Abstract
Rationale & Objective
Recent studies showed that antibody titers after vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the dialysis population are diminished as compared with the general population, suggesting the possible value of a third booster dose. We characterized the humoral response after 3 doses of the BNT162b2 vaccine in patients treated with either maintenance hemodialysis (HD) or peritoneal dialysis (PD).
Study Design
Case series.
Setting & Participants
69 French patients (38 HD and 31 PD) treated at a single center who received 3 doses of the BNT162b2 vaccine.
Findings
Humoral response was evaluated using plasma levels of anti-SARS-CoV-2 spike protein S1 immunoglobulin measured after the second dose and at least 3 weeks after the third dose of the BNT162b2 vaccine. Patients (median age 68 years [interquartile range (IQR), 53-76 years], 65% men) had a median anti-S1 antibody level of 284 [IQR, 83-1190] AU/mL after the second dose, and 7,554 [IQR, 2,268-11,736] AU/mL after the third dose. Three patients were nonresponders (anti-S1 antibody level < 0.8 AU/mL), and 12 were weak responders (anti-S1 antibody level 0.8-50 AU/mL) after the second vaccine dose. After the third dose, 1 of the 3 initial nonresponders produced anti-spike antibody, and all the 12 initial weak responders increased their antibody levels. Patients with a greater increase in anti-S1 antibody levels after a third dose had lower antibody levels after the second dose, and a longer time interval between the second and the third dose. Adverse events did not seem to be more common or severe after a third vaccine dose.
Limitations
Observational study, small sample size. Relationship between antibody levels and clinical outcomes is not well understood.
Conclusions
A third dose of the BNT162b2 vaccine substantially increased antibody levels in patients receiving maintenance dialysis and appeared to be as well tolerated as a second dose.
Index Words: Antibody levels, anti-spike serology, dialysis, coronavirus disease 2019 (COVID-19), end-stage renal disease (ESRD), immune response, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), third booster dose, BNT162b2, vaccine, vaccine adverse effects
Graphical abstract
Plain-Language Summary.
Since April 2021, the French National Authority for Health has recommended systematic use of a third dose of COVID-19 vaccine for dialysis patients to boost immunity. This study assessed vaccine response after the second and third doses by measuring patients’ antibody levels. Although most of the patients produced antibodies after 2 vaccine doses, some of them (mainly elderly patients and those receiving treatments that suppress immunity) had relatively low antibody levels. After a third booster dose, almost all patients increased their antibody levels, especially when the third dose was delayed. A third dose was not associated with greater side effects than the second dose. The relationship between increased antibody levels after a third dose and protection against clinical illness with COVID-19 remains to be evaluated.
Editorial, p. 162
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes high morbidity and mortality in patients with receiving kidney replacement therapy (KRT), whether they are receiving maintenance dialysis or have had a kidney transplant.1 , 2 Vaccination is one of the most important weapons in the fight against SARS-CoV-2. KRT patients have a reduced immune response.3 , 4 Vaccine response in patients treated with maintenance hemodialysis (HD) or peritoneal dialysis (PD) is attenuated, less effective, and shorter over time than in the general population, due to the accumulation of uremic toxins and the acceleration of immunosenescence induced by chronic inflammation.5 Dialysis patients often need a higher and more frequent vaccine dose, as has been found with the 14-valent pneumococcal capsular polysaccharide vaccine3 or hepatitis B virus (HBV) vaccine.4
Since the beginning of the epidemic, several vaccines have been approved to prevent acute respiratory distress syndrome induced by SARS-CoV-2. Among them, the BNT162b2 messenger RNA vaccine (Pfizer-BioNTech) is widely used in France, especially in hospitals where it is possible to store the doses at a sufficiently low temperature. A 2-dose regimen of BNT162b2 vaccine is 95% effective in preventing coronavirus disease 2019 (COVID-19) in the general population.6
It was reported in recent case series of dialysis patients vaccinated with BNT162b2 that seroconversion occurred in almost all patients although the antibody titer was lower than in controls.7, 8, 9, 10, 11, 12, 13 On this basis, the French National Authority for Health (Haute Autorité de Santé) has recommended that a third vaccine dose be systematically administered in dialysis patients.14 Recently, 2 small case series evaluating humoral response after a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients showed an increase in antibody titer in some patients.15 , 16 In dialysis patients, only 1 study has addressed the humoral response after a third vaccine dose and only in HD patients with a weak initial response, who showed an increase in antibody titer.17 Our study (1) assessed the humoral response after a third dose of the BNT162b2 vaccine both in HD and PD patients, (2) identified potential factors associated with vaccine response, and (3) determined whether a third dose is associated with adverse events.
Methods
Study Design and Participants
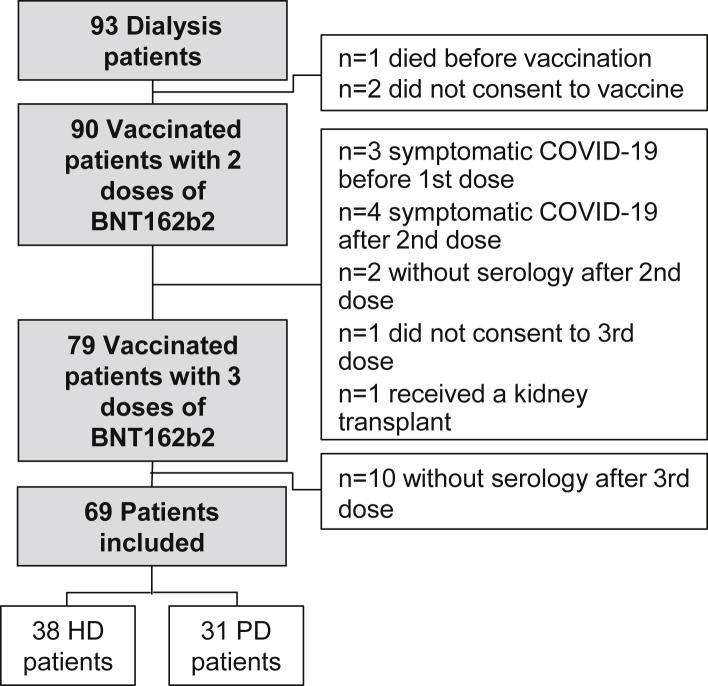
Following recommendation from the French National Authority for Health, vaccination of all dialysis patients began in January 2021. In April 2021, the systematic use of a third vaccine dose at least 4 weeks after the second dose was recommended in dialysis patients. Between January 13, 2021, and May 18, 2021, all HD and PD adult patients of the Nephrology Department of the Centre Hospitalier Sud-Francilien (Corbeil-Essonnes, France), who received 3 doses of the BNT162b2 vaccine were eligible for the study. We excluded patients who died before vaccination (n = 1), who did not consent to vaccination (n = 2) or to a third dose (n = 1), who did not receive a third dose because of symptomatic COVID-19 before the first dose (n = 3) or after the second dose (n = 4), who did not have serology results before (n = 2) or after (n = 10) the third dose, or who received a kidney transplant (n = 1) before the third dose. Sixty-nine dialysis patients (38 HD and 31 PD patients) did not meet the exclusion criteria and were included in the analysis (Fig 1 ). According to the French legislation (Loi Jardé), anonymous retrospective studies do not require institutional review board approval. All patients provided oral informed consent.
Figure 1.
Study flowchart. Abbreviations: HD, hemodialysis; peritoneal dialysis.
Data Collection
Clinical data, laboratory tests routinely performed in dialysis patients, and dialysis dose (measured by Kt/V) were collected from medical records. The level of immunosuppression was evaluated using gamma globulin concentration and lymphocyte and neutrophilic polymorphonuclear leukocyte counts. A history of hemopathy (plasma cell dyscrasia) and chemotherapy or immunomodulatory treatment (any treatment during the last year or prednisone ≥7.5 mg/d during ≥3 months during the last year) was also collected. Nutritional status was assessed by body mass index (calculated from the dry weight) and levels of serum albumin and prealbumin; inflammation was assessed by C-reactive protein level. When available, antinucleocapsid serology attesting a previous contact with SARS-CoV-2 was also collected.
Antibody Measurement
Humoral response was assessed using the Roche Elecsys Assay18 by the plasma concentration of anti-SARS-CoV-2 spike protein S1 total immunoglobulin antibodies (including IgG, IgM, and IgA), which are induced by vaccination; testing was performed after the second dose and at least 3 weeks after the third vaccine dose. Because the measurement range of this assay is of 0 to 250 arbitrary units per milliliter (AU/mL), samples giving results in excess of 250 AU/mL were diluted. According to the manufacturer’s protocol, patients with an antibody level below 0.8 AU/mL were considered nonresponders. Patients with a level between 0.8 and 50 AU/mL were defined as weak responders, as reported elsewhere.15
To assess humoral response after the third vaccine dose, we calculated the ratio of the antibody level measured after the third vaccine dose to the level measured after the second dose, and we analyzed in tertiles of increasing ratio (thus the first tertile has the lowest increase in antibody level between the second and the third dose). Patients with initial negative serology were excluded from the analysis, as by definition the ratio could not be calculated.
Adverse Events After Third Dose Vaccination
A questionnaire was provided to all patients to collect vaccine reactions after the third vaccine dose (Item S1). Overall experience with the third dose as compared with the second dose was self-reported by the patient. We also reported hospitalizations and visits to an emergency department within the 30 days after the administration of the third vaccine dose.
Statistical Analyses
Categorical variables are reported as frequencies and percentages, and continuous variables are reported as median and interquartile range (IQR). The patients’ characteristics were compared according to antibody response (< or ≥ 50 AU/mL) after the second and the third doses, and according to antibody increase after the third dose (across tertiles of the ratio of antibody levels measured after the third and second doses) using the Fisher exact test or the Mann-Whitney U test (or using the Kruskal-Wallis test, as appropriate) for qualitative and quantitative variables, respectively. A 2-tailed P < 0.05 was considered statistically significant. All statistical analyses were conducted using R 3.6 software (R Foundation for Statistical Computing).
Results
Humoral Response After Two Vaccine Doses
Among the 69 patients included, the median age was 68 (IQR, 53-76) years, 65% were men, and dialysis vintage was 3 (IQR, 1-6) years. Twelve patients (17%) had a history of plasma cell dyscrasia and/or immunosuppressive therapy. The median interval between administration of the second BNT162b2 vaccine dose and the first serology was 50 (IQR, 31-58) days. Overall, after 2 doses, the median anti-S1 antibody level was 284 (IQR, 83-1,190) AU/mL (Table 1 ). Three patients (4%) were nonresponders, and 12 patients (17%) were weak responders (Table S1). Factors associated with no or weak response after 2 doses included an older age, a history of immunosuppressive therapy, a lower lymphocyte count, a lower serum prealbumin level, and a lower serum gamma globulin level (Table 1). No difference was found according to dialysis modality.
Table 1.
Characteristics of the Study Population, According to the Antibody Response After the Second Vaccine Dose
| Total Population (N = 69) | Anti-Spike Antibody Titer |
P | ||
|---|---|---|---|---|
| <50 AU/mL (n = 15) | ≥50 AU/mL (n = 54) | |||
| Clinical characteristics | ||||
| Age, y | 68.0 [53.0-76.0] | 75.0 [68.5-81.0] | 64.5 [49.3-76.0] | 0.006 |
| Male sex | 45 (65%) | 7 (47%) | 38 (70%) | 0.1 |
| Body mass index, kg/m2 | 26.6 [22.2-30.6] | 25.89 [21.5-30.1] | 27.0 [22.2-30.6] | 0.8 |
| Diabetes | 22 (32%) | 7 (47%) | 15 (28%) | 0.2 |
| History of hemopathy | 6 (9%) | 3 (20%) | 3 (6%) | 0.1 |
| History of immunosuppressive therapy | 9 (13%) | 8 (53%) | 1 (2%) | <0.001 |
| HD as dialysis modality | 38 (55%) | 7 (47%) | 31 (57%) | 0.6 |
| Dialysis vintage, y | 3.0 [1.0-6.0] | 2.0 [1.0-5.0] | 3.5 [1.0-6.0] | 0.4 |
| Kt/V | 1.6 [1.2-1.9] | 1.7 [1.4-1.9] | 1.50 [1.2-1.9] | 0.4 |
| Biological parameters | ||||
| Lymphocytes, ×109/L | 1.2 [0.9-1.6] | 1.0 [0.7-1.2] | 1.3 [1.0-1.7] | 0.03 |
| Neutrophilic polymorphonuclear cells, ×109/L | 3.9 [2.8-5.0] | 3.6 [2.9-4.4] | 4.1 [2.8-5.2] | 0.3 |
| Gamma globulin, g/L | 9.6 [7.5-12.7] | 7.0 [4.6-9.2] | 10.0 [8.3-13.9] | 0.001 |
| Serum albumin, g/dL | 3.7 [3.5-4.1] | 3.5 [3.3-3.9] | 3.8 [3.5-4.2] | 0.07 |
| Serum prealbumin, mg/dL | 34 [30-41] | 30 [25-35] | 35 [31-41] | 0.02 |
| C-reactive protein, mg/L | 2.2 [0.9-3.7] | 2.8 [1.2-4.0] | 2.2 [0.9-3.7] | 0.8 |
| Humoral response after 2nd dose | ||||
| Interval between 2nd dose and serology, d | 50 [31-58] | 56 [45-70] | 50 [30-56] | 0.1 |
| Anti-S1 Ab after 2nd dose, AU/mL | 284 [83-1,190] | 7 [3-32] | 485 [158-1,572] | <0.001 |
Results are shown as median [interquartile range] for continuous variables, or as number of patients (percentage) for categorical variables. Mann-Whitney test and Fisher exact test were used to compare continuous and categorical variables according to the dialysis modality. Abbreviations: Ab, antibody; AU, arbitrary unit; HD, hemodialysis; S1, spike protein S1.
Humoral Response After Three Vaccine Doses
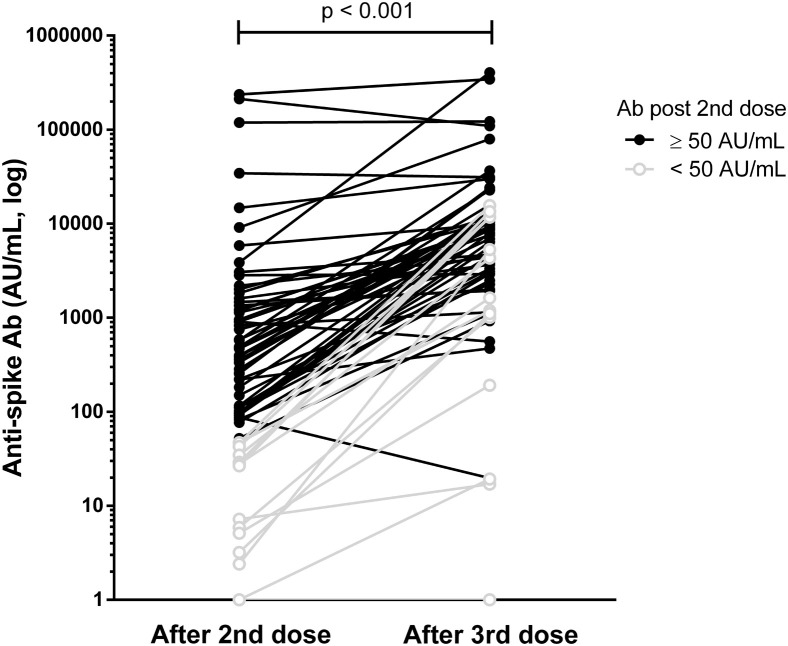
After 3 vaccine doses, there was a significant increase in antibody level, with a median anti-S1 antibody level of 7,554 (IQR, 2,268-11,736) AU/mL after a median interval between the third dose and serology of 30 (IQR, 27-36) days (Fig 2 ; Fig S1). Only 6 patients did not increase their antibody level (2 remained seronegative, 2 already had a very high antibody level, and 2 had a slight decrease) (Table S2). Among the 3 nonresponders after the second dose, only 1 produced anti-S1 antibody after the third dose. All the 12 weak responders increased their antibody level after the third dose, but 1 remained below the 50 AU/mL threshold (Table S1). All responders after the second dose were still seropositive at 1 month after the third vaccine dose. Factors associated with no or weak response after 3 doses included a history of immunosuppressive therapy, a history of hemopathy, a lower serum gamma globulin level, and an initial lower anti-S1 antibody level after 2 doses (Table S3). No difference between HD and PD patients was found.
Figure 2.
Humoral response after the second and the third vaccine doses. Abbreviations: Ab, antibody; AU, arbitrary unit.
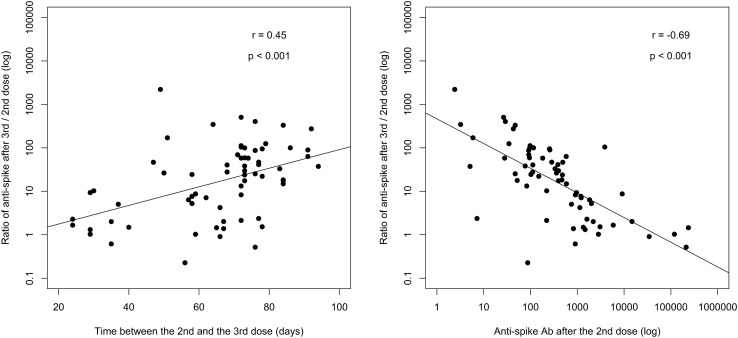
Patients who had the higher increase in anti-S1 antibody level had an initial lower antibody level after the second vaccine dose (94 [IQR, 30-237] vs 1,718 [IQR, 963-5,165] AU/mL [P < 0.001] in tertiles 3 and 1, respectively, of the ratio of antibody levels after the third and second dose) and a longer time interval between the second and the third vaccine doses (74 [IQR, 72-79] days for the third tertile vs 59 [IQR, 36-67] days for the first tertile, P < 0.001) (Fig 3 ; Table 2 ).
Figure 3.
Anti-spike antibody increase after the third vaccine dose according to the delay between the second and the third vaccine doses, and anti-spike antibody level after the second vaccine dose. Abbreviation: Ab, antibody.
Table 2.
Characteristics of the Patients According to Anti-Spike Antibody Increase After the Third Vaccine Dose
| Ratio of Anti-S1 Antibody Titer, 3rd vs 2nd Dose |
P | |||
|---|---|---|---|---|
| <7.5 (n = 22) | 7.5-43 (n = 22) | >43 (n = 22) | ||
| Clinical characteristics | ||||
| Age, y | 63.5 [45.5-73.3] | 68.5 [54.3-76.0] | 68.5 [59.0-79.0] | 0.2 |
| Male sex | 16 (73%) | 16 (73%) | 13 (59%) | 0.7 |
| Body mass index, kg/m2 | 28.2 [23.8-30.6] | 26.7 [23.2-30.6] | 26.1 [22.2-30.8] | 0.7 |
| Diabetes | 6 (27%) | 10 (46%) | 6 (27%) | 0.4 |
| History of hemopathy | 1 (5%) | 2 (9%) | 1 (5%) | 0.9 |
| History of immunosuppressive therapy | 1 (5%) | 1 (5%) | 4 (18%) | 0.4 |
| HD as dialysis modality | 11 (50%) | 14 (64%) | 10 (46%) | 0.6 |
| Dialysis vintage, y | 3.0 [0.6-3.8] | 3.50 [1.3-7.0] | 4.00 [1.0-6.0] | 0.3 |
| Kt/V | 1.6 [1.1-1.9] | 1.5 [1.3-2.0] | 1.7 [1.4-1.9] | 0.8 |
| Biological parameters | ||||
| Lymphocytes, ×109/L | 1.3 [1.0-1.5] | 1.3 [1.0-1.6] | 1.1 [0.8-1.7] | 0.7 |
| Neutrophilic polymorphonuclear cells, ×109/L | 3.7 [2.6-4.4] | 4.4 [3.3-5.2] | 3.7 [3.0-4.9] | 0.3 |
| Gamma globulin, g/L | 10.3 [8.3-14.8] | 9.0 [8.1-10.5] | 9.8 [7.3-12.3] | 0.6 |
| Serum albumin, g/L | 3.8 [3.5-4.0] | 3.9 [3.7-4.2] | 3.5 [3.3-3.9] | 0.2 |
| Serum prealbumin, g/L | 34 [30-41] | 37 [31-42] | 32 [27-38] | 0.2 |
| C-reactive protein, mg/L | 2.1 [1.0-4.5] | 1.7 [0.8-3.3] | 2.2 [1.0-3.8] | 0.9 |
| Humoral response | ||||
| Anti-S1 Ab after 2nd dose, AU/mL | 1,718 [963-5,165] | 347 [104-478] | 94 [30-237] | <0.001 |
| After the 3rd dose | ||||
| Interval between 2nd and 3rd doses, d | 59 [36-67] | 73 [61-78] | 74 [72-79] | <0.001 |
| Anti-S1 Ab after 3rd dose, AU/mL | 4,533 [1,950-11,351] | 7,237 [2,602-9,384] | 11,305 [5,800-15,027] | 0.08 |
| Ratio of Ab after 3rd vs 2nd dose | 1.6 [1.1-2.3] | 23.0 [13.7-29.3] | 99.9 [64.8-247.8] | <0.001 |
Results are shown as median [interquartile range] for continuous variables, or as number of patients (percentage) for categorical variables. Kruskal-Wallis test and Fisher exact test were used to compare continuous and categorical variables according to the dialysis modality. Abbreviations: Ab, antibody; AU, arbitrary unit; HD, hemodialysis; S1, spike protein S1.
Analysis of the antinucleocapsid serology status showed that seropositive patients were younger than those who were seronegative. They also displayed higher anti-S1 antibody levels after the second dose and a lower increase after third dose (Table S4).
Vaccine Reaction, Hospitalizations, and Emergency Department Visits After the Third Vaccine Dose
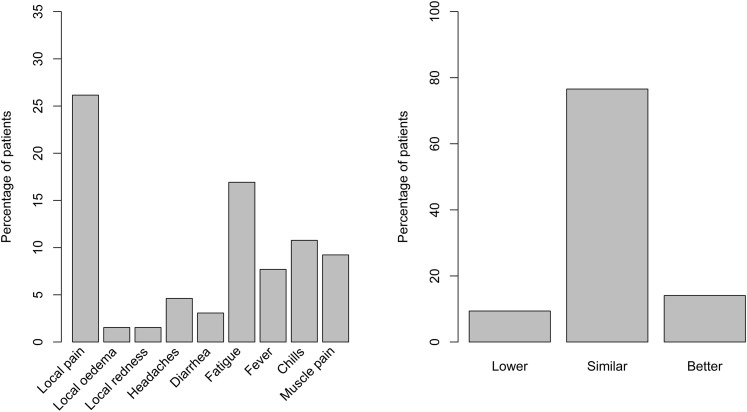
Sixty-six patients completed the vaccine reaction questionnaire after undergoing serology after the third dose. The most frequent self-reported reaction was pain at the injection site (27%); 23% of patients reported at least 1 systemic reaction, mostly fatigue (17%). Most patients reported tolerating the third vaccine dose as well as the second dose (Fig 4 ). In the 30 days after the third vaccine dose, 6 patients were hospitalized (causes were 3 bacterial and 1 aseptic peritonitis in PD patients; 1 pulmonary embolism; and 1 osteitis), and 2 visited the emergency department (1 for chest pain, and 1 for fatigue) (Table S5).
Figure 4.
Self-reported (left panel) reactions to vaccine after the third dose and (right panel) overall tolerance of the third vaccine dose.
COVID-19 Breakthrough Cases
In the study group, no cases of COVID-19 infection were diagnosed after the third vaccine dose, during a median follow-up period of 30 days. By contrast, 4 patients developed symptomatic COVID-19 infections after the second vaccine dose and thus were not included: 2 patients whose antibody levels were not available, 1 with anti-S1 antibody level of 709 AU/mL, and 1 with a negative serology after the 2 vaccine doses. Because the latter patient was at high risk of complications, he received monoclonal antibodies 4 days after symptom onset. All patients developed mild symptoms and had favorable outcomes.
Discussion
In this study, we describe the humoral response measured a median of 30 days after the third injection of the BNT162b2 vaccine in 69 patients on maintenance HD or PD. Administration of a third vaccine dose to dialysis patients substantially increased their anti-S1 antibody level, with acceptable vaccine reactions and an overall self-reported tolerance similar to the second dose. No cases of COVID-19 were reported.
Most of the weak responders after the second dose had a history of immunosuppression. Three patients in our HD series with plasma cell dyscrasia (2 with myeloma and 1 with amyloid light-chain amyloidosis) were undergoing chemotherapy at the time of vaccination. Two of them were nonresponders, and the remaining patient was a weak responder after 2 vaccine doses. After a third dose, the 2 nonresponders had still no detectable antibodies, and the remaining patient showed a 2.4-fold increase.
These results suggest an impact of comorbidities and immunosuppressive therapy on vaccine response in dialysis patients. They confirm a poor vaccine response in patients under therapy for hemopathy. A recent study reported that up to 48% of patients with myeloma under therapy developed a positive antibody response after SARS-CoV-2 vaccine.19 A third dose seems to have little or no effect in these patients, although this has to be confirmed, given the small sample size.
The recommendation from the French National Authority for Health to use a third vaccine dose as a booster 4 weeks after the second dose in dialysis patients was published in April 2021, and vaccination of dialysis patients began in January 2021. This delay explains the time interval between the 2 vaccine doses extending up to 13 weeks. When looking at the increase in antibody level, we found a greater increase after the third dose with a longer interval between second and third vaccine doses.
Moreover, it seems that patients with a high antibody titer after the second dose showed a lower increase after a third dose, especially in patients with positive antinucleocapsid serology. In terms of an increase in antibody level, our results do not show a benefit of a third dose in patients who already have high anti-S1 antibodies. Nevertheless, the third dose could have a benefit if it extends the duration of vaccine efficacy in a population known to have a rapid antibody decline after vaccination.20 , 21
Whether a higher anti-S1 antibody level titer confers better protection against COVID-19 remains to be determined. A recent study showed a correlation between postvaccination anti-S1 antibodies and neutralizing antibodies,22 implying a protective antibody response. It was also shown that the antibody titer after infection in health care workers was inversely correlated with the risk of reinfection.23 Another study showed that in patients who received convalescent plasma therapy, a higher antibody titer was associated with a lower risk of death.24 These results highlight the importance of the humoral response in COVID-19.
The clinical efficacy of a third vaccine dose could not be determined in our study. No cases of COVID-19 were diagnosed after the third vaccine dose in our center, but the follow-up period was limited to a median of 30 days and during a period with decreased incidence of COVID-19.
A cellular response can also play an important role and was not assessed in our study. A recent study in rituximab-treated patients22 found cellular response after SARS-CoV-2 vaccination in 58% of cases versus 39% of cases for humoral response, without correlation between the 2 immune responses. Another recent study conducted in HD patients found cellular response in 62% of cases versus 95.4% of patients with humoral response.25 The cellular response was not correlated with humoral response, and it could occur even in seronegative patients. The protective effect of the cellular response has yet to be determined.
How long vaccine-induced protection lasts in the dialysis population is another important question. Sakhi et al20 showed that after infection 25% of initially seropositive dialysis patients had undetectable antibodies at 6 months. Clarke et al21 confirmed a significant decline of antibody titers at 6 months in dialysis patients, but also showed that the majority of patients without antibody at 6 months still had SARS-CoV-2 antigen–specific T-cell responses. Overall, the effect of a third vaccine dose on the length of immune protection remains to be determined.
Tolerance of the second and third vaccine doses was considered to be similar by the patients. Four PD patients were hospitalized for infection of the peritoneal dialysate shortly after the third injection. Although this could be an incidental association, the question of a potential link between the 2 events can be raised. A direct effect of vaccination through immune-mediated peritonitis seems unlikely because bacteria were found in 3 cases (2 with Staphylococcus epidermidis and one with Streptococcus mitis), and all had a favorable outcome with antibiotic therapy. The most likely hypothesis is a behavioral change after the third vaccine dose. The COVID-19 pandemic has indeed changed behavior, and a decrease in Gram-positive peritonitis has been reported by Hu et al.26 In contrast, after the first vaccine dose, a part of the population observed less strict pandemic-related rules.27 Similarly, a supplementary vaccine dose could have increased the patients’ confidence in their protection, resulting in diminished carefulness in observing aseptic techniques. More studies are needed to confirm this observation, but it seems important to educate patients to maintain aseptic rules, especially with patients who started PD during the pandemic.
To our knowledge, ours is the first study to evaluate humoral response in both HD and PD patients who systematically receive a third dose of vaccine against SARS-CoV-2. Our study has some limitations. First, the humoral response to a third dose was assessed in a single-center, small-sized group, which limited the statistical analyses and could induce bias. Second, we included patients with positive antinucleocapsid serology, implying previous contact with SARS-CoV-2, but all patients with a history of symptomatic COVID-19 were excluded. This could induce biases because immunity elicited by infection versus vaccine cannot clearly be differentiated. We chose to give 3 vaccine doses to patients with positive antinucleocapsid antibodies and without history of symptomatic COVID-19 infections because COVID-19 serology was not mandatory or recommended before vaccination in dialysis patients. A third dose appears to have a diminished benefit in these patients, who already have developed a good humoral response after 2 vaccine doses.
Third, measurement of response to vaccination was only assessed using antibody levels, without taking into account cellular immunity or clinical outcomes. Fourth, we did not compare our results with a control group in which individuals did not receive a third vaccine dose. Therefore, we could not analyze whether the observed increase in antibody level was only due to the third vaccine dose or to a delayed immune response after the second vaccine dose. Nevertheless, recent studies have shown the relative stability of antibody titer between 2 and 3 weeks after vaccination in dialysis patients,10 and serology was measured a median of 50 days after the second vaccine dose in our study.
Fifth, there is no unequivocal means to assess the increase in antibody titer; we used titer ratio because it is classically used in other studies evaluating vaccine efficacy. However, this could have disproportionately emphasized the effect for those who had a poor antibody response after the second vaccine dose. Finally, because of the observational nature of our study, there is a variability in the time between the administration of the second dose and serology, which may be a potential source of bias.
To conclude, a third dose of BNT162b2 vaccine substantially increased antibody levels in dialysis patients, especially in patients with low antibody levels after the second dose and with a longer interval between second and third dose. In contrast, there was little evidence of an increase in antibody level after the third dose in patients with initial high anti-S1 antibodies or in those undergoing chemotherapy. Adverse events did not seem to be more common or severe after the third vaccine dose. The clinical efficacy of a third boost dose in dialysis patients remains to be determined.
Article Information
Authors’ Full Names and Academic Degrees
Ilias Bensouna, MD, Valérie Caudwell, MD, Sabah Kubab, MD, Sandra Acquaviva, Agathe Pardon, MD, Nathalie Vittoz, MD, Dogan-Firat Bozman, MD, Latifa Hanafi, MD, Anne-Laure Faucon, MD, PhD, and Pierre Housset, MD.
Authors’ Contributions
Study design and data interpretation: IB, PH, A-LF; statistical analysis: A-LF; anti-spike serology data collection: SA; anti-spike serology testing: SK; clinical and biological data collection: IB, PH, ALF, VC, AP, NV, D-FB, LH. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This study was sponsored by the Centre Hospitalier Sud-Francilien. The funder had no role in the study design, data collection, analysis, reporting, or the decision to submit the manuscript for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
The authors thank patients for their participation to the study.
Data Sharing
The data that support the findings of this study are available upon request.
Peer Review
Received July 02, 2021. Evaluated by 3 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form August 27, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1: Individual anti-S1 antibody titer according to dialysis modality.
Item S1: Questionnaire on vaccine reaction after the third vaccine dose.
Table S1: Nonresponders and weak responders after the second vaccine dose.
Table S2: Patients without increase in antibody titer after the third vaccine dose.
Table S3: Characteristics of the study population, according to the antibody response after the third vaccine dose.
Table S4: Characteristics of the study population, according to the antinucleocapsid serology status.
Table S5: Hospitalizations or visits to emergency department within 30 days after the third vaccine dose.
Supplementary Material
Figure S1. Item S1. Tables S1-S5.
References
- 1.Shimada N., Shimada H., Itaya Y., Tomino Y. Novel coronavirus disease in patients with end-stage kidney disease. Ther Apher Dial. 2021;25(5):544–550. doi: 10.1111/1744-9987.13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meziyerh S., Helm D., Vries A.P.J. Vulnerabilities in kidney transplant recipients with COVID-19: a single center experience. Transpl Int. 2020;33(11):1557–1561. doi: 10.1111/tri.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikoskelainen J., Koskela M., Forsström J., Kasanen A., Leinonen M. Persistence of antibodies to pneumococcal vaccine in patients with chronic renal failure. Kidney Int. 1985;28(4):672–677. doi: 10.1038/ki.1985.182. [DOI] [PubMed] [Google Scholar]
- 4.Pitchou Y Kengibe, Jean-Robert R Makulo, Yannick M Nlandu, et al. Response to single dose hepatitis B vaccine in Congolese non-HIV hemodialysis patients: a prospective observational study. Pan Afr Med J. 2019;34:122. doi: 10.11604/pamj.2019.34.122.19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crépin T., Legendre M., Courivaud C., et al. Premature immune senescence and chronic kidney disease: update and perspectives. Article in French. Nephrol Ther. 2020;16(1):9–18. doi: 10.1016/j.nephro.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grupper A, Sharon N, Finn T, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. Published online April 6, 2021. https://doi.org/10.2215/CJN.03500321 [DOI] [PMC free article] [PubMed]
- 8.Ikizler T.A., Coates P.T., Rovin B.H., Ronco P. Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int. 2021;99(6):1275–1279. doi: 10.1016/j.kint.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torreggiani M., Blanchi S., Fois A., Fessi H., Piccoli G.B. Neutralizing SARS-CoV-2 antibody response in dialysis patients after the first dose of the BNT162b2 mRNA COVID-19 vaccine: the war is far from being won. Kidney Int. 2021;99(6):1494–1496. doi: 10.1016/j.kint.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attias P., Sakhi H., Rieu P., et al. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021;99(6):1490–1492. doi: 10.1016/j.kint.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanay N.B., Freiman S., Shapira M., et al. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99(6):1496–1498. doi: 10.1016/j.kint.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agur T, Ben-Dor N, Goldman S, et al. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients—a prospective cohort study. Nephrol Dial Transplant. Published online April 11, 2021. https://doi.org/10.1093/ndt/gfab155 [DOI] [PMC free article] [PubMed]
- 13.Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared to healthy controls. Nephrol Dial Transplant. Published online May 17, 2021. https://doi.org/10.1093/ndt/gfab179 [DOI] [PMC free article] [PubMed]
- 14.Centre opérationnel de régulation et de réponse aux urgences sanitaires et sociales (CORRUSS) DGS-Urgent No. 2021-43. Vaccins contre la Covid-19: modalites d’administration des rappels. https://solidarites-sante.gouv.fr/IMG/pdf/dgs_urgent_n43_vaccination_modalites_d_administration_des_rappels.pdf April 11, 2021.
- 15.Werbel W.A., Boyarsky B.J., Ou M.T., et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frantzen L, Thibeaut S, Moussi-Frances J, et al. COVID-19 Vaccination in haemodialysis patients: good things come in threes. Nephrol Dial Transplant. Published online July 20, 2021. https://doi.org/10.1093/ndt/gfab224 [DOI] [PMC free article] [PubMed]
- 18.Mueller T. Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: a method comparison of two different commercially available serological assays from the same manufacturer. Clin Chim Acta. 2021;518:9–16. doi: 10.1016/j.cca.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bird S., Panopoulou A., Shea R.L., et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8(6):e389–e392. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakhi H., Dahmane D., Attias P., et al. Kinetics of anti–SARS-CoV-2 IgG antibodies in hemodialysis patients six months after infection. J Am Soc Nephrol. 2021;32(5):1033–1036. doi: 10.1681/ASN.2020111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke C.L., Prendecki M., Dhutia A., et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021;99(6):1470–1477. doi: 10.1016/j.kint.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. Published online July 20, 2021. https://doi.org/10.1136/annrheumdis-2021-220781 [DOI] [PubMed]
- 23.Lumley S.F., O’Donnell D., Stoesser N.E., et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2020;384(6):533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyner M.J., Carter R.E., Senefeld J.W., et al. Convalescent plasma antibody levels and the risk of death from COVID-19. N Engl J Med. 2021;384(11):1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broseta J.J., Rodríguez-Espinosa D., Rodríguez N., et al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78(4):571–681. doi: 10.1053/j.ajkd.2021.06.002. https://doi:10.1053/j.ajkd.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y., Xu L., Wang X., et al. Changes before and after COVID-19 pandemic on the personal hygiene behaviors and incidence of peritonitis in peritoneal-dialysis patients: a multi-center retrospective study. Int Urol Nephrol. Published online June 19, 2021 doi: 10.1007/s11255-021-02924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day M. COVID-19: Stronger warnings are needed to curb socialising after vaccination, say doctors and behavioural scientists. BMJ. 2021;372:n783. doi: 10.1136/bmj.n783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Item S1. Tables S1-S5.