PURPOSE
Therapies that produce deep and durable responses in patients with metastatic melanoma are needed. This phase II cohort from the international, single-arm PIVOT-02 study evaluated the CD122-preferential interleukin-2 pathway agonist bempegaldesleukin (BEMPEG) plus nivolumab (NIVO) in first-line metastatic melanoma.
METHODS
A total of 41 previously untreated patients with stage III/IV melanoma received BEMPEG 0.006 mg/kg plus NIVO 360 mg once every 3 weeks for ≤ 2 years; 38 were efficacy-evaluable (≥ 1 postbaseline scan). Primary end points were safety and objective response rate (blinded independent central review); other end points included progression-free survival, overall survival (OS), and exploratory biomarkers.
RESULTS
At 29.0 months' median follow-up, the objective response rate was 52.6% (20 of 38 patients), and the complete response rate was 34.2% (13 of 38 patients). Median change in size of target lesions from baseline was −78.5% (response-evaluable population); 47.4% (18 of 38 patients) experienced complete clearance of target lesions. Median progression-free survival was 30.9 months (95% CI, 5.3 to not estimable). Median OS was not reached; the 24-month OS rate was 77.0% (95% CI, 60.4 to 87.3). Grade 3 and 4 treatment-related and immune-mediated adverse events occurred in 17.1% (7 of 41) and 4.9% (2 of 41) of patients, respectively. Increased polyfunctional responses in CD8+ and CD4+ T cells were seen in blood after treatment, driven by cytokines with effector functions. Early on-treatment blood biomarkers (CD8+ polyfunctional strength difference and eosinophils) correlated with treatment response.
CONCLUSION
BEMPEG in combination with NIVO was tolerated, with relatively low rates of grade 3 and 4 treatment-related and immune-mediated adverse events. The combination had encouraging antitumor activity in first-line metastatic melanoma, including an extended median progression-free survival. Exploratory analyses associated noninvasive, on-treatment biomarkers with response, before radiologic evidence was observed.
BACKGROUND
Immune checkpoint inhibitors (ICIs), such as anti–programmed death-1 (PD-1) and PD-ligand 1 (PD-L1), have improved survival for patients with metastatic melanoma.1-4 ICIs are a standard of care in the first-line setting, either as a single agent or in combination with other therapies.5 However, not all patients respond to ICIs, with factors associated with a poor tumor response including a low density of baseline CD8+ tumor-infiltrating lymphocytes (TIL),6,7 low tumor PD-L1 expression,6-8 low tumor mutational burden (TMB),9,10 or low interferon-gamma (IFN-γ) gene expression profile (GEP).11,12 An unmet need remains for novel ICI combinations that achieve durable and deep responses in a broad population of patients with metastatic melanoma, without adding substantial toxicity. Combining an ICI with an agent that modulates the tumor microenvironment (TME) may address some of their known limitations.
CONTEXT
Key Objective
An unmet need remains for novel immune checkpoint inhibitor combinations that achieve durable and deep responses in patients with metastatic melanoma, without adding substantial toxicity. What is the clinical efficacy and safety profile of the interleukin-2 pathway agonist bempegaldesleukin (BEMPEG) plus nivolumab (NIVO) in previously untreated patients with metastatic melanoma from the PIVOT-02 phase II study?
Knowledge Generated
BEMPEG plus NIVO was well tolerated, and patients achieved deep and durable clinical responses, with a high rate of complete responses (34.2%) and a median progression-free survival of 30.9 months. Forty-seven percent of patients (18 of 38) achieved 100% reduction in target lesions. Exploratory on-treatment blood biomarkers were also associated with response.
Relevance
These data demonstrate encouraging safety and efficacy for this novel immunotherapy combination of BEMPEG plus NIVO in first-line metastatic melanoma. They provide rationale for the ongoing phase III study in this same patient population (PIVOT IO 001; NCT03635983).
Interleukin-2 (IL-2) plays an important role in promoting tumor cell death by enhancing the survival and expansion of CD4+ and CD8+ T cells and natural killer (NK) cells.13 High-dose IL-2 is approved for the treatment of metastatic melanoma, but its clinical use is limited by its short half-life, which necessitates a high dose leading to significant toxicities, such as vascular leak syndrome.14 As a result, high-dose IL-2 requires inpatient administration at specialized centers.15
Bempegaldesleukin (BEMPEG; NKTR-214) is a first-in-class, CD122-preferential IL-2 pathway agonist that leverages the clinically validated IL-2 pathway to stimulate an antitumor immune response.16-18 BEMPEG increases the proliferation and infiltration of CD8+ T cells and NK cells into the TME, without expansion of regulatory T cells, both in preclinical17,19-21 and in human studies.16,22 BEMPEG also increases PD-1 expression on lymphocytes in the TME (a marker of CD8+ tumor-reactive T cells)16 and PD-L1 expression on tumor cells.22 These attributes support evaluation of BEMPEG in combination with an ICI.
PIVOT-02 is an international, multicenter, phase I/II trial (NCT02983045) of the novel immunotherapy combination BEMPEG plus nivolumab (NIVO) in patients with advanced solid tumors.22 We report results from a phase II cohort of PIVOT-02, which evaluated BEMPEG plus NIVO as a first-line treatment for patients with metastatic melanoma.
METHODS
Patients
Eligible patients were ≥ 18 years of age and had histologically confirmed stage III (unresectable) or stage IV (metastatic) melanoma (per American Joint Committee on Cancer staging system version 7), measurable disease (per Response Evaluation Criteria in Solid Tumors, version 1.1 [RECIST v1.1]), a baseline Eastern Cooperative Oncology Group performance status of 0 or 1, tumor tissue available for biomarker testing, a known BRAF mutation (V600E or V600K) and PD-L1 immunohistochemistry status (patients with any status were eligible), and adequate organ function (hemoglobin ≥ 9.0 g/dL, serum creatinine ≤ 2 mg/dL, AST and ALT ≤ 3 × upper limit of normal [ULN], total bilirubin within normal limits, and lipase and amylase ≤ 1.5 × ULN). Patients were excluded if they had received prior treatment for melanoma in the neoadjuvant, adjuvant, locally advanced, or metastatic setting; had received prior IL-2 therapy; had uveal melanoma; or had active brain metastases.
Study Design and Treatment
PIVOT-02 (NCT02983045) was an international, multicenter, nonrandomized, open-label, phase I/II trial. Results from the phase I dose-escalation portion in advanced solid tumors have been published elsewhere.22 In this phase II cohort of patients with metastatic melanoma, intravenous BEMPEG 0.006 mg/kg plus NIVO 360 mg was to be given once every 3 weeks until disease progression, death, unacceptable toxicity, symptomatic deterioration, achievement of maximal response, investigator decision to discontinue treatment, patient withdrawal of consent, pregnancy, loss to follow-up, or study termination by the sponsor. Responding patients were treated for a maximum of 2 years.
The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The Protocol (online only) was approved by independent ethics committees and the relevant institutional review board at each site. All patients provided written informed consent.
End Points and Assessments
Safety and the objective response rate (ORR) per RECIST v1.1 were primary end points. Safety (National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.03) was evaluated in all patients who received ≥ 1 dose of study treatment. All patients with measurable disease per RECIST v1.1 at baseline and ≥ 1 postbaseline tumor response assessment were evaluable for efficacy (response-evaluable population). Response was assessed by RECIST v1.1 every 8 weeks by blinded independent central review (BICR; where radiographic examinations were reviewed by independent physician experts not involved in patient treatment) and the local investigator. The primary analysis for efficacy was by BICR.
Secondary end points included duration of response, clinical benefit rate or disease control rate (complete response [CR] or partial response [PR], or stable disease ≥ 7 weeks; per RECIST v1.1 in the response-evaluable population), progression-free survival (PFS), and overall survival (OS; intent-to-treat population). Long-term follow-up for survival occurred every 3 months.
Exploratory end points included biomarker analyses in the blood and tumor to determine associations with response (Data Supplement, online only). Tumor biomarkers associated with response to ICIs were selected for analyses (IFN-γ GEP, CD8+ TIL, PD-L1, TMB, CD74, HLA-A, HLA-B, and HLA-E).9,10,23,24 The polyfunctionality and polyfunctional strength index (PSI) of circulating CD4+ and CD8+ lymphocytes and NK cells were determined in peripheral blood mononuclear cells using single-cell cytokine analysis (IsoPlexis, New Haven, CT; Data Supplement).19 Polyfunctional strength difference (PSD; ie, the difference in PSI between cycle 1 day 8 and baseline; high v low by median cutoff) was evaluated for associations with response. Blood lymphocyte, eosinophil, and neutrophil concentrations (× 106/L) were evaluated at baseline, and on-treatment changes were evaluated for association with response.
A new or archival (within 6 months) tumor biopsy was obtained at baseline, with on-treatment biopsies collected during days 15-21 of cycle 1. Immunohistochemistry analysis for PD-L1 was performed centrally using PD-L1 IHC 28-8 PharmDx (Dako, an Agilent Technologies, Inc company, Santa Clara, CA), and tumors were defined as PD-L1–negative (< 1% tumor cell expression) or PD-L1–positive (≥ 1% tumor cell expression).
Statistical Analysis
The study was designed to enroll at least 28 patients with treatment-naïve metastatic melanoma who would be treated with the recommended phase II dose of BEMPEG plus NIVO (a subsequent protocol amendment allowed up to 39 patients to be enrolled). This sample size was based on the target ORR relative to a historic response rate1 and calculated using normal approximation to provide reasonable false-positive (< 10%) and false-negative (< 10%) rates. The Clopper–Pearson method was used to calculate two-sided 95% CIs for the response rate. Duration of response (response-evaluable population), PFS, and OS (both intent-to-treat population) are summarized by the Kaplan-Meier method. Data for patients without disease progression (RECIST v1.1) and alive, or with unknown status, were censored at the time of the last tumor assessment. All efficacy end points were analyzed at the cutoff date (September 1, 2020) for the primary analysis. Continuous data are summarized by descriptive statistics, and categorical data are summarized by the number and percentage of patients. Sum of lesions was defined using the normalization method of zeroing out any lymph nodes < 10 mm. Statistical analyses for biomarker evaluations included calculation of difference in response rates and hazard ratios for PFS with 95% CIs between biomarker-defined groups. P values for the comparison of on-treatment biomarker changes versus baseline were calculated by the two-sided Mann-Whitney test.
RESULTS
Patients
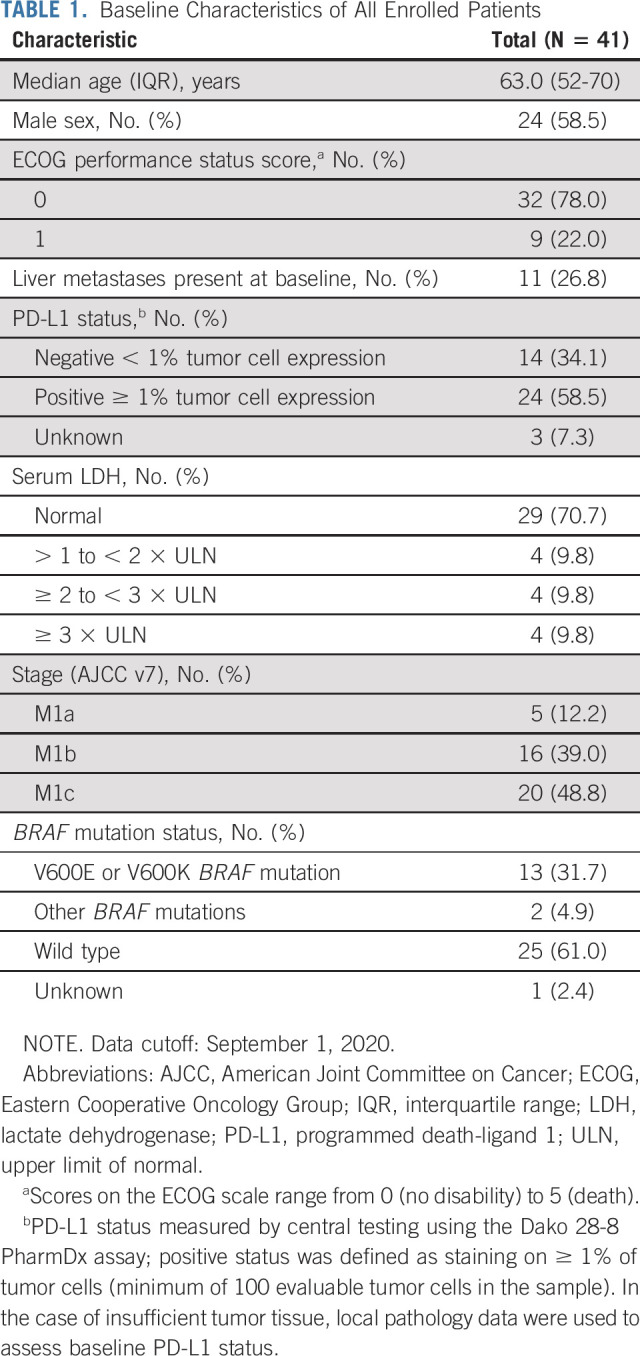
From March 27, 2017, through March 28, 2018, 41 patients with unresectable or metastatic melanoma were enrolled at 12 sites in three countries (United States, Italy, and Poland). At baseline, 12 of 41 (29.3%) patients had elevated serum lactate dehydrogenase (LDH; > ULN), 11 of 41 (26.8%) had liver metastases, and 14 of 41 (34.1%) were PD-L1–negative (Table 1). All patients received ≥ 1 dose of BEMPEG plus NIVO and were evaluable for safety. Thirty-eight patients had ≥ 1 postbaseline scan and were evaluable for response. Three patients discontinued prior to the first scan because of patient decision (n = 2) and an unrelated treatment-emergent adverse event (AE; n = 1).
TABLE 1.
Baseline Characteristics of All Enrolled Patients
At data cutoff, no patients remained on treatment. BEMPEG was discontinued because of the following reasons: progressive disease by RECIST (n = 14), AE (n = 9), patient decision (n = 9; reasons included withdrew consent [three], surgical resection [two], quality of life [one], return to work [one], travel burden [one], and entered hospice [one]), achievement of maximal response (n = 8), and clinical progression (n = 1). Reasons for NIVO discontinuation are shown in the Data Supplement. Ten patients died on study, all from disease progression. The median treatment duration was 6.2 months (interquartile range [IQR], 3.1-17.3 months), and the median number of cycles was 9.0 (IQR, 4-22).
Primary Analysis of Objective Response Rate
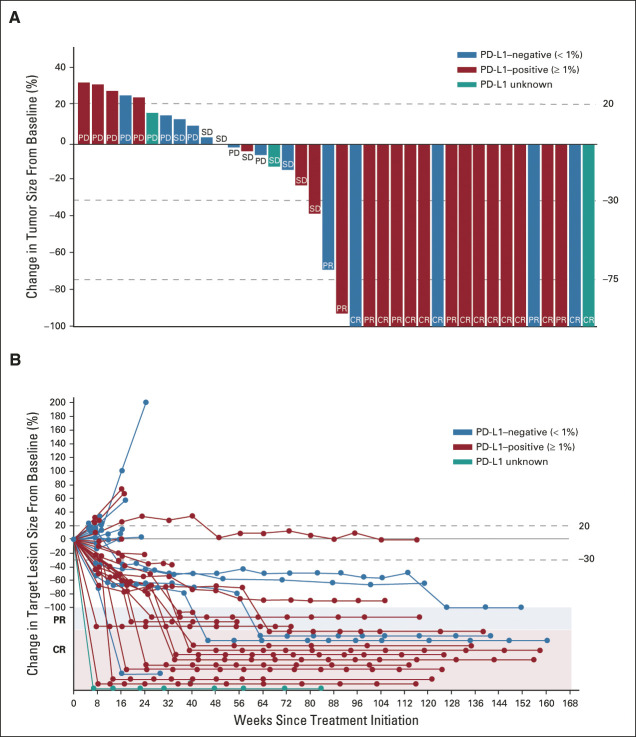
The median duration of follow-up was 29.0 months (IQR, 15.7-32.3 months). The confirmed ORR by BICR was 52.6% (95% CI, 35.8 to 69.0; 20 of 38; Data Supplement). CRs were seen in 13 patients (34.2%; Fig 1A). The median time to onset of first response was 2.0 months (range, 1.5-4.1 months) and to CR was 7.9 months (range, 1.5-15.2 months). Eight of 13 patients achieved a CR on or after the third postbaseline scan (after ≥ 8 treatment cycles; Data Supplement), suggesting deepening responses over time.
FIG 1.
Clinical response to BEMPEG plus NIVO by blinded independent central review (response-evaluable population). (A) Waterfall plot of the maximum change in tumor size. (B) Percent change in target lesion size over time. Data cutoff: September 1, 2020. Response-evaluable population includes eligible patients with measurable disease (per RECIST v1.1) at baseline and at least one postbaseline assessment of tumor response. All objective responses are confirmed. BEMPEG, bempegaldesleukin; CR, complete response; NIVO, nivolumab; PD, progressive disease (because of non–target lesion progression or presence of new lesion); PD-L1, programmed death-ligand 1; PR, partial response (complete response for target lesion; non–target lesion still present); SD, stable disease.
The ORR by investigator review was consistent with that determined by BICR (both 52.6%; 20 of 38; Data Supplement). CRs were reported for 13 of 38 (34.2%) patients by BICR and 7 of 38 (18.4%) patients by local investigator assessment (the reduction in tumor burden for the six patients who were differentially classified as having a PR versus a CR ranged from –68% to –100% by investigator assessment).
Secondary Analyses of Response
At data cutoff, 16 of 20 (80.0%) patients had an ongoing objective response. Responses lasted for ≥ 6 months in 18 of 20 (90.0%) patients and for ≥ 12 months in 16 of 20 (80.0%) patients. Median duration of response was not reached (IQR, 29.0 months-not reached).
The disease control rate by BICR was 73.7% (28 of 38 patients; Data Supplement). The median change in target lesion size from baseline was −78.5% (response-evaluable population; n = 38); 90% (18 of 20) of patients who responded to the combination (CR or PR) went on to achieve 100% reduction in their target lesions versus baseline (Fig 1B). Forty-seven percent of response-evaluable patients (18 of 38) achieved 100% reduction in target lesions. Baseline tumor burden is described in the Data Supplement: the median baseline tumor burden in patients who responded (CR or PR) to the combination was 30.5 mm, whereas in nonresponders (progressive disease or stable disease), it was 46.5 mm.
Objective responses by BICR in patients with traits typically indicative of poor prognosis were as follows: 5 of 10 patients with liver metastases (ORR 50.0%; all CRs) and 5 of 11 patients with elevated LDH > ULN (ORR 45.5%; three CRs and two PRs). Two of eight patients with LDH levels ≥ 2 ULN at baseline had an objective response. Objective responses by PD-L1 tumor status and BRAF mutation status are presented in the Data Supplement.
Secondary Analyses of PFS and OS
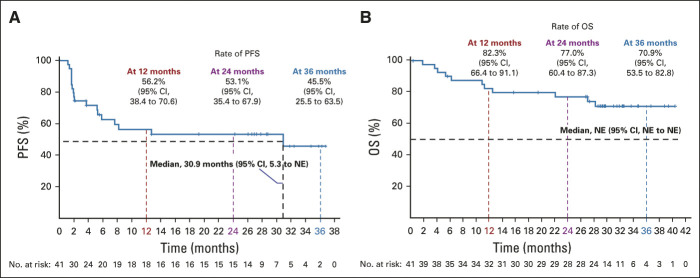
At data cutoff, median PFS in the intent-to-treat population was 30.9 months (95% CI, 5.3 to not estimable; Fig 2A). PFS rates were 56.2% (95% CI, 38.4 to 70.6) at 12 months, 53.1% (95% CI, 35.4 to 67.9) at 24 months, and 45.5% (95% CI, 25.5 to 63.5) at 36 months. Median OS was not reached (Fig 2B). OS rates were 82.3% (95% CI, 66.4 to 91.1) at 12 months, 77.0% (95% CI, 60.4 to 87.3) at 24 months, and 70.9% (95% CI, 53.5 to 82.8) at 36 months. Median PFS per baseline PD-L1 status by BICR is shown in the Data Supplement.
FIG 2.
Kaplan-Meier estimates of (A) PFS by blinded independent central review and (B) OS in all patients (intent-to-treat population; N = 41). Data cutoff: September 1, 2020. NE, not estimable; OS, overall survival; PFS, progression-free survival.
Exploratory Analyses of Tumor and Blood Biomarker Correlates With Response
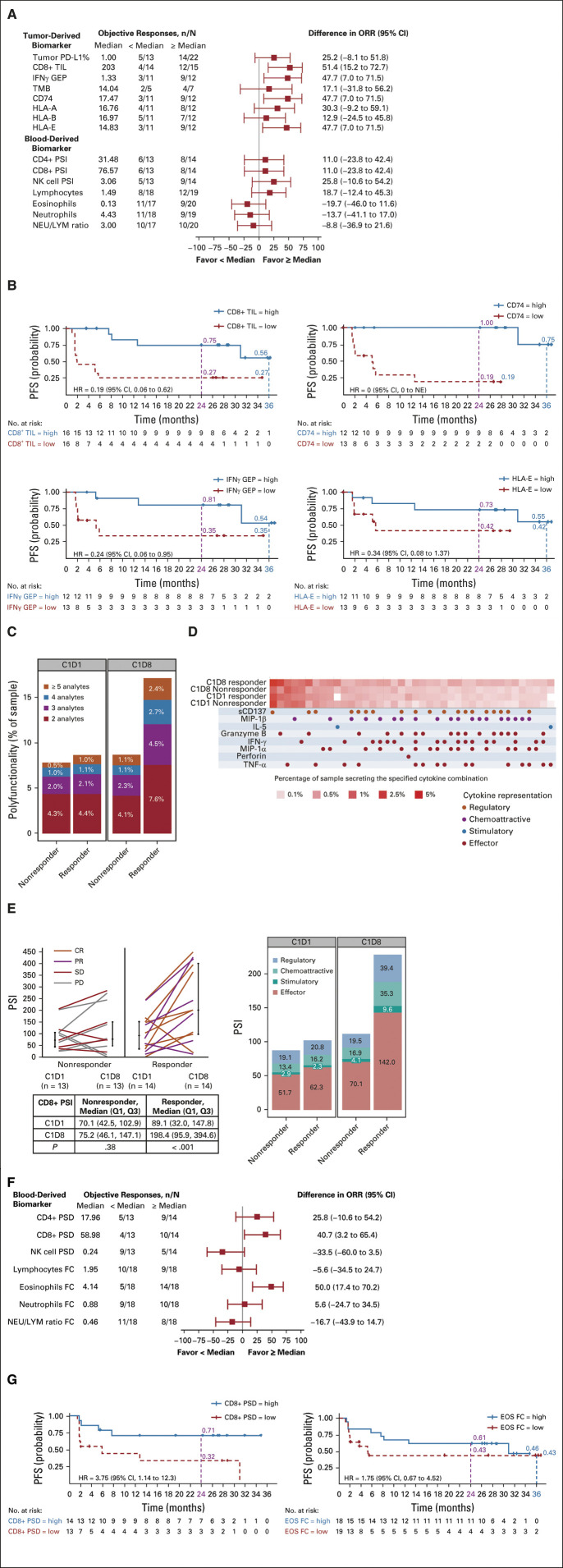
Baseline tumor biomarker analyses showed that tumor PD-L1 expression and TMB were not associated with objective response. However, high IFN-γ GEP, high CD8+ TIL, high CD74, and high HLA-E in baseline tumor biopsies were associated with a higher ORR (Fig 3A) and a longer PFS (Fig 3B).
FIG 3.
Single-cell cytokine analysis of biomarkers at baseline (C1D1) and on treatment (C1D8) and correlation with response by BICR. (A) Relationship between baseline tumor biomarkers and ORR.a,b (B) Relationship between CD8+ TIL, IFN-γ GEP, CD74, and HLA-E (high v low) in baseline tumor samples and PFS. (C) Change in polyfunctionality of CD8+ T cells on treatment by response. (D) Single-cell polyfunctional heatmap illustrating the single-cell cytokine combinations secreted by each sample. Each column corresponds to a specific cytokine or combination of cytokines, and the red squares represent the frequency at which the group was secreted by the corresponding sample. Cytokine groups are ordered by overall frequency across all the samples. (E) Change in single-cell PSI on treatment by response and cytokine representation (regulatory, chemoattractive, stimulatory, and effector). (F) Changes in the median values of blood biomarkers in paired samples on treatment versus baseline and relationship with the ORR.a,c (G) Relationship between CD8+ PSD and eosinophil FC and PFS. aFor each biomarker evaluated, the number of patients with an objective response (CR or PR; n), by BICR per RECIST v1.1, falling above and below the median biomarker measurement is presented. The denominator (N) is the number of patients evaluable for that biomarker. The difference in ORR for each biomarker on the basis of low (< median) or high (≥ median) is presented. bBiomarkers were evaluated in baseline tumor or blood samples: tumor PD-L1 expression by immunohistochemistry (PD-L1 IHC 28-8 PharmDx [Dako, an Agilent Technologies Inc company, Santa Clara, CA]), expressed as a percentage of tumor cell expression (negative, < 1% tumor cell expression; positive, ≥ 1% tumor cell expression); tumor CD8+ TIL by immunohistochemistry, cells/mm2; IFN-γ GEP, expression score; TMB, mutations per megabase; tumor CD74, HLA-A, HLA-B, and HLA-E expression score by immunohistochemistry; blood CD4+ PSI, CD8+ PSI, and NK cell PSI using single-cell cytokine analysis; blood lymphocytes, eosinophils, and neutrophils, all × 106/L; NEU/LYM ratio. cBiomarkers were evaluated in on-treatment blood biomarkers: CD4+, CD8+, and NK cell PSD (ie, difference in PSI between C1D1 and C1D8 measured using single-cell cytokine analysis); and the FC in levels of lymphocytes, eosinophils, neutrophils (all × 106/L), and NEU/LYM ratio between C1D1 and C1D8. BICR, blinded independent central review; C1D1, cycle 1 day 1 (baseline); C1D8, cycle 1 day 8 (on treatment); CR, complete response; EOS, eosinophils; FC, fold change; GEP, gene expression profile; HR, hazard ratio; IFN, interferon; IL-5, interleukin-5; MIP, macrophage inflammatory protein; NE, not estimable; NEU/LYM ratio, neutrophil to lymphocyte ratio; NK, natural killer; ORR, objective response rate; PD, progressive disease; PD-L1, programmed death-ligand 1; PFS, progression-free survival; polyfunctionality, cosecretion of two or more cytokines per cell; PR, partial response; PSD, polyfunctional strength difference; PSI, polyfunctional strength index (ie, percentage of polyfunctional cells in a sample, multiplied by the sum of secreted cytokine intensities of polyfunctional cells); SD, stable disease; TIL, tumor-infiltrating lymphocytes; TMB, tumor mutational burden; TNF, tumor necrosis factor.
We assessed blood-based biomarkers for the ability to predict response to treatment. Single-cell analysis of T cells and NK cells was done to determine their polyfunctional response after treatment (ie, the ability of a single cell to secrete multiple [≥ 2] cytokines). We observed robust upregulation of polyfunctional CD8+ T cells in the blood of patients with an objective response (Fig 3C) and of CD4+ T cells in patients with and without an objective response after treatment, whereas there was a decrease in polyfunctionality of NK cells after treatment (Data Supplement). A single-cell polyfunctional heatmap (Fig 3D) showed an increase in polyfunctional CD8+ T-cell subsets that coproduce combination cytokines. Treatment elicited an approximate 2.2-fold increase in the PSI of CD8+ T cells (Fig 3E) and CD4+ T cells in patients with an objective response, with no increase in the PSI of NK cells (Data Supplement). The increased polyfunctional response in CD8+ and CD4+ T cells appears to be driven by the production of cytokines with effector functions (Fig 3E and the Data Supplement).
High CD8+ PSD was associated with a higher ORR than low CD8+ PSD (Fig 3F) and a longer PFS (hazard ratio, 3.75 [95% CI, 1.1 to 12.3]; Fig 3G). Analysis of paired blood samples also demonstrated an early on-treatment increase in eosinophils (Data Supplement). High fold change from baseline to day 8 in eosinophils, consistent with IL-2 signaling,25 was associated with a higher ORR (Fig 3F), but not PFS (Fig 3G).
Safety
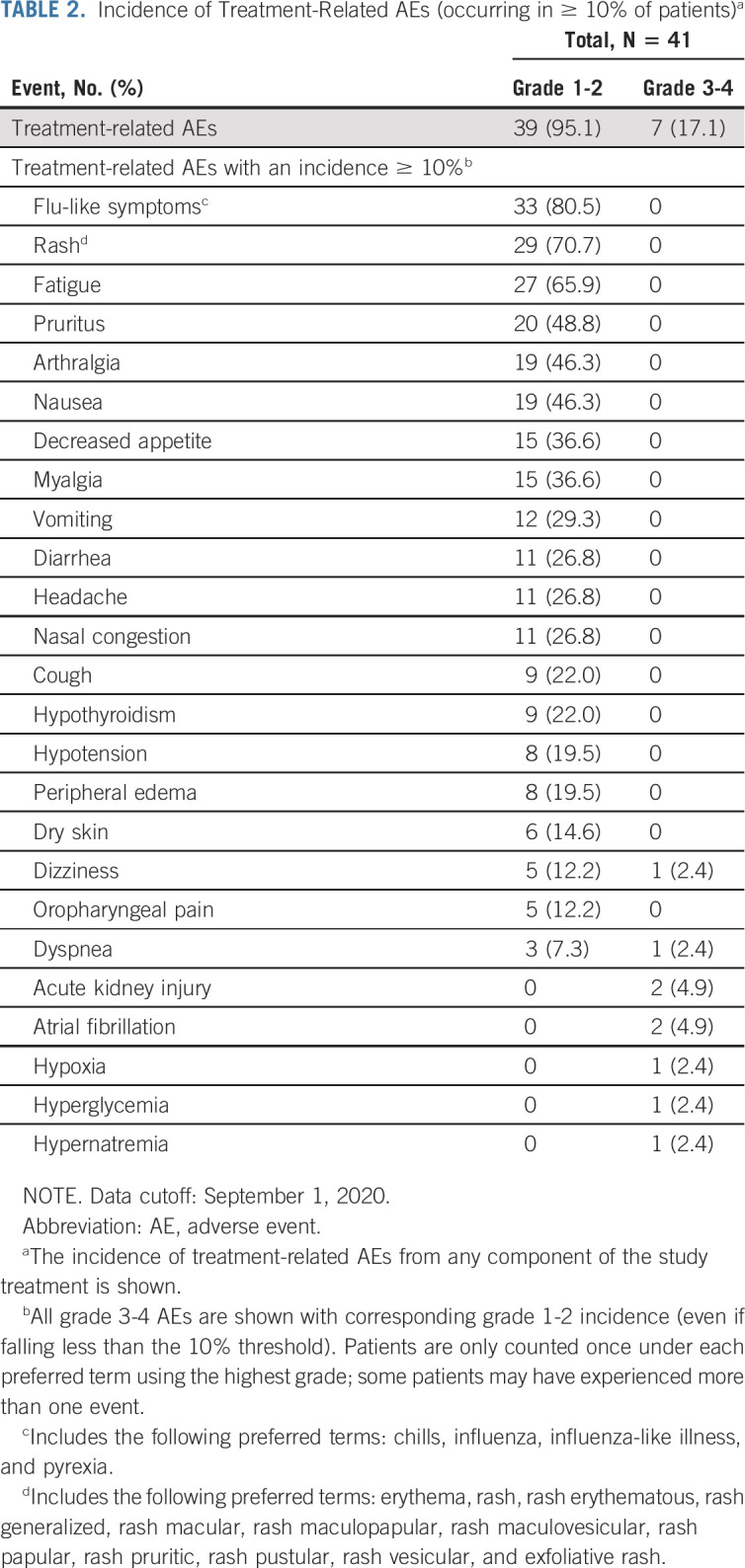
AEs related to treatment (determined by the investigator) occurred in 39 of 41 (95.1%) patients. The most frequent (≥ 40% of patients) any-grade events were flu-like symptoms, rash, fatigue, pruritus, arthralgia, and nausea (Table 2). Seven (17.1%) patients experienced grade 3 or 4 treatment-related AEs (Table 2), which were managed using standard treatment protocols. Two patients experienced atrial fibrillation: one patient with a history of atrial fibrillation since 2015 and a second patient 1 month after the last dose of study drug. Treatment-related AEs led to discontinuation in five patients (12.2%): blood creatinine increased, cerebrovascular accident, malaise, peripheral edema, and pharyngitis (n = 1 each). Immune-mediated AEs occurred in 13 patients (31.7%), of which two events (4.9%) were grade ≥ 3: nephritis and renal dysfunction (n = 1) and hyperglycemia related to diabetes mellitus (n = 1; Data Supplement). The incidence of cytokine-related AEs (flu-like symptoms, rash, pruritus, and hypotension) decreased with continued dosing (Data Supplement). There were no cases of cytokine release syndrome or hypereosinophilic syndrome and no grade ≥ 3 treatment-related hypotension. There were no treatment-related deaths.
TABLE 2.
Incidence of Treatment-Related AEs (occurring in ≥ 10% of patients)a
DISCUSSION
There is an unmet need for novel first-line combinations to extend the treatment benefit of immunotherapy to more patients with metastatic melanoma, without substantially adding toxicity. This phase II cohort from the international, single-arm PIVOT-02 study evaluated the novel IL-2 pathway agonist and ICI immunotherapy combination of BEMPEG plus NIVO in patients with previously untreated, unresectable, or metastatic melanoma. The combination was tolerable and patients achieved deep and durable clinical responses, as evidenced by the observed 52.6% (95% CI, 35.8 to 69.0) ORR, 34.2% CR rate, median 78.5% reduction in tumor burden from baseline, and median PFS of 30.9 months (95% CI, 5.3 to not estimable). Although comparisons cannot be formally made across trials, our preliminary findings indicate that BEMPEG plus NIVO has the potential to provide additional efficacy over PD-1 inhibition alone.2,26 At the primary analysis of the phase III CheckMate 067 trial (median duration of follow-up, 12.2-12.5 months), NIVO monotherapy achieved an ORR of 44% (95% CI, 38 to 49); 9% of patients had a CR, the median reduction in tumor burden was 34.5%, and median PFS was 6.9 months (95% CI, 4.3 to 9.5).26
An exploratory meta-analysis by the US Food and Drug Administration suggested a strong correlation between depth of response in first-line metastatic melanoma and OS,27 particularly for patients with a ≥ 75% reduction in target lesions.27 In our PIVOT-02 melanoma cohort, 47% of response-evaluable patients (18 of 38) achieved 100% reduction in target lesions. Median OS was not reached, but the Kaplan-Meier estimate for the OS rate at 2 years with BEMPEG plus NIVO was 77.0% (95% CI, 60.4 to 87.3). This compares favorably with the 2-year OS rate for NIVO at 2 years in CheckMate 067 (59% [95% CI, not reported]).28
Our cohort was limited by its small size and single-arm design. Similar early-phase, nonrandomized trials have produced encouraging results that were not reproduced in a phase III, randomized study (eg, epacadostat).29-31 Phase I/II trials have the potential for selection bias, creating a patient population with more favorable characteristics than would be expected in the phase III setting, or indeed in clinical practice. Nevertheless, analysis of the baseline characteristics of patients in our trial showed that they mirrored those found in published phase III trials in metastatic melanoma, with similar proportions of patients with traits typically associated with poorer outcomes on ICIs.9,10 With respect to baseline tumor biomarkers, our cohort of patients had a relatively low TMB (median 14.04 Mut/Mb v a cutoff of 16-23.1 Mut/Mb typically used to define high TMB by FoundationOne in other melanoma trials)32-34 and a relatively low CD8+ TIL count (median 203 cells/mm2) versus the values reported in the published literature.7 At baseline, 29.3% of patients in our trial had elevated LDH > ULN at baseline (compared with 29.0%-42.8% in published trials),1,3,26,29,35-38 whereas 19.5% had ≥ 2 × ULN at baseline (compared with 7.0%-12.8% in published trials).1,26,29,37 We observed objective responses with BEMPEG plus NIVO in patients with these poor baseline traits, notably those with high serum LDH, liver metastases, and PD-L1–negative tumor status. Of interest, historical data with high-dose IL-2 indicate that patients with liver metastases respond well to cytokine therapy.39
Baseline tumor biomarkers were associated with response to BEMPEG plus NIVO in our exploratory analyses. High IFN-γ GEP, high CD8+ TIL, high CD74 (CD74 is important in MHC Class II antigen presentation and has other MHC Class II–independent functions),40 and high HLA-E at baseline were associated with a higher ORR and a longer PFS, in line with published literature for ICIs.7,11,23,24,41
Additional exploratory biomarker analyses demonstrated that T cells induced by BEMPEG plus NIVO were highly polyfunctional, with increased PSI of CD4+ and CD8+ T cells on treatment versus baseline. The polyfunctional response by BEMPEG plus NIVO was driven by the production of cytokines with effector functions (eg, granzyme B, IFN-γ, macrophage inflammatory protein-1α, and tumor necrosis factor-α), which aligns with preclinical reports.19 Polyfunctional T cells provide a more effective immune response than T cells only producing a single cytokine.42 We hypothesize that BEMPEG may contribute to increased efficacy over NIVO alone not only by increasing the number of T cells in the blood and tumor16,22 but also by enhancing their fitness43 and functional capacity, as we have shown here. This concept is being further examined in an ongoing randomized phase III trial (PIVOT IO 001; NCT03635983). Increased CD8+ PSD was associated with a higher ORR and longer PFS. These findings are consistent with prior reports associating higher PSI with clinical response to immunotherapy,44 suggesting an anticancer potential of polyfunctional CD8+ T cells. Noninvasive, blood-based biomarkers of response that are detectable before radiologic evidence are highly desirable to identify patients who may benefit the most from treatment, and our exploratory findings warrant further exploration.
As an immunostimulatory IL-2 cytokine prodrug, BEMPEG was engineered to deliver a controlled and sustained IL-2 pathway signal, and thereby minimize toxicity versus high-dose IL-2, thus allowing for outpatient administration. The safety profile of BEMPEG plus NIVO in first-line metastatic melanoma was consistent with that of the individual compounds1,16 and no new safety signals were identified demonstrating the feasibility of an outpatient dosing regimen. The most frequent AEs were of grade 1 or 2 in severity and included flu-like symptoms, rash, fatigue, and pruritus. Most AEs were transient and resolved spontaneously without intervention or by using standard treatment protocols. Rates of cytokine-related AEs were typically higher in cycle 1, and declined over subsequent cycles, which was consistent with prior reports.22 The rate of grade 3 and 4 treatment-related AEs with BEMPEG plus NIVO (17.1%) aligns with that reported with PD-1 inhibitors in this setting (16%-17%)26,45 and compares favorably with reported rates for NIVO plus ipilimumab (55%)26 and BRAF and MEK inhibitors (54%-68%).46,47 The rate of grade 3 and 4 immune-mediated AEs with the combination (2 of 41; 4.9%) is consistent with that reported for anti–PD-1 monotherapy (24 of 313; 7.7%) and substantially lower than that reported for anti–PD-1 and anti–CTLA-4 combination therapy (124 of 313; 39.6%).26
In summary, these data provide preliminary evidence to support the safety and efficacy of BEMPEG plus NIVO in patients with previously untreated metastatic melanoma. The responses appeared deep and durable, with 90% (18 of 20) of responding patients achieving 100% reduction in their target lesions versus baseline, and rates of grade ≥ 3 events were within acceptable limits. Our exploratory biomarker findings advance our working hypothesis on the mechanism by which BEMPEG plus NIVO could provide additional efficacy over PD-1 inhibition alone—not only by increasing the number of T cells16,22 but also by boosting their fitness and functional capacity. Patients with a robust immune response experienced a greater treatment effect, with noninvasive, early on-treatment exploratory biomarkers predicting response. BEMPEG plus NIVO was awarded Breakthrough Therapy designation for previously untreated metastatic melanoma by the US Food and Drug Administration in 2019. The preliminary PIVOT-02 findings are being confirmed in an ongoing randomized, registrational, phase III trial in previously untreated patients with metastatic melanoma (PIVOT IO 001; NCT03635983).
ACKNOWLEDGMENT
We would like to thank all patients, their families, and the investigators who participated in this study. We thank Danni Yu and Arkopal Choudhury of Nektar Therapeutics for biostatistics input. We also thank Dako for collaborative development of the PD-L1 IHC 28-8 PharmDx assay, Bristol Myers Squibb (Princeton, NJ), and Jing Zhou from IsoPlexis (New Haven, CT). Medical writing assistance was provided by Alison Lovibond, PhD, CMPP, of BOLDSCIENCE Inc and was funded by Nektar Therapeutics.
Adi Diab
Honoraria: Array BioPharma
Consulting or Advisory Role: Nektar, CureVac, Celgene, Idera
Research Funding: Nektar, Idera, Celgene, Pfizer, Merck, Apexigen
Travel, Accommodations, Expenses: Nektar
Scott S. Tykodi
Consulting or Advisory Role: Merck, Intellisphere LLC, Natera, Bristol Myers Squibb, Exelixis
Research Funding: Genentech, Bristol Myers Squibb, Merck Sharp & Dohme, Calithera Biosciences, Pfizer, Jounce Therapeutics, Nektar, Exelixis, Clinigen Group
Patents, Royalties, Other Intellectual Property: Patent pending
Gregory A. Daniels
Honoraria: Sanofi/Regeneron
Consulting or Advisory Role: Sanofi/Regeneron
Speakers' Bureau: Regeneron, Array BioPharma, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb, Amgen, Viralytics, Nektar, Merck
Michele Maio
Stock and Other Ownership Interests: Theravance, Epigen Therapeutics
Honoraria: Bristol Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma, Sanofi, Lilly
Consulting or Advisory Role: Bristol Myers Squibb, Roche, AstraZeneca, MSD, Merck, Pierre Fabre, Alfasigma
Patents, Royalties, Other Intellectual Property: DNA hypomethylating agents for cancer therapy
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma
Brendan D. Curti
Honoraria: Clinigen Group, Nektar
Consulting or Advisory Role: Merck
Research Funding: Bristol Myers Squibb, Galectin Therapeutics, Clinigen Group
Patents, Royalties, Other Intellectual Property: Biomarkers for OX40 response
Travel, Accommodations, Expenses: Agonox
Karl D. Lewis
Honoraria: Array BioPharma, Iovance Biotherapeutics
Consulting or Advisory Role: Array BioPharma, Merck, Roche, Regeneron, Sanofi, Iovance Biotherapeutics
Research Funding: Roche/Genentech, Merck, Array BioPharma, Incyte, Nektar, Iovance Biotherapeutics, Bristol Myers Squibb, Kartos Therapeutics, OncoSec, Regeneron, Alkermes, Neon Therapeutics, Ultimovacs, Senhwa Biosciences, Replimune, Amgen
Travel, Accommodations, Expenses: Merck, Roche/Genentech, Regeneron, Neon Therapeutics, Alkermes
Uncompensated Relationships: Roche/Genentech, Regeneron
Sekwon Jang
Consulting or Advisory Role: Bristol Myers Squibb, EMD Serono, Novartis, Sanofi, Sun Biopharma, Genentech
Ewa Kalinka
Honoraria: Bristol Myers Squibb, MSD, AstraZeneca, Regeneron, Nektar, Roche
Consulting or Advisory Role: Bristol Myers Squibb
Speakers' Bureau: Bristol Myers Squibb, Roche
Research Funding: Bristol Myers Squibb, Merck Sharp & Dohme, Nektar, AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche
Igor Puzanov
Stock and Other Ownership Interests: Celldex
Consulting or Advisory Role: Amgen, Iovance Biotherapeutics, Merck, Roche, Nouscom, Seneca Therapeutics
Alexander I. Spira
Stock and Other Ownership Interests: Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer
Consulting or Advisory Role: Array BioPharma, Incyte, Amgen, Novartis, AstraZeneca/MedImmune, Mirati Therapeutics, Gritstone Oncology, Jazz Pharmaceuticals, Merck, Bristol Myers Squibb
Research Funding: Roche, AstraZeneca, Boehringer Ingelheim, Astellas Pharma, MedImmune, Novartis, Newlink Genetics, Incyte, AbbVie, Ignyta, LAM Therapeutics, Trovagene, Takeda, Macrogenics, CytomX Therapeutics, Astex Pharmaceuticals, Bristol Myers Squibb, Loxo, Arch Therapeutics, Gritstone Oncology, Plexxikon, Amgen, Daiichi Sankyo, ADC Therapeutics, Janssen Oncology, Mirati Therapeutics, Rubius Therapeutics
Daniel C. Cho
Consulting or Advisory Role: Nektar, Pfizer, Werewolf Therapeutics
Expert Testimony: Genentech, Abbott/AbbVie
Shanhong Guan
Employment: Nektar Therapeutics
Stock and Other Ownership Interests: Nektar Therapeutics
Erika Puente
Employment: Nektar
Stock and Other Ownership Interests: Nektar
Tuan Nguyen
Employment: Nektar, Theravance Therapeutics
Stock and Other Ownership Interests: Nektar, Theravance
Ute Hoch
Other Relationship: Nektar
Sue L. Currie
Employment: Nektar
Stock and Other Ownership Interests: Nektar
Wei Lin
Employment: Erasca Inc, Nektar
Leadership: Erasca Inc
Stock and Other Ownership Interests: Nektar, Erasca Inc
Travel, Accommodations, Expenses: Nektar, Erasca Inc
Mary A. Tagliaferri
Employment: Nektar
Leadership: Nektar, ENZO Biochem
Stock and Other Ownership Interests: Nektar
Patents, Royalties, Other Intellectual Property: US 10576121
Travel, Accommodations, Expenses: Nektar
Jonathan Zalevsky
Employment: Nektar
Leadership: Nektar
Stock and Other Ownership Interests: Nektar
Travel, Accommodations, Expenses: Nektar
Mario Sznol
Stock and Other Ownership Interests: Amphivena, Intensity Therapeutics, Adaptive Biotechnologies, Actym Therapeutics, Torque, Nextcure, EvolveImmune Therapeutics, Johnson & Johnson/Janssen, GlaxoSmithKline
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca/MedImmune, Nektar, Lilly, Adaptimmune, Seattle Genetics, Pierre Fabre, Molecular Partners, AbbVie, Pieris Pharmaceuticals, Innate Pharma, Immunocore, Genocea Biosciences, Anaeropharma, Zelluna, Boston Pharmaceuticals, Alligator Bioscience, Servier, Dragonfly Therapeutics, Verastem, Boehringer Ingelheim, Agenus, Numab, BioNTech AG, Genentech/Roche, Gilead Sciences, Jazz Pharmaceuticals, Targovax, Sapience Therapeutics, Pfizer, Tessa Therapeutics, OncoSec, Trillium Therapeutics, StCube, Simcha, ITeos Therapeutics
Other Relationship: Haymarket Media, Physicians' Education Resource, DAVAOncology, CEC Oncology
Michael E. Hurwitz
Employment: Pfizer, Gamida Cell, Arvinas
Consulting or Advisory Role: Nektar, Janssen, Crispr Therapeutics, Bristol Myers Squibb/Celgene, Exelixis
Research Funding: Apexigen, Astellas Pharma, AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, Corvus Pharmaceuticals, Lilly, Endocyte, Genentech, Genmab, Innocrin Pharma, Iovance Biotherapeutics, Merck, Nektar, Novartis, Pfizer, Progenics, Sanofi/Aventis, Seattle Genetics, Torque, Unum Therapeutics, Achilles Therapeutics
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at SITC, Virtual, November 11-14, 2020; Melanoma Bridge, Virtual (encore), December 3-5, 2020; Melanoma Research Alliance, Virtual (encore), February 22-24, 2021; and the World Congress of Melanoma, Virtual (encore), April 15-17, 2021.
SUPPORT
Supported by Nektar Therapeutics, San Francisco, CA.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The authors will consider requests for access to data from qualified researchers. Requests should be made directly to Dr Sue Currie (scurrie@nektar.com).
AUTHOR CONTRIBUTIONS
Conception and design: Adi Diab, Brendan D. Curti, Daniel C. Cho, Ute Hoch, Sue L. Currie, Wei Lin, Mary A. Tagliaferri, Jonathan Zalevsky, Michael E. Hurwitz
Administrative support: Wei Lin, Mary A. Tagliaferri, Jonathan Zalevsky
Provision of study materials or patients: Scott S. Tykodi, Gregory A. Daniels, Michele Maio, Brendan D. Curti, Alexander I. Spira, Erika Puente, Mary A. Tagliaferri, Jonathan Zalevsky, Mario Sznol, Michael E. Hurwitz
Collection and assembly of data: Adi Diab, Scott S. Tykodi, Gregory A. Daniels, Michele Maio, Brendan D. Curti, Karl D. Lewis, Sekwon Jang, Ewa Kalinka, Igor Puzanov, Alexander I. Spira, Daniel C. Cho, Shanhong Guan, Erika Puente, Ute Hoch, Sue L. Currie, Mary A. Tagliaferri, Mario Sznol, Michael E. Hurwitz
Data analysis and interpretation: Adi Diab, Scott S. Tykodi, Gregory A. Daniels, Michele Maio, Brendan D. Curti, Karl D. Lewis, Sekwon Jang, Ewa Kalinka, Igor Puzanov, Alexander I. Spira, Daniel C. Cho, Shanhong Guan, Erika Puente, Tuan Nguyen, Ute Hoch, Sue L. Currie, Wei Lin, Mary A. Tagliaferri, Jonathan Zalevsky, Mario Sznol, Michael E. Hurwitz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Bempegaldesleukin Plus Nivolumab in First-Line Metastatic Melanoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Adi Diab
Honoraria: Array BioPharma
Consulting or Advisory Role: Nektar, CureVac, Celgene, Idera
Research Funding: Nektar, Idera, Celgene, Pfizer, Merck, Apexigen
Travel, Accommodations, Expenses: Nektar
Scott S. Tykodi
Consulting or Advisory Role: Merck, Intellisphere LLC, Natera, Bristol Myers Squibb, Exelixis
Research Funding: Genentech, Bristol Myers Squibb, Merck Sharp & Dohme, Calithera Biosciences, Pfizer, Jounce Therapeutics, Nektar, Exelixis, Clinigen Group
Patents, Royalties, Other Intellectual Property: Patent pending
Gregory A. Daniels
Honoraria: Sanofi/Regeneron
Consulting or Advisory Role: Sanofi/Regeneron
Speakers' Bureau: Regeneron, Array BioPharma, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb, Amgen, Viralytics, Nektar, Merck
Michele Maio
Stock and Other Ownership Interests: Theravance, Epigen Therapeutics
Honoraria: Bristol Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma, Sanofi, Lilly
Consulting or Advisory Role: Bristol Myers Squibb, Roche, AstraZeneca, MSD, Merck, Pierre Fabre, Alfasigma
Patents, Royalties, Other Intellectual Property: DNA hypomethylating agents for cancer therapy
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma
Brendan D. Curti
Honoraria: Clinigen Group, Nektar
Consulting or Advisory Role: Merck
Research Funding: Bristol Myers Squibb, Galectin Therapeutics, Clinigen Group
Patents, Royalties, Other Intellectual Property: Biomarkers for OX40 response
Travel, Accommodations, Expenses: Agonox
Karl D. Lewis
Honoraria: Array BioPharma, Iovance Biotherapeutics
Consulting or Advisory Role: Array BioPharma, Merck, Roche, Regeneron, Sanofi, Iovance Biotherapeutics
Research Funding: Roche/Genentech, Merck, Array BioPharma, Incyte, Nektar, Iovance Biotherapeutics, Bristol Myers Squibb, Kartos Therapeutics, OncoSec, Regeneron, Alkermes, Neon Therapeutics, Ultimovacs, Senhwa Biosciences, Replimune, Amgen
Travel, Accommodations, Expenses: Merck, Roche/Genentech, Regeneron, Neon Therapeutics, Alkermes
Uncompensated Relationships: Roche/Genentech, Regeneron
Sekwon Jang
Consulting or Advisory Role: Bristol Myers Squibb, EMD Serono, Novartis, Sanofi, Sun Biopharma, Genentech
Ewa Kalinka
Honoraria: Bristol Myers Squibb, MSD, AstraZeneca, Regeneron, Nektar, Roche
Consulting or Advisory Role: Bristol Myers Squibb
Speakers' Bureau: Bristol Myers Squibb, Roche
Research Funding: Bristol Myers Squibb, Merck Sharp & Dohme, Nektar, AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche
Igor Puzanov
Stock and Other Ownership Interests: Celldex
Consulting or Advisory Role: Amgen, Iovance Biotherapeutics, Merck, Roche, Nouscom, Seneca Therapeutics
Alexander I. Spira
Stock and Other Ownership Interests: Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer
Consulting or Advisory Role: Array BioPharma, Incyte, Amgen, Novartis, AstraZeneca/MedImmune, Mirati Therapeutics, Gritstone Oncology, Jazz Pharmaceuticals, Merck, Bristol Myers Squibb
Research Funding: Roche, AstraZeneca, Boehringer Ingelheim, Astellas Pharma, MedImmune, Novartis, Newlink Genetics, Incyte, AbbVie, Ignyta, LAM Therapeutics, Trovagene, Takeda, Macrogenics, CytomX Therapeutics, Astex Pharmaceuticals, Bristol Myers Squibb, Loxo, Arch Therapeutics, Gritstone Oncology, Plexxikon, Amgen, Daiichi Sankyo, ADC Therapeutics, Janssen Oncology, Mirati Therapeutics, Rubius Therapeutics
Daniel C. Cho
Consulting or Advisory Role: Nektar, Pfizer, Werewolf Therapeutics
Expert Testimony: Genentech, Abbott/AbbVie
Shanhong Guan
Employment: Nektar Therapeutics
Stock and Other Ownership Interests: Nektar Therapeutics
Erika Puente
Employment: Nektar
Stock and Other Ownership Interests: Nektar
Tuan Nguyen
Employment: Nektar, Theravance Therapeutics
Stock and Other Ownership Interests: Nektar, Theravance
Ute Hoch
Other Relationship: Nektar
Sue L. Currie
Employment: Nektar
Stock and Other Ownership Interests: Nektar
Wei Lin
Employment: Erasca Inc, Nektar
Leadership: Erasca Inc
Stock and Other Ownership Interests: Nektar, Erasca Inc
Travel, Accommodations, Expenses: Nektar, Erasca Inc
Mary A. Tagliaferri
Employment: Nektar
Leadership: Nektar, ENZO Biochem
Stock and Other Ownership Interests: Nektar
Patents, Royalties, Other Intellectual Property: US 10576121
Travel, Accommodations, Expenses: Nektar
Jonathan Zalevsky
Employment: Nektar
Leadership: Nektar
Stock and Other Ownership Interests: Nektar
Travel, Accommodations, Expenses: Nektar
Mario Sznol
Stock and Other Ownership Interests: Amphivena, Intensity Therapeutics, Adaptive Biotechnologies, Actym Therapeutics, Torque, Nextcure, EvolveImmune Therapeutics, Johnson & Johnson/Janssen, GlaxoSmithKline
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca/MedImmune, Nektar, Lilly, Adaptimmune, Seattle Genetics, Pierre Fabre, Molecular Partners, AbbVie, Pieris Pharmaceuticals, Innate Pharma, Immunocore, Genocea Biosciences, Anaeropharma, Zelluna, Boston Pharmaceuticals, Alligator Bioscience, Servier, Dragonfly Therapeutics, Verastem, Boehringer Ingelheim, Agenus, Numab, BioNTech AG, Genentech/Roche, Gilead Sciences, Jazz Pharmaceuticals, Targovax, Sapience Therapeutics, Pfizer, Tessa Therapeutics, OncoSec, Trillium Therapeutics, StCube, Simcha, ITeos Therapeutics
Other Relationship: Haymarket Media, Physicians' Education Resource, DAVAOncology, CEC Oncology
Michael E. Hurwitz
Employment: Pfizer, Gamida Cell, Arvinas
Consulting or Advisory Role: Nektar, Janssen, Crispr Therapeutics, Bristol Myers Squibb/Celgene, Exelixis
Research Funding: Apexigen, Astellas Pharma, AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, Corvus Pharmaceuticals, Lilly, Endocyte, Genentech, Genmab, Innocrin Pharma, Iovance Biotherapeutics, Merck, Nektar, Novartis, Pfizer, Progenics, Sanofi/Aventis, Seattle Genetics, Torque, Unum Therapeutics, Achilles Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Robert C Long GV Brady B, et al. : Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320-330, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Larkin J Chiarion-Sileni V Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Robert C Schachter J Long GV, et al. : Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521-2532, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Ascierto PA Long GV Robert C, et al. : Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy. JAMA Oncol 5:187-194, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) : NCCN Clinical Practice Guideline: Cutaneous Melanoma (v4.2020). https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- 6.Plesca I Tunger A Müller L, et al. : Characteristics of tumor-infiltrating lymphocytes prior to and during immune checkpoint inhibitor therapy. Front Immunol 11:364, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumeh PC Harview CL Yearley JH, et al. : PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568-571, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daud AI Wolchok JD Robert C, et al. : Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 34:4102-4109, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buder-Bakhaya K, Hassel JC: Biomarkers for clinical benefit of immune checkpoint inhibitor treatment: A review from the melanoma perspective and beyond. Front Immunol 9:1474, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi M Jiao D Xu H, et al. : Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 17:129, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayers M Lunceford J Nebozhyn M, et al. : IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 127:2930-2940, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karachaliou N Gonzalez-Cao M Crespo G, et al. : Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol 10:1-23, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitra S, Leonard WJ: Biology of IL-2 and its therapeutic modulation: Mechanisms and strategies. J Leukoc Biol 103:643-655, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Klatzmann D, Abbas AK: The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 15:283-294, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Dutcher JP Schwartzentruber DJ Kaufman HL, et al. : High dose interleukin-2 (Aldesleukin)—Expert consensus on best management practices-2014. J Immunother Cancer 2:26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentebibel S-E Hurwitz ME Bernatchez C, et al. : A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov 9:711-721, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Charych DH Hoch U Langowski JL, et al. : NKTR-214: An engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res 22:680-690, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Charych D Khalili S Dixit V, et al. : Modeling the receptor pharmacology, pharmacokinetics, and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy. PLoS ONE 12:e017943S, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parisi G Saco JD Salazar FB, et al. : Persistence of adoptively transferred T cells with a kinetically engineered IL-2 receptor agonist. Nat Commun 11:660, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma M Khong H Fa’ak F, et al. : Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat Commun 11:661, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennessy M Wahba A Felix K, et al. : Bempegaldesleukin (BEMPEG; NKTR-214) efficacy as a single agent and in combination with checkpoint-inhibitor therapy in mouse models of osteosarcoma. Int J Cancer 148:1928-1937, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diab A Tannir NM Bentebibel S-E, et al. : Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: Phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov 10:1158-1173, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Grasso CS Tsoi J Onyshchenko M, et al. : Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell 38:500-515.e3, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodig SJ Gusenleitner D Jackson DG, et al. : MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med 10:eaar3342, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Van Gool F Molofsky AB Morar MM, et al. : Interleukin-5–producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood 124:3572-3576, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larkin J Chiarion-Sileni V Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osgood C Mulkey F Mishra-Kalyani PS, et al. : FDA analysis of depth of response (DpR) and survival across 10 randomized controlled trials in patients with previously untreated unresectable or metastatic melanoma (UMM) by therapy type. J Clin Oncol 37:9508, 2019 [Google Scholar]
- 28.Wolchok JD Chiarion-Sileni V Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345-1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long GV Dummer R Hamid O, et al. : Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol 20:1083-1097, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Hamid O Gajewski TF Frankel A, et al. : Epacadostat plus pembrolizumab in patients with advanced melanoma: Phase 1 and 2 efficacy and safety results from ECHO-202/KEYNOTE-037. Ann Oncol 28:428-448, 2017 [Google Scholar]
- 31.Mitchell TC Hamid O Smith DC, et al. : Epacadostat plus pembrolizumab in patients with advanced solid tumors: Phase I results from a multicenter, open-label Phase I/II trial (ECHO-202/KEYNOTE-037). J Clin Oncol 36:3223-3230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamid O Molinero L Bolen CR, et al. : Safety, clinical activity, and biological correlates of response in patients with metastatic melanoma: Results from a Phase I trial of atezolizumab. Clin Cancer Res 25:6061-6072, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Johnson DB Frampton GM Rioth MJ, et al. : Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res 4:959-967, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forschner A Battke F Hadaschik D, et al. : Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma – results of a prospective biomarker study. J Immunother Cancer 7:180, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dummer R Ascierto PA Gogas HJ, et al. : Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 19:603-615, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Long GV Stroyakovskiy D Gogas H, et al. : Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877-1888, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Lebbé C Meyer N Mortier L, et al. : Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: Results from the Phase IIIb/IV CheckMate 511 trial. J Clin Oncol 37:867-875, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutzmer R Stroyakovskiy D Gogas H, et al. : Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395:1835-1844, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Agarwala SS Glaspy J O’Day SJ, et al. : Results from a randomized phase III study comparing combined treatment with histamine dihydrochloride plus interleukin-2 versus interleukin-2 alone in patients with metastatic melanoma. J Clin Oncol 20:125-133, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Schröder B: The multifaceted roles of the invariant chain CD74--More than just a chaperone. Biochim Biophys Acta 1863:1269-1281, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Weber JS Del Vecchio M Mandala M, et al. : Adjuvant nivolumab (NIVO) versus ipilimumab (IPI) in resected stage III/IV melanoma: 3-year efficacy and biomarker results from the phase III CheckMate 238 trial. Ann Oncol 30:v533-v534, 2019 [Google Scholar]
- 42.Foley JF: Polyfunctional T cells. Sci Signal 5:ec42, 2012 [Google Scholar]
- 43.Gett AV Sallusto F Lanzavecchia A, et al. : T cell fitness determined by signal strength. Nat Immunol 4:355-360, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Rossi J Paczkowski P Shen Y-W, et al. : Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood 132:804-814, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schachter J Ribas A Long GV, et al. : Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390:1853-1862, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Robert C Grob JJ Stroyakovskiy D, et al. : Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626-636, 2019 [DOI] [PubMed] [Google Scholar]
- 47.Ascierto PA Dummer R Gogas HJ, et al. : Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600–mutant melanoma. Eur J Can 126:33-44, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors will consider requests for access to data from qualified researchers. Requests should be made directly to Dr Sue Currie (scurrie@nektar.com).