Abstract
Microbe exposure to pharmaceutical and non-pharmaceutical agents plays a role in the development of antibiotic resistance. The risks and consequences associated with extensive disinfectant use during the COVID-19 pandemic remain unclear. Some disinfectants, like sanitizers, contain genotoxic chemicals that damage microbial DNA, like phenol and hydrogen peroxide. This damage activates error-prone DNA repair enzymes, which can lead to mutations that induce antimicrobial resistance. Public health priority programs that have faced drug-resistance challenges associated with diseases, such as tuberculosis, HIV, and malaria, have given less attention to risks attributable to the COVID-19 pandemic. Pathogen-specific programs, like the directly observed treatment strategy designed to fight resistance against anti-tuberculosis drugs, have become impractical because COVID-19 restrictions have limited in-person visits to health institutions. Here, we summarized the key findings of studies on the current state of antimicrobial resistance development from the perspective of current disinfectant use. Additionally, we provide a brief overview of the consequences of restricted access to health services due to COVID-19 precautions and their implications on drug resistance development.
Keywords: Bacteria, AMR, COVID-19, Disinfectants, pharmaceuticals, non-pharmaceuticals
Abbreviations: BAC, Benzalkonium chloride; DNA, Deoxyribonucleic Acid; DOTs, Directly Observed Treatment Strategy; HIV, Human Immunodeficiency Virus; NDM1, New Delhi Metallo-β-lactamase 1; SARS-COV2, Severe Acute Respiratory Syndrome Coronavirus 2; TB, Tuberculosis; TLS, Translesion Synthesis Polymerase; WHO, World Health Organization
Introduction
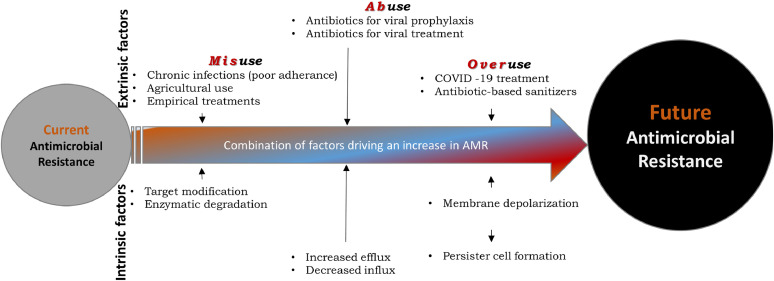
Antimicrobial resistance (AMR) is a global public health concern (Prestinaci, Pezzotti, & Pantosti, 2015). The main drivers of AMR include excess microbial exposure to antibiotic agents, mainly due to their overuse in agriculture and health facilities (Capozzi et al., 2013; Levy, 1998). On the other hand, progress in developing new antibiotics has remained stagnant due to scientific challenges, clinical hurdles, and low economic returns (Payne, Miller, Findlay, Anderson, & Marks, 2015). In addition to well-established factors that influence AMR, the overuse and misuse of existing antimicrobial agents have contributed to accelerating the spread of AMR during and beyond the COVID-19 pandemic (Fig. 1 ).
Figure 1.
The main drivers of antimicrobial resistance. Inappropriate use of antimicrobial agents for clinical and non-clinical applications accelerates the rate at which drug resistance develops.
Recent findings have identified mechanisms that link antibiotic-resistance dissemination to the use of non-antibiotic pharmaceuticals (Wang et al., 2020). Those findings are essential for understanding AMR in the context of the SARS-COV-2 pandemic, which has led to extensive use of non-pharmaceuticals (e.g., alcohol-based sanitizers) as a measure to prevent infection (Hui et al., 2020). The marked increase in the use of hand sanitizers and environmental cleaners, without proof of their short and long-term side effects, is a potential concern for human, animal, and environmental health (Campos et al., 2017; Mantlo et al., 2020). Developing countries and regions with limited resources, a flawed pharmaceutical supply chain, and poor health service management systems might contribute most to AMR spread because the quality of pharmaceutical products remains questionable (CDC., 2020) (Chokshi, Sifri, Cennimo, & Horng, 2019). Here, we briefly provide insight into how COVID-19 preventive measures, such as the use of disinfectants and disruptions in treatment modalities of AMR-targeted diseases, could potentially lead to an increase in AMR beyond the pandemic.
The current state of antimicrobial resistance
In 2017, the World Health Organization (WHO) released a report on drug-resistant bacterial pathogens that merit priority in research and development (WHO., 2017). Most pathogens on the list included bacteria that carry the enzyme New Delhi metallo-beta-lactamase 1 (NDM1), which confers resistance to a broader spectrum of mainstay antibiotics used for treating antibiotic-resistant bacteria (Kumarasamy et al., 2010; D. L. Paterson & Doi, 2007). Unfortunately, shortly after the release of the report, COVID-19 was recognized as a pandemic and required the full attention of the scientific community, which limited their capacity to fight AMR (Choudhury, Panda, & Singh, 2012; Ferri, Ranucci, Romagnoli, & Giaccone, 2017; Morehead & Scarbrough, 2018).
One of the WHO strategies for mitigating SARS-COV-2 infection was the use of hand sanitizers and disinfectants as frequently as, and whenever possible (CDC., 2020; Kratzel et al., 2020). As a result, the unseen microbial population is being continuously exposed to pharmaceutical and non-pharmaceutical agents at varying frequencies, concentrations, and doses (Haiderali, 2020). Regardless of composition, these products' long and short-term effects on microbial genetics and human health have apparently been overlooked; however, we believe that current practices may have a potential impact. Therefore, based on the lessons learned from biophysical and molecular responses of microbes to different stressors, we might infer that to the use of pharmaceutical and non-pharmaceutical agents may contribute to the emergence and spread of AMR via mutagenic mechanisms that introduce microbial genome instability (Booth et al., 2020; Cohen, Lobritz, & Collins, 2013; Dorr, Vulic, & Lewis, 2010; Gullberg et al., 2011; I. K. Paterson, Hoyle, Ochoa, Baker-Austin, & Taylor, 2016).
Mechanisms related to COVID-19 consequences that contribute to AMR
Previous studies have described the emergence of bacterial pathogens that became tolerant to alcohol-based sanitizers through unknown genetic and molecular mechanisms (Pidot et al., 2018). This tolerance could be induced by the constituents of sanitizers, such as alcohol, quaternary ammonium compounds, phenols, hydrogen peroxide, and surfactants, which cause microbial DNA damage, or benzalkonium chloride (BAC) and triclosan, which have antimicrobial properties (Carey & McNamara, 2015; McDonnell et al., 2013; Pereira & Tagkopoulos, 2019). BAC has a broad spectrum antimicrobial activity against bacteria, fungi, and viruses. It has been well documented that BACs create a selective environment that favors some microbial phenotypes, and thus, exposure to it can confer cross-resistance to various antimicrobial agents (Pereira & Tagkopoulos, 2019).
Most homemade and purchased cleaning and hand sanitizing products contain chemicals, such as phenol and hydrogen peroxides, that induce microbial DNA damage (da Silva, 2016; Akimitsu et al., 1999; WHO, 2010). The bacterial response to DNA damage by induction of translesion synthesis polymerases (TLS) tolerates and bypasses unrepaired DNA lesions creating mutations that contribute to the development of antimicrobial resistance (Choi, Kim, Motea, & Berdis, 2017; Fuchs & Fujii, 2013; Ghosal & Chen, 2013).
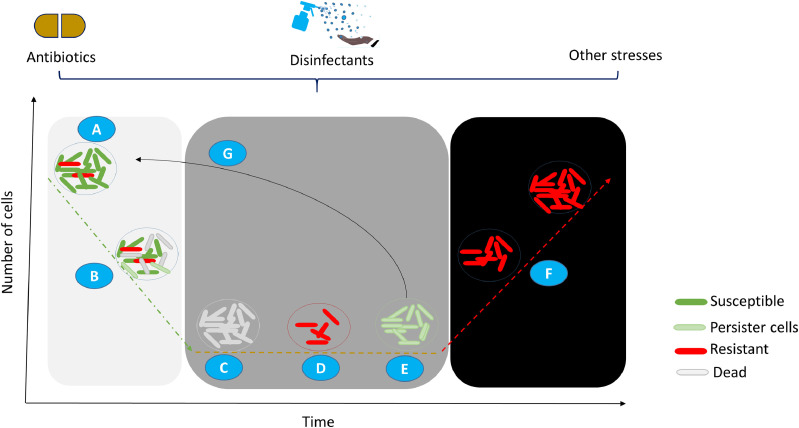
Upon exposure to antibiotic-based disinfectants, bacteria respond by forming a subpopulation that persists and can become highly tolerant to antibiotics. This “selected” subpopulation plays an essential role in the recalcitrance of biofilm infections (Fig. 2 )(Dorr et al., 2010). Therefore, microbes can undergo both phenotypic and genotypic changes that can alter antibiotic targets (Lewis, 2007).
Figure 2.
Classical curve showing biphasic killing by antibiotics that leaves a fraction of the bacterial subpopulation that enters into dormancy or becomes resistant. Pharmaceutical and non-pharmaceutical agents, such as antibiotics, sanitizers, and stress conditions, affect (A) actively replicating bacteria (green), which leads to (B and C) depletion of the sensitive clones (grey). A small proportion of surviving bacteria enters into (E) a dormant, persistent state (light green) or (D) a resistant state (red). The dormant, persistent fraction reactivates when antibacterial agents are removed (G), and become susceptible. The resistant subpopulation continues to multiply in the presence of the antimicrobial agent(s) that are designed to kill them (F).
Moreover, the use of antibiotics has increased in current efforts to ameliorate COVID-19. Indeed, antibiotics were administered in nearly 70% of COVID-19 related hospital admissions and 80-100% of COVID-19 related intensive care unit admissions (Langford et al., 2020). For instance, individuals received antibiotics when they presented with either mild COVID-19 without pneumonia, or moderate COVID-19 with pneumonia (WHO., 2020a).
Health system challenges related to COVID-19
The pandemic has posed a challenge to the preventive and control programs for public health priority diseases, such as tuberculosis (TB), HIV, and malaria. For instance, fighting the notoriously drug-resistant bacterial infection, TB, with a directly observed treatment strategy (DOTs), has become practically impossible because COVID-19 related restrictions have prevented patients with TB from visiting health service centers (WHO., 2020b).
In developing countries, where infectious diseases and other chronic illnesses are prevalent, the WHO global strategy and the recommendation for digital health interventions have remained elusive due to low and limited internet coverage (Labrique, Agarwal, Tamrat, & Mehl, 2020). These countries are burdened with far worse illnesses than COVID-19; thus, perhaps in that context, the COVID-19 measures are too strict compared to methods for managing other illnesses. Consequently, the number of empirical treatments has increased due to the lack of medical, physical, and laboratory examinations.
The consequences of undoing global efforts
Several global strategies have been established for combating AMR through the tremendous collaborative efforts of international agencies, including the US Center for Disease Control and Prevention, the European Center for Disease Prevention and Control, the WHO, the United Nations interagency coordination group on AMR, and the Global antibiotic resistance partnership (Chaudhary, 2016). If these achievements are undermined due to COVID-19 management strategies, we could experience even worse health, economic, social, and political crises.
Conclusions
Exposure of microbes to disinfectants and non-pharmaceutical agents contributes to the microbial ability to evolve mechanisms that increase AMR. Furthermore, the COVID-19 related restrictions to health services limited access for proper diagnosis, treatment, and management of patients with infectious diseases that need antibiotic-based interventions. Cognizance and actions are desperately needed to confront the existing and emerging public health threats from drug-resistant, multidrug-resistant, and total drug-resistant microbes.
Recommendations
Therefore, because the course of the COVID-19 pandemic remains enigmatic, we argue that the strategies for combating the emergence and spread of AMR should be incorporated into the pandemic response through different approaches.
A robust expert recommendation, as well as research on the formulations of disinfectants in current use, specifically for selecting, introducing, and regulating microbicides that have shown no or low selective pressure for inducing AMR, is critical. For example, investigations should focus on disinfectant constituents, mechanisms of action, genetic targets, and short and long-term effects on the environment, humans, and the microbial population. Furthermore, strategies should be designed and implemented to reach patients with chronic infections like TB that require antibiotic management. In this regard, it is indispensable to strengthen the capacity of community health care providers to follow and assist patients without compromising safety and infection prevention measures. In our opinion, alternatives to digital health (e-health) should be sought for areas with no or limited internet access. Here, we argue that internet expansion into remote areas is feasible provided there is an investment and political commitment.
The time is at hand to launch coordinated, collaborative measures to stem the further emergence and spread of untreatable infections and diseases, which could eventually lead to another public health emergency.
Acknowledgments
Availability of data and materials
Not applicable
Acknowledgments
We are indebted to the authors of articles used in this perspective.
Funding
This perspective was unfunded.
Authors’ contributions
TAL conceptualized, and TAL and AAR prepared the draft manuscripts. TAL wrote the mauscript. JAB, KIK, AA, KS, and MB contributed to revising the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Ethical approval and consent to participate are not required and not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
References
- Akimitsu N., Hamamoto H., Inoue R., Shoji M., Akamine A., Takemori K., Sekimizu K. Increase in resistance of methicillin-resistant Staphylococcus aureus to beta-lactams caused by mutations conferring resistance to benzalkonium chloride, a disinfectant widely used in hospitals. Antimicrobial Agents and Chemotherapy. 1999;43(12):3042–3043. doi: 10.1128/Aac.43.12.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.A., Spirek M., Lobie T.A., Skarstad K., Krejci L., Bjoras M. Antibiotic-induced DNA damage results in a controlled loss of pH homeostasis and genome instability. Sci Rep. 2020;10(1):19422. doi: 10.1038/s41598-020-76426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A.M.S., Bucaretchi F., Fernandes L.C.R., Fernandes C.B., Capitani E.M., Beck A.R.M. Toxic Exposures in Children Involving Legally and Illegally Commercialized Household Sanitizers. Rev Paul Pediatr. 2017;35(1):11–17. doi: 10.1590/1984-0462/;2017;35;1;00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi C., Volpi A., Maurici M., Lisena F.P., Visconti G., Pana A. Healthcare-associated infections and antibiotic resistance: a global challenge for the 21st century. Ig Sanita Pubbl. 2013;69(6):657–691. https://www.ncbi.nlm.nih.gov/pubmed/24548906 Retrieved from. [PubMed] [Google Scholar]
- Carey D.E., McNamara P.J. The impact of triclosan on the spread of antibiotic resistance in the environment. Frontiers in Microbiology. 2015;5 doi: 10.3389/fmicb.2014.00780. doi: ARTN 780 10.3389/fmicb.2014.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Hand Sanitizer Use Out and About. Handwashing in Community Settings, (2020); https://www.cdc.gov/handwashing/hand-sanitizer-use.html. Retrieved from https://www.cdc.gov/handwashing/hand-sanitizer-use.html
- Chaudhary A.S. A review of global initiatives to fight antibiotic resistance and recent antibiotics' discovery. Acta Pharmaceutica Sinica B. 2016;6(6):552–556. doi: 10.1016/j.apsb.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.S., Kim S., Motea E., Berdis A. Inhibiting translesion DNA synthesis as an approach to combat drug resistance to DNA damaging agents. Oncotarget. 2017;8(25):40804–40816. doi: 10.18632/oncotarget.17254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokshi A., Sifri Z., Cennimo D., Horng H. Global Contributors to Antibiotic Resistance. J Glob Infect Dis. 2019;11(1):36–42. doi: 10.4103/jgid.jgid_110_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R., Panda S., Singh D.V. Emergence and dissemination of antibiotic resistance: a global problem. Indian J Med Microbiol. 2012;30(4):384–390. doi: 10.4103/0255-0857.103756. [DOI] [PubMed] [Google Scholar]
- Cohen N.R., Lobritz M.A., Collins J.J. Microbial Persistence and the Road to Drug Resistance. Cell Host & Microbe. 2013;13(6):632–642. doi: 10.1016/j.chom.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva J. DNA damage induced by occupational and environmental exposure to miscellaneous chemicals. Mutat Res. 2016;770(Pt A):170–182. doi: 10.1016/j.mrrev.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Dorr T., Vulic M., Lewis K. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. Plos Biology. 2010;8(2) doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri M., Ranucci E., Romagnoli P., Giaccone V. Antimicrobial resistance: A global emerging threat to public health systems. Crit Rev Food Sci Nutr. 2017;57(13):2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- Fuchs R.P., Fujii S. Translesion DNA Synthesis and Mutagenesis in Prokaryotes. Cold Spring Harbor Perspectives in Biology. 2013;5(12) doi: 10.1101/cshperspect.a012682. doi:ARTN a012682 10.1101/cshperspect.a012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal G., Chen J. DNA damage tolerance: a double-edged sword guarding the genome. Transl Cancer Res. 2013;2(3):107–129. doi: 10.3978/j.issn.2218-676X.2013.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg E., Cao S., Berg O.G., Ilback C., Sandegren L., Hughes D., Andersson D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. Plos Pathogens. 2011;7(7) doi: 10.1371/journal.ppat.1002158. doi:ARTN e1002158 10.1371/journal.ppat.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiderali Z. Coming clean about sanitisers. British Dental Journal. 2020;228(7):564. doi: 10.1038/s41415-020-1521-y. [DOI] [Google Scholar]
- Hui D.S., E I.A., Madani T.A., Ntoumi F., Kock R., Dar O., …, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzel A., Todt D., V'Kovski P., Steiner S., Gultom M., Thao T.T.N., …, Pfaender S. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg Infect Dis. 2020;(7):26. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamy K.K., Toleman M.A., Walsh T.R., Bagaria J., Butt F., Balakrishnan R., …, Woodford N. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrique A., Agarwal S., Tamrat T., Mehl G. WHO Digital Health Guidelines: a milestone for global health. NPJ Digit Med. 2020;3:120. doi: 10.1038/s41746-020-00330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., Daneman N. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S.B. The challenge of antibiotic resistance. Sci Am. 1998;278(3):46–53. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5(1):48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- Mantlo E., Rhodes T., Boutros J., Patterson-Fortin L., Evans A., Paessler S. In vitro efficacy of a copper iodine complex PPE disinfectant for SARS-CoV-2 inactivation. F1000Res. 2020;9:674. doi: 10.12688/f1000research.24651.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell G., Dehen C., Perrin A., Thomas V., Igel-Egalon A., Burke P.A., …, Comoy E. Cleaning, disinfection and sterilization of surface prion contamination. J Hosp Infect. 2013;85(4):268–273. doi: 10.1016/j.jhin.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Morehead M.S., Scarbrough C. Emergence of Global Antibiotic Resistance. Prim Care. 2018;45(3):467–484. doi: 10.1016/j.pop.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Paterson D.L., Doi Y. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin Infect Dis. 2007;45(9):1179–1181. doi: 10.1086/522287. [DOI] [PubMed] [Google Scholar]
- Paterson I.K., Hoyle A., Ochoa G., Baker-Austin C., Taylor N.G.H. Optimising Antibiotic Usage to Treat Bacterial Infections. Scientific Reports. 2016;6 doi: 10.1038/srep37853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D.J., Miller L.F., Findlay D., Anderson J., Marks L. Time for a change: addressing R&D and commercialization challenges for antibacterials. Philos Trans R Soc Lond B Biol Sci. 2015;370(1670) doi: 10.1098/rstb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira B.M.P., Tagkopoulos I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Applied and Environmental Microbiology. 2019;85(13) doi: 10.1128/AEM.00377-19. doi:ARTN e00377-19 10.1128/AEM.00377-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidot S.J., Gao W., Buultjens A.H., Monk I.R., Guerillot R., Carter G.P., …, Stinear T.P. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci Transl Med. 2018;10(452) doi: 10.1126/scitranslmed.aar6115. [DOI] [PubMed] [Google Scholar]
- Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu J., Engelstadter J., Zhang S., Ding P., Mao L., Guo J. Non-antibiotic pharmaceuticals enhance the transmission of exogenous antibiotic resistance genes through bacterial transformation. ISME J. 2020;14(8):2179–2196. doi: 10.1038/s41396-020-0679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Guide to Local Production: WHO-recommended Handrub Formulations (2010).
- WHO Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva, Switzerland. 2017 https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ Retrieved from. [Google Scholar]
- WHO. Clinical management of COVID-19 Interim Guidance (2020a). https://apps.who.int/iris/rest/bitstreams/1278777/retrieve, Accessed March 12, 2021.
- WHO. Information note on Tuberculosis and COVID-19 (2020b). https://www.who.int/docs/default-source/documents/tuberculosis/infonote-tb-covid-19.pdf. Accessed March 12, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable