Abstract
Background
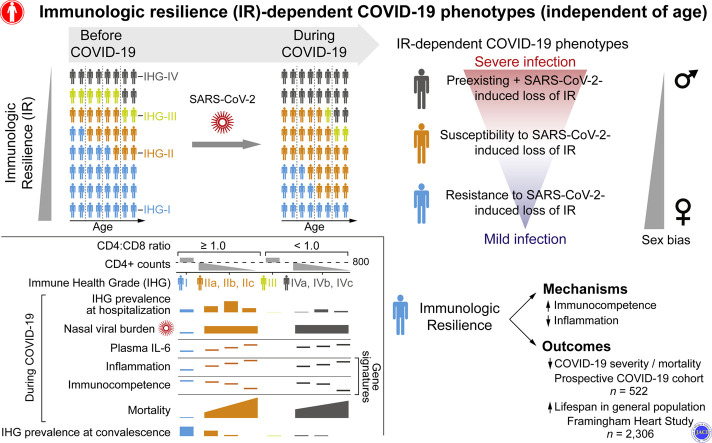
The risk of severe coronavirus disease 2019 (COVID-19) varies significantly among persons of similar age and is higher in males. Age-independent, sex-biased differences in susceptibility to severe COVID-19 may be ascribable to deficits in a sexually dimorphic protective attribute that we termed immunologic resilience (IR).
Objective
We sought to examine whether deficits in IR that antedate or are induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection independently predict COVID-19 mortality.
Methods
IR levels were quantified with 2 novel metrics: immune health grades (IHG-I [best] to IHG-IV) to gauge CD8+ and CD4+ T-cell count equilibrium, and blood gene expression signatures. IR metrics were examined in a prospective COVID-19 cohort (n = 522); primary outcome was 30-day mortality. Associations of IR metrics with outcomes in non–COVID-19 cohorts (n = 13,461) provided the framework for linking pre–COVID-19 IR status to IR during COVID-19, as well as to COVID-19 outcomes.
Results
IHG-I, tracking high-grade equilibrium between CD8+ and CD4+ T-cell counts, was the most common grade (73%) among healthy adults, particularly in females. SARS-CoV-2 infection was associated with underrepresentation of IHG-I (21%) versus overrepresentation (77%) of IHG-II or IHG-IV, especially in males versus females (P < .01). Presentation with IHG-I was associated with 88% lower mortality, after controlling for age and sex; reduced risk of hospitalization and respiratory failure; lower plasma IL-6 levels; rapid clearance of nasopharyngeal SARS-CoV-2 burden; and gene expression signatures correlating with survival that signify immunocompetence and controlled inflammation. In non–COVID-19 cohorts, IR-preserving metrics were associated with resistance to progressive influenza or HIV infection, as well as lower 9-year mortality in the Framingham Heart Study, especially in females.
Conclusions
Preservation of immunocompetence with controlled inflammation during antigenic challenges is a hallmark of IR and associates with longevity and AIDS resistance. Independent of age, a male-biased proclivity to degrade IR before and/or during SARS-CoV-2 infection predisposes to severe COVID-19.
Key words: Aging, AIDS, biomarkers, COVID-19, HIV, immune, inflammation, influenza, SARS-CoV-2
Abbreviations used: CMV, Cytomegalovirus; COVID-19, Coronavirus disease 2019; FHS, Framingham Heart Study; GO-BP, Gene ontology-biological process; HR, Hazard ratio; IC, Immunocompetence; IF, Inflammation; IHG, Immune health grade; IQR, Interquartile range; IR, Immunologic resilience; N, Viral nucleoprotein gene; OR, Odds ratio; RR, Rate ratio; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SLE, Systemic lupus erythematosus
Graphical abstract
Despite significant progress in the identification of host factors that influence coronavirus disease 2019 (COVID-19) outcomes,1, 2, 3, 4, 5, 6, 7 the reasons why persons of similar ages vary in their susceptibility to developing progressive COVID-19, characterized by hospitalization, respiratory failure, and death, are unclear. We sought to identify persons with this susceptibility through the articulation of a parsimonious basis for 3 conundrums. First, although the risk of progressive COVID-19 increases with age, some older persons resist progression, manifesting asymptomatic or mild (ie, nonprogressive) COVID-19,1 , 2 whereas some younger persons manifest progressive COVID-19.3 , 4 Second, females appear to resist progressive COVID-19 more effectively than males.5 , 6 , 8 , 9 This resistance aligns with evidence that females often manifest features of superior immunocompetence (IC) (resistance to some infections, longevity10, 11, 12, 13, 14, 15, 16, 17). Third, clearance rates of nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vary, with delays contributing to progressive COVID-19.18 , 19
We reasoned that a parsimonious basis for these conundrums is that, among persons of similar ages, progressive COVID-19 is ascribable to a deficit in a sexually dimorphic, immunity-optimizing attribute that antedates infection or is induced by SARS-CoV-2 infection. We termed this attribute as immunologic resilience (IR), defined as the capacity to preserve or restore IC and control inflammation (IF) in the face of antigenic challenges experienced throughout life. Thus, we posited that maintenance of a state of higher IC and lower IF (IChigh-IFlow) constitutively and/or during antigenic exposures is the hallmark of IR.
In this context, we hypothesized that individuals of similar ages may differ in their capacity to preserve IR before and during COVID-19, defining 3 major IR-dependent COVID-19 phenotypes (Table I ). In SARS-CoV-2–negative persons, a deficit in IR is characterized by an immunosuppressive, proinflammatory state (IClow-IFhigh) that is more common in males and predisposes to a shorter lifespan, as well as a progressive disease course during viral infections, such as influenza and HIV. If such persons acquire SARS-CoV-2 infection, the preexisting deficit in IR may increase susceptibility to development of progressive COVID-19 as well as amplify SARS-CoV-2–induced deficits in IR (phenotype 3; Table I). In contrast, resistance to developing deficits in IR before and during SARS-CoV-2 infection associates with nonprogressive COVID-19 (phenotype 1; Table I). Thus, persons with the IR-dependent COVID-19 phenotype 3 may manifest 2 distinct IClow-IFhigh states: one antedating infection and one induced by SARS-CoV-2.
Table I.
Projected IR-dependent COVID-19 phenotypes
| Characteristic | Phenotype 1 | Phenotype 2 | Phenotype 3 |
|---|---|---|---|
| Pre–COVID-19 IC-IF state∗ | IChigh-IFlow | IChigh-IFlow | IClow-IFhigh |
| SARS-CoV-2–induced IClow-IFhigh∗ state | Resistance to induction | Susceptibility to induction | Susceptibility to induction |
| Corollaries of pre–COVID-19 IC-IF state† | |||
| Longevity‡ | Survival advantage | Survival advantage | Survival disadvantage |
| Influenza infection severity§ | Asymptomatic or mild | Moderate | Severe |
| AIDS resistance¶ | High | Moderate | Low |
| Corollaries of pre–COVID-19 plus SARS-CoV-2–induced IC-IF states# | |||
| Resistance to progressive COVID-19# | High | Moderate | Low |
| Survival# | Pre–COVID-19 survival advantage preserved | Pre–COVID-19 survival advantage lost | Additive survival disadvantage |
| Sex bias†† | Females | Males | |
IC-IF (immunocompetence-inflammation) states: IChigh-IFlow signifies superior IR; IClow-IFhigh signifies a deficit in IR. In patients with COVID-19, phenotype 1 is defined by a pre–COVID-19 IChigh-IFlow state and resistance to induction of an IClow-IFhigh state in response to SARS-CoV-2–associated antigenic stimulation; phenotype 2 is defined by a pre–COVID-19 IChigh-IFlow state and susceptibility to induction of an IClow-IFhigh state in response to SARS-CoV-2–associated antigenic stimulation, and phenotype 3 is defined by a pre-COVID-19 IClow-IFhigh state and susceptibility to induction of an IClow-IFhigh state in response to SARS-CoV-2–associated antigenic stimulation. Corollaries (longevity, influenza infection severity, and AIDS risk) associated with pre–COVID-19 IC-IF states are contrasted with corollaries of pre–COVID-19 plus SARS-CoV-2–induced IC-IF states.
Evaluated in non-COVID-19 cohorts.
Evaluated in the non–COVID-19 HIV-seronegative, Offspring cohort of the FHS; survival advantage and disadvantage signify lower and higher hazards of mortality over 9 y, respectively.
Evaluated in non-COVID-19 HIV-seronegative, influenza infection challenge and natural influenza infection cohorts.
Evaluated in a non–COVID-19 HIV infection natural history cohort.
Evaluated in a COVID-19 cohort; survival advantage and disadvantage signify lower and higher hazards of mortality, respectively, during COVID-19. Additive survival disadvantage refers to the combined effects of pre–COVID-19 IClow-IFhigh state and the unique infection-induced IClow-IFhigh state induced in patients with COVID-19.
Evaluated in non-COVID-19 cohorts and a COVID-19 cohort.
To test our hypothesis, we developed novel laboratory (lymphocyte equilibrium–based) and transcriptomic (gene expression–based) metrics of IR. We juxtaposed the distributions and associations of IR metrics in a prospective COVID-19 cohort with those in cohorts of SARS-CoV-2–negative persons. This juxtaposition provided empiric evidence in support of 3 IR-dependent COVID-19 phenotypes (Table I). In addition, we evaluated the utility of IR metrics in the early prediction of all-cause 30-day COVID-19 mortality, the primary outcome of this study. We also examined secondary outcomes that track clinical indicators (eg, hospitalization and hospital length of stay) and biomarkers (eg, plasma IL-6 and nasopharyngeal SARS-CoV-2 levels) of progressive COVID-19.
Methods
Study design
Cohort details and analytical plan are available in this article’s Online Repository at www.jacionline.org. A prospective observational cohort of 522 SARS-CoV-2–positive adults was evaluated at the South Texas Veterans Health Care System from March 20, 2020, to October 15, 2020 (cohort features in Fig E1, A, and Table E1 in this article’s Online Repository at www.jacionline.org and Table II ). Hospitalized and nonhospitalized patients were included, and a subset was studied through to the convalescence phase of the infection. The distribution patterns of IR metrics and their associations with outcomes in non–COVID-19 cohorts provided the framework for understanding the contributions of pre–COVID-19 IR status to COVID-19 outcomes. We evaluated previously well-characterized non–COVID-19 cohorts of persons exposed to 4 types of antigenic stimulation: (1) varied causes across lifespan (4 cohorts; total n = 8201), including the community-based SardiNIA aging cohort (n = 389620) and the Offspring cohort of the Framingham Heart Study (FHS; n = 230621); (2) acute influenza infection (influenza virus challenge cohort of younger adults22 and 2 adult cohorts of natural infection23 , 24; total n = 218); (3) self-antigen–associated antigenic stimulation in younger adults with systemic lupus erythematosus (SLE)25 (n = 53); and (4) chronic HIV infection in the adult HIV Natural History Study26, 27, 28, 29, 30 (n = 4883) (see Tables E2 and E3 in this article’s Online Repository at www.jacionline.org). Health care workers at the South Texas Veterans Health Care System with risk of exposure to SARS-CoV-2 were also evaluated (n = 34).
Table II.
COVID-19 cohort characteristics by IHG status
| Characteristic | Overall | IHG-I | IHG-II | IHG-III | IHG-IV | P |
|---|---|---|---|---|---|---|
| n | 522 | 111 | 335 | 9 | 67 | |
| Median age (IQR) (y) | 62 (47-72) | 51 (42-65) | 63 (48-72) | 62 (41-67) | 69 (61-78) | <.001 |
| Age ≥ 60 y, n (%) | 284 (54) | 35 (32) | 192 (57) | 5 (56) | 52 (78) | <.001 |
| Male sex, n (%) | 468 (90) | 92 (83) | 309 (92) | 8 (89) | 59 (88) | .04 |
| Race or ethnic group, n (%)∗ | .26 | |||||
| White | 152 (29) | 43 (39) | 90 (27) | 4 (44) | 15 (22) | |
| Black | 72 (14) | 17 (15) | 45 (13) | 1 (11) | 9 (13) | |
| Hispanic or Latino American | 234 (45) | 36 (32) | 163 (49) | 4 (44) | 31 (46) | |
| Asian | 2 (0) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | |
| Indian or Alaska Native | 4 (1) | 1 (1) | 3 (1) | 0 (0) | 0 (0) | |
| Other | 51 (10) | 13 (12) | 27 (8) | 0 (0) | 11 (16) | |
| Unknown | 7 (1) | 1 (1) | 5 (1) | 0 (0) | 1 (1) | |
| Median BMI (IQR)† | 30 (27-34) | 31 (28-35) | 30 (27-34) | 29 (28-32) | 28 (25-32) | .02 |
| BMI ≥ 30, n (%) | 281 (56) | 70 (66) | 178 (54) | 4 (50) | 29 (45) | .04 |
| Median time from symptom onset (IQR) (d) | 6 (2-9) | 6 (2-17) | 6 (3-9) | 5 (2-7) | 5 (2-8) | .26 |
| Comorbidities, n (%) | ||||||
| Asthma | 28 (5) | 10 (9) | 17 (5) | 0 (0) | 1 (1) | .18 |
| Bronchiectasis | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | >.99 |
| Chronic obstructive pulmonary disease | 40 (8) | 4 (4) | 26 (8) | 1 (11) | 9 (13) | .09 |
| Interstitial lung disease | 9 (2) | 1 (1) | 3 (1) | 0 (0) | 5 (7) | .01 |
| Liver disease or cirrhosis | 38 (7) | 5 (5) | 25 (7) | 0 (0) | 8 (12) | .32 |
| Diabetes | 184 (35) | 33 (30) | 122 (36) | 3 (33) | 26 (39) | .59 |
| Chronic kidney disease | 110 (21) | 13 (12) | 70 (21) | 1 (11) | 26 (39) | <.001 |
| End-stage renal disease‡ | 8 (2) | 0 (0) | 4 (1) | 0 (0) | 4 (6) | .03 |
| Chronic heart failure | 45 (9) | 3 (3) | 33 (10) | 1 (11) | 8 (12) | .04 |
| Hypertension | 268 (51) | 46 (42) | 180 (54) | 3 (33) | 39 (58) | .06 |
| Coronary artery disease | 64 (12) | 8 (7) | 45 (13) | 1 (11) | 10 (15) | .27 |
| Connective tissue disease | 13 (2) | 3 (3) | 6 (2) | 0 (0) | 4 (6) | .26 |
| Myocardial infarction | 4 (1) | 0 (0) | 3 (1) | 1 (11) | 0 (0) | .09 |
| Atrial fibrillation | 37 (7) | 7 (6) | 24 (7) | 0 (0) | 6 (9) | .91 |
| Peripheral vascular disease | 30 (6) | 4 (4) | 18 (5) | 0 (0) | 8 (12) | .15 |
| Cerebrovascular accident | 33 (6) | 5 (5) | 18 (5) | 1 (11) | 9 (13) | .06 |
| Active, lymphoma | 3 (1) | 0 (0) | 1 (0) | 0 (0) | 2 (3) | .12 |
| Active, leukemia | 2 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (1) | .31 |
| Active, solid tumor | 11 (2) | 1 (1) | 5 (1) | 0 (0) | 5 (7) | .03 |
| History of cancer | 34 (7) | 7 (6) | 14 (4) | 2 (22) | 11 (16) | .001 |
| HIV | 9 (2) | 1 (1) | 7 (2) | 0 (0) | 1 (1) | .90 |
| Dementia | 43 (8) | 5 (5) | 25 (7) | 0 (0) | 13 (19) | .009 |
| Asplenia | 2 (0) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | .09 |
| Smoking status | .006 | |||||
| Current smoker | 45 (10) | 17 (20) | 24 (8) | 2 (33) | 2 (3) | |
| Former smoker | 82 (19) | 12 (14) | 55 (19) | 0 (0) | 15 (24) | |
| Clinical ordinal scale, n (%)§ | <.001 | |||||
| 1 | 29 (6) | 19 (17) | 8 (2) | 2 (22) | 0 (0) | |
| 2 | 137 (26) | 53 (48) | 70 (21) | 4 (44) | 10 (15) | |
| 3 | 5 (1) | 3 (3) | 2 (1) | 0 (0) | 0 (0) | |
| 4 | 225 (43) | 31 (28) | 162 (48) | 3 (33) | 29 (43) | |
| 5 | 94 (18) | 4 (4) | 68 (20) | 0 (0) | 22 (33) | |
| 6 | 7 (1) | 0 (0) | 4 (1) | 0 (0) | 3 (4) | |
| 7 | 24 (5) | 1 (1) | 20 (6) | 0 (0) | 3 (4) | |
| 8 | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | |
| CMV seropositivity, n (%) | 231 (47) | 52 (51) | 123 (39) | 7 (88) | 49 (74) | <.001 |
| Initial setting, n (%) | <.001 | |||||
| Nonhospitalized | 173 (33) | 76 (68) | 79 (24) | 7 (78) | 11 (16) | |
| Hospitalized | 349 (67) | 35 (32) | 256 (76) | 2 (22) | 56 (84) |
BMI, Body mass index.
Race or ethnicity and sex were as recorded in electronic medical record.
BMI is the weight (kilograms) divided by the square of the height (meters).
End-stage renal disease was defined as requiring hemodialysis.
Scores on the clinical ordinal scale are as follows: 1, not hospitalized, no limitations of activities; 2, not hospitalized, limitation of activities, home oxygen requirement, or both; 3, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; 4, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (COVID-19–related or other medical conditions); 5, hospitalized, requiring any supplemental oxygen; 6, hospitalized, receiving noninvasive ventilation or use of high-flow oxygen devices; 7, hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation; and 8, death.
The study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for observational studies (see Strengthening the Reporting of Observational Studies in Epidemiology table in this article’s Online Repository at www.jacionline.org).31 This study was approved by the University of Texas Health Science Center at San Antonio, Texas, Institutional Review Board. All authors vouch for the completeness of the data and adherence to the analytic plan articulated a priori (see this article’s Methods section in the Online Repository at www.jacionline.org; Fig E1, B).
Procedures
Clinical and laboratory assessments (eg, scores of a clinical ordinal scale,32 CD4+ and CD8+ T-cell counts, and plasma IL-6 levels) were recorded (see this article’s Methods section in the Online Repository). These assessments were evaluated at baseline (presentation to the hospital or home visit) as well as prospectively (daily) during hospitalization and at least once in the convalescence phase. Cytomegalovirus (CMV) serostatus was evaluated at baseline. RNA from nasal cells collected from hospitalized patients was sequenced (RNA-Seq) to quantify SARS-CoV-2 levels as well as identify human genes whose expression levels were associated with SARS-CoV-2 levels and survival (see this article’s Methods section in the Online Repository). RNA from peripheral blood cells was also sequenced.
Definitions and outcomes
Progressive COVID-19 was defined as progression along the continuum from hospitalization to need for any respiratory support and/or death. The primary outcome was all-cause 30-day COVID-19 mortality. Secondary outcomes included hospitalization, requirement of any respiratory support or mechanical ventilation; progression quantified by an increase in an 8-category clinical ordinal scale (see this article’s Methods section in the Online Repository); hospital length of stay (recovery rate); all-cause 120-day mortality; plasma IL-6 levels (biomarker of IF and mortality33, 34, 35, 36, 37); and nasal SARS-CoV-2 clearance, defined as time to achievement of fewer than 10 counts of the viral nucleoprotein (N) gene. Clinical indicators of nonprogressive versus progressive COVID-19 disease course were nonhospitalized versus hospitalized patients, as well as survivors versus nonsurvivors within 120 days of presentation. Prespecified outcomes in the non–COVID-19 cohorts were all-cause age-adjusted mortality over 9 years in the FHS, severity of influenza infection, and hazard of AIDS. A lower hazard of mortality in the COVID-19 cohort or the FHS was defined as a survival advantage.
Measurements/predictors: Metrics of IR
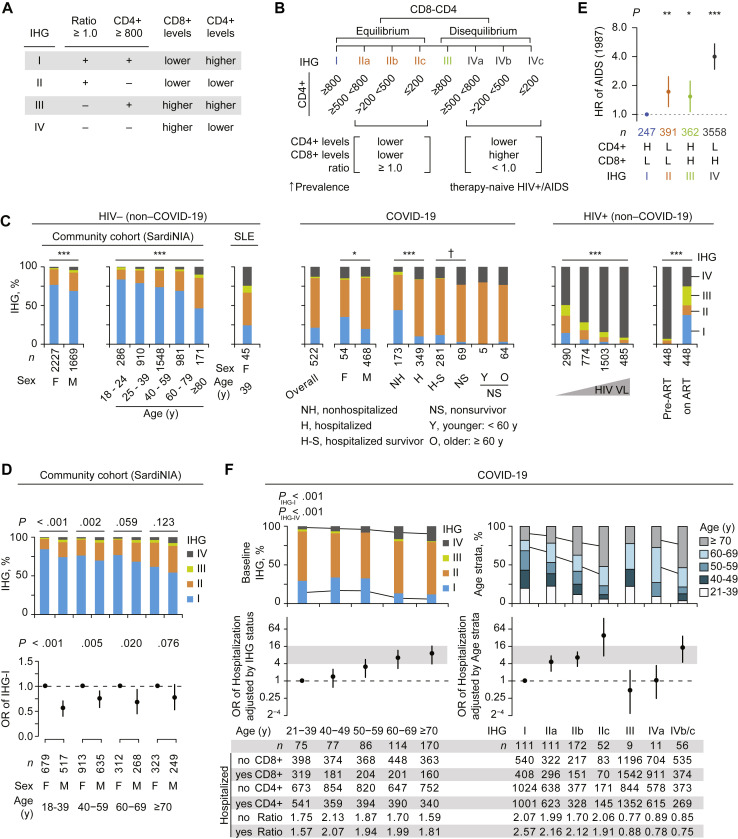
To capture the cellular and molecular events that may associate with IR status, we derived laboratory and transcriptomic metrics of IR, which served as predictors in this study. Laboratory metrics of IR, termed immune health grades (IHGs), were derived on the principles that (1) IR tracks superior IC, (2) peripheral blood CD8+ and CD4+ T-cell levels influence immunity, and (3) CD4+ lymphopenia is a characteristic of aging, COVID-19, SLE, and HIV infection.38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 We posited that the level of equilibrium versus disequilibrium between peripheral blood CD8+ and CD4+ T-cells, rather than their absolute values, may be more precise metrics of IC. Four IHGs (I-IV) tracking the equilibrium between CD8+ and CD4+ T-cell counts were computed (Fig 1 , A; Fig E1, C). Laboratory cutoffs were the lower interquartile bounds of the median CD4+ count in otherwise-healthy HIV-seronegative persons (800 cells/mm3)26 , 27 and an inverted CD4:CD8 T-cell ratio (<1.0) (Fig 1, A). The CD4+ cutoff was used as greater than or equal to 800 cells/mm3 associated with near-normalized immune status in HIV+ persons.27 An inverted ratio was used as a cutoff because it is a mathematical reflection of expanded CD8+ T-cell levels uncompensated by a concomitant increase in CD4+ T-cell levels. Thus, an inverted ratio marks CD8-CD4 disequilibrium, signifying higher CD8+ levels with relatively higher (IHG-III) or lower (IHG-IV) CD4+ counts (Fig 1, A and B). To metricize CD4+ T-cell levels during SARS-CoV-2 versus HIV infections, we derived subgrades (suffixes a, b, and c) of IHG-II and IHG-IV using CD4+ cutoffs demarcating AIDS (≤200 cells/mm3)50 and the median CD4+ count during primary/early HIV infection (500 cells/mm3).26 , 27 Although the CD8-CD4 profiles of IHG-IIc and IHG-IVc mark profound CD4+ lymphopenia (≤200 cells/mm3), IHG-IVc defines the profile of AIDS (Fig 1, B).
Fig 1.
IHG associations in study cohorts. A and B, IHG features. Ratio, CD4:CD8 ratio. C,Left: IHG prevalence in SardiNIA cohort by sex and age strata and females with SLE. Middle: IHG at presentation in the COVID-19 cohort by sex, hospitalization and survivor status, and nonsurvivors by age. Right: IHG in therapy-naive HIV+ participants (with known dates of seroconversion) at entry into the Natural History Study by entry viral load (VL; <1K, 1-10K, 10-100K, ≥100K copies/mL; K, ×103). Rightmost: IHGs before and after at least 5 years of antiretroviral therapy (ART). D, IHG prevalence (top) and OR with 95% CI for prevalence of IHG-I (bottom) by sex within age strata. E, HRs with 95% CI of developing AIDS (1987 criteria51) in HIV+ participants by entry IHG. H, High; L, low. F,Top-left: IHG prevalence by age strata. PIHG-I and PIHG-IV is for the proportion of IHG-I vs rest and IHG-IV vs rest across age strata. Top-right: Age strata distribution by IHG subgrades. Middle-left and right: OR with 95% CI for hospitalization. OR by age strata (left) adjusted by baseline IHG status and OR by baseline IHG status (right) adjusted by age strata. Bottom: Median values of CD4+ and CD8+ counts and CD4:CD8 ratio by hospitalization status (yes/no) and (left) age strata and (right) IHG subgrades. F, Female; M, male. †P = .06. ∗P < .05. ∗∗P < .01. ∗∗∗P < .001.
RNA obtained from peripheral blood samples was sequenced and transcriptomic metrics of IR comprised gene expression signatures associated with mortality hazards during COVID-19 (Fig E1, B; see this article’s Methods section in the Online Repository); bioinformatic approaches were similar to our previous studies.52 , 53 Genes with significantly higher expression levels in study groups signifying nonprogressive COVID-19 were defined as beneficial, whereas genes with higher levels in study groups signifying progressive COVID-19 were defined as detrimental. Significantly enriched gene ontology biologic process (GO-BP) terms were derived on the basis of differentially expressed genes. Correspondingly, the GO-BP terms were defined as beneficial and detrimental traits. Gene scores were computed by averaging the z-score–normalized expression of all genes comprising the GO-BP term. Gene scores of the traits (GO-BP terms) that associated with 30-day mortality hazards during COVID-19 were examined for their associations with mortality in the FHS. Traits that associated with mortality in the COVID-19 cohort and FHS were examined for their associations with influenza infection severity.
Statistical analyses
On the basis of chi-square distribution, 522 patients provided 92% power to detect a moderate effect size (w = 0.17) based on the incidence of death within 30 days of presentation with baseline IHG status at a significance level of .05. The statistical tests used for each panel in the figures are detailed in this article’s Online Repository at www.jacionline.org. Kaplan-Meier survival curves were derived to illustrate cumulative events. Cox proportional hazards models were used to calculate hazard ratios (HRs) for mortality in the COVID-19 cohort and the FHS, and rate ratios (RRs) for recovery rates (hospital length of stay) as well as nasal SARS-CoV-2 clearance in the COVID-19 cohort. Multivariable logistic and linear regression models were used to compare secondary outcomes. RNA-sequencing analysis was performed using DESeq.54 All P values are 2-sided and shown without adjustment for multiple testing unless specified. All analyses were performed using R, v3.5.0.
Results
IHG distributions and associations in non–COVID-19 cohorts
Based on median CD8+ and CD4+ counts, the IHGs tracked distinct CD8-CD4 equilibrium/disequilibrium profiles: IHG-I tracked lower CD8-higher CD4, IHG-II tracked lowest CD8-lower CD4, IHG-III tracked highest CD8-higher CD4, and IHG-IV tracked higher CD8-lowest CD4 (Fig E1, D). In a general population cohort (SardiNIA), across all age ranges, IHG-I was the most common grade and IHG-I was uniformly underrepresented in males (Fig 1, C and D). The overall prevalence of IHG-I in the SardiNIA cohort was 73%. IHG-I frequency declined progressively with age (84% and 46% in 18-24 and ≥80 years age strata, respectively). Reciprocally, the prevalence of the other IHGs, especially IHG-II and IHG-IV, increased substantially with age (Fig 1, C, left). The IHG distribution patterns in younger women with SLE mirrored those of older persons (≥80 years) in the SardiNIA cohort. In therapy-naive HIV-seropositive adults, higher plasma HIV viral loads induced IHG-IV (Fig 1, C, right). The hierarchy of the hazard of developing AIDS according to the IHG at presentation was IHG-IV > IHG-III ∼ IHG-II > IHG-I, despite IHG-I and IHG-III marking higher CD4+ counts and IHG-II and IHG-IV marking lower CD4+ counts (Fig 1, E; Fig E1, E). Initiation of antiretroviral therapy was associated with reconstitution of IHG-I (Fig 1, C, far-right).
Thus, the juxtaposition of the IHG distributions from 3 non–COVID-19 cohorts suggested that IHG-I is the primordial IHG from which the other IHGs emerged in settings of increased antigenic stimulation (eg, SLE, HIV, and aging). Correspondingly, in HIV-seropositive persons, preservation of IHG-I was an indicator of superior IR (ie, resistance to AIDS progression). Our finding that mitigation of antigenic stimulation was associated with reconstitution of IHG-I (Fig 1, C, far-right) corroborated that degradation of IHG-I was related to increased antigenic stimulation rather than chronologic age per se. Instead, age may serve as an imperfect proxy for cumulative antigenic experience throughout life. This framework was used to examine whether (1) SARS-CoV-2 infection–associated antigenic stimulation induced similar shifts in IHG distributions across age ranges, and (2) mitigation of SARS-CoV-2 infection–associated antigenic stimulation was associated with reconstitution of IHGs to levels observed in age-matched persons in the SardiNIA cohort.
IHG distribution and associations in COVID-19
The COVID-19 cohort comprised 522 patients, mostly males (n = 468 [90%]) with a median age of 62 (interquartile range [IQR], 47-72) years; 349 (67%) were hospitalized and 173 (33%) were nonhospitalized (Table II; Fig E1, A, and Table E1, A). A total of 224 (43%) patients required respiratory support, including 59 (11%) with mechanical ventilation (Fig E1, A, and Table E1, B). Forty-eight (9%) and 69 (13%) patients died within 30 and 120 days of presentation, respectively. The median (IQR) time to death within 30 and 120 days of presentation was 13 (6-17) and 17 (11-36) days, respectively (Table E1, B).
Cohort characteristics by baseline IHG status are described (Table II). Overall, 21%, 64%, 2%, and 13% individuals presented with IHG-I, IHG-II, IHG-III, and IHG-IV, respectively (n = 111, 335, 9, and 67, respectively; Fig 1, C, middle). The median time from symptom onset to presentation (baseline) was 6 (IQR, 2-9) days and did not differ significantly by baseline IHG (P = .26). Individuals presenting with IHG-I tended to be younger with fewer comorbidities. Individuals presenting with IHG-IV versus IHG-I were older (median age [IQR], 69 [61-78] vs 51 [42-65] years, respectively; P < .001), and a greater proportion had comorbidities, including chronic kidney disease and dementia (Table II; Table E1, D). No persons were receiving COVID-19–directed therapies at baseline; significantly more patients presenting with IHG-II and IHG-IV versus IHG-I subsequently received COVID-19–directed therapies (eg, remdesivir and corticosteroids; 41% vs 9%, P < .001; Table E1, E).
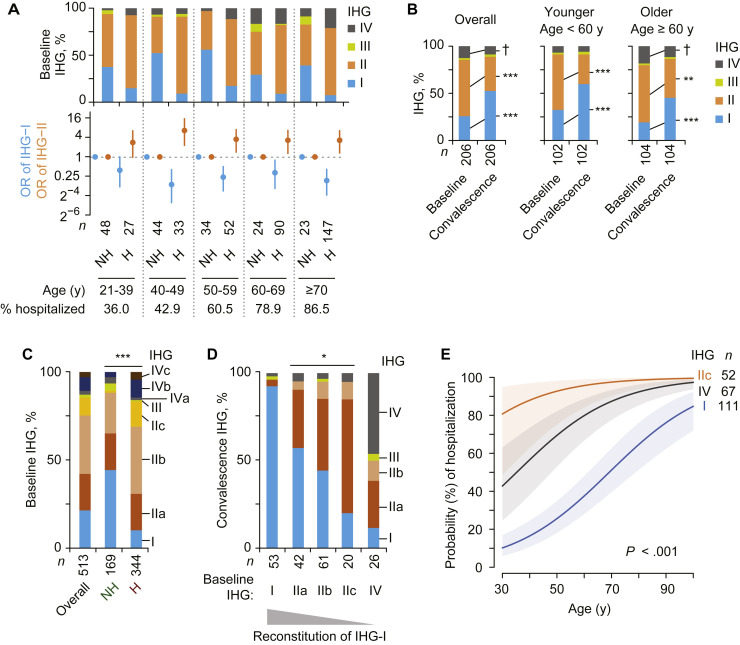
Akin to the SardiNIA cohort, IHG-I was overrepresented in females versus males in the COVID-19 cohort (35% vs 20%; P = .01; Fig 1, C). Conversely, a lower proportion of females versus males presented with IHG-II (48% vs 66%; P = .01; Fig 1, C). As in the SardiNIA cohort (Fig 1, C, left), IHG-I frequency declined and IHG-II and IHG-IV frequencies increased with age in the COVID-19 cohort (Fig 1, F, upper-left stacked bars). However, at both the overall cohort level and within comparable age ranges, IHG-I was underrepresented, whereas IHG-II and IHG-IV were overrepresented in the COVID-19 versus SardiNIA cohort (Fig 1, C; Fig 1, F, upper-left stacked bars). Overall cohort-level differences in proportions were 21% versus 73% for IHG-I, 64% versus 22% for IHG-II, and 13% versus 3% for IHG-IV (P < .001 for COVID-19 vs SardiNIA intercohort differences). In addition, although hospitalization rates increased with age, within each age stratum, hospitalized patients were more likely to have IHG-II and IHG-IV at presentation (Fig 2 , A). Conversely, nonhospitalized patients were more likely to have IHG-I (Fig 2, A). IHG distributions were similar in younger and older patients who required respiratory support and/or died (Fig 1, C; Fig E1, F).
Fig 2.
Association of IHGs at presentation (baseline) with hospitalization status and reconstitution of IHG during convalescence in the COVID-19 cohort. A,Top: IHG prevalence at baseline by hospitalized (H) and nonhospitalized (NH) status and age strata. Bottom: OR with 95% CI for having IHG-I or IHG-II at baseline by hospitalization status. Percent hospitalized shown by age strata. B, Paired IHG distributions at baseline and during convalescence among 206 COVID-19 survivors overall and stratified by age. C, Distribution of IHG subgrades (as in Fig 1, B) in HIV-seronegative persons in the COVID-19 cohort categorized as hospitalized (H) and nonhospitalized (NH). D, Distribution of IHGs reconstituted during convalescence by baseline IHG status. E, The probability (with 95% confidence bands) of hospitalization according to age and baseline IHG. Overall IHG-IV was used in this model because all patients with IHG-IVc were hospitalized and numbers of patients in the other IHG-IV subgrades were small. †P = .07. ∗P < .05. ∗∗P < .01. ∗∗∗P < .001.
The underrepresentation of IHG-I versus overrepresentation of IHG-II and IHG-IV was attributable to SARS-CoV-2 infection because, during convalescence, younger and older COVID-19 survivors reconstituted IHG frequencies to levels found in their corresponding age groups in non–COVID-19 cohorts (SardiNIA cohort and health care workers; Fig 2, B, vs Fig 1, C; see Fig E2, A, in this article’s Online Repository at www.jacionline.org). The IHG reconstitution patterns assigned patients to the IR-dependent COVID-19 phenotypes (Table I). Phenotype 1 corresponded to preservation of superior IR (IHG-I) before and during early COVID-19. Phenotype 2 corresponded to presentation with a deficit in IR (IHG-II or IHG-IV) but reconstitution of IHG-I (reflecting a diathesis to degrade IHG-I during COVID-19). Phenotype 3 corresponded to presentation with and maintenance of IHG-II or IHG-IV during convalescence, suggesting that these grades may have antedated COVID-19.
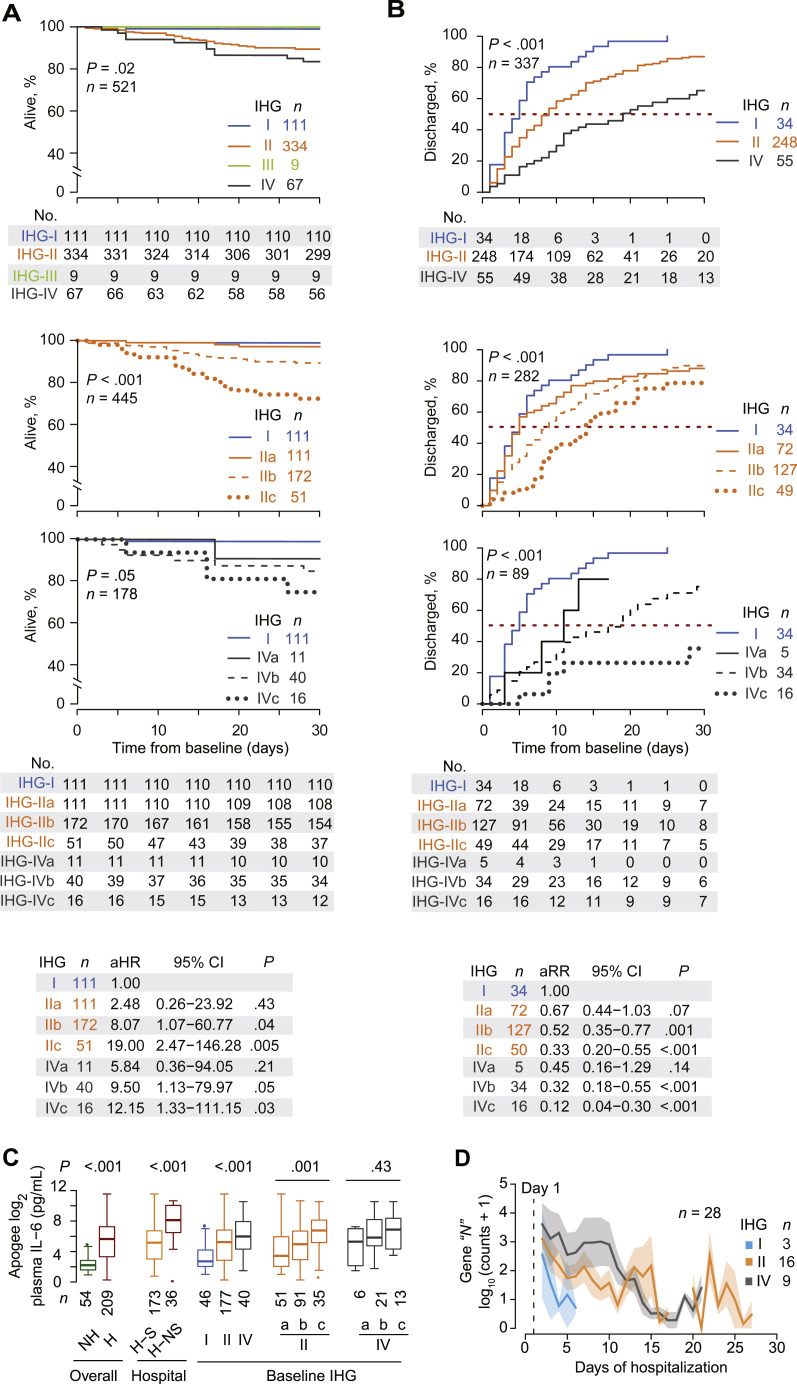
The 30-day mortality was lowest among patients with IHG-I compared with IHG-IV and IHG-II (1%, 16%, and 11%, respectively, P < .001; Table E1, B; Fig E2, B). Each 1-year increase in age was independently associated with mortality (IHG-adjusted HR, 1.05; 95% CI, 1.03-1.08), as well as likelihood of hospitalization (IHG-adjusted odds ratio [OR], 1.06; 95% CI, 1.01-1.07) and need for respiratory support (IHG-adjusted OR, 1.04; 95% CI, 1.02-1.05). An independent association of sex with these outcomes was not observed (data not shown). After adjusting for age and sex, presentation with IHG-I versus other grades was associated with a lower HR for 30-day mortality (adjusted HR, 0.12; 95% CI, 0.02-0.87) and 120-day mortality (adjusted HR, 0.17; 95% CI, 0.04-0.69) as well as a lower OR for hospitalization (adjusted OR, 0.16; 95% CI, 0.10-0.26) and mechanical ventilation (adjusted OR, 0.06; 95% CI, 0.01-0.45) and a more rapid recovery rate (adjusted RR, 2.08; 95% CI, 1.40-3.07) (Fig E2, B).
Compared with presentation with IHG-I, the age-adjusted HR for 30-day mortality in patients presenting with IHG-II or IHG-IV was 8.05 (95% CI, 1.10-59.09) and 9.08 (95% CI, 1.16-71.36), respectively. Despite similar mortality HRs, IHG-II and IHG-IV were tracking individuals with distinct features and outcomes. A greater proportion of individuals presenting with IHG-IV versus IHG-II were older (≥60 years; 78% vs 57%; P = .002), progressed according to the clinical ordinal scale (61% vs 41%; P = .003, Table E1, B), and placed on mechanical ventilation (24% vs 13%; P = .02). For these reasons, and because IHG-IV is the hallmark CD8-CD4 profile of therapy-naive HIV-seropositive persons (Fig 1, B and C), we examined the differences in the origins and associations of IHG-II and IHG-IV subgrades (Fig 1, B).
Most (98% [513 of 522]) patients in the COVID-19 cohort were HIV-seronegative; of these, 13% (n = 67) presented with IHG-IIc or IHG-IVc. A greater proportion of hospitalized versus nonhospitalized HIV-seronegative patients presented with IHG-IIc (15% vs 1%, respectively; P < .001) or IHG-IVc (4% vs 0%, respectively; P = .004; Fig 2, C). During convalescence, no patient had IHG-IIc or IHG-IVc and few patients manifested IHG-IIb (6%) and IHG-IVb (4%) (Fig 2, D; Fig E2, C). This finding suggests that these subgrades were induced during COVID-19. In addition, there was high correspondence between the IR level (IHG status) reconstituted during convalescence and the IR level during acute COVID-19 (Fig 2, D). Nearly all (92%) patients who presented with IHG-I preserved IHG-I during convalescence. The proportion reconstituting IHG-I was progressively lower in individuals presenting with IHG-IIa, IHG-IIb, and IHG-IIc (57%, 44%, and 20%, respectively). Meanwhile, 46% of the patients who presented with IHG-IV persisted with IHG-IV during convalescence (Fig 2, D). These paired baseline-convalescence IHG distribution patterns indirectly indicate the possibility that pre–COVID-19 IHG status impacts IHG status at presentation during acute COVID-19.
The age-adjusted OR for hospitalization associated with IHG-II subgrades, and IHG-IVb/c versus IHG-I at baseline were comparable to or greater than the IHG-adjusted OR of hospitalization associated with older age (Fig 1, F; Fig E2, D). Although the probability of presenting with IHG-I declined with age (Fig E2, E), the modeled probability of hospitalization for a 30-year-old patient presenting with IHG-IIc (80%; 95% CI, 48%-95%) was similar to that modeled for a 95-year-old patient with IHG-I (81%; 95% CI, 67%-90%; Fig 2, E). Presentation with IHG-IV showed a similar increased probability of hospitalization but lower than that associated with IHG-IIc (Fig 2, E). The age-independent prognostication of hospitalization by baseline IHG status was attributable to our finding that, unlike aggregated lymphocyte values by age, lymphocyte values by IHG status are range-bound across age (Fig 1, F). Hence, IHGs provide a uniform metric of IR across age.
The age-adjusted hazard of 30-day mortality was increased by a factor of 19.00 (95% CI, 2.47-146.28) with baseline IHG-IIc and 12.15 (95% CI, 1.33-111.15) with baseline IHG-IVc compared with baseline IHG-I (Fig 3 , A; see Fig E3, A, in this article’s Online Repository at www.jacionline.org). Correspondingly, 30-day mortality rates were highest among persons presenting with IHG-IIc [0.29 (n = 15 deaths out of 52 patients), 95% CI, 0.17-0.43] and IHG-IVc [0.25 (4 deaths out of 16 patients); 95% CI, 0.07-0.52] yielding absolute risk differences in mortality of 0.28 (0.16-0.40) and 0.24 (0.03-0.45), respectively. In persons who presented with IHG-I, the risk difference was 0.01 (n = 1 death out of 111 patients with IHG-I; 95% CI, 0.00-0.05).
Fig 3.
COVID-19 outcomes by baseline IHG status. A,Top 3 plots: Kaplan-Meier curves for 30-day all-cause mortality from days since presentation by baseline IHG status (top), IHG-I and IHG-II subgrades (middle), and IHG-I and IHG-IV subgrades (bottom). Middle: Number at risk (No.) indicates patients who had not died or been censored before that time point. Bottom: Age-adjusted HRs (aHRs) with 95% CIs (reference: IHG-I). B,Top 3 plots: Cumulative hospital discharge estimates within 30 days of presentation among hospitalized patients by baseline IHG status (top), IHG-I and IHG-II subgrades (middle), and IHG-I and IHG-IV subgrades (bottom). Middle: Number at risk (No.) indicates patients who had not been discharged or been censored before that time point. Bottom: Age-adjusted RRs (aRRs) with 95% CIs (reference: IHG-I). C, Peak plasma IL-6 levels by hospitalization status, survivorship status in hospitalized patients, and baseline IHG status. D, Levels of SARS-CoV-2 viral transcript burden proxied by log10-normalized counts of SARS-CoV-2 nucleoprotein “N” gene sequence in nasal transcriptomes of hospitalized patients by baseline IHG status, depicted as mean ± SEM (bands). H, Hospitalized; H-S, hospitalized survivor; H-NS, hospitalized nonsurvivor; NH, nonhospitalized; SEM, standard error of mean.
Compared with baseline IHG-I, recovery rates were slowest with IHG-IVc (adjusted RR, 0.12; 95% CI, 0.04-0.30) followed by IHG-IIc (adjusted RR, 0.33; 95% CI, 0.20-0.55; Fig 3, B; Fig E3, B). In addition, the likelihood of (1) hospitalization, (2) manifesting as an increase in the clinical ordinal scale, (3) need for any respiratory support, and (4) dying within 30 or 120 days of presentation, was lowest to highest in patients presenting with IHG-IVa followed by IHG-IVb, IHG-IIb, IHG-IVc, and IHG-IIc (see Fig E3, C, and Fig E4, A-C, in this article’s Online Repository at www.jacionline.org; Table E1, B and G). The strength of the associations between baseline IHG status and these outcomes was comparable to or greater than those of older age and was independent of age and body mass index (see Online Repository Notes 1 and 2 and Table E1, F and G, in this article’s Online Repository at www.jacionline.org).
Correspondingly, although peak IL-6 levels during the disease course were higher in hospitalized patients and nonsurvivors, IL-6 levels were highest with baseline IHG-IIb/c and IHG-IVb/c (Fig 3, C; see Online Repository Note 3 in this article’s Online Repository at www.jacionline.org). In addition, daily monitoring of IHGs revealed varied IHG reconstitution patterns, and improvements in IHG status presaged declines in plasma IL-6 levels during recovery (see Figs E5 and E6 and Online Repository Note 4 in this article’s Online Repository at www.jacionline.org).
Seropositivity for CMV has been associated with the immunosenescence of age as well as changes in CD4+ and CD8+ lymphocyte levels (eg, the immune risk phenotype that tracks an inverted ratio and CMV seropositivity).55, 56, 57, 58 Because of these associations, we examined whether the associations of IHGs with outcomes were attributable to CMV seropositivity. Predictably, CMV seropositivity rates increased with age in the COVID-19 cohort; however, CMV serostatus did not independently associate with the outcomes of mortality, hospitalization, or respiratory support (see Online Repository Note 5 in this article’s Online Repository at www.jacionline.org). In contrast, baseline IHGs were associated with these outcomes independent of CMV serostatus (Online Repository Note 5). This independent association may relate to our finding that CMV seropositivity was differentially distributed between IHG-II and IHG-IV (Online Repository Note 5). A greater proportion of individuals presenting with IHG-II (61%) were CMV-seronegative, whereas a greater proportion of persons presenting with IHG-IV (74%) were CMV-seropositive, and nearly 50% with IHG-I were CMV-seropositive (Table II).
Baseline IHG, nasal SARS-CoV-2 levels, and local host response
Baseline IHG-I, IHG-II, and IHG-IV were associated with relatively rapid, intermediate, and delayed clearance, respectively, of nasal SARS-CoV-2 levels (Fig 3, D; see Fig E7, A, and Table E4 in this article’s Online Repository at www.jacionline.org). Compared with baseline IHG-I, SARS-CoV-2 clearance rates were significantly slower by 87% (RR, 0.13; 95% CI, 0.02-0.82) with IHG-IV and trended to be slower by 72% (RR, 0.28; 95% CI, 0.05-1.44) with IHG-II (Fig E7, B). Correspondingly, SARS-CoV-2 levels were higher in nonsurvivors and patients requiring mechanical ventilation (Fig E7, C). Genes in the nasal transcriptome whose expression levels were associated with both lower SARS-CoV-2 levels and increased survival related to preservation of epithelial integrity as well as robust antiviral immune responses and controlled inflammatory responses (see Fig E8, Tables E5, A-C, and E6, A, and Online Repository Note 6 in this article’s Online Repository at www.jacionline.org). Genes associated with higher SARS-CoV-2 levels also correlated with higher levels of ACE2, the SARS-CoV-2 receptor59; these genes related to virus-induced inflammation (see Fig E8, B, in this article’s Online Repository at www.jacionline.org; Table E5, D).
Unique and shared traits influencing mortality
Comparisons of study groups signifying nonprogressive and progressive COVID-19 identified 42 detrimental and 9 beneficial traits (GO-BP terms) and 28 beneficial genes (see Fig E8, A, and Tables E6, B, and E7 in this article’s Online Repository at www.jacionline.org). These traits/genes segregated into 4 categories, depending on whether higher levels of detrimental traits and lower levels of the beneficial traits/genes associated with increased mortality hazards in either, both, or neither the COVID-19 cohort and the FHS (Fig 4 , A; see Fig E9 and Table E8, A and B, in this article’s Online Repository at www.jacionline.org). Category 1 comprised 17 detrimental and 6 beneficial traits, along with 28 beneficial genes whose levels defined a unique IClow-IFhigh state associated with increased mortality hazards only in the COVID-19 cohort (Table E8, A and B). The unique IClow state related to lower levels of the beneficial traits/genes linked to MHC class II–dependent antigen processing/presentation, the tyrosine signaling pathway, and HLA genes involved in T-cell costimulation (eg, GO-BP terms 28, 29, and 33, respectively; Fig 4, A). The unique IFhigh state related to higher levels of the detrimental traits linked to the inflammatory response to virus (eg, GO-BP terms 1, 5, and 15 corresponding to type I interferon signaling pathway, response to virus, and IFN-γ–mediating signaling pathway, respectively; Fig 4, A).
Fig 4.
Beneficial and detrimental traits in the COVID-19 cohort and the FHS. A,Center: HRs (95% CI) of mortality in the COVID-19 cohort (30-day) and the FHS (survival over 9 years adjusted by age as a continuous variable). HRs are depicted for 34 of the 52 traits identified in baseline peripheral blood transcriptome from 48 patients that were significantly associated with progressive COVID-19. GO-BP terms (left) were classified as detrimental (#1-26) or beneficial (#27-34) traits, and representative genes (right) in each trait are shown. The association of these traits was analyzed in the FHS for 9-year survival. Forest plots are categorized and color-coded to represent shared and unique significant associations (FDR < 0.1) in these 2 cohorts. Fig E9 and Table E8 depict the entire list of traits. B, Gene expression scores for a representative detrimental and beneficial trait in baseline peripheral blood transcriptomes (n = 48) by hospitalization and survivorship status (NH-S, nonhospitalized survivors; H-S, hospitalized survivors; NS, nonsurvivors), age strata, and baseline IHG status. FDR, False-discovery rate.
Category 2 comprised 12 detrimental and 3 beneficial traits whose levels defined a shared IClow-IFhigh state associated with mortality hazards in both the COVID-19 cohort and the FHS (Table E8, C). The shared IClow state related to lower levels of shared beneficial traits linked to T-cell costimulation, T-cell signaling, and immune responses with genes (eg, CCR5, CCR7, and IL7R) essential for the integrity of T-cell homeostasis (eg, GO-BP terms 30, 31, and 32). The shared IFhigh state related to higher levels of shared detrimental traits related to inflammatory responses commonly elicited during antigenic challenges experienced during aging (eg, GO-BP terms 4 and 13 corresponding to defense response to gram-positive bacteria and response to LPS, respectively; Fig 4, A; see Fig E10 in this article’s Online Repository at www.jacionline.org; Table E8, C). Category 3 comprised 8 detrimental traits associated with an increased mortality hazard that achieved statistical significance only in the FHS (eg, GO-BP terms 24 and 25; Fig 4, A; Fig E8, A). Category 4 comprised 5 detrimental traits that did not achieve statistical significance for mortality hazards in either the COVID-19 cohort or the FHS (Fig E9).
The unique and shared IClow-IFhigh states were associated with nonsurvivors, patients requiring mechanical ventilation, older individuals, and those presenting with IHG-II or IHG-IV (Fig 4, B; see Fig E11 and Table E9, A, in this article’s Online Repository at www.jacionline.org,). Similar associations were observed with trait levels measured during the disease course (see Fig E12 in this article’s Online Repository at www.jacionline.org; Table E9, B and C). In addition, baseline levels of 48 of the 51 traits and the 28 beneficial genes were associated with baseline IHG status after controlling for age (Table E9, D).
Our discovery of a shared IChigh-IFlow state that was associated with a survival advantage in both the COVID-19 cohort and the FHS was congruent with 4 additional salutary associations in non–COVID-19 cohorts. First, akin to our findings in the COVID-19 cohort (Fig 4, B; Fig E11), in non–COVID-19 cohorts (see Fig E13 in this article’s Online Repository at www.jacionline.org), the shared IChigh-IFlow state that was associated with COVID-19 survival marked individuals with IHG-I, whereas the shared IClow-IFhigh state that was associated with COVID-19 mortality marked persons with IHG-IIc and IHG-IVc. Second, the shared IChigh-IFlow state was overrepresented in females versus males across age in the FHS (see Fig E14, A, in this article’s Online Repository at www.jacionline.org). In both sexes, levels of the shared IChigh-IFlow state declined proportionately during aging; however, comparable levels of this state were associated with a greater survival benefit in females (Fig E14, B; Table E8, D). Third, beneficial traits that defined the shared IChigh state coclustered at high correlation levels with a gene signature (termed IMM-AGE [immune age])38 associated with lower hazards of cardiovascular disease and mortality in the FHS (see Fig E15 in this article’s Online Repository at www.jacionline.org). Correspondingly, higher expression levels of the IMM-AGE signature were associated with a lower mortality hazard in the COVID-19 cohort (HR, 0.91; 95% CI, 0.84-0.98). Notably, higher levels of the IMM-AGE signature38 were associated with a lower abundance of immune markers signifying immunosenescence in the FHS. Fourth, the shared IChigh-IFlow state was associated with less-severe influenza infection, including after nasal inoculation of influenza virus in otherwise-healthy younger adults (see Fig E16 in this article’s Online Repository at www.jacionline.org).
Discussion
Through evaluations of COVID-19 and non–COVID-19 cohorts, we uncovered the conserved features of a suspected but previously uncharacterized sexually dimorphic, antigen-activated, immunologic program that associates independently with lower IC and increased IF at any age. We propose that relative resistance to activation of this immunologic program is more common in females and signifies IR. Laboratory (CD8-CD4 equilibrium/disequilibrium–based) and transcriptomic (gene expression signatures) metrics signifying preservation of IR were associated with salutary outcomes: a survival advantage in persons either with or without COVID-19, as well as resistance to development of a progressive disease course during infections with SARS-CoV-2, influenza viruses, or HIV. We report on 3 observations relevant to understanding the basis for the age-independent and sex-biased differences in susceptibility to developing severe COVID-19, as well as improving clinical care and identifying therapeutic targets for mitigation of progressive COVID-19.
First, we derived readily measurable metrics of IR that predicted all-cause, 30-day COVID-19 mortality independent of age, sex, body mass index, and CMV serostatus. Correspondingly, use of these metrics predicted secondary outcomes that included clinical indicators (eg, hospitalization and mechanical ventilation) and biomarkers (eg, plasma IL-6 and nasopharyngeal SARS-CoV-2 levels) indicative of progressive COVID-19. Second, we distinguished and quantified the individual contributions to COVID-19 outcomes of age versus age-independent deficits in IR that antedated SARS-CoV-2 infection versus age-independent deficits in IR that are uniquely induced in response to infection. These findings provide support for 3 novel, IR-dependent COVID-19 phenotypes linked to differential survival risks (Table I), predicated on whether persons had the proclivity to preserve IR before and during COVID-19, at any age. Across age strata, persons preserving metrics indicative of superior IR before and during early COVID-19 were more likely to manifest a survival advantage and nonprogressive COVID-19 (phenotype 1; Table I). Females were more likely to manifest phenotype 1. The distinction between the negative effects of age and preinfection or infection-induced deficits in IR at any age has clinical implications for the prediction and prevention of progressive COVID-19, as well as for the personalization of care (see Online Repository Note 7 and Figs E17 and E18 in this article’s Online Repository at www.jacionline.org). Third, we identified 2 sets of IR-associated traits linked to IC or IF that were associated with survival: one set comprised traits that were uniquely associated with survival during COVID-19, whereas the other set comprised shared traits that were associated with mortality in persons with or without COVID-19. Both sets of traits may proffer therapeutic targets for mitigation of COVID-19 mortality, whereas the latter set of traits points to therapeutic targets for extending longevity.
IHG-I signifies high-grade CD8-CD4 equilibrium and represents the primordial IHG from which other IHGs emerge during antigenic challenges (see Online Repository Note 7). The relative prevalence of the various IHGs in a study group is unique to the type and level of antigenic experience or exposures. Consequently, IHG distribution patterns across cohorts differed: SardiNIA (IHG-I > II > IV > III), SLE (IHG-II > IV > I > III), COVID-19 (IHG-II > I > IV > III), and HIV+ therapy-naive patients with low HIV viral load (IHG-IV > II > I∼III). Hence, HIV-associated and SARS-CoV-2–associated antigenic stimulation preferentially induce CD8-CD4 profiles corresponding to IHG-IV and IHG-II, respectively. Underscoring that IHG-I marks superior IR, in the HIV cohort, presentation with IHG-I, IHG-II, and IHG-IV was associated with the lowest, intermediate, and highest AIDS susceptibility, respectively. Paralleling these HIV-related associations, presentation with IHG-I versus IHG-II or IHG-IV was associated in an age-independent manner with a survival advantage versus disadvantage, as well as other features of nonprogressive versus progressive COVID-19.
The intercohort differences in IHG distributions underscore that age is an imperfect proxy for antigenic experience. In response to antigenic experience, the prevalence of IHG-I declines with age, increasing the pool of at-risk persons with IHG-II and IHG-IV. Thus, at any age, the presence of IHG-II or IHG-IV versus IHG-I before COVID-19 likely heightens the risk of developing progressive COVID-19. This is a parsimonious basis for our finding that although hospitalization rates increased with age, irrespective of age, patients who were hospitalized, required respiratory support, and/or died were more likely to present with IHG-II or IHG-IV versus IHG-I. Moreover, presentation with IHG-II or IHG-IV versus IHG-I was associated with delayed nasal SARS-CoV-2 clearance, a feature associated with mortality in our cohort. In a general HIV-seronegative community population, nearly 12% versus 27% of younger versus older adults (age <30 vs ≥60 years) had IHG-II, and about 1 in 25 older adults had IHG-IV. Thus, we suggest that irrespective of age, persons with a diathesis to develop IHG-II or IHG-IV, before or during COVID-19, likely contribute the most to the overall burden of progressive COVID-19.
SARS-CoV-2 infection is unique in that, in the susceptible host, it may transiently perturb preexisting IR status to levels observed in HIV-seropositive persons. To metricize these perturbations and their contributions to COVID-19 outcomes, we evaluated IHG-II and IHG-IV subgrades, with IHG-IIc and IHG-IVc corresponding to CD8-CD4 profiles linked to profound CD4+ lymphopenia (≤200 cells/mm3) and IHG-IVc reflecting the profile of AIDS. In HIV-seronegative non–COVID-19 contexts, most persons with IHG-II or IHG-IV manifest the IHG-IIa or IHG-IVa subgrades; in the setting of COVID-19, some persons degrade preexisting IHG-I, IHG-IIa, or IHG-IVa to lower grades/subgrades. However, some persons resisted this degradation, accounting for our finding that, independent of age, baseline IHG status predicted survival and secondary outcomes, generally following a hierarchical pattern (better to worse): IHG-I > IHG-IIa > IHG-IVa > IHG-IVb ≥ IHG-IIb > IHG-IVc ≥ IHG-IIc. The extremes of this spectrum, that is, IHG-I versus IHG-IIc or IHG-IVc, represent persons with high-grade resistance versus susceptibility to degrade IR (phenotypes 1 vs 3, respectively). During COVID-19, these extremes were associated with lower versus higher plasma IL-6 levels. Nearly 40% of the individuals who died presented with IHG-IIc or IHG-IVc.
Persons presenting with IHG-IIc and IHG-IVc differed. Compared with persons presenting with IHG-I, presentation with IHG-IIc or IHG-IVc was associated with an approximately 19-fold or approximately 12-fold higher hazard of death in 30 days, respectively, and recovery times that were 67% and 88% longer, respectively. Persons presenting with IHG-IVc versus IHG-IIc tended to be older, have more comorbidities, were CMV-seropositive, and displayed contrasting IHG reconstitution patterns during convalescence. Although CMV seropositivity increased with age, CMV serostatus was not associated with mortality. Thus, the IHG at presentation was not stochastic, but likely reflected the antigenic experience of the host before COVID-19 and response to SARS-CoV-2 infection.
Four findings highlight the substantial impact of baseline IHG status on COVID-19 outcomes: (1) compared to presentation with IHG-I, presentation with IHG-IIc increased the absolute risk of 30-day mortality by 28 additional deaths per 100 persons, (2) compared to presentation with IHG-IIa, presentation with IHG-IIc was associated with an excess of 26 additional deaths per 100 persons, (3) although more older persons presented with IHG-IIc or IHG-IVc, the age-adjusted associations of these grades with COVID-19 outcomes were at least as strong as those associated with older age, and (4) the associations of IHG status with COVID-19 mortality were independent of both age and CMV serostatus, as well as body mass index, a risk factor for severe COVID-19.60 , 61
Transcriptomic data support that a switch from IHG-I to IHG-II or IHG-IV before or during COVID-19 may associate with an immunosuppressive, proinflammatory state that contributes to mortality. We identified 2 distinct IClow-IFhigh states that were associated with COVID-19 mortality. The shared IClow-IFhigh state likely antedated COVID-19, because it was associated with mortality not only in persons with COVID-19 but also in participants in the FHS, independent of age. In addition, trait features of the shared IClow-IFhigh state corresponded to maladaptive host responses to antigenic challenges typically experienced throughout life. The unique IClow-IFhigh state corresponded to unregulated host responses to SARS-CoV-2 infection and was associated with a survival disadvantage exclusively in patients with COVID-19. The unique and shared IClow-IFhigh states were enriched in persons with IHG-II or IHG-IV. Conversely, the shared IChigh-IFlow state was associated with a survival advantage in both the COVID-19 cohort and the FHS, and was enriched in persons with IHG-I, regardless of COVID-19 status. The shared IChigh-IFlow state was also associated with less-severe influenza infection.
Thus, irrespective of COVID-19 status, superior IR was indicated by IHG-I linked to a shared IChigh-IFlow state, whereas deficits in IR were indicated by IHG-II or IHG-IV linked to shared IClow-IFhigh states. Females were more successful than males in preserving indicators of superior IR, as well as in manifesting a longevity advantage compared with males in the FHS, even after correction for differences in the levels of IR between the sexes. Congruently, during COVID-19, more females presented with IHG-I, potentially explaining why the risk of developing progressive COVID-19 is lower in females.
Because the baseline IHGs independently predict COVID-19–associated mortality and recovery, balancing IHG status across trial intervention versus placebo arms may reduce confounding in clinical studies (see Online Repository Note 7; Fig E18). Moreover, assessment of IHGs may allow for individualized care and tailoring therapies. During COVID-19, levels of the unique and shared IClow-IFhigh states were highest in persons presenting with IHG-IIc or IHG-IVc and corresponded to an immunodeficient-hyperinflammatory state characterized by uncontrolled inflammation, inadequate MHC class II responses, deficits in T-cell costimulation/signaling, higher plasma IL-6 levels, and higher nasal SARS-CoV-2 levels (see Online Repository Note 7; Fig E18). Gene expression analysis suggests that the failure to mount effective antiviral immune responses coupled with loss of epithelial integrity in the nasal compartment may underlie both higher SARS-CoV-2 burden and increased mortality, as well as correlate with higher expression of ACE2, the receptor for SARS-CoV-259 (see Online Repository Note 6). Thus, presentation with or rapid advancement to IHG-IIc or IHG-IVc serves as an early indicator of progressive disease, even before worsening respiratory function. Early targeting of the IClow-IFhigh state in persons presenting with or advancing to IHG-IIc and IHG-IVc states may mitigate disease progression. This can potentially be achieved via combinatorial therapies with immunomodulators (eg, baricitinib) and anti-inflammatory agents (eg, IL-6 signaling inhibitors).
The IHGs were more precise measures of immune status versus conventional metrics (CD4+ count or CD4:CD8 ratio used alone; see Online Repository Note 8). This observation in conjunction with the strength of the associations of IHGs with COVID-19 outcomes suggest that IHGs hold promise as a pragmatic clinical tool to assess, monitor, and predict clinical outcomes and inflammatory status during COVID-19. Daily monitoring of IHGs (eg, Fig E6) was implemented at our institution to support algorithms in conjunction with clinical assessment for admission, anticipating levels of care and duration, and directing therapies. Based on our findings, examples of clinical utility of the IHGs include (1) initial staging of COVID-19 severity, (2) monitoring progress of COVID-19 severity, (3) improved differential diagnosis of worsening oxygenation status attributable to COVID-19 versus other sources (eg, patients with improving IHG status would suggest COVID-19 was less likely the cause, suggesting alternative possibilities such as pulmonary embolism or hospital-acquired pneumonia), (4) identification of patients with poor recovery or persistent COVID-19 (eg, persistently poor oxygenation, unresolved radiographic findings consistent with COVID-19, positive PCR test result for SARS-CoV-2 with low cycle threshold values, and failure to improve IR metrics). We found that all patients in our cohort with presumed persistent COVID-19 had persistent IHG-IIc or IHG-IVc, suggesting that a failure to clear SARS-CoV-2 burden, possibly due to deficits in IR, may contribute to this persistence. In such patients, IHGs were used to help identify those more likely to have persistent COVID-19, warranting aggressive/alternative therapeutic approaches including prolongation or reinitiation of remdesivir, corticosteroids, and/or initiation of immunomodulators (eg, baricitinib).
At the time of this article, the US Food and Drug Administration is strategizing COVID-19 vaccine booster doses for those who are “immunocompromised” and face a greater risk from infection or reinfection.62 Yet, identifying persons who are “immunocompromised” beyond qualifying comorbidities (eg, history of cancer, transplant recipients, and receiving immunosuppressant medications), or those with advanced age, remains obscure and limited. IHGs track IC and IF status independent of age. For example, up to 14% of a general population manifested IHG-IV, a grade that is highly prevalent in therapy-naive HIV+ persons. Hence, assessment of IHG status may provide a means to accurately and precisely gauge “immunocompromised” status relevant for prioritization of booster doses and other preventative therapies, regardless of age.
There are limitations of our study. The COVID-19 cohort comprised mostly male veterans, many of whom were older and had comorbidities; these features may not be generalizable. Second, therapies for COVID-19 could confound associations. Treatment decisions in our cohort were not dictated by participation in any concomitant clinical trials, and baseline IHG status was used for defining associations. Third, we were unable to obtain viral cultures. Fourth, defining the association of IHG status with postacute sequelae of SARS-CoV-2 infection was beyond the scope of this study.
Conclusions
We identified age-independent, female-biased processes linked to higher IC and lower IF, signifying IR that bridge longevity, COVID-19 survival, and resistance to AIDS and severe influenza. Thus, the IR metrics have value for precision immune health monitoring across lifespan, irrespective of COVID-19 status.
Our data favor a mechanistic model wherein gain of detrimental function (higher IF) and loss of beneficial functions (lower IC) signifying IR deficits independently and synergistically contribute to mortality in persons with or without COVID-19 (see Online Repository Note 7). Across all age ranges, the relative burden of progressive COVID-19 is dependent on the prevalence of individuals with a proclivity to preserve versus degrade IR before and during COVID-19, with males having a diathesis to degrade IR. The magnitude of the absolute risk differences for 30-day mortality associated with IHG status at presentation underscores the need for a combinatorial approach with immunomodulators and anti-inflammatory agents to mitigate progression of preexisting IR deficits and/or promote the rapid restoration of the IR status that antedated SARS-CoV-2 infection.
Clinical implications.
Biomarkers tracking IR may have broad prognostic utility because they are associated with both longevity and resistance to a progressive disease course during SARS-CoV-2, influenza, or HIV infection.
Acknowledgments
This article is dedicated to the patients with COVID-19 who died at our institution and Dr Tarun Kaul who also succumbed to this disease. We thank Alvaro Gaitan for critical discussions and Kimberly Summers for assistance with study approvals. Additional members of the South Texas Veterans Health Care System COVID-19 Team (Affiliated to South Texas Veterans Health Care System, San Antonio, Tex) include Mohamed I. Abdalla, Sandra G. Adams, Joseph Agnew, Saleem Ali, Jennifer Barker, Angela Birdwell, Stephen Bradford, Heather Briggs, Judith Marin Corral, Jennifer J. Dacus, Patrick J. Danaher, Scott A. DePaul, Jill Dickerson, Jollynn Doanne, Samantha Elbel, Corina Escamilla, Robert Farrar, David Feldman, Julianne Flynn, Delvina Ford, Joanna D. Foy, Megan Freeman, Samantha Galley, Maritza Garza, Sherraine Gilman, Jennifer Gomez, Varun K. Goyal, Sally Grassmuck, Joshua Hanson, Brande Harris, Gabrielyd Hastings, Audrey Haywood, Cecilia Hinojosa, Tony T. Ho, Teri Hopkins, Pamela Jewell, Thomas B. Johnson, Vasiliki Kotogiannes, Austin C. Lawler, Chadwick S. Lester, Stephanie M. Levine, Haidee V. Lewis, Angel Louder, Charmaine Mainor, Rachel Maldonado, Yvette Martinez, Neil McElligott, Laura Medlin, Myra Mireles, Kathleen Morneau, Samuel B. Munro, Anoop Nambiar, Daniel Nassery, Robert Nathanson, Jane O’Rorke, Cheryl Padgett, Sergi Pascual-Guardia, Marisa Patterson, Rogelio Perez, Robert E. Phillips, Patrick B. Polk, Michael A. Pomager, Kristy J. Preston, Kevin C. Proud, Michelle Rangel, Temple A. Ratcliffe, Renee L. Reichelderfer, Evan M. Renz, Jeanette Ross, Teresa Rudd, Maria E. Sanchez, Tammy Sanders, Kevin C. Schindler, David Schmit, Claudio Solorzano, Nilam Soni, Win S. Tam, Edward J. Tovar, Anna R. Tyler, Anjuli Vasquez, Maria C. Veloso, Steven G. Venticinque, Jorge A. Villalpando, Melissa Villanueva, Lauren Villegas, Andrew Wallace, Emily Wang, Andreia Williamson, Sadie A. Trammell Velasquez, Andrea Yunes, and Katharine H. Zentner.
Footnotes
The main sources of funding for the data presented herein are those awarded to S.K.A., M.I.R., and J.F.O. S.K.A. was supported by grants from the Veterans Affairs (VA) (VA Center for Personalized Medicine, grant no. IP1 CX000875-01A1), by the National Institutes of Health (NIH) MERIT award (grant no. R37AI046326), by the Doris Duke Distinguished Clinical Scientist Award, by the Burroughs Wellcome Clinical Scientist Award in Translational Research, and by the Elizabeth Glaser Pediatric AIDS Foundation. The work was also supported, in part, by an award jointly funded by the National Institute of Allergy and Infectious Diseases (NIAID)/NIH (grant no. AAI20042-001) and the Department of Veterans Affairs (grant no. COVID19-8100-01) awarded to S.K.A. and M.I.R. A portion of the material presented is based on research sponsored by the US Air Force under agreement number FA8650-17-2-6816 (US Air Force 59th Medical Wing Intramural Award to J.F.O.). The HIV cohort was supported by the Infectious Disease Clinical Research Program (grant no. IDCRP-000-03), a Department of Defense program executed by the Uniformed Services University of the Health Sciences through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. The latter project has been supported with federal funds from the NIAID/NIH (under Inter-Agency Agreement no. Y1-AI-5072) and from the Defense Health Program, US Department of Defense (under award no. HU0001190002). The SardiNIA study was supported in part by the Intramural Research Program of the NIH, National Institute on Aging (with contract nos. N01-AG-1-2109 and HHSN271201100005C); by Italian grants (grant no. FISM 2011/R/13, grant no. FaReBio2011, Funds MIUR/CNR for Rare Diseases and Molecular Screening, CNR/DSB flagship INTEROMICS, PNR/CNR Aging Program 2012-2014); European Union’s Horizon 2020 Research and Innovation Programme (under grant agreement no. 633964); Giovani Ricercatori 2007 (D.lgs 502/92); and Legge Regionale 30 giugno 2011 n.12, articolo 3, comma 3 (FC). G.C.L. was supported by the NIH (grant no. K23-AG066933). A.M.S. was supported by the NIH (grant no. T32DE014318 COSTAR) institutional research training grant. This work was also supported by pilot project funding to S.K.A. (from 1UL1 TR002645 Clinical and Translational Science Award, San Antonio Claude D. Pepper Older Americans Independence Center grant no. P30 AG044271, and Long School of Medicine, UTHSCA).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest. The views expressed are those of the authors and do not reflect the official views of the Uniformed Services University of the Health Sciences, the National Institutes of Health, or the Department of Health and Human Services, the Department of Defense, or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46. The US government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright notation thereon.
Contributor Information
South Texas Veterans Health Care System COVID-19 Team:
Mohamed I. Abdalla, Sandra G. Adams, Joseph Agnew, Saleem Ali, Jennifer Barker, Angela Birdwell, Stephen Bradford, Heather Briggs, Judith Marin Corral, Jennifer J. Dacus, Patrick J. Danaher, Scott A. DePaul, Jill Dickerson, Jollynn Doanne, Samantha Elbel, Corina Escamilla, Robert Farrar, David Feldman, Julianne Flynn, Delvina Ford, Joanna D. Foy, Megan Freeman, Samantha Galley, Maritza Garza, Sherraine Gilman, Jennifer Gomez, Varun K. Goyal, Sally Grassmuck, Joshua Hanson, Brande Harris, Gabrielyd Hastings, Audrey Haywood, Cecilia Hinojosa, Tony T. Ho, Teri Hopkins, Pamela Jewell, Thomas B. Johnson, Vasiliki Kotogiannes, Austin C. Lawler, Chadwick S. Lester, Stephanie M. Levine, Haidee V. Lewis, Angel Louder, Charmaine Mainor, Rachel Maldonado, Yvette Martinez, Neil McElligott, Laura Medlin, Myra Mireles, Kathleen Morneau, Samuel B. Munro, Anoop Nambiar, Daniel Nassery, Robert Nathanson, Jane O’Rorke, Cheryl Padgett, Sergi Pascual-Guardia, Marisa Patterson, Rogelio Perez, Robert E. Phillips, Patrick B. Polk, Michael A. Pomager, Kristy J. Preston, Kevin C. Proud, Michelle Rangel, Temple A. Ratcliffe, Renee L. Reichelderfer, Evan M. Renz, Jeanette Ross, Teresa Rudd, Maria E. Sanchez, Tammy Sanders, Kevin C. Schindler, David Schmit, Claudio Solorzano, Nilam Soni, Win S. Tam, Edward J. Tovar, Anna R. Tyler, Anjuli Vasquez, Maria C. Veloso, Steven G. Venticinque, Jorge A. Villalpando, Melissa Villanueva, Lauren Villegas, Andrew Wallace, Emily Wang, Andreia Williamson, Sadie A. Trammell Velasquez, Andrea Yunes, and Katharine H. Zentner
Supplementary data
References
- 1.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham J.W., Vaduganathan M., Claggett B.L., Jering K.S., Bhatt A.S., Rosenthal N., et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020;181:379–381. doi: 10.1001/jamainternmed.2020.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Team CC-R Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi T., Iwasaki A. Sex differences in immune responses. Science. 2021;371:347–348. doi: 10.1126/science.abe7199. [DOI] [PubMed] [Google Scholar]
- 7.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 8.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 11.Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen Y.F., Hu H.Y., Lee Y.L., Ku P.W., Ko M.C., Chuang P.H., et al. Sexual inequality in incident tuberculosis: a cohort study in Taiwan. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nhamoyebonde S., Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209:S100–S106. doi: 10.1093/infdis/jiu147. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel G., Arck P.C. Sex, immunity and influenza. J Infect Dis. 2014;209:S93–S99. doi: 10.1093/infdis/jiu020. [DOI] [PubMed] [Google Scholar]
- 15.Austad S.N., Fischer K.E. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarulli V., Barthold Jones J.A., Oksuzyan A., Lindahl-Jacobsen R., Christensen K., Vaupel J.W. Women live longer than men even during severe famines and epidemics. Proc Natl Acad Sci U S A. 2018;115:E832–E840. doi: 10.1073/pnas.1701535115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostan R., Monti D., Gueresi P., Bussolotto M., Franceschi C., Baggio G. Gender, aging and longevity in humans: an update of an intriguing/neglected scenario paving the way to a gender-specific medicine. Clin Sci (Lond) 2016;130:1711–1725. doi: 10.1042/CS20160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X., et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydillo T., Gonzalez-Reiche A.S., Aslam S., van de Guchte A., Khan Z., Obla A., et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orru V., Steri M., Sole G., Sidore C., Virdis F., Dei M., et al. Genetic variants regulating immune cell levels in health and disease. Cell. 2013;155:242–256. doi: 10.1016/j.cell.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmood S.S., Levy D., Vasan R.S., Wang T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383:999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y., Zaas A.K., Rao A., Dobigeon N., Woolf P.J., Veldman T., et al. Temporal dynamics of host molecular responses differentiate symptomatic and asymptomatic influenza A infection. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang B.M., Shojaei M., Teoh S., Meyers A., Ho J., Ball T.B., et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat Commun. 2019;10:3422. doi: 10.1038/s41467-019-11249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunning J., Blankley S., Hoang L.T., Cox M., Graham C.M., James P.L., et al. Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza. Nat Immunol. 2018;19:625–635. doi: 10.1038/s41590-018-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiche L., Jourde-Chiche N., Whalen E., Presnell S., Gersuk V., Dang K., et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014;66:1583–1595. doi: 10.1002/art.38628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le T., Wright E.J., Smith D.M., He W., Catano G., Okulicz J.F., et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okulicz J.F., Le T.D., Agan B.K., Camargo J.F., Landrum M.L., Wright E., et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med. 2015;175:88–99. doi: 10.1001/jamainternmed.2014.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okulicz J.F., Marconi V.C., Landrum M.L., Wegner S., Weintrob A., Ganesan A., et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis. 2009;200:1714–1723. doi: 10.1086/646609. [DOI] [PubMed] [Google Scholar]
- 29.Marconi V.C., Grandits G.A., Weintrob A.C., Chun H., Landrum M.L., Ganesan A., et al. Outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the U.S. Military HIV Natural History Study. AIDS Res Ther. 2010;7:14. doi: 10.1186/1742-6405-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marconi V.C., Grandits G., Okulicz J.F., Wortmann G., Ganesan A., Crum-Cianflone N., et al. Cumulative viral load and virologic decay patterns after antiretroviral therapy in HIV-infected subjects influence CD4 recovery and AIDS. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 32.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laguna-Goya R., Utrero-Rico A., Talayero P., Lasa-Lazaro M., Ramirez-Fernandez A., Naranjo L., et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:799–807.e9. doi: 10.1016/j.jaci.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wikby A., Ferguson F., Forsey R., Thompson J., Strindhall J., Lofgren S., et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 36.Puzianowska-Kuznicka M., Owczarz M., Wieczorowska-Tobis K., Nadrowski P., Chudek J., Slusarczyk P., et al. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing. 2016;13:21. doi: 10.1186/s12979-016-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utrero-Rico A., Ruiz-Hornillos J., Gonzalez-Cuadrado C., Rita C.G., Almoguera B., Minguez P., et al. IL-6-based mortality prediction model for COVID-19: validation and update in multicenter and second wave cohorts. J Allergy Clin Immunol. 2021;147:1652–1661.e1. doi: 10.1016/j.jaci.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alpert A., Pickman Y., Leipold M., Rosenberg-Hasson Y., Ji X., Gaujoux R., et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med. 2019;25:487–495. doi: 10.1038/s41591-019-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urra J.M., Cabrera C.M., Porras L., Rodenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369 doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter M.J., Fish M., Jennings A., Doores K.J., Wellman P., Seow J., et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 42.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hue S., Beldi-Ferchiou A., Bendib I., Surenaud M., Fourati S., Frapard T., et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 ARDS. Am J Respir Crit Care Med. 2020;202:1509–1519. doi: 10.1164/rccm.202005-1885OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor J.M., Fahey J.L., Detels R., Giorgi J.V. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. J Acquired Immune Deficiency Syndromes. 1989;2:114–124. [PubMed] [Google Scholar]
- 48.Vila L.M., Alarcon G.S., McGwin G., Jr., Bastian H.M., Fessler B.J., Reveille J.D., et al. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum. 2006;55:799–806. doi: 10.1002/art.22224. [DOI] [PubMed] [Google Scholar]
- 49.Liu M.F., Wang C.R., Fung L.L., Wu C.R. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Suppl. 1987;36:1S–15. [PubMed] [Google Scholar]
- 52.Ahuja S.K., Manoharan M.S., Harper N.L., Jimenez F., Hobson B., Martinez H., et al. Preservation of epithelial cell barrier function and muted inflammation in resistance to allergic rhinoconjunctivitis from house dust mite challenge. J Allergy Clin Immunol. 2017;139:844–854. doi: 10.1016/j.jaci.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 53.Smith A.M., Harper N., Meunier J.A., Branum A.P., Jimenez F., Pandranki L., et al. Repetitive aeroallergen challenges elucidate maladaptive epithelial and inflammatory traits that underpin allergic airway diseases. J Allergy Clin Immunol. 2021;148:533–549. doi: 10.1016/j.jaci.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Effros R.B. The silent war of CMV in aging and HIV infection. Mech Ageing Dev. 2016;158:46–52. doi: 10.1016/j.mad.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu W., Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol. 2016;7:2111. doi: 10.3389/fmicb.2016.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp Gerontol. 2014;54:1–5. doi: 10.1016/j.exger.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tartof S.Y., Qian L., Hong V., Wei R., Nadjafi R.F., Fischer H., et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson M.R., Geleris J., Anderson D.R., Zucker J., Nobel Y.R., Freedberg D., et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med. 2020;173:782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall V.G., Ferreira V.H., Ku T., Ierullo M., Majchrzak-Kita B., Chaparro C., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.