Supplemental Digital Content is available in the text.
Background.
The coronavirus 2019 (COVID-19) pandemic has disrupted health systems worldwide, including solid organ donation and transplantation programs. Guidance on how best to screen patients who are potential organ donors to minimize the risks of COVID-19 as well as how best to manage immunosuppression and reduce the risk of COVID-19 and manage infection in solid organ transplant recipients (SOTr) is needed.
Methods.
Iterative literature searches were conducted, the last being January 2021, by a team of 3 information specialists. Stakeholders representing key groups undertook the systematic reviews and generation of recommendations using a rapid response approach that respected the Appraisal of Guidelines for Research and Evaluation II and Grading of Recommendations, Assessment, Development and Evaluations frameworks.
Results.
The systematic reviews addressed multiple questions of interest. In this guidance document, we make 4 strong recommendations, 7 weak recommendations, 3 good practice statements, and 3 statements of “no recommendation.”
Conclusions.
SOTr and patients on the waitlist are populations of interest in the COVID-19 pandemic. Currently, there is a paucity of high-quality evidence to guide decisions around deceased donation assessments and the management of SOTr and waitlist patients. Inclusion of these populations in clinical trials of therapeutic interventions, including vaccine candidates, is essential to guide best practices.
INTRODUCTION
All aspects of global healthcare systems have been strained in response to the coronavirus 2019 (COVID-19) pandemic. In addition to a general lack of capacity, organ donation and transplantation (ODT) systems have been forced to contend with specific issues such as possible donor-to-recipient transmission and immunosuppression in transplant recipients. In the early stages of the pandemic, many centers closed their living donor programs, and deceased donor referrals dropped significantly.1 Questions were raised as to how best to proceed with lifesaving transplants while balancing the risks posed by COVID-19.
In response, Canadian Blood Services, the Canadian Donation and Transplantation Research Program, the Canadian Society of Transplantation, and the Peter Morris Centre for Evidence in Transplantation came together to undertake a literature review and recommendation generation process to offer guidance to donation and transplant programs and clinicians. The first step of the process was a summary of international recommendations that created an overview of the breadth of topics addressed by other organizations.2
The goal of this collaboration is to create rigorously developed clinical practice recommendations that address the priorities of ODT activity during the pandemic. The planning and scientific committees agreed that the most urgent need was to create recommendations regarding (1) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) screening methods of patients who are potential deceased donors and (2) treatment and protection of transplant recipients and patients awaiting transplantation. This article describes the methods used and provides a summary of recommendations.
MATERIALS AND METHODS
Development of Recommendations
Recommendations were developed using a rapid response approach.3-5 Our process also emphasized involvement of patient partners and we informed all recommendations with transparent and systematic literature reviews according to the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) process6 while endeavoring to incorporate and address all domains of the Appraisal of Guidelines for Research and Evaluation II framework.7
Guideline panel members were selected through an informal process of purposeful sampling. We emphasized the inclusion of (1) authors of the systematic reviews informing our recommendations, (2) patient partners who have lived experience with solid organ transplantation (SOT), (3) a full spectrum of transplant clinicians who are involved in the management of solid organ transplant recipients (SOTr) at risk for or infected with COVID-19, and (4) methodologists with expertise in health research methodology and guideline development. During panel member selection, we managed financial and intellectual conflicts of interest by requiring disclosure statements from all participants. Research ethics approval was not requested for the systematic reviews of the literature or guideline development.
Clinical questions thought to merit recommendations were identified by working group members, and with the help of an information specialist, we conducted a systematic literature search. A single search strategy was used for both the deceased donation and recipient treatment and protection clinical questions (see Supplemental Material S1, SDC, http://links.lww.com/TXD/A351 for full details). Studies were included that provided direct evidence for the donation and transplantation population as well as indirect evidence from several systematic reviews of the general population. The first search strategy was designed and executed June 18–21, 2020, and updated once August 22–28, 2020, and again January 9–10, 2021. Multiple electronic databases were searched for references published since 2019 without language or publication type limits. Due to the sparsity of evidence on management of COVID-19 in SOT, we retained peer-reviewed reports (excluding preprints) using most study designs, including case series. Two case reports of donor to recipient transmission following lung transplantation8,9 were added after the formal search based on continued informal literature surveillance.
For each search, teams of reviewers screened the titles and abstracts of the identified citations independently and in duplicate using prespecified eligibility criteria. Criteria for eligibility were adapted for specific questions where updated searches retrieved studies of higher quality (see Supplemental Material S1, SDC, http://links.lww.com/TXD/A351 for full details of included studies). After the title and abstract screening, the same process was repeated for the eligible full texts. From the final set of eligible articles, reviewers extracted the data relevant to the characteristics of the cohorts, interventions, comparative groups, and outcomes. A total of 1930 unique references were screened from 9 distinct databases, and evidence profiles were created to support the generation of each recommendation (see Supplemental Material S1, SDC, http://links.lww.com/TXD/A351 for full details of this process).
We assessed the risk of bias of each eligible study guided by the Risk of Bias in Non-Randomized Studies – Interventional (ROBINS-I) tool.10 In assessing our overall certainty in the body of evidence, we used the GRADE process.6 Table 1 provides a guide to the difference in interpretation between strong (we recommend) and weak (we suggest) recommendations in the GRADE framework for different intended stakeholders.11 Evidence profiles and detailed evidence to decision tables generated in the web-based application MAGICapp are available at https://app.magicapp.org/#/guideline/ERWQ1j
TABLE 1.
Interpretation of strong and weak recommendations for different stakeholders
| Implications | Strong recommendationa | Conditional (weak) recommendation |
|---|---|---|
| For patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | The majority of individuals in this situation, if fully informed, would choose the suggested course of action, but some would not. |
| For clinicians | Most individuals should receive the intervention. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. | The care needs of individual donors may vary as a result of comorbidities, and the practice of individual clinicians may vary in these circumstances, largely due to the lack of evidence to address these situations. |
| For policymakers | The recommendation can be adopted as policy in most situations. | Policymaking will require substantial debate and involvement of various stakeholders. |
Modified from Guyatt GH, Oxman AD, Kunz R, et al.11
aNote: Good Practice Statements should be interpreted similarly to strong recommendations.
In the assessment of the clinical question of proceeding to transplantation versus remaining on organ replacement therapy, we conducted a meta-analysis of proportions for each organ group and outcome separately. Due to the paucity of studies conducting a direct comparison, we conducted a random effect meta-analysis of proportions and subclassified the studies based on the patient group evaluated (studies evaluating the risk of COVID-19 in the kidney transplantation as compared to studies evaluating the risk of COVID-19 in the renal replacement therapy [RRT] population). In doing so, we conducted an indirect comparison of 2 groups of interest, in the form of between study subgroup analyses. To avoid minimizing the weight attributed to studies with few events (few events are informative as they may represent a true low-risk population), we used the Freeman–Tukey transformation12 to equalize the weights across studies. Metaprop13 package of STATA provided the platform for the conduct of our meta-analyses.
Recommendations
This guidance is intended to inform clinical and administrative ODT stakeholders operating during the COVID-19 pandemic (Table 1). The included evidence pertains to both adult and pediatric populations, although very few reports including pediatric patients were identified in the search. A total of 11 recommendations (4 strong, 7 weak) and 3 Good Practice Statements were generated (Table 2). Three other questions were considered but resulted in statements of no recommendation. We acknowledge that some evidence has been published since the execution of our searches, but based on informal scans, no study would have changed the direction of our recommendations.
TABLE 2.
Clinical practice guideline recommendationsa
| Screening of Patients Who are Potential Deceased Organ Donors |
|---|
| Transplantation from potential organ donors positive for COVID-19 |
| • We recommend against transplantation of organs retrieved from deceased donors with active COVID-19 infection, particularly in the case of lung transplantation (strong recommendation, very low certainty of evidence). |
| • We suggest proceeding with solid organ transplantation from living and deceased donors with a resolved COVID-19 infection (weak recommendation, low certainty of evidence). |
| PCR methods and repeat testing for diagnosis of COVID-19 in potential deceased organ donors |
| • We recommend PCR testing of all patients who are potential deceased organ donors (strong recommendation, low certainty of evidence). |
| • We recommend PCR testing of both upper and lower respiratory tract samples of all patients who are potential deceased organ donors within 24 h before organ recovery (strong recommendation, low certainty of evidence). |
| • Lower respiratory samples should be collected by methods that produce the least risk of aerosol generation (Good Practice Statement). |
| • We suggest against repeat PCR testing from the same collection site of patients who are potential donors (weak recommendation, low certainty of evidence). |
| • Screening of patients who are potential donors and recipients should include pre-recovery or pre-transplant evaluation for COVID-19 risk factors such as absence of symptoms, risk of potential exposure, and travel history (Good Practice Statement). |
| CT scan accuracy for diagnosis of COVID-19 in potential deceased organ donors |
| • We recommend against routine thoracic CT scans for COVID-19 screening for potential deceased organ donors (strong recommendation, low certainty of evidence). |
| • We suggest that the results of PCR testing supersede any contradictory information from available thoracic CT scan results (weak recommendation, moderate certainty of evidence) |
| SARS-CoV-2 antibodies post-infection with COVID-19 in potential deceased organ donors |
| • We make no recommendation regarding the use of antibody screening to evaluate the risk of COVID-19 transmission from potential deceased organ donors to organ recipients. |
| Recipient Treatment and Protection |
| Modifications to induction immunosuppression and rejection treatment in solid organ transplant recipients |
| • We suggest no modification to induction immunosuppression to prevent COVID-19 acquisition or severity (weak recommendation, very low certainty of evidence). |
| Immunosuppression therapy in the setting of COVID-19 |
| • We suggest temporary adjustment of maintenance immunosuppression may be considered for patients with COVID-19 (weak recommendation, very low certainty of evidence). |
| • We suggest against preemptive adjustment of maintenance immunosuppression to prevent acquisition of COVID-19 (weak recommendation, very low certainty of evidence). |
| Decision to proceed with organ transplant or organ replacement therapy in the setting of COVID-19 |
| • We suggest proceeding with transplantation over remaining on organ replacement therapies in the setting of COVID-19 activity in the community (weak recommendation, very low certainty of evidence). |
| Prophylaxis against COVID-19 in solid organ transplant recipients |
| • We make no recommendation for or against prophylactic treatment for SARS-CoV-2. |
| • Transplant recipients and those waiting for transplant should follow public health guidance, including but not limited to, physical distancing, hand hygiene, and wearing a mask (Good Practice Statement). |
| Anti-COVID-19 therapy in solid organ transplant recipients |
| • We make no recommendation for specific therapy for COVID-19. We suggest following national guidance pertaining to treatments in the general population. |
aRationales for bolded recommendations are included in this article. To access the rationales of the remaining recommendations, please consult the Supplemental Material S1 (SDC, http://links.lww.com/TXD/A351).
COVID-19, coronavirus disease 2019; CT, computed tomography; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Due to article constraints, we have only included summary rationales for the recommendations determined to be of greatest clinical relevance. Rationales for all recommendations, for example, the rationales against making a recommendation regarding the use of antibody screening in patients who are potential donors or the prophylactic treatment for SARS-CoV-2 in recipients, are detailed in the Supplemental Material S1 (SDC, http://links.lww.com/TXD/A351). A summary version of the recommendations was published as part of broader COVID recommendations on the Canadian Blood Services website (https://professionaleducation.blood.ca/en/organs-and-tissues/covid-19-update-organ-donation-and-transplantation-services).
Screening of Patients Who Are Potential Deceased Organ Donors
Transplantation From Potential Organ Donors Positive for COVID-19
We suggest proceeding with transplantation of solid organs retrieved from living and deceased donors after confirmation of resolution of COVID-19 infection (weak recommendation, low certainty of evidence).
Key Literature
While the recommendation to avoid transplantation from donors with active COVID-19 (see Table 2) is based on direct evidence of transmission in the case of lung transplantation8,9 and laboratory evidence of plausible transmission from other organs,14 the panel felt it was important to address the situation of a previously positive and currently asymptomatic patient who is a potential donor. We included 4 published reports15-18 of previously positive COVID-19 donors (n = 7 deceased donors; 32 living donors) who proceeded to donation following resolution of their infection and testing COVID-19 negative. Successful recovery and transplantation were reported to be between 4 wk after symptom resolution to 14 wk following the initial infection. Donors were all COVID-19 negative at time of organ donation. There were no reports of transmission to healthcare workers, and none of the recipients developed active COVID-19 infection at the last follow-up. At the last follow-up, both graft and patient survival was 99%, with no deaths attributed to COVID-19 in the recipients.
Rationale
The aforementioned evidence supports our weak recommendation that patients with resolved COVID-19 can safely be considered as organ donors. However, it is important to note that our process did not include a search regarding the optimal laboratory methods to determine when a previously infected patient has recovered adequately to be considered eligible and safe for organ donation. Furthermore, although the evidence demonstrates safe transplantation after waiting at least 28 d after symptom resolution, the minimum amount of time to wait between symptom or laboratory-confirmed resolution before organ recovery is currently unknown. Each case should be carefully evaluated, and considering the complexity of these decisions, it would be preferable to consult transplant-focused infectious disease specialists before organ recovery from previously COVID-19–positive patients.
PCR Methods and Repeat Testing for Diagnosis of COVID-19 in Potential Deceased Organ Donors
We recommend polymerase chain reaction (PCR) testing of both upper and lower respiratory tract samples of all patients who are potential deceased organ donors in 24 h before organ recovery (strong recommendation, low certainty of evidence).
We recommend that lower respiratory samples be collected by methods that produce the least risk of aerosol generation (Good Practice Statement).
Key Literature
Although PCR-based testing is recommended for all donors based on substantial indirect19-22 and direct evidence,23-37 recent data suggest that anatomic collection site is of critical importance.8,9,22 Two recent reports describe cases of donor-derived SARS-CoV-2 infection in lung transplant recipients.8,9 In both cases, although the donors had been screened by nasopharyngeal PCR samples, no lower respiratory sample was collected before lung recovery. In both cases, later analyses from bronchioalveolar lavage (BAL) fluid9 or in the recipient8 were highly suggestive of donor-derived transmission. Indirect evidence from 1 systematic review analyzing different donor screening sites also suggests that lower respiratory tract samples had improved diagnostic accuracy compared with nasal or oropharyngeal samples.22 No reports compared the sensitivity or specificity of different lower respiratory secretion collection techniques (BAL versus closed-circuit endotracheal aspiration).
Rationale
The aforementioned evidence supports our recommendation to collect lower respiratory samples before organ recovery. Though not directly addressed by the evidence, we also recommend collection of upper respiratory samples in the form of nasopharyngeal swabs to exclude the possibility of a recent infection not yet detectable in lower respiratory secretions. This is consistent with the strong preference to avoid possible transmission to recipients and the assumption that rapid PCR testing is now readily available in most intensive care unit (ICU) settings.
Although we recommend lower respiratory samples from all patients who are potential donors, the evidence does not inform optimal sampling technique. We recommend that samples be collected by methods that produce the least risk of aerosol generation (eg, closed-circuit endotracheal aspirate as opposed to BAL), consistent with the strong value of protecting healthcare workers from potential harm. Finally, the collection of upper and lower samples in 24 h before organ recovery should be done in addition to any other routine screening that was done for infection surveillance during the patient’s ICU admission. When the capacity to perform PCR testing within 24 h before recovery is limited, we encourage collection of PCR samples as closely as possible to the scheduled recovery to limit the potential of interim acquisition of SARS-CoV-2.
Computed Tomography Accuracy for Diagnosis of COVID-19 in Potential Deceased Organ Donors
We recommend against routine thoracic computed tomography (CT) scans for COVID-19 screening for potential deceased organ donors (strong recommendation, low certainty of evidence).
We suggest that the results of PCR testing supersede any contradictory information from available thoracic CT scan results (weak recommendation, moderate certainty of evidence).
Key Literature
Indirect evidence, in the form of a large systematic review, suggests that the addition of thoracic CT scan to screen for COVID-19 in any patient population may increase sensitivity but decrease the specificity of a COVID-19 diagnosis.38 This suggests the possibility of elevated rates of false-positive CT scans. Six studies (case series or cohort studies) from the donation and transplantation population included direct evidence, but no study directly compared protocols with or without routine CT scans.26-29,33,34 All patients who were potential donors (n = 4) found to be positive for COVID-19 tested positive by PCR testing; none were excluded solely based on CT scan results. The impact of routine thoracic CT donor screening results on decision making was not explicitly described. In both cases of donor-derived COVID-19 following lung transplantation, prerecovery CT scans were performed and did not prevent transmission.8,9
Rationale
We determined that there is no compelling benefit for the use of routine thoracic CT imaging for diagnosis of COVID-19 in potential deceased donors. We also valued cost and harm avoidance (eg, cost of imaging, transport of an unstable patient to CT, infection control considerations for diagnostic imaging personnel, and potential harm from contrast materials). Thus, a strong recommendation was made against the routine use of routine thoracic CT scans for COVID-19 screening among potential deceased organ donors.
Furthermore, indirect evidence indicates that thoracic CT scans are sensitive but only moderately specific in the diagnosis of COVID-19 in suspected patients, meaning thoracic CT findings have limited capability in differentiating between SARS-CoV-2 infection and other causes of respiratory illness. False-positive COVID-19 diagnoses related to equivocal CT findings could lead to missed donation opportunities by excluding donors without COVID-19. This is the basis for our recommendation that regardless of CT evidence, PCR status should be the primary paraclinical data used to evaluate risk of SARS-CoV-2 infection in a patient who is a potential donor.
Recipient Treatment and Protection
Modifications to Induction Immunosuppression and Rejection Treatment in Solid Organ Transplant Recipients
We suggest no modification to induction immunosuppression to prevent COVID-19 acquisition or severity (weak recommendation, very low certainty of evidence).
Key Literature
This recommendation is based on 9 publications of individual case reports and small case series.39-47 None of the reviewed publications reported on the risk of acquisition of COVID-19 as an outcome. All reports were of SOTr who had developed COVID-19 within the first 6 mo posttransplant. Additional data on patient-level outcomes were requested and obtained from authors of 2 of the included articles.39,44 The most commonly reported and most pertinent outcomes for this intervention were patient survival and development of acute rejection. Although numbers were small, there was no appreciable trend in mortality based on type of induction therapy for transplant recipients who developed COVID-19. There are no available data on the risk of developing COVID-19 stratified by induction immunosuppression.
Rationale
Given the potential harms of acute and chronic allograft rejection that may occur with reduction in standard induction immunosuppression, this risk is felt to outweigh any theoretical benefit that this strategy may have on reduction of COVID-19 disease and severity for recipients. This position is supported by findings from 1 large US center, which showed that during the early phases of the pandemic, use of lymphocyte depleting induction therapy was not associated with an increase in mortality; however, withholding of lymphocyte depleting induction therapy was associated with an increased risk of rejection.48
Many factors are considered in the selection of an induction immunosuppression strategy, and clinicians should choose a regimen that they believe offers the greatest chance of recipient and graft survival while minimizing risks of over immunosuppression. These decisions take into consideration the best available evidence as well as the individual patient circumstances and values and preferences. For this reason, clinicians may choose, in certain candidates, for example, to reduce induction immunosuppression. However, at a programmatic level, we suggest against broad reduction in induction immunosuppression purely to mitigate against COVID-19.
Immunosuppression Therapy in Solid Organ Transplant Recipients
We suggest that temporary adjustment of maintenance immunosuppression may be considered for patients with COVID-19 (weak recommendation, very low certainty of evidence).
We suggest against preemptive adjustment of maintenance immunosuppression to prevent acquisition of COVID-19 (weak recommendation, very low certainty of evidence).
Key Literature
The recommendation for temporary adjustment of maintenance immunosuppression is based on 31 publications (case reports and case series); 18 included kidney transplant recipients,39,49-65 5 included liver transplant recipients,44,57,66-68 6 included heart transplant recipients,23,47,57,69-71 and 2 included lung transplant recipients.43,72 All studies reported some form of modification to the patients’ immunosuppressive regimen. None of the studies relied on an experimental design, and there was no control group of patients without changes to their immunosuppressive agents. The reports considered SOTr who developed COVID-19 at various intervals posttransplant and follow-up was relatively short. Modifications to immunosuppression regimens were temporary. In addition to reducing or holding antimetabolites, several studies reported on simultaneous reduction in doses of calcineurin inhibitors and mammalian target of rapamycin inhibitors, and administration of steroids as well as other supplementary immune modulating therapies. Thus, the observed outcomes may not be solely attributable from the temporary reduction in maintenance immunosuppression.
The weak recommendation against preemptive adjustment of maintenance immunosuppression is based on indirect evidence from 8 publications, limited to cohort studies.52,53,64,65,68,70,73,74 Four studies reported on incidence of COVID-19 in kidney transplant recipients.52,53,64,65 Immunosuppression therapy was not modified preemptively and the incidence of COVID-19 ranged from 0% to 0.67%. One study reported on a cohort of liver transplant recipients.68 In the absence of preemptive modification of immunosuppression in this cohort, the incidence of COVID-19 in this population was 0.11%. Two studies reported on incidence of COVID-19 in heart transplant recipients.53,70 The incidence of COVID-19 ranged between 3.5% and 5% while on standard immunosuppression therapy. One study reported on the incidence of COVID-19 in a cohort of lung transplant recipients.53 Maintenance of standard immunosuppression regimen was associated with COVID-19 incidence of 3.3% over a follow-up period of 116 d (46–187). Finally, 2 studies reported on cohorts of all SOTr on standard immunosuppression regimens and estimated the incidence of COVID-19 to be <1%.73,74
Rationale
Many factors are considered in the modification of maintenance immunosuppression strategy. These decisions should take into consideration the best available evidence as well as the individual patient circumstances alongside their values and preferences. Given the potential harms of acute and chronic allograft rejection, which may occur with reduction in maintenance immunosuppression, adjustment of maintenance immunosuppression in patients infected with COVID-19 is suggested to be implemented as a temporary measure. Although we suggest that this may be done, and, as demonstrated by the referenced reports, it is common practice, it is unknown if reduction of maintenance immunosuppression in transplant recipients with COVID-19 results in improved outcomes from the infection. The severe manifestations of COVID-19 are believed to be due to an amplified and aberrant immune response.75 As such, it is unknown if reduction of immunosuppression will improve response to infection or conversely, if this will worsen the immune response to the infection and lead to worse outcomes. In the absence of evidence, we are prioritizing a preference for potential decreased COVID-19-related morbidity and mortality, accepting the potential increased short-term risk of rejection. Further data on the efficacy of temporary reductions to maintenance immunosuppression and the attributable impact of immunosuppression on COVID-19 morbidity and mortality may change this recommendation significantly.
We suggest against preemptive reduction in maintenance immunosuppression therapy in an effort to prevent COVID-19 because we weighed the increased risk of rejection to be higher than what is believed to be a small potential benefit of a reduction of immunosuppression on the risk of acquiring COVID-19, especially in light of the evidence demonstrating a low incidence of COVID-19 in transplant patients on standard immunosuppression regimens.
Decision to Proceed With Organ Transplant or Organ Replacement Therapy in the Setting of COVID-19
We suggest proceeding with transplantation over remaining on organ replacement therapies in the setting of COVID-19 activity in the community (weak recommendation, very low certainty of evidence).
Key Literature
This recommendation is based on several studies encompassing both kidney- and liver-specific groups as well as several large studies of all SOTr.23,40,50,63,66,71,76-84 These studies were primarily retrospective cohorts from single centers. However, more recent reports included in the last iteration of the literature search included higher quality prospective cohorts.85-87 There is currently more literature for the renal group than other SOT groups.
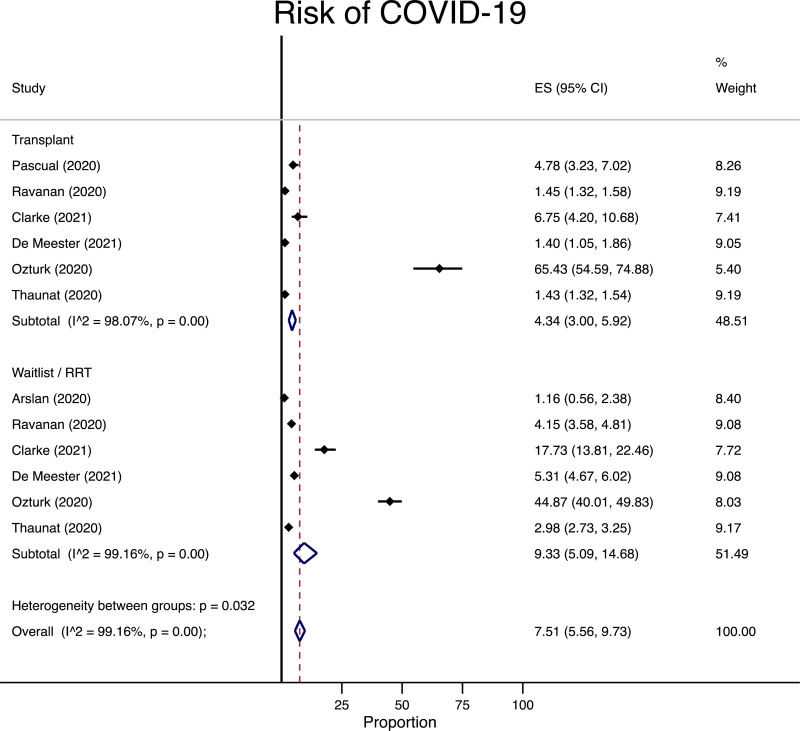
In the renal group, we identified 7 studies following 104 811 patients who were either on the waitlist/RRT or underwent transplantation.76-78,85,86,88,89 Some of the studies reported the incidence of COVID-19 in each group separately, whereas others conducted a direct comparison between the 2 groups. We combined all of the studies in a meta-analysis of proportions and subgrouped the studies/cohorts based on the patient group (transplant versus waitlist/RRT). Among the patients on waitlist/RRT, the risk of COVID-19 was 93 per 1000 persons followed compared with 43 per 1000 in the transplant group (Figure 1). The absolute risk difference between the 2 groups was 50 fewer cases of COVID-19, with a 95% confidence interval (CI) of 117 fewer cases to 9 more cases per 1000 persons followed.
FIGURE 1.
Meta-analysis for risk of infection with COVID-19 among transplant, waitlist, or renal replacement therapy patients at risk. CI, confidence interval; COVID-19, coronavirus disease-2019; ES, effect size, representing the risk of COVID-19 infection as a percentage; RRT, renal replacement therapy.
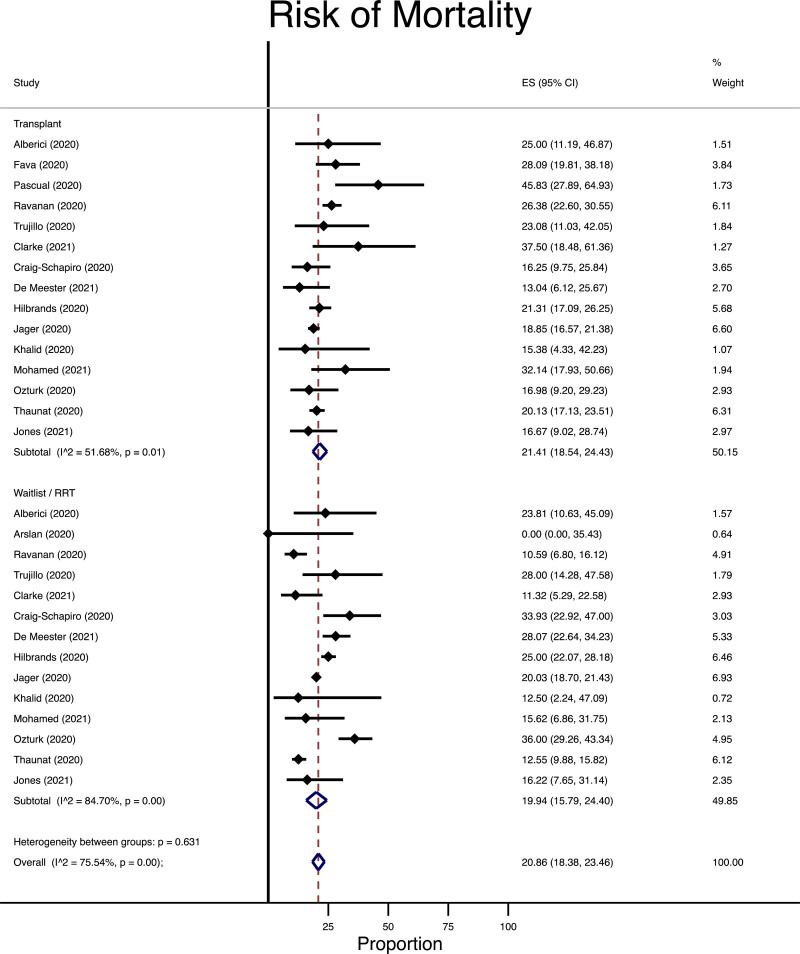
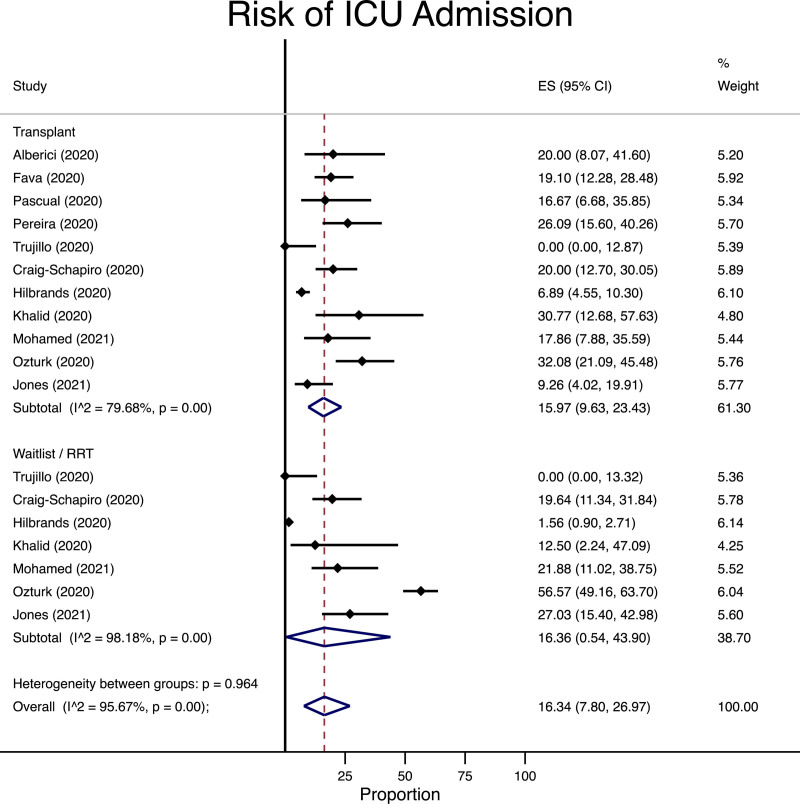
Among the patients who were diagnosed with COVID-19, the risk of mortality (16 studies following 8186 patients63,76-79,85-95) and admission to the ICU (11 studies following 1839 patients63,77,79,80,87,88,90,91,93-95) was similar between patients undergoing transplantation as compared with those remaining on the waitlist/RRT. In the waitlist/RRT group, the risk of mortality was 199 per 1000 persons followed compared with 214 per 1000 in the transplant recipient group (Figure 2) with an absolute risk difference of 15 more cases in the transplant groups (95% CI of 59 fewer to 86 more cases per 1000 persons). Similarly, the incidence of ICU admission was 163 per 1000 in the waitlist/RRT group (Figure 3) but 160 per 1000 in the transplant group (absolute risk difference of 3 fewer per 1000 in the transplant group, 95% CI 343 fewer to 229 more per 1000 persons followed).
FIGURE 2.
Meta-analysis for risk of death among transplant, waitlist, or renal replacement therapy patients diagnosed with COVID-19. CI, confidence interval; COVID-19, coronavirus disease-2019; ES, effect size, representing the risk of COVID-19 infection as a percentage; RRT, renal replacement therapy.
FIGURE 3.
Meta-analysis for risk of ICU admission among transplant, waitlist, or renal replacement therapy patients diagnosed with COVID-19. CI, confidence interval; COVID-19, coronavirus disease-2019; ES, effect size, representing the risk of COVID-19 infection as a percentage; ICU, intensive care unit; RRT, renal replacement therapy.
Meta-analysis was not possible for data from other organ groups. Based on a small number of studies,44,66,78,80-83 liver recipients affected with COVID-19 appear to have low mortality compared with other organ recipient groups. Mortality was higher in those who were longer posttransplantation, which may be confounded by the fact that COVID-19 mortality risk is higher in older individuals.81 Most of the early posttransplantation infections were mild, and all patients survived. This was in stark contrast to the cohorts of patients with end-stage liver disease based on 1 study.82
Limited data from pediatric heart and kidney recipients indicated that survival was 100% for children affected with COVID-19 even in the setting of transplantation.71,84,96,97
Rationale
For the kidney group specifically, based on the meta-analysis performed, there does not appear to be a trend toward transplant improving or worsening the risk of COVID-19. Among those with COVID-19, the meta-analysis did not show a difference in mortality or admission to the ICU between individuals undergoing transplantation compared with those on the waitlist/RRT. We acknowledge that there is imprecision around the point estimates and the indirect nature of our comparisons. However, despite the low certainty in the evidence, the panel felt that the overall balance of benefits, however, favored continuing with transplantation, particularly if it could reduce the patient’s overall need to access healthcare. For the other organ groups, the panel reached the same conclusions and rationales and favored proceeding with transplantation. This includes pediatric patients, though data were even more limited for this group.
The panel, however, felt that the decision to proceed with transplantation may vary across different transplant programs and across different candidates in need of transplantation. The decision to proceed with transplantation will also be dependent on the local status of the pandemic. Although at the individual patient level we favor proceeding with transplant, this may not be feasible if healthcare resources are overwhelmed by the pandemic response. Local hospital administration will need to be involved in the allocation of surgical and medical resources and ultimately in the decision as to whether proceeding with transplantation is feasible for the system. To reflect this variability in practice and values and preferences, the strength of the recommendation remains weak.
In an effort to optimize transplant outcomes and maintain transplant activity, centers should have a planned COVID-19-free pathway, which minimizes the risk of nosocomial COVID-19 infection. This should include pretransplant testing of the recipient, isolation precautions for staff and recipient while in hospital, minimization of laboratory testing postdischarge, and postdischarge virtual care when feasible.
DISCUSSION
The aforementioned recommendations represent rigorously developed guidance on how to manage key aspects of ODT systems during the COVID-19 pandemic. Although the content of these recommendations is similar to many of the existing national and international ODT organizations, we believe them to be the first created using accepted, transparent methods to link the quality of the available evidence to the strength of the recommendations.2
The early stages of the pandemic were characterized by steep increases in cases and an urgent need to focus the vast majority of resources and attention on clinical care. As such, the overall quality of the existing evidence was both very low and largely indirect. For this reason, the major limitation of these recommendations is the low certainty in evidence and the inability to provide a recommendation for some questions. As studies of the impact of COVID-19 on the ODT system continue to be published, the GRADE framework will allow for updating of these recommendations, as appropriate.
This review identified considerable knowledge gaps as highlighted in Table 3. One area in which literature is particularly limited is pediatric transplant populations. In a report of registry data from the United States, kidney transplant centers reported an incidence rate of COVID-19 of 0.6% among pediatric kidney transplant recipients with no cases of respiratory failure or death.96 Similar favorable outcomes for pediatric recipients were reported by others.97 When considering the recommendations we put forth, the risks and benefits of various interventions, particularly modulation of immunosuppression, will need to take into account the generally more benign clinical manifestations of COVID-19 in the pediatric population. To this end, the Canadian Society of Transplantation Pediatric Group has developed targeted guidance related to COVID-19 in pediatric kidney transplant recipients.98
TABLE 3.
Knowledge gaps and areas of future research in deceased donor screening and recipient treatment and protection
| Screening of Patients Who are Potential Deceased Organ Donors |
| Laboratory tests and time from initial infection required to confirm resolution of COVID-19 to safely consider organ recovery from patient was previously infected with COVID-19. |
| The impact of vaccination or previous COVID-19 infection in potential recipients on accepting organs from previously COVID-19–infected donors. |
| Further research into specific radiologic findings either from CT scans or other modalities that may increase both the sensitivity and specificity of COVID-19 diagnosis in ways that are additive to PCR screening. |
| Further understanding of biologic mechanisms that would either support or refute the possibility of transmission of SARS-CoV-2 from nonpulmonary transplanted organs. |
| Quantifying the variable risk of COVID-19 transmission from different organ transplantation (eg, lungs vs abdominal organs). |
| Short- and long-term outcomes of recipients who either accidentally or deliberately receive organs from patients with active COVID-19 infections. |
| Recipient Treatment and Protection |
| Assessment of reduction vs no reduction to induction and maintenance immunosuppression on COVID-19 and graft-related outcomes. |
| Data on COVID-19 risk and outcomes in transplant candidates from non-liver, non-kidney organ groups, specifically candidates for lung, heart, and pancreas transplant. |
| In solid organ transplant recipients and organ transplant candidates, knowledge of efficacy and safety of both preexposure prophylaxis in areas of high prevalence or postexposure prophylaxis after a confirmed exposure. |
| Efficacy and safety of antiviral and immunomodulatory COVID-19 therapies in SOT recipients. |
| Data on COVID-19 risk and outcomes in both pediatric organ transplant candidates and pediatric organ transplant recipients. |
| Efficacy of vaccinations against SARS-CoV-2 in solid organ transplant recipients. |
| Assessment of vaccine complications such as VITT on quality of organs and appropriateness for donation. |
COVID-19, coronavirus disease 2019; CT, computed tomography; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SOT, solid organ transplant; VITT, vaccine-induced immune thrombotic thrombocytopenia.
Although the strength of this work is the rigor of the applied methods, its principal weakness is the delay in being able to respond to a rapidly evolving situation. Currently, no international governing body or professional society is recognized as responsible for the development of ODT guidelines. Each national or regional organization is left with the responsibility of defining questions, reviewing the literature, and creating recommendations, often at significant cost in terms of person hours or consultant fees. The result is a substantial duplication of effort with dozens of entities reviewing and summarizing the same collection of references. We partially overcame this challenge by combining both Canadian and British team members. While increasing our efficiency and expertise, this represents a tiny fraction of the global potential to create international guidelines that would respond to aspects of the ODT system. As illustrated in systems such as the International Liaison Committee on Resuscitation, the early stage work that is focused on systematic literature reviews and evidence profile generation can be distributed across partners, followed by adaptation of initial recommendations to local specific contexts.99 International ODT organizations could consider the creation of a similar structure in the future to create timely, trustworthy guidelines that would require fewer resources from individual partners.
CONCLUSION
These rigorously developed recommendations were created to address clinical practice questions facing ODT programs. The challenge of dealing with the COVID-19 pandemic remains. Despite efforts to vaccinate populations as rapidly as possible, as the virus moves from a pandemic to an endemic phase, the clinical scenarios addressed here will be combined with new challenges. All ODT stakeholders must continue their efforts to confront these challenges using the best available evidence, evaluated in a comprehensive manner. Doing so will increase our capacity to protect the extremely vulnerable populations awaiting or having received a transplant from further harm from this unprecedented virus.
ACKNOWLEDGMENTS
The authors would like to thank Abdullah Malik, who assisted with screening citations and the included meta-analyses, and information specialists Robin Featherstone, Marc-Andre Simard, and Liset Pengel for designing, executing, and updating the search strategy. Project oversight and management was provided by Leanne Stalker, Chelsea Patriquin-Stoner, Melanie Dieude and David Hartell. Project steering committee members included Lori West, Sam Shemie, Peter Nickerson, Marie-Chantal Fortin, Michel Paquet, and Rosanne Dawson. External review was provided by Simon Knight and Michael Ison.
Supplementary Material
Footnotes
Published online 7 September, 2021.
The authors have no relevant conflicts of interest to disclose.
The work was supported financially by Canadian Blood Services through a contribution from Health Canada, and in kind by the Canadian Donation and Transplantation Research Program and the Peter Morris Centre for Evidence in Transplantation.
Canadian Blood Services is a national, not-for-profit charitable organization. In the domain of organ and tissue donation and transplantation, it provides national services in the development of leading practices, system performance measurement, interprovincial organ sharing registries, and public awareness and education. Canadian Blood Services is not responsible for the management or funding of any Canadian organ donation organizations or transplant programs. Canadian Blood Services receives its funding from the provincial and territorial Ministries of Health and from the federal government (through Health Canada).
M.J.W., L.H., F.F. L.C.W., and A.M. participated in project design, systematic review, and article preparation. S.B., M.B., C.A.B., M.G., G.H., M.I., C.L., M.L.L., R.M., A.R.M. R.SP. S.S., T.S., J.M.S., S.S., I.T., M.W., A.W., and S.B. participated in systematic review and article preparation.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Kumar D. C4 article: implications of COVID-19 in transplantation. Am J Transplant. 2021;21:1801–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss MJ, Lalani J, Patriquin-Stoner C, et al. Summary of international recommendations for donation and transplantation programs during the coronavirus disease pandemic. Transplantation. 2021;105:14–17. [DOI] [PubMed] [Google Scholar]
- 3.Garritty CM, Norris SL, Moher D. Developing WHO rapid advice guidelines in the setting of a public health emergency. J Clin Epidemiol. 2017;82:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RL, Florez I, Falavigna M, et al. Development of rapid guidelines: 3. GIN-McMaster guideline development checklist extension for rapid recommendations. Health Res Policy Syst. 2018;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haut Autorité de Santé. Rapid responses in the context of COVID-19: accelerated guidelines method. 2020. Available at https://www.has-sante.fr/jcms/p_3168771/en/methode-d-elaboration-des-reponses-rapides-dans-le-cadre-du-covid-19. Accessed April 15, 2020.
- 6.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 7.Brouwers MC, Kho ME, Browman GP, et al. ; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar D, Humar A, Keshavjee S, et al. A call to routinely test lower respiratory tract samples for SARS-CoV-2 in lung donors. Am J Transplant. 2021;21:2623–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. [Epub ahead of print. February 10, 2021]. doi: 10.1111/ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group. Going from evidence to recommendations. BMJ. 2008;336:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat. 1978;32:138. [Google Scholar]
- 13.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaussen A, Hornby L, Rockl G, et al. Evidence of SARS-CoV-2 infection in cells, tissues, and organs and the risk of transmission through transplantation. Transplantation. 2021;105:1405–1422. [DOI] [PubMed] [Google Scholar]
- 15.Ceulemans LJ, Van Slambrouck J, De Leyn P, et al. Successful double-lung transplantation from a donor previously infected with SARS-CoV-2. Lancet Respir Med. 2021;9:315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neidlinger NA, Smith JA, D’Alessandro AM, et al. Organ recovery from deceased donors with prior COVID-19: a case series. Transpl Infect Dis. 2021;23:e13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong HL, Kim SH, Choi DL, et al. A case of coronavirus disease 2019-infected liver transplant donor. Am J Transplant. 2020;20:2938–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kute VB, Godara S, Guleria S, et al. Is it safe to be transplanted from living donors who recovered from COVID-19? Experience of 31 kidney transplants in a multicenter cohort study from India. Transplantation. 2021;105:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. 2020;296:E145–E155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floriano I, Silvinato A, Bernardo WM, et al. Accuracy of the polymerase chain reaction (PCR) test in the diagnosis of acute respiratory syndrome due to coronavirus: a systematic review and meta-analysis. Rev Assoc Med Bras (1992). 2020;66:880–888. [DOI] [PubMed] [Google Scholar]
- 21.Jarrom D, Elston L, Washington J, et al. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evid Based Med. [Epub ahead of print. October 1, 2020]. doi: 10.1136/bmjebm-2020-111511. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi A, Esmaeilzadeh E, Li Y, et al. SARS-CoV-2 detection in different respiratory sites: a systematic review and meta-analysis. EBioMedicine. 2020;59:102903. [DOI] [PMC free article] [PubMed]
- 23.Boffini M, Pidello S, Simonato E, et al. An effective protocol for heart transplantation during COVID-19 outbreak. Transpl Int. 2020;33:1326–1328. [DOI] [PubMed] [Google Scholar]
- 24.Boyarsky BJ, Massie AB, Love AD, et al. Early experiences with COVID-19 testing in transplantation. Transplant Direct. 2020;6:e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandorkar A, Coro A, Natori Y, et al. Kidney transplantation during coronavirus 2019 pandemic at a large hospital in Miami. Transpl Infect Dis. 2020;22:e13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galvan NTN, Moreno NF, Garza JE, et al. Donor and transplant candidate selection for solid organ transplantation during the COVID-19 pandemic. Am J Transplant. 2020;20:3113–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman JA, Mays JA, Wells C, et al. Expedited SARS-CoV-2 screening of donors and recipients supports continued solid organ transplantation. Am J Transplant. 2020;20:3106–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller X, Tilmans G, Chenevas-Paule Q, et al. Strategies for liver transplantation during the SARS-CoV-2 outbreak: preliminary experience from a single center in France. Am J Transplant. 2020;20:2989–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Yang H, Liu H, et al. Strategies to halt 2019 novel coronavirus (SARS-CoV-2) spread for organ transplantation programs at the Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital, China. Am J Transplant. 2020;20:1837–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domínguez-Gil B, Coll E, Fernández-Ruiz M, et al. COVID-19 in Spain: transplantation in the midst of the pandemic. Am J Transplant. 2020;20:2593–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauterio A, De Carlis R, Belli L, et al. How to guarantee liver transplantation in the north of Italy during the COVID-19 pandemic: a sound transplant protection strategy. Transpl Int. 2020;33:969–970. [DOI] [PubMed] [Google Scholar]
- 32.Cannavò A, Passamonti SM, Martinuzzi D, et al. The impact of COVID-19 on solid organ donation: the North Italy transplant program experience. Transplant Proc. 2020;52:2578–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akdur A, Karakaya E, Ayvazoglu Soy EH, et al. Liver and kidney transplant during a 6-month period in the COVID-19 pandemic: a single-center experience. Exp Clin Transplant. 2020;18:564–571. [DOI] [PubMed] [Google Scholar]
- 34.Bo W, Man H, Guohui J, et al. Lung transplantation during the outbreak of coronavirus disease 2019 in China. J Thorac Cardiovasc Surg. [Epub ahead of print. December 1, 2020]. doi: 10.1016/j.jtcvs.2020.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georgiades F, Summers DM, Butler AJ, et al. Renal transplantation during the SARS-CoV-2 pandemic in the UK: experience from a large-volume center. Clin Transplant. 2021;35:e14150. [DOI] [PubMed] [Google Scholar]
- 36.Halpern SE, Olaso DG, Krischak MK, et al. Lung transplantation during the COVID-19 pandemic: safely navigating the new “normal.” Am J Transplant. 2020;20:3094–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siniscalchi A, Vitale G, Morelli MC, et al. Liver transplantation in Italy in the era of COVID 19: reorganizing critical care of recipients. Intern Emerg Med. 2020;15:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salameh JP, Leeflang MM, Hooft L, et al. ; Cochrane COVID-19 Diagnostic Test Accuracy Group. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst Rev. 2020;9:CD013639. [DOI] [PubMed] [Google Scholar]
- 39.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dube GK, Husain SA, McCune KR, et al. COVID-19 in pancreas transplant recipients. Transpl Infect Dis. 2020;22:e13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fung M, Chiu CY, DeVoe C, et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: a case series from the United States. Am J Transplant. 2020;20:3225–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller BC, Le A, Sobhanie M, et al. Early COVID-19 infection after lung transplantation. Am J Transplant. 2020;20:2923–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myers CN, Scott JH, Criner GJ, et al. ; Temple University COVID-19 Research Group. COVID-19 in lung transplant recipients. Transpl Infect Dis. 2020;22:e13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patrono D, Lupo F, Canta F, et al. Outcome of COVID-19 in liver transplant recipients: a preliminary report from Northwestern Italy. Transpl Infect Dis. 2020;22:e13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shingare A, Bahadur MM, Raina S. COVID-19 in recent kidney transplant recipients. Am J Transplant. 2020;20:3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vilaro J, Al-Ani M, Manjarres DG, et al. Severe COVID-19 after recent heart transplantation complicated by allograft dysfunction. JACC Case Rep. 2020;2:1347–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20:1916–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bae S, McAdams-DeMarco MA, Massie AB, et al. Early changes in kidney transplant immunosuppression regimens during the COVID-19 pandemic. Transplantation. 2021;105:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrishami A, Samavat S, Behnam B, et al. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. 2020;78:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lubetzky M, Aull MJ, Craig-Schapiro R, et al. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35:1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angeletti A, Trivelli A, Magnasco A, et al. Risk of COVID-19 in young kidney transplant recipients. Results from a single-center observational study. Clin Transplant. 2020;34:e13889. [DOI] [PubMed] [Google Scholar]
- 53.Cavagna L, Seminari E, Zanframundo G, et al. Calcineurin inhibitor-based immunosuppression and COVID-19: results from a multidisciplinary cohort of patients in North Italy. Microorganisms. 2020;8:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crespo M, Pérez-Sáez MJ, Redondo-Pachón D, et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20:2883–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devresse A, Belkhir L, Vo B, et al. COVID-19 infection in kidney transplant recipients: a single-center case series of 22 cases from Belgium. Kidney Med. 2020;2:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15:1174–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mella A, Mingozzi S, Gallo E, et al. Case series of six kidney transplanted patients with COVID-19 pneumonia treated with tocilizumab. Transpl Infect Dis. 2020;22:e13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montagud-Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single center cohort of kidney recipients. Am J Transplant. 2020;20:2958–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair V, Jandovitz N, Hirsch JS, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Cubillo B, de la Higuera MAM, Lucena R, et al. Should cyclosporine be useful in renal transplant recipients affected by SARS-CoV-2? Am J Transplant. 2020;20:3173–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trujillo H, Caravaca-Fontán F, Sevillano Á, et al. SARS-CoV-2 infection in hospitalized patients with kidney disease. Kidney Int Rep. 2020;5:905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee BT, Perumalswami PV, Im GY, et al. ; COBE Study Group. COVID-19 in liver transplant recipients: an initial experience from the US epicenter. Gastroenterology. 2020;159:1176–1178. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verma A, Khorsandi SE, Dolcet A, et al. Low prevalence and disease severity of COVID-19 in post-liver transplant recipients-a single centre experience. Liver Int. 2020;40:1972–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ketcham SW, Adie SK, Malliett A, et al. Coronavirus disease-2019 in heart transplant recipients in Southeastern Michigan: a case series. J Card Fail. 2020;26:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020;5:1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee H, Mantell BS, Richmond ME, et al. Varying presentations of COVID-19 in young heart transplant recipients: a case series. Pediatr Transplant. 2020;24:e13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morlacchi LC, Rossetti V, Gigli L, et al. COVID-19 in lung transplant recipients: a case series from Milan, Italy. Transpl Infect Dis. 2020;22:e13356. [DOI] [PubMed] [Google Scholar]
- 73.Tschopp J, L’Huillier AG, Mombelli M, et al. ; Swiss Transplant Cohort Study (STCS). First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20:2876–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yi SG, Rogers AW, Saharia A, et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020;104:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arslan H, Musabak U, Ayvazoglu Soy EH, et al. Incidence and immunologic analysis of coronavirus disease (COVID-19) in hemodialysis patients: a single-center experience. Exp Clin Transplant. 2020;18:275–283. [DOI] [PubMed] [Google Scholar]
- 77.Pascual J, Melilli E, Jiménez-Martín C, et al. ; Spanish Society of Nephrology COVID-19 Group. COVID-19-related mortality during the first 60 days after kidney transplantation. Eur Urol. 2020;78:641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravanan R, Callaghan CJ, Mumford L, et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20:3008–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Favà A, Montero N, Cucchiari D, et al. SARS-CoV-2 in kidney transplant recipients: a multicentric prospective cohort study. Am J Transplant. 2020;20:3030–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belli LS, Duvoux C, Karam V, et al. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry . Lancet Gastroenterol Hepatol. 2020;5:724–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iavarone M, D’Ambrosio R, Soria A, et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Massoumi H, Rocca J, Frager S, et al. COVID-19 infection in early post-operative period after liver transplantation. Liver Transpl. [Epub ahead of print. June 5, 2020]. doi: 10.1002/lt.25811. [Google Scholar]
- 84.Melgosa M, Madrid A, Alvarez O, et al. SARS-CoV-2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol. 2020:1–4;35: 1521–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clarke C, Lucisano G, Prendecki M, et al. ; ICHNT Renal COVID Group. Informing the risk of kidney transplantation versus remaining on the waitlist in the coronavirus disease 2019 era. Kidney Int Rep. 2021;6:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Meester J, De Bacquer D, Naesens M, et al. ; NBVN Kidney Registry Group. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J Am Soc Nephrol. 2021;32:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ozturk S, Turgutalp K, Arici M, et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35:2083–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thaunat O, Legeai C, Anglicheau D, et al. ; French Nationwide Registry of Solid Organ Transplant Recipients with COVID-19. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int. 2020;98:1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alberici F, Delbarba E, Manenti C, et al. ; Brescia Renal COVID Task Force. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Craig-Schapiro R, Salinas T, Lubetzky M, et al. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant. 2021;21:1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones ESW, Davidson BJ, Barday Z, et al. COVID-19 and the kidney: a South African state healthcare experience. Clin Nephrol. 2021;95:171–181. [DOI] [PubMed] [Google Scholar]
- 94.Khalid UIMA, Nagaraja PEDAA. SARS-CoV-2 in kidney transplant and wait-listed patients during the first peak: the Welsh experience. Transplant Proc. 2021;53:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mohamed IH, Chowdary PB, Shetty S, et al. Outcomes of renal transplant recipients with SARS-CoV-2 infection in the eye of the storm: a comparative study with waitlisted patients. Transplantation. 2021;105:115–120. [DOI] [PubMed] [Google Scholar]
- 96.Varnell CD, Al-Akash SI, Belsha CW, et al. Incidence of COVID-19 disease in pediatric kidney transplant recipients: a report of the improving renal outcomes collaborative. Am J Transplant. 2020;31:277. [Google Scholar]
- 97.Goss MB, Galván NTN, Ruan W, et al. The pediatric solid organ transplant experience with COVID-19: an initial multi-center, multi-organ case series. Pediatr Transplant. 2021;25:e13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teoh CW, Gaudreault-Tremblay MM, Blydt-Hansen TD, et al. Management of pediatric kidney transplant patients during the COVID-19 pandemic: guidance from the Canadian Society of Transplantation Pediatric Group. Can J Kidney Health Dis. 2020;7:2054358120967845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.International Liaison Committee on Resuscitation. Available at https://www.ilcor.org. Accessed April 9, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.