Abstract
OBJECTIVE:
To develop a set of clinical criteria that identifies patients with a potential autoinflammatory IFNopathy.
METHODS:
Based on a literature review, a set of clinical criteria identifying genetically confirmed monogenic IFNopathies was selected. For validation, the clinical score was assessed in healthy controls (HCs) and 18 disease controls, including 2 known autoimmune IFNopathies, juvenile systemic lupus erythematosus (JSLE, n = 4) and dermatomyositis (JDM, n = 4); adenosine deaminase 2 deficiency (DADA2, n = 4); and oligoarticular juvenile idiopathic arthritis (oJIA, n = 6). We assessed an IFN score (IRG-S) in whole blood by NanoString using a previously published 28-gene-IRG-S and a reduced 6-gene-IRG-S.
RESULTS:
The 12 patients with a possible IFNopathy had higher clinical scores (3–5) than the patients with sJLE, JDM, DADA2, and oJIA and in HCs. Both the 28-IRG-S and 6-IRG-S were significantly higher in the autoinflammatory IFNopathy patients compared to HCs and oJIA and DADA2 patients but not different from patients with JSLE and JDM. Subsequently, genetic analysis revealed mutations in genes previously reported in genes related to the IFN pathway in 9 of the 12 patients.
CONCLUSION:
We developed a clinical score to identify patients with possible autoinflammatory IFNopathies. A clinical score was associated with a high IRG-S and may serve to identify patients with an autoinflammatory IFNopathy.
INTRODUCTION
Systemic autoinflammatory diseases are an expanding group of immunedysregulatory diseases that present typically in early childhood with flares of sterile inflammation that are driven by innate immune pathway activation without significant contribution of autoantibodies or antigen-specific T cells.1 While the “classic autoinflammatory diseases,” which include the hereditary fever syndromes and the cryopyrinopathies, illustrated the role of interleukin (IL)-1-activating inflammasome dysregulation as pathomechanism that causes these diseases, the description of autoinflammatory type-1 interferonopathies (IFNopathies) in the past decade has expanded the spectrum of autoinflammatory diseases beyond IL-1. This new group of autoinflammatory diseases is caused by dysregulation in innate immune pathways that regulate type I interferon (IFN) production.2,3 These “autoinflammatory type-1 IFNopathies” include chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE), Aicardi–Goutières syndrome (AGS), and STING-associated vasculopathy with onset in infancy (SAVI) and present with chronically elevated expression of IFN-response genes (IRGs) in peripheral blood.4 These diseases share clinical and immunological features that are not present in the previously defined IL-1-mediated “classic autoinflammatory diseases.” The autoinflammatory IFNopathies differ from autoimmune diseases with a high IFN-related gene expression, such as systemic lupus erythematosus (SLE) and juvenile dermatomyositis (JDM), who present with disease-specific autoantibodies and adaptive immune response dysregulation including T and B lymphocytes and dendritic cells, have a leading role in disease pathogenesis, and have evidence of immune complex deposition-mediated organ damage;4 these conditions have been termed autoimmune IFNopathies.5 The autoinflammatory IFNopathies may present with peripheral vasculitis and other vasculopathies, cerebral calcifications, and variable pulmonary disease. They often have fluctuating low-titer antinuclear antigen (ANA) and other autoantibodies, but the presence of autoantibodies does not correlate with the disease severity and immune complex deposition-mediated tissue damage (i.e., renal disease is not a typical feature). However, some patients with autoinflammatory IFNopathies, such as spondyloenchondrodysplasia (SPENCD), may develop classical features of SLE.6,7
As genetic testing and the assessment of the IFN-related gene expression that can be quantified in an IFN-response gene score (IRG-S) are not widely accessible, we set out to develop a clinical score that may allow to clinically define a disease subset with a high likelihood of having an autoinflammatory IFNopathy or help stratify patients who may benefit from further work-up, including an assessment of an IRG-S, genetic testing, and or treatment with Janus kinase (JAK) inhibitors.
First, we conducted a systematic literature review to define features of the known autoinflammatory IFNopathies (described in detail below) and recruited a cohort of probable autoinflammatory IFNopathies. Using the data from the literature review, we defined a list of criteria that were common to the genetically defined autoinflammatory IFNopathies. Each symptom was assigned a score of 1. We then calculated a clinical score by adding the symptoms in the patients with a probable autoinflammatory IFNopathy.
To validate the score, we assessed an IFN score by NanoString technology. When elevated, this would be consistent with ongoing IFN signaling8 and measurement of IRG expression is considered as a diagnostic tool in these diseases.8,9 We compared the performance and correlation of a previously developed 28-IRG-S with a more limited 6-IRG-S8 that assesses six IFN-induced genes only as well as the expression of C-X-C chemokine motif ligand 10 (CXCL10) alone.
Finally, we carried out genotyping to define the underlying genetic defect in these patients. Thus we were able to assess the practical performance of the clinical score and the IFN score for defining patients with monogenic autoinflammatory IFNopathy.
PATIENTS AND METHODS
Patients and defining clinical manifestations associated with type I IFNopathies
In our large clinical cohort of patients with autoinflammatory diseases, we defined patients who had clinical features suggestive of an IFNopathy such as CANDLE-like, AGS-like, and SAVI-like diseases (n = 12). They will be referred to as “probable autoinflammatory IFNopathy” patients.
A systematic literature review was conducted through MEDLINE and PubMed databases, from inception to January 2017, using the following keywords: “type 1 IFNopathies,” “type 1 IFNopathy,” “mendelian IFNopathies,” “mendelian IFNopathy,” “autoinflammation and IFN.” Case reports, original research articles, and review articles with a focus on type I IFNopathies were analyzed. Both searches were limited to English language. We also reviewed the references of these studies and review articles for additional publications. Literature review was performed by two investigators (H.E.S. and E.D.B.) independently. Discrepancies were resolved by discussion between the authors and with a third author (S.O.). Twenty-five articles were included after elimination steps through title, abstract, and full-text reviews (Supplementary Fig. S1).2,5,6,8,10–30 The clinical findings that were reported to be associated with monogenic type 1 IFNopathies are summarized in Table 1.
Table 1.
Clinical findings reported to be associated with monogenic type 1 interferonopathies (results from the systematic literature review)
| Clinical findings | |
|---|---|
| Skin involvement and vasculopathic changes | Panniculitis Nail dystrophy Ulcers, rash in acral surfaces OR acral skin infarcts OR violaceous plaques/nodules in cold-sensitive acral areas OR gangrene/ulcers/infarcts in acral areas Nodular erythema Violaceous periorbital rash Sparse/thin hair Livedo reticularis Periungal erythema Onychodystrophy Immature PMNLs in skin biopsy Chilblain-like rash OR cold-induced acral dermatitis Raynaud’s phenomena Microangiopathic vasculopathy |
| Mucosal involvement | Oral ulcers |
| Musculoskeletal involvement | Myositis (patchy) Axial bone dysplasia OR skeletal dysplasia OR enchondromatous nonossifying metaphyseal and spondylar lesions Short stature Joint contractures Non-erosive arthritis Lipodystrophy |
| Central nervous system involvement | Basal ganglia calcifications Leukoencephalopathy Progressive cerebral atrophy White matter disease Intellectual disability Microcephaly Polymicrogyria Spasticity Seizures L/P findings (neutrophilic vs lymphocytic) CNS vasculitis |
| Pulmonary and cardiac involvement | Interstitial lung disease OR pulmonary fibrosis Pulmonary hypertension |
| Hematologic involvement | Leukopenia (or cytopenia) with flares Dyserithropoesis Thrombocytosis Anemia |
| Ophtalmologic involvement | Keratoconjunctivitis Glaucoma Episcleritis |
| Metabolic involvement | Metabolic manifestations exaggerated by steroid (truncal obesity, dyslipidemia, insulin resistance, acanthosis nigricans) |
| Autoimmune findings | Positive autoantibodies Presence of autoimmune-mediated organ disease (e.g., SLE, autoimmune thyroiditis, DC+hemolytic anemia) Presence of low-titer autoantibodies |
| Other | High CRP value only in severe flares Recurrent fever Nasal septal perforation HSM Congenital infection-like syndrome OR pseudo-TORCH syndrome |
CNS central nervous system, CRP C-reactive protein, DC direct Coombs, L/P lumbar puncture, PMNL, polymorphonuclear leukocyte, SLE systemic lupus erythematosus
Subsequently, data from 25 articles reporting monogenic IFNopathies were used in the development of the “clinical” score for these diseases. The underlying monogenic IFNopathies in the patients described in these 25 articles varied from AGS, SAVI, CANDLE, SPENCD to ISG15 deficiency. We have classified the clinical findings into seven categories according to the system involvement in this novel set of criteria: (1) skin manifestations (nodular erythema, violaceous plaques in cold-sensitive acral areas); (2) vasculopathy (chilblain-like rash, microangiopathic vasculopathy, gangrene/ulcers/infarcts in acral areas); (3) lipodystrophy, (4) joint manifestations (contractures, non-erosive arthritis), (5) myositis (patchy), (6) central nervous system (CNS) manifestations (basal ganglia calcifications, leukoencephalopathy, white matter disease, lumbar puncture lymphocytic findings); (7) pulmonary involvement (interstitial lung disease, pulmonary fibrosis, pulmonary hypertension); and (8) leukopenia/lymphopenia with flares. For any criteria, the patient received only one point for the category.
All 12 patients with a probable autoinflammatory IFNopathy were then scored.
Control population
We included eight healthy controls (HCs) and four patients with juvenile SLE (JSLE), four with JDM, six with oligoarticular juvenile idiopathic arthritis (oJIA), and four with adenosine deaminase 2 deficiency (DADA2) as disease controls. HCs were age matched with the patient group. There was no evidence of infection during the blood sampling. Acute phase reactants were negative in all HCs.
Patients were diagnosed as having JSLE according to the International Systemic Lupus Clinics Cooperation criteria.31 All JDM patients met the Bohan and Peter criteria;32,33 oJIA patients were classified according to the International League of Associations for Rheumatology classification criteria.34 Diagnosis of all DADA2 patients was confirmed by genetic analysis.
The demographic data, clinical manifestations, laboratory findings, and treatment were assessed as well. SLE and oJIA patients were all newly diagnosed and treatment naive. All DADA2 patients were on etanercept treatment. JDM patients were active and/or were resistant to first-line conventional treatment.35 Written consents of the patients were obtained according to the Declaration of Helsinki (1964). The study was approved by the ethics committee of Hacettepe University (Nov. 04, 2015; GO15/678-28).
RNA isolation, measurement of gene expression, and calculation of IRG-S
28-IRG-S and 6-IRG-S were assessed in the 12 patients with probable autoinflammatory IFNopathy and in the HCs and disease control groups.
RNA isolation and IRG-S calculation were performed as previously reported.9,36 In short, peripheral blood samples from all patients and controls were stored in PAX gene blood RNA tubes (Qiagen, Germantown, MD). PAX gene RNA tubes are designed for the collection of 2.5 ml of blood and contain 6.9 ml of a proprietary RNA-stabilizing reagent. PAX gene blood RNA tubes were frozen first at −20 °C for 24 h, then transferred to be stored at −80 °C. Total RNA was isolated by using the PAX gene RNA Isolation Kit following the manufacturer’s recommendations. After RNA isolation, RNA concentration was measured with a spectrophotometer. Gene expression was measured using NanoString technology (nCounter FLEX Analysis System) according to the manufacturer’s instructions (www.nanostring.com). NanoString technology is a quick and easy method to assess IFN gene expression. Recently, this method was validated in treatment-naive patients with autoinflammatory IFNopathy.9 NanoString technology provides a method for detecting mRNAs with molecular barcodes called nCounter Reporter Probes without the use of reverse transcription or amplification. Briefly, this technology includes three steps as follows: hybridization, nCounter Prep Station, and nCounter Digital Analyzer.37 Subsequently, IFN6 and IFN28 scores were calculated according to mRNA expression levels of the type I IFN-inducible gene using the nSolver software. First, the raw copy number of mRNA transcripts of each gene was normalized to the geometric mean of the four housekeeping genes (ALAS1, HPRT1, TBP, TUBB) for each individual. A Z-score for each IFN-inducible gene was calculated using the following formula:9
Then 28-IRG-S and 6-IRG-S scores were calculated by summing the 6 and 28 Z-scores for each sample, respectively. 6-IRG-S includes six IFN-related genes (IFI27, IFI44L, IFIT1, ISG5, RSAD2, SIGLEC1) and 28-IRG-S evaluates 28 IFN-related genes (CXCL10, DDX60, EPSTI1, GBP1, HERC5, HERC6, IFI27, IFI44, IFI44L, IFI6, IFIT1, IFIT2, IFIT3, IFIT5, ISG5, LAMP3, LY6E, MX1, OAS1, OAS2, OAS3, OASL, RSAD2, RTP4, SIGLEC1, SOCS1, SPATS2L, USP18). CXCL10 (IP10) score was also calculated by normalizing the CXCL10 counts to the geometric mean of the 4 housekeeping genes.9
Genetic analysis
Genetic analysis was carried out in the 12 patients with a probable autoinflammatory IFNopathy. All patients were initially analyzed with the immune deficiency-dysregulation panel in our department of immunology including the well-known IFN-related genes (TREX1, RNASEH2B, RNASEH2C, RNASEH2A, SAMHD1, ADAR, IFIH1, PSMB8, TMEM173, ISG15, ACP5) (n = 12). In patients who did not have any of the above mutations, whole-exome sequencing (WES) including variant filtering was performed (n = 3, 1 at NIH).
Statistical analysis
The SPSS version 21.0 (SPSS, Inc., Chicago, IL) was used for statistical analysis. The variables were investigated using visual (histogram, probability plots) and analytic methods (Kolmogorov–Smirnov/Shapiro–Wilk’s test) to determine whether or not they are normally distributed. Descriptive analyses were presented using proportions, medians, minimum (min), and maximum (max) values where appropriate. Differences in proportions between groups were evaluated by the Chi-square test or Fisher’s exact test where appropriate. Mann–Whitney U test was used to compare the non-normally distributed continuous data between two groups. p Value < 0.05 was considered as significant. Kruskal–Wallis tests were conducted to compare the non-normally distributed parameters between groups. Mann–Whitney U test was performed to test the significance of pairwise differences using Bonferroni corrections to adjust for multiple comparisons. For investigating the association between non-normally distributed and/or ordinal variables, the correlation coefficients and their significance were calculated using Spearman test.
RESULTS
Demographic and clinical features
A total of 12 patients were included in the autoinflammatory IFNopathy group, 9 (75%) were male. The median (min–max) age at symptom onset and current age were 3 (0.5–9) years and 10 (4–20) years, respectively. Skin involvement was present in all patients (Table 2). The most common skin findings were ulcers and rash in acral surfaces in nine, panniculitis in four, livedo reticularis in four, chilblain-like rash in four, gangrene/infarcts of fingers/toes in three, and Raynaud’s phenomena in two patients. One patient had classical features of SAVI with severe tissue loss [published in ref. 17].
Table 2.
Clinical features of the patients and controls
| Autoinf. IFNopathy (n = 12) | HC (n = 8) | oJIA (n = 6) | JSLE (n = 4) | DADA2 (n = 5) | JDM (n = 4) | |
|---|---|---|---|---|---|---|
| Gender (F/M) | 3/9 | 3/5 | 5/1 | 4/0 | 1/4 | 3/1 |
| Skin involvement, n (%) | 12 (100) | 0 (0) | 0 (0) | 4 (100) | 5 (100) | 3 (75) |
| Musculoskeletal involvement, n (%) | 8 (41.7) | 0 (0) | 6 (100) | 4 (100) | 5 (100) | 2 (50) |
| Renal involvement, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) |
| CNS involvement, n (%) | 8 (66.7) | 0 (0) | 0 (0) | 0 (0) | 2 (40) | 0 (0) |
| Pulmonary involvement, n (%) | 3 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) |
| Cardiac involvement, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) |
| Hematologic involvement, n (%) | 2 (16.7) | 0 (0) | 0 (0) | 4 (100) | 2 (40) | 0 (0) |
CNS central nervous system, DADA2 adenosine deaminase 2 deficiency, F female, HC healthy control, IFNopathy interferonopathy, JDM juvenile dermatomyositis, M male, oJIA oligoarticular juvenile idiopathic arthritis, SLE systemic lupus erythematosus
Eight patients had CNS involvement, including basal ganglia calcifications (n = 5), white matter disease (n = 3), progressive cerebral atrophy (n = 2), and CNS vasculitis (n = 2) (Table 2). Four of these patients had motor-mental retardation and were also followed by the neurology department, whereas the remaining three did not have a neurological deficit and the CNS findings were picked up by magnetic resonance imaging (MRI) examinations. One had optic neuritis.
Four patients had joint contractures, four had lipodystrophy, and one had non-erosive arthritis. Three patients had interstitial lung disease established through computerized tomography (CT). One patient also had pulmonary fibrosis. Chest CTs were not obtained for the remaining nine patients since their clinical lung exam and X-rays were normal. One patient had autoimmune hepatitis.
Fluctuating low-titer autoantibodies were detected among 11 patients, including ANA (n = 11), anti-double-stranded DNA (n = 2), and anti-neutrophil cytoplasmic antibodies (n = 2), all at low titers. All except one patient (one of the sibs with an AGS mutation) had occasional elevation of acute-phase reactants (APRs). However, at the time of the blood sample collection, only two patients in this “autoinflammatory IFNopathy group” had elevated APRs. Two patients had leukopenia. The comparison of the clinical findings of the patients and controls is summarized in Table 2.
Developing a clinical score suggesting the presence of an IFNopathy
Subsequently with the systematic literature review, we developed a preliminary clinical score to classify the autoinflammatory IFNopathy patients (Table 3). Table 1 shows the main clinical findings associated with monogenic IFNopathies. Preliminary clinical score consisted of eight items and all items were scored as no (0) or yes (1).
Table 3.
Preliminary clinical classification criteria to classify patients with interferon (IFN)-mediated autoinflammatory diseases: a score of at least 3 is required
| Clinical criteria for autoinflammatory interferonopathies (derived from the literature) |
|---|
| 1. Skin manifestations (nodular erythema, violaceous plaques in cold-sensitive acral areas) |
| 2. Vasculopathy (chilblain-like rash, microangiopathic vasculopathy, gangrene/ulcers/infarcts in acral areas) |
| 3. Lipodystrophy |
| 4. Joint manifestations (contractures, non-erosive arthritis) |
| 5. Myositis (patchy) |
| 6. CNS manifestations (basal ganglia calcifications, leukoencephalopathy, white matter disease, L/P lymphocytic findings) |
| 7. Pulmonary involvement (interstitial lung disease, pulmonary fibrosis, pulmonary hypertension) |
| 8. Leukopenia/lymphopenia with flares |
CNS central nervous system, L/P lumbar puncture
The clinical score that we propose was able to differentiate the autoinflammatory IFNopathy patients since it was high in only them but not in autoimmune IFNopathies such as JSLE and JDM and in DADA2 patients (Table 4). All of the patients in the autoinflammatory IFNopathy group had clinical scores ≥3.
Table 4.
Application of preliminary clinical score to patients and controls
| Clinical preliminary score | Probable Autoinflammatory interferonopathies, n = 12 | JIA, n = 6 | JSLE, n = 4 | DADA2, n = 5 | JDM, n = 4 |
|---|---|---|---|---|---|
| Skin manifestations | 12/12 | 0/6 | 0/4 | 1/5 | 1/4 |
| Vasculopathy | 6/12 | 0/6 | 0/4 | 1/5 | 0/4 |
| Lipodystrophy | 2/12 | 0/6 | 0/4 | 0/5 | 0/4 |
| Joint manifestations | 5/12 | 0/6 | 0/4 | 0/5 | 2/4 |
| Myositis | 3/12 | 0/6 | 0/4 | 0/5 | 0/4 |
| CNS manifestations | 8/12 | 0/6 | 0/4 | 0/5 | 0/4 |
| Pulmonary involvement | 2/12 | 0/6 | 0/4 | 0/5 | 1/4 |
| Leukopenia/lymphopenia with flares | 4/12 | 0/6 | 4/4 | 1/5 | 0/4 |
| Clinical score, median (min–max) | 3 (3–5) | 0 (0) | 1 (0–1) | 0 (0–1) | 1 (0–2) |
All items were scored as no (0) or yes (1)
CNS central nervous system, min–max minimum–maximum
Assessment of the IFN score confirmed elevation in all patients with presumed autoinflammatory IFNopathy
The 28-IRG-S and 6-IRG-S were calculated for the “autoinflammatory IFNopathy” patients and the HCs as well as the disease control groups. The clinical scores significantly correlated with IFN6, IFN28, and CXCL10 scores (r = 0.628, p = 0.001; r = 0.638, p = 0.001; r = 0.608, p = 0.001, respectively).
Both the 28-IRG-S and 6-IRG-S were significantly higher in the autoinflammatory IFNopathy group compared to the HC and oJIA and DADA2 patients. However, there was no significant difference between the probable IFNopathy group and the autoimmune IFNopathies, the JSLE and JDM patients. Interestingly, the CXCL10 (IP10) score was significantly higher in the probable autoinflammatory IFNopathy patients compared to patients with JSLE and JDM (Fig. 1; Supplementary Table S1). The 28-IRG-S positively correlated with the 6-IRG-S (r = 0.969, p < 0.001). The CXCL10 (IP10) score correlated both with the 28-IRG-S and 6-IRG-S (r = 0.521, p = 0.001 and r = 0.544, p = 0.001, respectively).
Fig. 1.
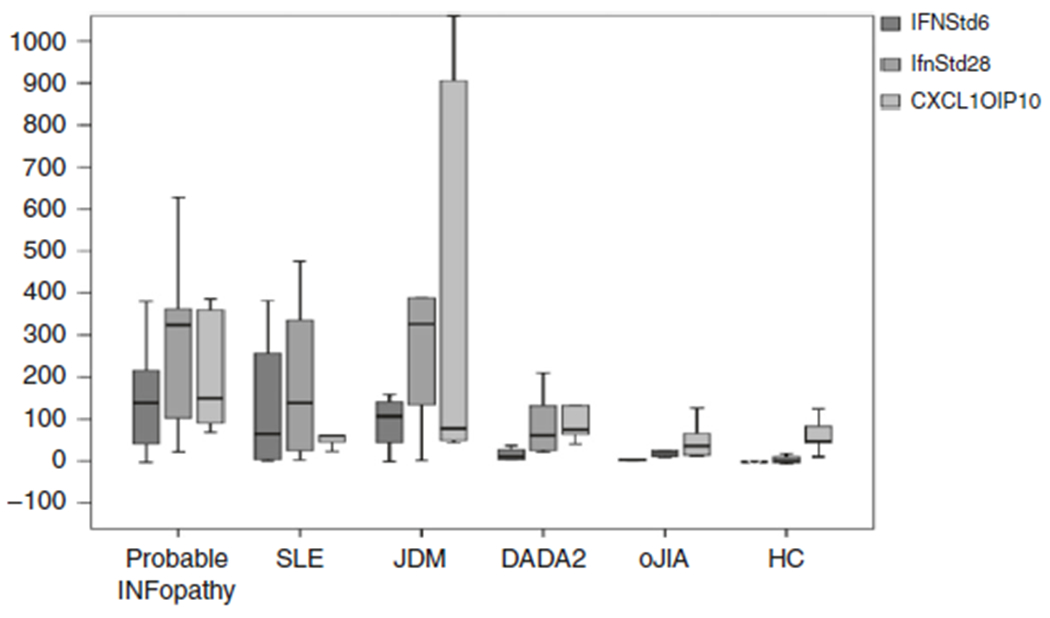
Interferon (IFN) scores of patients and controls
Genetic analysis confirms a genetic diagnosis in 9 out of 12 patients with a presumed autoinflammatory disease
Genetic analyses provided a diagnosis for 9 of the 12 patients. Three patients with a CANDLE-like disease manifestations (patients 3, 5, and 8) such as panniculitis, lipodystrophy, and rash had mutations associated with AGS in IFIH1 (p.Thr702Ile) and RNASEH2B (n = 2; p.Ala177Thr homozygous), respectively. The patients with a CANDLE-like phenotypes had no apparent neurologic symptoms, and their mental–motor development was not delayed. However, all three had basal ganglia calcifications, and in contrast to CANDLE patients, all had white matter disease on MRI (Table 5). Two siblings (patients 6 and 7) with a typical AGS phenotype had cerebral vasculitis and both had AGS-related mutations in TREX1 (homozygous p.Arg114Cys) (Table 5). One patient (patient 2) had a homozygous intronic c.3443+8G>A mutation in a splice site of the ADAR1 gene. One AGS patient (patient 11) was homozygous for the p.Ala177Thr mutation in RNASEH2B, which was also present in two unrelated patients with a CANDLE-like phenotypes (patients 5 and 8) as described above (Table 5). All of these mutations were previously described. One patient (patient 10) had a previously described SAVI-causing mutation (p.Asn154Ser).17,38
Table 5.
Genetic analysis and main clinical findings of the “autoinflammatory interferonopathy” patients
| Patients | Current age/symptom-onset age | Clinical phenotype | Radiologic cranial MRI findings | Genotype | High APR |
|---|---|---|---|---|---|
| Patient 1 | 16/1 | Digital necrosis, livedo reticularis, contractures, MMR, contracture, autoimmune hepatitis | Periventricular calcification | No definite disease-causing variants found in WESa | Yes |
| Patient 2 | 18/3 | Chilblain, digital ulcers, MMR | Calcification in globus pallidus | Homozygous for common SNP in ADAR1b (c.3443+8G>A) |
Yes |
| Patient 3 (CANDLE-like) | 4/0.5 | Fever, cold-induced rash, panniculitis, lipodystrophy, contracture | Calcification in globus pallidus, white matter disease | Heterozygous mutation in IFIH1c c.2105C>T p.Thr702Ile |
Yes |
| Patient 4 | 6/3 | Fever, rash, panniculitis, HSM, fluctuating cytopenia | Normal | Heterozygous mutation in STAT3c c.893T>A, p.Ile298Asn |
Yes |
| Patient 5 (CANDLE-like) | 5/2 | Fever, elevated transaminase, panniculitis | Basal ganglia calcification, white matter disease | Homozygous mutation in RNASEH2Bd c.529G>A p.Ala177Thr |
Yes |
| Patient 6 (Sib 1) | 10/3.5 | Chilblain, digital ulcers, MMR, contracture | Cerebral vasculitis | Homozygous mutation in TREX1d c.340C>T p.Arg114Cys |
Yes |
| Patient 7 (Sib 2) | 5/3.5 | Chilblain | Cerebral vasculitis | Homozygous mutation in TREX1d c.340C>T p.Arg114Cys |
No |
| Patient 8 (CANDLE-like) | 10/2 | Cold-induced rash, panniculitis, lipodystrophy | Calcification in globus pallidus, white matter disease | Homozygous mutation in RNASEH2Bd c.529G>A p.Ala177Thr | Yes |
| Patient 9 | 13/4 | Livedo reticularis, lipodystrophy, interstitial lung disease, ulcers, pulmonary fibrosis | Optic neuritis | No definite disease-causing variants found in WESa | Yes |
| Patient 10e | 20/0.5 | Digital necrosis, autoamputation, interstitial lung disease, fluctuating cytopenia | Normal | SAVI-causing mutation in TMEM173e c.461A>G p.Asn154Ser | Yes |
| Patient 11 | 5/2.5 | Cold-induced rash, chilblain, livedo reticularis, MMR, panniculitis | Not available | Homozygous mutation in RNASEH2Bd c.529G>A p.Ala177Thr | Yes |
| Patient 12 | 14/9 | Cold-induced rash, digital necrosis, contracture, lipodystrophy, interstitial lung disease | Normal | Heterozygous mutation in STIM1c c.1299C>T p.Ile433Ile Synonymous SNP (possible splice variant) |
Yes |
APR acute phase reactants, MMR mental, motor retardation, gnomAD Genome Aggregation Database (https://gnomad.broadinstitute.org)
In two patients, no diseases-causing mutations were identified in WES
ADAR1 single-nucleotide polymorphism (SNP) (patient 2, gnomAD allele frequency 0.46, 30,793 homozygotes) functional impact not clear but likely not disease-causing
Mutations not previously associated with disease in IFIH1 (pt.3, gnomAD allele frequency 0.0023, zero homozygotes), STAT3 (patient 4, not present in gnomAD), STIM1 (patient12, gnomAD allele frequency 0.000021, zero homozygotes)
Patients with previously reported mutations in RNASEH2B (patients 2, 5, 8, and 11), TREX1 (patients 6 and 7), and TMEM173 (patient10)
This patient was previously published17
One patient (patient 4) without disease-causing mutations on the genetic panel who subsequently had WES harbors a gain-of-function mutation in the STAT3 gene (c.893T>A, p.Ile298Asn) (Table 5). Another patient (patient 12) had a heterozygous mutation in STIM1 (c.1299C>T), which was recently linked to activation of the STING pathway.39 This mutation has a minor allele frequency of 0.000021. But functional impact on IFN signaling needs to be evaluated. The patient with the STIM1 mutation (patient 12) resembled SAVI with the digital necrosis, interstitial lung disease, and rash, whereas the suggestive features of the patient with STAT3 mutation (patient 4) were rash, fever, panniculitis, and hepatosplenomegaly (Table 5).
No mutations were identified in WES performed on two patients (patients 1 and 9), one (patient 1) with an AGS-like phenotype with motor-mental retardation and the other (patient 9) with interstitial lung disease, lipodystrophy, and livedo reticularis. Furthermore, patient 2 had a mutation in ADAR1, however, we were not able to confirm whether this was a disease-causing mutation or a common single-nucleotide polymorphism (Table 5).
Treatment
Almost all of the “autoinflammatory IFNopathy” patients received corticosteroids, five patients also received hydroxychloroquine, and two patients received azathioprine. Five of the 12 patients with autoinflammatory IFNopathy were treated with JAK inhibitors. After the elevated IRG-S were obtained, three patients with CANDLE-like phenotype (patients 3, 5, and 8) and one patient with TREX1 mutation (patient 6) were started on tofacitinib (compassionate use). All of them were stable and had no new symptoms. One SAVI patient has been on baricitinib for 4 years.40
DISCUSSION
Autoinflammatory IFNopathies have emerged as a new group of inflammatory diseases that changed our concept of cytokines pathways that cause autoinflammatory diseases.5 Patients with autoinflammatory IFNopathies present with a range of clinical symptoms that are distinct from those found in IL-1-mediated autoinflammatory disease and the most common autoimmune pediatric rheumatologic diseases, such as JSLE and JDM with chronic elevation of an IRG signature in the blood. The diagnosis of this novel group of diseases is challenging as the assessment of an IFN score or IRG-S and sophisticated genetic testing are not widely available. In the present study, our first objective was to provide the spectrum of clinical manifestations and address the challenge in recognizing these patients by attempting to develop a clinical score that will encourage physicians to request and order analysis of the IFN pathway. This clinical score distinguished autoinflammatory IFNopathies from SLE and JDM. Furthermore, it predicted a known genetically defined autoinflammatory IFNopathy in 75% (9/12) of the patients. On the other hand, the features of our patients and the genetic results (Tables 4 and 5) highlight the clinical overlaps between AGS and CANDLE and SAVI that is not well described in the literature. Three of the patients who clinically were thought to have CANDLE (with neutrophilic panniculitis, etc.) and who were without any clinical neurological features up-front had white matter disease. This raises the suspicion that patients with white matter disease may be more likely to have mutations associated with AGS. We have also presented one patient who resembled SAVI but had a mutation in STIM1 (Table 5), another gene that was recently linked to activation of the IFN pathway.39,41
Our clinical score had an excellent correlation with the IFN score. Direct detection of type I IFN protein in biologic samples has proved challenging, and while IFN have been measured in serum, reliable assays for IFN are still not available. Thus indirect methods are often used to infer the presence of type I IFN via quantification of IFN-responsive genes.37 In their review of available methods for IFN detection, Lamot et al.37 conclude that none have proven feasible for everyday clinical practice. We therefore assessed the 28-IRG-S using a previously published NanoString panel.9,36 We have found that the more reduced panel of 6 genes (IFN6-IRG-S) and a 28-gene panel (IFN28-IRG-S) scores and the CXCL10 expression alone correlated well suggesting that in the context of a suggestive clinical disease pattern expression of a small number of IRGs may be sufficient to document elevation of IFN-stimulated genes. Although our cohort was small, we believe that these results will have important implications for routine use. All of these scores were significantly lower in diseases where IFN has not been implicated in pathogenesis, such as oJIA. On the other hand, the 8-IRG-S and 6-IRG-S were mildly elevated in DADA2 patients. As expected, these IFN scores were also high in the other autoimmune diseases with high IFN, JSLE and JDM. Interestingly, we have noted that CXCL10 was significantly higher among patients with autoinflammatory IFNopathies when compared to SLE: there was a threefold difference that differentiated the two groups of patients. Furthermore, CXCL10 scores were 2.5 times higher than in the four JDM patients. Given the small size of the our JSLE and JDM cohort, studies in larger number of patients are needed to reproduce and validate these findings.
The gold standard for the diagnosis of an autoinflammatory IFNopathy is identifying the mutated gene. We had a very high yield in the genetic analysis of the presented patients, thus our clinical score allowed us to detect patients with a high likelihood to have a genetically defined IFNopathy. Whether a score >3 also identified patients with yet genetically unidentified IFNopathies who may respond to IFN signal-blocking treatments needs to be assessed in larger studies.
Our data suggest that a positive clinical score plus a high IFN score is highly suggestive of a known Mendelian autoinflammatory IFNopathy. Nine of our patients subsequently had a genetic diagnosis of a monogenic IFNopathy. Three of our patients with predominantly CANDLE-like phenotypes had mutations previously associated with AGS (Table 5). All these patients had basal ganglia calcifications and white matter disease without neurologic symptoms.
One patient had SAVI with a disease-causing mutation in TMEM173, and one had a STAT3 gain of function mutation. We await this patient’s response to JAK inhibitors. Although Sanchez et al.40 have reported favorable outcome with baricitinib in CANDLE patients, the jury is out for the best treatment for many of these patients, particularly those who do not have CANDLE.
A disease-causing genetic defect was not found in three patients who again had features suggestive of an autoinflammatory IFNopathy. It remains intriguing to speculate that non-monogenic genetic pattern may need to be considered as well.
Furthermore, one patient with a positive clinical score and high IFN score harbored a heterozygous mutation in a gene associated with an immune dysregulation, the sensor stromal interaction molecule 1 (STIM1) gene. Most recently, Srikanth et al.39 have demonstrated that deficiency in the Ca2+ STIM1 caused spontaneous activation of STING and enhanced the expression of type I IFNs. However, more functional studies are needed to determine the functional impact of this mutation and the pathways that induce the high IFN score need to be assessed in future studies. Our data also suggest that, should an extended IRG panel of 28-IRG-S or 6-IRG-S not be available, then even CXCL10 assessment by itself in the context of suggestive clinical findings was a good indicator of an elevated more extensive IFN score given the high correlation of the CXCL10 with the 28-IRG-S and 6-IRG-S.
Fluctuating levels of autoantibodies were observed in 11 of the patients with autoinflammatory IFNopathies, which did not discriminate them from JSLE and JDM. However, the score easily differentiates these patients from patients with the autoimmune IFNopathies, including JSLE and JDM. This may not be surprising as our patients have early-onset disease and lack characteristic features for the well-known pediatric autoimmune diseases including clinical features of immune-complex disease (i.e., butterfly rash and immune-complex vasculitis or nephritis) that are typically present in SLE patients. On the other hand, JSLE and JDM patients lack the clinical criteria of autoinflammatory IFNopathies.
Given the recent encouraging treatment data in patients with autoinflammatory IFNopathies, a “proper diagnosis” of an autoinflammatory IFNopathy can direct treatment decisions and prevent the use of improper treatments. A total of four patients have been started on a JAK inhibitor, mostly tofacitinib, since baricitinib is not available in Turkey. None of the patients treated have so far achieved complete remission of their symptoms, which is consistent with other case reports in AGS and SAVI.36,42 The patients started on JAK kinase inhibitor treatment stabilized their disease and have not developed new symptoms. However, JAK inhibitor treatment in patients with AGS and SAVI may not be as effective as anti-IL-1 treatment in the inflammasomopathies; we await further long-term observations. None of our patients had mutations of CANDLE, which is the only monogenic disease where prolonged remission with JAK inhibitor treatment has been reported in 50% of patients.36
Our study is limited by the small sample size. The clinical score was based on the clinical findings of our review of the relevant literature. In our cohort, the sensitivity and specificity of the test was 75%, but as proper sensitivity analysis cannot be performed in this small cohort, validations needs to be performed in much larger and more diverse cohorts.
In our expanding spectrum of rare rheumatic diseases, the autoinflammatory IFNopathies are a new class of diseases that we need to consider. The availability of measuring and quantifying the IFN score expands the armamentarium of immunologic tests that assist in characterizing dysregulated immune responses. The diagnosis of these patients by genetic testing is still challenging in many parts of the world or a genetic analysis may be unrevealing. On the other hand, we need to identify these diseases since the current first-line treatment is JAK inhibition and the availability of treatment(s) that block the IFN signaling pathway is expanding. The clinical score we developed identified patients who had elevated IFN scores and may be helpful for the clinicians in identifying patients with autoinflammatory IFNopathies. The clinical score alone or in combination with a high IFN score should prompt genetic screening for mutations in the IFN pathway and may serve as indications for a genetic analysis to be covered by the Health Authorities. However, 3 of the 12 patients did not have a genetic diagnosis of a previously identified gene causing autoinflammatory IFNopathy and studies to suggest whether these patients would respond to IFN-blocking therapies needs to be determined. In summary, our data show that the clinical score was high only among patients with autoinflammatory IFNopathies and distinguished them from autoimmune diseases, such as SLE. Furthermore, a clinical score of >3 was associated with a high 28-IRG-S or 6-IRG-S that was higher than healthy and diseased controls but not different from JSLE and JDM. Clinical criteria predicted a known genetically defined autoinflammatory IFNopathy in 9/12 (75%) of patients with a probable IFNopathy and may be used in identifying patients who need genetic testing, IFN score assessments, and possibly access to IFN-blocking treatments.
CONCLUSION
The suggested clinical score may guide clinicians in their clinical practice. An autoinflammatory IFNopathy should be considered in patients with a positive clinical score and high IFN score, even if the genetic analysis is not confirmatory. Finally, the pediatric community needs to design controlled studies with well-defined outcomes to define the best management and treatment of these rare but important diseases.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the Tubitak project (116S707).
Footnotes
The online version of this article (https://doi.org/10.1038/s41390-019-0614-2) contains supplementary material, which is available to authorized users.
Competing interests: The authors declare no competing interests.
REFERENCES
- 1.McDermott MF et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97, 133–144 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Crow YJ & Type I IFNopathies: a novel set of inborn errors of immunity. Ann. NY Acad. Sci 1238, 91–98 (2011). [DOI] [PubMed] [Google Scholar]
- 3.de Jesus AA, Canna SW, Liu Y & Goldbach-Mansky R Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu. Rev. Immunol 33, 823–874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kretschmer S, Lee-Kirsch MA & Type I IFN-mediated autoinflammation and autoimmunity. Curr. Opin. Immunol 49, 96–102 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Sanchez GA & Goldbach-Mansky R Insights from Mendelian IFNopathies: comparison of CANDLE, SAVI with AGS, monogenic lupus. J. Mol. Med. (Berl.) 94, 1111–1127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canna SW & Goldbach-Mansky R New monogenic autoinflammatory diseases-a clinical overview. Semin. Immunopathol 37, 387–394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jesus AA et al. Novel proteasome assembly chaperone mutations in PSMG2/PAC2 cause the autoinflammatory IFNopathy CANDLE/PRAAS4. J. Allergy Clin. Immunol 143, 1939.e1938–1943.e1938 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice GI et al. Assessment of type I IFN signaling in pediatric inflammatory disease. J. Clin. Immunol 37, 123–132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H et al. Development of a validated IFN score using NanoString technology. J. IFN Cytokine Res 38, 171–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston JH et al. A type I IFN score identifies bilateral striatal necrosis due to mutations in ADAR1. J. Med. Genet 51, 76–82 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Crow YJ, Vanderver A, Orcesi S, Kuijpers TW & Rice GI Therapies in Aicardi-Goutieres syndrome. Clin. Exp. Immunol 175, 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice GI et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I IFN signaling. Nat. Genet 46, 503–509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meuwissen ME et al. Human USP18 deficiency underlies type 1 IFNopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med 213, 1163–1174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livingston JH & Crow YJ Neurologic phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi-Goutieres syndrome and beyond. Neuropediatrics 47, 355–360 (2016). [DOI] [PubMed] [Google Scholar]
- 15.van Kempen TS, Wenink MH, Leijten EF, Radstake TR & Boes M Perception of self: distinguishing autoimmunity from autoinflammation. Nat. Rev. Rheumatol 11, 483–492 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Lee-Kirsch MA, Wolf C, Kretschmer S, Roers A & Type I IFNopathies-an expanding disease spectrum of immunodysregulation. Semin. Immunopathol 37, 349–357 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Chia J et al. Failure to thrive, interstitial lung disease, and progressive digital necrosis with onset in infancy. J. Am. Acad. Dermatol 74, 186–189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee-Kirsch MA, Gunther C & Roers A Nucleic acid-mediated autoinflammation and autoimmunity-type I IFNopathies. J. Mol. Med 94, 1081–1084 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Pathak S, McDermott MF & Savic S Autoinflammatory diseases: update on classification diagnosis and management. J. Clin. Pathol 70, 1–8 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Torrelo A CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front. Immunol 8, 927 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crow YJ Type I IFNopathies: mendelian type I IFN up-regulation. Curr. Opin. Immunol 32, 7–12 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Munoz J et al. Stimulator of IFN genes-associated vasculopathy with onset in infancy: a mimic of childhood granulomatosis with polyangiitis. JAMA Dermatol. 151, 872–877 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Lee-Kirsch MA The type I IFNopathies. Annu. Rev. Med 68, 297–315 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Rodero MP, Crow YJ & Type I IFN-mediated monogenic autoinflammation: The type I IFNopathies, a conceptual overview. J. Exp. Med 213, 2527–2538 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X et al. Human intracellular ISG15 prevents IFN-alpha/beta over-amplification and auto-inflammation. Nature 517, 89–93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crow YJ & Manel N Aicardi-Goutieres syndrome and the type I IFNopathies. Nat. Rev. Immunol 15, 429–440 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Picard C et al. Inherited anomalies of innate immune receptors in pediatric-onset inflammatory diseases. Autoimmun. Rev 14, 1147–1153 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Munoz J et al. IFNopathies. Ann. Dermatol Venereol 142, 653–663 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Buers I, Nitschke Y & Rutsch F Novel IFNopathies associated with mutations in RIG-I like receptors. Cytokine Growth Factor Rev. 29, 101–107 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Volpi S et al. IFNopathies in pediatric rheumatology. Pediatr. Rheumatol. Online J 14, 35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petri M et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohan A & Peter JB Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med 292, 344–347 (1975). [DOI] [PubMed] [Google Scholar]
- 33.Bohan A & Peter JB Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med 292, 403–407 (1975). [DOI] [PubMed] [Google Scholar]
- 34.Petty RE et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol 31, 390–392 (2004). [PubMed] [Google Scholar]
- 35.Bellutti Enders F et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann. Rheum. Dis 76, 329–340 (2017). d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H et al. Pharmacokinetics, pharmacodynamics, and proposed dosing of the oral JAK1 and JAK2 inhibitor baricitinib in pediatric and young adult CANDLE and SAVI patients. Clin. Pharm. Ther 104, 364–373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamot L, Niemietz I & Brown KL Methods for type I interferon detection and their relevance for clinical utility and improved understanding of rheumatic diseases. Clin. Exp. Rheumatol (In Press, 2019) [PubMed] [Google Scholar]
- 38.Liu Y et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med 371, 507–518 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srikanth S et al. The Ca(2+) sensor STIM1 regulates the type I IFN response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat. Immunol 20, 152–162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez GAM et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory IFNopathies. J. Clin. Invest 128, 3041–3052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parry DA et al. A homozygous STIM1 mutation impairs store-operated calcium entry and natural killer cell effector function without clinical immunodeficiency. J. Allergy Clin. Immunol 137, 955–957 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kothur K et al. An open-label trial of JAK 1/2 blockade in progressive IFIH1-associated neuroinflammation. Neurology 90, 289–291 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


