OBJECTIVES:
The development of thrombocytopenia and thrombosis after the administration of the AstraZeneca and Johnson & Johnson/Janssen vaccines has been recently described. This new condition has been called vaccine-induced immune thrombotic thrombocytopenia. The objective of this review is to summarize the clinical characteristics and therapeutic options of vaccine-induced immune thrombotic thrombocytopenia based on available published case series. Furthermore, we provide a comparison of the diagnostic pathway and treatment recommendations provided by six major medical societies.
DATA SOURCES:
We searched MEDLINE, PubMed, and Cochrane Central Register of Controlled Trials databases.
STUDY SELECTION:
We included case series and case reports on patients who developed vaccine-induced immune thrombotic thrombocytopenia. We also included guidelines for the diagnosis and management of vaccine-induced immune thrombotic thrombocytopenia from major medical societies.
DATA EXTRACTION:
We examined baseline risk factors, symptoms, physical signs, laboratory and imaging findings, and treatment in patients with vaccine-induced immune thrombotic thrombocytopenia reported in the case series. We also analyzed the diagnostic and treatment recommendations provided by major societal guidelines on the management of vaccine-induced immune thrombotic thrombocytopenia.
DATA SYNTHESIS:
Patients who developed vaccine-induced immune thrombotic thrombocytopenia were more likely to be young women (age 20–50) who were given the AstraZeneca or Johnson & Johnson/Janssen 4–28 days prior to presentation. Patients showed signs, symptoms, and imaging findings consistent with cerebral venous sinus thrombosis and splanchnic thrombosis. Laboratory findings showed thrombocytopenia, low fibrinogen, and elevate d-dimer levels, while positive platelet factor 4 antibodies were always positive. Major societal guidelines recommend avoidance of heparin and platelets. Treatment with nonheparin anticoagulants and IV immunoglobulin is also recommended.
CONCLUSIONS:
Vaccine-induced immune thrombotic thrombocytopenia is a rare but highly morbid complication related to the administration of the AstraZeneca and Johnson & Johnson/Janssen vaccines. Clinicians should be prepared for the early identification of patients with suspicious symptoms and prompt treatment should be initiated to avoid catastrophic deterioration. Major societal guidelines provide useful recommendations for the diagnosis and management of patients with vaccine-induced immune thrombotic thrombocytopenia.
Keywords: AstraZeneca, Johnson & Johnson/Janssen, thromboembolism, thrombosis with thrombocytopenia syndrome, vaccine-associated thrombocytopenia, vaccine-induced immune thrombotic thrombocytopenia, vaccine-induced prothrombotic immune thrombocytopenia
The global pandemic of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to the rapid development of vaccines with the aim to limit the spread of SARS-CoV-2 and control the disease. Clinical trials involving tens of thousands of individuals receiving COVID-19 vaccines have demonstrated few adverse events (1–3) and vaccines were rapidly deployed. As of May 11, 2021, over 1.3 billion doses of newly developed COVID-19 vaccines have been administered worldwide (4). In the United States, three brands of vaccines are now available: the Pfizer-BioNTech, the Moderna, and the Johnson & Johnson/Janssen vaccines. In Europe, available vaccines include the Pfizer-BioNTech and the AstraZeneca vaccines. Despite the overall safe profile of these vaccines, recently, a rare condition characterized by the development of thrombotic events in women age 18–49 who were exposed to the AstraZeneca and Johnson & Johnson/Janssen vaccines has been described (5–8). This condition is called vaccine-induced immune thrombotic thrombocytopenia (VITT), vaccine-induced prothrombotic immune thrombocytopenia, and more recently as thrombosis with thrombocytopenia syndrome (TTS). From a clinical standpoint, VITT is characterized by thrombocytopenia and the presence of symptoms related to the development of thrombosis often in unusual sites such as cerebral venous sinus thrombosis (CVST) and splanchnic vein thrombosis (SVT), although other locations may be involved (9).
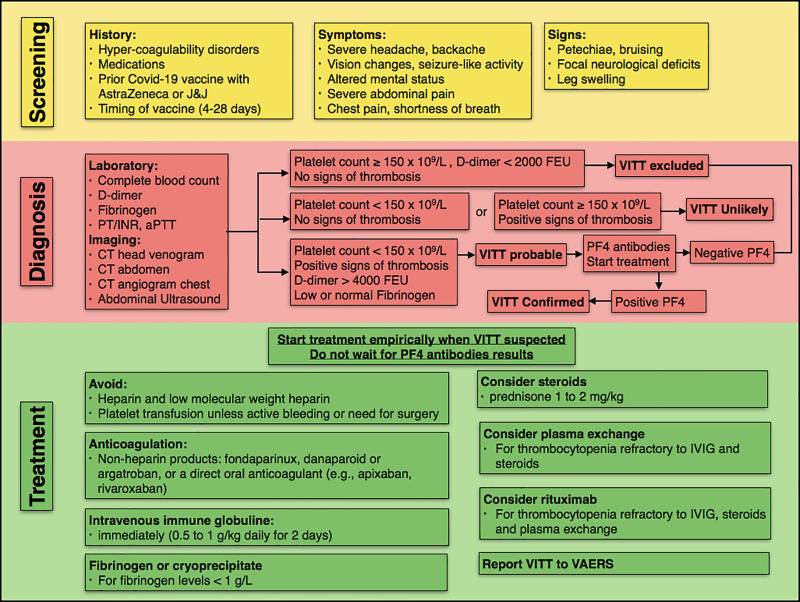
Given the high morbidity associated with the development of VITT, a number of guidelines and recommendations are now available to guide identification and management of patients with this rare condition. In this article, we summarize the clinical features, risk factors, diagnostic pathway, and management of VITT based on current evidence and recommendations from six major societal guidelines (Fig. 1). These societies include the Centers for Disease Control and Prevention (CDC) (10), the International Society on Thrombosis and Haemostasis interim guidance (ISTH-IG) (11), the American Society of Hematology (ASH) (12), the American College of Cardiology (ACC) (13), the Expert Haematology Panel (EHP) from the British Society for Haematology (14), and the American Heart Association and American Stroke Association (AHA/ASA) (15).
Figure 1.
Flow chart describing screening, diagnostic approach, and management of patients with vaccine-induced immune thrombotic thrombocytopenia (VITT). aPTT = activated partial thromboplastin time, COVID-19 = coronavirus disease 2019, FEU = fibrinogen-equivalent units, INR = international normalized ratio, IVIG = IV immunoglobulin, PF4 = platelet factor 4, PT = prothrombin time, VAERS = Vaccine Adverse Event Reporting System.
WHAT BRAND OF VACCINE IS ASSOCIATED WITH VITT?
Current evidence suggests that only the ChAdOx1nCoV-19 (AstraZeneca) and the Ad26.COV2.S (Johnson & Johnson/Janssen) vaccines are associated with the development of VITT (Table 1). Three independent reports describe a total of 39 patients who presented with both thrombocytopenia and thrombosis after receiving the AstraZeneca vaccine (5–7). One case of thrombocytopenia and diffuse intravascular coagulation after the Johnson & Johnson/Janssen vaccine has also been reported (8).
TABLE 1.
Demographic Features, Laboratory Findings, and Clinical Course of Patients With Vaccine-Induced Immune Thrombotic Thrombocytopenia Based on Published Case Series
| Source | No. of Cases | Vaccine Received | Sex Female, n (%) | Age | Timing Onset (d) | Laboratory Findings | Symptoms and Imaging on Presentation | Prior Hematologic Disease | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Schultz et al (5) | 5 | ChAdOx1 nCoV-19 (AstraZeneca) | 4 (80) | 39 (32–54) | 7–10 | Platelet count range: 10–70 × 109/L, low fibrinogen, elevated d-dimer, positive PF4 antibodies (n = 5) | Headache (3), abdominal pain (1), back pain (1), hemiparesis (1) | None | Corticosteroids (n = 4), (IVIG, n = 12) | Mortality 60% |
| Imaging: (CVST, n = 3), cerebral hemorrhage (1), splanchnic veins thrombosis (2) | ||||||||||
| Greinacher et al (6) | 11 | ChAdOx1 nCoV-19 (AstraZeneca) | 9 (82) | 36 (22–49) | 5–16 | Platelet count range: 8–107 (×109/L), positive PF4 antibodies (n = 11) | Fever, chills, abdominal pain | One patient: von Willebrand disease, anticardiolipin antibodies, and factor V Leiden | Heparin (n = 4) recommend anticoagulation and IVIG | Mortality 55% |
| CVST (n = 9), cerebral hemorrhage (1), splanchnic veins thrombosis (3), pulmonary embolism (3), other thromboses (4) | ||||||||||
| Scully et al (7) | 23 | ChAdOx1 nCoV-19 (AstraZeneca) | 14 (61) | 46 (21–77) | 6–24 | Low or normal fibrinogen, elevated d-dimer, positive PF4 antibodies (22/23), negative SARS-CoV-2 PCR (23/23), negative SARS-CoV-2 serologic test for antibodies (10/10 tested), positive lupus anticoagulant (5/10 tested) | Petechiae, bruising, CVST (n = 13), pulmonary embolism (4), DVT (10), ischemic stroke (2), portal vein thrombosis (2), hemorrhage (1) | One patient with history of DVT | Not mentioned. Recommend avoiding platelet transfusion, IVIG, glucocorticoids, nonheparin anticoagulant. Consider treatment to increase fibrinogen level to > 1.0 g/L | Mortality 30% |
| One patient on combined oral contraceptive pills | ||||||||||
| Muir et al (8) | 1 | Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) | 1 (100) | 48 | 14 | Anemia, thrombocytopenia (13× 109/L), low fibrinogen, elevated d-dimer, negative SARS-CoV-2 PCR, positive PF4 antibodies ELISA | Malaise, abdominal pain | None | Heparin IVIG | Patient critically ill at the time of report |
| Imaging: Splanchnic vein thrombosis and CVST | ||||||||||
| Clinical Immunization Safety Assessment project (10) | 15 | Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) | 15 (100) | 37 (18–59) | 6–15 | Thrombocytopenia, positive PF4 antibodies (11/11), negative SARS-CoV-2 PCR (10), negative SARS-CoV-2 serology (4/4) | Headache, chills, fever, malaise, abdominal pain | Oral contraceptives (2) | Heparin (6) | Three deaths |
| Imaging: CVST (n = 12), pulmonary embolism, splanchnic vein thrombosis | Nonheparin anticoagulants (12) | Seven patients hospitalized and five discharged home as of April 21 | ||||||||
| Platelet transfusion (7) | ||||||||||
| IVIG (8) | ||||||||||
| Bersinger et al (17) | 1 | ChAdOx1 nCoV-19 (AstraZeneca) | 1 (100%) | 21 | 9 | Thrombocytopenia (61 × 109/L), positive PF4 antibodies ELISA on hospital day 1 and negative on hospital day 6, serotonin-release assay positive | Headache, seizures, hemiplegia, aphasia | Oral contraceptive | Heparin | Transferred from ICU to rehabilitation on hospital day 27 |
| Imaging: Thrombosis in the deep and superficial cerebral veins, left jugular vein, and splanchnic vein, pulmonary embolism, DVT, left frontoparietal venous hemorrhagic infarction | Nonheparin anticoagulants | |||||||||
| IVIG |
CVST = cerebral venous sinus thrombosis, DVT = deep vein thrombosis, ELISA = enzyme-linked immunosorbent assay, IVIG = IV immunoglobulin, PCR = polymerase chain reaction, PF4 = platelet factor 4, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
In addition to cases published in scientific journals, as of April 4, 2021, a total of 169 cases of CVST and 53 cases of SVT out of 34 million people vaccinated with the ChAdOx1nCoV-19 (AstraZeneca) vaccine were reported to the European Medicines Agency. According to the Vaccine Adverse Event Reporting System (VAERS), as of April 12, 2021, there were six cases of CVST with thrombocytopenia among 6.86 million people who received the Johnson &Johnson/Janssen vaccine in the United States. Additionally, the Clinical Immunization Safety Assessment (CISA) project that includes seven medical research centers in the United States identified 15 cases of TTS in people receiving the Johnson & Johnson/Janssen vaccine. No reports were submitted to VAERS among the 97.9 million people who received the Pfizer-BioNTech vaccine. Three cases of CVST out of 84.7 million Moderna vaccines were reported to VAERS, but all presented without thrombocytopenia, thus excluding a diagnosis of VITT (10).
WHY SOME VACCINES ARE ASSOCIATED WITH VITT AND SOME OTHERS ARE NOT?
The main difference between the AstraZeneca and the Johnson & Johnson/Janssen vaccines and other vaccines is that the former vaccines are nonreplicating adenovirus vector-based DNA vaccines (chimpanzee [ChAdOx1] and human [Ad26.COV2.S], respectively), while other vaccines such as the Pfizer-BioNTech and Moderna vaccines are messenger RNA-based vaccines. The fact that VITT has been observed only after administration of adenoviral-based vaccines suggests that adenovirus vectors may be implicated in the pathogenesis of VITT, which is characterized by the development of platelet factor 4 (PF4) antibodies, platelet activation, and thrombosis. Of note, thrombocytopenia without thrombosis has also been reported after the administration of Pfizer-BioNTech and Moderna vaccines, but these cases (n = 20) do not meet criteria for VITT diagnosis (16) and rather represent cases of immune thrombocytopenic purpura (ITP).
WHAT ARE THE RISK FACTORS ASSOCIATED WITH THE DEVELOPMENT OF VITT?
The main demographic and clinical characteristics of patients who have developed VITT and been reported in case series are summarized in Table 1. The majority of patients with VITT were females with a median age of 40 years. Overall, patients were generally healthy with no major preexisting medical conditions. One patient had confirmed von Willebrand disease, anticardiolipin antibodies, and factor V Leiden; one had a history of deep vein thrombosis (DVT); and one was on combined oral contraceptive pills. None of the patients had a history of heparin-induced thrombocytopenia (HIT). These findings suggest that preexisting medical conditions do not predispose an individual to developing VITT.
DOES PRIOR COVID-19 INFECTION MATTER?
Existing evidence suggests that current or prior exposure to SARS-CoV-2 is not a risk factor for developing VITT. In two reports (7, 8), all patients with VITT (n = 24) underwent SARS-CoV-2 polymerase chain reaction testing with negative results. In the CISA project (10), 10 patients out of 15 were tested for SARS-CoV-2 on presentation with negative results. Four patients also underwent SARS-CoV-2 serologic testing and resulted negative. In the study by Scully et al (7), 10 patients underwent serologic testing for antibodies against the SARS-CoV-2 nucleocapsid protein and resulted negative. These findings suggest that VITT is not associated with concurrent or recent infection with SARS-CoV-2.
WHAT ARE THE SIGNS AND SYMPTOMS OF VITT?
The common presenting physical signs in patients with VITT are linked to the presence of thrombocytopenia and thrombosis in some vascular beds. Petechiae and bruising are common findings on physical examination. The presence of headache, altered mental status, and focal neurologic deficits suggest CNS involvement with thrombosis of venous sinuses and possible cerebral ischemia or hemorrhage, while abdominal pain at presentation suggests thrombosis of the splanchnic veins. The pulmonary circulation is another location in which thrombosis has been described, in which patients presented with shortness of breath and chest pain. Other less common symptoms on presentation were fever, chills, and back pain.
LABORATORY AND IMAGING FINDINGS
Mild to moderate thrombocytopenia is the main laboratory feature, and its presence is required for the diagnosis of VITT. Elevated d-dimer and low fibrinogen levels are also common. Based on reported cases (Table 1), approximately 70% of patients had positive imaging for CVST, while about 20% of patients were found with SVT. Other locations of thrombosis such as pulmonary embolism (PE) were also present but less common. While hemorrhagic features at presentation were observed only in three patients (8%), more patients showed secondary hemorrhagic conversion of the cerebral venous thrombosis. Schultz et al (5) described one patient with cerebellar hemorrhage, while three more patients showed hemorrhagic conversion of the CVST. Greinacher et al (6) reported one patient who presented with fatal intracranial hemorrhage without any specification about the location of the hemorrhage. In the report by Scully et al (7), some patients showed cerebral hemorrhage secondary to cerebral venous thrombosis. Bersinger et al (17) described one patient who presented with left frontoparietal venous hemorrhagic infarction.
RISK STRATIFICATION AND INITIAL TRIAGE ACCORDING TO MAJOR SOCIETAL GUIDELINES
Table 2 summarizes major societal recommendations regarding risk stratification and early identification of patients with possible VITT. New onset of severe headache, backache, vision changes, seizure-like activity, altered mental status, severe abdominal pain, chest pain, or shortness of breath in patients recently (within 28 d) exposed to the AstraZeneca or the Johnson & Johnson/Janssen vaccines should be screened for VITT. Often past medical history is negative, and patients do not have a prior history of hypercoagulability disorders. Careful physical examination should be performed, as it may reveal signs of thrombocytopenia such as petechiae and bruising. A neurologic physical examination should be performed to identify neurologic deficits related to CNS involvement, while leg swelling or leg pain may suggest DVT of the lower extremities.
TABLE 2.
Major Societal Considerations Regarding Risk Stratification and Initial Screening of Patients With Suspect Vaccine-Induced Immune Thrombotic Thrombocytopenia
| Society | Risk Factors | Presenting Signs and Symptoms |
|---|---|---|
| Centers for Disease Control and Prevention guidelines (10) | Recent exposure to the Johnson & Johnson/Janssen COVID-19 vaccine | Maintain a high index of suspicion for symptoms that might represent serious thrombotic events or thrombocytopenia such as severe headache, backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, petechiae (tiny red spots on the skin), or new or easy bruising |
| International Society for Thrombosis and Haemostasis’s interim guidance (11) | COVID-19 vaccination 4–28 d prior to onset of symptoms? VITT has only been identified following AstraZeneca or Johnson & Johnson/Janssen vaccine. It has not been identified after other vaccines | Severe, persistent headache ± vision change, seizure-like activity |
| Severe, persistent abdominal pain | ||
| Leg swelling or pain | ||
| Chest pain and/or shortness of breath | ||
| American Society of Hematology (12) | COVID-19 vaccine (Johnson & Johnson/AstraZeneca only to date) 4 to 30 d previously | Urgent medical evaluation for thrombosis with thrombocytopenia syndrome is indicated if any of the following develop 4 to 30 d after vaccination: |
| Chest pain and/or shortness of breath | ||
| Severe headache | ||
| Visual changes | ||
| Abdominal pain, nausea, and vomiting | ||
| Back pain | ||
| Leg pain or swelling | ||
| Petechiae, easy bruising, or bleeding | ||
| American College of Cardiology (13) | Early data suggest that VITT occurs only following a COVID-19 vaccination with Johnson & Johnson/Janssen or AstraZeneca vaccine. Note that AstraZeneca vaccine is NOT available in the United States. Furthermore, the small number of reported events occurred between 5 and 28 d following the vaccine. They have not been reported to occur immediately (within 1–2 d) or longer term (beyond 3–4 wk) after vaccination. It will be important to reevaluate the “at risk” window as more is learned about VITT | Not mentioned |
| Demographic and clinical risk factors for the development of VITT are uncertain. Most patients who developed VITT were younger (age < 60 yr). While all of the cases following Johnson & Johnson/Janssen vaccine in the United States occurred in women, both men and women have been diagnosed with VITT following AstraZeneca vaccine in other parts of the world | ||
| Specific clinical risk factors for VITT have not been identified. There is no evidence that patients with a history of thrombosis, thrombophilia, or heparin-induced thrombocytopenia are at increased risk for VITT | ||
| Expert Haematology Panel from the British Society for Haematology (14) | Not mentioned | Not mentioned |
| American Heart Association and American Stroke Association (15) | Risk factors for CVST in the general population include young age (35–40 yr), predominantly women of childbearing age. Risk factors for CVST are similar to those for venous thromboembolism; over 80% of patients with CVST have at least one identifiable risk factor for thrombosis, and half have multiple predisposing factors. Most common transient risks factors include temporary medical conditions, such as pregnancy and puerperium, exposure to drugs (oral contraceptives, chemotherapy), CNS or ear and face infections, and head trauma. Chronic risk factors include hereditary or acquired thrombophilia, autoimmune diseases, and cancer. Thrombocytopenia is an uncommon primary cause of CVST | Symptoms of CVST reflect the location of the vein or sinus affected; in some cases, multiple locations may be affected simultaneously. Presentations of CVST may be roughly divided into four syndromes: 1) isolated headache or increased intracranial pressure; 2) focal neurologic presentations; 3) subacute encephalopathy; and 4) cavernous sinus syndrome/multiple cranial neuropathies |
COVID-19 = coronavirus disease 2019, CVST = cerebral venous sinus thrombosis, VITT = vaccine-induced immune thrombotic thrombocytopenia.
LABORATORY TESTING AND IMAGING ACCORDING TO MAJOR SOCIETAL GUIDELINES
Table 3 shows the major societal considerations regarding diagnosis of VITT. According to existing guidelines, an urgent complete blood count and appropriate imaging tests to confirm thrombocytopenia and thromboembolism should be performed. Based on the symptoms at presentation, a CT venogram of the head should be obtained in patients presenting with headache or neurologic signs, a CT venogram of the abdomen should be obtained in patients presenting with abdominal pain, and a CT angiogram of the chest should be obtained in patients presenting with respiratory symptoms. If the platelet count is normal or imaging studies do not reveal thrombosis, VITT can be excluded, as both criteria are required for the diagnosis. Isolated thrombocytopenia might be found and more likely represents an ITP that has also been described after vaccination with Pfizer-BioNTech and Moderna vaccines (16). If thrombocytopenia and thrombosis are confirmed, additional laboratory studies should be obtained to confirm the diagnosis of VITT. These tests include d-dimer, prothrombin time, activated partial thromboplastin time, fibrinogen, and an immunoassay for PF4 antibodies. Since not all assays detect the PF4 antibody, the enzyme-linked immunosorbent assay is the preferred and most reliable test. If a reliable PF4 antibody immunoassay test is negative, VITT can likely be excluded and thrombosis can be treated according to standard practice.
TABLE 3.
Major Societal Considerations Regarding Diagnosis of Vaccine-Induced Immune Thrombotic Thrombocytopenia
| Society | Laboratory Tests and Imaging | Diagnostic Pathway |
|---|---|---|
| Centers for Disease Control and Prevention guidelines (10) | Obtain platelet counts and screen for evidence of immune thrombotic thrombocytopenia | Not mentioned |
| Screening PF4 ELISA assay as would be performed for autoimmune HIT | ||
| Consultation with a hematologist is strongly recommended | ||
| International Society for Thrombosis and Haemostasis’s interim guidance (11) | Order appropriate imaging tests to confirm thromboembolism based on symptom presentation (e.g., CT venogram head for headache, CT venogram abdomen for abdominal pain) | If no thrombosis on imaging → this is not VITT |
| If platelet count ≥ 150 × 109/L → VITT unlikely | ||
| If reliable PF4 antibody immunoassay test is negative, VITT is excluded. Treat thrombosis according to standard practice | ||
| Order an urgent complete blood count | If PF4 antibody immunoassay test is positive, particularly if the optical density reading is high, VITT is likely; arrange confirmatory functional assay for PF4 antibodies (if available) and treat as per VITT | |
| Order standard coagulation laboratory studies (d-dimer, prothrombin time, activated partial thromboplastin time, and Clauss fibrinogen) | ||
| Order immunoassay for PF4 antibodies (not all assays detect this antibody. HIT ELISA is the most reliable) | If PF4 antibody immunoassay is not rapidly available, check d-dimer level. Markedly elevated d-dimer levels (e.g., > 4× threshold for venous thromboembolism exclusion) is highly suggestive of VITT. Treat as per VITT | |
| American Society of Hematology (12) | Complete blood count with platelet count and peripheral smear | Must meet all criteria |
| Imaging for thrombosis based on signs/symptoms | Covid vaccine (Johnson & Johnson/Janssen and AstraZeneca only to date) 4 to 30 d previously | |
| PF4-ELISA (HIT assay); draw blood prior to any therapies | Venous or arterial thrombosis (often cerebral or abdominal) | |
| Thrombocytopenia | ||
| Fibrinogen and d-dimer | Positive PF4 “HIT” ELISA | |
| American College of Cardiology (13) | Imaging appropriate to symptom presentation (e.g., CT or MR venogram of the head for suspected cerebral sinus vein thrombosis) | If either test is normal (no thrombosis or thrombocytopenia), then VITT can be excluded |
| Urgent complete blood count (including platelet count) is appropriate | If both tests are abnormal (acute thrombosis and thrombocytopenia), hospitalization for further evaluation and treatment guided by a hematologist or other thrombosis expert is appropriate | |
| Expert Haematology Panel from the British Society for Haematology (14) | Full blood count—specifically to confirm thrombocytopenia < 150 × 109/L | Definite case: Cases usually present 5–28 d after vaccination and are characterized by thrombocytopenia, raised d-dimers and thrombosis, which is often rapidly progressive. There is a high preponderance of cerebral venous sinus thrombosis. Portal vein and splanchnic vein thrombosis, pulmonary embolism and arterial ischemia are also common, as well as adrenal infarction and hemorrhage. Intracranial hemorrhage can be significant and unexpected |
| Coagulation screen, including Clauss fibrinogen and d-dimers | ||
| Blood film to confirm true thrombocytopenia and identify alternative causes | ||
| Send serum sample for PF4 antibody assay (ELISA HIT assay). | ||
| Ultrasound abdomen for portal and splanchnic vein thrombosis | ||
| Possible case: Any patient presenting with acute thrombosis and new onset thrombocytopenia within 28 d of receiving coronavirus disease 2019 vaccination | ||
| Look carefully for CVST, initial imaging may be negative but may be seen on subsequent imaging | ||
| Unlikely case: | ||
| Reduced platelet count without thrombosis with d-dimer at or near normal and normal fibrinogen | ||
| Thrombosis with normal platelet count and d-dimer < 2,000 and normal fibrinogen | ||
| Probable case: d-dimers > 4,000 µg/L (or d-dimers > 2,000 with strong clinical suspicion) | ||
| American Heart Association and American Stroke Association (15) | MRI with venogram or CT with venogram can accurately detect CVST | Not mentioned |
| A conventional angiogram is rarely necessary | ||
| Complete blood count with platelet count and peripheral smear | ||
| Prothrombin time, partial thromboplastin time, fibrinogen, d-dimer | ||
| PF4 antibody ELISA and a confirmatory PF4 platelet activation assay (serotonin release assay, P-selectin expression assay, or heparin-induced platelet antibody) can be obtained if locally available and the PF4 ELISA is low positive or if there is uncertainty regarding the diagnosis |
CVST = cerebral venous sinus thrombosis, ELISA = enzyme-linked immunosorbent assay, HIT = heparin-induced thrombocytopenia, PF4 = platelet factor 4, VITT = vaccine-induced immune thrombotic thrombocytopenia.
If a PF4 antibody immunoassay test is positive and patients do not have a history of heparin exposure, VITT is likely. Since results of a PF4 antibody immunoassay may not be rapidly available, d-dimer levels may guide the clinical management and according to the ISTH-IG guidelines, markedly elevated d-dimer levels are highly suggestive of VITT.
MANAGEMENT AND TREATMENT OF PATIENTS WITH SUSPECT OR CONFIRMED VITT BASED ON MAJOR SOCIETAL GUIDELINES
Recommendations from major societal guidelines differ somewhat in their format and the details provided for management of patients with suspect or confirmed VITT (Table 4). While there are some major recommendations on which they all societies agree, some other recommendations are mentioned only in some of the guidelines. Major societies guidelines recommend that patients with confirmed thrombocytopenia and acute thrombosis should be admitted to the hospital, and if VITT is confirmed, patients should be transferred to a tertiary care center. If CVST is suspected or confirmed, transfer to a center with a neurosurgical expertise is strongly encouraged. The ISTH-IG, the ACC, and the AHA/ASA recommend that a hematologist or other thrombosis expert should be consulted to guide treatment and that in the presence of CVST or SVT, neurosurgical and vascular consultations should also be obtained to determine whether surgical intervention is indicated. All of the societies recommend avoiding heparin products until VITT is excluded. Based on the evidence reported by the recently published case series and the similarities of VITT to HIT, the ISTH-IG, the ASH, and the AHA/ASA recommend avoiding platelet transfusion unless patients are experiencing life-threatening bleeding or need for urgent surgery. Given the thrombotic nature of the disease and the similarities with HIT, all societies recommend administration of nonheparin anticoagulants. These nonheparin anticoagulants include fondaparinux, direct thrombin inhibitors such as argatroban or bivalirudin, or direct oral anticoagulants such as apixaban or rivaroxaban. Argatroban at a dose of 0.5 to 2.2 µg/kg/min has been successfully employed in a patient with life-threatening extensive thrombosis in the cerebral and splanchnic veins as well as PE and DVT (17). Bleeding and thrombotic risk should be carefully balanced and the EHP recommends that low-dose fondaparinux or critical illness dosing of argatroban may be appropriate while the platelet count is less than 30 × 109/L. Based on all guidelines, high-dose IV immunoglobulin (IVIG) should also be administered irrespective of the degree of thrombocytopenia (e.g. g/kg for 2 d). Given the rapidly evolving nature of this condition and the high rates of morbidity and mortality, if the clinical suspicion for VITT is high, IVIG treatment should be started promptly while awaiting confirmatory diagnosis with PF4 antibody testing, as based on the guidelines available, this intervention may positively affect clinical outcomes, although data to support this are still lacking.
TABLE 4.
Major Societal Recommendations Regarding Management and Treatment of Patients With Vaccine-Induced Immune Thrombotic Thrombocytopenia
| Society | Recommendations | Additional Considerations |
|---|---|---|
| Centers for Disease Control and Prevention guidelines | Do not treat patients with heparin unless HIT testing is negative | Not mentioned |
| Administer nonheparin anticoagulants if HIT testing is positive or unable to be performed | ||
| High-dose IVIG should be strongly considered | ||
| Report adverse events to Vaccine Adverse Event Reporting System, including serious and life-threatening adverse events and deaths in patients following receipt of COVID-19 vaccines as required under the Emergency Use Authorizations for COVID-19 vaccines | ||
| Aspirin should be continued if part of the patient’s routine medication regimen, but it is not recommended to start aspirin before or after vaccination given that inhibition of thromboxane does not block platelet activation in HIT, there is an associated risk of bleeding, and the incidence of VITT is rare | ||
| International Society for Thrombosis and Haemostasis’s interim guidance | Do not wait for results if diagnosis of VITT seems likely | Consider steroids (e.g., prednisone 1 to 2 mg/kg) if platelet count is less than 50 × 109/L |
| Give IVIG immediately (0.5–1 g/kg daily for 2 d) | ||
| Avoid platelet transfusions (unless patient requires urgent surgery), heparin, low-molecular-weight heparin, and vitamin K antagonists | Consider early plasma exchange or fibrinogen substitution to > 1.0 g/L if platelet count remains less than 30 × 109/L despite IVIG and steroid treatment or fibrinogen level is less than 1 g/L | |
| Give a nonheparin anticoagulant such as fondaparinux, argatroban, or a DOAC (e.g., apixaban, rivaroxaban) if platelet count is over 50 × 109/L and there is no serious bleeding | ||
| Consult an expert in thrombosis, such as hematology or vascular medicine | ||
| No mention regarding aspirin use | ||
| American Society of Hematology | Urgent consultation from hematologist with expertise in hemostasis | Consider referral to tertiary care center if TTS is confirmed |
| Avoid use of heparin until TTS has been ruled out or until an alternative other plausible diagnosis has been made | ||
| Initiate therapy with IVIG | ||
| Administer nonheparin anticoagulation pending PF4 ELISA results if signs or symptoms of serious thrombosis and positive imaging or low platelets are present | ||
| Avoid platelet transfusions unless other treatments have been initiated and life-threatening bleeding or imminent surgery | ||
| If PF4 ELISA returns negative and there is no thrombocytopenia, TTS is ruled out; treat as standard venous thromboembolism | ||
| Aspirin should be avoided as treatment or prophylaxis for TTS as it is not effective in preventing HIT antibodies from activating platelets and could increase risk of bleeding | ||
| American College of Cardiology | Treatment by a hematologist or other thrombosis expert is appropriate | Not mentioned |
| Recommends against the use of medications such as aspirin to prevent VITT given the lack of evidence and the rare incidence of VITT | ||
| Expert Haematology Panel from the British Society for Haematology | Treat first while awaiting confirmatory diagnosis | Steroids may also be helpful, and although this is unknown, the benefit is likely to outweigh risks of harm |
| Give IVIG urgently as this is the treatment most likely to influence the disease process. Give 1 g/kg (divided into 2 d if needed), irrespective of the degree of thrombocytopenia, and review clinical course. Repeated IVIG may be required | Plasma exchange may be considered if very severe or resistant disease. This may be required daily for up to 5 d if recovery is slow | |
| Anticoagulate with nonheparin-based therapies such as DOACs, fondaparinux, danaparoid, or argatroban depending on the clinical picture. Bleeding and thrombotic risk needs to be carefully balanced and low-dose fondaparinux or critical illness dose argatroban may be appropriate while platelet count is < 30 × 109/L | Transfer patients with cerebral venous thrombosis to a center with a neurosurgical unit and consider early recourse to neuroradiology and/or neurosurgery if deterioration/progressive bleed | |
| If urgent neurosurgery is required, transfuse platelets to > 100 × 109/L and cryoprecipitate to maintain fibrinogen > 1.5 g/L | It is unclear whether platelet transfusions will exacerbate the condition, the risk/benefit in supporting | |
| Replace fibrinogen if needed, to ensure level does not drop below 1.5 g/L, using fibrinogen concentrate or cryoprecipitate | Patients with platelets < 50 × 109/L on anticoagulation who have a secondary cerebral bleed and not requiring procedures is unknown, and therefore, clear advice cannot be given at present | |
| No mention regarding aspirin use | ||
| It is unknown whether heparin exacerbates the condition but until further evidence is available, as the syndrome mimics HIT, heparin is best avoided, including heparin flushes | ||
| For patients who are refractory to repeat doses of IVIG and plasma exchange | ||
| Rituximab can be considered, although there is no evidence of its efficacy in VITT at present | ||
| American Heart Association and American Stroke Association | It is strongly suggested that care be provided collaboratively by vascular neurology and hematology, vascular medicine, or other consultant with expertise in managing HIT with cerebral or systemic thrombosis | Consider administration of steroids |
| IVIG 1 g/kg body weight daily for 2 d | ||
| No heparin products in any dose should be given | ||
| Anticoagulation should follow recent guidelines on HIT with thrombosis that recommend alternative anticoagulants to heparin including argatroban, bivalirudin, danaparoid, fondaparinux, or a DOAC at therapeutic anticoagulant dose intensity. Dosing strategy may require alteration if there is severe thrombocytopenia (i.e., < 20,000 per mm3) or low fibrinogen | ||
| Anticoagulation should be used in cerebral venous sinus thrombosis even in the presence of secondary intracranial hemorrhage, as it is necessary to prevent progressive thrombosis to control this bleeding. In severely ill patients, parenteral agents with short half-life are preferred | ||
| Platelet transfusion should be avoided | ||
| Report thrombosis cases after SARS-CoV-2 vaccines (as well as any suspected adverse events) to the Department of Health and Human Services Vaccine Adverse Event Reporting System | ||
| No mention regarding aspirin use |
COVID-19 = coronavirus disease 2019, DOAC = direct oral anticoagulant, ELISA = enzyme-linked immunosorbent assay, HIT = heparin-induced thrombocytopenia, IVIG = IV immunoglobulin, PF4 = platelet factor 4, TTS = thrombocytopenia syndrome, VITT = vaccine-induced immune thrombotic thrombocytopenia.
Other recommendations found in major societal guidelines regard treatment with steroids. Whether steroids improve outcome is unknown; however, the ISTH-IG, the EHP, and the AHA/ASA recommend consideration of steroid treatment (e.g., prednisone 1 to 2 mg/kg) since the benefit likely outweighs risks of harm. The same societies (the ISTH-IG, the EHP, and the AHA/ASA) also recommend replacement of fibrinogen to ensure a level above 1 g/L using fibrinogen concentrate or cryoprecipitate.
Although there are no reports available on the efficacy of plasma exchange for the treatment of VITT, the ISTH-IG and the EHP guidelines suggest that in resistant disease in which platelet count remains low and there is continuous thrombosis despite IVIG and steroid treatment, plasma exchange should be considered. For patients who are refractory to repeat administration of IVIG and plasma exchange, the EHP suggests the use of rituximab, although there is no evidence of its efficacy in VITT at present.
None of the major societal guidelines recommend aspirin as treatment or prophylaxis for VITT. The CDC, ASH, and ACC recommend against the use of aspirin as treatment and/or prophylaxis for VITT given a lack of evidence including that its inhibition of thromboxane does not block platelet activation in HIT, an increased risk of bleeding, and the rare occurrence rate of VITT. The CDC recommends that aspirin should be continued if already part of the patient’s routine medical regimen.
Finally, since VITT represents a serious and life-threatening adverse event following receipt of certain COVID-19 vaccines, it should be reported to VAERS that is required by the Food and Drug Administration (FDA) and its rules on the Emergency Use Authorizations for COVID-19 vaccines.
UNANSWERED QUESTIONS AND FUTURE RESEARCH
Although clinicians are now provided with basic tools for the identification and management of patients at risk of VITT, there are still many questions that need to be answered.
First, the pathophysiological mechanisms underlying the development of VITT with positive PF4 antibodies in the absence of prior heparin exposure have not been fully elucidated. The reason why adenoviral vaccines and not messenger RNA (mRNA) vaccines are associated with VITT also needs explanation. Additionally, the prevalence of positive PF4 antibodies in patients who underwent vaccination and who did not develop thrombosis or thrombocytopenia is unknown; therefore, the relationship between the pathogenesis of VITT and the presence of PF4 antibodies deserves further study. Further research on these topics will help with the selection of vaccine for a given patient and potentially the development of safer vaccines.
A second important question is whether the incidence of VITT in patients’ post-vaccination with AstraZeneca or Johnson & Johnson/Janssen vaccines is higher than in the general population. The existing reporting systems adopted by the FDA and the European Medicines Agencies to identify vaccine-related adverse effects may overemphasize the relevance of rare conditions that would otherwise be unnoticed in the general population.
A third unanswered question is whether the risk of developing complications such as CVST, SVT, or PE after vaccination is higher than the risk of developing similar complications in COVID-19 disease. Reports of vaccine-associated side effects such as CVST and VITT may induce fear of vaccination in the general population. However, coagulopathy and thrombophilia have often been reported during SARS-CoV-2 infection and the risk of developing CVST with COVID-19 has been shown to be higher than in subjects receiving mRNA vaccines (18). Further studies to comparing the incidence of thrombotic complications in patients with COVID-19 and in patients who received adenoviral vaccines will be required.
CONCLUSIONS
In summary, VITT is a rare but highly morbid complication related to the administration of the AstraZeneca and Johnson & Johnson/Janssen vaccines. Clinicians should be prepared for the early identification of patients with suspicious symptoms and prompt treatment should be initiated to avoid catastrophic deterioration. Major societal guidelines provide useful recommendations for the diagnosis and management of patients with VITT. Clinicians should remain updated on emerging literature to optimize the care of patients presenting with suspected VITT.
Footnotes
All authors wrote and reviewed the article.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383:2603–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384:403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The New York Times. Tracking Coronavirus Vaccinations Around the World. Accessed May 11, 2021
- 5.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384:2124–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021; 384:2092–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384:2202–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muir KL, Kallam A, Koepsell SA, et al. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021; 384:1964–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cines DB, Bussell JB. SAR-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021; 384:2254–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Johnson & Johnson/Janssen COVID-19 Vaccine and Thrombosis With Thrombocytopenia Syndrome (TTS): Update for Clinicians. 2021. Available at: https://emergency.cdc.gov/coca/calls/2021/callinfo_042721.asp. Accessed April 30, 2021
- 11.International Society for Thrombosis and Haemostasis. The ISTH Releases Interim Guidance on Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT). 2021. Available at: https://www.isth.org/news/561406/The-ISTH-Releases-Interim-Guidance-on-Vaccine-Induced-Immune-Thrombotic-Thrombocytopenia-VITT-.htm. Accessed April 22, 2021
- 12.American Society of Hematology. Thrombosis With Thrombocy topenia Syndrome (Also Termed Vaccine-Induced Thrombotic Thrombocytopenia). 2021. Available at: https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia. Accessed June 9, 2021
- 13.American College of Cardiology. Vaccine-Induced Thrombotic Thrombocytopenia (VITT) and COVID-19 Vaccines: What Cardiovascular Clinicians Need to Know. Cardiology Magazine. 2021. Available at: https://www.acc.org/latest-in-cardiology/articles/2021/04/01/01/42/vaccine-induced-thrombotic-thrombocytopenia-vitt-and-covid-19-vaccines. Accessed June 9, 2021
- 14.British Society for Haematology. Guidance Produced by the Expert Haematology Panel (EHP) Focused on Vaccine Induced Thrombosis and Thrombocytopenia (VITT). 2021. Available at: https://b-s-h.org.uk/about-us/news/guidance-produced-by-the-expert-haematology-panel-ehp-focussed-on-vaccine-induced-thrombosis-and-thrombocytopenia-vitt/. Accessed June 9, 2021
- 15.Furie KL, Cushman M, Elkind MSV, et al. ; On Behalf of the American Heart Association/American Stroke Association Stroke Council Leadership: Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced thrombotic thrombocytopenia. Stroke. 2021; 52:2478–2482 [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021; 96:534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bersinger S, Lagarde K, Marlu R, et al. Using nonheparin anticoagulant to treat a near-fatal case with multiple venous thrombotic lesions during ChAdOx1 nCoV-19 vaccination-related vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021. Jun 1. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Tacquet M, Husain M, Geddes JR, et al. Cerebral venous thrombosis: A retrospective cohort study of 513,284 confirmed COVID-19 cases and a comparison with 489,871 people receiving a COVID-19 mRNA vaccine. medRxiv. 2021. doi: 10.1101/2021.04.27.21256153 [Google Scholar]