And Natural Killer Cell Engager Based On A 2 + 1 Common Light Chain Antibody Architecture. Front Immunol. 2021;12:669496. PubMed PMID: 34040611; PubMed Central PMCID: PMCPMC8141644. doi: 10.3389/fimmu.2021.669496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bogen JP, Storka J, Yanakieva D, et al. Isolation of common light chain antibodies from immunized chickens using yeast biopanning and fluorescence-activated cell sorting. Biotechnol J. 2021Mar;16(3):e2000240. 10.1002/biot.202000240: PubMed PMID: 32914549 [DOI] [PubMed] [Google Scholar]
- 206.Chiu D, Tavare R, Haber L, et al. A PSMA-Targeting CD3 Bispecific Antibody Induces Antitumor Responses that Are Enhanced by 4-1BB Costimulation. Cancer Immunol Res. 2020May;8(5):596–608. 10.1158/2326-6066.CIR-19-0518: PubMed PMID: 32184296 [DOI] [PubMed] [Google Scholar]
- 207.Crawford A, Haber L, Kelly MP, et al. A Mucin 16 bispecific T cell-engaging antibody for the treatment of ovarian cancer. Sci Transl Med. 2019Jun19;11(497): 10.1126/scitranslmed.aau7534. PubMed PMID: 31217340. [DOI] [PubMed] [Google Scholar]
- 208.Skokos D, Waite JC, Haber L, et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci Transl Med. 2020Jan8;12:525. 10.1126/scitranslmed.aaw7888: PubMed PMID: 31915305 [DOI] [PubMed] [Google Scholar]
- 209.Waite JC, Wang B, Haber L, et al. Tumor-targeted CD28 bispecific antibodies enhance the antitumor efficacy of PD-1 immunotherapy. Sci Transl Med. 2020Jun24;12(549): 10.1126/scitranslmed.aba2325. PubMed PMID: 32581132. [DOI] [PubMed] [Google Scholar]
- 210.Geuijen C, Tacken P, Wang LC, et al. A human CD137xPD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat Commun. 2021Jul21;12(1):4445. PubMed PMID: 34290245. doi: 10.1038/s41467-021-24767-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Geuijen CAW, De Nardis C, Maussang D, et al. Unbiased combinatorial screening identifies a bispecific igg1 that potently inhibits her3 signaling via her2-guided ligand blockade. Cancer Cell. 2018May14;33(5):922–936e10. PubMed PMID: 29763625. doi: 10.1016/j.ccell.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 212.Van Loo PF, Hangalapura BN, Thordardottir S, et al. MCLA-117, a CLEC12AxCD3 bispecific antibody targeting a leukaemic stem cell antigen, induces T cell-mediated AML blast lysis. Expert Opin Biol Ther. 2019Jul;19(7):721–33. 10.1080/14712598.2019.1623200: PubMed PMID: 31286786 [DOI] [PubMed] [Google Scholar]
- 213.Shiraiwa H, Narita A, Kamata-Sakurai M, et al. Engineering a bispecific antibody with a common light chain: identification and optimization of an anti-CD3 epsilon and anti-GPC3 bispecific antibody, ERY974. Methods. 2019Feb1;154:10–20. 10.1016/j.ymeth.2018.10.005: PubMed PMID: 30326272 [DOI] [PubMed] [Google Scholar]
- 214.Bonisch M, Sellmann C, Maresch D, et al. Novel CH1: cLinterfaces that enhance correct light chain pairing in heterodimeric bispecific antibodies. Protein Eng Des Sel. 2017Sep1;30(9):685–96. PubMed PMID: 28981885; PubMed Central PMCID: PMCPMC5914326. doi: 10.1093/protein/gzx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dillon M, Yin Y, Zhou J, et al. Efficient production of bispecific IgG of different isotypes and species of origin in single mammalian cells. MAbs. 2017;9(2):213–30. PubMed PMID: 27929752; PubMed Central PMCID: PMCPMC5297516. doi: 10.1080/19420862.2016.1267089. Feb/Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]


