Abstract
Background
This study was designed to determine the epidemiological trends and adverse outcomes of hepatopulmonary syndrome (HPS).
Methods
This retrospective interrupted trend study analyzed data from the Nationwide Inpatient Sample (NIS) for the years 2012, 2014, 2016 and 2018 to identify adult (≥ 18 years) hospitalizations with a diagnosis of HPS. We highlighted epidemiological trends for HPS. Inpatient mortality, mean length of stay (LOS) and mean total hospital charge (THC) were estimated using multivariate regression trend analysis.
Results
We observed an increase in the total number of HPS hospitalizations from 1,565 in 2012 to 2,495 in 2018, with mean age ranging from 55.8 to 58.1 years. There was a trend towards increasing hospitalizations (P-trend < 0.001) with increasing mean age (P-trend = 0.003) for HPS. Whites made up most of the study population. The inpatient mortality for HPS ranged from 12.4% to 12.6%, but there was no statistically significant trend for mortality (P-trend = 0.534) between 2012 and 2018. Additionally, there was no change in both mean LOS (P-trend = 0.545) and mean THC (P-trend = 0.534) for HPS for these years.
Conclusions
Hospitalizations and mean age for HPS were on the rise. Inpatient mortality ranged from 12.4% to 12.6%; however, a statistically significant trend for mortality was absent.
Keywords: Hepatopulmonary syndrome, Epidemiology, Outcomes, Total hospital charge, Length of stay, Mortality
Introduction
Hepatopulmonary syndrome (HPS) is a characterized by abnormalities in blood oxygenation secondary to the presence of intrapulmonary vascular dilations (IPVD) in the setting of end-stage liver disease [1, 2]. The complex pathogenic mechanism revolves around overproduction of vasoactive mediators such as tumor necrosis factor-alpha, heme oxygenase-derived carbon monoxide and nitric oxide, and angiogenesis within the pulmonary circulation leading to intrapulmonary shunting and ventilation-perfusion mismatch in the background of liver cirrhosis and portal hypertension [3]. Furthermore, based on the alveolar-arterial oxygen gradient (A-a gradient) and partial pressure oxygen (PaO2), it can be classified into four distinct categories ranging from mild (PaO2 ≥ 80 mm Hg), moderate (PaO2 60 - 79 mm Hg), severe (PaO2 50 - 59 mm Hg) to very severe (PaO2 ≤ 50 mm Hg) disease [4]. The prevalence of HPS is estimated to range from 4% to 47% in patients with cirrhosis [1, 5]. There is no clear effective medical therapy for HPS, but liver transplantation (LT) has shown to improve survival even in patients with severe disease [6]. Overall, in patients with liver cirrhosis, HPS significantly impacts quality of life and survival outcomes. However, there is significant paucity of data on the disease entity, particularly in an inpatient setting. Hence, in this study, we use the National Inpatient Sample (NIS) database from 2012 to 2018 to assess hospitalization characteristics, adverse outcomes, and the burden of HPS on the US healthcare system. Additionally, we also attempt to identify trends in hospitalizations, outcomes, and healthcare utilization for HPS.
Materials and Methods
Design and data source
This was a retrospective interrupted trends study involving HPS hospitalizations in the USA from 2012 to 2018. Data was collected from the NIS for the years 2012, 2014, 2016 and 2018. The NIS database was developed by the Healthcare Cost and Utilization Project (HCUP), a Federal-State-Industry partnership sponsored by the Agency for Healthcare Research and Quality (AHRQ). The NIS is a database of inpatient hospital stays derived from billing data submitted by hospitals to state-wide data collection organizations across the USA, covering more than 97% of the US population [7]. It approximates a 20% stratified sample of discharges from US community hospitals, excluding rehabilitation and long-term acute care hospitals. This dataset was weighted to obtain national estimates [8]. The NIS dataset prior to September 2015 was coded using the International Classification of Diseases, Ninth Revision, Clinical Modification/Procedure Coding System (ICD-9-CM/PCS), whereas the dataset from the last quarter of 2015 onwards was coded using the International Classification of Diseases, Tenth Revision, Clinical Modification/Procedure Coding System (ICD-10-CM/PCS). Hence, in this study, both ICD-9 and ICD-10 codes were used for data collection.
Study population
The study included all adult (≥ 18 years) hospitalizations with a primary diagnosis of HPS from 2012 to 2018 using the ICD codes 573.5 and K76.81. Patients younger than 18 years were excluded from the study.
Outcome measures
The outcome measures included epidemiological trends, inpatient mortality, and the burden of the disease on the US healthcare system in terms of mean length of stay (LOS) and mean total hospital charge (THC). The trends of inpatient mortality, LOS and THC were calculated using multivariate logistic trend analysis. Total hospital charge was obtained using the HCUP Cost-to-Charge Ratio files and adjusted for inflation using the Medical Expenditure Panel Survey index for hospital care with 2018 as the reference point [9, 10].
Statistical analysis
Stata® Version 16 software (StataCorp, Texas, USA) was used for data analysis. All analyses were conducted using weighted samples for national estimates in adjunct with HCUP regulations for use of the NIS database. Multivariate regression analysis was used to calculate the adjusted odds of trend in inpatient mortality, LOS and THC following adjustment for age, sex, race, grouped Charlson comorbidity Index (CCI), insurance type, mean household income, and hospital characteristics. All P-values were two-sided with 0.05 set as the threshold for statistical significance.
Ethical considerations
The NIS database lacks patient and hospital-specific identifiers. Therefore, this study was exempt from Institutional Review Board (IRB) approval as per guideline put forth by our institutional IRB for analysis of HCUP databases and this study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Data availability statement
NIS is a large, publicly available all-payer inpatient database which contains hospitalization data on more than 7 million hospital stays. The large sample size is ideal for assessing national and regional estimates while also enabling analysis of rare conditions, uncommon treatments, and special populations. The NIS database is available at: https://www.hcup-us.ahrq.gov.
Results
Epidemiological characteristics
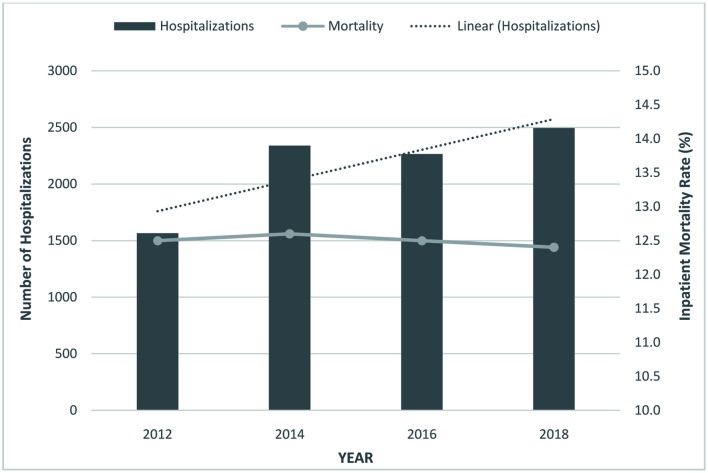
From 2010 to 2018, we noted an increase in the total number of HPS hospitalizations from 1,565 in 2012 to 2,495 in 2018 (Table 1). The mean age for these hospitalizations ranged from 55.8 to 58.1 years. After a trend analysis, we noted a rising trend for total HPS hospitalizations (P-trend < 0.001) and mean age (P-trend = 0.003) for the study period (Fig. 1). A slight decrease in HPS hospitalizations from 50.5% in 2012 to 47.3% in 2018 was observed for women. Furthermore, Whites made up a majority of the study population followed by Hispanics, Blacks, and other races (Table 1). Additionally, most patients had a CCI score ≥ 3 throughout the study period.
Table 1. Biodemographic Characteristics for Hepatopulmonary Syndrome Hospitalizations From 2012 to 2018.
| Variables | Year |
|||
|---|---|---|---|---|
| 2012 | 2014 | 2016 | 2018 | |
| Total number of hospitalizations | 1,565 | 2,340 | 2,265 | 2,495 |
| Mean age (years) | 56.7 | 55.8 | 56.4 | 58.1 |
| Women (%) | 50.5 | 50.2 | 46.7 | 47.3 |
| Racial distribution (%) | ||||
| White | 67.7 | 67.5 | 62 | 69.2 |
| Black | 6.4 | 3.8 | 4.6 | 6.4 |
| Hispanic | 16.0 | 18.0 | 20.8 | 14.4 |
| Others | 9.9 | 10.7 | 12.6 | 10.0 |
| Charlson comorbidity index (CCI) score (%) | ||||
| 1 | 10.2 | 9.0 | 7.7 | 5.6 |
| 2 | 10.5 | 8.8 | 9.9 | 7.6 |
| ≥ 3 | 79.3 | 82.2 | 82.4 | 86.8 |
| Insurance type (%) | ||||
| Medicaid | 45.6 | 38.5 | 39.8 | 44.4 |
| Medicare | 23.2 | 23.8 | 27.5 | 25.4 |
| Private | 25.5 | 34.1 | 30.9 | 26.7 |
| Uninsured | 5.7 | 3.6 | 1.8 | 3.5 |
Figure 1.
Trends for hepatopulmonary syndrome (HPS) hospitalizations and inpatient mortality.
Inpatient mortality
The inpatient mortality for HPS ranged from 12.4% to 12.6% from 2010 to 2018 (Table 2). However, we did not find a statistically significant trend for mortality in these patients (P-trend = 0.534).
Table 2. Outcomes for Hepatopulmonary Syndrome From 2012 to 2018.
| Outcome | Year |
|||
|---|---|---|---|---|
| 2012 | 2014 | 2016 | 2018 | |
| Inpatient mortality (%) | 12.5 | 12.6 | 12.5 | 12.4 |
| Mean length of stay (days) | 7.9 | 10.0 | 8.7 | 9.2 |
| Mean total hospital cost ($) | 28,373 | 36,976 | 34,717 | 33,965 |
Hospitalization characteristics
The THC for the management of HPS was noted to be $33,965 in 2018 compared to $28,373 in 2012, and the LOS was 9.2 days in 2018 compared to 7.9 days in 2012 (Table 1). However, no change was noted for mean THC (P-trend = 0.534) and mean LOS (P-trend = 0.545) for the study period. Furthermore, from a payment perspective, Medicaid was the largest insurer followed by Private Insurance and Medicare. In 2018, 3.5% of these patients were uninsured (Table 2).
Discussion
HPS is characterized by the classical triad of liver disease, IPVD, and arterial hypoxemia secondary to abnormalities in pulmonary gas exchange without the presence of an intrinsic lung pathology [11]. The term HPS was first coined in 1977 by Kennedy and Knudson; however, the first case of HPS may have been described much earlier in 1884 by Fluckiger [12]. Much of the literature available on HPS has been obtained from animal studies [13, 14]. The current diagnostic criteria proposed for HPS include the presence of chronic liver disease, an A-a gradient ≥ 15 mm Hg in individuals < 64 years of age or ≥ 20 mm Hg in individuals ≥ 65 years of age on arterial blood gas analysis in a seated position, and the detection of IPVD by either contrast-enhanced echocardiography or a macroaggregated albumin lung perfusion scan (99mTc-MAA) [15, 16]. Over the years, literature has reported a varied prevalence range for HPS due to differences in diagnostic criteria. However, to our knowledge, there have only been a handful of studies assessing the epidemiology and outcomes of HPS in an inpatient setting. Therefore, this study was designed to identify biodemographic characteristics, and estimate inpatient mortality and burden of HPS on the US healthcare system.
Hospitalization characteristics for HPS
The epidemiology of HPS is relatively unknown due to a significant dearth of prospective studies on the disease entity. For the US population, an incidence rate has not been estimated in literature. Additionally, the prevalence of HPS is highly dependent on the diagnostic criteria, methods used to establish diagnosis and the study population. In patients with end-stage liver disease, studies have reported a prevalence ranging from 4% to 32% in adults and 9% to 20% in children [17-21]. In terms of patient profiles, differences in the prevalence rates for HPS have also been observed. The average prevalence of HPS in patients with Budd-Chiari syndrome was noted to be 28%, for those with chronic viral hepatitis (with or without cirrhosis), it was about 10%, and in patients enrolled for LT, it ranged from 4% to 32% [2]. Overall, aggregations of studies in literature have estimated the prevalence rate ranging from 4% to 47% [1, 5].
In our study, there was a rising trend of HPS hospitalizations from 1,565 in 2012 to 2,495 in 2018 (Table 1). This may in part be due to the increasing prevalence of liver cirrhosis in the general population in combination with an increasing awareness about the disease entity [22-24]. The mean age of our study population was noted to be 55.8 - 58.1 years. This finding was in line with literature which has reported a mean age of 53.3 ± 10.3 for HPS. There was an increasing trend of the mean age for HPS during our study period (Fig. 1). This may, in part, be due to the fact that patients above 65 years of age are being increasingly diagnosed with liver cirrhosis and the elderly demographic (≥ 65 years) is on the rise in the USA as per data available from the US Census Bureau [25, 26]. Furthermore, we noted a slight decrease in HPS hospitalizations for women from 50.5% in 2012 to 47.3% in 2018. The exact reason for this finding is unknown, but may be attributed to a declining prevalence of cirrhosis in women [27, 28]. An inequitable racial distribution was noted in our study with majority of our study cohort being White, followed by Hispanics, Blacks, and other races. This finding reflected current literature which reports a White predominance for HPS [29, 30].
Inpatient mortality for HPS
Studies assessing the impact of HPS on mortality are fairly limited despite a relatively high prevalence in cirrhotics. It has been well established that the presence of HPS is associated with worse clinical outcomes and decreased quality of life in patients with liver cirrhosis [29, 31]. A retrospective study noted a 41% mortality rate for HPS over a 2.5-year study period [32]. Another study investigating the impact of HPS on liver cirrhosis reported a 23% 5-year survival for patients with HPS and 67% for those without HPS in a setting of cirrhosis [16]. Numerous studies assessing survival outcomes for patients with cirrhosis and HPS who underwent LT have reported worse survival in patients with HPS compared to patients without HPS [1, 24]. Moreover, PaO2 < 50 mm Hg has also been associated with worse survival. However, the data available in literature on mortality may not reflect true mortality rates for HPS due to the differences in the diagnostic criteria and the methods used to establish diagnosis.
In our study, after a multivariate regression trend analysis for the 2012 to 2018 study period, inpatient mortality for HPS ranged from 12.4% to 12.6% (Table 2), but a statistically significant trend for inpatient mortality was absent. The lower percentage of mortality compared to other prospective studies may stem from early diagnosis, improved management and better follow-up for HPS. Furthermore, an increase in LT over the past decade may also have had a role to play [33]. It is also worth noting that inpatient mortality for HPS may be different from mortality secondary to HPS in the general population. Additionally, the mortality rates noted in our study may primarily reflect inpatient mortality for patients with advance liver disease requiring hospitalization and LT. Nonetheless, additional studies are needed to further assess the relationship between HPS and mortality as it may govern therapeutic intervention.
Burden of HPS on the US healthcare system
HPS not only impacts the quality of life of individual patients but also places a significant burden on the US healthcare system in terms of cost and healthcare utilization. However, literature on the impact of the disease entity on the US healthcare system is limited. Studies have reported 27% higher hospital costs for the management of HPS compared to non-HPS patients [34]. HPS has also been observed to be independently associated with longer intensive care unit (ICU) and hospital stay [34]. Nursing cost was reported to be six times higher and respiratory care cost was about 2.6 times higher for patients with HPS compared to non-HPS patients [34]. LT is currently the only known treatment option that may reverse HPS in a period of 6 to 12 months [1]. Hence, these patients would eventually require LT which further increases hospitalization costs, ICU stay post-transplant and healthcare resource utilization [35]. In our study, the mean THC for management of HPS was noted to be $33,965 in 2018 compared to $28,373 in 2012 and the mean LOS in 2018 was found to be 9.2 days compared to 7.9 days for 2012. However, after a trend analysis, no change was noted for both mean THC and mean LOS. This may be because the management approach for these patients was standardized and has remained constant over the years. Moreover, as discussed earlier, we did not find a trend of inpatient mortality which further supports this hypothesis. Furthermore, in the US healthcare system, insurance plays a key role in the payment for hospital charges. From a payment perspective for HPS hospitalizations, Medicaid was the largest insurer followed by Private Insurance and Medicare. About 3.5% of these patients were uninsured in 2018 compared to 5.7% in 2012 (Table 2).
Strength and limitations
This study has several key strengths and numerous limitations. A considerable strength of the study is the study population, which is derived from one of the largest, publicly available databases in the USA developed through a Federal-State-Industry partnership. It contains data on inpatient admissions from hospitals all over the USA. Hence, the outcomes are applicable to almost all hospitals across the USA. Moreover, to our knowledge, this is one of the very few studies which use the NIS database to determine the epidemiology, adverse outcomes, and burden of HPS on the US healthcare system. Due to the design of the NIS database, it is perfect to estimate the trends of HPS and allows for addition of meaningful information to current literature.
However, we do acknowledge the limitations. The NIS database does not provide data on the severity of the disease, time to diagnosis, methods used to establish diagnosis, and the treatment aspects of the disease. Additionally, the study population only included patients with a primary diagnosis of HPS; hence, individuals with liver cirrhosis or its complications with HPS in the background were not identified for the analysis. Furthermore, all biases present in retrospective studies are applicable to this study. The hospitalizations identified for the study were based on a diagnosis of HPS rather than individual patients. Hence, individuals admitted numerous times for the same chief complaint may have been included several times in the data set. Lastly, NIS is an administrative database that uses specific codes to gather and store information; therefore, possibility of coding errors cannot be excluded. Despite these limitations, we believe that the sample size, study design and comprehensive analysis technique used, helps us better understand the topic in question. Through this study, we aim to encourage intellectual conversation and promote future research on HPS.
Conclusions
HPS is frequent complication of cirrhosis; however, there is substantial paucity of knowledge about the disease entity. An increasing number and trend of hospitalizations for HPS was observed in our study. The mean age for these individuals was 55.8 - 58.1 years with a trend towards increasing mean age. Inpatient mortality for HPS was 12.4-12.6% from 2010 to 2018. In 2018, the THC for HPS was noted to be $33,965 and the LOS was 9.2 days. However, there was no significant change for mean THC and mean LOS over the study period.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no financial disclosures.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
The NIS database lacks patient identifiers. Hence, no consent is required for NIS studies.
Author Contributions
Substantial contributions to the conception and design of the work: Dushyant Singh Dahiya, Asim Kichloo and Hafeez Shaka. Acquisition, analysis, or interpretation of data for the work: all authors. Literature search and review: all authors. Drafting the work or revising it critically for important intellectual content: all authors. Final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work: all authors.
Data Availability
We used and analyzed the NIS database from 2012 to 2018, available online at http://www.hcup- us.ahrq.gov. The NIS is a large publicly available all-payer inpatient care database in the USA containing data on more than 7 million hospital stays yearly. Its large sample size is ideal for developing national and regional estimates and enables analyses of rare conditions, uncommon treatments, and special populations.
Abbreviations
- A-a gradient
alveolar-arterial oxygen gradient
- AHRQ
Agency for Healthcare Research and Quality
- CCI
Charlston comorbidity index
- HCUP
Healthcare Cost and Utilization Project
- HPS
hepatopulmonary syndrome
- ICD-9-CM/PCS
International Classification of Diseases, Ninth Revision, Clinical Modification/Procedure Coding System
- ICD-10-CM/PCS
International Classification of Diseases, Tenth Revision, Clinical Modification/Procedure Coding System
- ICU
intensive care unit
- IPVD
intrapulmonary vascular dilations
- IRB
Institutional Review Board
- LT
liver transplantation
- LOS
length of stay
- 99mTc-MAA
macroaggregated albumin lung perfusion scan
- NIS
Nationwide Inpatient Sample
- PaO2
partial pressure of oxygen
- THC
total hospital charge
References
- 1.Soulaidopoulos S, Cholongitas E, Giannakoulas G, Vlachou M, Goulis I. Review article: Update on current and emergent data on hepatopulmonary syndrome. World J Gastroenterol. 2018;24(12):1285–1298. doi: 10.3748/wjg.v24.i12.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grilo-Bensusan I, Pascasio-Acevedo JM. Hepatopulmonary syndrome: What we know and what we would like to know. World J Gastroenterol. 2016;22(25):5728–5741. doi: 10.3748/wjg.v22.i25.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho V. Current concepts in the management of hepatopulmonary syndrome. Vasc Health Risk Manag. 2008;4(5):1035–1041. doi: 10.2147/vhrm.s3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuhrmann V, Drolz A, Rutter K, Horvatits T. HPS: Diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken) 2014;4(2):46–49. doi: 10.1002/cld.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira PP, Camara EJ, Paula RL, Zollinger CC, Cavalcanti AR, Bittencourt PL. Prevalence of hepatopulmonary syndrome in patients with decompensated chronic liver disease and its impact on short-term survival. Arq Gastroenterol. 2008;45(1):34–37. doi: 10.1590/s0004-28032008000100007. [DOI] [PubMed] [Google Scholar]
- 6.Cosarderelioglu C, Cosar AM, Gurakar M, Dagher NN, Gurakar A. Hepatopulmonary syndrome and liver transplantation: a recent review of the literature. J Clin Transl Hepatol. 2016;4(1):47–53. doi: 10.14218/JCTH.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Healthcare Cost and Utilization Project. Introduction to the HCUP National Inpatient Sample (NIS). The national (nationwide) inpatient sample database documentation. Rockville, MD: Agency for Healthcare Research and Quality. Available at: https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2018.jsp. Accessed February 10, 2021.
- 8.Houchens R, Ross D, Elixhauser A, Jiang J. Nationwide Inpatient Sample (NIS) Redesign Final Report. 2014. HCUP Methods Series Report # 2014-04 ONLINE. April 4, 2014. U.S. Agency for healthcare research and quality. Available: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. Accessed February 10, 2021.
- 9. HCUP Cost-to-Charge Ratio (CCR). 2008 - 2018. Agency for healthcare research and quality, Rockville, MD. https://www.hcup-us.ahrq.gov/db/ccr/costtocharge.jsp. Accessed February 10, 2021.
- 10.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2018;53(1):175–196. doi: 10.1111/1475-6773.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshraghian A, Kamyab AA, Yoon SK. Pharmacological treatment for hepatopulmonary syndrome. Biomed Res Int. 2013;2013:670139. doi: 10.1155/2013/670139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy TC, Knudson RJ. Exercise-aggravated hypoxemia and orthodeoxia in cirrhosis. Chest. 1977;72(3):305–309. doi: 10.1378/chest.72.3.305. [DOI] [PubMed] [Google Scholar]
- 13.Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol. 1997;272(4 Pt 1):G779–784. doi: 10.1152/ajpgi.1997.272.4.G779. [DOI] [PubMed] [Google Scholar]
- 14.Assimakopoulos SF, Vagianos CE. Bile duct ligation in rats: a reliable model of hepatorenal syndrome? World J Gastroenterol. 2009;15(1):121–123. doi: 10.3748/wjg.15.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Roisin R, Krowka MJ, Herve P, Fallon MB, ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-Hepatic vascular Disorders (PHD) Eur Respir J. 2004;24(5):861–880. doi: 10.1183/09031936.04.00010904. [DOI] [PubMed] [Google Scholar]
- 16.Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41(5):1122–1129. doi: 10.1002/hep.20658. [DOI] [PubMed] [Google Scholar]
- 17.Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Muller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51(6):853–859. doi: 10.1136/gut.51.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varghese J, Ilias-basha H, Dhanasekaran R, Singh S, Venkataraman J. Hepatopulmonary syndrome - past to present. Ann Hepatol. 2007;6(3):135–142. [PubMed] [Google Scholar]
- 19.Tumgor G, Arikan C, Yuksekkaya HA, Cakir M, Levent E, Yagci RV, Kilic M. et al. Childhood cirrhosis, hepatopulmonary syndrome and liver transplantation. Pediatr Transplant. 2008;12(3):353–357. doi: 10.1111/j.1399-3046.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 20.Sari S, Oguz D, Sucak T, Dalgic B, Atasever T. Hepatopulmonary syndrome in children with cirrhotic and non-cirrhotic portal hypertension: a single-center experience. Dig Dis Sci. 2012;57(1):175–181. doi: 10.1007/s10620-011-1832-6. [DOI] [PubMed] [Google Scholar]
- 21.Noli K, Solomon M, Golding F, Charron M, Ling SC. Prevalence of hepatopulmonary syndrome in children. Pediatrics. 2008;121(3):e522–527. doi: 10.1542/peds.2007-1075. [DOI] [PubMed] [Google Scholar]
- 22.Zhai M, Liu Z, Long J, Zhou Q, Yang L, Zhou Q, Liu S. et al. The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci Rep. 2021;11(1):5195. doi: 10.1038/s41598-021-84577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. doi: 10.1001/jamanetworkopen.2020.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenk P, Schoniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Muller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003;125(4):1042–1052. doi: 10.1016/s0016-5085(03)01207-1. [DOI] [PubMed] [Google Scholar]
- 25.Orman ES, Roberts A, Ghabril M, Nephew L, Desai AP, Patidar K, Chalasani N. Trends in characteristics, mortality, and other outcomes of patients with newly diagnosed cirrhosis. JAMA Netw Open. 2019;2(6):e196412. doi: 10.1001/jamanetworkopen.2019.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Available from: https://www.census.gov/newsroom/press-releases/2020/65-older-population-grows.html. Accessed on March 27, 2021.
- 27.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27(3):209–219. [PMC free article] [PubMed] [Google Scholar]
- 29.Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, Shah VH. et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135(4):1168–1175. doi: 10.1053/j.gastro.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bommena S, Gerkin RD, Agarwal S, Raevens S, Glassberg MK, Fallon MB. Diagnosis of hepatopulmonary syndrome in a large integrated health system. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.09.050. [DOI] [PubMed] [Google Scholar]
- 31.Iyer VN. Liver transplantation for hepatopulmonary syndrome. Clin Liver Dis (Hoboken) 2014;4(2):38–41. doi: 10.1002/cld.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome. Clinical observations and lack of therapeutic response to somatostatin analogue. Chest. 1993;104(2):515–521. doi: 10.1378/chest.104.2.515. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Toy M, Hang Pham TT, So S. Causes and trends in liver disease and hepatocellular carcinoma among men and women who received liver transplants in the U.S., 2010-2019. PLoS One. 2020;15(9):e0239393. doi: 10.1371/journal.pone.0239393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cywinski JB, Makarova N, Arney A, Liu Q, Fujiki M, Menon KVN, Quintini C. Resources utilization after liver transplantation in patients with and without hepatopulmonary syndrome: Cleveland clinic experience. Transplant Direct. 2020;6(4):e545. doi: 10.1097/TXD.0000000000000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S, Castel H, Rao RV, Picard M, Lilly L, Faughnan ME, Pomier-Layrargues G. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10(2):354–363. doi: 10.1111/j.1600-6143.2009.02822.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NIS is a large, publicly available all-payer inpatient database which contains hospitalization data on more than 7 million hospital stays. The large sample size is ideal for assessing national and regional estimates while also enabling analysis of rare conditions, uncommon treatments, and special populations. The NIS database is available at: https://www.hcup-us.ahrq.gov.
We used and analyzed the NIS database from 2012 to 2018, available online at http://www.hcup- us.ahrq.gov. The NIS is a large publicly available all-payer inpatient care database in the USA containing data on more than 7 million hospital stays yearly. Its large sample size is ideal for developing national and regional estimates and enables analyses of rare conditions, uncommon treatments, and special populations.