Abstract
SARS-CoV-2 monoclonal antibodies (mAbs) have been proposed as a treatment for mild to moderate COVID-19, with favorable outcomes reported in clinical trials and an emergency use authorization granted by the Food and Drug Administration. Real-world data remain limited, however, and thus this analysis presents findings from over 6,500 outpatient administrations of mAb at facilities affiliated with a large healthcare organization in the United States. Within 48 hours of mAb infusion, 15.6% (1,043) of patients received a drug that was indicative of a possible reaction to the infusion; the majority of these were mild (e.g., acetaminophen). Approximately 5.2% of patients who received mAb (n=347) had a post-infusion emergency department visit or admission for COVID-19 disease progression. The results of this analysis indicate that patients who receive mAb have a low likelihood of both an immediate negative reaction to the treatment as well as future inpatient admission related to COVID-19 disease progression.
Keywords: COVID-19, Sars-Co-V-2, monoclonal antibody
Background
As the COVID-19 pandemic progressed and the capacity to care for severely ill patients stabilized, there was an increased interest in the development of treatments for mild to moderate COVID-19. Among these are SARS-CoV-2 monoclonal antibodies (mAbs). These lab-manufactured antibodies are engineered to bind to and neutralize SARS-CoV-2 under the assumption that this could reduce viral load or activity and thus reduce the severity of disease and the consequences, such as hospitalization.(Chen et al., 2021, Weinreich et al., 2021)
Clinical trials of mAbs suggest that these therapies may reduce hospitalization when administered to outpatients with mild to moderate COVID-19.(Gottlieb et al., 2021) As a result, the Food and Drug Administration (FDA) approved emergency use authorization (EUA) for mAbs.(Food and Drug Administration, 2020, 2021) However, the data supporting the use of mAbs are limited and the EUA authorizes the use of this treatment in situations beyond those studied in clinical trials.
Insight from the real-world use of mAbs for early COVID-19 disease could complement clinical trial data. Here we present findings from over 6,500 outpatient administrations of mAb at facilities affiliated with a large healthcare organization in the United States. We assessed medications administered after mAb infusion as a marker of post-infusion reaction. In addition, we analyzed the rate of emergency department admissions or visits in the post-infusion period to gauge the effect of mAb on COVID-19 disease progression.
Methods
Data for this analysis were obtained from the electronic health records of patients at acute care facilities, outpatient care locations, and freestanding emergency departments associated with 58 hospitals affiliated with a large healthcare system in the United States. Data include mAb administrations from November 20, 2020 through April 23, 2021. All patients 18 and older who received an mAb infusion as outpatients or emergency department patients were included in the analysis. All mAb available at the time were included in the analysis; this includes bamlanivimab, bamlanivimab/etesevimab, and casirivimab/imdevimab. Patients with mAb administrations as inpatients or inpatient observation were excluded from the analysis.
Reactions following mAb infusion were analyzed by 1) medication administration for an infusion-related reaction, and 2) emergency department visits or admissions for COVID-19 progression in the post-infusion period. Drugs administered between five minutes to 48 hours following mAb infusion, either during the mAb encounter or a subsequent encounter, were categorized by the severity of reaction the medication would address, as determined by a clinical pharmacist: mild (e.g., acetaminophen), moderate (e.g., antihistamine or steroid), or severe (adrenergic). Patients were categorized by the highest severity medication in cases where multiple medications were administered.
Emergency department visits or admissions in the post-infusion period were considered in the analysis if they occurred after a patient was discharged from the encounter in which mAb was administered. These visits or admissions must have occurred more than 24 hours post-mAb but within 14 days of infusion, have a length of stay greater than 1 day if admitted as an inpatient, and required the use of oxygen. Dexamethasone use, chest X-ray/CT, and tests for D-dimer or C-reaction protein were also flagged as other clinical indicators of visits for disease progression.
Statistical analysis
Due to the descriptive nature of the study, statistical analysis was primarily limited to descriptive statistics in the form of frequencies and percentages. Statistical analysis was done in R (R Foundation for Statistical Computing; Vienna, Austria) and Microsoft Excel.
Results
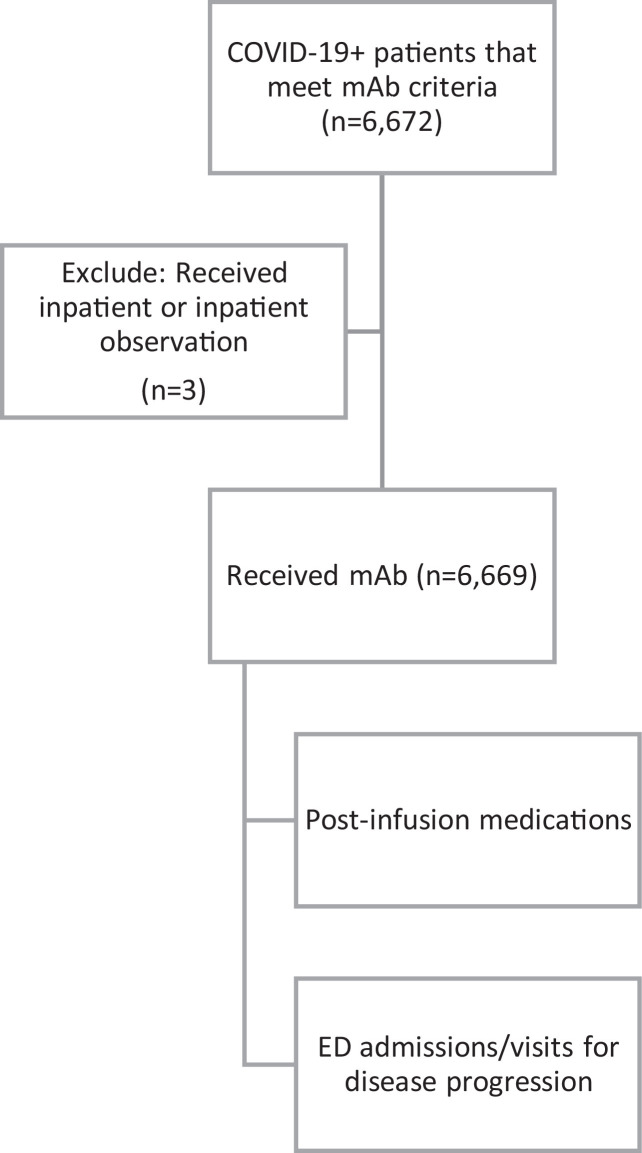
From November 20, 2020 through April 23, 2021, there were 6,672 COVID-19 mAb administrations at affiliated facilities. Of these, 3 occurred in patients with inpatient or inpatient observation status at the time of infusion, and were thus excluded from the subsequent analysis (Figure 1 ). The resulting data set consisted of 6,669 mAb infusions; 14% (n=925) of these mAb infusions occurred in the emergency department and 86% (n=5744) occurred in outpatient clinics.
Figure 1.
Analysis population.
The majority of patients in this data set received bamlanivimab alone (n=5,722, 86.5%). The remaining patients received either bamlanivimab/etesevimab (n=276, 4.1%) or casirivimab/indevimab (n=621, 9.3%). The overall population was 52.5% female (n=3,499) and 64.3% (n=4,289) identified as non-Hispanic white. The majority of patients were between 50 and 79 years of age (50-59, n=1,328, 20.2%; 60-69, n=1,868, 28.0%; 70-79, n=1,463, 21.9%).
Within 48 hours of mAb infusion, 15.6% (1,043) of patients received a drug that was indicative of a possible reaction to the infusion. Of the patients that received medication indicating a reaction post-mAb infusion, the highest class received by the majority of these patients (60.8%, n=635) was medication classified a response to a mild reaction (acetaminophen); 32.5% (n=339) were classified as moderate (antihistamine and/or steroid) and 0.9% (n=9) as severe (adrenergic).
Patients were flagged for emergency department visits or admission for an adverse reaction to mAb infusion if, within 48 hours of mAb infusion, patient had a drug administered for infusion reaction (any severity) and an emergency department visit or admission with length of stay less than or equal to one day. With these criteria, 0.9% (n=57) of mAb patients had an ED visit or admission potentially due to an adverse reaction to the infusion.
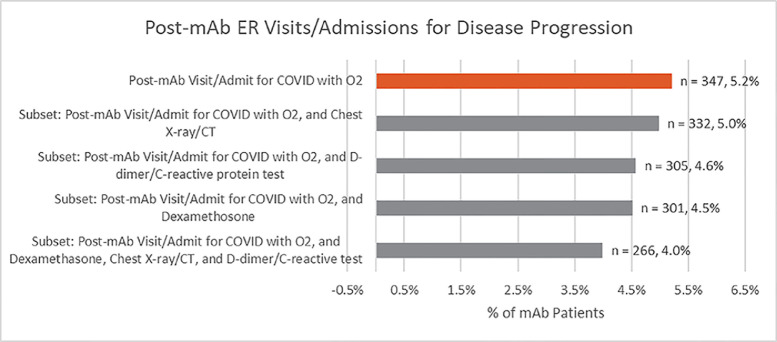
Post-mAb emergency department visits or admissions were considered related to COVID-19 progression if they occurred more than 24 hours but less than 14 days post-infusion, had a length of stay greater than 1 day if admitted, and required the use of oxygen. With this criteria, 5.2% of patients who received mAb (n=347) had a post-infusion emergency department visit or admission for COVID-19 disease progression. Additional analyses using stricter definitions and more clinical criteria for COVID-19 disease progression are displayed in figure 2 . Using the most clinically strict definition to indicate potential encounters for COVID-19 progression, the post-mAb ED visit/admission rate was reduced even further to 4.0% (266/6,669).
Figure 2.
Post-mAb Emergency department visits or admissions for COVID-19 disease progression.
Distribution of race/ethnicity was similar between patients with a post-mAb ED visit/admission and those without. The median age of patients with a post-mAb ED visit/admission was 68; the median age of patients without was 63. Distribution of Elixhauser comorbidities scores were similar between the groups. The most common comorbidities in both groups were diabetes and hypertension. Among those with a post-mAb ED visit/admission 16.1% had diabetes and 15.9% had hypertension; among those without a post-mAb visit, 13.7% had diabetes and 18.9% had hypertension.
Discussion
Administration of mAb is associated with a low rate of immediate reactions and subsequent emergency department visits or admission for COVID-19 disease progression. Reactions to mAb were relatively rare and, when occurring, mild in severity. The majority of patients who received mAb did not have a subsequent emergency department visit or admission for COVID-19 progression.
These real-world findings compare favorably to clinical trial results. The BLAZE-1 trials (bamlanivimab) had a hospitalization rate following mAb of 4% in a posthoc analysis of high-risk patients; patients who met the same criteria but received placebo had a hospitalization rate of 15%. The attributes of the high-risk patients in this analysis are similar to the inclusion criteria required of the EUA. Similar results have been reported regarding disease progression in patients with mild to moderate COVID-19 as well.(Homsher and Yamada, 1988)
As post-infusion reactions and subsequent ED visit/admissions for COVID-19 disease progression were rare among this population of patients, we were limited in the analyses that could be performed on these data. In general, there were few differences between patients with a post-mAb ED visit/admission and those with no visits following mAb infusion. Patients with a post-mAb ED visit/admission were older on average. As age is a known risk factor for more severe COVID-19 disease, additional analyses are necessary to determine the independent effect of mAb.(Sands et al., 2021a)
As is the case with all real-world observational studies, our analysis is limited due to constraints of the data. Analysis versus a historical comparison group, i.e. patients who would have met criteria for mAb but presented prior to the EUA, would be confounded by known changes in the patient population from the early phase of the pandemic.(Sands et al., 2021b) Inclusion of patients within the timeframe of our data set who met criteria but did not receive mAb may have introduced additional bias related to accessibility/availability of mAb, provider preference, or other social or cultural factors that influence consent.(Bierle et al., 2021) It should be noted that in our data set there was no mechanism for distinguishing patients who declined mAb from those who were not offered mAb among the outpatient/ED COVID-19 population.
In total, this analysis demonstrates that patients with COVID-19 who receive mAb in an outpatient or emergency department setting have a low likelihood of both an immediate negative reaction to the treatment as well as future inpatient admission related to COVID-19 disease progression. This supports the continued consideration of mAb for early treatment of COVID-19 patients.
Disclosures
Authors declare no conflicts of interest.
Funding
This work was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Drs. Jonathan Perlin and Kenneth Sands
References
- Bierle DM, Ganesh R, Wilker CG, Hanson SN, Moehnke DE, Jackson TA, et al. Influence of Social and Cultural Factors on the Decision to Consent for Monoclonal Antibody Treatment among High-Risk Patients with Mild-Moderate COVID-19. J Prim Care Community Health. 2021;12 doi: 10.1177/21501327211019282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 (casirivimab and imdevimab) 2020. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19.
- Food and Drug Administration. FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 (bamlanivimab and etesevimab); 2021. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0.
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. Jama. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E, Yamada T. The effect of shortening velocity on the shortening heat and its relationship to the distance shortened. Adv Exp Med Biol. 1988;226:689–700. [PubMed] [Google Scholar]
- Sands KE, Wenzel RP, McLean LE, Korwek KM, Roach JD, Miller KM, et al. Patient characteristics and admitting vital signs associated with coronavirus disease 2019 (COVID-19)-related mortality among patients admitted with noncritical illness. Infect Control Hosp Epidemiol. 2021;42(4):399–405. doi: 10.1017/ice.2020.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands KE, Wenzel RP, McLean LE, Korwek KM, Roach JD, Poland RE, et al. Changes in hospitalized coronavirus disease 2019 (COVID-19) patient characteristics and resource use in a system of community hospitals in the United States. Infect Control Hosp Epidemiol. 2021;42(2):228–229. doi: 10.1017/ice.2020.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]